Quality assessment of Andrographis paniculata products reveals significant labelling inaccuracies and contaminations
DOI: https://doi.org/https://doi.org/10.57187/s.4728
Angélique Bourquiab,
Hugo Morincd,
Robin Hubercd,
Chantal Csajkabcd,
Clara Podmorea,
Jean-Luc Wolfendercd,
Emerson Ferreira Queirozcd*,
Pierre-Yves Rodondia*
a Institute
of Family Medicine, Faculty of Science and Medicine, University of Fribourg,
Fribourg, Switzerland
b Centre for Research and Innovation in Clinical Pharmaceutical Sciences, University
Hospital and University of Lausanne, Lausanne, Switzerland
c School of
Pharmaceutical Sciences, University of Geneva, CMU, Geneva, Switzerland
d Institute of Pharmaceutical Sciences
of Western Switzerland, University of Geneva, CMU, Geneva, Switzerland
* Equal
contribution as last authors
Summary
BACKGROUND: Andrographis paniculata products
have gained in popularity for the management of respiratory infections since the
COVID-19 pandemic. None of these products holds marketing authorisation and all
are sold as herbal food supplements. Current herbal food supplement regulations
generally do not impose quality assessments prior to commercialisation, such that
the quality of herbal food supplements available to consumers is largely unknown.
STUDY
AIM: To assess the quality, purity and labelling accuracy of A. paniculata-containing
products, focusing on andrographolide content (the pharmaceutically active component)
and the presence of contaminants and residues.
METHODS: Forty A. paniculata-containing
products were purchased from 13 countries: 13 from pharmacies and 27 from online
retailers readily accessible to consumers in Switzerland. Samples were analysed
using ultra-high-performance liquid chromatography–ultraviolet (UHPLC-UV) and ultra-high-performance
liquid chromatography–mass spectrometry (UHPLC-MS) based on the European Pharmacopoeia
method. Contaminants and residues were assessed using inductively coupled plasma
mass spectrometry and gas chromatography–mass spectrometry, respectively.
RESULTS: All samples except one contained A.
paniculata. The measured daily dose of andrographolide was compared to the labelled dose.
Andrographolide content ranged from 29% to 174% of the labelled dose, with only
2 products accurately labelled, while 20 were underdosed and 1 overdosed. Two products
contained quercetin, which interfered with UHPLC-UV analysis. Additionally, three
online-purchased products contained toxic contaminants, including a heavy metal
(mercury) or pesticides (strychnine, butralin).
CONCLUSION: This study reveals widespread mislabelling
and underdosing in A. paniculata-containing food supplements marketed internationally,
along with the presence of impurities that pose risks to consumers in products bought
online. Regulatory authorities must implement stringent quality controls to ensure
consumer safety and product transparency.
Abbreviations
- A. paniculata
-
Andrographis paniculata
- HPLC
-
high-performance liquid chromatography
- MS
-
mass spectrometry
- UHPLC
-
ultra-high-performance liquid chromatography
Introduction
One in four people in Switzerland take food supplements, including
herbal products [1]. Herbal
products are consumed for a variety of purposes, often driven by the perception
that they are ‘natural’, so less harmful than conventional pharmaceutical medicines.
Herbal products
are considered herbal medicinal products or herbal food supplements depending
on whether they are marketed with a health or with a nutritional claim, respectively,
as well as the dosage of the pharmacologically active substance [2]. This in turn
determines whether they are subject to drug or food regulations [3, 4]. In Europe,
several governments, including Switzerland, hold a list of botanicals that can only
be commercialised as a herbal medicinal product and not a herbal food supplement,
mainly because of safety concerns [5].
Despite their widespread use,
herbal food supplements are generally not subject to safety or quality assessment
before commercialisation, so their quality depends on the manufacturer [3]. Also,
the regulatory framework for herbal food supplements differs from country to country
and is generally less rigorous than that of herbal medicinal products [3]. Concerns
have been raised
about the safety of various herbal products, including unsanitary manufacturing
conditions, adulteration, plant misidentification, contamination with heavy metals
or pesticides, and inaccurate labelling [6, 7]. Despite this, few high-quality studies
have assessed
the quality and safety of herbal food supplements.
Andrographis paniculata is an Asian medicinal plant primarily used in
traditional medicine to treat respiratory infections [8, 9]. A. paniculata is currently available worldwide, but only as a herbal food supplement
[10]. Andrographolide, the
main component and the pharmaceutically active substance, is thought to have anti-inflammatory,
antiviral and immunity-stimulating
properties [11]. A systematic review and meta-analysis of randomised controlled trials
suggested
that A. paniculata extract (an andrographolide dose of 60 mg/day) may reduce
the severity and frequency of cough symptoms of upper respiratory tract infections
[12, 13], but robust clinical evidence is still lacking [9]. More recently, the
Thai government recommended A. paniculata as a remedy for mild cases of SARS-CoV-2
infections [14], leading to a surge in consumption, including in Western countries
where it was already used for the symptomatic treatment of the common cold [15],
sometimes outpacing regulatory oversight.
We investigated the quality
and purity of herbal products marketed as containing A. paniculata, obtained
from pharmacies or purchased online internationally – including from Switzerland
– focusing on contaminants (heavy metals) and residues (pesticides). We developed
an analytical
approach based on ultra-high-performance liquid chromatography (UHPLC) combined with
two independent
detection methods – mass
spectrometry (MS) and ultraviolet (UV) – to analyse 40 commercially available A.
paniculata-containing products.
Materials and methods
The sample of herbal products containing Andrographis paniculata
Between 3 February and 18 August
2023, 40 products containing A. paniculata were purchased by the investigators
and analysed before their expiry date. Of these, 13 were purchased opportunistically
directly from pharmacies (Ph) in seven countries, including Switzerland, and 27
were ordered from online sources (On) accessible from Switzerland (table 1). To
be included in the study, the product had to mention A. paniculata on its
labelling and had to be intended for oral use. One pack of each product was purchased,
and the brand name, place of purchase, formulation, indications, labelled dose of
A. paniculata and andrographolide per serving, recommended dosages, Good
Manufacturing Practice certification from the manufacturer, and cost per pack were
recorded.
