Passive RSV immunisation using nirsevimab in neonatal care: a structured multidisciplinary
approach and immunisation data from a Swiss tertiary centre
DOI: https://doi.org/https://doi.org/10.57187/s.4689
Katalin
Kalyaa,
Jehudith
Fontijnb,
Flurina Famosa,
Dirk Basslerb,
Nicole Ochsenbein-Kölblea,
Ladina Vonzuna
a Department of Obstetrics, University Hospital Zurich,
Switzerland
b Department of Neonatology,
University Hospital Zurich, Switzerland
Summary
BACKGROUND: Respiratory syncytial virus (RSV) infection is a major cause of
severe lower respiratory tract infections in newborns and young infants,
especially during the winter season from October to March. In Switzerland, RSV infection
represents a leading cause of hospital admissions among newborns. Since October
2024, a long-acting monoclonal antibody, nirsevimab (Beyfortus®), has been
available in Switzerland for standard care in newborns.
OBJECTIVES: This paper presents a multidisciplinary, standardised protocol for
the administration of nirsevimab, as well as immunisation data
from the first season of application in 2024/25 at a tertiary centre in
Switzerland.
METHODS: A protocol for implementing the RSV immunisation strategy was
developed at University Hospital Zurich by a multidisciplinary team of obstetricians,
neonatologists, nurses, from in- and outpatient services. The focus was on prenatal
counselling during outpatient consultations as well as inpatient procedures on
maternity and neonatology wards. The goal was to provide expectant parents with
consistent information by different healthcare professionals. Neonatal immunisation
data from the first season in 2024/25 (25 October to 31 March ) were retrieved
from patient charts (Yes/No) in a retrospective, quality control, observational
cohort study. All newborns discharged from the maternity ward or the
neonatology unit of our centre were included in the analysis.
RESULTS: The protocol included early and multidisciplinary parental
education, offering consistent oral and written information, as well as
opportunities to discuss their questions regarding the new immunisation, ensured
informed consent and enabled timely administration of nirsevimab by healthcare professionals
in the obstetrics and neonatology units. Over the 2024/25 season, 78% of the newborns
were immunised before leaving hospital care: 78% (588/758) of newborns discharged
home from the maternity ward were immunised and 82% (125/153) of those
discharged home from the neonatology unit were immunised.
CONCLUSION: The implementation of passive RSV immunisation was overall
successful with an immunisation rate of around 80% for the first season in 2024/25.
Introduction
Respiratory syncytial virus (RSV) is a ubiquitous seasonal virus and a leading cause
of bronchiolitis and pneumonia in infants aged below one year, particularly during
the winter season. National and international epidemiological data confirm that RSV
contributes to significant paediatric morbidity, accounting for a large proportion
of hospitalisations among newborns [1]. The clinical burden and risk of severe RSV
infection is particularly high in the first month of life, due to the immature immune
system and narrow airways of newborns. Traditionally prevention has relied on hygiene
measures and administration of monoclonal antibodies approved for high-risk infants
only [2].
The recent development and regulatory approval of nirsevimab (Beyfortus®), a long-acting monoclonal antibody with demonstrated efficacy and safety, provides
a transformative opportunity in neonatal infectious disease prevention. Clinical trials
and observational studies have demonstrated an up to 80% reduction in severe RSV-related
lower respiratory tract infections, 77% fewer hospitalisations and 86% fewer intensive
care admissions among infants who received nirsevimab [3–6]. These benefits were consistent
across populations, including full-term and preterm infants. Safety data from over
3700 infants showed no increase in adverse events compared to placebo [7].
Based on these findings, the Swiss Federal Office of Public Health (FOPH / Bundesamt für Gesundheit [BAG]) and the Federal Vaccination Commission (FVC / Eidgenössische Kommission für Impffragen [EKIF]) in collaboration with the various expert societies have recommended among
others a routine passive immunisation strategy with nirsevimab for all newborns born
between October and March, to be administered within the first week of life or as
soon as possible thereafter [7]. The costs of nirsevimab are covered by mandatory
health insurance and embedded within the Swiss Diagnosis-Related Groups (DRG) system
[8]. The latter incorporation was confirmed on 5 September 2024, leaving very little
time to inform relevant healthcare workers and expectant parents about, and prepare
them for, this new passive immunisation option for newborns in the upcoming 2024/25
RSV season. To achieve high immunisation coverage without generating extensive workload
in the inpatient setting, clear parental communication and seamless interdisciplinary
collaboration were required.
This paper presents a pragmatic implementation report developed at University Hospital
Zurich, integrating the RSV immunisation strategy into routine perinatal care, emphasising
parental education, interprofessional collaboration and clinical efficiency to minimise
administrative burden. Additionally, immunisation data of the first RSV season with
nirsevimab, 2024/25, are shown and discussed in detail.
