Endocrine care of transgender and gender-diverse adults: Swiss recommendations
DOI: https://doi.org/https://doi.org/10.57187/5059
Hüseyin Cihanabc,
Barbara Bischofberger-Baumannd,
Julie Buchere,
Verdiana Caironif,
Fabien Claudeg,
Federico del Ventoh,
Michelle Egloffi,
Niklaus Flütschj,
David Garcia Nuñezc,
Ursula Gobrecht-Kellerk,
Ulrike Itenl,
Martine Jacot-Guillarmodm,
Johannes Kliebhanbc,
Maddalena Masciocchik,
Maria Mavromatin,
Christian Meierb,
Georgios
E. Papadakiso,
Carole Riebene,
Lea Slahorj,
Lorenzo Soldatip,
Maria-Isabelle Streuliq,
Sébastien Thalmannr,
Vincent Vibertp,
Bettina Winzelercs
a Faculty of Medicine,
Zurich University, Zurich, Switzerland
b Department of Endocrinology,
Basel University Hospital, Basel, Switzerland
c Innovation-Focus Gender Variance, Basel
University Hospital, Basel, Switzerland
d Department of Endocrinology, HOCH Health
Ostschweiz, St. Gallen, Switzerland
e EndoDia Centre, Biel, Switzerland
f Department of Endocrinology, Regional
Hospital of Bellinzona and Valli, Bellinzona, Switzerland
g Department of Paediatric Endocrinology,
University Children’s Hospital Basel (UKBB), Basel, Switzerland
h Department of Gynaecology, Geneva
University Hospitals (HUG), Geneva, Switzerland
i Department of Endocrinology, Cantonal
Hospital Baden, Baden, Switzerland
j Department of Endocrinology and
Diabetology, Cantonal Hospital Lucerne, Lucerne, Switzerland
k Department of Reproductive Medicine and
Gynaecological Endocrinology, University Hospital Basel, Basel, Switzerland
l Swiss Society for Endocrinology and
Diabetology (SGED), Baden, Switzerland
m Department of Gynaecology, Lausanne
University Hospital (CHUV), Lausanne, Switzerland
n Department of Endocrinology, Geneva
University Hospitals (HUG), Geneva, Switzerland
o Department of Endocrinology, Diabetology
and Metabolism, Lausanne University Hospital (CHUV), Lausanne, Switzerland
p Department of Psychiatry, Geneva
University Hospitals (HUG), Geneva, Switzerland
q Department of Reproductive Medicine and
Gynaecological Endocrinology, Geneva University Hospitals (HUG), Geneva,
Switzerland
r Ärztezentrum Sihlcity, Zurich, Switzerland
s Department of Endocrinology, Zurich
University Hospital, Zurich, Switzerland
Summary
Transgender and gender-diverse
individuals require standardised, evidence-based and culturally sensitive
endocrine care. International guidelines such as
those from the World Professional Association for Transgender
Health
(WPATH) and the Endocrine Society provide a strong foundation. These Swiss recommendations
build on them and offer
context-specific recommendations adapted to Switzerland’s healthcare system, insurance
policies and social considerations.
These recommendations
were developed by the “Working Group Transgender” of the Swiss Society of Endocrinology
and Diabetology (SSED/SGED) in collaboration with multidisciplinary experts. They
primarily focus on gender-affirming hormone therapy, including indications,
monitoring and long-term management. They also address fertility preservation, bone
and sexual health, and relevant psychological and legal aspects.
Interdisciplinary collaboration
among endocrinologists, primary care providers, mental health professionals, reproductive
specialists and surgeons is recommended to ensure cohesive patient-centred care.
Introduction
Transgender and gender-diverse
individuals are those whose gender identity does not align with sex assigned
at birth. For example, transfeminine individuals were assigned male at birth and
transmasculine individuals were assigned female at birth [1, 2].
For many transgender and gender-diverse individuals,
medical transition is an important aspect of aligning their physical characteristics
with their gender identity. Medical transition commonly involves gender-affirming
hormone therapy and gender-affirming surgeries, both of which have
been demonstrated to significantly improve psychological and physical health outcomes
[1, 3, 4]. Two diagnostic terms are used: International Classification of Diseases,
11th Revision (ICD-11) “gender incongruence”, which describes incongruence
between gender identity and sex assigned at birth and includes the desire to undergo
treatment as part of the diagnosis while Diagnostic and Statistical Manual of Mental
Disorders, 5th Edition (DSM-5) “gender dysphoria” describes distress
or impairment associated with this incongruence [5–7].
Health systems-based
studies estimate the prevalence of gender incongruence at 0.02–0.1%, while survey-based
studies report 0.3–4.5% [8]. In the 2023 IPSOS survey across 30 countries, the average
proportion of individuals self-identifying as transgender, non-binary, gender non-conforming,
gender-fluid or other gender identities was 3%, with Switzerland having one of the
highest rates at 6% [9]. The growing visibility of transgender and gender-diverse
people has been accompanied by an increasing
demand for equitable, high-quality healthcare. While international
guidelines such as the Standards of Care, Version 8 (SOC 8) from the World Professional
Association for Transgender Health (WPATH) and the Endocrine Society’s “Gender Incongruence
Guideline” offer an evidence-based framework for care, adaptations are required
to account for Switzerland’s healthcare organisation, insurance regulations, legal
gender recognition processes and access to specific medications. The purpose of
this document is to equip healthcare professionals in Switzerland with useful
and practical guidance for transgender care. It integrates international best practices
with Swiss-specific clinical realities, legal frameworks and sociopolitical contexts.
Methods
The “Working Group Transgender” of the Swiss Society of Endocrinology
and Diabetology (SSED/SGED), composed of Swiss endocrinologists with expertise in
transgender
health, developed these recommendations in collaboration with invited multidisciplinary
guest authors. To establish evidence-based recommendations, the authors reviewed
relevant scientific literature, national resources and international guidelines.
Each contributor drafted one or more chapters within their area of expertise, which
were then reviewed by at least one other expert from a different institution or
geographical region.
Following this peer-review
process, members of the editorial board (ME, LS, ST, BW) revised each chapter to
ensure accuracy, consistency and clarity. The complete draft was then circulated
among all contributing authors for critical appraisal and feedback. Revisions were
made iteratively until consensus was achieved. The final version underwent internal
peer review by the SSED/SGED executive committee.
The full recommendations are available on the SSED/SGED website.
Clear terminology in
transgender and gender-diverse healthcare supports respectful and accurate description
of diverse gender identities
[10]. A glossary of terms
to aid clinical communication is provided in table 1.
Table 1Definitions of key terms for clinical communication in gender-affirming care.
| Sex |
Term relating to biological characteristics (i.e.
chromosomes, genitals, hormones) |
| Gender |
Term relating to personal, social and cultural
concepts |
| Sex assigned at birth |
Categorisation at birth, mostly based on phenotypic
presentation (i.e. genitals) |
| Transgender |
Gender identity does not correspond to sex assigned at birth |
| Cisgender |
Gender identity corresponds to sex assigned at
birth |
| Non-binary |
Gender identity outside binary categorisation of women and men |
| Gender-diverse |
Gender identity not constrained by binary concept of
gender |
| Gender-fluid |
Gender identity that is not fixed to a specific
gender and may change over time |
| Gender incongruence |
Marked and persistent incongruence between a person’s
experienced gender and that assigned at birth |
| Gender dysphoria |
Distress caused by gender incongruence |
| Transfeminine |
Feminine identity of someone who was assigned male
at birth. This includes
trans women and other gender identities |
| Transmasculine |
Masculine identity of someone who was assigned
female at birth. This
includes trans men and other gender identities |
A clinical checklist
summarising all key recommendations is provided at the end of the article to facilitate
implementation
in clinical practice.
Diagnosis, mental health and transition support
According
to the World Professional Association for Transgender Health’s SOC 8, healthcare
professionals experienced in transgender medicine may assess adults for gender-affirming
hormone therapy if they can identify co-existing mental health or psychosocial issues,
assess capacity to consent, evaluate clinical aspects of gender dysphoria and engage
in ongoing education. Diagnosis of gender incongruence is based on clinical history,
as no psychometric, laboratory or imaging methods exist for this purpose. Gender-affirming
hormone therapy can be indicated when there is a marked and sustained experience
of incongruence. Gender-affirming hormone therapy repeatedly demonstrated a decrease
in depressive symptoms and psychological discomfort [1]. Medical or psychosocial
conditions that could impact gender-affirming hormone therapy outcomes and the individual’s
expectations must be identified and addressed. The SSED/SGED working group recommends
that an assessment by a mental health professional experienced in gender incongruence
be offered to all individuals before gender-affirming hormone therapy. This evaluation
can help explore personal resources, identify mental health comorbidities and, if
desired, initiate longer-term support. It is particularly recommended in cases of
diagnostic uncertainty where incongruence may be primarily due to underlying psychopathology
rather than a transgender identity per se. Once the diagnosis is established, an
individual medical transition plan is created through shared decision-making [11,
12].
Because the transition process affects core physical, psychological and social factors,
support by a professional experienced in gender incongruence should be offered.