Table 1Characteristics of Andrographis
paniculata-containing products obtained from labelling and estimated daily dose
of Andrographis paniculata and andrographolide.
| Product code |
Country of purchase/dispatch |
Formulation |
Manufacturer claims
GMP compliance? |
Number of other ingredients |
Maximum daily dosage |
Daily dose of, mg/day * |
Daily dose of andrographolide,
mg/day * |
| Ph1 |
Switzerland |
Powder |
No |
0 |
NA |
NA |
NA |
| Ph2 |
Switzerland |
Capsule |
No |
0 |
6 |
720 |
NA |
| Ph3 |
Switzerland |
Tablet |
No |
4 |
2 |
200 |
20 |
| Ph4 |
France |
Capsule |
No |
11 |
4 |
200 |
10 |
| Ph5 |
USA |
Capsule |
Yes |
0 |
1 |
400 |
80 |
| Ph6 |
USA |
Capsule |
No |
0 |
2 |
1600 |
48 |
| Ph7 |
Germany |
Capsule |
No |
0 |
2 |
700 |
35 |
| Ph8 |
USA |
Capsule |
No |
0 |
2 |
800 |
60 |
| Ph9 |
Luxembourg |
Capsule |
No |
0 |
2 |
1000 |
100 |
| Ph10 |
USA |
Capsule |
Yes |
0 |
3 |
1200 |
120 |
| Ph11 |
Thailand |
Capsule |
No |
0 |
3 |
600 |
180 |
| Ph12 |
Canada |
Capsule |
Yes |
0 |
3 |
1200 |
120 |
| Ph13 |
Switzerland |
Liquid |
No |
0 |
NA |
NA |
NA |
| On1 |
USA |
Capsule |
Yes |
0 |
6 |
3000 |
NA |
| On2 |
Belgium |
Capsule |
Yes |
0 |
6 |
NA |
NA |
| On3 |
Germany |
Capsule |
No |
0 |
2 |
800 |
NA |
| On4 |
Netherlands |
Capsule |
No |
0 |
2 |
800 |
NA |
| On5 |
UK |
Capsule |
No |
0 |
4 |
1880 |
NA |
| On6 |
Germany |
Capsule |
No |
0 |
10 |
2700 |
NA |
| On7 |
Germany |
Capsule |
No |
0 |
2 |
700 |
35 |
| On8 |
Poland |
Capsule |
No |
0 |
4 |
2000 |
NA |
| On9 |
Austria |
Capsule |
No |
0 |
2 |
700 |
35 |
| On10 |
Germany |
Capsule |
Yes |
0 |
1 |
400 |
40 |
| On11 |
USA |
Capsule |
Yes |
0 |
1 |
400 |
80 |
| On12 |
Lithuania |
Capsule |
Yes |
0 |
2 |
800 |
NA |
| On13 |
USA |
Capsule |
Yes |
0 |
1 |
400 |
40 |
| On14 |
France |
Capsule |
No |
1 |
6 |
2250 |
132 |
| On15 |
USA |
Capsule |
Yes |
0 |
2 |
900 |
150 |
| On16 |
France |
Capsule |
No |
0 |
2 |
1000 |
100 |
| On17 |
Belgium |
Capsule |
No |
0 |
2 |
1000 |
100 |
| On18 |
USA |
Capsule |
Yes |
0 |
4 |
1600 |
40 |
| On19 |
Austria |
Capsule |
No |
0 |
3 |
900 |
90 |
| On20 |
USA |
Capsule |
Yes |
0 |
3 |
1200 |
120 |
| On21 |
France |
Capsule |
No |
0 |
6 |
1800 |
180 |
| On22 |
USA |
Liquid |
No |
0 |
2.8 ml |
700 |
NA |
| On23 |
Austria |
Liquid |
No |
0 |
60 drops |
NA |
NA |
| On24 |
Poland |
Liquid |
No |
0 |
3 ml |
NA |
NA |
| On25 |
USA |
Liquid |
No |
0 |
360 drops |
NA |
NA |
| On26 |
USA |
Liquid |
No |
0 |
80 drops |
2857 |
NA |
| On27 |
USA |
Liquid |
No |
0 |
30 drops |
NA |
NA |
Pharmacy-purchased products
Pharmacies selling products
containing A. paniculata were identified opportunistically in seven countries
in Europe, Asia and North America. Countries, and consequently pharmacies, were
selected based on their proximity to the authors’ places of residence or locations
visited for professional or personal reasons. Researchers, acting as lay customers,
purchased A. paniculata over the counter for health reasons. Pharmacy staff
were unaware that these samples would be analysed for research purposes. Products
were purchased based on the pharmacist’s recommendation; the investigators did not
request a specific brand. In Switzerland, some formulations of A. paniculata
require a medical prescription, so these products were obtained directly by traditional
Chinese medicine physicians or therapists.
Online-purchased products
A. paniculata-containing products were also
ordered online for delivery to Switzerland. The online search was conducted using
three different search engines: Google, Ecosia and Bing. Search terms used were:
“Andrographis”, “Andrographis paniculata”, “Kalmegh” (the Thai name of A. paniculata),
“Buy Andrographis” and “Andrographis dietary supplements”. The first author clicked
on the hyperlinks from the first results page
of each search engine, which led directly to company websites selling A. paniculata-containing
products. All such products available on these websites were purchased, without
setting a maximum number of products per site. Product labels were not considered
during the selection process.
Chemicals and extracts
In this study, the reference
extract of A. paniculata was sourced from the United States Pharmacopeia
(USP; Rockville, MD, United States). Standard reference compounds, including andrographolide,
neoandrographolide and andrographiside, were purchased from Merck Sigma Aldrich
(Saint Louis, MO, United States). Quercetin was procured from Fluka (Buchs, Switzerland).
All references are reported in supplementary file 1 in the appendix, available for
download as separate file at https://doi.org/10.57187/s.4728.
Laboratory analyses
For each A. paniculata
product, analyses were performed in triplicate.
Qualitative assessment of Andrographis paniculata
The qualitative evaluation
of A. paniculata was carried out in all 40 products (solids and liquids)
using ultra-high-performance liquid chromatography
(UHPLC)–high-resolution mass spectrometry (HRMS), which identifies compounds based
on their retention time (RT) and exact mass (m/z) (method detailed in supplementary
file 2 in the appendix (available for download as a separate file at https://doi.org/10.57187/s.4728).
Sample preparation is described in the appendix, supplementary file 3. A reference
extract of A. paniculata was used to authenticate products by providing a
characteristic chemical signature (supplementary file 1 in the appendix). The
identity of the three most intense peaks was confirmed using exact mass and pure
standards of andrographolide, neoandrographolide and andrographiside.
