A new cardiovascular tissue bank in Switzerland
Giorgio Franciosi, Enrico Ferrari, Tiziano Torre, Lucia Turchetto, Stefanos Demertzis
Human homograft valves (allografts) have been in clinical use for several decades because they guarantee an excellent hemodynamic performance together with anticoagulation-free therapy. In spite of unfulfilled promises of the early days, they still maintain a primary role in clinical cardiac surgery, and represent the golden standard in the treatment of peculiar cardiac settings such as active aortic endocarditis and pulmonary trunk replacement in children. A new tissue bank has been created at Cardiocentro Ticino, Switzerland, to prepare, stock and deliver human homografts, potentially to all cardiac centres in Switzerland and, for the time being, is the only accredited human tissue bank available in our country. Moreover, the restrictions to cross borders required during a pandemic can limit the delivery of homografts within European countries and, therefore, justify even more the presence of a tissue bank within the Swiss territory. During the COVID-19 lockdown, we were allowed by the Swiss authorities to treat two critical patients by using aortic homografts stored in our bank. Beside the usefulness of such a sophisticated health structure in treating complicated cases, tissue banks are essential to sustain cardiovascular research and we believe on the strategic value of a Swiss tissue bank.
Introduction
Clinical application of autologous human heart valves, “homografts”, dates back more than 60 years. In 1956, the first clinical homograft was implanted into the descending aorta by Dr Gordon Murray to treat a case of aortic valve insufficiency, at a time when no man-made prostheses were available [1]. In the early days of cardiac surgery, to the heroic surgeons of that era a human valve represented the ideal substitute: haemodynamic superiority compared with early biological prostheses, no need for permanent anticoagulation (required with mechanical valves) and the promise of long-term durability ensured that homografts represented the gold standard [2].
Time, slowly but surely, has eroded the perception of the homograft’s superiority within the cardiac surgery community, partly because of the relentless improvement in performance of both biological and mechanical valves, but even more because of the persistence of the original factor limiting their widespread use, i.e., the inadequate number of donors in relation to requirements [3].
Nonetheless, throughout the decades homografts have preserved a valuable role, albeit a niche within specific surgical indications. In this respect, the uses of homografts are for the treatment of infected valves and correction of complex congenital heart defects.
For years, it was assumed that homografts would provide better protection against recurrent infection when preserved in antibiotics [4]. Methods of preservation aiming to maintain viability were devised, on the assumption this would enable fibroblasts to repair tissue damage, conferring long lasting durability. In this respect, cryopreservation techniques had more success, besides allowing long-term “on-the-shelf” availability [5, 6].
Although these assumptions have never been completely confirmed, and indeed time has shown that structural degeneration ensues, nonetheless the real technical advantage conveyed by human homografts stems from their plasticity, allowing a radical excision of all infected tissue with replacement of the entire aortic root, with limited or no use of synthetic material, hence limiting the risk of relapsing infection [7, 8].
In the field of congenital defects, early expectations envisaged a progressive growth of the graft along with the child’s development. Unfortunately this proved incorrect, as progressive degeneration of the graft is expected. Still, in all forms of congenital defects involving the right or left ventricular outflow, such as tetralogy of Fallot or for Norwood procedures, allografts are well suited for reconstruction, although leaving open the prospect of future reoperation [9, 10].
The surgical procedure that stands out as the hallmark of homograft utilisation is the Ross procedure, whereby the patient’s own pulmonary valve and pulmonary trunk are used to replace the patient’s aortic root, while a homograft is interposed on the right side to reconstruct the right ventricular outflow tract [11, 12]. This procedure, although technically more complex, is suitable for young patients and confers long-lasting durability of the native valve autograft on the left outflow tract, avoiding life-long anticoagulation.
