Treatment patterns and clinical outcomes in stage III non-small-cell lung cancer:
a long-term institutional experience in Switzerland
DOI: https://doi.org/https://doi.org/10.57187/s.4522
Altay Turunça,
David Königb,
Judith
Haferb,
Spasenija
Savic Princec,
Kathleen
Jahnd,
Jens
Bremeriche,
Didier Lardinoisf,
Sacha I. Rothschildbg,
Tobias
Finazziah
a Clinic of Radiotherapy and Radiation
Oncology, University Hospital Basel, Basel, Switzerland
b Department of Medical Oncology, University
Hospital Basel, Basel, Switzerland
c Institute of Medical Genetics and
Pathology, University Hospital Basel, Basel, Switzerland
d Department of Pulmonary Medicine,
University Hospital Basel, Basel, Switzerland
e Department of Radiology, University
Hospital Basel, Basel, Switzerland
f Department of Thoracic Surgery,
University Hospital Basel, Basel, Switzerland
g Department of Oncology / Haematology,
Cantonal Hospital Baden, Baden, Switzerland
h Department of Radiation Oncology,
Cantonal Hospital Baden, Baden, Switzerland
Summary
STUDY AIM: Treatment of stage III non-small-cell
lung cancer (NSCLC) has evolved rapidly in recent years. To improve our
understanding of real-world outcomes in Switzerland, we report on our
institutional experience at an academic lung cancer centre and describe treatment
patterns and clinical outcomes over a multi-year period.
METHODS: Patients diagnosed with stage III
NSCLC between 2013 and 2023 were included in an ethics-approved institutional
database. Based on tumour board decisions, the initial treatment strategy was
defined for each patient. Overall and progression-free survival were calculated
using the Kaplan-Meier method. A multivariate Cox regression analysis was
performed to study the impact of different factors on clinical outcomes.
RESULTS: A total of 315 patients with stage
III NSCLC were included. Patients were a median of 68 years old, and two-thirds
were male. The most common stage at diagnosis was IIIA (56%), followed by stage
IIIB (36%) and IIIC (8%). A curative treatment approach was pursued in 88% of
patients, and over 90% of these received definitive local treatment (surgery
and/or radiotherapy). Rates of 1-year overall and progression-free survival
improved from 64% and 47%, respectively, in 2013–2016, to 82% and 70% in 2020–2023.
However, 49% of patients developed locoregional and/or distant recurrence.
Results of the multivariate analysis are presented in the manuscript.
CONCLUSIONS: Almost 90% of patients with
stage III NSCLC underwent treatment with curative intent, with rates of
treatment adherence that compared favourably to the literature. Although
survival outcomes appear to have improved in recent years, the rates of disease
recurrence remain high, reflecting a need for further improvements.
Introduction
Lung cancer is the most commonly diagnosed
cancer worldwide, accounting for around 2.5 million new cases (or 12.4% of all
cancers) in 2022. Lung cancer is also the leading cause of cancer mortality,
responsible for around 1.8 million deaths (or 18.7% of all cancer deaths)
annually [1]. Population-level analyses suggest that lung cancer mortality has
decreased in some countries, which may be attributed to substantial advances in
lung cancer treatment in recent years [2]. However, the global burden of
disease remains high, and clinicians and patients alike are faced with a
treatment landscape of increasing complexity.
Non-small-cell lung cancer (NSCLC) accounts
for approximately 85% of all lung cancers [3]. Around 30% of patients with
NSCLC are diagnosed with stage III disease, which comprises a heterogeneous
group of patients, including tumours with advanced local infiltration, or
mediastinal lymph node metastases, among other criteria [4, 5]. The treatment
of stage III NSCLC has evolved rapidly in recent years. In particular, the
introduction of immune checkpoint inhibitors (ICI) has changed the standard of
care for both resectable and unresectable patients [6–9]. Despite these
advances, the management of patients with locally advanced NSCLC remains
challenging, as most patients will eventually develop a recurrence. In
addition, patients are often elderly and comorbid, and large variations exist
in real-world treatment patterns [5, 10, 11]. Treatment decision-making may
thus differ between institutions based on local expertise, as well as expert
opinion, in this rapidly evolving field.