Quantification of andrographolide
The quantity
of andrographolide was measured in 33 solid products, while liquid samples were
excluded due to insufficient content information. Analysis was performed using ultraviolet
and mass spectrometry detection, as detailed in the appendix (supplementary file
4 in the appendix).
HPLC-UV, the European Pharmacopoeia reference method, is widely used by manufacturers
for quantifying bioactive compounds in plant-based supplements due to its accessibility
[16]. Mass spectrometry
detection was employed to resolve co-eluting signals inherent to complex matrices,
ensuring greater selectivity.
Analysis of contaminants and residues
All 40 samples were analysed
for the presence of contaminants and residues. Measurements for heavy metals and
pesticides were performed at an accredited laboratory (ISO 17025). For the identification
and quantification of heavy metals, the samples were prepared using a method involving
high-pressure microwave-assisted acid digestion. Arsenic, lead, cadmium, mercury
and nickel were analysed using inductively coupled plasma mass spectrometry (single-quadrupole
inductively coupled plasma–mass spectrometry [ICP-MS]). The assay
detection range for arsenic was 0.01–0.4 mg/kg dry matter; for lead, it was 0.003–0.4
mg/kg (dry matter); for cadmium, 0.002–0.4 mg/kg mg/kg (dry matter); for mercury,
0.005–0.4 mg/kg (dry matter); and for nickel, 0.1–0.4 mg/kg (dry matter). More-concentrated
samples were diluted in accordance with these detection ranges. Pesticides were
extracted using the QuEChERS method and subsequently analysed by liquid chromatography-mass
spectrometry/mass spectrometry [17].
Labelled and measured daily doses of andrographolide
For each solid A. paniculata product with
complete labelling, we calculated the labelled daily dose by multiplying the single
serving dose by the maximum daily servings (dosage) given on the labelling. We calculated
the labelled andrographolide daily dose based on its proportion in the extract.
For instance, product Ph10’s labelling indicated 400 mg of A. paniculata
per capsule with 10% andrographolide; with three capsules daily, the labelled doses
were 1200 mg and 120 mg, respectively. Similarly, we calculated the measured daily
dose of andrographolide for each sample using the average of the triplicate values
for both ultraviolet and mass spectrometry methods. For comparison of measured versus
labelled daily dose of andrographolide, and versus the daily therapeutic dose, we
considered results obtained using UHPLC-UV. However, mass spectrometry results were
prioritised when there were substantial differences between these two methods. HPLC-UV
is insufficient when it comes to analysing complex matrices such as those based
on several plant extracts.
Statistical analyses
We first compared andrographolide
quantification results from UHPLC-UV and UHPLC-MS methods by analysing the measured
daily dose of andrographolide using a two-tailed student’s t-test, Pearson correlation
and Bland–Altman analysis, considering p-values <0.05 as statistically significant.
We then compared the measured and labelled daily doses, considering any deviations
beyond the ±10% pharmacopoeial tolerance as inaccurately labelled [18]. Finally,
we assessed whether the measured daily dose in each product met the recommended
therapeutic dose (60 mg/day) for upper respiratory tract infections [8].
Ethics
Ethics approval was not required for this study
as it did not involve human participants, animal subjects or any other ethical concerns.
Results
Andrographis paniculata-containing
products
In this quality control study
of 40 herbal products containing A. paniculata, 13 were purchased from pharmacies
(Ph) and 27 online (On), as detailed in table 1; 24 came from European countries,
15 from the USA or Canada and 1 from Thailand. The cost of a 7-day supply ranged
from CHF 10.30 to 67.15 with an average of CHF 27.40.
The majority of products (33/40) were sold in a
solid form (mainly capsules), while the remaining 7 were in a liquid form. According
to labelling, 37 products had been prepared exclusively with an extract of A.
paniculata, 1 with a mixture of A. paniculata and Eleutherococcus
senticosus (On14) and 2 with a mixture of plants and vitamins (Ph3 and Ph4).
Seventeen products lacked labelling information about the quantity of andrographolide;
14 of these had been purchased online and 3 from pharmacies.
One product (identical brand,
packaging and labelling) was unintentionally purchased three times from three different
sources (On20, Ph12 and Ph10), and three other products were purchased twice (On7
and Ph7; On11 and Ph5; On17 and Ph9). Despite this, each sample was analysed as
an individual product. One product (On1) was received unlabelled, and another product
(solid) was excluded from the analysis as it had expired by the time the quality
control analyses were performed.
Quality assessment of Andrographis paniculata extract
Before analysing products containing
A. paniculata, we characterised a standardised reference methanolic extract
of this plant using UHPLC-HRMS. This analysis revealed the presence of signals attributable
to characteristic compounds already described from this plant, belonging to the
diterpene lactone chemical family [19] (figure S1 in the appendix,
available for download as a separate file at https://doi.org/10.57187/s.4728). The
chromatogram showed andrographolide as the most intense peak (RT = 2.06 min, m/z
395.2070 [M+HCOO]−, Δ: 0.25 ppm), followed by neoandrographolide
(RT = 2.48 min, m/z 525.2706 [M+HCOO]−, Δ: 1.20 ppm) and andrographiside
(RT = 1.73 min, m/z 557.2601 [M+HCOO]−, Δ: 0.54 ppm). These identities
were confirmed by comparisons with pure reference standards. Additionally, ultraviolet
detection at 220 nm highlighted andrographolide as the compound with significant
absorption. Given its status as the literature-established bioactive constituent
and its chromatographic predominance, andrographolide was selected as the primary
chemical marker for quality control of A. paniculata-based products [15].
Among the 40 products analysed,
only one (On1) lacked the 3 characteristic peaks presented in the appendix (supplementary
file 5, figure S1).
Most products had a smaller andrographolide peak and a larger neoandrographolide
peak than the reference standard. In five products (Ph1, Ph2, On2, On12, On25),
neoandrographolide was the most intense analyte.
In addition
to the A. paniculata extract, Ph3 and Ph4 contained an abundant flavonoid,
quercetin. Using high-resolution mass spectrometry, an intense signal of
m/z: 301.0351 ([M−H]−) was recorded at the very same retention
time of andrographolide and unexpectedly corresponded to the exact mass of quercetin.
This was corroborated by the ultraviolet detection, which revealed a typical flavonoid
ultraviolet spectrum for this peak (figure 1). Finally, the injection of a pure
standard of quercetin confirmed co-elution of this compound and andrographolide
(detailed further in the appendix, supplementary file 5). To prevent bias in measurement,
a mass spectrometry detection method was developed in addition to the standard ultraviolet
method.
