Modelling the health and cost implications of expanded access to HIV, HCV and sexually
transmitted infection testing in Switzerland
DOI: https://doi.org/https://doi.org/10.57187/s.4581
Harsh Vivek Harkareab,
Marina Antillonab,
Axel J. Schmidtc,
Fabrizio Tediosiabd
a Swiss Tropical and Public Health Institute,
Allschwil, Switzerland
b University of Basel, Basel,
Switzerland
c Sigma Research, Department of
Public Health, Environments and Society, London School of Hygiene and Tropical
Medicine, London, United Kingdom
d University of Milan, Milan, Italy
Summary
BACKGROUND: This study was conducted as part of the Swiss
National Programme to Stop HIV, Hepatitis B Virus, Hepatitis C Virus and Sexually
Transmitted Infections (NAPS), which aims to reduce the spread of sexually transmitted
infections in Switzerland. The goal was to identify the most effective and cost-efficient
screening strategies to lower the incidence of human immunodeficiency virus (HIV),
hepatitis C virus (HCV), syphilis, Neisseria gonorrhoeae and Chlamydia
trachomatis by improving access to screening.
METHODS: A Markov model
was developed to assess the impact of various screening strategies among key populations
over two years, including men who have sex with men (MSM), female sex workers
(FSW) and people who inject drugs (PWID). The model further stratifies individuals
based on partner number (MSM) and injection-equipment sharing (PWID). Comprehensive
cost estimates for screening and treatment were derived from insurance data, literature
and expert opinions. The effectiveness of screening interventions was evaluated
by measuring reductions in disease incidence and cost savings, comparing the costs
of screening to those of acute and chronic care for prevented infections.
RESULTS: Increased screening frequency among key populations
led to a reduction in incidence for all five infections studied. The largest effect
was seen in people who inject drugs who share injecting equipment, where HCV incidence
fell by up to 76% with four annual screens. However, only screening for HIV, HCV
and syphilis proved to be cost-saving. Screening for Chlamydia trachomatis
and Neisseria gonorrhoeae consistently incurred net costs due to the high
screening costs and relatively low treatment costs.
CONCLUSION: Targeted expansion of screening among key populations
can reduce the incidence of HIV, HCV and syphilis in Switzerland, with regular screening
offering potential cost savings to insurers under specific coverage and treatment
scenarios.
Abbreviations
- FSW
-
female sex worker
- MSM
-
men who have sex with men
- PWID
-
people who inject drugs
Introduction
The Swiss National Programme to Stop HIV, Hepatitis B Virus, Hepatitis C Virus
and Sexually Transmitted Infections (NAPS) aims to eliminate
HIV and HCV transmission and reduce the spread of sexually transmitted infections
in Switzerland by 2030 [1]. Previous research has shown that increasing screening
frequency can significantly lower the prevalence of certain bacterial sexually
transmitted infections in Switzerland, such as syphilis [2]. However, the optimal
screening intervals for other sexually transmitted infections, such as Neisseria
gonorrhoeae and Chlamydia trachomatis, remain uncertain. Currently,
sexually transmitted infection screening and testing in Switzerland is not covered
by compulsory health insurance unless symptoms are present or there is a justified
suspicion of infection. HIV testing is covered under provider-initiated counselling
and testing (PICT) guidelines, while HCV screening is not covered [3].
With the increasing recognition of asymptomatic sexually
transmitted infection transmission, assessing the effectiveness of various screening
strategies within the Swiss context has become increasingly important [4]. Previous
studies have highlighted the heightened infection risk among specific populations
such as men who have sex with men (MSM) and female sex workers (FSW) [5, 6]. In
response, NAPS prioritises expanding screening efforts and improving access to testing
for at-risk groups. This includes revising testing strategies based on evidence
and ensuring equitable access for all, including individuals with limited financial
resources.
The present modelling study was conducted with two key objectives:
to assess the impact of increased screening frequencies and to provide guidance
for official sexually transmitted infection screening recommendations by the Swiss
Federal Office of Public Health (FOPH). Currently, screening guidelines are available
only from the Swiss AIDS Federation (Aids-Hilfe Schweiz [AHS]) [7].
The study seeks to identify the most effective screening
strategy, considering key populations and optimal screening frequencies to reduce
the incidence of HIV, HCV, syphilis, Neisseria gonorrhoeae and Chlamydia
trachomatis in Switzerland. Additionally, it evaluates the economic impact of
integrating such a strategy into the Swiss compulsory health insurance benefit package.
Methods
Screening strategies
Screening strategies are determined based on a combination
of factors, including key population, type of infection and screening frequency.
Following the recommendations outlined by the Swiss AIDS Federation and considering
data availability, the study focuses on three key populations:
- men who have sex with men (MSM)
- female sex workers (FSW)
- people who inject drugs (PWID)
MSM and PWID were further subdivided into two subgroups: those at higher
risk and those at lower risk of sexually transmitted infections. According to recommendations
from the Swiss AIDS Federation, MSM in Switzerland are considered at higher risk
if they had 12 or more non-steady partners per year [8]. For PWID, the Swiss
Federal Office of Public Health LoveLife campaign defines higher risk of HIV and
HCV as having ever shared needles or injection equipment [9].
Screening frequencies for all five infections under study
– HIV, hepatitis C virus, syphilis, Neisseria gonorrhoeae and Chlamydia
trachomatis – were modelled based on the behavioural risk level of each
target group: individuals at lower risk undergo annual screening, while those at
higher risk are screened two to four times per year [7]. All FSW were classified
as being at high risk of sexually transmitted infections due to their typically
high number of partners.
A significant proportion of pre-exposure prophylaxis (PrEP)
users in Switzerland are enrolled in the Swiss PrEPared study, where they undergo
frequent screening for sexually transmitted infections [10]. Consequently, pre-exposure
prophylaxis users were excluded from our analysis.
We modelled screening for different combinations of key populations
and infections based on Swiss AIDS Federation recommendations [7]. Specifically,
we simulated the impact of screening for all infections except HCV among MSM and
FSW, while for PWID, screening was modelled only for HIV and HCV.
Screening frequencies were defined in increments, starting
from a baseline level (0×) and increasing by one additional annual screen up to
a maximum of four screens per year (4×). The baseline screening frequency (0×) represents
the current status quo of testing within the key populations in Switzerland, without
any interventions offering screening at reduced prices. A 1× screening scenario
refers to the provision of one annual screen for everyone within a given key population,
taking the actual testing uptake rate into account.
Based on the above recommendations, a total of 36 screening
strategies were defined and simulated. Additionally, 16 comparator scenarios were
established, each representing a baseline screening frequency for each disease and
key population, simulating the current observed screening uptake in Switzerland.
The modelling conventions for the key populations are as follows: MSM with higher
partner numbers (MSM_HR), MSM with lower partner numbers (MSM_LR), female sex
workers, people who inject drugs with equipment sharing (PWID_HR) and other PWID
(PWID_LR). A list of all simulated screening frequencies is provided in the appendix
(table S2).
Modelling approach and outcomes
A Markov model was developed to simulate the impact of increased
screening within each target population. The model runs on weekly time steps and
comprises four health states: (1) susceptible, untested, (2) susceptible, tested,
(3) infected, diagnosed, and (4) infected, undiagnosed, as outlined in figure 1.
The transition probabilities for each infection were defined separately and were
informed by a variety of data sources as well as published literature as outlined
in the appendix. The observed values of the population were fitted
to a Dirichlet distribution – a conjugate prior to the multinomial distribution
– and sampled 1000 times to incorporate parameter uncertainty.
