Magnetic resonance imaging in pulmonary hypertension: an overview of current applications and future perspectives
DOI: https://doi.org/10.4414/SMW.2022.w30055
Benoit
Lechartierabc, Ari
Chaouatd, John-David
Aubertbc, Juerg
Schwittercef, on behalf of the Swiss Society for Pulmonary Hypertension (SSPH)
aAP-HP, Department of Respiratory and Intensive Care Medicine, Hôpital Bicêtre, Le Kremlin-Bicêtre, France
bPulmonary Division, Lausanne University Hospital, Lausanne, Switzerland
cFaculty of Biology and Medicine, University of Lausanne, UniL, Lausanne, Switzerland
dCHRU de Nancy, Pôle des spécialités médicales/département de pneumologie, Vandoeuvre-les-Nancy, France
eDivision of Cardiology, Cardiovascular Department, University Hospital Lausanne, CHUV, Lausanne, Switzerland
fCardiac MR Centre of the CHUV, Lausanne University Hospital, Lausanne, Switzerland
Summary
Pulmonary hypertension is an heterogeneous group of diseases characterised by increased pulmonary arterial pressures which impact on the upstream right ventricle. Pulmonary hypertension can be challenging to diagnose, classify and monitor when specific therapies are applicable. Cardiac magnetic resonance (CMR) imaging has greatly evolved in the last decades and is a promising tool to non-invasively follow pulmonary hypertension patients. CMR provides a comprehensive evaluation of the heart and is therefore the gold standard for quantification of right ventricular volumes, mass and function, which are critical for pulmonary hypertension prognosis. In addition, innovative MR techniques allow an increasingly precise evaluation of pulmonary haemodynamics and lung perfusion. This review highlights the main advantages offered by CMR in pulmonary hypertension and gives an overview of putative future applications. Although right heart catheterisation remains mandatory in the diagnostic algorithm, CMR could play an increasingly important role in the coming years in monitoring pulmonary hypertension patients.
Introduction
Pulmonary hypertension is a pathophysiological manifestation of a heterogeneous group of diseases characterised by abnormally elevated pulmonary arterial pressures [1]. Chronic heart and lung diseases are the most common aetiologies of pulmonary hypertension in Western countries. Since these are increasing in an ageing population, recent studies indicated that the prevalence of pulmonary hypertension greatly increased in the last decades [2–4]. The clinical manifestations of pulmonary hypertension, which encompass exercise dyspnoea, lower limb oedema and even syncope in severe cases, are generally related to right ventricular failure and reduced cardiac output due to right ventricle (RV)/ left ventricle (LV) interdependence [5], as well as RV / pulmonary artery uncoupling [6]. Early diagnosis of RV failure is critical, and therefore cardiac magnetic resonance imaging (CMR) could play a pivotal role in the diagnosis and management of pulmonary hypertension.
The purpose of the review is to discuss current knowledge about CMR in the diagnosis and follow-up assessment of patients with pulmonary hypertension, as well as to compare the benefits of CMR with right heart catheterisation (RHC) for haemodynamic evaluation. Although right heart catheterisation will remain mandatory in the diagnostic algorithm, CMR could play an increasingly important role in monitoring pulmonary hypertension patients in the coming years.
Pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is a severe, incurable, cardiovascular disorder characterised by a diffuse remodelling of the pulmonary arteries causing precapillary pulmonary hypertension with increased pulmonary vascular resistance (PVR), in the absence of other causes of precapillary pulmonary hypertension such as chronic lung diseases, pulmonary artery obstructions or other rarer diseases [7]. The recently updated haemodynamic definition of PAH is a mean pulmonary arterial pressure (mPAP) >20 mm Hg together with PVR ≥3 Wood units in the presence of a normal left heart filling pressure (≤15 mm Hg) [7].
The mPAP is defined by the formula: mPAP = PVR x cardiac output + left atrial pressure. This formula reveals the main pathophysiological mechanisms underlying pulmonary hypertension: (a) elevation of the PVR due to various levels of vasculopathy; (b) increased cardiac output due to hyperdynamic states; (c) elevated pressures in the left heart causing post-capillary pulmonary hypertension.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) has greatly evolved in the past decades and thus emerges as an important imaging technique for pulmonary vascular diseases and pulmonary hypertension [8–10]. Regular evaluation of RV structure and function allows assessment of the response to treatment, which was shown to reflect PAH patient prognosis [11, 12]. CMR is the gold standard for accurate and reproducible quantification of RV volumes, mass and function, using high spatiotemporal resolution imaging sequences [13–15]. CMR thus provides critical prognostic information in PAH patients at baseline, as well as during follow-up [8].
Pulmonary arterial hypertension and the right ventricle
Although pulmonary arterial hypertension is a disease of the lung vasculature itself, irreversible pulmonary vascular remodelling leads to upstream right heart failure with a poor outcome once the diagnosis is set [16]. Vasoconstriction together with vascular remodelling contribute to a progressive increase in PVR and PAP, causing increased RV afterload, which has deleterious effects on the RV, such as distension and systolic or diastolic dysfunction. It is well known that the pathophysiology of the RV allows this cavity to cope with increased volume, whereas it is less well adapted to an elevated downstream resistance [16]. Changes due to pressure overload are detrimental as increased RV wall stress leads to ventricular dilation and hypertrophy, finally with RV maladaptation as a consequence of increased PVR (fig. 1). Monitoring RV size and function with a reliable method is thus crucial in the assessment of pulmonary hypertension.