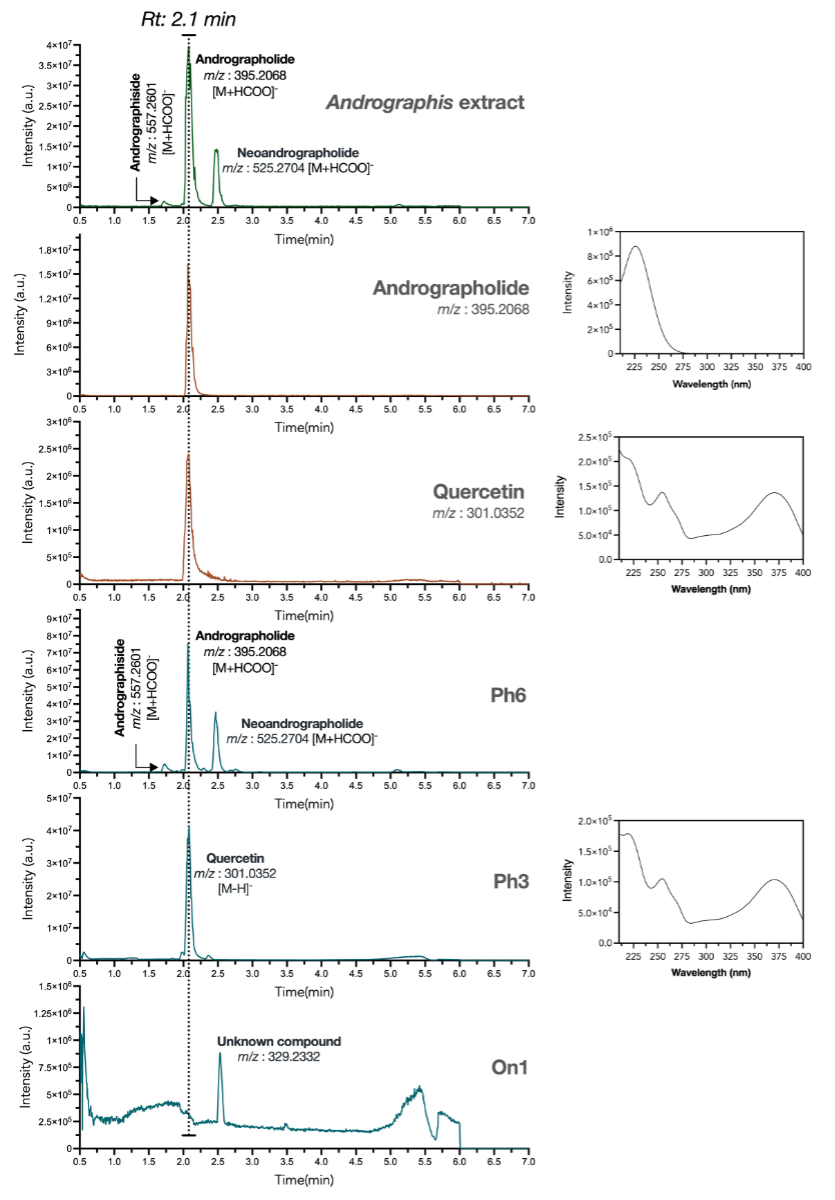
Figure 1UHPLC-PDA-HRMS chromatograms, comparing
standardised extract of Andrographis paniculata (green trace) vs marketed herbal products
(blue traces) and pure standards (orange traces). Andrographolide and quercetin
were found to elute at the same retention time (rt: 2.1 min) but were unequivocally
discriminated after their exact mass. Various herbal products differed in chemical
content: Ph6 resembled A. paniculata extract, Ph3 contained andrographolide
with a dominant quercetin signal and On1 lacked characteristic A. paniculata
signals. HRMS: high-resolution mass spectrometry; On: online; PDA: photo diode array;
Ph: pharmacy; UHPLC: ultra-high-performance liquid chromatography.
Quantification of andrographolide
A Bland-Altman plot comparing
the measured daily dose of andrographolide using the UHPLC-UV and UHPLC-MS methods
is presented in figure 2. These results are comparable (t(62) = 0.83, p = 0.39)
using the two methods, except for two products, Ph3 and Ph4. The Pearson correlation
coefficient (r) between the results obtained using the UHPLC-UV and UHPLC-MS methods
was 0.44 overall (p ≤0.01) and 0.97 after removing these two outliers (p <0.01). The
ultraviolet and mass spectrometry methods produced similar results for most products,
independently confirming andrographolide levels (supplementary file 6 in the appendix,
figure S2). The UHPLC-UV method
was deemed reliable for the quantification of andrographolide, except for samples
Ph3 and Ph4, where quercetin interference skewed the results. In such cases, the
UHPLC-MS method proved more accurate.
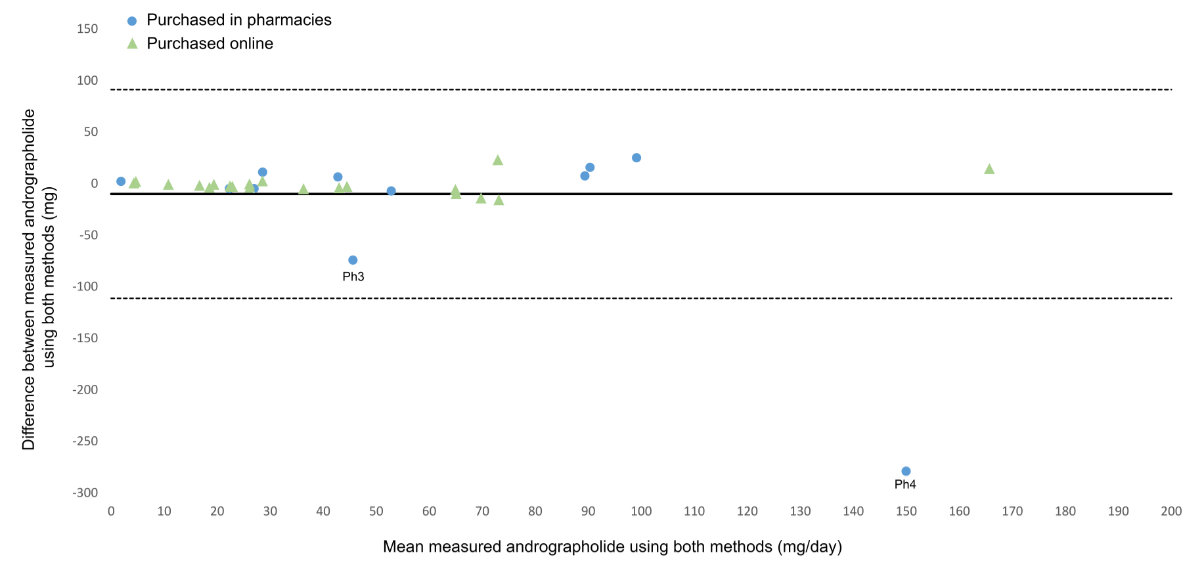
Figure 2Bland-Altman plot of measured
andrographolide using UHPLC-MS versus UHPLC-UV expressed in daily dose for each
product purchased in a pharmacy or online. The solid line represents the mean difference
and the dashed lines represent the limits of agreement (mean difference ± 1.96 standard
deviations). MS: mass spectrometry; UHPLC: ultra-high-performance liquid chromatography;
UV: ultraviolet.