Innovative approaches, such as advanced practice nurse-supported models, can facilitate
coordination among specialists and help adapt the transition plan without pressure
from the medical system [13].
Legal aspects
Since
January 2022, individuals aged over 16 years in Switzerland can legally register
a change of recorded gender and first name by self-declaration at the civil registry
office without medical or psychological assessments [14]. The register
allows only male or female entries. A male gender entry before age 24 requires assessment
by the military medical service to determine compulsory military service eligibility,
considering transition stage and comorbidities [15]. Under the
informed consent model, transgender and gender-diverse individuals can start gender-affirming
therapies without
mandatory psychological evaluation, though such evaluations may
still be recommended.
Costs for medical and surgical procedures are covered by Swiss health insurance
regardless of civil register gender status. Transgender and gender-diverse individuals
are protected from workplace
discrimination under the Swiss gender equality act [16]. While using
correct names, pronouns and appropriate facilities is strongly recommended in schools
and workplaces, no specific anti-discrimination law for transgender and gender-diverse
individuals exists as
of 2025.
Gender-affirming hormone therapy
Indications and general principles of gender-affirming
hormone therapy
The primary indication for gender-affirming
hormone therapy is a diagnosis of gender incongruence. By suppressing endogenous
sex hormones and administering gender-affirming hormones, gender-affirming
hormone therapy supports aligning physical characteristics with gender identity. Gender-affirming
hormone therapy has demonstrated safety and efficacy to achieve desired physical
changes and to reduce gender incongruence for transgender and gender-diverse individuals
in short- and medium-term
follow-up studies [3].
Baseline assessment includes a discussion of expected
physical changes, potential irreversible effects and possible adverse effects. Counselling
on the impact of gender-affirming hormone therapy on fertility, as well as fertility
preservation options, is essential prior to initiation. Table 2 outlines the recommended
assessments at baseline and during follow-up. In the first year, clinical evaluations
are advised every three months, followed by monitoring every 6–12 months. This includes
assessment of body weight, body mass index (BMI), blood pressure, cardiovascular
risk and laboratory parameters such as hormone levels, glucose, HbA1c, and renal
and liver function [8, 17].
Table 2Overview of recommended clinical, laboratory and psychosocial evaluations
prior to and during gender-affirming hormone therapy.
| Baseline |
Discuss
expectations from gender-affirming hormone therapy |
| Explain
onset and time course of physical changes including irreversible effects as
well as side effects |
| Provide counselling
for impact on fertility and fertility-preservation options |
| Assess
psychosocial setting and resources |
| Check
relative contraindication (i.e. thromboembolic disease, hormone-sensitive
cancer and for transmasculine individuals erythrocytosis and obstructive
sleep apnoea syndrome) |
| Clinical
evaluation, i.e. measure body weight, BMI, blood pressure; smoking cessation
counselling |
| Laboratory
evaluation: sex hormones, liver and renal parameters, lipids, glucose/HbA1c,
blood count, 25-OH-vitamin D |
| Every 3
months for the 1st year, then every 6–12 months |
Clinical
evaluation to monitor signs of feminisation/virilisation and
undesired/adverse effects |
| Monitor
cardiovascular risk factors such as body weight, body mass
index (BMI), blood pressure; smoking
cessation counselling |
| Laboratory evaluation: sex hormones, liver and renal parameters, lipids,
glucose/HbA1c, blood count |
As gender-affirming hormone therapy represents a
lifelong treatment for most individuals, long-term follow-up is essential. Satisfaction
with therapy should be evaluated continuously through shared decision-making, respecting
patient autonomy and individual preferences.
Feminising hormone therapy
Feminising
hormone therapy aims to suppress endogenous testosterone and induce feminisation.
Therapy uses 17β-oestradiol, usually combined with an anti-androgen [8, 17].
Ethinyl oestradiol and conjugated equine oestrogens are not recommended due to higher
thromboembolism risk (table 3). Clinicians review relative contraindications like
thromboembolic disease, liver dysfunction and hormone-sensitive malignancies before
initiation.
Table 3Summary of oestradiol options used
in feminising hormone regimens, with their pharmacological characteristics.
| Route of administration |
Active ingredient |
Formulation |
Main characteristics |
| Transdermal |
Oestradiol (Estradot®) |
Transdermal patch: 50–300
µg every 72 hours |
Slow
release; oestradiol values stable; avoids first-pass effect; ↓ thrombotic
risk compared to oral oestradiol; half-life 24 hours |
| Oestradiol hemihydricum
(Oestrogel®) |
Transdermal gel: 0.75–3
mg/day (= 1–6 pushes/day) |
| Oral |
Oestradiol valerate
(Progynova®, Estrofem®) |
Oestradiol tablets: 2–6
mg/day |
Accumulation of oestrone as
first-pass effect; fluctuation of plasma levels; half-life 12 hours |
| Parenteral |
Oestradiol valerate or
cypionate |
Not available in
Switzerland |
Transdermal
oestradiol via patches or gels is preferred due to a better thromboembolic risk
profile compared to oral forms [8, 18, 19]. However oral oestradiol remains an option
especially for younger individuals (<45 years) without cardiovascular risks. Parenteral
oestradiol is not available in Switzerland.
Anti-androgens
are generally used in combination with oestradiol, as monotherapy rarely suppresses
testosterone sufficiently [18]. Available agents in Switzerland include
cyproterone acetate, spironolactone and gonadotropin-releasing hormone (GnRH)
analogues (table 4). Cyproterone acetate is effective but linked to hyperprolactinaemia,
hepatotoxicity and increased meningioma risk with cumulative doses above 10 grams
[20].
Spironolactone blocks androgen receptors and may reduce testosterone synthesis but
can cause hyperkalaemia, especially in older adults or those with renal impairment
[21].
GnRH analogues provide potent suppression but are limited
by cost and need for bone health monitoring. Current guidelines do not give a clear
preference for any of the three options [22].
Table 4Description of available
anti-androgens used to suppress testosterone production in feminising therapy.
| Route of administration |
Active substance |
Dose |
Main characteristics |
Side effects |
| Oral
|
Cyproterone acetate
(Androcur®) |
Approximately 10 mg/day (no
benefit with higher doses); 1/4 of 50 mg every day or every other day if 10 mg
tablets not available |
Hepatic metabolism; half-life 48–72
hours |
Negative effects on lipid
profile, weight; Increase in prolactin values; Increased incidence of
meningiomas |
| Spironolactone (Aldactone®) |
Tablets: 100–300 mg/day |
Hepatic metabolism; half-life 16–22
hours |
Hyperkalaemia (greater in patients >45 years old/with specific risk
factors; dehydration; hyponatraemia |
| Parenteral
|
GnRH agonist: triptorelin (Pamorelin LA®) or leuprolide (Lucrin®) |
3.75 mg/monthly s.c.
injection: 11.25 mg/ every 3 months s.c. injection |
Hepatic metabolism;
half-life 3 hours |
|
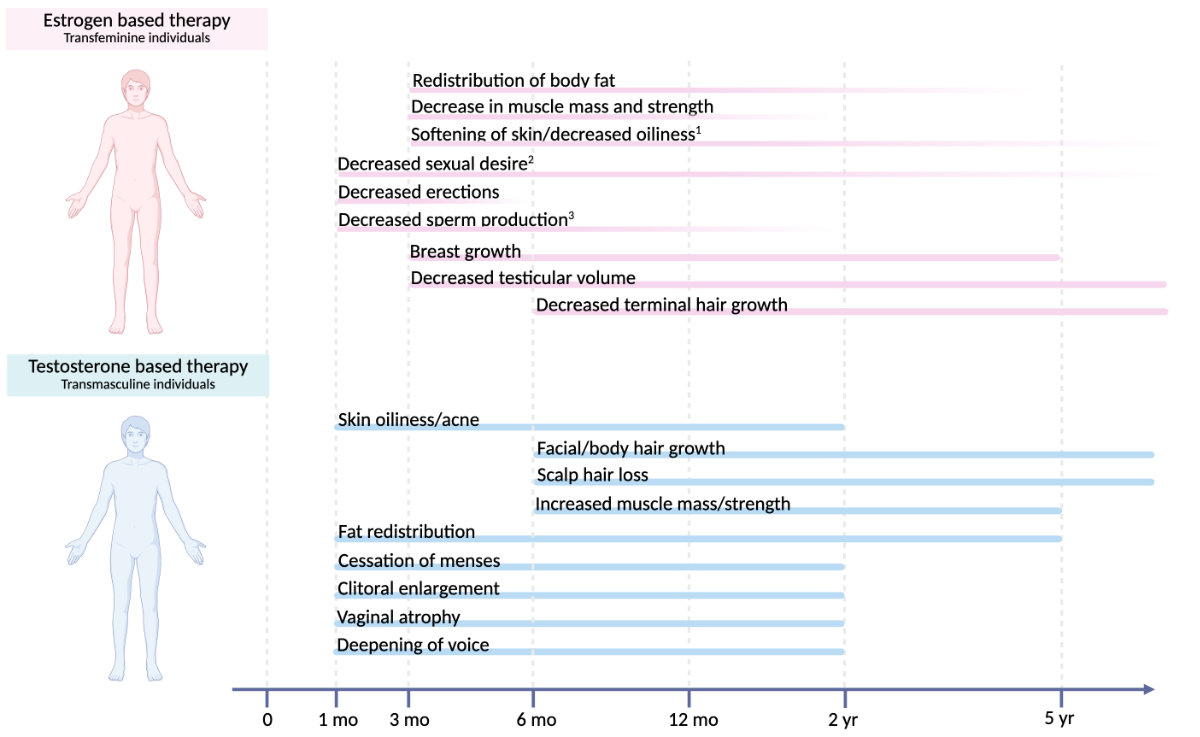
Figure 1Time course of physical changes
in gender-affirming hormone therapy. Estimated onset and time to maximum effect
of common physical changes induced by oestrogen-based therapy in transfeminine individuals
and testosterone-based therapy in transmasculine individuals, showing considerable
interindividual variability (1 maximum effect is unknown, 2 onset is unknown, 3 maximum effect is
variable). Created with BioRender.com.