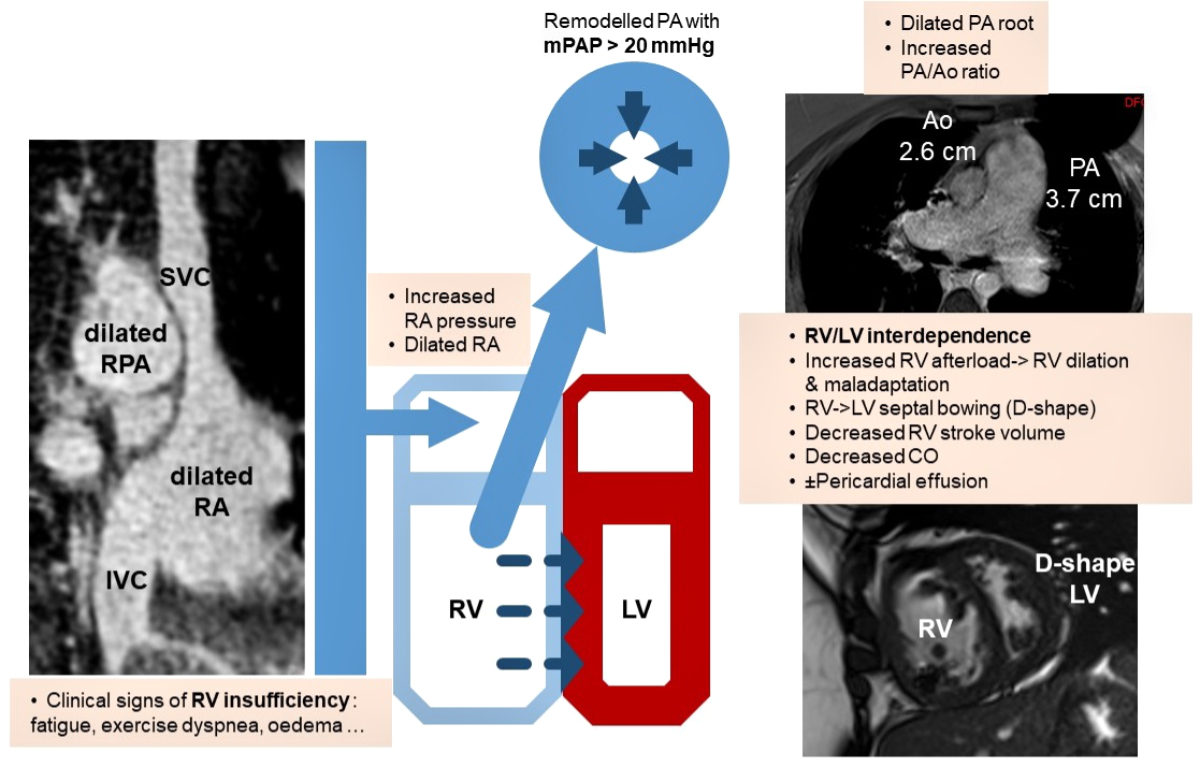
Figure 1 Schematic pathophysiological interactions in precapillary pulmonary hypertension and consecutive clinical and MRI manifestations. RV: right ventricle; LV: left ventricle; RA: right atrium; SVC: superior vena cava; IVC: inferior vena cava; PA: pulmonary artery; RPA: right pulmonary artery; mPAP: mean PA pressure; Ao: aorta; CO: cardiac output.
Diagnostic approach for pulmonary hypertension
Patients with suspected pulmonary hypertension typically undergo several noninvasive and invasive examinations, including right heart catheterisation, before a final diagnosis can be reached [17]. Several diseases, such as systemic sclerosis, can encompass simultaneously precapillary and postcapillary causes of pulmonary hypertension, thereby complicating the PAH diagnostic workflow [17, 18].
Doppler echocardiography is safe, widely available and therefore represents an inevitable screening tool to raise suspicion of pulmonary hypertension. Although transthoracic echocardiography (TTE) is an invaluable test for pulmonary hypertension evaluation, it has some limitations. Precise assessment of the right chambers is not always possible as the ultrasound window may be compromised, particularly in patients with lung diseases. Quantitative TTE measurements are operator-dependent and are limited as different thresholds for RV parameters exist. A thorough review of the pivotal role of TTE in pulmonary hypertension was recently published in this journal [19].
When pulmonary hypertension is suspected, right heart catheterisation is the gold standard examination to confirm the diagnosis and is thus mandatory in the diagnostic algorithm to distinguish between precapillary and postcapillary forms of pulmonary hypertension. However, right heart catheterisation does not allow a comprehensive evaluation of the heart chambers or RV-LV interdependence [20]. Right heart catheterisation should only be performed in expert centres as serious complications may happen [21, 22]. Even in expert centres, invasive haemodynamic measurements can be burdensome for patients. In the multicentre RePHerral study, many incident pulmonary hypertension patients referred to an expert centre did not undergo a right heart catheterisation during the diagnostic procedure, which led in a high percentage of patients to an erroneous diagnosis [23]. Although right heart catheterisation is the central examination for pulmonary hypertension evaluation and will most certainly remain mandatory in the near future, noninvasive diagnostic techniques such as CMR reinforce the evaluation procedure and are likely to play an increasing role in patient monitoring.
Computed tomography pulmonary angiography (CTPA) is a common imaging modality for pulmonary hypertension assessment. It allows precise evaluation of pulmonary structures together with indirect cardiac assessment [24]. CTPA can highlight indirect signs of RV dysfunction and elevated pulmonary pressures, such as ventricular hypertrophy or dilation, abnormal interventricular septum curvature and pericardial effusion [24]. CTPA can point towards several pulmonary hypertension aetiologies, for example when advanced interstitial lung disease is present (putative pulmonary hypertension due to lung diseases) or oesophageal abnormalities, which can be seen in systemic sclerosis (possible PAH associated with connective tissue disease) [24]. CTPA can display chronic thrombi in the pulmonary arteries, suggesting the diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH). However, a negative CTPA does not completely rule out the diagnosis of CTEPH, since perfusion scintigraphy generally has a higher sensitivity [25]. CTPA has considerable drawbacks compared with MRI or TTE, as it does not allow haemodynamic assessment unless radiation intensity is increased. In addition, repetitive CTPA, for example for treatment monitoring, causes significant radiation exposure. The CTPA application can be limited by the risk of nephrotoxicity and the risk of iodinated contrast media allergy.