For 20 of 23 products with
complete labelling, the measured daily dose of andrographolide was lower than that
claimed on the labelling (figure 3). Products purchased online had a higher median
labelled daily dose of andrographolide (90 mg/day; range: 35–180 mg) than those
from pharmacies (70 mg/day; range: 10–180 mg). The measured dose of andrographolide
ranged from 29% to 174% of the labelled dose. Only two products (Ph4 and On13) were
accurately labelled, while 91% of products were inaccurately labelled. In one case
(On18), the measured dose exceeded the labelled amount; this discrepancy was not
explained by a high-dosage regimen.
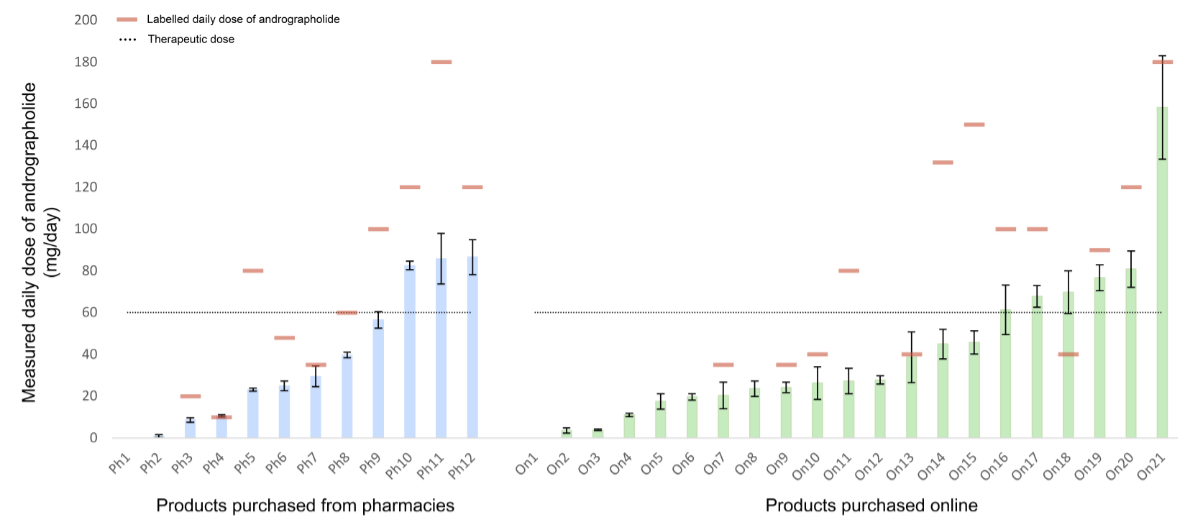
Figure 3Daily dose of andrographolide
measured with UHPLC-UV in pharmacy products (light blue bars) and online products
(light green bars) compared with those on the label (red dash) and with the therapeutic
dose (dotted line). The data are from three product replicates ± standard deviation
(SD). UHPLC: ultra-high-performance liquid chromatography; UV: ultraviolet. * no
information about daily serving; ** no andrographolide detected.
Of the 33 products in solid
form, the labelled daily dose of andrographolide ranged from 10 mg to 180 mg per
day. As displayed in figure 3, the measured daily dose of nine products (27%) reached
at least 60 mg of andrographolide, which is the recommended therapeutic dose for
upper respiratory tract infections [8]. Even though Ph4 and On13were labelled
correctly, they did not reach the therapeutic dose. The daily dose of andrographolide
varied from batch to batch within products of the same brand, as shown with products
On7 and Ph7(20.3 mg vs 29.4 mg); On11 and Ph5 (27.2 mg vs 23.1 mg); On17
and Ph9(67.8 mg vs 56.5 mg). Twelve of the 33 solid products claimed
to have been manufactured in accordance with Good Manufacturing Practice in their
labelling. Of these, only On18 and On20 reached the therapeutic dose of andrographolide,
and On13 was accurately labelled. There was no pattern observed between labelling
accuracy and country of origin.
Analysis of contaminants and residues
Of the 40 A. paniculata-containing
products analysed, we identified levels of mercury that exceeded the maximum authorised
levels in one product and another two products contained contaminants (pesticides)
(table 2). These three products had all been purchased online. Product On5 contained
0.153 mg/kg of mercury (maximum tolerated according to the European legislation:
0.100 mg/kg), product On19 contained 0.023 mg/kg of butralin and On8 contained 0.058
mg/kg of strychnine. Butralin and strychnine are both pesticides banned throughout
Europe [20, 21].
Table 2Levels of heavy metals and pesticide
residues detected in products containing Andrographis paniculata.