Follow-up
is essential and includes key parameters such as cardiovascular risk factors, sex
hormones, blood count, and liver and renal function tests. Target serum testosterone
levels are usually below 2 nmol/l, and oestradiol levels between 370–730 pmol/l.
Importantly, laboratory values are used as a guide during treatment, but the goal
is not to reach specific targets; rather, clinical wellbeing and desired physical
changes are prioritised. Feminising effects typically start within 3–6 months, with
maximal changes over 2–5 years (figure 1). Effects include breast development, fat
redistribution, decreased erections, softer skin, and reduced facial and body hair,
which often requires additional measures like laser or electrolysis. Degree and
rate of change vary depending on age, genetic predisposition and adherence. Bone
health surveillance is important, especially on GnRH analogues
or in individuals who stop oestrogen after gonadectomy.
Masculinising hormone therapy
The
approach of masculinising hormone therapy parallels treatment for hypogonadal cisgender
men and includes injectable and transdermal preparations [17, 23, 24].
Testosterone enanthate subcutaneously (monthly) or undecanoate intramuscularly (every
three months) are common regimens, though
intervals vary with individual response. Transdermal preparations like testosterone
gel are other options (table 5) [25, 26].
Table 5Commonly used testosterone
preparations for masculinising hormone therapy.
| Testosterone |
Dose |
| Parenteral |
Testosterone
enanthate (Testoviron®) |
125–250 mg
i.m. every 3–4 weeks |
| Testosterone
undecanoate (Nebido®) |
500–1000 mg
i.m. every 8–14 weeks, then according to plasma testosterone levels |
| Transdermal |
Testosterone
gel (Testogel®, Tostran®) |
10–80 mg
testosterone/day |
Pre-therapy
evaluation should assess cardiovascular risk, haematological parameters, sleep apnoea
and liver function [8]. While testosterone alone is usually sufficient
to suppress ovulation and menstrual cycles, it does not provide contraception, so
counselling is essential.
Physiological
effects begin within 1–6 months and continue over years, including increased lean
mass, decreased fat mass, voice deepening, clitoromegaly, increased hair growth,
cessation of menses and skin changes (figure 1) [17, 27, 28].
About 10% may continue uterine bleeding, and benefit from progestogens or GnRH analogues
in case of distress associated with gender incongruence [28, 29].
Serum
testosterone is monitored every 3 months during titration: for enanthate, mid-cycle
levels should be 14–24 nmol/l; for undecanoate, levels assessed at the end of the
interval <14 nmol/l may indicate a need for interval adjustment; for transdermal
therapy, levels obtained ≥2 hours post-application after ≥1 week of use should demonstrate
stable physiological concentrations. Follow-up also includes haematocrit, lipid
profile, liver enzymes and renal function; see table 3. Haematocrit >0.50–0.54 may
indicate erythrocytosis, an adverse effect which may occur in approximately 11%
[8, 17,
23].
Other
potential adverse effects include hypertension, dyslipidaemia or liver enzyme elevations.
Bone health surveillance is important, especially on GnRH analogues or in individuals
who stop testosterone after gonadectomy [30–33].
Therapy of non-binary people
No
standardised gender-affirming hormone therapy protocols exist specifically for non-binary
individuals. Clinical principles from binary transition can be adapted, recognising
that non-binary people may seek only partial feminisation or masculinisation based
on their goals. Individualised plans may include oestradiol alone or in combination
with low-dose androgen blockers for partial feminisation, and low-dose testosterone
for partial masculinisation. Sometimes suppression of the
menstrual cycle (e.g. with oral, subcutaneous or intrauterine progestogens) may
be sufficient. These approaches aim to achieve specific effects
like breast development, voice deepening or amenorrhoea while avoiding undesired
changes.
Due
to lack of long-term outcome data, therapy should use shared decision-making with
thorough counselling on expected changes, fertility and health risks [8].
Minimal exposure to endogenous or exogenous sex hormones is needed to support bone
and cardiovascular health, maintaining hormone levels at least within the lower
physiological range for cisgender individuals [34].
Fertility protection
Gender-affirming hormone therapy affects fertility; it is therefore strongly
recommended to discuss the topic of fertility preservation early and before initiating
gender-affirming hormone therapy, and to generously refer patients to fertility
preservation experts if indicated [35–37].
In transfeminine individuals, oestrogens and antiandrogens often reduce
spermatogenesis or cause azoospermia, though recovery may occur after stopping therapy
[38]. Standard preservation involves sperm cryopreservation
via masturbation, or testicular sperm extraction if needed [39].
In transmasculine individuals, testosterone and/or GnRH analogues typically induce
anovulation and amenorrhoea. Ovarian function
may return after discontinuation [40]. Fertility
options include spontaneous conception after gender-affirming hormone therapy discontinuation,
oocyte or embryo cryopreservation, and third-party reproduction (surrogate). Cryopreservation
procedures require ovarian stimulation and retrieval, which may be psychologically
distressing and provoke distress related to gender incongruence [41]. Data from transgender
individuals with ovaries
who have used testosterone suggest that testosterone use does not significantly
impact oocyte retrieval, follicular function or oocyte maturation. Notably, the duration
of testosterone use did not correlate with
mature oocyte outcomes [42]. Adnexectomy leads
to irreversible loss of ovarian function, so ovarian tissue cryopreservation before
surgery may be considered [43].
Despite comparable parenthood desires to cisgender individuals, uptake
of fertility preservation is low [35, 36, 44].
Barriers include high costs (not covered by basic health insurance), lack of legal
frameworks and insufficient information.
Long-term management and special populations
Long-term risks of gender-affirming hormone
therapy and cancer screening
Gender-affirming hormone therapy is typically lifelong and generally safe
when properly monitored. However, long-term effects on somatic health and cancer
risk remain under study. No consistent evidence shows gender-affirming hormone
therapy increases overall mortality. Higher morbidity and mortality rates, including
high rates of suicide and homicide, may largely reflect psychosocial factors such
as minority stress, limited access to care and mental health issues. Cause-related
mortality from lung cancer, cardiovascular disease, HIV and suicide does not show
a direct link to hormone therapy, but it does highlight the need to monitor and
manage comorbidities and lifestyle factors [45, 46].
Cancer screening should follow local guidelines based on anatomy and hormone exposure.
In Switzerland, national screening guidelines for breast and cervical cancer are
applicable to all individuals having a uterus or mammary glands, regardless of gender
identity or gender-affirming hormone therapy use. For breast cancer, data suggest
no increased incidence in transfeminine individuals compared to cisgender populations,
though prospective studies are limited. Transfeminine individuals and transmasculine
individuals with retained breast tissue should follow cisgender women’s screening.
After chest masculinisation surgery (mastectomy), annual chest wall exams are recommended
[8, 47]. Cervical cancer screening applies to all transgender and gender-diverse
individuals with a cervix via Pap smear per cisgender women intervals: every three
years from ages 21 to 70, or human papilloma virus (HPV) testing starting at 30
years [48].
For transfeminine individuals on long-term oestrogen, prostate cancer risk
is low but present, and screening should be individualised [49–51]. As shown in table
6, routine screening should
align with general population practices, with attention to individual risks related
to hormones, age and comorbidities.
Table 6Suggested screening procedures
based on present anatomy and hormone exposure in individuals receiving gender-affirming
hormone therapy.
| Screening |
Transfeminine individuals |
Transmasculine individuals |
| Cardiovascular disease |
Screening for risk factors |
Screening for risk factors |
| Diabetes mellitus type 2 |
Screening according to cis individuals |
Screening according to cis individuals |
| Dyslipidaemia |
Annual screening |
Annual screening |
| Breast cancer |
Screening according to cis women |
Screening according to cis women in individuals
with breasts not having gender-affirming chest surgery.
After mastectomy: annual sub- and periareolar breast examinations. |
| Cervical cancer |
Not applicable |
Screening according to cis women in sexually
active individuals if cervical tissue is present. |
| Prostate cancer |
Screening according to cis men |
Not applicable |
Cardiovascular health and health risk behaviours
Transgender and gender-diverse individuals are at elevated risk of cardiovascular
disease. A recent meta-analysis of 10 studies (15,781 trans women and 11,304 trans
men) showed a 40% higher risk for major cardiovascular
events in transgender and gender-diverse individuals compared with individuals of
the same birth sex [52]. This increased
risk appears to be multifactorial. While the contribution of gender-affirming
hormone therapy remains uncertain, it intersects with minority stress, lifestyle
behaviours and classic cardiovascular risk factors.