We analysed treatment patterns and clinical
outcomes in patients with stage III NSCLC who underwent treatment at our
institution over a multi-year period. Considering the evolution of treatment
approaches over this time, we aimed to understand how this has influenced the
treatment of stage III NSCLC in a real-world setting. Furthermore, we studied survival
and recurrence patterns to improve our understanding of treatment outcomes in the
contemporary setting.
Materials and methods
We conducted a retrospective analysis of
patients who underwent treatment for stage III NSCLC at the University Hospital
of Basel in Basel, Switzerland. The University Hospital of Basel is a tertiary
academic centre, and the largest provider of thoracic oncology services in
Northwestern Switzerland. The project was approved by the Ethics Committee of
Northwestern and Central Switzerland (BASEC ID 2023-01712). No external funding
was received for the planning or conduct of this study.
All patients newly diagnosed with stage III
NSCLC between 1 January 2013 and 31 December 2023 were considered eligible for
the analysis. Patients with a documented refusal of consent for data analysis
for research purposes were excluded. Patients were identified through a
multi-stage process, based on the thoracic tumour board reports. Electronic tumour
board reports were searched for terms associated with stage III NSCLC
(including “stage III”, “locally advanced” and “locoregionally advanced”). The
resulting list as well as all individual tumour board reports (including
non-searchable documents generated before 2017) were manually cross-checked for
validity and completion.
All patient data was anonymised using an
external data catalogue and stored on a study-specific Castor EDC platform
(Castor, USA). Patient and tumour characteristics, as well as follow-up data,
were manually collected from electronic medical records. Patients were
generally staged using 18F-fluorodeoxyglucose (FDG) positron emission
tomography (PET) and magnetic resonance imaging (MRI) of the brain. Mediastinal
staging was performed using bronchoscopy ± endobronchial
ultrasound (EBUS) and/or mediastinoscopy in accordance with clinical practice
guidelines [12]. NSCLC stage was defined based on the 8th edition of
TNM staging for lung cancer [13]. Cases that had been staged using the 7th
edition (prior to 2017) were manually verified and redefined according to the 8th
edition, if necessary. Pathological stage was considered in patients undergoing
primary surgery, but not after any neoadjuvant treatment due to possible
downstaging. Based on tumour board recommendations and/or individual consultations,
the treatment strategy was documented for each patient: primary surgery with
adjuvant therapy, neoadjuvant treatment before surgery, definitive
chemoradiotherapy, palliative therapy (radiotherapy, systemic therapy or both) or
best supportive care.
Overall survival was calculated from the
time of diagnosis (based on histology/cytology) to death using the Kaplan-Meier
method. Progression-free survival was defined as the time from diagnosis until
disease progression or death. Sites of first recurrence were manually confirmed
and categorised as follows: locoregional (ipsilateral lung and/or regional lymph
node), distant lung, brain metastases, other extracranial metastases or new
primary lung tumours. A multivariate Cox regression analysis was performed to
calculate the hazard ratio (HR) of progression-free survival in patients who
were treated with curative intent and followed for at least two years. Statistical
analyses were performed using RStudio version 2024.04.1+748 (RStudio, USA).
Results
Patient characteristics
A total of 315 patients were included in
the analysis. Baseline patient characteristics are summarised in table 1. Patients
were a median of 68 years old (range: 35–92) at time of diagnosis. The
proportion of male and female patients was 66% and 34%, respectively, and most
patients (92%) had a smoking history. Patients were most commonly diagnosed
with adenocarcinoma (50%), followed by squamous cell carcinoma (43%), NSCLC-Not
Otherwise Specified (4%) and large cell neuroendocrine carcinoma (LCNEC; 3%). The
most common stage at diagnosis was IIIA (56%), followed by stage IIIB (36%) and
IIIC (8%). Predictive biomarker testing was performed in 82% of patients with
non-squamous histology (n = 180) and 19% of patients with squamous histology (n
= 135). Among patients with non-squamous histology who underwent testing (n =
148), targetable driver mutations in the epidermal growth factor receptor (EGFR)
or anaplastic lymphoma kinase (ALK) genes were found in 8 (5%) and 4
(3%) patients, respectively. Other tested patients had either no actionable
driver alterations, or other mutations that were not actionable at that time.