| Product code |
Heavy metal (mg/kg) (ICP-MS) |
Pesticides (mg/kg) (LC-MS/MS) |
| Arsenic (As) (max: NA) |
Lead (Pb) (max: 3.0) |
Cadmium (Cd) (max: 1.0) |
Mercury (Hg) (max: 0.1) |
Nickel (Ni) (max: NA) |
Strychnine |
Butralin |
| Ph1 |
0.16 |
0.113 |
0.085 |
0.006 |
2.1 |
ND |
ND |
| Ph2 |
0.17 |
0.116 |
0.086 |
ND |
2.2 |
ND |
ND |
| Ph3 |
0.02 |
0.017 |
ND |
ND |
0.6 |
ND |
ND |
| Ph4 |
0.06 |
0.044 |
0.015 |
0.010 |
0.3 |
ND |
ND |
| Ph5 |
0.03 |
0.098 |
0.005 |
ND |
1.3 |
ND |
ND |
| Ph6 |
0.22 |
0.183 |
0.132 |
ND |
2.2 |
ND |
ND |
| Ph7 |
0.02 |
0.008 |
ND |
ND |
1.0 |
ND |
ND |
| Ph8 |
0.04 |
0.091 |
0.012 |
ND |
1.3 |
ND |
ND |
| Ph9 |
0.07 |
0.015 |
0.004 |
ND |
2.8 |
ND |
ND |
| Ph10 |
0.03 |
0.062 |
0.003 |
ND |
0.3 |
ND |
ND |
| Ph11 |
0.01 |
0.038 |
0.003 |
ND |
1.0 |
ND |
ND |
| Ph12 |
0.18 |
0.177 |
0.018 |
ND |
1.3 |
ND |
ND |
| Ph13 |
0.02 |
0.017 |
0.010 |
ND |
0.3 |
ND |
ND |
| On1 |
0.91 |
1.46 |
0.181 |
0.015 |
2.5 |
ND |
ND |
| On2 |
0.33 |
0.209 |
0.087 |
ND |
2.6 |
ND |
ND |
| On3 |
0.03 |
0.006 |
0.005 |
ND |
1.0 |
ND |
ND |
| On4 |
0.71 |
1.50 |
0.040 |
0.020 |
10.3 |
ND |
ND |
| On5 |
0.14 |
0.766 |
0.051 |
0.153 |
2.3 |
ND |
ND |
| On6 |
0.08 |
0.266 |
0.038 |
0.009 |
1.8 |
ND |
ND |
| On7 |
0.02 |
0.010 |
0.004 |
ND |
1.0 |
ND |
ND |
| On8 |
1.36 |
0.953 |
0.428 |
0.023 |
1.4 |
0.058 |
ND |
| On9 |
0.02 |
0.007 |
0.003 |
ND |
1.0 |
ND |
ND |
| On10 |
0.03 |
0.115 |
0.008 |
ND |
1.2 |
ND |
ND |
| On11 |
0.03 |
0.134 |
0.006 |
ND |
1.7 |
ND |
ND |
| On12 |
0.04 |
0.027 |
ND |
ND |
0.2 |
ND |
ND |
| On13 |
0.08 |
0.023 |
0.010 |
ND |
0.8 |
ND |
ND |
| On14 |
0.11 |
0.054 |
0.096 |
ND |
3.2 |
ND |
ND |
| On15 |
0.17 |
0.052 |
0.107 |
ND |
5.0 |
ND |
ND |
| On16 |
0.03 |
0.056 |
0.005 |
ND |
0.2 |
ND |
ND |
| On17 |
0.07 |
0.017 |
0.005 |
ND |
3.0 |
ND |
ND |
| On18 |
0.03 |
0.065 |
0.004 |
0.007 |
0.4 |
ND |
ND |
| On19 |
0.06 |
0.030 |
0.002 |
ND |
0.9 |
ND |
0.023 |
| On20 |
0.03 |
0.060 |
0.005 |
0.006 |
0.4 |
ND |
ND |
| On21 |
0.03 |
0.056 |
0.004 |
ND |
1.0 |
Below LOQ |
ND |
| On22 |
0.01 |
0.010 |
0.013 |
ND |
0.9 |
ND |
ND |
| On23 |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
| On24 |
ND |
0.003 |
ND |
ND |
ND |
ND |
ND |
| On25 |
ND |
ND |
ND |
ND |
0.9 |
ND |
ND |
| On26 |
ND |
0.015 |
0.004 |
ND |
0.4 |
ND |
ND |
| On27 |
ND |
ND |
ND |
ND |
0.2 |
ND |
ND |
Discussion
In this quality control study
of 40 A. paniculata herbal products sourced from pharmacies worldwide and
online retailers available to Swiss consumers, the majority were found to be of poor
quality, including
products claiming to have been made according to Good Manufacturing Practice guidelines.
All products except one, which was delivered without a label, contained A. paniculata.
For most products, the quantity of
andrographolide was substantially lower than that indicated on the product’s labelling
and only one third met the recommended daily therapeutic dose of andrographolide
for upper respiratory tract infection. The presence of quercetin in two products
further complicated accurate andrographolide quantification. Quercetin, which was
listed as an ingredient on the label of these two products, is commonly used as
a food supplement for its potential antioxidant and anti-inflammatory properties
and is regarded as safe at moderate doses (≤1000 mg/day) [22]. However, it may have
been used as an
adulterant to falsely enhance the peak of andrographolide, as both compounds share
the same retention time when
analysed with the pharmacopoeial reference method. Alarmingly, three products purchased
online contained toxic contaminants: one exceeded European safety limits for mercury
and two others contained pesticide residues of strychnine and butralin, substances
banned or heavily regulated in the European Union, Switzerland and the US [21].
Even though the measured doses of each contaminant were well below the lethal dose,
this finding represents a serious breach of safety standards [20]. As no safe threshold
has been established for butralin or strychnine, it cannot be excluded that the
two products in which these pesticides were detected may pose health risks, even
at low exposure levels.
The levels of the characteristic
compounds – namely andrographolide, andrographiside and neoandrographolide – varied
among the products analysed. This finding is consistent with previous studies, and
is likely attributable to differences in extraction methods and plant parts used
[23, 24]. Standardising the extraction process would help ensure consistent composition
and quality of plant-based products. Our findings of inaccurate labelling of A.
paniculata-containing products are consistent with previous studies on other
food supplement products purchased online or from a single country. In these studies,
25% to 88% of herbal products, including Curcuma longa (turmeric)and
Lavandula angustifolia (lavender), were inaccurately labelled due to suspected
adulteration and/or a quantity of the active substance that was not within ±10%
of the labelled dose [25–27]. Other studies of products containing Hypericum
perforatum and Rhodiola rosea showed inaccurate labelling and the presence
of contaminants in products sold as herbal food supplements, but not in products
containing the same herb but sold as herbal medicinal products [28, 29]. Regarding
contaminants, a case of strychnine contamination has previously been reported in
Panax ginseng (ginseng)-containing herbal products [30]. In our study, although the
mercury level exceeded the maximum authorised limit in one herbal product, the overall
proportion of affected samples remained lower than that reported in a large-scale
study assessing the quality of herbal products purchased in China, where nearly
one third of products contained at least one heavy metal above safety thresholds
[31]. A similar difference was previously reported, with herbal food supplements
manufactured in China showing higher heavy metal levels than those from North America
[32]. While our findings
only apply to products containing A. paniculata, similar issues are likely
to affect other herbal food supplements.