Materials and methods
Multidisciplinary implementation strategy for nirsevimab
The protocol for implementation of the RSV immunisation
strategy was developed at University Hospital Zurich by a multidisciplinary and
interprofessional team of obstetricians, neonatologists and nurses regarding in-
and outpatient services. The goal was to achieve, from the first day of the protocol’s
implementation, high immunisation rates with nirsevimab for newborns leaving
our centre. To this end, we focused on the following:
- Consistent
information for the expectant parents by different healthcare workers (doctors,
midwives, nurses), based on existing information material, i.e. the fact sheets
of the Federal Office of Health [9].
- Consistent
information for the expectant parents at multiple time points (prenatally,
after delivery at the ward, during the stay at the maternity ward or
neonatology).
- Activation
of various information channels (oral, written i.e. leaflets, smartphone applications).
- Minimalised
use of administrative and time resources for each discipline (especially
considering primary information and subsequent questions by parents).
- Clinical
efficiency of the workflows of nirsevimab administration (written parental consent).
- Easy
access to nirsevimab for parents who had not reached a decision by the time of
hospital discharge, by implementing an outpatient consultation at University
Hospital Zurich’s neonatology department.
- Reduction
of workload of paediatric outpatient clinics by implementing an
outpatient consultation at University Hospital Zurich’s neonatology department for
undecided parents (this consideration was made with special regard to the beginning
of the programme, where paediatricians in the outpatient practices were responsible
for the vaccination of all children born between April and September 2024 in
their practice).
Nirsevimab immunisation data for the 2024/25 season
This is a retrospective, quality control, observational
cohort study. All newborns born alive at University Hospital Zurich between 25 October
2024 and 31 March 2025, discharged home from the maternity ward as well as all
newborns discharged from the neonatology unit in this time period, were offered
nirsevimab, and were included in this data analysis. Exclusion criteria
included stillbirths, neonatal deaths, newborns receiving palliative care and
those transferred to other hospitals for follow-up.
Following the recommendations of the Swiss
Federal Office of Public Health / Federal Vaccination Commission, all newborns
without contraindications (e.g. haemophilia or thrombocytopenia) were
recommended nirsevimab 50 mg intramuscularly in their first days of life
(application possible immediately after birth or as soon as possible thereafter).
For newborns discharged home from the
maternity ward, data were retrieved every second week from patient charts. For newborns
discharged home from
the neonatology unit, an overall immunisation coverage was calculated, as the length
of stay varied and thus the application of immunisation did not occur in a timely
standardised manner. Documented administration of nirsevimab (Yes/No) before discharge
was coded. Additionally, maternal vaccination with Abrysvo®, the maternal RSV
vaccine, was coded if application occurred between the 32nd and 36th
week of pregnancy and at least two weeks before delivery. If Abrysvo® had been
given according to the mentioned conditions, nirsevimab was not additionally
administered to the newborns and they were coded immunised. Newborns immunised
during their first two weeks of life at the outpatient service of University
Hospital Zurich’s neonatology department, as described in the implementation
strategy, were considered ‘immunised’ for analysis too.
Data were retrieved anonymously from the
electronic patient chart KISIM© (version V5.6.0.14, Cistec AG©) and / or
Perinat (Klinisches Informationssystem, version 7.0.0.89, © University Hospital
Zurich 2025).
Statistical analysis was performed using Microsoft®
Excel® for Microsoft 365 MSO (Version 2402 Build 16.0.17328.20550) 64-bit. Categorical
data are presented as counts (n) with percentages (%).
This study does not fall within the scope
of the Swiss Human Research Act. The study was reported, but approval of the
project by a Swiss Ethics Committee was not required as it was designed as a retrospective,
quality control, observational study and only targeted anonymous data were
obtained (Req-2025-00202).
Results
Multidisciplinary protocol for nirsevimab
The protocol developed at University Hospital Zurich by a multidisciplinary team from
obstetrics, neonatology, nursing and outpatient services is schematically shown in
figure 1 and its key features are as follows:
Prenatal information and counselling of parents
During pregnancy consultations by obstetricians and/or midwives, all expectant parents
are informed about passive RSV immunisation. Printed fact sheets by the Swiss Federal
Office of Public Health are included in the maternity documents sent by post to every
woman planning to deliver at our centre. Additionally, these fact sheets are placed
in the hospital’s digital maternity pass/application, available to every woman who
has had a consultation at our centre [10].
Delivery and postpartum period in the delivery room
After delivery, however still in the delivery room, midwives reinforce the information
about RSV immunisation during the initial newborn assessments (ideally as a reminder
with the standardised vitamin K administration). Parents receive an RSV consent form,
containing key information about nirsevimab, and are asked to return it signed before
the neonatal routine check before discharge from the hospital. The form contains the
following options for parents to choose from: “Yes, I want nirsevimab to be administered”,
“No, I do not want nirsevimab to be administered” or “I have not decided yet”.
Maternity ward (postpartum care)
Any remaining parental concerns are addressed by the attending obstetricians and/or
nurses/midwives during the daily rounds. Nurses verify that the signed consent form
is present at discharge. At the routine neonatal examination by neonatology before
discharge (usually day two or three after birth), parents are again informed and asked
about their decision concerning nirsevimab administration. If parents agree, nirsevimab
is prescribed and documented in the vaccination booklet by the neonatologist and administered
by the ward nurses/midwives. If parents decline the nirsevimab administration, a note
is made in the report for the paediatrician, asking that the topic be brought up
in the follow-up visits. If parents remain undecided, they are referred to the outpatient
neonatology clinic (see point 5).