CMR provides three-dimensional, high resolution images of the four chambers of the heart (fig. 2). Hence, it allows a precise evaluation of the right heart structure and function, which are only indirectly assessed with right heart catheterisation. CMR quantifies blood flow with high precision yielding RV stroke volume, cardiac output, or pulmonary arterial distensibility [1, 8–10] (fig. 3). Several CMR signs, such as the presence of tissue fibrosis assessed by late gadolinium enhancement or decreased pulmonary arterial distensibility with retrograde pulmonary flow, have a high value for pulmonary hypertension identification and prognosis [24, 26]. Evaluation of the interventricular septum by CMR can distinguish with moderate sensitivity and excellent specificity between pre- or postcapillary forms from isolated postcapillary pulmonary hypertension as an increased angle of ≥160° is associated with mixed pulmonary hypertension forms with poorer prognosis [27]. CMR provides excellent anatomical and functional information due to improved spatial and temporal resolutions, without ionising radiation or the need to administer nephrotoxic contrast media. A summarised, but not exhaustive, comparison between TTE, CTPA and MRI is provided in table 1.
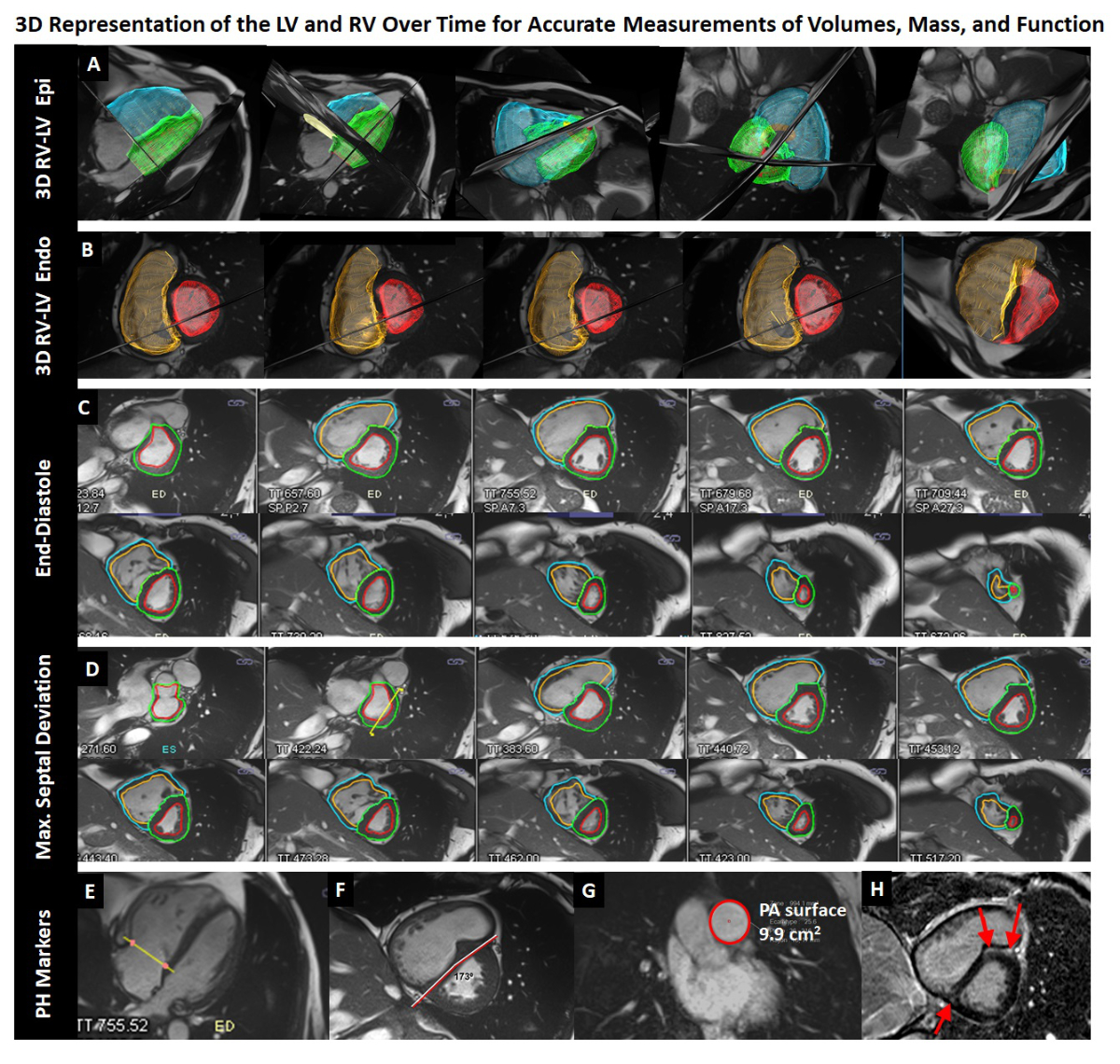
Figure 2 Example of a 45-year old man with idiopathic PAH suffering from dyspnoea on exertion.
The cardiac MR (CMR) examination consists of cine short-axis (C, D) and long-axis acquisitions (E) covering the entire right and left ventricles (RV, LV). Post-processing allows reconstruction of three-dimensional representations of the RV and LV (A, B) for accurate quantification of end-diastolic and end-systolic volumes, stroke volumes, and RV and LV myocardial mass. In this patient, the RV is dilated and hypertrophied (239 ml, 126 ml/m2 at end-diastole, 83 g, 44 g/m2) and the ejection fraction is severely reduced to 14%. A typical D-shape deformation of the septum is observed at end-systole / early diastole. The interventricular septal angle was 173⁰, the RV/LV mass ratio was 83 g/159 g, and diastolic pulmonary artery area was 9.9 cm2. According to the model developed by Johns et al. [38] these measurements translate into an approximate mean PA pressure of 44 mm Hg; a right heart catheterisation performed 16 months later yielded 42 mm Hg. These data indicate that the RV converted from a volume to a pressure pump. Accordingly, the ventricular-arterial coupling (the ratio of RV maximum end-systolic elastance over PA effective elastance) is unfavourable in this patient and estimated by the CMR examination to be 6.2 according to Sanz et al. [33]; normal values with optimal coupling are close to 1.0.