In most countries, herbal food
supplements can be purchased over the counter in pharmacies, health food stores
or on the internet, like other food supplements. Unlike herbal medicinal products,
which are regulated by the same drug regulators as any other medical drug, herbal
food supplements do not require proof of safety or efficacy before being marketed
in Switzerland, Europe or the US [3]. In addition, once marketed, the quality of
herbal food supplements is not controlled, in contrast to herbal products marketed
as herbal medicinal products. Manufacturers in certain countries such as the US
are expected to comply with Good Manufacturing Practice guidelines and to display
this on their labelling. However, our findings showed that even A. paniculata-containing
products with Good Manufacturing Practice labels can be inaccurate. The distinction
between regulations applied to herbal food supplements and herbal medicinal products
may not be clear to the general public, making it difficult for consumers to assess
the quality of herbal food supplements they buy. Physicians and pharmacists should
be informed of this and educate patients about the risks of buying herbal food supplements
or other products online, particularly due to the risk of ingesting contaminants
and residues that they would not otherwise encounter. Clinicians should also actively
enquire about herbal
food supplement consumption by their patients, particularly in situations of unexplained
symptoms or suspected intoxication. Moreover, it is
possible that a prescribed herbal product fails to have the
expected effect on the clinical course of a disease because
it is underdosed and the prescriber and the patient have no
way of knowing that this is the reason for lack of efficacy.
To improve consumer safety, stronger regulation
of locally marketed herbal food supplements is needed. Since A. paniculata
is only available as a herbal food supplement, introducing a herbal medicinal product
version would ensure higher product quality. However, applying the same strict standards
as herbal medicinal products could disadvantage small manufacturers, reducing product
availability and pushing consumers towards lower-quality alternatives. A balanced
solution would be third-party certification to verify product quality, helping consumers
make informed choices. In the US, voluntary certification programmes such as ConsumerLab.com,
NSF International and US Pharmacopeia already help ensure the quality of herbal
food supplements. Expanding similar initiatives globally could enhance consumer
protection while maintaining accessibility to herbal products.
We acknowledge several limitations of our study.
First, these 40 products are not meant to be representative of all herbal products
sold online or in pharmacies in Switzerland or abroad. The overall sample size was
modest, with most brands of A. paniculata-containing products represented
by only one batch sample, which may not capture overall variability. Second, the
pharmacies and websites where these samples were obtained were selected opportunistically
rather than randomly. While it is not feasible to analyse all such products on the
market at such a high standard, our results indicate a pattern that we believe would
hold with a larger sample size. Additionally, our analyses were conducted with on
an international sample using two robust analytical methods, ensuring the accuracy
and validity of our results. Finally, since we only quantified andrographolide in
solid forms, our findings may not apply to products in other forms.
Our study highlights the poor quality of most A.
paniculata-containing herbal products sold as herbal food supplements, even
when compliance with Good Manufacturing Practice is claimed. The frequent mislabelling
of A. paniculata-containing products should alert clinicians, pharmacists
and regulatory authorities. The general public should be aware of the risks associated
with ordering products online, including ingesting potential contaminants and residues.
We emphasise the need for stricter yet pragmatic regulation of the global herbal
food supplements market and, amid varying regulatory frameworks worldwide, we propose
that a standardised labelling system be used to identify products whose quality
has been independently verified.
Data sharing statement
Deidentified data derived
from analysed samples will be made available upon reasonable request from qualified
researchers whose proposed use of the data has been approved by the investigators
and participating institutions. Data will be shared after publication, under a formal
data sharing agreement, and only for samples collected and processed in accordance
with applicable ethical and regulatory approvals.
Declaration of use of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors
– who are not native English speakers – used ChatGPT for proofreading. After using
this tool, the authors reviewed and edited the content as needed and take full responsibility
for the content of the publication.
Professor Pierre-Yves Rodondi
Institute
of Family Medicine
University
of Fribourg
Route des Arsenaux 4
CH-1700 Fribourg
pierre-yves.rodondi[at]unifr.ch
and
Dr Emerson Ferreira Queiroz
Institute of Pharmaceutical Sciences
of Western Switzerland
University of Geneva
CMU
CH-1206 Geneva
emerson.ferreira[at]unige.ch
References
1. Patriota P, Guessous I, Marques-Vidal P. Dietary patterns according to vitamin supplement
use. A cross-sectional study in Switzerland. Int J Vitam Nutr Res. 2022 Oct;92(5-6):331–41.
10.1024/0300-9831/a000679
2. Health Products Regulatory Authority [Internet]. Herbal medicines: regulatory information
Dublin (Ireland): HPRA; 2014 [cited 2025 Oct 14]. Available from: http://www.hpra.ie/homepage/medicines/regulatory-information/medicines-authorisation/herbal-medicines/information-for-the-public-thmps
3. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002
on the Approximation of the Laws of the Member States Relating to Food Supplements. Off
J Eur Union. L 183 No. 51-5 (2002).
4. Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004
amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on
the Community code relating to medicinal products for human use. Off J Eur Union.
L 136 No. 85-90 (2004).
5. Finnish Food Authority [Internet]. Establishing novel food status of a food Helsinki
(Finland): Finnish Food Authority; 2023 [cited 2025 Oct 14]. Available from: https://www.ruokavirasto.fi/en/foodstuffs/food-sector/ainesosat-ja-sisalto/novel-foods/establishing-novel-food-status-of-a-food/
6. Orhan N, Gafner S, Blumenthal M. Estimating the extent of adulteration of the popular
herbs black cohosh, echinacea, elder berry, ginkgo, and turmeric - its challenges
and limitations. Nat Prod Rep. 2024 Oct;41(10):1604–21. 10.1039/d4np00014e doi: https://doi.org/10.1039/D4NP00014E
7. Gafner S, Blumenthal M, Foster S, Cardellina JH 2nd, Khan IA, Upton R. Botanical Ingredient
Forensics: Detection of Attempts to Deceive Commonly Used Analytical Methods for Authenticating
Herbal Dietary and Food Ingredients and Supplements. J Nat Prod. 2023 Feb;86(2):460–72.
10.1021/acs.jnatprod.2c00929
8. Coon JT, Ernst E. Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety
and efficacy. Planta Med. 2004 Apr;70(4):293–8. 10.1055/s-2004-818938
9. Hu XY, Wu RH, Logue M, Blondel C, Lai LY, Stuart B, et al. Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in
adults and children: A systematic review and meta-analysis. PLoS One. 2017 Aug;12(8):e0181780.