This included all patients with squamous histology, although these were only
selectively tested (e.g. young age, non-smokers).
Table 1Characteristics of patients
diagnosed with stage III non-small-cell lung cancer (NSCLC) between 2013 and
2023.
|
Patients (n = 315) |
| Age in years, median (range) |
68 (35–92) |
| Sex, n (%) |
Male |
209 (66) |
| Female |
106 (34) |
| Smoking history, n (%) |
Never |
23 (7) |
| Former |
111 (35) |
| Current |
181 (57) |
| Histology, n (%) |
Adenocarcinoma |
157 (50) |
| Squamous cell carcinoma |
135 (43) |
| Large cell neuroendocrine carcinoma |
11 (3) |
| NSCLC-Not Otherwise Specified |
12 (4) |
| Driver mutation, n (%) |
EGFR (Epidermal Growth Factor Receptor) |
8 (3) |
| ALK (Anaplastic Lymphoma Kinase) |
4 (1) |
| Other / None |
157 (50) |
| Unknown |
146 (46) |
| UICC stage (TNM 8th edition), n (%) |
IIIA |
177 (56) |
| IIIB |
112 (36) |
| IIIC |
26 (8) |
| Treatment strategy, n (%) |
Curative |
Primary surgery with adjuvant therapy |
115 (37) |
| Neoadjuvant treatment before surgery |
109 (35) |
| Definitive chemoradiotherapy |
53 (17) |
| Palliative |
Palliative systemic therapy |
13 (4) |
| Palliative radiotherapy |
8 (3) |
| Palliative systemic and radiotherapy |
3 (1) |
| Best supportive care |
14 (4) |
Treatment patterns
Baseline treatment characteristics are
summarised in table 1. Primary surgery followed by adjuvant therapy (37% of all
patients) and neoadjuvant treatment before surgery (35%) were the most frequent
treatment approaches used, followed by definitive chemoradiotherapy (17%) and
palliative treatments (12%; see table 1 for details). Twenty-three patients
(7%) were treated within clinical trials. The distribution of treatment
strategies over time is visualised in appendix figure S1.
A summary of patients who underwent
treatment with curative intent (n = 277) is shown in figure 1, with details on
systemic therapies provided in appendix figure S2. The median time from
diagnosis to treatment was 26 days. In the primary surgery group (n = 115), 96
patients (83%) had stage IIIA disease and 114 (99%) did undergo definitive surgery,
with one patient dying prior to the planned procedure. Seventy-one patients
(62%) subsequently received adjuvant chemotherapy, most commonly with cisplatin
/ vinorelbine, and 20 patients (18%) underwent postoperative radiotherapy
(PORT). In 42 patients who did
not receive adjuvant chemotherapy, reasons were patient preference (n = 17),
poor general condition (n = 17), death (n = 5), delay >6 months due to
protracted course with repeated surgeries (n = 1), atypical carcinoid histology
(n = 1) and preference for adjuvant tyrosine kinase inhibitor (TKI) alone (n = 1;
patient with epidermal
growth factor receptor mutation).