Neonatology unit (inpatient newborns)
Parents of hospitalised newborns are informed about RSV immunisation as part of discharge
planning. With consent, nirsevimab is administered prior to discharge.
Outpatient neonatology clinic
For parents who are undecided or prefer more time to consider, an appointment is scheduled
with neonatology specialists within the week after discharge. After counselling, if
consent is obtained, the immunisation is administered and documented in the child’s
immunisation booklet.
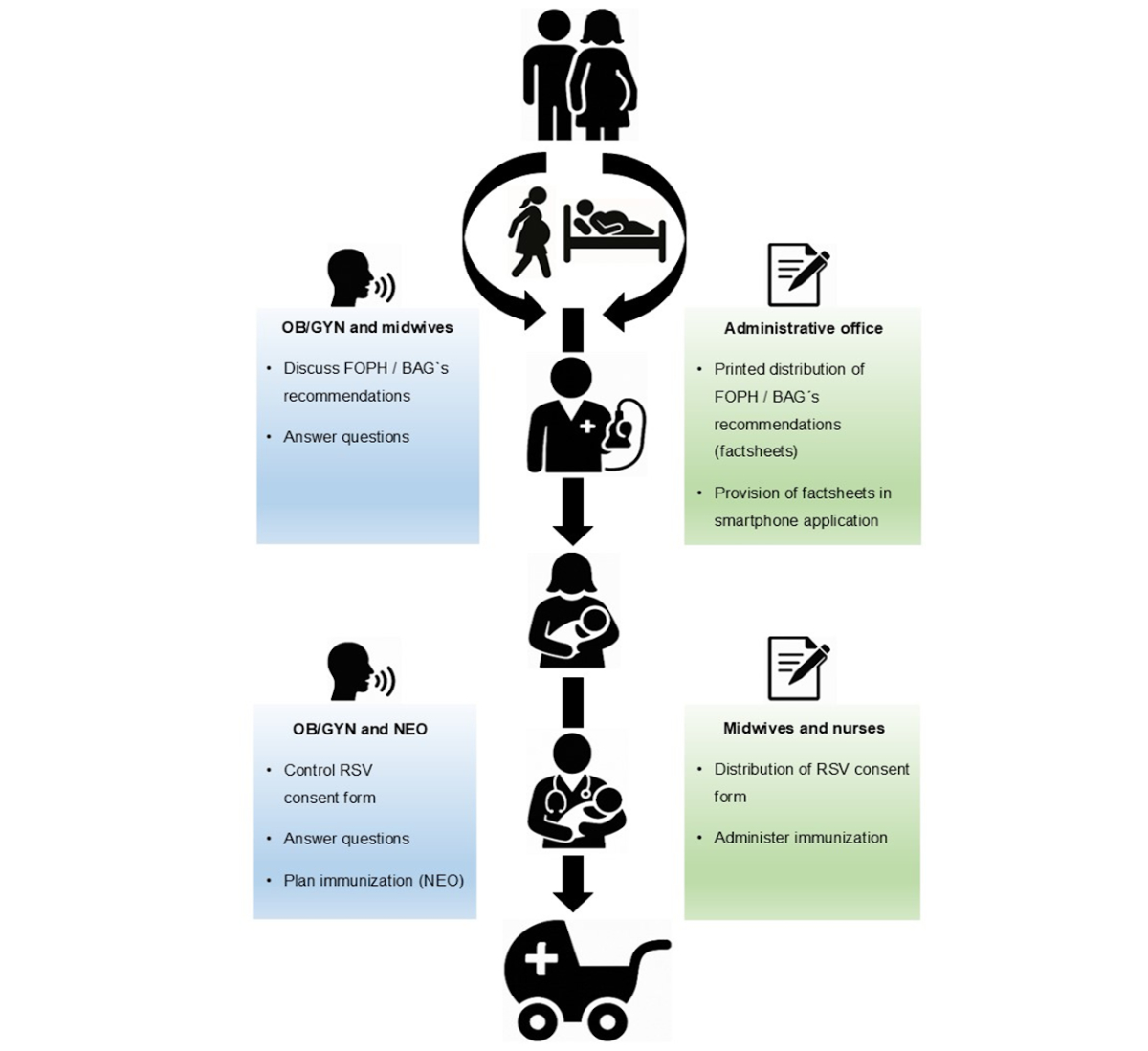
Figure 1Schematic presentation of the passive
respiratory syncytial virus (RSV) immunisation strategy for newborns
implemented at University Hospital Zurich in inpatients and outpatients during
the 2024/25 season, with emphasis on parental information by multiple channels,
healthcare workers and time points in pregnancy and postpartum. *FOPH / BAG:
Federal Office of Public Health / Bundesamt für Gesundheit; ** OB/GYN:
obstetrics/gynaecology physicians; *** NEO: neonatology physicians.
RSV immunisation data for the 2024/25 season
During the study period, 758 newborns were discharged
home from the maternity ward with an RSV immunisation coverage of 78% (n = 588).