In parallel to these cardiac procedures, the field of vascular surgery has benefited enormously from the procurement and storage of vascular homografts. Once again the surgeon is confronted with complicated cases involving prosthetic infections or visceral fistulas extremely difficult to eradicate, forcing patients to take long-lasting antibiotic treatment and to have complicated vascular reconstructions. Here again, cryopreserved homografts excel because of their plasticity and resistance to infection [13].
Homografts in Switzerland
According to data from Swisstransplant, the Swiss National Foundation for organ donation and transplantation, the number of donors following brain death has risen from 96 per year in 2015 to 127 in 2019. These numbers, however, are testimony of inadequate availability, forcing the country to import increasing numbers of organs from abroad (from 27 in 2015 to 61 in 2019) to meet the growing internal demand. For information on the number of cadaveric donors and transplanted homografts, see the Swisstransplant report for the past three years in table 1. These data once again highlight the limited number of donors compared with the number of homografts needed annually, making it necessary to rely on foreign tissue banks.
|
Table 1: Donations and transplants of hearts, valves and arteries in Switzerland. |
|||
|
|
2016 |
2017 |
2018 |
|
Donations |
|
|
|
|
Heart |
11 |
19 |
18 |
|
Arteries |
6 |
8 |
6 |
|
Transplants |
|
|
|
|
Valves |
26 |
30 |
19 |
|
Arteries |
21 |
37 |
69 |
During 25 years of activity, the European Tissue Bank of Brussels (ETBB) has distributed 6000 heart valves and 3000 allogeneic vascular homografts throughout Europe, with an annual average of 400 donor organs and 450 valves and vascular homografts [14]. Among the various centres that supply donor material to the ETBB are 10 hospitals in Switzerland. These figures underline the importance of human allografts and their value in specific clinical contexts that frequently are urgent. The recent COVID-19 pandemic has negatively impacted on the number of donors, compounding the fragility of the international trade in the case of severe exchange limitations at national borders, and has emphasised the need for a national supplier of primary goods and medical devices [15]. Switzerland completely depends on European countries for the supply of human homografts. So far.
The new cardiovascular tissue bank
In a medium-volume cardiac surgical unit such as our centre, the Cardiocentro Ticino in Lugano, Switzerland, the use of homografts is mainly confined to patients with endocarditis, specifically prosthetic valve endocarditis. In this setting, the advantages outlined above are greatly appreciated by the surgeon and of great advantage to the patient.
In previous years, the Cardiocentro has satisfactory relied on homografts imported from abroad (ETBB), but at the same time has had to contend with the shortage of the product and with considerable costs, not to mention the worst case scenario: having to do without. Moreover, the recent limitations imposed by the COVID-19 pandemic on the international transport have even more emphasised the limits of this approach. We were repeatedly astonished to face the reality that a country so technologically advanced in any scientific field should not have its own tissue bank and be dependent from foreign imports.
From this reality stemmed the endeavour to establish a tissue bank at Cardiocentro Ticino in Lugano. An opportunity to foster research in the field of bio-engineering was also envisaged.
Our Swiss tissue bank can offer the great advantages of in-house preparation and storage of human homografts (including aortic root, aortic arch, pulmonary artery and valve, pericardium) with the possibility of providing cryopreserved tissues within Switzerland in a fast and potentially unlimited way.
Tissue banks are health structures with the task of storing and distributing human tissues intended for transplantation and certifying their suitability and safety. The Cardiocentro Ticino Tissue Bank was established in 2016 within the Lugano Cell Factory (LCF), following certification by SwissMedic (Ref. n. BS2015-nTxG108-N0-V00), the national authorisation and supervisory authority for drugs and medical products, on behalf of the Federal Office of Public Health (fig. 1).
Figure 1: Swissmedic Certificate of Authorisation for a Tissue Bank at Cardiocentro Ticino, Lugano, Switzerland.

The daunting task of obtaining certification was greatly facilitated by having access to the clean room of the cell factory, present and operational at Cardiocentro Ticino since 2008, with personnel qualified for tissue removal, processing and conservation (fig. 2).