A: blue and green 3D-meshes represent RV and LV surfaces at end-diastole, respectively, B: orange and red 3D-meshes represent RV and LV endocardial surfaces at maximum septal deviation, respectively. Corresponding contours on short-axis cine images (out of a series of 25 frames per heart beat) are given in C and D, respectively. F: short-axis cine image at maximum septal deviation, G: Diastolic area of the pulmonary artery, H: short-axis late-gadolinium enhancement image demonstrating intramyocardial fibrosis (bright areas) in the anterior and posterior insertion point of the RV at the septum.
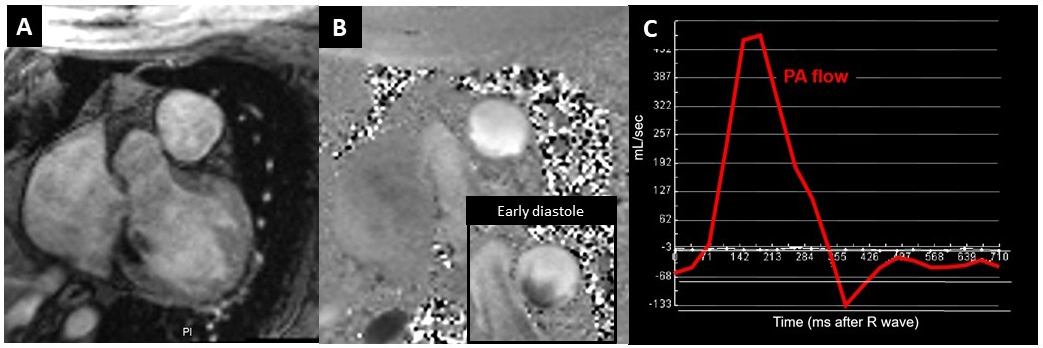
Figure 3 Flow measurement in the pulmonary artery (PA). A: Magnitude image, B: Phase contrast image at peak systole. Each voxel is velocity-encoded (with grey levels representing velocities). The inlet in B shows a flow map in early diastole with forward flow along the outer curvature of the PA (white pixels), while flow is retrograde at the inner curvature (dark pixels) compatible with vortex flow in the PA at this cardiac phase. C: Multiplying velocities of all corresponding voxels (spatial resolution: 1.9 x 1.9 mm2) within the vessel surface with temporal resolution (40 ms) yields volume flow.
Table 1Comparison of selected noninvasive imaging modalities for pulmonary hypertension, adapted from [24, 26].
|
Parameters
|
Echocardiography
|
CTPA
|
Magnetic resonance imaging
|
| pulmonary hypertension detection |
+++ |
+ |
+ |
| Lung evaluation |
|
– |
|
|
| Parenchyma |
|
+++ |
+/– |
| Vasculature |
|
+++ |
++ |
| Assessment of pulmonary hypertension aetiology |
+ |
+++ |
++ |
| Evaluation of cardiac chambers and shunts |
++ |
+ |
+++ |
| Pulmonary pressure evaluation |
++ |
– |
+/– |
| Strengths |
No radiation, widely available, noninvasive |
Lung parenchyma and vessels evaluation |
No radiation, precise evaluation of cardiac structure/function and pulmonary flow quantification |
| Weaknesses |
Difficult assessment of the right chambers; only indirect signs of pulmonary hypertension; interobserver variability |
Limited haemodynamic assessment, radiation and iodine contrast administration |
Limited evaluation of the lungs |
Assessing RV function with CMR and PAH follow-up under specific therapy
Approved therapies for PAH have been developed to favour pulmonary vasodilatation and slow down the progression of pulmonary vascular remodelling, via one of three well-characterised pathways, namely the nitric oxide, the endothelin-1 and the prostacyclin (PGI2) signalling pathways [28]. The therapeutic strategy for PAH has greatly evolved in the last two decades and will likely benefit from novel molecules in the coming years [29].
Most treatment goals are targeted on maintaining normal or improving RV function rather than to normalise PA pressures per se. General measures such as intensive diuretic treatment palliates RV insufficiency in order to act as supportive therapy for PAH. Although the current treatment options have markedly improved overall quality of life, exercise capacity and long-term outcomes, the 5-year survival remains low (~60%) [30]. Lung transplantation is the last therapeutic option if previous therapy failed.
Regular evaluation of RV structure and function are fundamental for clinical monitoring of the therapeutic response as well as evaluation of patient prognosis [1]. Several key clinical parameters are obtained noninvasively, such as New York Heart Association (NYHA) functional class, 6-minute walk distance, and N-terminal pro-B-type natriuretic peptide (NT pro-BNP) levels [30]. Following the ERS/ESC risk assessment strategy, haemodynamic parameters assessed by right heart catheterisation, such right atrial pressure, cardiac index and mixed venous oxygen saturation (SvO2) should be measured if deterioration is suspected [1]. Imaging parameters such as an increased right atrial size as well as the presence of a pericardial effusion, either on TTE or CMR, are included in the ERS/ESC risk assessment. However, numerous important functional parameters related to the RV are not covered by current guidelines, but can be obtained noninvasively with CMR without the need for an invasive haemodynamic evaluation.
CMR is generally recognised as an accurate technique to measure RV volumes and its accuracy has further improved in recent years as a result of pulse sequences with increased temporo-spatial resolutions [10]. Short-axis images allow a three-dimensional reconstruction of the ventricular anatomy where postprocessing of the endocardial contours delineate end-systolic volume (ESV), end-diastolic volume (EDV), stroke volume and stroke volume index, referred to the body surface area) together with RV ejection fraction. The same 3-dimensional postprocessing measures ventricular wall masses accurately (see fig. 2).