A further 153 newborns were discharged home from the neonatology unit, of whom 125
were immunised (82%) (figures 2 and 3).
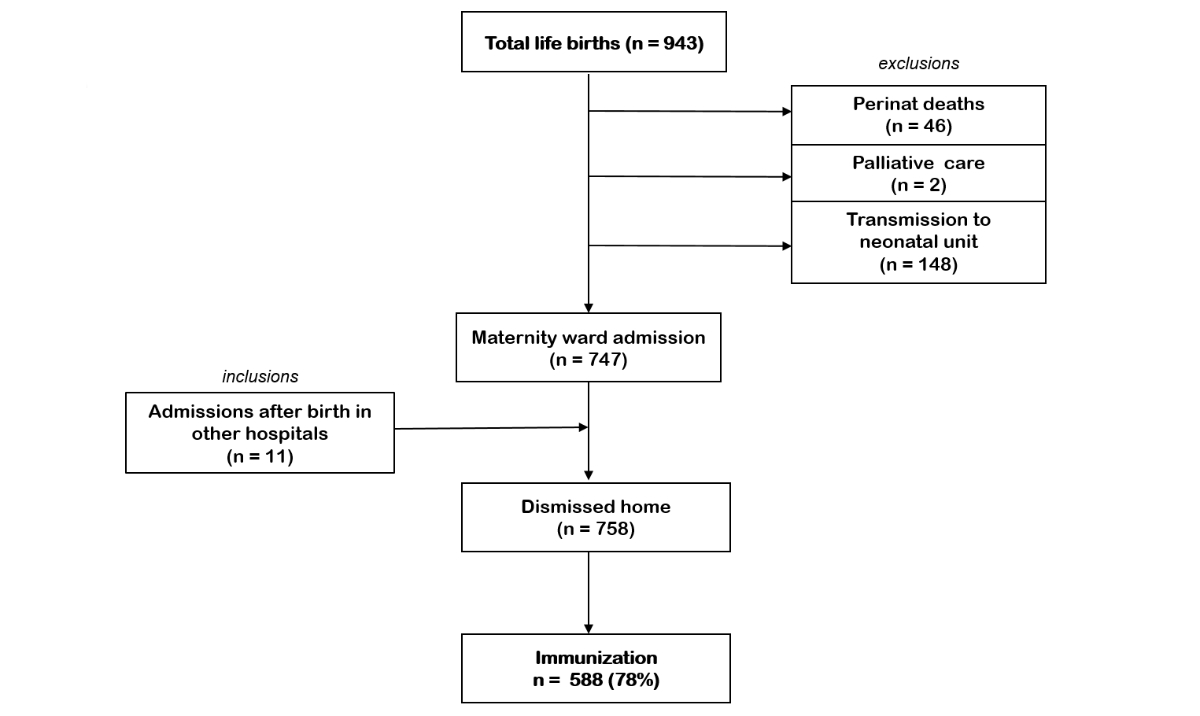
Figure 2Inclusions and exclusions of immunisation
with nirsevimab during the respiratory syncytial virus (RSV) 2024/25 season in the
maternity ward at University
Hospital Zurich.
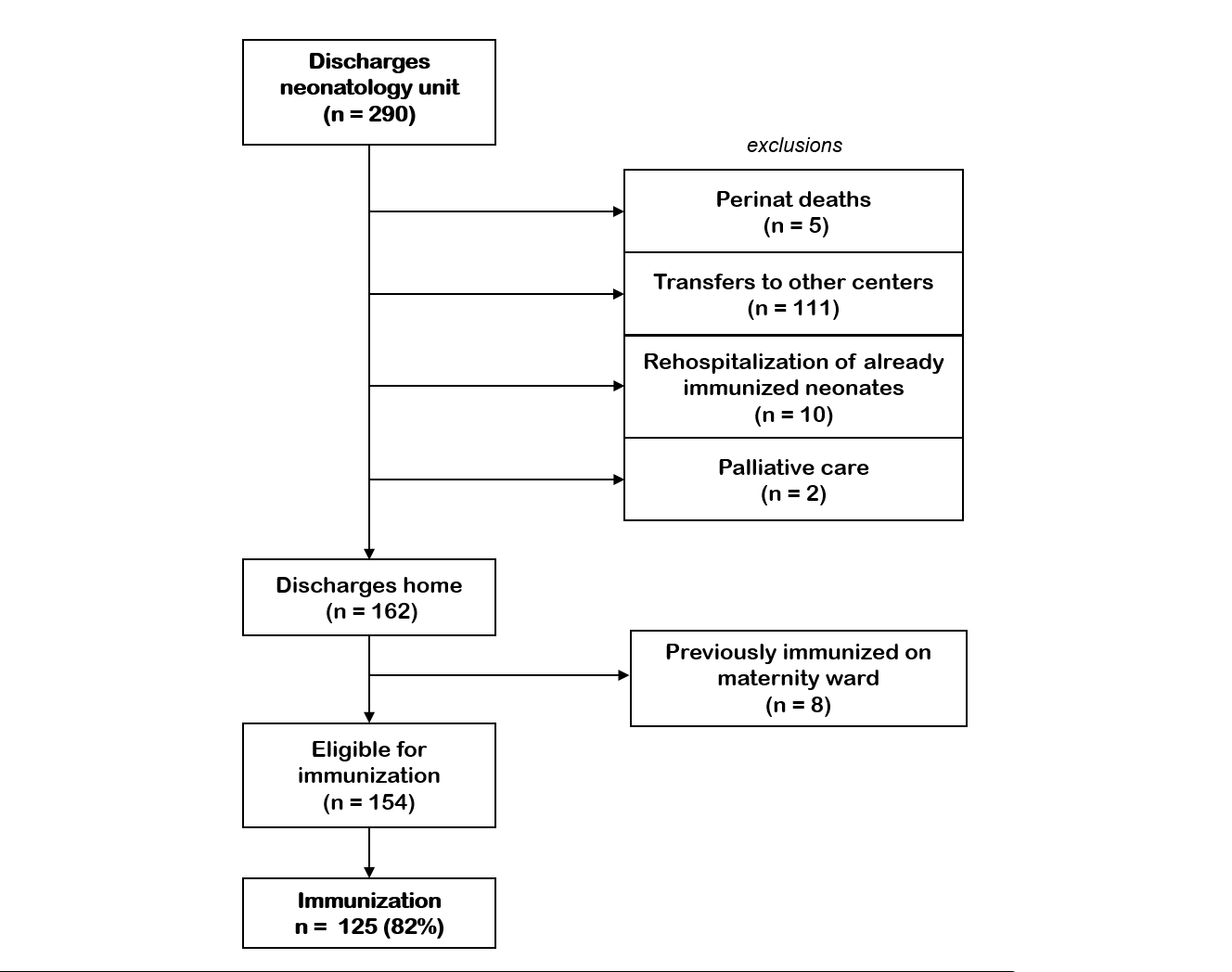
Figure 3Inclusions and exclusions of immunisation
with nirsevimab during the respiratory syncytial virus (RSV) 2024/25 season in the
neonatology unit at University
Hospital Zurich.
In summary, the overall immunisation rate
of all newborns discharged home from our centre was 78%. Four (0.01%) newborns
were passively immunised by maternal vaccination (Abrysvo®). A small percentage
of all immunisations (1.6% or n = 15) occurred in the outpatient setting.
The monthly immunisation rates of the
neonates discharged from the maternity ward are shown in figure 4A. Immunisation
rates in the first weeks of implementation were 82%. The highest rates were
observed in October 2024 and January 2025 – 82% and 81%, respectively – and the
lowest in February 2025 with 73%.
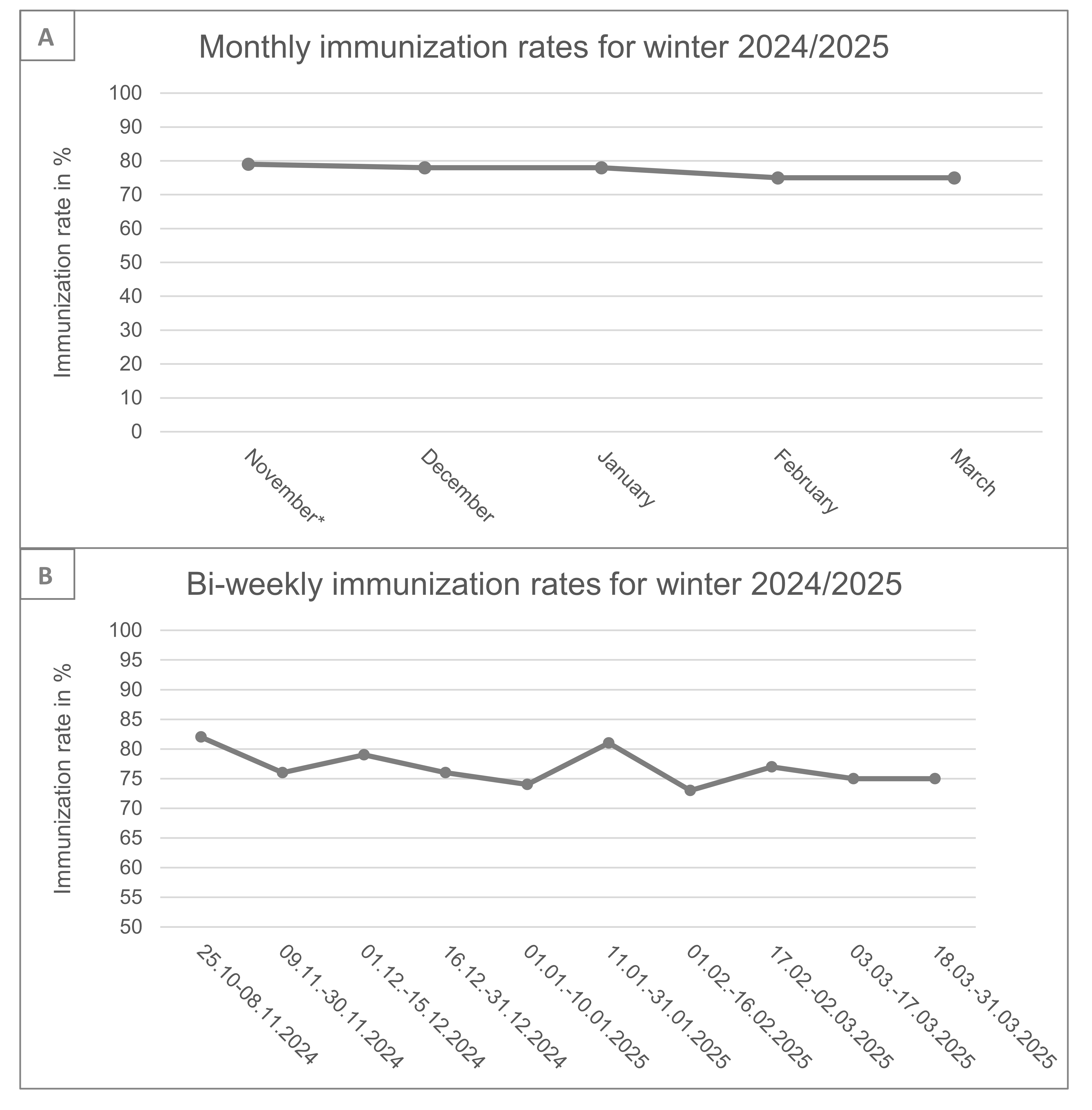
Figure 4Monthly (A) and fortnightly (B) immunisation rates for RSV with nirsevimab or maternal
vaccination (Abrysvo®) in newborns discharged home from the maternity ward during
the 2024/25 winter season at University Hospital Zurich. * includes immunisations
from 28 to 31 October.
Discussion
This study reports on an interdisciplinary and interprofessional implementation strategy
for nirsevimab as well as on the immunisation coverage of newborns born during RSV
season 2024/25 at University Hospital Zurich. The immunisation rate was 78% for the
newborns discharged from the maternity ward and 82% for newborns leaving the neonatology
unit.