Figure 2: Pictures from the clean room of the cell factory at Cardiocentro Ticino, Lugano, Switzerland.

Donor identification and screening
Harvesting of cardiac valves began in 2018. Patients are considered for explantation according to the European Committee of Organ Transplantation inclusion and exclusion criteria, and family members’ written consent is required. Our protocol stipulates that the organ explantation be performed in the operating room, under sterile conditions, within 15 hours from cardiac arrest, or within 24 hours if the body is refrigerated within 6 hours from cardiac arrest and death.
Non-beating/non-transplantable hearts only are considered for retrieval. The upper age limit of the patients is 65 years and exclusion criteria include patients with valve lesions evident on echocardiography prior to death. Patients positive for transmissible diseases, cancer, active infection, sepsis, or on long-term immunosuppressant or steroid treatment also are excluded (table 2).
|
Table 2: Exclusion criteria for homograft donors. |
|
Neoplasia |
|
Transmissible disease |
|
Active infection / sepsis |
|
Immunodeficiency status (disease or chemical origin) |
|
Unknown cause of death |
|
Dysfunctional heart valves or dilated aortic root at echocardiogram |
Our original certification from SwissMedic has made possible explanting homografts from cadavers of patients who died within our premises. Thanks to the generosity of their families, we have explanted, so far, aortic and pulmonary valves, together with pericardium, from the hearts of four patients. From one patient, the corneas were also explanted with collaboration from the ophthalmic department of the hospital.
Processing procedure
The pericardium and heart explanted under totally sterility conditions in the operating room are placed in a sterile recipient filled with cardioplegic solution (Del Nido Solution: Plasma-Lyte A® 1000 ml, mannitol 15% 20 ml, MgSO4 20% 10 ml, NaHCO3 1 mEq/ml 13ml, KCl 2 mEq/l 13 ml) and inserted into a double bag for transfer to the Lugano Cell Factory to be processed.
In the clean room, once the specimens are extracted from the bag, two samples of the transport fluid are taken for microbiological culture. Tissue samples from all cardiac specimens are taken for histology and microbiology testing. The processing proceeds with trimming of the cardiac specimens to the optimum, testing for valve continence, sizing and classification in terms of overall quality. A technical data sheet is prepared for each specimen and stored in an electronic database (fig. 3).
The specimens are then stored in antibiotic solution (Sol base 128) for 24 hours at 4°C. After this time a second fragment of tissue is taken for repeat microbiology, and then the aortic, pulmonary and pericardial homograft are ready for cryopreservation.
Following the decontamination procedure, the specimens are transferred into a double-cryobag immersed in cryo-protective solution (BASE + 10% DMSO) and inserted into a first pouch; 40 ml of fluid is aspirated from the cryobag and tested for sterility. The primary pouch is then transferred into a secondary pouch, ready for cryopreservation.
The freezing programme scheduled to achieve the goal temperature is implemented at a rate of −1°C/min, down to a probe temperature of −40°C, followed by deeper freezing at the rate of −5°C/min to reach −100°C. Forty days after microbiology testing, if the results are negative, the homograft is labelled ready for use within the following 5 years.
Figure 3: Data sheet describing in detail the explanted and processed human homograft stored in the tissue bank (Italian version).

Clinical activity
In 2020, at Cardiocentro Ticino, two patients with devastating prosthetic aortic valve endocarditis were successfully operated on, with replacement of the bioprosthesis and the entire aortic root according to the Bentall technique using two homografts from our tissue bank (fig. 4).