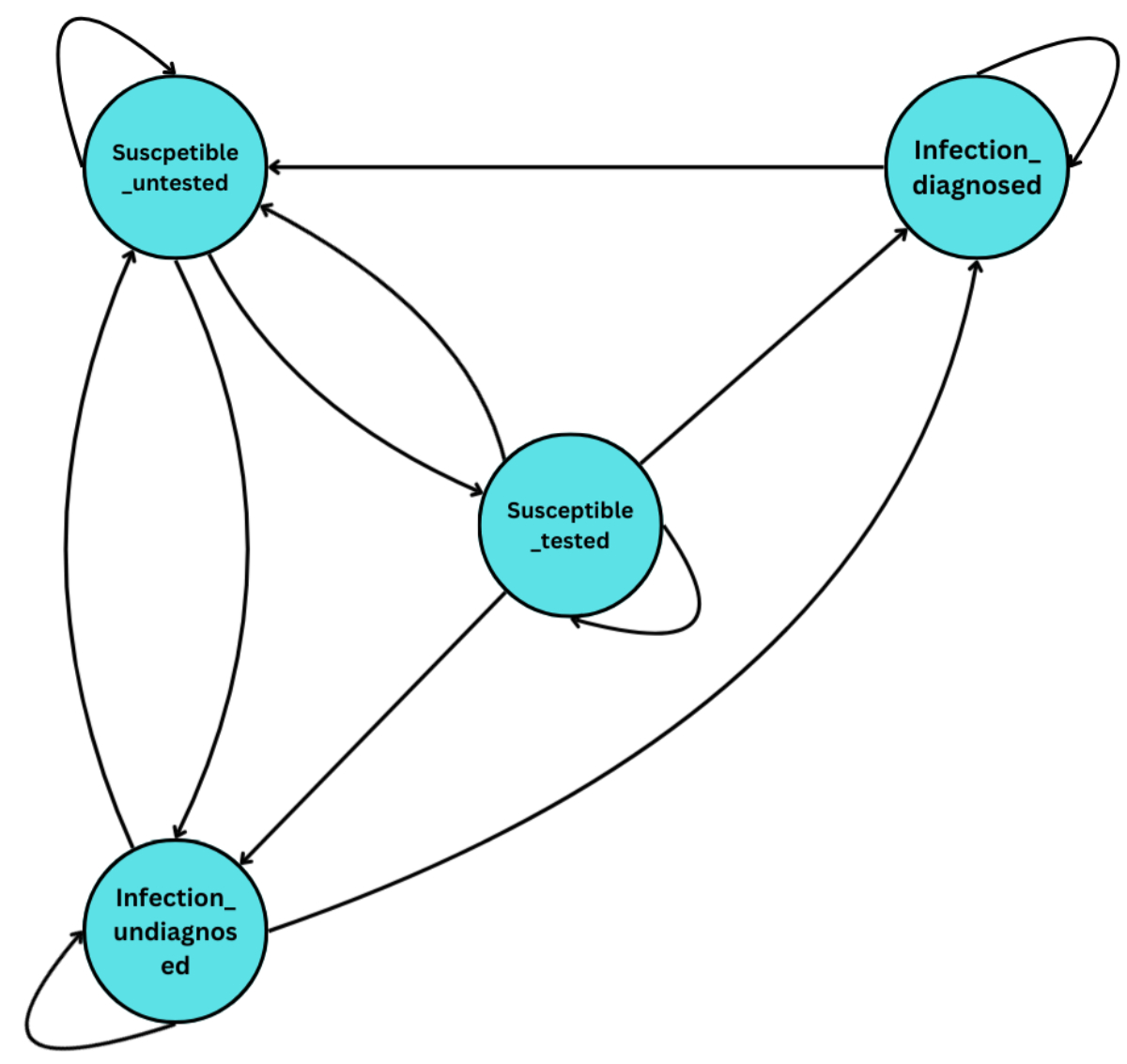
Figure 1Markov model structure. The model consists of four health states: (1) susceptible
and untested, (2) susceptible
and tested, (3) infected but undiagnosed, and (4) infected and diagnosed. Individuals
can transition between these states on a weekly basis. Not all infections involve
all transitions. For example, in the case of HIV, the transition from “Infection,
diagnosed” to “Susceptible, untested” is zero, as individuals do not return to an
untested susceptible state after diagnosis. Similarly, transition probabilities
may differ by infection type depending on natural clearance or treatment efficacy.
For each screening strategy, we assessed two primary outcomes:
- Health benefit: Decrease in incidence
of infection in the first and the second year after implementing the expanded screening
strategy, as compared to the baseline incidence under the status quo screening frequency.
- Cost to insurance providers: This
will include comparing the cost of screening with the cost of acute- and chronic-care
treatment for HIV and HCV, or treatment of severe complications for syphilis, Neisseria
gonorrhoeae and Chlamydia trachomatis.
Healthcare in Switzerland is funded through
mandatory basic insurance, with individuals paying monthly premiums and covering
medical costs up to a set deductible (franchise). Beyond this, insurance covers
expenses minus a co-payment, until a maximum out-of-pocket limit is reached. Except
for HIV, screening for sexually transmitted infections and HCV is covered by insurance
when symptoms are present or an infection is suspected. Otherwise, screening is
subject to the deductible, which may discourage access [11].
To assess the cost implications for insurance providers,
we analysed two distinct scenarios of insurance coverage: one with and one without
the waiver of the deductible component (referred to here as with or without franchise-waiver).
The decision to offer screening with or without a franchise-waiver substantially
affects the estimated financial burden on insurers, as implementing a franchise-waiver
leads to higher costs for them. A screening strategy is considered cost-saving if
its total implementation cost is lower than the projected expenses for treating
the infections it prevents. Additionally, we assumed that the screening uptake rate
remained constant, regardless of whether a franchise-waiver is in place. We considered
the full range of deductible thresholds offered in Switzerland (CHF 300 to CHF 2500)
for the franchise-waiver scenario. Detailed assumptions on the franchise-level enrolment
and insurer cost shares can be found in the appendix.
The cost implications to insurance providers
under different screening evaluation strategies were assessed in terms of their
cost-saving potential. Cost-saving
outcomes were evaluated across four scenarios, ordered by increasing cost assumptions:
(1) acute treatment with franchise-waiver, (2) acute treatment without franchise-waiver,
(3) chronic treatment with franchise-waiver, and (4) chronic treatment without franchise-waiver.
This hierarchical structure reflects the logic that if a screening strategy is cost-saving
under the most conservative assumption (acute treatment with franchise-waiver),
it will also be cost-saving under all less-conservative scenarios. Conversely, if
a strategy is only cost-saving under the final scenario (chronic treatment without
franchise-waiver), it will not be cost-saving under any of the cheaper evaluation
strategies. This framework allows us to assess the robustness of each strategy’s
cost-saving potential across increasing cost assumptions.
To assess the validity of our model,
we compared the model-estimated incidence rates under baseline screening conditions
with empirically observed incidence data for key populations, including MSM and
FSW.
Data sources
Multiple data sources were used to inform model parameters.
Empirical data on screening uptake, test positivity, disease prevalence and risk
behaviours were drawn from BerDa, the Swiss STAR trial and EMIS-2017 [5, 12]. BerDa
is an electronic tool developed by the Swiss Federal Office of Public Health for
history-taking, counselling and recording related to HIV, HCV and sexually
transmitted infection [3]. It captures self-reported information on sexual risk,
behaviours and previous infections. Additionally, most test centres using BerDa
also reported laboratory-confirmed diagnoses of HIV, HCV and sexually
transmitted infections. For our analysis, we used BerDa data for one year, from
June 2023 to June 2024. The data was collected from 19 voluntary counselling and
testing centres across Switzerland, of which only two did not report laboratory-confirmed
outcomes. The Swiss STAR trial also provides laboratory-confirmed diagnoses, whereas
EMIS-2017 relies on self-reported data.
Additional parameters – such as treatment
success and uptake rates, diagnostic sensitivity and specificity, proportion of
symptomatic and asymptomatic infections and population estimates – were obtained
from published literature. Population estimates were updated to reflect the current
year based on Switzerland’s overall population growth trend. SWICA provided data
on health expenditure and insurance demographic profiles. For model validation,
empirically observed incidence rates in Switzerland were taken from the most recent
population-specific, laboratory-confirmed incidence estimates available, derived
from the Swiss STAR trial, which was conducted in 2017 [5, 6]. Data from the Swiss
HIV Cohort Study and Swiss PrEPared could not be obtained. A summary of key model
parameters and data sources is presented in table 1, while further details, including
a complete list of model parameters and their sources, are provided in appendix
table S1.
Table 1Overview of modelling parameters and key data sources. Notes:
Exact values for each parameter can be found in appendix table S1.
| Parameter category |
Parameters |
Key data sources |
| Population size |
Size of the populations
of men who have sex with men, female sex workers and people who inject drugs |
Schmidt
& Altpeter (2019) [37]; Vernazza et al. (2020) [6]; Bihl et al. (2021) [35]; UNAIDS
[38] |
| Risk behaviour |
Proportion engaging
in high-risk behaviour (e.g. multiple partners, equipment sharing) |
BerDa EMIS-2017 [39];
Swiss STAR Trial [40] |
| Disease prevalence |
HIV, syphilis, Neisseria
gonorrhoeae, Chlamydia trachomatis, HCV prevalence by group |
Bigler et al. (2023)
[41]; Schmidt & Altpeter (2019) [37]; Swiss STAR Trial [40]; UNODC [42] |
| Screening uptake |
Proportion tested
in past year by infection and risk group |
BerDa EMIS-2017 [39];
Expert opinion |
| Test characteristics |
Test-positivity rate
and diagnostic sensitivity and specificity by infection |
BerDa EMIS-2017 [39]; Nevin et al. (2008) [43]; Park et al. (2020) [44]; Vetter et
al. (2020) [45] |
| Treatment parameters |
Treatment uptake
and success rates by infection |
Expert opinion; Kohler et al. (2015) [46]; Nevin et al. (2008) [43]; Wandeler et al.