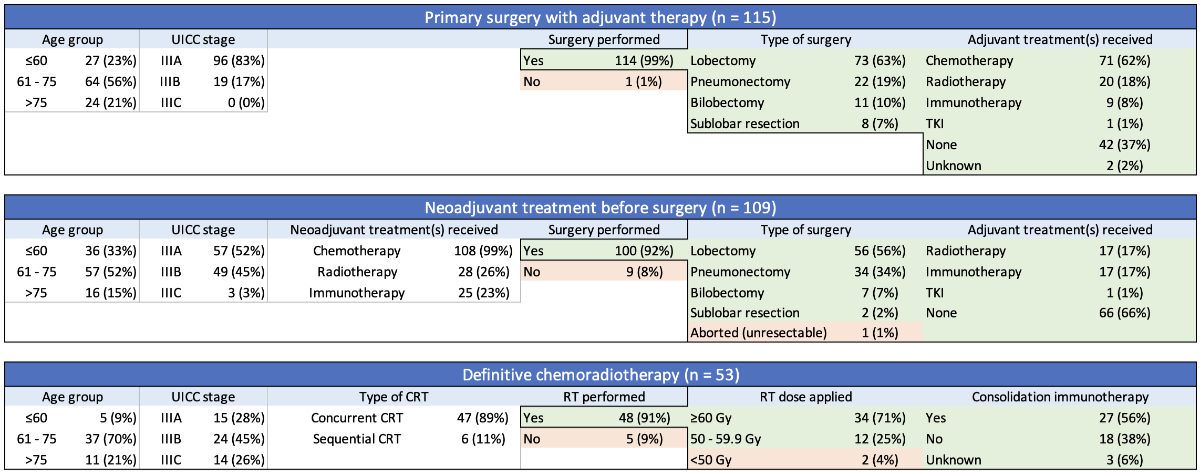
Figure 1 Characteristics of curative
treatment in stage III non-small-cell lung cancer (NSCLC). An overview of
patients with stage III NSCLC who underwent treatment with curative intent (n =
277). Details on systemic therapies are provided in appendix figure S2. CRT:
chemoradiotherapy; RT: radiation therapy; TKI: tyrosine kinase inhibitor; UICC:
Union for International Cancer Control.
In the neoadjuvant group (n = 109), 57
patients (52%) had stage IIIA disease. The most common regimen for induction
chemotherapy was cisplatin/docetaxel, and additional neoadjuvant immune
checkpoint inhibitors and/or radiotherapy were administered in 23% and 26% of
patients, respectively. Following induction, 100 patients (92%) underwent definitive
surgery, with 9 patients not undergoing surgery due to unresectable disease (n
= 3), medical inoperability (n = 3), progressive disease (n = 1), patient
refusal (n = 1) and death (n = 1). One additional patient was found to be
unresectable during surgery. Following resection, 17% of patients received
adjuvant immune checkpoint inhibitors and/or postoperative radiotherapy
(details see figure 2 and appendix figure S2).
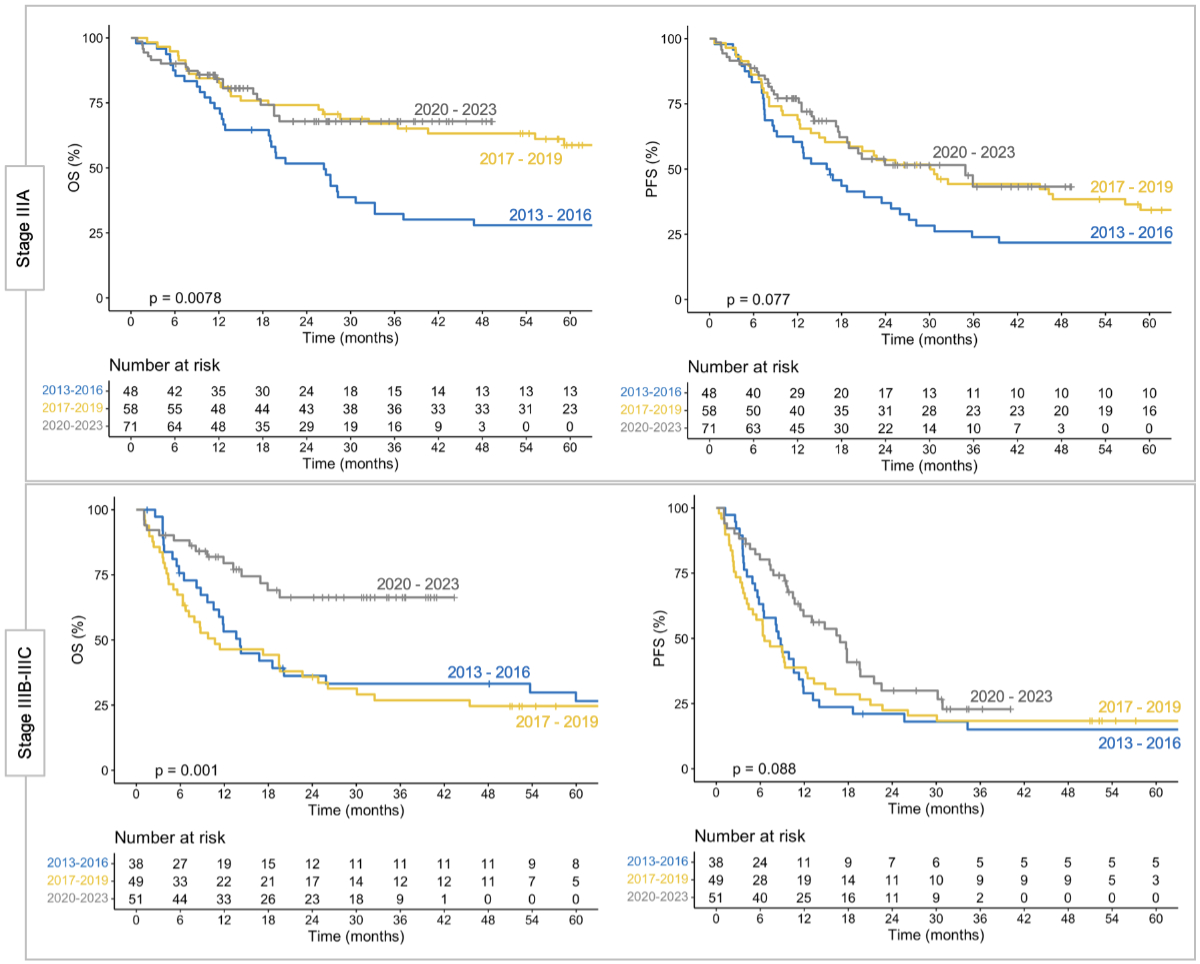
Figure 2 Clinical outcomes in stage III non-small-cell
lung cancer (NSCLC) during different time periods. Overall survival (OS) and
progression-free survival (PFS) in stage IIIA (upper panels) and stages IIIB–IIIC
(lower panels) for patients treated during different time periods. Outcomes
appear to have improved in recent years, although confounding factors cannot be