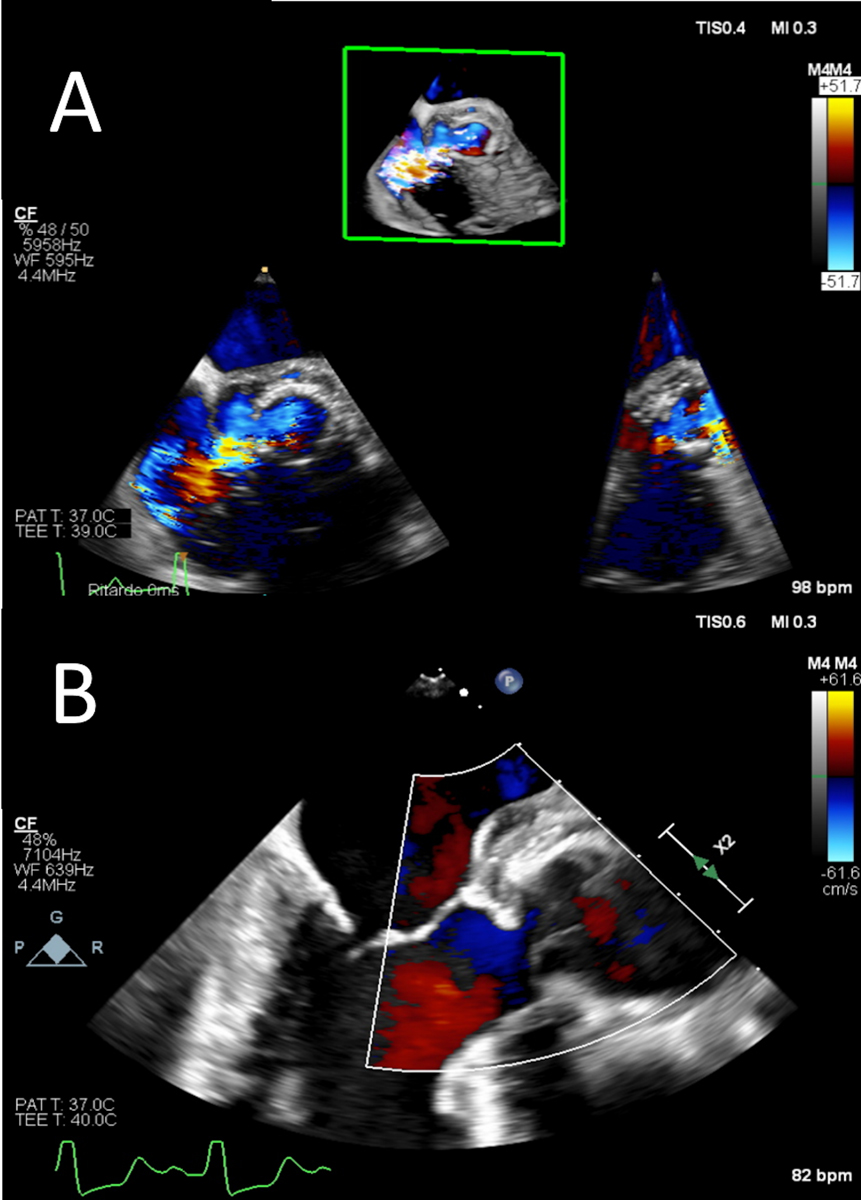
Both cases presented within an emergency clinical setting owing to severe heart failure secondary to an aortic root to the right atrium fistula in the first case, and torrential aortic valve insufficiency in the second (fig. 5). Both patients were redo cases who had previously been operated on for isolated aortic valve replacement and the Bentall procedure with biological prostheses. Both cases occurred during the COVID-19 lockdown, and a 26-mm and a 25-mm diameter aortic root homograft stored in our tissue bank were very convenient, at a time when importing from abroad was tough given the restrictions on crossing borders imposed during the pandemic in February to May 2020.
Following uneventful surgery, both patients had a full recovery with repeated echocardiographic checks confirming excellent haemodynamic performance of the homografts, with negligible trans-valvular gradients and absence of residual trans-aortic regurgitation.
Figure 4: Intraoperative trimming of thawed aortic homograft.