Masculinising therapy is linked to lower HDL and higher
LDL cholesterol and triglycerides. Blood pressure may rise with testosterone, though
findings are inconsistent.
Feminising therapy has variable effects on lipids and blood pressure depending on
the agents used (e.g. cyproterone acetate vs spironolactone), often modestly reducing
LDL cholesterol at the beginning [53, 54].
Important contributing behavioural factors are higher rates of tobacco
use, physical inactivity and obesity. Lifestyle counselling is therefore important,
including encouraging people to quit smoking and engage in regular physical activity
[55–57].
Bone health
Both medical and surgical gender-affirming interventions can influence
bone health [58]. Bone mineral density (BMD) might
be assessed using dual-energy X-ray absorptiometry (DXA) prior to gender-affirming
hormone therapy in individuals at risk particularly in transfeminine individuals,
where studies suggest up to 30% may have low bone mineral density at baseline [59].
Further indications for dual-energy X-ray absorptiometry include planned
gonadectomy without gender-affirming hormone therapy, long-term suppression of endogenous
sex hormones (e.g. via GnRH analogues) and poor adherence
to gender-affirming hormone therapy. The 2019 ISCD Position Paper advises that
z-scores for transgender individuals should be calculated using reference data (mean
and standard deviation) matched to the individual’s affirmed gender [60].
Despite methodological limitations, existing studies indicate that gender-affirming
hormone therapy has a neutral effect on bone mineral density in transmasculine individuals
and may slightly improve bone mineral density at the lumbar spine in transfeminine
individuals [61–63]. In addition, lifestyle counselling
to support bone health should include adequate calcium and vitamin D intake and
weight-bearing exercise, though long-term studies are needed to determine their
impact on fracture risk [64].
The management of osteoporosis diagnosed in transgender and gender-diverse individuals
follows clinical
guidelines that apply to the general population [65, 66].
Treatment of older or medically complex individuals
In
older individuals and those with a history of complex or severe concomitant diseases,
close surveillance of hormonal therapy is essential (table 7). Age is not a contraindication
for initiation of gender-affirming hormone therapy. While studies on gender-affirming
hormone therapy in older trans individuals are limited, evidence suggests that transitioning
improves quality of life in this population [67].
Table 7Recommended modifications of
hormone therapy in the presence of older age, impaired organ function or
elevated cardiovascular and thromboembolic risk.
| Condition |
Masculinising
hormone therapy |
Feminising
hormone therapy |
| Older age; andro-/Menopause |
Monitor for cardiovascular risk factors (see section
“Cardiovascular
health and health risk behaviours”). Monitor for
osteoporosis (see section “Bone health”). Consider dose reduction analogous
cis-individuals. |
Transdermal oestradiol (>45 years). Monitor electrolytes/kidney function with
spironolactone use. Monitor for cardiovascular risk factors (see section
“Cardiovascular
health and health risk behaviours”). Monitor for osteoporosis (see section “Bone health”).
Consider dose reduction analogous cis-individuals |
| Severe liver disease |
Consider dose reduction/adjustment. Consider
estimating free testosterone for therapy guidance |
No
oral oestradiol or cyproterone acetate. No
dose adjustment. |
| Severe kidney disease (eGFR
<30 ml/min) |
Consider measuring free testosterone for therapy guidance
|
Avoid spironolactone. Decrease dose of oestradiol in end-stage renal
disease (eGFR <15 ml/min) |
| Risk factors for venous thromboembolism |
No
dose adjustment. |
Switch to transdermal oestradiol. Avoid cyproterone acetate. Avoid supraphysiological
oestradiol levels. Consider haematology referral. |
| Breast
cancer |
Conflicting
data. Consider stop due to possible aromatisation to oestradiol. Shared decision-making
person/gynaecologic-oncologist. |
Withhold
therapy, refer for shared decision-making with person/gynaecologic oncologist. If
therapy is continued, aim for lowest possible dose of oestradiol. |
| High cardiovascular risk |
Continuation seems safe; see section “Cardiovascular health
and health risk behaviours” |
Switch to transdermal oestradiol oestrogen. See section “Cardiovascular health and
health
risk behaviours”. |
Similar
to physiological changes in cis individuals, a reduction in gender-affirming
hormone therapy dosage may be considered [1].
There are no specific guidelines for discontinuing gender-affirming hormone
therapy at any specific age. In the absence of research evidence, a shared decision-making
approach is recommended to achieve individual goals while minimising potential adverse
effects. Given that approximately 50% of testosterone is metabolised by the liver,
dose reduction should be considered in individuals with severe liver conditions.
Oral forms of testosterone and oestrogen are not recommended. Chronic kidney disease
is associated with mild hypogonadotropic hypogonadism, so a mild dose reduction
might be indicated in cases of severe kidney disease [68].
Comprehensive multidisciplinary care
Collaboration with other disciplines
Successful gender-affirming care relies on ongoing interdisciplinary dialogue
and patient-centred coordination to optimise health outcomes and improve quality
of life [69]. While endocrinologists, gynaecologists
and mental health specialists are typically involved early in the transition process,
many other disciplines contribute to comprehensive care. Dermatologists provide
treatment for unwanted facial and body hair, often through laser or electrolysis,
and also address acne and other hormone-related skin changes [70]. Speech therapists
support transgender and gender-diverse individuals in voice
modification and communication style alignment, working on pitch, resonance and
expression early in transition. Surgeons specialising in maxillofacial, plastic
and reconstructive procedures play a central role in delivering gender-affirming
surgical interventions [8].
In the following sections, we will expand on key aspects of gender-affirming
care, including sexual health, gender-affirming surgery and the importance of collaboration
with paediatric teams to support young adults as they transition to adult care.
Sexual health including sexually transmitted infections
Gender-affirming therapies can profoundly impact sexual health. Before
gender-affirming hormone therapy or surgery, many transgender and gender-diverse individuals
report a negative body image, which may limit their sexual satisfaction. While transition
can improve this, surgeries
may cause loss of erogenous zones and sensory changes affecting pleasure [8, 71, 72].
Providers should engage in patient-centred
discussions about expectations and potential sexual side effects, covering anatomical,
physiological and psychosocial aspects. Counselling and sex therapy can help manage
distress or dysfunction. Pharmacological options like phosphodiesterase-5 inhibitors
for erectile dysfunction, topical oestrogen for vaginal dryness or low-dose testosterone
for low sexual desire disorders may support sexual functioning [73, 74].
Routine genital examinations may be distressing and are not necessary unless
there is evidence-based screening [75]. STI screening
is based on personalised risk evaluation, considering sexual practices, anatomy
and behaviours, rather than identity labels. HIV, Chlamydia trachomatis,
Neisseria gonorrhoeae, hepatitis B/C and syphilis are some of the most important
infections to check for [76]. Chlamydia screening
is not routine but may be indicated for sexually active adolescents or symptomatic
individuals. Vaginal self-sampling is preferred while urine testing is less sensitive
and specific. Screening in individuals with neovaginas is questionable, as infections
seem rare. In such cases, anorectal swabs or first-catch morning urine are more reliable
[77, 78].
The Safer Sex Check
tool is a valuable aid to personalised risk assessment and can be consulted in complete
confidentiality [79]. HIV pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir
disoproxil (Truvada®)
has been available since 2016 in Switzerland for individuals at high HIV risk and
is relevant in sexually active transgender and gender-diverse populations [80].
Contraceptive counselling is important for all transgender and gender-diverse individuals
engaging
in sexual relations at risk of pregnancy. Of note, testosterone does not reliably
prevent ovulation. No contraceptive method is contraindicated in people undergoing
masculinising hormone therapy as a result of this treatment [81, 82].
Gender-affirming surgery
Gender-affirming surgery is an important part of medical transition for
many transgender and gender-diverse individuals. Endocrinologists involved in gender-affirming
hormone
therapy should be familiar with common procedures and participate in presurgical
assessment and perioperative planning. Gender-affirming surgery varies by gender
identity and may include breast augmentation, facial feminisation, chondrolaryngoplasty,
vocal cord surgery, vaginoplasty or vulvoplasty for transfeminine individuals, and
chest reconstruction, phalloplasty, metoidioplasty or hysterectomy/oophorectomy
for transmasculine individuals [8].
A confirmed diagnosis of persistent, well-documented gender incongruence/dysphoria
is generally required
by health insurance companies, supported by psychiatric and/or endocrinological
reports. Informed shared decision-making is essential and preoperative hormone therapy
is not generally mandatory for surgery, but it may be needed or beneficial for certain
procedures [8, 83].
Preoperative assessments include comorbidities, cardiovascular and cancer
risks, and mental health evaluation. Fertility preservation must be addressed before
gonadectomy if not previously discussed. Surgeon selection is important, with recommended
criteria including experience in transgender and gender-diverse specific procedures,
supervised training and
continuing education.