excluded.
In the chemoradiotherapy group (n = 53), 15
patients (28%) had stage IIIA disease. Concurrent and sequential chemoradiotherapy
was administered in 89% and 11% of patients, respectively. Patients received a
median of 4 cycles (range: 1–7) of chemotherapy, and radiotherapy was delivered
with a median of 60 Gy (range: 0–69). Cisplatin-based chemotherapy regimens
were administered in 19% of patients. The most frequently used chemotherapy
regimen was carboplatin/paclitaxel (51%), followed by carboplatin/pemetrexed
(21%) and cisplatin/etoposide (15%). Five patients (9%) did not receive
radiotherapy, and two additional patients received less than 50 Gy. Following chemoradiotherapy,
27 patients (56%) received consolidation immune checkpoint inhibitors. This
rate was 76% after approval of durvalumab in 2018.
Clinical outcomes
The Kaplan-Meier estimates of overall and progression-free
survival for the full cohort are visualised in appendix figure S3. Median
follow-up was 1.7 years (range: 21 days to 10.6 years). For all patients,
median overall survival was 33.3 months (95% confidence interval [CI]: 26.3–63.7),
and median progression-free survival was 16.2 months (95% CI: 12.8–19.1). Median
overall survival in stage IIIA, IIIB and IIIC was 59.2 months (95% CI: 33.3–NR [upper
limit of
the 95% confidence interval was not reached]),
19.6 months (95% CI: 11.9–53.8) and 17.9 months (95% CI: 13.6–NR), respectively.
Median progression-free survival was 22.8 months (95% CI: 18.0–32.5), 10.6
months (95% CI: 8.8–14.0) and 9.0 months (95% CI: 6.6–21.0) for the respective
stages.