Hence, CMR offers the advantage of precisely assessing RV volumes as well as noninvasively obtaining prognostic indices in pulmonary hypertension. Stroke volume or stroke volume index were demonstrated to be key haemodynamic parameters in pulmonary hypertension as they are directly correlated to RV function. A large RV with a low stroke volume, as well as a reduced LV volume, are strong independent predictors of treatment failure or mortality [11]. After 1 year of PAH-treatment (mainly an endothelin receptor antagonist), a significant increase in stroke volume was demonstrated with CMR [31]. A 10-ml change in stroke volume was correlated to a difference in 6-minute walk distance, which is widely used in pulmonary hypertension to assess clinical significance [31].
In the pan-European EURO-MR study, comprising 91 pulmonary hypertension patients, CMR-derived variables, at baseline versus after 12 months of disease-targeted therapy, reflected changes in functional class and predicted survival [32].
In a retrospective study comprising 139 adults referred for pulmonary hypertension evaluation, right ventriculo-arterial coupling was quantified as the ratio of pulmonary artery effective elastance (as an index of arterial load) to RV maximum end-systolic elastance (index of contractility). RVESV and stroke volume were measured by CMR; these measures were compared with standard right heart catheterisation parameters and the authors demonstrated that pulmonary artery elastance increases with pulmonary hypertension severity, whereas contractility failed to progress leading to right ventriculo-arterial uncoupling [33].
Lewis et al. recently evaluated 438 PAH patients from the ASPIRE MRI database to determine thresholds for PAH risk stratification [34]. A RV-ESV index threshold of 227% or a LV-EDV index of 58 ml/m2 identified patients at low (<5%) and high (>10%) risk of 1-year mortality, respectively. RV-ESV index independently predicted PAH outcome and improved risk stratification when added to the REVEAL 2.0 risk calculator or the French Pulmonary Hypertension Registry strategy [34].
In a recent meta-analysis encompassing 22 studies with nearly 2000 PAH patients, CMR was a robust predictor of clinical deterioration and mortality [35]. The pooled hazard ratios indicated that every 1% decrease in RV ejection fraction was associated with a 4.9% increase in the risk of clinical worsening as well as an increased risk of death, respectively, over 22 and 54 months of follow-up. With every increase in RV-ESV or -EDV index by 1 ml/m2, the risk of clinical worsening increased by 1.3% and 1%, respectively. With every 1 ml/m2 decrease in LV- stroke volume index, the mortality increased by 2.5% over 54 months.
CMR for vascular and haemodynamic evaluations and comparison with right heart catheterisation
RV anatomy/function and pulmonary artery anatomy for pulmonary hypertension assessment
Several CMR studies demonstrated that absolute or relative main pulmonary artery size can be directly correlated with PAP values measured by right heart catheterisation [36, 37]. In a recent retrospective study, Johns et al. developed regression models to predict pulmonary pressure based on CMR measurements in 600 patients with right heart catheterisation and CMR testing [38]. In one half of the patients a regression model was developed to estimate mPAP and its performance was then tested in the remaining patients (the validation cohort). This model, using interventricular septum angle, RV-LV mass ratio, and pulmonary artery anatomy, had a sensitivity of 93% with a specificity of 79% to detect pulmonary hypertension noninvasively [38]. This model was also adapted to detect mPAP at a lower threshold of 20 mm Hg, according to the 6th World Symposium on Pulmonary Hypertension definition [39].
Pulmonary artery haemodynamics for pulmonary hypertension assessment
Two-dimensional flow acquisitions are standard components of pulmonary hypertension studies by CMR. However, 3-dimensional flow acquisitions (also called 4D-flow acquisitions as they acquire 3D-volumes over time) have the potential to provide more detailed flow pattern analyses. For example, aberrant flow patterns in the main pulmonary artery, namely vortex formations, can be associated with pulmonary hypertension. These 2D and 3D flow data assign velocities to each pixel (or voxel), thus allowing accurate measurement of volume flow in non-homogeneous flow fields, such as in vessels with skewed flow profiles (fig. 3). This technique allows a precise analysis of vascular flows as well as transvalvular or intra-cavity flows, which can be difficult by ultrasound. Appearance of the vortex flows in the main pulmonary artery was linearly associated with mPAP measurements. Reiter et al. recently compared 4D-flow imaging with right heart catheterisation in 44 pulmonary hypertension patients. In their work, they converted pulmonary artery flow patterns into mPAP estimations [40]. In patients with mPAP >16 mm Hg, 4D-flow imaging accurately predicted mPAP changes [40]. In another elegant study by Ikoma et al., 4D-flow was found to have the potential to detect early haemodynamic change in systemic sclerosis, since wall shear stress and shear stress index were correlated with PAH in systemic sclerosis when compared with controls [41].
These novel 4D-flow sequences proved to be of value for noninvasive estimations of mPAP in smaller studies. Accordingly, larger studies are currently warranted to confirm these findings. Noninvasive determination of mPAP and PVR could also benefit from models that combine RV/LV and pulmonary artery anatomical findings with pulmonary haemodynamics and flow patterns assessed by 4D-flow acquisitions.
Interventional CMR for pulmonary hypertension assessment
With the advent of interventional CMR, the performance of CMR-guided right heart catheterisation is another innovative technique, which combines MRI with simultaneous pressure measurements yielding a comprehensive and highly precise assessment of RV and pulmonary haemodynamics [42, 43]. This radiation-free technique was successfully evaluated in both adults and children [44]. These experimental findings require further evaluation in larger studies to assess their application in clinical practice.
Promising future directions: the example of chronic thromboembolic pulmonary hypertension
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare but severe cause of pulmonary hypertension where incomplete resolution of a thrombus localised in the pulmonary arteries leads to precapillary pulmonary hypertension [25]. Pulmonary vascular evaluation with MRI emerges as a useful imaging technique in CTEPH. Contrast-enhanced MR pulmonary angiography is promising, especially when iodinated contrast media are contraindicated. Several innovative MRI techniques are emerging, which incorporate pulse sequences for lung perfusion evaluation. As only few multicentre validation studies are available so far, these techniques are not yet ready to replace ventilation/perfusion scintigraphy to confirm or rule out CTEPH [24, 25].