10.1371/journal.pone.0181780
10. Tanwettiyanont J, Piriyachananusorn N, Sangsoi L, Boonsong B, Sunpapoa C, Tanamatayarat P,
et al. Use of Andrographis paniculata (Burm.f.) Wall. ex Nees and risk of pneumonia in hospitalised patients with mild
coronavirus disease 2019: A retrospective cohort study. Front Med (Lausanne). 2022 Aug;9:947373.
10.3389/fmed.2022.947373
11. Puri A, Saxena R, Saxena RP, Saxena KC, Srivastava V, Tandon JS. Immunostimulant agents
from Andrographis paniculata. J Nat Prod. 1993 Jul;56(7):995–9. 10.1021/np50097a002
12. Songvut P, Suriyo T, Panomvana D, Rangkadilok N, Satayavivad J. A comprehensive review
on disposition kinetics and dosage of oral administration of Andrographis paniculata, an alternative herbal medicine, in co-treatment of coronavirus disease. Front Pharmacol.
2022 Aug;13:952660. 10.3389/fphar.2022.952660
13. Wagner L, Cramer H, Klose P, Lauche R, Gass F, Dobos G, et al. Herbal Medicine for
Cough: a Systematic Review and Meta-Analysis. Forsch Komplementmed. 2015;22(6):359–68.
10.1159/000442111
14. Raynor DK, Dickinson R, Knapp P, Long AF, Nicolson DJ. Buyer beware? Does the information
provided with herbal products available over the counter enable safe use? BMC Med.
2011 Aug;9(1):94. 10.1186/1741-7015-9-94
15. Karioti A, Timoteo P, Bergonzi MC, Bilia AR. A Validated Method for the Quality Control
of Andrographis paniculata Preparations. Planta Med. 2017 Oct;83(14-15):1207–13. 10.1055/s-0043-113827
16. Fibigr J, Šatínský D, Solich P. Current trends in the analysis and quality control
of food supplements based on plant extracts. Anal Chim Acta. 2018 Dec;1036:1–15. 10.1016/j.aca.2018.08.017
17. Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ. Fast and easy multiresidue
method employing acetonitrile extraction/partitioning and “dispersive solid-phase
extraction” for the determination of pesticide residues in produce. J AOAC Int. 2003;86(2):412–31.
10.1093/jaoac/86.2.412
18. Allen LV, Bassani GS, Elder EJ, Parr AF. Strength and stability testing for compounded
preparations. Rockville (USA) [USP]. U S Pharmacop. 2015.
19. Kumar S, Singh B, Bajpai V. Andrographis paniculata (Burm.f.) Nees: traditional uses, phytochemistry, pharmacological properties and
quality control/quality assurance. J Ethnopharmacol. 2021 Jul;275:114054. 10.1016/j.jep.2021.114054
20. Parker AJ, Lee JB, Redman J, Jolliffe L. Strychnine poisoning: gone but not forgotten.
Emerg Med J. 2011 Jan;28(1):84. 10.1136/emj.2009.080879
21. Smith E, Azoulay D, Tuncak B. Lowest common denominator : how the proposed EU-US trade
deal threatens to lower standards of protection from toxic pesticides. London, UK:
Center for International Environmental Law; 2015.
22. Aghababaei F, Hadidi M. Recent Advances in Potential Health Benefits of Quercetin.
Pharmaceuticals (Basel). 2023 Jul;16(7):1020. 10.3390/ph16071020
23. Avula B, Katragunta K, Tatapudi KK, Wang YH, Ali Z, Chittiboyina AG, et al. Phytochemical
analysis of phenolics and diterpene lactones in Andrographis paniculata plant samples and dietary supplements. J Pharm Biomed Anal. 2025 Sep;262:116866.
10.1016/j.jpba.2025.116866
24. Ruan LJ, Yan BX, Song SS, Yun-Qiu W, Liu XH, Yao CY, et al. Harmonizing international
quality standards for Andrographis paniculata: A comparative analysis of content determination methods across pharmacopeias. J
Pharm Biomed Anal. 2024 Mar;240:115924. 10.1016/j.jpba.2023.115924
25. Booker A, Frommenwiler D, Reich E, Horsfield S, Heinrich M. Adulteration and Poor
Quality of Ginkgo biloba Supplements. J Herb Med. 2016;6(2):79–87. 10.1016/j.hermed.2016.04.003
26. Sorng S, Balayssac S, Danoun S, Assemat G, Mirre A, Cristofoli V, et al. Quality assessment
of Curcuma dietary supplements: complementary data from LC-MS and 1H NMR. J Pharm
Biomed Anal. 2022 Apr;212:114631. 10.1016/j.jpba.2022.114631
27. Jalil B, Heinrich M. Pharmaceutical quality of herbal medicinal products and dietary
supplements - a case study with oral solid formulations containing Lavandula species.
Eur J Pharm Sci. 2025 May;208:107042. 10.1016/j.ejps.2025.107042
28. Booker A, Agapouda A, Frommenwiler DA, Scotti F, Reich E, Heinrich M. St John’s wort
(Hypericum perforatum) products - an assessment of their authenticity and quality. Phytomedicine. 2018 Feb;40:158–64.
10.1016/j.phymed.2017.12.012
29. Booker A, Jalil B, Frommenwiler D, Reich E, Zhai L, Kulic Z, et al. The authenticity
and quality of Rhodiola rosea products. Phytomedicine. 2016 Jun;23(7):754–62. 10.1016/j.phymed.2015.10.006
30. Filipiak-Szok A, Kurzawa M, Szłyk E. Determination of toxic metals by ICP-MS in Asiatic
and European medicinal plants and dietary supplements. J Trace Elem Med Biol. 2015 Apr;30:54–8.
10.1016/j.jtemb.2014.10.008
31. Luo L, Wang B, Jiang J, Fitzgerald M, Huang Q, Yu Z, et al. Heavy metal contaminations
in herbal medicines: determination, comprehensive risk assessments, and solutions.
Front Pharmacol. 2021 Jan;11:595335. 10.3389/fphar.2020.595335
32. Genuis SJ, Schwalfenberg G, Siy AK, Rodushkin I. Toxic element contamination of natural
health products and pharmaceutical preparations. PLoS One. 2012;7(11):e49676. 10.1371/journal.pone.0049676
Appendix
The appendix is available for download as a separate file at https://doi.org/10.57187/s.4728.