For patients diagnosed between 2013 and 2016
(n = 86), the 1-year rates of overall and progression-free survival were 64% (95%
CI: 55–76%) and 47% (95% CI: 37–58%), respectively. These rates were 66% (95%
CI: 58–76%) and 55% (95% CI: 46–65%) in patients diagnosed between 2017 and 2019
(n = 107), and 82% (95% CI: 76–89%) and 70% (95% CI: 62–78%) in patients
diagnosed between 2020 and 2023 (n = 122; p <0.01 for overall survival, p <0.05
for progression-free survival). The Kaplan-Meier estimates of overall and progression-free
survival for these different time periods (grouped by disease stages IIIA and
IIIB–IIIC) are shown in figure 2.
Patient outcomes separated by treatment
strategy are shown in figure 3. In stage IIIA, progression-free survival
at 1 and 2 years was 71.6% (95% CI: 63.0–81.2%) and 46.5% (95% CI: 37.2–58.1%)
with primary surgery, 77.2% (95% CI: 67.0–88.9%) and 60.0% (95% CI: 47.9–75.2%)
with neoadjuvant treatment, and 66.7% (95% CI: 46.6–95.3%) and 50.0% (95% CI: 29.2–85.5%)
with definitive chemoradiotherapy. In stages IIIB–IIIC, progression-free
survival at 1 and 2 years was 63.2% (95% CI: 44.8–89.0%) and 42.1% (95% CI:
24.9–71.3%) with primary surgery, 51.2% (95% CI: 39.1–66.9%) and 28.4% (95% CI:
18.2–44.3%) with neoadjuvant treatment, and 41.6% (95% CI: 28.4–60.8%) and
25.9% (95% CI: 14.6–45.7%) with definitive chemoradiotherapy. Patients of any
stage treated with palliative intent had a poorer prognosis, with a median overall
survival of 9.1 months (95% CI: 4.1–19.6) in patients receiving palliative
systemic and/or radiotherapy, and 2.4 months (95% CI: 1.6–13.2) with best
supportive care.
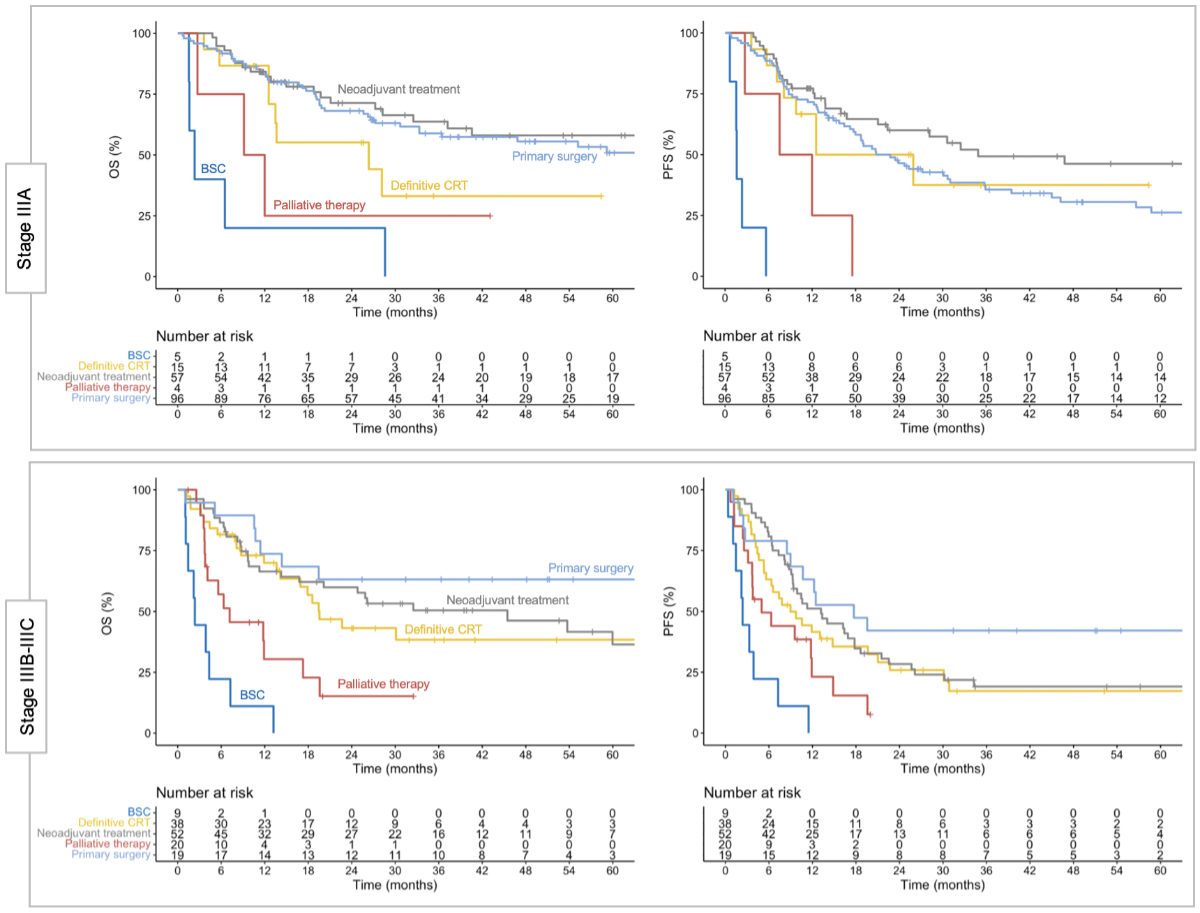
Figure 3 Clinical outcomes in stage III non-small-cell