Three-dimensional dynamic contrast-enhanced lung perfusion provided a dynamic analysis of regional pulmonary perfusion by tracking the passage of a contrast bolus into the pulmonary arteries. It can assess parenchymal hypoperfusion as seen in CTEPH, together with quantification of regional parenchymal hypoperfusion, before and after pulmonary endarterectomy, the curative treatment option in operable CTEPH, where proximal marginal thrombi are surgically removed [45].
Meanwhile, several papers elegantly demonstrated the benefit of 4D-MRI in CTEPH. Ota et al. reported in 2015 the first demonstration of normalisation of the main pulmonary artery flow patterns (vortex flow) after pulmonary angioplasty [46]. Enhanced pulmonary blood flow was demonstrated via MRI two weeks after pulmonary endarterectomy in CTEPH patients with a significant correlation between the 6-minute walk test and perfusion improvement [45]. The same authors demonstrated perfusion improvement after balloon pulmonary angioplasty, which may allow a longitudinal noninvasive imaging follow-up in distal forms of CTEPH [47].
A recent post-processing technique, called phase-resolved functional lung MRI, includes the reconstruction of the pulmonary arterial pulse wave during the cardiac cycle, thereby deriving dynamic information about ventilation and perfusion with increased temporal resolution [48]. This technique emerges as an innovative noninvasive tool for quantification of regional perfusion, not only for CTEPH but also in patients with chronic lung diseases [48]. The same procedure was recently demonstrated to correlate with outcome after pulmonary endarterectomy as well as mPAP evaluation, which is of interest for longitudinal follow-up of CTEPH patients after surgery [49].
Conclusion
MRI enables a comprehensive evaluation of both sides of the heart together with the assessment of pulmonary artery dynamics, which is critical for pulmonary hypertension diagnostic workup as well as for disease monitoring. CMR offers noninvasive prognostic values in PAH, where serial right heart catheterisations are burdensome. Innovative MRI techniques also provide increasingly precise evaluation of the lung vasculature; therefore, they will have a growing importance for pulmonary vascular disease evaluation in the coming years. Indirect PAP evaluation and monitoring via CMR paves the way for noninvasive, radiation-free patient follow-up, although right heart catheterisation remains mandatory for pulmonary hypertension diagnosis. The relatively limited availability of CMR should positively evolve in parallel with the numerous innovations observed in the last years.
Prof. Juerg Schwitter
Service de Cardiologie
Centre Hospitalier Universitaire Vaudois
Rue du Bugnon 46
CH-1011 Lausanne
jurg.schwitter[at]chuv.ch
References
1.
Galiè N
,
Humbert M
,
Vachiery JL
,
Gibbs S
,
Lang I
,
Torbicki A
, et al.
2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015 Oct;46(4):903–75. https://doi.org/10.1183/13993003.01032-2015
2.
Wijeratne DT
,
Lajkosz K
,
Brogly SB
,
Lougheed MD
,
Jiang L
,
Housin A
, et al.
Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018 Feb;11(2):e003973. https://doi.org/10.1161/CIRCOUTCOMES.117.003973
3.
Nathan SD
,
Barbera JA
,
Gaine SP
,
Harari S
,
Martinez FJ
,
Olschewski H
, et al.
Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019 Jan;53(1):1801914. https://doi.org/10.1183/13993003.01914-2018
4.
Vachiéry JL
,
Tedford RJ
,
Rosenkranz S
,
Palazzini M
,
Lang I
,
Guazzi M
, et al.
Pulmonary hypertension due to left heart disease. Eur Respir J. 2019 Jan;53(1):1801897. https://doi.org/10.1183/13993003.01897-2018
5.
Naeije R
,
Badagliacca R
. The overloaded right heart and ventricular interdependence. Cardiovasc Res. 2017 Oct;113(12):1474–85. https://doi.org/10.1093/cvr/cvx160
6.
Fourie PR
,
Coetzee AR
,
Bolliger CT
. Pulmonary artery compliance: its role in right ventricular-arterial coupling. Cardiovasc Res. 1992 Sep;26(9):839–44. https://doi.org/10.1093/cvr/26.9.839
7.
Simonneau G
,
Montani D
,
Celermajer DS
,
Denton CP
,
Gatzoulis MA
,
Krowka M
, et al.
Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019 Jan;53(1):1801913. https://doi.org/10.1183/13993003.01913-2018
8.
Aryal SR
,
Sharifov OF
,
Lloyd SG
. Emerging role of cardiovascular magnetic resonance imaging in the management of pulmonary hypertension. Eur Respir Rev. 2020 Jul;29(156):190138. https://doi.org/10.1183/16000617.0138-2019
9.
Alabed S
,
Garg P
,
Johns CS
,
Alandejani F
,
Shahin Y
,
Dwivedi K
, et al.
Cardiac Magnetic Resonance in Pulmonary Hypertension-an Update. Curr Cardiovasc Imaging Rep. 2020;13(12):30. https://doi.org/10.1007/s12410-020-09550-2
10.
Wessels JN
,
de Man FS
,
Vonk Noordegraaf A
. The use of magnetic resonance imaging in pulmonary hypertension: why are we still waiting? Eur Respir Rev. 2020 Jul;29(156):200139. https://doi.org/10.1183/16000617.0139-2020
11.
van Wolferen SA
,
Marcus JT
,
Boonstra A
,
Marques KM
,
Bronzwaer JG
,
Spreeuwenberg MD
, et al.
Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007 May;28(10):1250–7. https://doi.org/10.1093/eurheartj/ehl477
12.
van de Veerdonk MC
,
Kind T
,
Marcus JT
,
Mauritz GJ
,
Heymans MW
,
Bogaard HJ
, et al.
Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011 Dec;58(24):2511–9. https://doi.org/10.1016/j.jacc.2011.06.068
13.
Swift AJ
,
Wild JM
,
Nagle SK
,
Roldán-Alzate A
,
François CJ
,
Fain S
, et al.