(2015) [47] |
| Transmission parameters |
Onward infections
per untreated case |
Garnett et al. (1997, 1999) [48, 49]; Paltiel et al. (2006) [50]; Potterat et al.
(1999) [51] |
| Symptomatology |
Proportion of asymptomatic
infections |
Cui et al. (2024) [52]; ECDC [53]; Maheshwari et al. (2008) [54]; Martin-Sanchez et
al. (2020)
[56] |
| Self-clearance (HCV) |
Proportion of untreated
infections that self-clear |
Grebely et al. (2014)
[56] |
Health insurance cost estimates
To estimate the cost implications of screening and treatment,
we also estimated the cost of a single screening test as well as the costs of acute-care
treatment for all five infections, chronic-care treatment for HIV and HCV, and of
severe complications of Neisseria gonorrhoeae, Chlamydia trachomatis,
syphilis. The estimated cost of screening is inclusive of the cost of consultation
as well as the actual screening cost. Consultation costs for screening for any test
include 20 minutes of consultation time with an infection specialist as well as
personnel time for blood draw, for preparing the test, and for communicating results
to the patient (appendix table S5). The cost of screening is estimated from data from
SWICA insurance, which covers 20% of the Swiss population and has data on the actual
number of tests undertaken and costs incurred by different test centres. Aggregated
SWICA data from 2021–2022 provide information on insured individuals, completed
screening tests, and associated costs, stratified by age group and franchise level.
The mean and 95% CI of the cost of a single screening test was calculated by aggregating
over the age group, franchise and year. In line with approved practices, costs for
Neisseria gonorrhoeae and Chlamydia trachomatis screening include
testing from three separate swab sites [13].
The cost of medication and treatment were added to the cost
of consultation to obtain the final cost of treating one infection. Treatment and
medication costs were derived from the official tariff structure of medical services
and from the Swiss Federal Office of Public Health-mandated costs for speciality
medical products and services [14, 15]. Treatment guidelines for each infection
were taken from the Swiss Society for Infectiology [16].
While the costs used in this analysis are estimated from
a macroeconomic societal perspective, we also calculated the costs of treating a
single infection – both acute and chronic care. A summary of the screening and treatment
costs are tabulated in appendix table S3. The costing methodology and guidelines
are further detailed in the tables S4 and S5 in the appendix.
Sensitivity analysis
To assess the robustness of our model results to variations
in key input parameters, we ran sensitivity analyses, comparing changes to the status
quo values based on the observed data. The choice of these sensitivity analyses
was based on the parameters that had the most uncertainty and the greatest potential
to influence results, as well as with the least available data:
- a
20% increase in the screening uptake rate;
- a
10% increase in the test-positivity rate;
- a
25% decrease in the onward infection transmission rate.
A detailed description of the modelling methods can be found
in the appendix.
Results
Impact on incidence
To validate the Markov transition dynamics
of our model, we compared the model-estimated incidence of HIV, syphilis, Neisseria
gonorrhoeae and Chlamydia trachomatis under the baseline status quo screening
frequency with the incidence observed in the STAR trial for the key populations
of MSM and FSW [5, 6]. Figure 2 presents a comparison of the model-estimated incidence
against the observed incidence. Except for Neisseria gonorrhoeae in the high-partner-number
MSM group, our model accurately estimates the observed incidence. Due to a lack
of data on population-representative incidence estimates, we were unable to compare
our results for PWID.
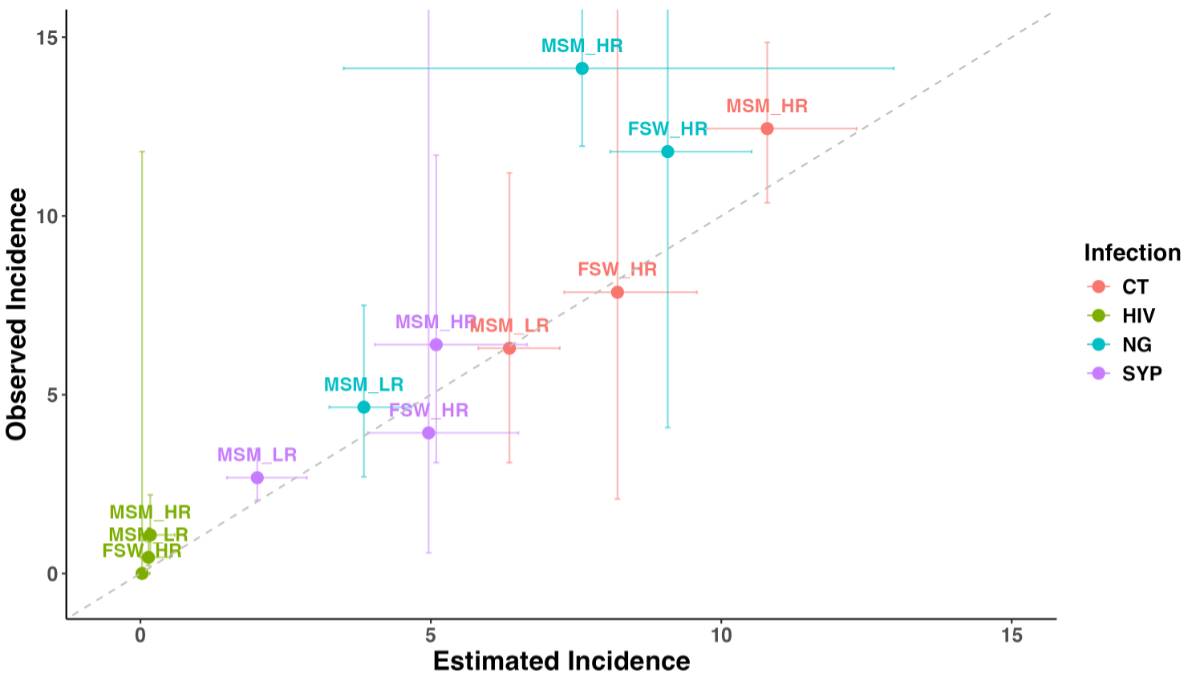
Figure 2Comparison of model-estimated and observed incidence per 100 person-years.
The model-estimated incidence was compared with observed incidence across
various populations for human immunodeficiency virus (HIV), syphilis (SYP),
Chlamydia trachomatis and Neisseria gonorrhoeae. Observed incidence
data was obtained from the Swiss STAR trial. The vertical bars show the 95%
confidence intervals of the incidence in the trial (observed incidence) and the
horizontal bars show the 95% predictive intervals of our model with re-sampling
of parameter values (estimated incidence). FSW: female
sex workers; MSM_HR: men who have sex with men with higher partner numbers; MSM_LR:
men who have
sex with men with lower partner numbers.
Figure
3 illustrates the impact of expanded sexually transmitted infection screening on
incidence in Switzerland. As the screening frequency increases, incidence declines
across all infections as compared to the baseline incidence under the status quo
screening frequency. The most prominent reduction is observed for HCV among high-risk
PWID: a 76% decrease with 4× annual screens by year 2.