Immunisation strategies
The integration of nirsevimab into standard neonatal care represents a major advancement
in RSV infection prevention. Real-world data from the previous RSV season have shown
that it reduces severe RSV infections and related hospitalisations in infants, with
70–83% efficacy across several RCTs [6, 10, 11]. Unlike vaccines, passive immunisation
provides immediate protection, making it particularly valuable for newborns who are
at highest risk during their first months of life. Clearly this made the quick implementation
of nirsevimab essential; however several challenges were to be expected. First, the
particularly short time interval between regulatory approval and financial coverage
of nirsevimab in Switzerland and the start of the RSV season made implementation a
challenge for all disciplines providing care, whether inpatient or outpatient care.
Furthermore, when starting the programme for the newborns, the focus of the paediatricians
had to be the catch-up immunisation of all children born between April and September
2024, thus leaving little space for extra consultations of newborns. On the other
hand, maternity wards and neonatologists faced the challenge of an additional and
potentially time-consuming task, without reimbursement, other than the costs of nirsevimab
itself.
Previous experiences in Switzerland show that vaccine acceptance rates are rather
low in comparison to most neighbouring countries [12, 13]. Among 2-year-olds, full
coverage of the mandatory vaccines (such as measles and diphtheria, pertussis [whooping
cough] and tetanus [DPT]) in Switzerland was reported as under 90%, vs 93% in France
and 92% in Italy. Vaccine hesitancy has been reported to be influenced by a complex
interplay of emotional, cultural, religious, political, logistical and cognitive factors