lung cancer (NSCLC) by treatment strategy. Clinical outcomes separated by
initial treatment strategy for stage IIIA (upper panels) and stages IIIB–IIIC
(lower panels). Stage IIIA was most frequently treated with primary surgery,
whereas neoadjuvant treatment and definitive chemoradiotherapy (CRT) were more
common in stages IIIB–IIIC. Of note, stage IIIC was predominantly treated with
definitive chemoradiotherapy, and no patient in that group underwent primary
surgery (see figure 1). BSC: best supportive care; OS: overall survival; PFS:
progression-free survival.
Disease recurrence was observed in 152
patients (49%). In these patients, the first site(s) of recurrence were
locoregional (n = 57; 38%), distant (n = 47; 31%), both locoregional and
distant (n = 41; 27%), or new primary lung cancers (n = 7; 5%; with 4 additional
new primaries diagnosed in conjunction with locoregional and/or distant
recurrence). The rate of locoregional failure at first recurrence (with or
without other metastases) was 32% with primary surgery, 28% with neoadjuvant
treatment and 34% with definitive chemoradiotherapy (p = 0.78; χ2). Of all 315 patients, 30 (10%) were diagnosed with brain
metastases at any time point (27 at first recurrence).
A total of 250 patients were treated with
curative intent and followed for at least 2 years. Results of the multivariate
Cox regression analysis of progression-free survival performed in this subgroup
are shown in figure 4. Age group
>75 years (HR: 1.64; p = 0.046) and Union for International Cancer Control (UICC)
stage IIIB (HR: 1.71; p = 0.002) were factors associated with an increased risk
of progression and/or death following treatment. Other factors did not reach
statistical significance in the model.