Quantitative magnetic resonance imaging of pulmonary hypertension: a practical approach to the current state of the art. J Thorac Imaging. 2014 Mar;29(2):68–79. https://doi.org/10.1097/RTI.0000000000000079
14.
Kiely DG
,
Levin D
,
Hassoun P
,
Ivy DD
,
Jone PN
,
Bwika J
, et al.
EXPRESS: Statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ. 2019 Mar;9(3):2045894019841990. https://doi.org/10.1177/2045894019841990
15.
Schwitter J
,
Nagel E
. Volumes, Function, and Deformation: Left and Right Ventricles and Atria. In: CMR-Update, 3rd edition [Internet]. Lausanne, Switzerland; 2020. Available from: www.herz-mri.ch
16.
Vonk Noordegraaf A
,
Chin KM
,
Haddad F
,
Hassoun PM
,
Hemnes AR
,
Hopkins SR
, et al.
Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019 Jan;53(1):1801900. https://doi.org/10.1183/13993003.01900-2018
17.
Frost A
,
Badesch D
,
Gibbs JS
,
Gopalan D
,
Khanna D
,
Manes A
, et al.
Diagnosis of pulmonary hypertension. Eur Respir J. 2019 Jan;53(1):1801904. https://doi.org/10.1183/13993003.01904-2018
18.
Lechartier B
,
Humbert M
. Pulmonary arterial hypertension in systemic sclerosis. Presse Med. 2021 Feb;50(1):104062. https://doi.org/10.1016/j.lpm.2021.104062
19.
Brugger N
,
Lichtblau M
,
Maeder M
,
Muller H
,
Pellaton C
,
Yerly P
; Swiss Society For Pulmonary Hypertension SSPH
. Two-dimensional transthoracic echocardiography at rest for the diagnosis, screening and management of pulmonary hypertension. Swiss Med Wkly. 2021 Jun;151(2122):w20486
https://smw.ch/article/doi/smw.2021.20486
20.
Ibrahim SH
,
White RD
. Cardiovascular magnetic resonance for the assessment of pulmonary arterial hypertension: toward a comprehensive CMR exam. Magn Reson Imaging. 2012 Oct;30(8):1047–58. https://doi.org/10.1016/j.mri.2012.03.001
21.
Hoeper MM
,
Lee SH
,
Voswinckel R
,
Palazzini M
,
Jais X
,
Marinelli A
, et al.
Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006 Dec;48(12):2546–52. https://doi.org/10.1016/j.jacc.2006.07.061
22.
Ginoux M
,
Cottin V
,
Glérant JC
,
Traclet J
,
Philit F
,
Sénéchal A
, et al.
Safety of right heart catheterization for pulmonary hypertension in very elderly patients. Pulm Circ. 2018 Oct-Dec;8(4):2045894018799272. https://doi.org/10.1177/2045894018799272
23.
Deaño RC
,
Glassner-Kolmin C
,
Rubenfire M
,
Frost A
,
Visovatti S
,
McLaughlin VV
, et al.
Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med. 2013 May;173(10):887–93. https://doi.org/10.1001/jamainternmed.2013.319
24.
Remy-Jardin M
,
Ryerson CJ
,
Schiebler ML
,
Leung AN
,
Wild JM
,
Hoeper MM
, et al.
Imaging of Pulmonary Hypertension in Adults: A Position Paper from the Fleischner Society. Radiology. 2021 Mar;298(3):531–49. https://doi.org/10.1148/radiol.2020203108
25.
Delcroix M
,
Torbicki A
,
Gopalan D
,
Sitbon O
,
Klok FA
,
Lang I
, et al.
ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021 Jun;57(6):2002828. https://doi.org/10.1183/13993003.02828-2020
26.
Ascha M
,
Renapurkar RD
,
Tonelli AR
. A review of imaging modalities in pulmonary hypertension. Ann Thorac Med. 2017 Apr-Jun;12(2):61–73. https://doi.org/10.4103/1817-1737.203742
27.
Johns CS
,
Wild JM
,
Rajaram S
,
Tubman E
,
Capener D
,
Elliot C
, et al.
Identifying At-Risk Patients with Combined Pre- and Postcapillary Pulmonary Hypertension Using Interventricular Septal Angle at Cardiac MRI. Radiology. 2018 Oct;289(1):61–8. https://doi.org/10.1148/radiol.2018180120
28.
Humbert M
,
Lau EM
,
Montani D
,
Jaïs X
,
Sitbon O
,
Simonneau G
. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014 Dec;130(24):2189–208. https://doi.org/10.1161/CIRCULATIONAHA.114.006974
29.
Sitbon O
,
Gomberg-Maitland M
,
Granton J
,
Lewis MI
,
Mathai SC
,
Rainisio M
, et al.
Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019 Jan;53(1):1801908. https://doi.org/10.1183/13993003.01908-2018
30.
Boucly A
,
Weatherald J
,
Savale L
,
Jaïs X
,
Cottin V
,
Prevot G
, et al.
Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017 Aug;50(2):1700889. https://doi.org/10.1183/13993003.00889-2017
31.
van Wolferen SA
,
van de Veerdonk MC
,
Mauritz GJ
,
Jacobs W
,
Marcus JT
,
Marques KM
, et al.
Clinically significant change in stroke volume in pulmonary hypertension. Chest. 2011 May;139(5):1003–9. https://doi.org/10.1378/chest.10-1066
32.
Peacock AJ
,
Crawley S
,
McLure L
,
Blyth KG
,
Vizza CD
,
Poscia R
, et al.
Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circ Cardiovasc Imaging. 2014 Jan;7(1):107–14. https://doi.org/10.1161/CIRCIMAGING.113.000629
33.
Sanz J
,
García-Alvarez A
,
Fernández-Friera L
,
Nair A
,
Mirelis JG
,
Sawit ST
, et al.
Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart. 2012 Feb;98(3):238–43. https://doi.org/10.1136/heartjnl-2011-300462
34.
Lewis RA
,
Johns CS
,
Cogliano M
,
Capener D
,
Tubman E
,
Elliot CA
, et al.