[12, 14, 15]. While lack of knowledge and insufficient information have been consistently
linked to low vaccination rates, particularly in Switzerland, other elements such
as trust, personal stories and opportunities for dialogue with peers also play a crucial
role [13–16]. Although our study does not allow conclusions to be drawn on the above-mentioned
factors, these may still contribute to hesitancy in certain groups of our patients
and the 20% of parents who did not accept nirsevimab at our centre. Addressing vaccine
hesitancy therefore requires not only factual education but also empathetic and context-sensitive
communication strategies. A framework for open, non-judgemental discussion with vaccine-hesitant
parents from a trusted resource has been shown to positively influence vaccine acceptance
[13, 15].
Taken together, a good and quickly conceptualised strategy had to be brought up in
order to optimise the implementation of the passive immunisation against RSV for newborns.
The introduction of the multidisciplinary and interprofessional strategy at University
Hospital Zurich demonstrates how existing clinical routines can be adapted to incorporate
new preventive measures without significantly increasing workload for any discipline.
Immunisation rates in the first weeks of the programme, as high as 82%, show that
the concept was immediately effective. We believe that a key factor in the successful
implementation was the early and consistent communication with parents, which fostered
understanding and acceptance. Standardised information material endorsed by federal
authorities (Swiss Federal Office of Public Health / Federal Vaccination Commission)
ensured clarity and coherence across disciplines. Additionally, the interprofessional
approach including midwives and nurses might have raised acceptance by the parents.