Perioperative continuation of gender-affirming hormone therapy is generally
safe and does not significantly increase venous thromboembolism risk, though caution
is advised in older or high-risk individuals [84, 85].
Postoperative hormone therapy is needed after gonad removal to prevent hypogonadism
and long-term complications like cardiovascular disease or osteoporosis [47, 86].
Transition from adolescent to adult care
Transitioning transgender and gender-diverse individuals to adult care requires early,
structured
planning, ideally starting ≥2 years before transfer. Multidisciplinary follow-up
during adolescence and joint visits with adult providers help prevent treatment
gaps, especially in those with psychosocial or psychiatric comorbidities [8, 17].
Puberty blockers (GnRH agonists) may be offered to transgender and gender-diverse
adolescents after starting puberty (i.e.
at least Tanner stage 2); gender-affirming hormone therapy may be started later
during adolescence (around age 14–16 or later), and evaluation of indication and
follow-up by a specialised interdisciplinary team of at least paediatric endocrinologists
and mental health specialists are recommended. Fertility
counselling should be offered to all patients. However, genital surgery should be
postponed until the age of majority. Interdisciplinary management must be continued
into adulthood in a process known as “transition”. The transition process concludes
with the transfer of patient care from a specialised team for adolescents to one
for adults. The transition process must be individualised and lead to patient empowerment,
involving both patients and their relatives [87, 88].
Insurance coverage of gender-affirming hormone therapy
In Switzerland, gender-affirming hormone therapy is considered medically
necessary for individuals diagnosed with gender dysphoria and is covered by basic
health insurance.
Coverage includes consultations, medications, follow-up evaluations and laboratory
monitoring [89].
Reimbursement requires that treatment meets criteria for medical necessity
and effectiveness: (1) a medically relevant condition, (2) provision by certified
providers within Switzerland, (3) absence of explicit exclusion from insurance coverage
by legal regulation, and (4) the intervention being efficient and cost-effective [90].
Although coverage is standardised, some insurers may impose additional
requirements not permitted under Swiss law, such as “everyday life tests”, mandatory
sequencing of steps, minimum age limits, prior psychiatric treatments or exclusion
of non-binary individuals [91, 92].
Gender-affirming hormone therapy medications are typically prescribed off-label,
as none currently has formal approval for this indication [93]. Medications on the
official specialty list are
reimbursed without prior authorisation. When off-list or imported medications are
used, such as transdermal testosterone products, some insurers may ask for justification
and it is recommended to confirm reimbursement eligibility in advance [94].
Knowledge gaps and future directions
Additional research is needed to strengthen the evidence base for transgender
healthcare. Main areas include investigating the long-term effects of gender-affirming
hormone therapy on bone density, cardiometabolic health and fertility. Similarly,
more focus needs to be placed on documenting individuals who are ageing and nonbinary,
as well as those who have stopped or paused hormone therapy. The effect of medical
transition on quality of life and other outcomes in patients is also underexplored.
To address these knowledge gaps and advance evidence-based care, Switzerland
would benefit from the establishment of a national data collection infrastructure,
ideally through multicentre cohorts and long-term registries. This method would
allow for systematic surveillance of health outcomes, promote high-quality research
and form a base for clinical guidelines. Collaboration among healthcare providers,
academic institutions, professional organisations and patient advocacy groups will
be needed to promote knowledge exchange and ensure that emerging evidence is rapidly
translated into clinical practice.
Equally important is the integration of transgender health education into
medical school curricula and postgraduate training programmes. Currently, most healthcare
professionals in Switzerland receive little or no formal training in this area.
Improving education would enhance the competence of providers and contribute to
a more equitable and inclusive healthcare system for transgender and gender-diverse
individuals.
Checklist
These recommendations have been created to
provide transgender and gender-diverse people with safe, evidence-based and
affirming care. Every person’s goals, health needs and circumstances should be
taken into consideration when creating a plan for therapy. Flexibility, respect
for autonomy, and shared decision-making are essential.
Communication
- Use clear, affirming
language in all interactions.
- Record and use preferred names and pronouns.
Mental
health
- Offer a mental
health assessment before initiating hormone therapy.
- Explore coping
strategies, resilience and any support needs.
Fertility
preservation
- Discuss fertility
impact of hormone therapy and surgery before starting gender-affirming hormone
therapy and gender-affirming surgeries.
- Review sperm, egg,
embryo or gonadal tissue preservation options.
- If interest shown, refer
promptly to reproductive specialists.
Baseline
evaluation before hormone therapy
- Record weight,
height, BMI and vital signs.
- Perform baseline
labs: hormone levels (oestradiol, testosterone, LH, FSH); full blood count; lipids,
glucose and HbA1c; liver and renal function; electrolytes (especially if considering
spironolactone)
- Review medical
history for cardiovascular risk factors and relative contraindications.
- Advise about weight control
and smoking cessation, if applicable.
- Discuss expected
physical changes, timelines, reversibility and risks.
- Clarify
contraception needs and options (testosterone is not a contraceptive).
Hormone
therapy initiation and regimen
- Tailor dosing to
individual goals, risks and preferences.
- Transfeminine
patients: Prefer transdermal oestradiol if higher clot risk.
- Avoid ethinyl oestradiol
and conjugated oestrogens.
- Oestrogen therapy combined
with antiandrogen (cyproterone, spironolactone or GnRH analogues).
- Transmasculine
patients: Intramuscular or transdermal testosterone regimen based on the patient.
- Discuss menstrual
suppression options if desired.
Non-binary
care considerations
- Offer standard care
or low-dose or partial hormone regimens as appropriate.
- Clearly explain
expected effects and uncertainties.
- Monitor bone health
if prolonged hypogonadism occurs.
Follow-up
monitoring
- Schedule follow-up
visits: every 3 months during the first year; every 6–12 months thereafter.
- At each visit: review goals and satisfaction; measure weight, BMI and blood pressure;
discuss lifestyle behaviours including smoking, nutrition, physical activity; check
hormone levels to confirm targets; monitor lipids, glucose, liver and renal function;
assess for side effects (e.g. erythrocytosis, hyperkalaemia, thromboembolism); adjust
dosing based
on labs and clinical response.
Cancer
screening and preventive care
- Schedule cancer
screening based on retained organs: Breast: follow cis female guidelines if breast
tissue present. Cervix: Pap smears per guideline if cervix present. Prostate: individualise
screening in transfeminine patients.
- Discuss STI
screening tailored to sexual behaviour, not identity labels.
- Offer HIV PrEP if
indicated.
- Provide smoking
cessation counselling if applicable.
Older
or medically complex patients
- Use lower hormone
doses and prefer transdermal preparations.
- Monitor
cardiovascular risks closely.
- Avoid spironolactone
if eGFR <30 ml/min.
- If history of
cancer, coordinate with oncologist before restarting hormones.
Sexual
health support
- Discuss potential
effects on libido, function and sensitivity.
- Offer support for
sexual concerns, including sex therapy if needed.
- Consider
pharmacological options (PDE-5 inhibitors, topical oestrogens, low-dose
testosterone).
Gender-affirming
surgery
- Confirm diagnosis
and readiness with appropriate documentation.
- Ensure fertility
preservation discussed prior to gonadectomy.
- Refer to qualified
surgeons experienced in gender-affirming procedures.
- Plan
perioperative hormone management (generally continue hormones unless high VTE
risk).
Transition
from adolescence to adult care
- Start planning at
least 1–2 years before transfer to adult services.
- Use joint visits
with paediatric and adult teams if possible.
- Ensure continuous
care and clear handover.
Multidisciplinary
coordination
- Involve
endocrinology, primary care, mental health, reproductive health, dermatology,
speech therapy and surgery as needed.
- Assign a care
coordinator if available.
Documentation
and legal aspects
- Provide clear
records supporting medical necessity for insurance reimbursement.
- Confirm coverage for
non-standard treatments.
- Inform patients of
their rights to legal gender recognition and protections.
Research
and continuous improvement
- Encourage
participation in registries or studies when appropriate.
- Support ongoing
professional development in transgender healthcare.
Acknowledgments
Minor
stylistic revisions were assisted by ChatGPT (OpenAI, GPT-4, GPT-5), under the authors’
supervision. The SSED/SGED provided administrative
support for guideline development.