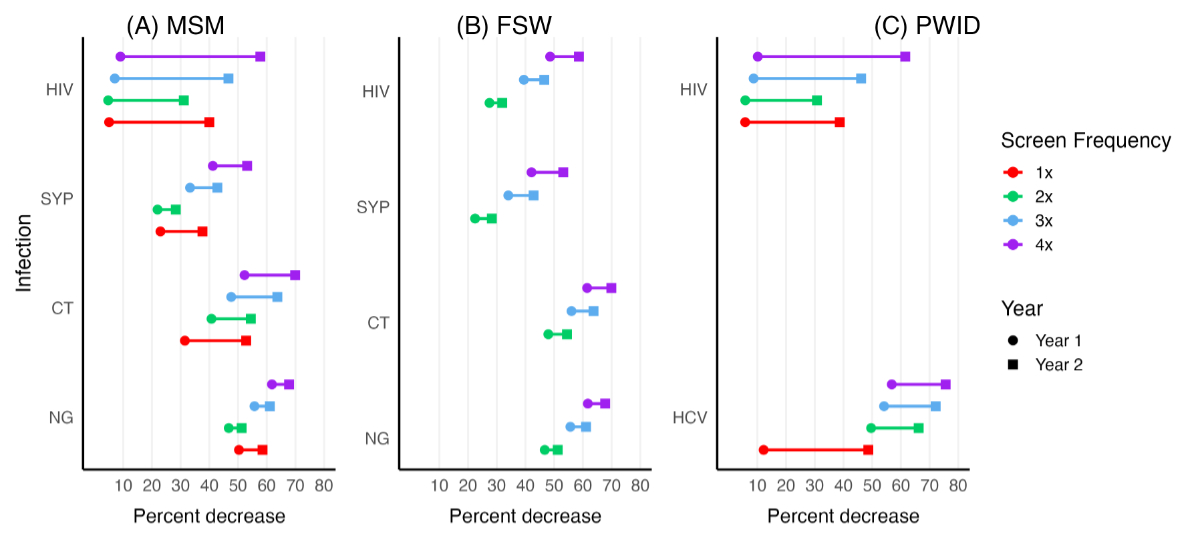
Figure 3Percentage decrease in incidence by infection and key population over time. The percentage
reduction is estimated for different screening frequencies
over a two-year period. In this dumbbell plot, the circle denotes the average
reduction in year 1, while the square represents the corresponding reduction in
year 2. Annual screening (1×) was available exclusively for men who have sex with
men (MSM) and people who inject drugs (PWID) in the low-risk groups, with
no 1× screen provided for the female sex workers (FSW) group. In contrast, screening
frequencies of 2×, 3× and 4× per year were offered only to MSM and PWID in the
high-risk groups and to all FSW. CT: Chlamydia trachomatis; HIV: human
immunodeficiency virus; NG: Neisseria gonorrhoeae; SYP: syphilis.
Among
MSM, Neisseria gonorrhoeae incidence shows a substantial decline, primarily
due to its already high baseline observed incidence. Similarly, HIV also exhibits
a significant reduction, largely driven by the high screening uptake rate. This
effect is, however, smaller in magnitude as compared to other infections due to
its already low prevalence. Screening strategies with 1× annual screening for any
infection have a more pronounced effect on incidence reduction, as they target a
larger population – MSM with lower partner numbers – whereas higher screening frequencies
(2×, 3× and 4×) are limited to MSM with higher partner numbers, a much smaller population.
Among
FSW, where observed incidence of Neisseria gonorrhoeae and Chlamydia
trachomatis is high, screening has the greatest impact on reducing incidence
for these two infections. Despite the already low HIV incidence in FSW, the high
testing uptake rate results in a significant reduction in incidence.
For HCV
in PWID, the impact of 1× annual screening is relatively smaller, reflecting the
high test-positivity rate among PWID who don’t report sharing of drug use equipment.
The impact of 1× annual screening on HIV is higher than 2× but smaller than 3× and
4× annual screening because of the larger number of PWID not sharing drug use equipment
as well as the low prevalence in the population.
Impact on costs to insurance providers
Figure 4 presents the estimated
annual cost savings under different screening strategies by key population from
the perspective of insurers. Cost savings are evaluated based on the type of insurance
coverage – franchise-waiver or no franchise-waiver – and treatment costs (acute
or chronic care). Given that acute-care treatment costs are typically the lowest,
cost-savings are more limited under this framework.
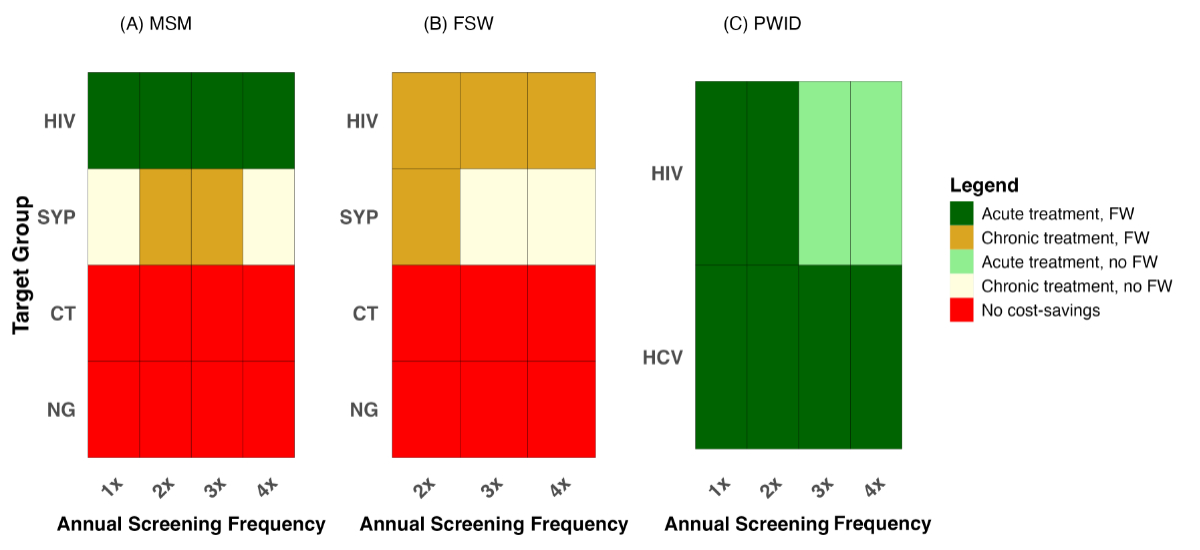
Figure 4Cost savings under different
screening strategies and insurance coverage. Each screening
strategy reflects a combination of screening frequency and infection type. Cost
savings are shown separately for key populations: (A) men who have sex with men
(MSM), (B) female sex workers (FSW) and (C) people who inject drugs (PWID).
Estimates are presented across insurance scenarios – with or without a
franchise-waiver (FW) – and treatment coverage assumptions (acute or chronic
care). Importantly, if a strategy is cost-saving under the “acute treatment, franchise-waiver”
scenario, it remains cost-saving under all other, more expensive combinations.
In contrast, strategies that are only cost-saving under the “chronic treatment,
no franchise-waiver” scenario are not cost-saving in any other case. Red cells
indicate strategies that are not cost-saving. All other colours represent
cost-saving strategies, classified by cost and insurance assumptions as
detailed in the figure legend. Please also note: 1× screening is offered only
to low-risk MSM and PWID groups, which represent larger population segments
compared to their high-risk counterparts. CT: Chlamydia trachomatis; HIV: human
immunodeficiency virus; NG: Neisseria gonorrhoeae; SYP: syphilis.
Cost-saving outcomes were assessed across
four evaluation scenarios, ordered by increasing cost assumptions: (1) acute treatment
with franchise-waiver, (2) acute treatment without franchise-waiver, (3) chronic
treatment with franchise-waiver, and (4) chronic treatment without franchise-waiver.
A strategy deemed cost-saving under a more conservative cost scenario (acute treatment
with franchise-waiver) is also considered cost-saving under all scenarios that are
more costly to insurance providers. In figure 4, dark green indicates strategies
that are cost-saving under the most conservative assumption – acute treatment with
franchise-waiver – and thus also under all other evaluation scenarios. Light green
reflects strategies that are cost-saving under acute treatment without franchise-waiver
and under all subsequent scenarios, but not under the most conservative. Ochre denotes
strategies that are cost-saving under both chronic treatment scenarios but not under
either acute scenario. Pale yellow indicates strategies that are cost-saving only
under the most generous and costly evaluation condition: chronic treatment without
franchise-waiver. Red represents strategies that are not cost-saving under any of
the four evaluation scenarios.
From a macroeconomic societal
perspective, a cost saving is more likely when considering chronic-care costs, as
these are generally higher than acute-care costs – except in the case of Neisseria
gonorrhoeae and Chlamydia trachomatis. With a franchise-waiver, insurers
bear a larger share of screening costs, making it more challenging for screening
strategies to be cost-saving. Conversely, integrating screening into the health
insurance framework without a franchise-waiver would impose a lower financial burden
on insurers. Thus, strategies that are cost-saving under the “chronic treatment,
no franchise-waiver” scenario are not cost-saving under any other scenario.
Overall, offering 1–4× annual
screens to each key population is expected to be cost-saving for HIV, HCV and syphilis
at different levels of treatment and insurance coverage. However, for Neisseria
gonorrhoeae and Chlamydia trachomatis, cost savings are not anticipated
under any combination of treatment and insurance coverage. These findings were robust
under the different sensitivity scenarios conducted.
HIV had the lowest reduction
in the absolute number of infections due to its already low prevalence, but the
cost of treatment was the highest, thus making it cost-saving. Despite the large
reductions in incidence for Neisseria gonorrhoeae and Chlamydia
trachomatis, the high screening costs coupled with relatively low treatment
costs make them unlikely to be cost-saving. Detailed results of screening impact
on absolute values of infections prevented, screening and treatment costs are shown
in the appendix in tables S7–S9.