Even though not specifically studied, subjectively the strategy was quickly incorporated
into routine standard care at our centre. By embedding the administration of nirsevimab
within routine workflows, such as postpartum assessments and discharge planning, the
protocol avoided additional strain on staff while maximising immunisation rates. This
approach may also serve as a model for introducing future innovations in neonatal
care.
RSV immunisation rates of newborns
Overall acceptance of nirsevimab at our centre was very high (80%). This rate is comparable
with the previously reported average overall vaccination rates of 70–90% from Galicia
(Spain), France, Luxembourg and the USA for the 2023/24 winter season [3–5, 17]. Most
likely, some children were additionally immunised at the paediatrician’s practice
at the 1-month follow-up.
The reasons for this high acceptance of the passive RSV immunisation of newborns are
speculative; however it seems likely that the perceived risk and heightened awareness
of contracting RSV can motivate parents to seek immunisation. Previous studies on
influenza vaccination found that the immediate risk of illness influenced individuals’
perception of vaccine efficacy and decision to vaccinate [18–20]. Seasonal interest
for other vaccines has been shown before. Krasselt et al. [14], for example, showed
significantly higher interest in the seasonal tick-born encephalitis vaccine during
the spring and summer months. Our results could be partly explained by these influences,
as we found particularly high immunisation rates at the beginning of the season and
during the peak of RSV in January. Hsieh et. al reported similar observations for
the maternal RSV vaccination in the current season [21]. Fewer observed RSV cases
in their environments might have led parents to decline immunisation in March. On
the other hand, it has been previously observed that health authorities often intensify
vaccination campaigns and public messaging during or leading up to peak disease seasons,
which affects vaccine uptake and reduces vaccination hesitancy [19, 22]. We cannot
rule out the possibility that our campaign was more rigorous at the beginning and
during the RSV peak season and that this led to reduced immunisation uptakes.
Strengths and limitations
This study presents an interdisciplinary and interprofessional approach for implementing
a new RSV immunisation strategy. Overall, high acceptance and immunisation of nirsevimab
was observed. However, the study design does not allow any conclusions to be drawn
on the acceptance of the implementation strategy itself; nor did the design compare
the strategy with other strategies. Furthermore, no direct correlation can be made
with the decreased incidence of severe RSV-related infections or hospitalisations.
Further studies, especially emphasising the individual and socioeconomic burden of
disease over the next two RSV seasons, are needed to respond to this question. Finally
yet importantly, this study does not allow any conclusions to be drawn as to why parents/families
accepted or declined immunisation of their newborn. Further research should specifically
address vaccine hesitancy and its underlying determinants such as language barriers,
parental education and health literacy, prior experiences with the healthcare system
and other relevant sociodemographic variables.
Conclusion
This is a pragmatic report on a feasible multidisciplinary and interprofessional implementation
strategy for neonatal RSV immunisation with nirsevimab that had an immediate high
immunisation rate. This study further presents an overall successful immunisation
rate of nearly 80% for all newborns discharged home from a tertiary centre in Switzerland
during the 2024/25 season.
Data sharing statement
All
data generated or analysed during this study are included in this article and
its supplementary material files. Further enquiries can be directed to the
corresponding author.
PD Dr
med. Ladina Vonzun
Department
of Obstetrics
University
Hospital Zurich
Frauenklinikstrasse 10
CH-8091 Zurich
ladina.vonzun[at]usz.ch
References
1. Mochizuki H, Kusuda S, Okada K, Yoshihara S, Furuya H, Simões EA, et al.; Scientific
Committee for Elucidation of Infantile Asthma. Palivizumab Prophylaxis in Preterm
Infants and Subsequent Recurrent Wheezing. Six-Year Follow-up Study. Am J Respir Crit
Care Med. 2017 Jul;196(1):29–38. doi: https://doi.org/10.1164/rccm.201609-1812OC
2. Simões EA, Madhi SA, Muller WJ, Atanasova V, Bosheva M, Cabañas F, et al. Efficacy
of nirsevimab against respiratory syncytial virus lower respiratory tract infections
in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital
heart disease and chronic lung disease: a pooled analysis of randomised controlled
trials. Lancet Child Adolesc Health. 2023 Mar;7(3):180–9. doi: https://doi.org/10.1016/S2352-4642(22)00321-2
3. Ernst C, Bejko D, Gaasch L, Hannelas E, Kahn I, Pierron C, et al. Impact of nirsevimab
prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations
during the initial 2023/24 season in Luxembourg. Euro Surveill. 2024 Jan;29(4):2400033.
doi: https://doi.org/10.2807/1560-7917.ES.2024.29.4.2400033
4. Lassoued Y, Levy C, Werner A, Assad Z, Bechet S, Frandji B, et al. Effectiveness of
nirsevimab against RSV-bronchiolitis in paediatric ambulatory care: a test-negative
case-control study. Lancet Reg Health Eur. 2024 Jul;44:101007. doi: https://doi.org/10.1016/j.lanepe.2024.101007
5. López-Lacort M, Muñoz-Quiles C, Mira-Iglesias A, López-Labrador FX, Mengual-Chuliá B,
Fernández-García C, et al. Early estimates of nirsevimab immunoprophylaxis effectiveness
against hospital admission for respiratory syncytial virus lower respiratory tract
infections in infants, Spain, October 2023 to January 2024. Euro Surveill. 2024 Feb;29(6):2400046.
doi: https://doi.org/10.2807/1560-7917.ES.2024.29.6.2400046
6. Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, et al.; MELODY Study
Group. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants.