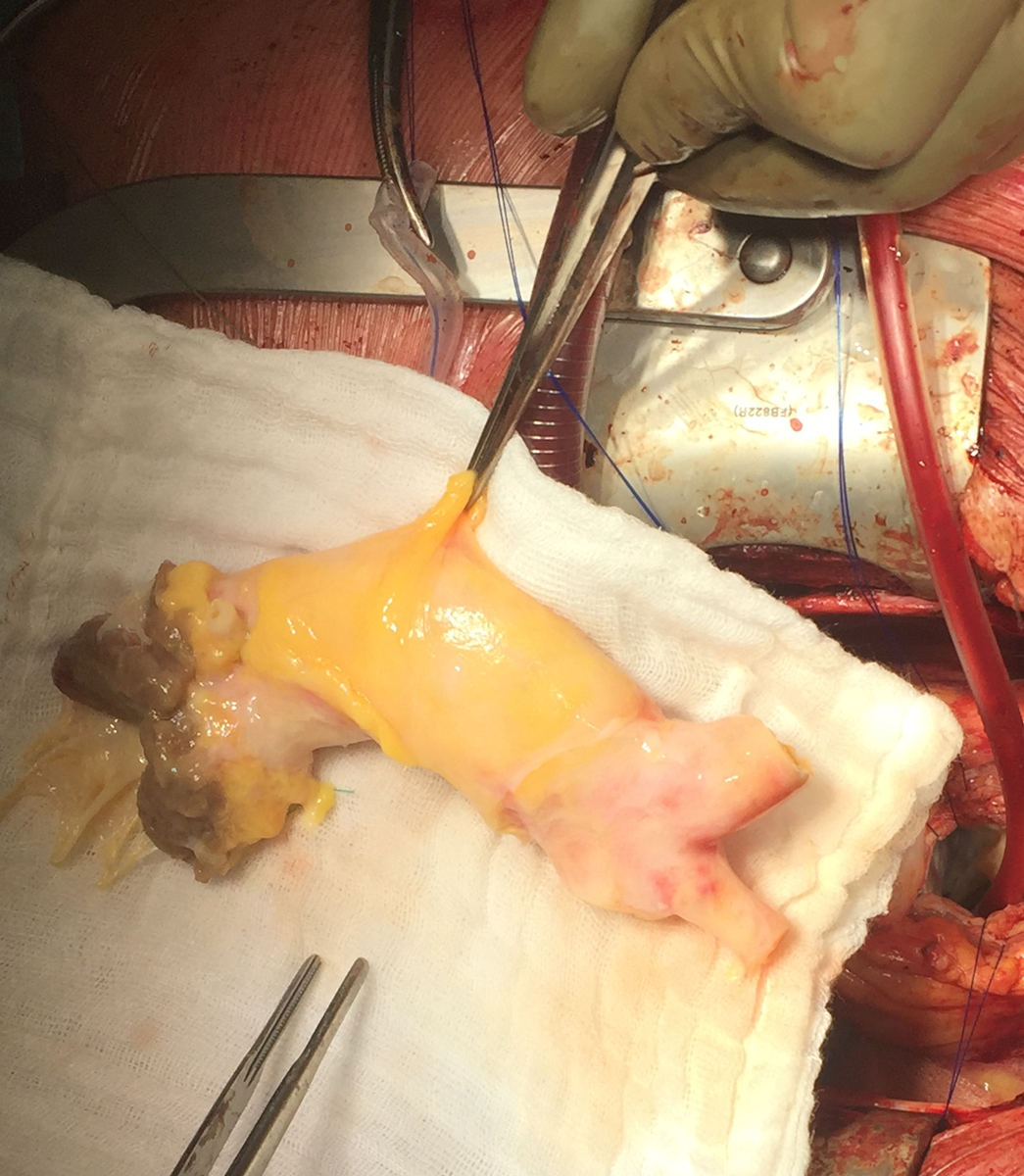
Figure 5: Transoesophageal echocardiogram showing (A) prosthetic aortic valve detachment due to valve endocarditis with a wide aortic to right atrium fistula and (B) the postoperative result with the aortic homograft in place (Bentall procedure).