Table 2 presents the estimated annual cost savings (in thousands
of CHF) for each screening strategy, disaggregated by infection, key population,
screening frequency and evaluation scenario over one year. Cost-saving potential
is greatest for HIV, particularly among MSM with fewer than 12 sexual partners a
year (MSM_LR), where annual screening (1×) yields over CHF 261 million in savings
under the chronic treatment without franchise-waiver scenario. Substantial savings
are also observed for high-frequency screening among MSM_HR and for HCV screening
among PWID, especially those not sharing injection equipment. In contrast, screening
for Neisseria gonorrhoeae and Chlamydia trachomatis consistently results
in net financial losses, reflecting high screening costs relative to treatment costs.
For syphilis, cost savings are modest and scenario-dependent, with benefits observed
primarily under more generous cost evaluation assumptions.
Table 2Estimated cost savings
by comparing screening costs with treatment costs prevented for different
combinations of screening and treatment coverage and screening scenarios over
one year.
| Infection |
Key
population |
Screening frequency |
Estimated
cost savings (in thousands of CHF) |
| Acute
treatment, franchise-waiver screening |
Acute
treatment, no franchise-waiver screening |
Chronic
treatment, franchise-waiver screening |
Chronic
treatment, no franchise-waiver screening |
| Human immunodeficiency virus (HIV) |
MSM_LR |
1× |
17,111 |
19,616 |
258,818 |
261,323 |
| MSM_HR |
2× |
6,,203 |
6,940 |
88,700 |
89,437 |
| 3× |
5967 |
6,713 |
86,411 |
87,157 |
| 4× |
5,800 |
6,550 |
84,751 |
85,501 |
| Female sex workers |
2× |
−1,447 |
−493 |
13,111 |
14,065 |
| 3× |
−1,721 |
−760 |
10,411 |
11,372 |
| 4× |
−1,913 |
−945 |
8,539 |
9,507 |
| PWID_LR |
1× |
2,013 |
2,368 |
32,249 |
32,604 |
| PWID_HR |
2× |
631 |
737 |
9,963 |
10,069 |
| 3× |
591 |
698 |
9,550 |
9,657 |
| 4× |
566 |
675 |
9,339 |
9,448 |
| Syphilis |
MSM_LR |
1× |
−7,884 |
−5,221 |
−3,044 |
−381 |
| MSM_HR |
2× |
−2,225 |
−1,440 |
580 |
1,365 |
| 3× |
−2,257 |
−1,471 |
142 |
928 |
| 4× |
−2,278 |
−1,492 |
−162 |
624 |
| Female sex workers |
2× |
−3123 |
−2023 |
702 |
1,802 |
| 3× |
−3171 |
−2070 |
88 |
1,189 |
| 4× |
−3,200 |
−2,098 |
−342 |
760 |
| Neisseria gonorrhoeae |
MSM_LR |
1× |
−78,535 |
−53,125 |
−78,821 |
−53,411 |
| MSM_HR |
2× |
−24,873 |
−16,796 |
−25,039 |
−16,962 |
| 3× |
−26,197 |
−17,705 |
−26,334 |
−17,842 |
| 4× |
−26,981 |
−18,243 |
−27,099 |
−18,361 |
| Female sex workers |
2× |
−35,072 |
−23,684 |
−35,306 |
−23,918 |
| 3× |
−36,945 |
−24,968 |
−37,139 |
−25,162 |
| 4× |
−38,045 |
−25,724 |
−38,213 |
−25,892 |
| Chlamydia trachomatis |
MSM_LR |
1× |
−89,210 |
−60,244 |
−89,818 |
−60,852 |
| MSM_HR |
2× |
−28,758 |
−19,411 |
−28,978 |
−19,631 |
| 3× |
−30,273 |
−20,448 |
−30,468 |
−20,643 |
| 4× |
−31,156 |
−21,053 |
−31,334 |
−21,231 |
| Female sex workers |
2× |
−36,976 |
−24,988 |
−37,183 |
−25,195 |
| 3× |
−38,925 |
−26,321 |
−39,100 |
−26,496 |
| 4× |
−40,059 |
−27,098 |
−40,213 |
−27,252 |
| Hepatitis C virus (HCV) |
PWID_LR |
1× |
17,467 |
19,210 |
−2,765 |
−1,022 |
| PWID_HR |
2× |
7,259 |
7,790 |
−620 |
−89 |
| 3× |
6,675 |
7,237 |
−772 |
−210 |
| 4× |
6,281 |
6,861 |
−868 |
−288 |
Savings are maximised under the chronic treatment without franchise-waiver
scenario, which combines high treatment costs with lower insurer contributions to
screening. Chronic treatment costs – such as lifelong antiretroviral therapy or
HCV-related complications – substantially exceed those of acute care, enhancing
the value of prevention. In the absence of a franchise-waiver, screening costs are
partially borne by the insured, further reducing the financial burden on insurers.
Consequently, strategies that prevent high-cost infections while limiting insurer
liability for screening yield the greatest net savings.
However, it is important to note that the acute treatment with
franchise-waiver scenario represents the lowest overall cost to insurers, as outlined
in the aforementioned hierarchy of scenarios for evaluating cost savings. Interpretation
of cost-saving results should therefore be contextualised alongside the absolute
screening and treatment costs presented in tables S8 and S9 in the appendix.
Sensitivity analysis
Figure S1 demonstrates that our findings
are generally robust to variations in assumptions regarding screening uptake rate,
test positivity and onward transmission rate. Among MSM, the results remain unchanged.
For FSW, a 10% increase in the test positivity rate renders syphilis screening
cost-saving. Likewise, for PWID, a 10% increase in test positivity results in screening
becoming cost-saving for HIV under the franchise-waiver scheme.
Discussion
Our model accurately replicates the baseline incidence
of the five infections under study at the current screening uptake rate and simulates
a reduction in incidence across all key populations with increased screening. The
most substantial decline is observed for human immunodeficiency virus (HIV) among
men who have sex with men (MSM)
and people who inject drugs (PWID) over time. Incorporating screening for HIV, syphilis
and hepatitis C virus (HCV) into the health insurance framework is expected to be
cost-saving. While screening has a notable impact on the incidence of Neisseria
gonorrhoeae and Chlamydia trachomatis, it is not enough to offset the
relatively low treatment cost and the high cost of screening. Therefore, integrating
voluntary screening for these infections into the health insurance framework is
unlikely to be cost-saving.
Our findings support the implementation of more frequent
HIV screening for MSM and female sex workers (FSW), as well as at least biannual
screening for people who inject drugs under a franchise-waiver. Although the number
of cases prevented annually is relatively small, the associated cost savings are
significant. The importance of expanding HIV screening coverage, particularly among
vulnerable populations, has been well documented in the literature [17, 18]. Research
also emphasises the benefits of frequent annual screenings in reducing incidence
and improving viral suppression among people living with HIV, particularly within
key populations such as MSM [19, 20]. Additionally, studies underscore the need
for increased screening frequency among groups at higher risk within vulnerable
populations such as MSM [21].
Similarly, for HCV, our results support the provision
of more frequent screening for PWID, aligning with existing literature. Previous
studies have highlighted the benefits of expanded general population screening in
reducing prevalence and mitigating severe complications for individuals who were
infected with contaminated blood products or injectables prior to the discovery
of HCV [22, 23]. Since its discovery in 1989, HCV has primarily been transmitted
through injectables. As a result, targeted and comprehensive screening strategies,
combined with improved access to treatment, have been identified as crucial for
eliminating HCV in vulnerable populations such as PWID and MSM living with HIV [24–26].
Importantly, expanded screening among PWID should be implemented in tandem with
harm-reduction interventions such as needle and syringe programmes and opioid substitution
therapy as these remain the cornerstone of primary prevention efforts [33].
Our study also aligns with findings from a recent
modelling study, which showed that expanding screening from once to twice annually
among HIV-positive MSM resulted in a 63.5% reduction in syphilis incidence. In contrast,
the same increase in screening frequency among HIV-negative MSM led to a 12.8% reduction
in incidence [2]. Similarly, quarterly syphilis screening (four times a year) was
found to be the most effective strategy for reducing syphilis incidence among MSM
with a high frequency of unprotected intercourse [27].