PD Dr med. Bettina Winzeler
Department of Endocrinology, Diabetology and Clinical Nutrition
Zurich University
Hospital
Raemistrasse 100
CH-8091 Zurich
bettina.winzeler[at]usz.ch
References
1. Coleman E, Radix AE, Bouman WP, Brown GR, de Vries AL, Deutsch MB, et al. Standards
of Care for the Health of Transgender and Gender Diverse People, Version 8. Int J
Transgender Health. 2022 Sep;23 Suppl 1:S1–259. doi: https://doi.org/10.1080/26895269.2022.2100644
2. Fiani CN, Han HJ. Navigating identity: Experiences of binary and non-binary transgender
and gender non-conforming (TGNC) adults. Int J Transgend. 2018;20(2–3):181–94. doi:
https://pubmed.ncbi.nlm.nih.gov/32999605/
3. D’hoore L, T’Sjoen G. Gender-affirming hormone therapy: an updated literature review
with an eye on the future. J Intern Med. 2022 May;291(5):574–92. doi: https://doi.org/10.1111/joim.13441
4. Cocchetti C, Ristori J, Romani A, Maggi M, Fisher AD. Hormonal Treatment Strategies
Tailored to Non-Binary Transgender Individuals. J Clin Med . 2020 Jun 1;9(6). doi:
/pmc/articles/PMC7356977/
5. Gender incongruence and transgender health in the ICD. [cited 2025 Aug 6]. Available
from: https://www.who.int/standards/classifications/frequently-asked-questions/gender-incongruence-and-transgender-health-in-the-icd
6. Fernández Rodríguez M. Gender Incongruence is No Longer a Mental Disorder. J Ment
Health Clin Psychol. 2018 Sep;2(5):6–8. doi: https://doi.org/10.29245/2578-2959/2018/5.1157
7. Nokoff NJ. Medical Interventions for Transgender Youth. [Updated 2022 Jan 19]. In:
Feingold KR, Ahmed SF, Anawalt B, et al., editors. South Dartmouth (MA): MDText.com,
Inc.; 2000. Table 2. [DSM-5 Criteria for Gender Dysphoria]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK577212/table/pediat_transgender.T.dsm5_criteria_for_g/
8. Coleman E, Radix AE, Bouman WP, Brown GR, de Vries AL, Deutsch MB, et al. Standards
of Care for the Health of Transgender and Gender Diverse People, Version 8. Int J
Transgender Health. 2022 Sep;23 Suppl 1:S1–259. 10.1080/26895269.2022.2100644
9. Ipsos. A 30-Country Ipsos Global Advisor Survey LGBT+ PRIDE 2023. 2023 [cited 2025
Aug 6]; Available from: https://www.ipsos.com/en/pride-month-2023-9-of-adults-identify-as-lgbt
10. Bouman WP, Schwend AS, Motmans J, Smiley A, Safer JD, Deutsch MB, et al. Language
and trans health. International Journal of Transgenderism. 2017 Jan 2;18(1):1–6. doi:
https://www.tandfonline.com/doi/abs/10.1080/15532739.2016.1262127
11. van de Grift TC, Mullender MG, Bouman MB. Shared Decision Making in Gender-Affirming
Surgery. Implications for Research and Standards of Care. J Sex Med. 2018 Jun;15(6):813–5.
doi: https://doi.org/10.1016/j.jsxm.2018.03.088
12. Clark BA, Virani A, Marshall SK, Saewyc EM. Conditions for shared decision making
in the care of transgender youth in Canada. Health Promot Int. 2021 Apr;36(2):570–80.
doi: https://doi.org/10.1093/heapro/daaa043
13. Rudolph H, Burgermeister N, Schulze J, Gross P, Hbscher E, Garcia Nuez D. Von der
Psychopathologisierung zum affirmativen Umgang mit Geschlechtervielfalt. Swiss Medical
Forum ‒ Schweizerisches Medizin-Forum. 2023. Available from: https://www.researchgate.net/publication/367411586_Von_der_Psychopathologisierung_zum_affirmativen_Umgang_mit_Geschlechtervielfalt doi: https://doi.org/10.4414/smf.2023.09300
14. SR 210 - Schweizerisches Zivilgesetzbuch vom 10. Dezember 1907. Fedlex. [cited 2025
Aug 6]. Available from: https://www.fedlex.admin.ch/eli/cc/24/233_245_233/de
15. Die Gruppe Verteidigung. [cited 2025 Aug 6]. Available from: https://www.vtg.admin.ch/de
16. SR 151.1 - Bundesgesetz vom 24. März 1995 über die Gleichstellung von Frau und Mann
(Gleichstellungsgesetz, GlG). Fedlex. [cited 2025 Aug 6]. Available from: https://www.fedlex.admin.ch/eli/cc/1996/1498_1498_1498/de#art_3
17. Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine
Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical
Practice Guideline. J Clin Endocrinol Metab. 2017 Nov;102(11):3869–903. 10.1210/jc.2017-01658
18. Haupt C, Henke M, Kutschmar A, Hauser B, Baldinger S, Saenz SR, et al. Antiandrogen
or estradiol treatment or both during hormone therapy in transitioning transgender
women. Cochrane Database Syst Rev. 2020 Nov;11(11):CD013138.
19. Glintborg D, T’Sjoen G, Ravn P, Andersen MS. MANAGEMENT OF ENDOCRINE DISEASE: optimal
feminizing hormone treatment in transgender people. Eur J Endocrinol. 2021 Jun;185(2):R49–63.
doi: https://doi.org/10.1530/EJE-21-0059
20. Kuijpers SM, Wiepjes CM, Conemans EB, Fisher AD, T’Sjoen G, den Heijer M. Toward a
Lowest Effective Dose of Cyproterone Acetate in Trans Women: Results From the ENIGI
Study. J Clin Endocrinol Metab. 2021 Sep;106(10):e3936–45. doi: https://doi.org/10.1210/clinem/dgab427
21. Hayes H, Russell R, Haugen A, Nagavally S, Sarvaideo J. The Utility of Monitoring
Potassium in Transgender, Gender Diverse, and Nonbinary Individuals on Spironolactone.
J Endocr Soc. 2022 Sep;6(11):bvac133. doi: https://doi.org/10.1210/jendso/bvac133
22. Angus LM, Nolan BJ, Zajac JD, Cheung AS. A systematic review of antiandrogens and
feminization in transgender women. Clin Endocrinol (Oxf). 2021 May;94(5):743–52. doi: https://doi.org/10.1111/cen.14329
23. Irwig MS. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017 Apr;5(4):301–11.
doi: https://doi.org/10.1016/S2213-8587(16)00036-X
24. Meriggiola MC, Gava G. Endocrine care of transpeople part I. A review of cross-sex
hormonal treatments, outcomes and adverse effects in transmen. Clin Endocrinol (Oxf).
2015 Nov;83(5):597–606. doi: https://doi.org/10.1111/cen.12753
25. Gava G, Mancini I, Cerpolini S, Baldassarre M, Seracchioli R, Meriggiola MC. Testosterone
undecanoate and testosterone enanthate injections are both effective and safe in transmen
over 5 years of administration. Clin Endocrinol (Oxf). 2018 Dec;89(6):878–86. doi: https://doi.org/10.1111/cen.13821
26. Wierckx K, Van de Peer F, Verhaeghe E, Dedecker D, Van Caenegem E, Toye K, et al. Short-
and long-term clinical skin effects of testosterone treatment in trans men. J Sex
Med. 2014 Jan;11(1):222–9. doi: https://doi.org/10.1111/jsm.12366
27. Fisher AD, Castellini G, Ristori J, Casale H, Cassioli E, Sensi C, et al. Cross-Sex
Hormone Treatment and Psychobiological Changes in Transsexual Persons: Two-Year Follow-Up
Data. J Clin Endocrinol Metab. 2016 Nov;101(11):4260–9. doi: https://doi.org/10.1210/jc.2016-1276
28. van Dijk D, Dekker MJ, Conemans EB, Wiepjes CM, de Goeij EG, Overbeek KA, et al. Explorative
Prospective Evaluation of Short-Term Subjective Effects of Hormonal Treatment in Trans
People-Results from the European Network for the Investigation of Gender Incongruence.
J Sex Med. 2019 Aug;16(8):1297–309. doi: https://doi.org/10.1016/j.jsxm.2019.05.009
29. Dickersin K, Munro MG, Clark M, Langenberg P, Scherer R, Frick K, et al.; Surgical
Treatments Outcomes Project for Dysfunctional Uterine Bleeding (STOP-DUB) Research
Group. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding:
a randomized controlled trial. Obstet Gynecol. 2007 Dec;110(6):1279–89. Available
from: https://pubmed.ncbi.nlm.nih.gov/18055721/ doi: https://doi.org/10.1097/01.AOG.0000292083.97478.38
30. Antun A, Zhang Q, Bhasin S, Bradlyn A, Flanders WD, Getahun D, et al. Longitudinal
Changes in Hematologic Parameters Among Transgender People Receiving Hormone Therapy.