N Engl J Med. 2022 Mar;386(9):837–46. doi: https://doi.org/10.1056/NEJMoa2110275
7. Svizzera; P.S.P.S.P., et al., Consensus statement / recommendation on the prevention
of respiratory syncytial virus (RSV) infections with the monoclonal antibody Nirsevimab
(Beyfortus®) 2024: https://www.infovac.ch
8. Swiss DR. Klarstellung zur Vergütung von Nirsevimab (RSB-Immunisierung für Neugeborene).
2025 15.01.2025]; Available from: https://www.swissdrg.org/application/files/6917/3693/8899/Klarstellung_zur_Verguetung_von_Nirsevimab.pdf
9. Bundesamt für Gesundheit. S., Respiratorisches Synzytial-Virus. RSV; 2024.
10. Drysdale SB, Cathie K, Flamein F, Knuf M, Collins AM, Hill HC, et al.; HARMONIE Study
Group. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N Engl
J Med. 2023 Dec;389(26):2425–35. doi: https://doi.org/10.1056/NEJMoa2309189
11. Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, et al.; Nirsevimab
Study Group. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N Engl
J Med. 2020 Jul;383(5):415–25. doi: https://doi.org/10.1056/NEJMoa1913556
12. Zürcher SJ, Signorell A, Léchot-Huser A, Aebi C, Huber CA. Childhood vaccination coverage
and regional differences in Swiss birth cohorts 2012-2021: are we on track? Vaccine.
2023 Nov;41(48):7226–33. doi: https://doi.org/10.1016/j.vaccine.2023.10.043
13. Sabatini S, Kaufmann M, Fadda M, Tancredi S, Noor N, Van Der Linden BW, et al. Factors
Associated With COVID-19 Non-Vaccination in Switzerland: A Nationwide Study. Int J
Public Health. 2023 May;68:1605852. doi: https://doi.org/10.3389/ijph.2023.1605852
14. Krasselt J, Robin D, Fadda M, Geutjes A, Bubenhofer N, Suzanne Suggs L, et al. Tick-Talk:
parental online discourse about TBE vaccination. Vaccine. 2022 Dec;40(52):7538–46.
doi: https://doi.org/10.1016/j.vaccine.2022.10.055
15. Lafnitzegger A, Gaviria-Agudelo C. Vaccine Hesitancy in Pediatrics. Adv Pediatr. 2022 Aug;69(1):163–76.
doi: https://doi.org/10.1016/j.yapd.2022.03.011
16. Wagner A, Juvalta S, Speranza C, Suggs LS, Drava J; COVIDisc study group. Let’s talk
about COVID-19 vaccination: relevance of conversations about COVID-19 vaccination
and information sources on vaccination intention in Switzerland. Vaccine. 2023 Aug;41(36):5313–21.
doi: https://doi.org/10.1016/j.vaccine.2023.07.004
17. Bundesamt für Gesundheit. S., Nirsevimab zur Immunisierung gegen das Respiratorische
Synzytial-Virus (RSV), in BAG-Bulletin, S. Bundesamt für Gesundheit, Editor. 2024.
p. 8-18.
18. Bhugra P, Grandhi GR, Mszar R, Satish P, Singh R, Blaha M, et al. Determinants of
Influenza Vaccine Uptake in Patients With Cardiovascular Disease and Strategies for
Improvement. J Am Heart Assoc. 2021 Aug;10(15):e019671. doi: https://doi.org/10.1161/JAHA.120.019671
19. Ng QX, Ng CX, Ong C, Lee DY, Liew TM. Examining Public Messaging on Influenza Vaccine
over Social Media: Unsupervised Deep Learning of 235,261 Twitter Posts from 2017 to
2023. Vaccines (Basel). 2023 Sep;11(10):1518. doi: https://doi.org/10.3390/vaccines11101518
20. Chao DL, Dimitrov DT. Seasonality and the effectiveness of mass vaccination. Math
Biosci Eng. 2016 Apr;13(2):249–59. doi: https://doi.org/10.3934/mbe.2015001
21. Jin Hsieh TY, Cheng-Chung Wei J, Collier AR. Investigation of Maternal Outcomes Following
Respiratory Syncytial Virus Vaccination in the Third Trimester: Insights from a Real-World
U.S. Electronic Health Records Database. Am J Obstet Gynecol. 2025.
22. Kafadar AH, Sabatini S, Jones KA, Dening T. Categorising interventions to enhance
vaccine uptake or reduce vaccine hesitancy in the United Kingdom: A systematic review
and meta-analysis. Vaccine. 2024 Nov;42(25):126092. doi: https://doi.org/10.1016/j.vaccine.2024.06.059