For Neisseria gonorrhoeae and Chlamydia
trachomatis, although pooled swab testing can help lower screening costs, it
would not be sufficient to make the intervention cost-saving, even with a substantial
reduction in incidence observed in our results. A more targeted strategy is needed
to identify the groups at highest risk within key populations and improve cost-effectiveness
by combining focused screening with lower-cost strategies. The evidence in the literature
on the effectiveness of Neisseria gonorrhoeae and Chlamydia
trachomatis screening is inconclusive. While a hospital-based screening strategy
for rectal Neisseria gonorrhoeae/Chlamydia trachomatis in MSM led
to a 43% reduction in incidence, other studies found no strong evidence that Neisseria
gonorrhoeae / Chlamydia trachomatis screening in MSM significantly impacts
disease prevalence, nor that more frequent screening is more effective than annual
screening [28, 29]. A randomised controlled trial (RCT) further showed no impact
of Neisseria gonorrhoeae and Chlamydia trachomatis screening on incidence
among pre-exposure prophylaxis-using MSM [30]. For Chlamydia trachomatis
screening in the general population, a separate RCT found a limited impact, suggesting
that broad population-based screening may not be an effective strategy for reducing
incidence [31]. Moreover, some studies have questioned the clinical rationale for
screening Chlamydia trachomatis infections in general, citing a lack of evidence
for preventing long-term sequelae such as infertility, particular in MSM [34].
Notably, many of these studies focused either on
general populations, which would have a lower infection prevalence than the groups
in our study, or on populations with dense sexual networks, such as MSM on pre-exposure
prophylaxis. Our study excludes pre-exposure prophylaxis users from the key populations
and thus does not capture the densest segment of the MSM sexual networks. This distinction
may explain why our results show a stronger impact of screening in reducing Neisseria
gonorrhoeae and Chlamydia trachomatis prevalence in MSM and FSW who
do not use pre-exposure prophylaxis in Switzerland. These two infections are more
evenly distributed in the overall general MSM and FSW populations compared to syphilis,
which is concentrated among pre-exposure prophylaxis-using and HIV-positive MSM.
Our findings highlight how cost savings from different
screening strategies vary not only by infection type and key population but also
by the structure of insurance coverage and underlying treatment cost assumptions.
A strategy that appears cost-saving under chronic treatment scenarios without a
franchise-waiver may not remain cost-saving under more conservative assumptions,
such as acute care with a franchise-waiver. This gradient underscores the importance
of clearly specifying the evaluation perspective and cost scenario when assessing
the financial implications of sexually transmitted infection screening interventions.
These distinctions are critical for informing health policy decisions and designing
financially sustainable and equitable screening programmes. In addition, while more
frequent screening leads to greater reductions in incidence, these benefits occur
at increasing cost and with diminishing returns.
Cost savings also depend on the underlying incidence
of infection within each key population. For example, HIV screening among MSM remains
cost-saving even under more conservative assumptions, such as acute care with a
franchise-waiver, whereas the same is not true for FSW. This likely reflects the
higher baseline incidence and prevalence of HIV among MSM, which increases the potential
for screening to detect and prevent more cases. Thus, the optimal frequency of screening
should be determined based on local epidemiology, available resources and programme
priorities.
Our study contributes to the growing, albeit small,
body of research evaluating the impact of sexually transmitted infection screening
on disease incidence in the Swiss context [2, 5, 6]. It is the first to examine
this relationship for Neisseria gonorrhoeae and Chlamydia trachomatis
while considering the cost implications for insurance providers. A key strength
of our model is the use of a common Markov framework to simulate both viral and
bacterial sexually transmitted infections, while accounting for their distinct transmission
dynamics and natural histories. Additionally, we provide a comprehensive cost estimate
for screening five different sexually transmitted infections in Switzerland, as
well as the costs associated with treating both acute and chronic conditions or
complications resulting from these infections. Another strength of our approach
is the incorporation of risk-behaviour stratification within the defined key populations,
enabling a more nuanced analysis of disease dynamics between the two risk groups.
Finally, although pre-exposure prophylaxis users were excluded from the analysis,
this does not compromise the generalisability of our findings within the non–pre-exposure
prophylaxis-using MSM population. While pre-exposure prophylaxis users constitute
a small subgroup – less than 7% of the estimated MSM population in Switzerland –
with higher sexual risk behaviours and sexually transmitted infection prevalence,
they are already systematically screened through established programmes [32]. In
contrast, our analysis focuses on expanding access to screening for vulnerable populations
with limited access to structured sexually transmitted infection screening.
There are also some limitations to our analysis.
First, due to data limitations, we are unable to account for variations in disease
dynamics and behaviour within key populations, which may not accurately capture
the intragroup differences. Second, we do not consider the site of swabbing for
Neisseria gonorrhoeae and Chlamydia trachomatis screening, which is
an important factor, as genital infections are more likely to lead to complications
and, therefore, have higher expected treatment costs. Additionally, our assumption
that a reduction in the cost of screening, whether offered with or without a franchise-waiver,
has the same effect on screening behaviour may not hold. Offering asymptomatic screening
without a franchise-waiver through the insurance framework may be more expensive
for the test-takers, potentially leading to a lower impact on screening uptake.
Finally, we assume a linear relationship between screening frequency and its impact
on transition probabilities, due to a lack of data to inform a non-linear relationship,
where increasing screening frequency might result in diminishing returns in uptake.
Future research could focus on more specific subgroups,
particularly for infections with less evidence, such as Neisseria
gonorrhoeae and Chlamydia trachomatis among HIV-positive or pre-exposure
prophylaxis-using MSM. Additionally, future modelling studies may consider the timing
of screening, as diagnostic sensitivity varies depending on the duration of infection.
Finally, since most decision-making is driven by cost-effectiveness rather than
just cost-savings, a comprehensive cost-effectiveness analysis, especially for Neisseria
gonorrhoeae and Chlamydia trachomatis, considering both the costs
prevented through case prevention and the quality-of-life improvements from earlier
diagnosis and treatment, could provide stronger evidence for the economic efficiency
of screening these infections.
Conclusion
Expanding access to screening among key populations
in Switzerland can substantially reduce the incidence of HIV, HCV, syphilis, Neisseria
gonorrhoeae and Chlamydia trachomatis. Our model indicates that incorporating
regular screening for HIV, HCV and, in selected contexts, syphilis into the national
health insurance framework could yield meaningful cost savings for insurers, especially
when long-term treatment costs are considered and screening is offered without a
franchise-waiver by maintaining deductible thresholds for individuals.
Although our model showed a substantial reduction
in Neisseria gonorrhoeae and Chlamydia trachomatis incidence with
increased screening, the high screening costs combined with relatively low treatment
costs meant these strategies were not financially viable under the scenarios evaluated.
This highlights the potential value of more targeted screening approaches, possibly
using finer risk stratifications than applied in this study, and the use of cost-saving
diagnostic methods such as pooled testing.
Data sharing statement
The data used in this study was obtained from participating
Voluntary Counselling and Testing centres (VCTs) in Switzerland (BerDa) and were
provided to the research team under strict data use agreements for the duration
of the study. The datasets were deidentified but remain the property of the individual
VCTs and were deleted from our systems following completion of the analysis, in
accordance with these agreements. As such, we are unable to share the data publicly
or upon request. Interested researchers may contact the relevant VCTs directly to
enquire about access, subject to their institutional data governance policies and
approvals. Data dictionaries and related documents are not publicly available.
Open science statement: This study is a secondary analysis of
anonymised data collected through routine sexually transmitted infection testing
and service delivery. As it does not constitute a clinical trial, registration in
a trial registry was not applicable. Although no formal protocol was prepared, a
detailed analysis plan was included in the original funding proposal and shared
with both the funders and collaborating data-providing centres. No deviations from
this analysis plan occurred.
All analytical code used in the study will be made publicly
available via GitHub prior to publication, including details of the software environment,
packages and versioning. The code will be released under an open-source licence
and linked to in the final manuscript.
Acknowledgments
We sincerely thank Athos Staub for his assistance
in organising the study, for offering high-level feedback and for his continued
support with the project. We also acknowledge Barbara Jakopp and the Federal Commission
for Issues relating to Sexually Transmitted Infections, Working Group 3 for their
significant contributions to estimating the costs of screening and treatment for
different infections. We would also like to extend our thanks to Benjamin Hampel
for sharing his expertise on the clinical context of sexually transmitted
infection screening in Switzerland.
We are grateful to Claudia Scheuter and Guido Biscontin
for their guidance in refining the analytical approach and shaping the direction
of the analysis. We also thank Marcel Tanner and Hannah Tough for their high-level
guidance and feedback on the project. Finally, we are grateful to the entire EKSI
for their valuable feedback on the modelling process and results.