J Endocr Soc. 2020 Aug;4(11):bvaa119. doi: https://doi.org/10.1210/jendso/bvaa119
31. Hashemi L, Zhang Q, Getahun D, Jasuja GK, McCracken C, Pisegna J, et al. Longitudinal
Changes in Liver Enzyme Levels Among Transgender People Receiving Gender Affirming
Hormone Therapy. J Sex Med. 2021 Sep;18(9):1662–75. doi: https://doi.org/10.1016/j.jsxm.2021.06.011
32. Stangl TA, Wiepjes CM, Defreyne J, Conemans E, D Fisher A, Schreiner T, et al. Is
there a need for liver enzyme monitoring in people using gender-affirming hormone
therapy? Eur J Endocrinol. 2021 Apr;184(4):513–20. doi: https://doi.org/10.1530/EJE-20-1064
33. Kristensen TT, Christensen LL, Frystyk J, Glintborg D. T’Sjoen G, Roessler KK, et
al. Effects of testosterone therapy on constructs related to aggression in transgender
men: A systematic review. Horm Behav. 2021 Feb 1;128. https://pubmed.ncbi.nlm.nih.gov/33309817/
34. Cocchetti C, Ristori J, Romani A, Maggi M, Fisher AD. Hormonal Treatment Strategies
Tailored to Non-Binary Transgender Individuals. J Clin Med. 2020 May;9(6):1609. doi: https://doi.org/10.3390/jcm9061609
35. Riggs DW, Bartholomaeus C. Fertility preservation decision making amongst Australian
transgender and non-binary adults. Reprod Health. 2018 Oct;15(1):181. 10.1186/s12978-018-0627-z
36. Amir H, Yaish I, Oren A, Groutz A, Greenman Y, Azem F. Fertility preservation rates
among transgender women compared with transgender men receiving comprehensive fertility
counselling. Reprod Biomed Online. 2020 Sep;41(3):546–54. doi: https://doi.org/10.1016/j.rbmo.2020.05.003
37. Wiepjes CM, Nota NM, de Blok CJ, Klaver M, de Vries AL, Wensing-Kruger SA, et al. The
Amsterdam Cohort of Gender Dysphoria Study (1972-2015): Trends in Prevalence, Treatment,
and Regrets. J Sex Med. 2018 Apr;15(4):582–90. doi: https://doi.org/10.1016/j.jsxm.2018.01.016
38. de Nie I, van Mello NM, Vlahakis E, Cooper C, Peri A, den Heijer M, et al. Successful
restoration of spermatogenesis following gender-affirming hormone therapy in transgender
women. Cell Rep Med. 2023 Jan;4(1):100858. doi: https://doi.org/10.1016/j.xcrm.2022.100858
39. Sterling J, Garcia MM. Fertility preservation options for transgender individuals.
Transl Androl Urol. 2020 Mar;9(S2 Suppl 2):S215–26. doi: https://doi.org/10.21037/tau.2019.09.28
40. Cho K, Harjee R, Roberts J, Dunne C. Fertility preservation in a transgender man without
prolonged discontinuation of testosterone: a case report and literature review. F
S Rep. 2020 Apr;1(1):43–7. doi: https://doi.org/10.1016/j.xfre.2020.03.003
41. Barbe JE, Del Vento F, Moussaoui D, Crofts VL, Yaron M, Streuli I. Oocyte cryopreservation
for fertility preservation in transgender and gender diverse individuals: a SWOT analysis.
J Assist Reprod Genet. 2025 Jul:1–9. 10.1007/s10815-025-03579-2
42. De Roo C, Schneider F, Stolk TH, van Vugt WL, Stoop D, van Mello NM. Fertility in
transgender and gender diverse people: systematic review of the effects of gender-affirming
hormones on reproductive organs and fertility. Hum Reprod Update. 2025 May;31(3):183–217.
10.1093/humupd/dmae036
43. Park SU, Sachdev D, Dolitsky S, Bridgeman M, Sauer MV, Bachmann G, et al. Fertility
preservation in transgender men and the need for uniform, comprehensive counseling.
F S Rep. 2022 Jul;3(3):253–63. doi: https://doi.org/10.1016/j.xfre.2022.07.006
44. Durcan E, Turan S, Bircan BE, Yaylamaz S, Okur I, Demir AN, et al. Fertility Desire
and Motivation Among Individuals with Gender Dysphoria: A Comparative Study. J Sex
Marital Ther. 2022;48(8):789–803. doi: https://doi.org/10.1080/0092623X.2022.2053617
45. Jackson SS, Brown J, Pfeiffer RM, Shrewsbury D, O’Callaghan S, Berner AM, et al. Analysis
of Mortality Among Transgender and Gender Diverse Adults in England. JAMA Netw Open.
2023 Jan;6(1):e2253687. doi: https://doi.org/10.1001/jamanetworkopen.2022.53687
46. de Blok CJ, Wiepjes CM, van Velzen DM, Staphorsius AS, Nota NM, Gooren LJ, et al. Mortality
trends over five decades in adult transgender people receiving hormone treatment:
a report from the Amsterdam cohort of gender dysphoria. Lancet Diabetes Endocrinol.
2021 Oct;9(10):663–70. doi: https://doi.org/10.1016/S2213-8587(21)00185-6
47. Robinson IS, Rifkin WJ, Kloer C, Parker A, Blasdel G, Shakir N, et al. Perioperative
Hormone Management in Gender-Affirming Mastectomy: Is Stopping Testosterone before
Top Surgery Really Necessary? Plast Reconstr Surg. 2023 Feb;151(2):421–7. [cited 2025
Aug 6] Available from: https://www.researchgate.net/publication/365403495_Perioperative_Hormone_Management_in_Gender-Affirming_Mastectomy_Is_Stopping_Testosterone_Before_Top_Surgery_Really_Necessary doi: https://doi.org/10.1097/PRS.0000000000009858
48. gynécologie suisse. Algorithmen zum Expertenbrief Nr. 50. 2018. Available from: https://www.sggg.ch/fileadmin/user_upload/Formulardaten/Algorithmen_zum_Expertenbrief_Nr_50_D.pdf
49. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual
diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544–6.
doi: https://doi.org/10.5489/cuaj.175
50. Miksad RA, Bubley G, Church P, Sanda M, Rofsky N, Kaplan I, et al. Prostate cancer
in a transgender woman 41 years after initiation of feminization. JAMA. 2006 Nov;296(19):2316–7.
doi: https://doi.org/10.1001/jama.296.19.2316
51. Nik-Ahd F, De Hoedt AM, Butler C, Anger JT, Carroll PR, Cooperberg MR, et al. Prostate-Specific
Antigen Values in Transgender Women Receiving Estrogen. JAMA. 2024 Jul;332(4):335–7.
doi: https://doi.org/10.1001/jama.2024.9997
52. van Zijverden LM, Wiepjes CM, van Diemen JJ, Thijs A, den Heijer M. Cardiovascular
disease in transgender people: a systematic review and meta-analysis. Eur J Endocrinol.
2024 Feb;190(2):S13–24. doi: https://doi.org/10.1093/ejendo/lvad170
53. Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, Davidge-Pitts CJ, Nippoldt TB, Prokop LJ,
et al. Sex Steroids and Cardiovascular Outcomes in Transgender Individuals: A Systematic
Review and Meta-Analysis. J Clin Endocrinol Metab. 2017 Nov;102(11):3914–23. doi: https://doi.org/10.1210/jc.2017-01643
54. Leemaqz SY, Kyinn M, Banks K, Sarkodie E, Goldstein D, Irwig MS. Lipid profiles and
hypertriglyceridemia among transgender and gender diverse adults on gender-affirming
hormone therapy. J Clin Lipidol. 2023;17(1):103–11. doi: https://doi.org/10.1016/j.jacl.2022.11.010
55. Kcomt L, Evans-Polce RJ, Veliz PT, Boyd CJ, McCabe SE. Use of Cigarettes and E-Cigarettes/Vaping
Among Transgender People: Results From the 2015 U.S. Transgender Survey. Am J Prev
Med. 2020 Oct;59(4):538–47. doi: https://doi.org/10.1016/j.amepre.2020.03.027
56. Fredriksen-Goldsen KI, Cook-Daniels L, Kim HJ, Erosheva EA, Emlet CA, Hoy-Ellis CP,
et al. Physical and mental health of transgender older adults: an at-risk and underserved
population. Gerontologist. 2014 Jun;54(3):488–500. doi: https://doi.org/10.1093/geront/gnt021
57. Streed CG Jr, Beach LB, Caceres BA, Dowshen NL, Moreau KL, Mukherjee M, et al.; American
Heart Association Council on Peripheral Vascular Disease; Council on Arteriosclerosis,
Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council
on Cardiovascular Radiology and Intervention; Council on Hypertension; and Stroke
Council. Assessing and Addressing Cardiovascular Health in People Who Are Transgender
and Gender Diverse: A Scientific Statement From the American Heart Association. Circulation.
2021 Aug;144(6):e136–48. doi: https://doi.org/10.1161/CIR.0000000000001003
58. Vlot MC, Wiepjes CM, de Jongh RT, T’Sjoen G, Heijboer AC, den Heijer M. Gender-Affirming
Hormone Treatment Decreases Bone Turnover in Transwomen and Older Transmen. J Bone
Miner Res. 2019 Oct;34(10):1862–72. doi: https://doi.org/10.1002/jbmr.3762
59. Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, et al. Body
composition, bone turnover, and bone mass in trans men during testosterone treatment:
1-year follow-up data from a prospective case-controlled study (ENIGI). Eur J Endocrinol.