The pandemic has strongly underlined this critical issue, which only the presence in Switzerland of a human tissue bank can limit, by providing in loco the necessary homografts for adult and paediatric cardiac surgery.
Future perspective
In spite of unmet expectations regarding viability and durability, human homograft valves have managed to retain a role of excellence in the surgical armamentarium, and to date they still stand out as a model of perfection. The unfulfilled promises of the early days have not overshadowed the advantages of perfect haemodynamic performance together without the need of anticoagulation treatment, which is traditionally required with mechanical valve prostheses.
With the objective of achieving perfection, the problem of structural valve degeneration hindering durability has been tackled. On the assumption that decellularisation would curtail the immune response of the graft recipient, techniques have been developed to engineer decellularised grafts, and clinical use seems to validate the concept and confirm superior haemodynamic performance and durability compared with xenografts in the pulmonary position. Even more surprisingly, they have shown adaptive growth [15]. A step further in reducing immune reactions, has been accomplished by re-seeding decellularised grafts with cultured cells or autologous progenitor cells from the recipient, providing valves with biomechanical properties similar to native valves [16].
Such achievements have arguably opened the door to bioengineered valves with life-long durability, fulfilling the dream of an ideal valve/conduit that would be the perfect solution in the paediatric realm of cardiac surgery, as well as for adult patients. Modern national tissue banks and cell factories can offer to our researchers the opportunity to rapidly explore alternative solutions and techniques in the important and expanding fields of human tissue preservation, bioengineering and cryopreservation.
Moreover, beside the preparation and storage of human valvular homografts and pericardium, a further clinical application of the Swiss tissue bank could be the preservation of medium-size explanted arteries for vascular surgery. In this growing field, vascular homografts, including the distal aorta with its bifurcation or the ileo-femoral arteries, are useful for the surgical treatment of infected intra-abdominal aortic grafts or endografts, or in cases where the autologous venous capital is absent or of poor quality. The presence of an accredited national tissue bank can easily spread the use of these human vascular homografts in Switzerland, with great clinical benefits, in terms of quality of life and repeated hospitalisation, for patients suffering from major cardiovascular diseases.
Conclusion
The history of human homografts has spanned several decades and has been fed by the endeavour to provide ideal valve and vascular substitutes. This effort has contributed considerably to the progress of tissue bioengineering and applications can now be extended to collateral fields of research, such as small calibre vascular conduits and even more complex decellularised and re-seeded tissues. The viability of a Swiss tissue bank is a treasure that should be seriously considered, not only to meet the clinical needs of our country but also to foster scientific advances in the field of tissue bioengineering.