Finally, we extend our warmest thanks to the following
voluntary counselling and testing centres for their cooperation with data sharing:
Aids-Hilfe beider Basel, LadyCheck; Aids-Hilfe beider Basel, Checkpoint; Seges,
Sexuelle Gesundheit Aargau; Checkin Zollstrasse, Zurich; Stadtmission ZH / Isla
Victoria; Centre de santé sexuelle Fribourg; Kantonsspital St. Gallen; Unisanté;
Perspektive Thurgau; Générations Séxualités Neuchâtel; Checkpoint Luzern / S&X
Sexuelle Gesundheit Zentralschweiz; Planning Familial (La Chaux de Fonds); Checkpoint
Zürich; Test-in Zürich; Aids-Hilfe Graubünden; Centre Empreinte; Spital Thurgau;
Checkpoint Vaud; and SIPE Valais.
Harsh Vivek Harkare, MSc
Swiss Tropical and
Public Health Institute
Kreuzstrasse 2
CH-4123 Allschwil
harshvivek.harkare[at]swisstph.ch
References
1. Federal Office of Public Health. National Programme on HIV and other STIs [Internet].
2024 [cited 2025 Feb 14]. Available from: https://www.bag.admin.ch/bag/de/home/strategie-und-politik/nationale-gesundheitsstrategien/nationales-programm-hiv-hep-sti-naps.html
2. Balakrishna S, Salazar-Vizcaya L, Schmidt AJ, Kachalov V, Kusejko K, Thurnheer MC,
et al.; Swiss HIV Cohort Study (SHCS). Assessing the drivers of syphilis among men
who have sex with men in Switzerland reveals a key impact of screening frequency:
A modelling study. PLOS Comput Biol. 2021 Oct;17(10):e1009529. doi: https://doi.org/10.1371/journal.pcbi.1009529
3. Federal Office of Public Health (FOPH). Voluntary counselling and testing [Internet].
Swiss Confederation; 2024 [cited 2025 Apr 4]. Available from: https://www.bag.admin.ch/bag/en/home/krankheiten/krankheiten-im-ueberblick/sexuell-uebertragbare-infektionen/freiwillige-beratung-und-testung.html
4. Schmidt AJ, Marcus U. What’s on the rise in Sexually Transmitted Infections? Lancet
Reg Health Eur. 2023 Oct;34:100764. doi: https://doi.org/10.1016/j.lanepe.2023.100764
5. Schmidt AJ, Rasi M, Esson C, Christinet V, Ritzler M, Lung T, et al. The Swiss STAR
trial - an evaluation of target groups for sexually transmitted infection screening
in the sub-sample of men. Swiss Med Wkly. 2020 Dec;150(5153):w20392. doi: https://doi.org/10.4414/smw.2020.20392
6. Vernazza PL, Rasi M, Ritzler M, Dost F, Stoffel M, Aebi-Popp K, et al. The Swiss STAR
trial - an evaluation of target groups for sexually transmitted infection screening
in the sub-sample of women. Swiss Med Wkly. 2020 Dec;150(5153):w20393. doi: https://doi.org/10.4414/smw.2020.20393
7. AIDS.ch. Leitfaden Safer Sex [Internet]. [cited 2024 Mar 21]. Available from: https://shop.aids.ch/de/A~1911~45/2~110~Shop/Infomaterial/Schutz-vor-HIV-und-STI/Leitfaden-Safer-Sex/deu-fra
8. Dr. Gay. Testing advice [Internet]. 2024 [cited 2025 Feb 14]. Available from: https://drgay.ch/en/safer-sex/tested-and-vaccinated/testing-advice
9. Love Life. STI tests: Important for your sexual health [Internet]. 2024 [cited 2025
Feb 14]. Available from: https://lovelife.ch/de/testen
10. SwissPrepared. SwissPrepared: Pandemic preparedness in Switzerland [Internet]. 2025
[cited 2025 Feb 21]. Available from: https://www.swissprepared.ch/en/
11. Federal Office of Public Health (FOPH). HIV-Test auf Initiative der Ärztin oder des
Arztes (Provider Initiated Counselling and Testing, PICT): Medizinische Empfehlungen
(BAG Bulletin 21/15) [Internet]. 2021 [cited 2025 Feb 25]. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/p-und-p/richtlinien-empfehlungen/pict-hiv-test-auf-initiative-des-arztes.pdf.download.pdf/bu-21-15-pict-hiv.pdf
12. Weatherburn P, Hickson F, Reid DS, Marcus U, Schmidt AJ. European Men-Who-Have-Sex-With-Men
Internet Survey (EMIS-2017): design and methods. Sex Res Soc Policy. 2020;17(4):543–57.
doi: https://doi.org/10.1007/s13178-019-00413-0
13. Swissmedic. Swissmedic - The Swiss Agency for Therapeutic Products [Internet]. 2024
[cited 2025 Feb 14]. Available from: https://www.swissmedic.ch/swissmedic/de/home.html
14. Spezialitätenliste. Spezialitätenliste (SL) und Geburtsgebrechen-Spezialitätenliste
(GGSL) by the BAG/FOPH [Internet]. 2024 [cited 2025 Feb 14]. Available from: https://www.spezialitätenliste.ch
15. TARMED. Tarifstruktur TARMED by the BAG/FOPH [Internet]. 2018 [cited 2025 Feb 14].
Available from: https://www.bag.admin.ch/bag/de/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Aerztliche-Leistungen-in-der-Krankenversicherung/Tarifsystem-Tarmed.html
16. Swiss Society of Infectious Diseases. SSI guidelines [Internet]. 2024 [cited 2024
May 11]. Available from: https://ssi.guidelines.ch
17. Wainberg MA, Hull MW, Girard PM, Montaner JS. Achieving the 90-90-90 target: incentives
for HIV testing. Lancet Infect Dis. 2016 Nov;16(11):1215–6. doi: https://doi.org/10.1016/S1473-3099(16)30383-8
18. UNAIDS. 90–90–90: An ambitious treatment target to help end the AIDS epidemic [Internet].
2014 [cited 2025 Feb 14]. Available from: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf
19. Neilan AM, Bulteel AJ, Hosek SG, Foote JH, Freedberg KA, Landovitz RJ, et al. Cost-effectiveness
of Frequent HIV Screening Among High-risk Young Men Who Have Sex With Men in the United
States. Clin Infect Dis. 2021 Oct;73(7):e1927–35. doi: https://doi.org/10.1093/cid/ciaa1061
20. Phillips AN, Cambiano V, Miners A, Lampe FC, Rodger A, Nakagawa F, et al. Potential
impact on HIV incidence of higher HIV testing rates and earlier antiretroviral therapy
initiation in MSM. AIDS. 2015 Sep;29(14):1855–62. doi: https://doi.org/10.1097/QAD.0000000000000767
21. DiNenno EA, Prejean J, Delaney KP, Bowles K, Martin T, Tailor A, et al. Evaluating
the Evidence for More Frequent Than Annual HIV Screening of Gay, Bisexual, and Other
Men Who Have Sex With Men in the United States: Results From a Systematic Review and
CDC Expert Consultation. Public Health Rep. 2018;133(1):3–21. doi: https://doi.org/10.1177/0033354917738769
22. Cramp ME, Rosenberg WM, Ryder SD, Blach S, Parkes J. Modelling the impact of improving
screening and treatment of chronic hepatitis C virus infection on future hepatocellular
carcinoma rates and liver-related mortality. BMC Gastroenterol. 2014 Aug;14(1):137.
doi: https://doi.org/10.1186/1471-230X-14-137
23. Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes
of general population screening for hepatitis C. Clin Infect Dis. 2012 May;54(9):1259–71.
doi: https://doi.org/10.1093/cid/cis011
24. Durham DP, Skrip LA, Bruce RD, Vilarinho S, Elbasha EH, Galvani AP, et al. The impact
of enhanced screening and treatment on hepatitis C in the United States. Clin Infect
Dis. 2016 Feb;62(3):298–304. doi: https://doi.org/10.1093/cid/civ894
25. Houghton M. Discovery of the hepatitis C virus. Liver Int. 2009 Jan;29(s1 Suppl 1):82–8.
doi: https://doi.org/10.1111/j.1478-3231.2008.01925.x
26. Kusejko K, Salazar-Vizcaya L, Shah C, Stöckle M, Béguelin C, Schmid P, et al.; Swiss
HIV Cohort Study. Sustained Effect on Hepatitis C Elimination Among Men Who Have Sex
With Men in the Swiss HIV Cohort Study: A Systematic Re-Screening for Hepatitis C
RNA Two Years Following a Nation-Wide Elimination Program. Clin Infect Dis. 2022 Nov;75(10):1723–31.
doi: https://doi.org/10.1093/cid/ciac273
27. Tuite AR, Fisman DN, Mishra S. Screen more or screen more often? Using mathematical
models to inform syphilis control strategies. BMC Public Health. 2013 Jun;13(1):606.
doi: https://doi.org/10.1186/1471-2458-13-606
28. Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness
of screening men who have sex with men for rectal chlamydial and gonococcal infection
to prevent HIV Infection. Sex Transm Dis. 2013 May;40(5):366–71. doi: https://doi.org/10.1097/OLQ.0b013e318284e544
29. Tsoumanis A, Hens N, Kenyon CR. Is Screening for Chlamydia and Gonorrhea in Men Who
Have Sex With Men Associated With Reduction of the Prevalence of these Infections?