Identification of Cardiac Magnetic Resonance Imaging Thresholds for Risk Stratification in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2020 Feb;201(4):458–68. https://doi.org/10.1164/rccm.201909-1771OC
35.
Alabed S
,
Shahin Y
,
Garg P
,
Alandejani F
,
Johns CS
,
Lewis RA
, et al.
Cardiac-MRI Predicts Clinical Worsening and Mortality in Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging. 2020 Sep.
36.
Swift AJ
,
Rajaram S
,
Condliffe R
,
Capener D
,
Hurdman J
,
Elliot CA
, et al.
Diagnostic accuracy of cardiovascular magnetic resonance imaging of right ventricular morphology and function in the assessment of suspected pulmonary hypertension results from the ASPIRE registry. J Cardiovasc Magn Reson. 2012 Jun;14(1):40. https://doi.org/10.1186/1532-429X-14-40
37.
Creuzé N
,
Hoette S
,
Montani D
,
Günther S
,
Lau E
,
Ternacle J
, et al.
Usefulness of Cardiovascular Magnetic Resonance Indices to Rule In or Rule Out Precapillary Pulmonary Hypertension. Can J Cardiol. 2015 Dec;31(12):1469–76. https://doi.org/10.1016/j.cjca.2015.04.014
38.
Johns CS
,
Kiely DG
,
Rajaram S
,
Hill C
,
Thomas S
,
Karunasaagarar K
, et al.
Diagnosis of Pulmonary Hypertension with Cardiac MRI: Derivation and Validation of Regression Models. Radiology. 2019 Jan;290(1):61–8. https://doi.org/10.1148/radiol.2018180603
39.
Whitfield AJ
,
Solanki R
,
Johns CS
,
Kiely D
,
Wild J
,
Swift AJ
. MRI Prediction of Precapillary Pulmonary Hypertension according to the Sixth World Symposium on Pulmonary Hypertension. Radiology. 2020 Feb;294(2):482–482. https://doi.org/10.1148/radiol.2019192078
40.
Reiter U
,
Kovacs G
,
Reiter C
,
Kräuter C
,
Nizhnikava V
,
Fuchsjäger M
, et al.
MR 4D flow-based mean pulmonary arterial pressure tracking in pulmonary hypertension. Eur Radiol. 2021 Apr;31(4):1883–93. https://doi.org/10.1007/s00330-020-07287-6
41.
Ikoma T
,
Suwa K
,
Sano M
,
Ushio T
,
Saotome M
,
Ogawa N
, et al.
Early changes of pulmonary arterial hemodynamics in patients with systemic sclerosis: flow pattern, WSS, and OSI analysis with 4D flow MRI. Eur Radiol. 2021 Jun;31(6):4253–63. https://doi.org/10.1007/s00330-020-07301-x
42.
Rogers T
,
Ratnayaka K
,
Khan JM
,
Stine A
,
Schenke WH
,
Grant LP
, et al.
CMR fluoroscopy right heart catheterization for cardiac output and pulmonary vascular resistance: results in 102 patients. J Cardiovasc Magn Reson. 2017 Jul;19(1):54. https://doi.org/10.1186/s12968-017-0366-2
43.
Knight DS
,
Kotecha T
,
Martinez-Naharro A
,
Brown JT
,
Bertelli M
,
Fontana M
, et al.
Cardiovascular magnetic resonance-guided right heart catheterization in a conventional CMR environment - predictors of procedure success and duration in pulmonary artery hypertension. J Cardiovasc Magn Reson. 2019 Sep;21(1):57. https://doi.org/10.1186/s12968-019-0569-9
44.
Ratnayaka K
,
Kanter JP
,
Faranesh AZ
,
Grant EK
,
Olivieri LJ
,
Cross RR
, et al.
Radiation-free CMR diagnostic heart catheterization in children. J Cardiovasc Magn Reson. 2017 Sep;19(1):65. https://doi.org/10.1186/s12968-017-0374-2
45.
Schoenfeld C
,
Cebotari S
,
Hinrichs J
,
Renne J
,
Kaireit T
,
Olsson KM
, et al.
MR Imaging-derived Regional Pulmonary Parenchymal Perfusion and Cardiac Function for Monitoring Patients with Chronic Thromboembolic Pulmonary Hypertension before and after Pulmonary Endarterectomy. Radiology. 2016 Jun;279(3):925–34. https://doi.org/10.1148/radiol.2015150765
46.
Ota H
,
Sugimura K
,
Miura M
,
Shimokawa H
. Four-dimensional flow magnetic resonance imaging visualizes drastic change in vortex flow in the main pulmonary artery after percutaneous transluminal pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Eur Heart J. 2015 Jul;36(25):1630. https://doi.org/10.1093/eurheartj/ehv054
47.
Schoenfeld C
,
Hinrichs JB
,
Olsson KM
,
Kuettner MA
,
Renne J
,
Kaireit T
, et al.
Cardio-pulmonary MRI for detection of treatment response after a single BPA treatment session in CTEPH patients. Eur Radiol. 2019 Apr;29(4):1693–702. https://doi.org/10.1007/s00330-018-5696-4
48.
Kaireit TF
,
Voskrebenzev A
,
Gutberlet M
,
Freise J
,
Jobst B
,
Kauczor HU
, et al.
Comparison of quantitative regional perfusion-weighted phase resolved functional lung (PREFUL) MRI with dynamic gadolinium-enhanced regional pulmonary perfusion MRI in COPD patients. J Magn Reson Imaging. 2019 Apr;49(4):1122–32. https://doi.org/10.1002/jmri.26342
49.
Pöhler GH
,
Klimes F
,
Voskrebenzev A
,
Behrendt L
,
Czerner C
,
Gutberlet M
, et al.
Chronic Thromboembolic Pulmonary Hypertension Perioperative Monitoring Using Phase-Resolved Functional Lung (PREFUL)-MRI. J Magn Reson Imaging. 2020 Aug;52(2):610–9. https://doi.org/10.1002/jmri.27097