Giorgio Franciosi, Cardiac Surgery Unit, Cardiocentro Ticino, Lugano, Switzerland
Enrico Ferrari, Cardiocentro Ticino, Via Tesserete 48, CH-6900 Lugano, enrico.ferrari[at]cardiocentro.org
Tiziano Torre, Cardiac Surgery Unit, Cardiocentro Ticino, Lugano, Switzerland
Lucia Turchetto, Lugano Cell Factory, Cardiocentro Ticino, Lugano, Switzerland
Stefanos Demertzis, Cardiac Surgery Unit, Cardiocentro Ticino, Lugano, Switzerland
References
- Murray G. Aortic valve transplants. Angiology. 1960;11(2):99–102. doi:https://doi.org/10.1177/000331976001100201. PubMed
- Ross DN. Homograft replacement of the aortic valve. Lancet. 1962;280(7254):487. doi:https://doi.org/10.1016/S0140-6736(62)90345-8. PubMed
- Delmo Walter EM, de By TM, Meyer R, Hetzer R. The future of heart valve banking and of homografts: perspective from the Deutsches Herzzentrum Berlin. HSR Proc Intensive Care Cardiovasc Anesth. 2012;4(2):97–108. PubMed
- Lau JK, Robles A, Cherian A, Ross DN. Surgical treatment of prosthetic endocarditis. Aortic root replacement using a homograft. J Thorac Cardiovasc Surg. 1984;87(5):712–6. doi:https://doi.org/10.1016/S0022-5223(19)38453-3. PubMed
- Lockey E, Ai-Janabi N, Gonzalez-Lavin L, Ross DN. A method of sterilizing and preserving fresh allograft heart valves. Thorax. 1972;27(4):398–400. doi:https://doi.org/10.1136/thx.27.4.398. PubMed
- O’Brien MF, Stafford G, Gardner M, Pohlner P, McGiffin D, Johnston N, et al. The viable cryopreserved allograft aortic valve. J Card Surg. 1987;2(1, Suppl):153–67. doi:https://doi.org/10.1111/jocs.1987.2.1s.153. PubMed
- Musci M, Weng Y, Hübler M, Amiri A, Pasic M, Kosky S, et al. Homograft aortic root replacement in native or prosthetic active infective endocarditis: twenty-year single-center experience. J Thorac Cardiovasc Surg. 2010;139(3):665–73. doi:https://doi.org/10.1016/j.jtcvs.2009.07.026. PubMed
- Solari S, Mastrobuoni S, De Kerchove L, Navarra E, Astarci P, Noirhomme P, et al. Over 20 years experience with aortic homograft in aortic valve replacement during acute infective endocarditis. Eur J Cardiothorac Surg. 2016;50(6):1158–64. doi:https://doi.org/10.1093/ejcts/ezw175. PubMed
- McElhinney DB, Reddy VM, Hanley FL. Homografts in congenital heart disease: current applications and future directions. Isr J Med Sci. 1996;32(10):880–5. PubMed
- Khwaja S, Nigro JJ, Starnes VA. The Ross procedure is an ideal aortic valve replacement operation for the teen patient. Semin Thorac Cardiovasc Surg Annu. 2005;8(1):173–5. doi:https://doi.org/10.1053/j.pcsu.2005.01.012.
- Ross D. Replacement of the aortic valve with a pulmonary autograft: the “switch” operation. Ann Thorac Surg. 1991;52(6):1346–50. doi:https://doi.org/10.1016/0003-4975(91)90032-L. PubMed
- Bell D, Prabhu S, Betts KS, Chen Y, Radford D, Whight C, et al. Long-term performance of homografts versus stented bioprosthetic valves in the pulmonary position in patients aged 10-20 years. Eur J Cardiothorac Surg. 2018;54(5):946–52. doi:https://doi.org/10.1093/ejcts/ezy149. PubMed
- Brown KE, Heyer K, Rodriguez H, Eskandari MK, Pearce WH, Morasch MD. Arterial reconstruction with cryopreserved human allografts in the setting of infection: A single-center experience with midterm follow-up. J Vasc Surg. 2009;49(3):660–6. doi:https://doi.org/10.1016/j.jvs.2008.10.026. PubMed
- Villalba R, Santos S, Martinez MJ, Díaz M, Pevida M, Cemborain A, et al. Analysis of impact on tissue activity during COVID-19 outbreak: a survey of 8 banks in Spain. Cell Tissue Bank. 2020;21(4):557–62. doi:https://doi.org/10.1007/s10561-020-09853-0. PubMed
- Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg. 2011;92(4):1308–14. doi:https://doi.org/10.1016/j.athoracsur.2011.06.009. PubMed
- Cebotari S, Tudorache I, Ciubotaru A, Boethig D, Sarikouch S, Goerler A, et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation. 2011;124(11, Suppl):S115–23. doi:https://doi.org/10.1161/CIRCULATIONAHA.110.012161. PubMed