A Systematic Review of Observational Studies. Sex Transm Dis. 2018 Sep;45(9):615–22.
doi: https://doi.org/10.1097/OLQ.0000000000000824
30. Vanbaelen T, Rotsaert A, Van Landeghem E, Nöstlinger C, Vuylsteke B, Platteau T, et
al. Do pre-exposure prophylaxis (PrEP) users engaging in chemsex experience their
participation as problematic and how can they best be supported? Findings from an
online survey in Belgium. Sex Health. 2023 Oct;20(5):424–30. doi: https://doi.org/10.1071/SH23037
31. Hocking JS, Temple-Smith M, Guy R, Donovan B, Braat S, Law M, et al.; ACCEPt Consortium.
Population effectiveness of opportunistic chlamydia testing in primary care in Australia:
a cluster-randomised controlled trial. Lancet. 2018 Oct;392(10156):1413–22. doi: https://doi.org/10.1016/S0140-6736(18)31816-6
32. Aids-Hilfe Schweiz. Data on HIV and AIDS [Internet]. 2025 Jul 24 [cited 2025 Feb 14].
Available from: https://aids.ch/en/knowledge/topics/data/
33. Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle and syringe
programmes and opioid substitution therapy for preventing HCV transmission among people
who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction. 2018 Mar;113(3):545–63.
doi: https://doi.org/10.1111/add.14012
34. Williams E, Williamson DA, Hocking JS. Frequent screening for asymptomatic chlamydia
and gonorrhoea infections in men who have sex with men: time to re-evaluate? Lancet
Infect Dis. 2023 Dec;23(12):e558–66. 10.1016/S1473-3099(23)00356-0
35. Bihl F, Bruggmann P, Castro Batänjer E, Dufour JF, Lavanchy D, Müllhaupt B, et al. HCV
disease burden and population segments in Switzerland. Liver Int. 2021;41(12):2803–14.
10.1111/liv.15111
36. Bruggmann P, Blach S, Deltenre P, Fehr J, Kouyos R, Lavanchy D, et al. Hepatitis C
virus dynamics among intravenous drug users suggest that an annual treatment uptake
above 10% would eliminate the disease by 2030. Swiss Med Wkly. 2017 Nov;147(4546):w14543.
10.4414/smw.2017.14543
37. Schmidt AJ, Altpeter E. The Denominator problem: estimating the size of local populations
of men-who-have-sex-with-men and rates of HIV and other STIs in Switzerland. Sex Transm
Infect. 2019 Jun;95(4):285–91. 10.1136/sextrans-2017-053363
38. UNAIDS. Switzerland [Internet]. Geneva: UNAIDS; 2021 [cited 2024 Mar 5]. Available
from: https://www.unaids.org/en/regionscountries/countries/switzerland
39. EMIS Project. The European Men-Who-Have-Sex-With-Men Internet Survey [Internet]. 2024 Jul 20
[cited 2025 Feb 14]. Available from: https://www.emis-project.eu
40. Schmidt AJ, Rasi M, Esson C, Christinet V, Ritzler M, Lung T, et al. The Swiss STAR
trial - an evaluation of target groups for sexually transmitted infection screening
in the sub-sample of men. Swiss Med Wkly. 2020 Dec;150(5153):w20392. doi: https://doi.org/10.4414/smw.2020.20392
41. Bigler D, Surial B, Hauser CV, Konrad T, Furrer H, Rauch A, et al. Prevalence of STIs
and people’s satisfaction in a general population STI testing site in Bern, Switzerland.
Sex Transm Infect. 2023 Jun;99(4):268–71. 10.1136/sextrans-2022-055596
42. United Nations Office on Drugs and Crime (UNODC). Ending an epidemic: HCV treatment
for people who inject drugs (PWID) [Internet]. 2018 [cited 2025 Feb 14]. Available
from: https://www.unodc.org/documents/commissions/CND/2019/Contributions/Civil_Society/2018_IAS_Brief_Ending_an_epidemic_HCV_treatment_for_PWID.pdf
43. Nevin RL, Shuping EE, Frick KD, Gaydos JC, Gaydos CA. Cost and effectiveness of Chlamydia
screening among male military recruits: markov modeling of complications averted through
notification of prior female partners. Sex Transm Dis. 2008 Aug;35(8):705–13. 10.1097/OLQ.0b013e31816d1f55
44. Park IU, Tran A, Pereira L, Fakile Y. Sensitivity and specificity of treponemal-specific
tests for the diagnosis of syphilis. Clin Infect Dis. 2020 Jun;71 Suppl 1:S13–20.
10.1093/cid/ciaa349
54. Vetter BN, Ongarello S, Tyshkovskiy A, Alkhazashvili M, Chitadze N, Choun K, et al. Sensitivity
and specificity of rapid hepatitis C antibody assays in freshly collected whole blood,
plasma and serum samples: A multicentre prospective study. PLoS One. 2020 Dec;15(12):e0243040.
10.1371/journal.pone.0243040
46. Kohler P, Schmidt AJ, Cavassini M, Furrer H, Calmy A, Battegay M, et al.; Swiss HIV
Cohort Study. The HIV care cascade in Switzerland: reaching the UNAIDS/WHO targets
for patients diagnosed with HIV. AIDS. 2015 Nov;29(18):2509–15. 10.1097/QAD.0000000000000878
47. Wandeler G, Dufour JF, Bruggmann P, Rauch A. Hepatitis C: a changing epidemic. Swiss
Med Wkly. 2015 Feb;145(506):w14093. 10.4414/smw.2015.14093
48. Garnett GP, Aral SO, Hoyle DV, Cates W Jr, Anderson RM. The natural history of syphilis.
Implications for the transmission dynamics and control of infection. Sex Transm Dis.
1997 Apr;24(4):185–200. 10.1097/00007435-199704000-00002
49. Garnett GP, Mertz KJ, Finelli L, Levine WC, St Louis ME. The transmission dynamics
of gonorrhoea: modelling the reported behaviour of infected patients from Newark,
New Jersey. Philos Trans R Soc Lond B Biol Sci. 1999 Apr;354(1384):787–97. 10.1098/rstb.1999.0432 doi: https://doi.org/10.1098/rstb.1999.0431
50. Paltiel AD, Weinstein MC, Kimmel AD, Seage GR 3rd, Losina E, Zhang H, et al. Expanded
screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J
Med. 2005 Feb;352(6):586–95. 10.1056/NEJMsa042088
51. Potterat JJ, Zimmerman-Rogers H, Muth SQ, Rothenberg RB, Green DL, Taylor JE, et al. Chlamydia
transmission: concurrency, reproduction number, and the epidemic trajectory. Am J
Epidemiol. 1999 Dec;150(12):1331–9. 10.1093/oxfordjournals.aje.a009965
52. Cui M, Qi H, Zhang T, Wang S, Zhang X, Cao X, et al. Symptomatic HIV infection and
in-hospital outcomes for patients with acute myocardial infarction undergoing percutaneous
coronary intervention from national inpatient sample. Sci Rep. 2024 Apr;14(1):9832.
10.1038/s41598-024-59920-9
53. European Centre for Disease Prevention and Control. Chlamydia infection [Internet].
2024 Aug 5 [cited 2025 Feb 14]. Available from: https://www.ecdc.europa.eu/en/chlamydia-infection
54. Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008 Jul;372(9635):321–32.
10.1016/S0140-6736(08)61116-2
55. Martín-Sánchez M, Fairley CK, Ong JJ, Maddaford K, Chen MY, Williamson DA, et al. Clinical
presentation of asymptomatic and symptomatic women who tested positive for genital
gonorrhoea at a sexual health service in Melbourne, Australia. Epidemiol Infect. 2020 Sep;148:e240.
10.1017/S0950268820002265
56. Grebely J, Matthews S, Causer LM, Feld JJ, Cunningham P, Dore GJ, et al. We have reached
single-visit testing, diagnosis, and treatment for hepatitis C infection, now what? Expert
Rev Mol Diagn. 2024 Mar;24(3):177–91. 10.1080/14737159.2023.2292645
Appendix
The appendix is available in the PDF version of the manuscript at https://doi.org/10.57187/s.4581.