2015 Feb;172(2):163–71. doi: https://doi.org/10.1530/EJE-14-0586
60. Rosen HN, Hamnvik OR, Jaisamrarn U, Malabanan AO, Safer JD, Tangpricha V, et al. Bone
Densitometry in Transgender and Gender Non-Conforming (TGNC) Individuals: 2019 ISCD
Official Position. J Clin Densitom. 2019;22(4):544–53. doi: https://doi.org/10.1016/j.jocd.2019.07.004
61. Singh-Ospina N, Maraka S, Rodriguez-Gutierrez R, Davidge-Pitts C, Nippoldt TB, Prokop LJ,
et al. Effect of Sex Steroids on the Bone Health of Transgender Individuals: A Systematic
Review and Meta-Analysis. J Clin Endocrinol Metab. 2017 Nov;102(11):3904–13. doi: https://doi.org/10.1210/jc.2017-01642
62. Fighera TM, Ziegelmann PK, Rasia da Silva T, Spritzer PM. Bone Mass Effects of Cross-Sex
Hormone Therapy in Transgender People: Updated Systematic Review and Meta-Analysis.
J Endocr Soc. 2019 Mar;3(5):943–64. doi: https://doi.org/10.1210/js.2018-00413
63. Wiepjes CM, Vlot MC, Klaver M, Nota NM, de Blok CJ, de Jongh RT, et al. Bone Mineral
Density Increases in Trans Persons After 1 Year of Hormonal Treatment: A Multicenter
Prospective Observational Study. J Bone Miner Res. 2017 Jun;32(6):1252–60. doi: https://doi.org/10.1002/jbmr.3102
64. Giacomelli G, Meriggiola MC. Bone health in transgender people: a narrative review.
Ther Adv Endocrinol Metab. 2022 May;13:20420188221099346. doi: https://doi.org/10.1177/20420188221099346
65. Ferrari S, Lippuner K, Lamy O, Meier C. 2020 recommendations for osteoporosis treatment
according to fracture risk from the Swiss Association against Osteoporosis (SVGO).
Swiss Med Wkly. 2020 Sep;150(3940):w20352. doi: https://doi.org/10.4414/smw.2020.20352
66. Verroken C, Collet S, Lapauw B, T’Sjoen G. Osteoporosis and Bone Health in Transgender
Individuals. Calcif Tissue Int. 2022 May;110(5):615–23. 10.1007/s00223-022-00972-2
67. Cai X, Hughto JM, Reisner SL, Pachankis JE, Levy BR. Benefit of Gender-Affirming Medical
Treatment for Transgender Elders: Later-Life Alignment of Mind and Body. LGBT Health.
2019 Jan;6(1):34–9. doi: https://doi.org/10.1089/lgbt.2017.0262
68. Collister D, Saad N, Christie E, Ahmed S. Providing Care for Transgender Persons With
Kidney Disease: A Narrative Review. Can J Kidney Health Dis. 2021 Jan;8:2054358120985379.
doi: https://doi.org/10.1177/2054358120985379
69. Hancock AB, Siegfriedt LL. Transforming Voice and Communication with Transgender and
Gender-Diverse People: An Evidence-Based Process. San Diego (CA): Plural Publishing
Inc.; 2020.
70. Ginsberg BA. Dermatologic care of the transgender patient. Int J Womens Dermatol.
2016 Dec;3(1):65–7. doi: https://doi.org/10.1016/j.ijwd.2016.11.007
71. Miroshnychenko A, Ibrahim S, Roldan Y, Kulatunga-Moruzi C, Montante S, Couban R, et
al. Gender affirming hormone therapy for individuals with gender dysphoria aged <26
years: a systematic review and meta-analysis. Arch Dis Child. 2025 May;110(6):437–45.
doi: https://doi.org/10.1136/archdischild-2024-327921
72. van de Grift TC, Elaut E, Cerwenka SC, Cohen-Kettenis PT, De Cuypere G, Richter-Appelt H,
et al. Effects of Medical Interventions on Gender Dysphoria and Body Image: A Follow-Up
Study. Psychosom Med. 2017 Sep;79(7):815–23. doi: https://doi.org/10.1097/PSY.0000000000000465
73. Krakowsky Y, Grober ED. A practical guide to female sexual dysfunction: an evidence-based
review for physicians in Canada. Can Urol Assoc J. 2018 Jun;12(6):211–6. doi: https://doi.org/10.5489/cuaj.4907
74. Steers WD. Pharmacologic treatment of erectile dysfunction. Rev Urol. 2002;4(Suppl
3 Suppl 3):S17–25. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC1476024/
75. Qaseem A, Humphrey LL, Harris R, Starkey M, Denberg TD; Clinical Guidelines Committee
of the American College of Physicians. Screening pelvic examination in adult women:
a clinical practice guideline from the American College of Physicians. Ann Intern
Med. 2014 Jul;161(1):67–72. doi: https://doi.org/10.7326/M14-0701
76. Bockting WO, Robinson BE, Forberg J, Scheltema K. Evaluation of a sexual health approach
to reducing HIV/STD risk in the transgender community. AIDS Care. 2005 Apr;17(3):289–303.
10.1080/09540120412331299825
77. Aaron KJ, Griner S, Footman A, Boutwell A, Van Der Pol B. Vaginal Swab vs Urine for
Detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis: A Meta-Analysis. Ann Fam Med. 2023;21(2):172–9. doi: https://doi.org/10.1370/afm.2942
78. Radix AE, Harris AB, Belkind U, Ting J, Goldstein ZG. Chlamydia trachomatis Infection of the Neovagina in Transgender Women. Open Forum Infect Dis. 2019 Nov;6(11):ofz470.
doi: https://doi.org/10.1093/ofid/ofz470
79. Home: LOVE LIFE - Sex aber sicher. [cited 2025 Aug 6]. Available from: https://lovelife.ch/de
80. Recommendations of the Swiss Federal Commission for Sexual Health (FCSH) on pre-exposure
prophylaxis (PrEP) for HIV prevention. [cited 2025 Aug 6]; Available from: www.who.int/hiv/pub/
81. PROFA | Contraception transmasculine: un mémo pour les pros. [cited 2025 Aug 6]. Available
from: https://www.profa.ch/contraception-transmasculine
82. Okano SH, Pellicciotta GG, Braga GC. Contraceptive Counseling for the Transgender
Patient Assigned Female at Birth. RBGO Gynecology & Obstetrics. 2022 Sep 1;44(9):884.
doi: https://doi.org/10.1055/s-0042-1751063
83. Gelles-Soto D, Ward D, Florio T, Kouzounis K, Salgado CJ. Maximizing surgical outcomes
with gender affirming hormone therapy in gender affirmation surgery. J Clin Transl
Endocrinol. 2024 May;36:100355. doi: https://doi.org/10.1016/j.jcte.2024.100355
84. Hontscharuk R, Alba B, Manno C, Pine E, Deutsch MB, Coon D, et al. Perioperative Transgender
Hormone Management: Avoiding Venous Thromboembolism and Other Complications. Plast
Reconstr Surg. 2021 Apr;147(4):1008–17. doi: https://doi.org/10.1097/PRS.0000000000007786
85. Kozato A, Fox GW, Yong PC, Shin SJ, Avanessian BK, Ting J, et al. No Venous Thromboembolism
Increase Among Transgender Female Patients Remaining on Estrogen for Gender-Affirming
Surgery. J Clin Endocrinol Metab. 2021 Mar;106(4):e1586–90. doi: https://doi.org/10.1210/clinem/dgaa966
86. Herndon J, Gupta N, Davidge-Pitts C, Imhof N, Gonzalez C, Carlson S, et al. Genital
Surgery Outcomes Using an Individualized Algorithm for Hormone Management in Transfeminine
Individuals. J Clin Endocrinol Metab. 2024 Oct;109(11):2774–83. doi: https://doi.org/10.1210/clinem/dgae269
87. Salas-Humara C, Sequeira GM, Rossi W, Dhar CP. Gender affirming medical care of transgender
youth. Curr Probl Pediatr Adolesc Health Care. 2019 Sep;49(9):100683. doi: https://doi.org/10.1016/j.cppeds.2019.100683
88. Mellerio H, Jacquin P, Trelles N, Le Roux E, Belanger R, Alberti C, et al. Validation
of the “Good2Go”: the first French-language transition readiness questionnaire. Eur
J Pediatr. 2020 Jan;179(1):61–71. 10.1007/s00431-019-03450-4
89. SR 832.102 - Verordnung vom 27. Juni 1995 über die Krankenversicherung (KVV). Fedlex.
[cited 2025 Aug 6]. Available from: https://www.fedlex.admin.ch/eli/cc/1995/3867_3867_3867/de
90. 07 - WZW - SGAIM - SSMIG - SSGIM. [cited 2025 Aug 6]. Available from: https://www.sgaim.ch/de/themen/swissdrg-blog/07-wzw
91. SCHLUMPF c. SUISSE. [cited 2025 Aug 6]. Available from: https://hudoc.echr.coe.int/fre#{%22itemid%22:[%22001-90476%22]}
92. Recht | TGNS Transgender Network Switzerland. [cited 2025 Aug 6]. Available from:
https://www.tgns.ch/de/information/rechtliches/recht/
93. Remuneration of medicinal products in individual cases. [cited 2025 Aug 21]. Available
from: https://www.bag.admin.ch/de/verguetung-von-arzneimitteln-im-einzelfall
94. Fedlex. Verordnung über die Krankenversicherung / Ordonnance
sur l’assurance-maladie. Available from: https://www.fedlex.admin.ch/eli/cc/1995/3867_3867_3867/de