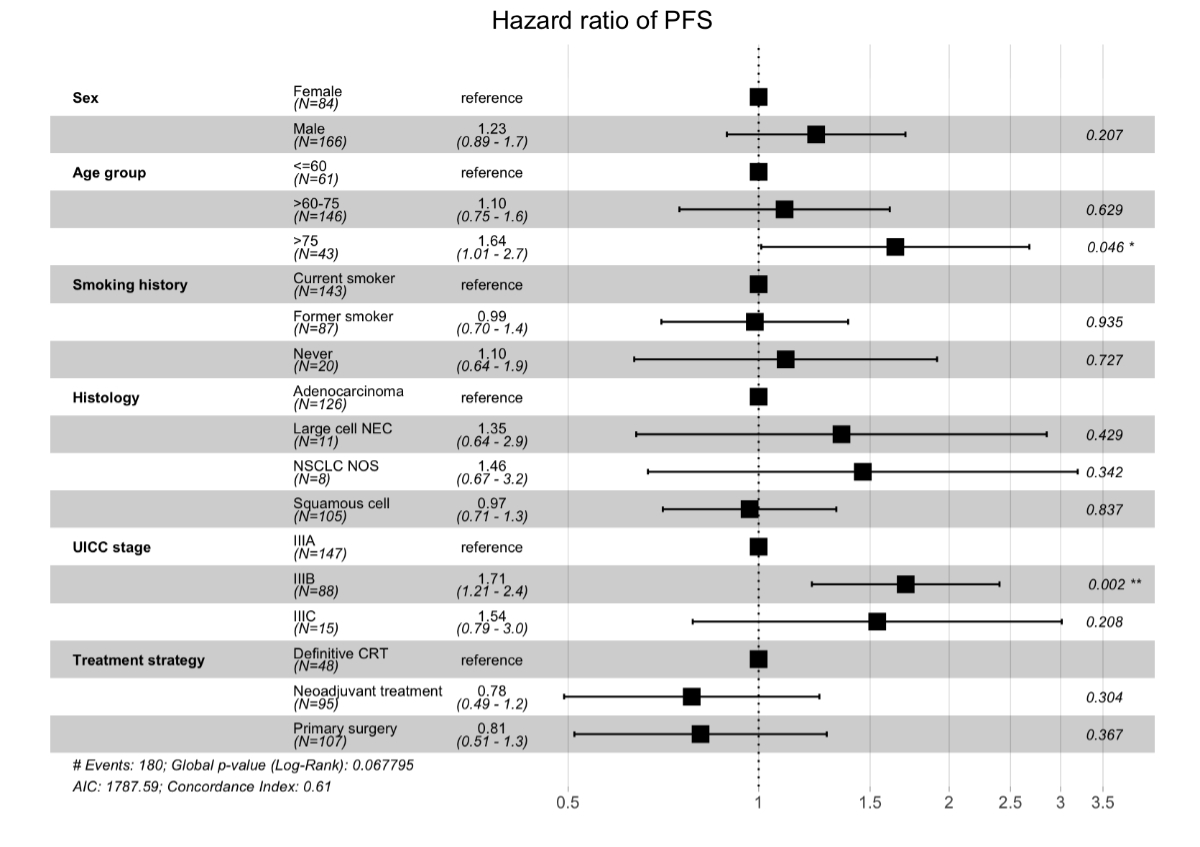
Figure 4 Multivariate analysis of
progression-free survival (PFS) in stage III non-small-cell lung cancer
(NSCLC). Multivariate Cox regression analysis of progression-free survival in
patients treated with curative intent with a minimum follow-up of 2 years (n =
250). Age group >75 years and Union for International Cancer Control (UICC)
stage IIIB were associated with a higher risk of progression and/or death.
Other factors did not reach statistical significance in the model, although
visual trends can be observed. CRT: chemoradiotherapy; NEC: neuroendocrine
carcinoma; NOS: not otherwise specified.
Discussion
Treatment of stage III non-small-cell lung
cancer (NSCLC) has evolved rapidly in recent years. Despite the availability of
local and international guidelines, a large heterogeneity in clinical practice
persists, reflecting the complexity of multidisciplinary management in these
patients [11, 14, 15]. Furthermore, differences in local expertise and expert
opinion will affect treatment patterns in the real-world setting. For these
reasons, we decided to analyse our institutional experience, both as a method
of quality assurance in this challenging setting, and to enhance our
understanding of treatment patterns and clinical outcomes in the contemporary era.
In our cohort, a curative treatment
approach was pursued in 88% of patients. Although not all patients were able to
receive local and/or systemic therapy as initially planned, treatment adherence
appeared overall high (figure 1). For example, 91% of patients underwent resection
after neoadjuvant treatment. For reference, the rate of definitive surgery was
83% and 75%, respectively, after chemo-immunotherapy and chemotherapy in the landmark
CheckMate 816 trial, which also included earlier stages of disease (IB–IIIA) [7].
A relatively high proportion of our patients underwent primary surgery, which
included patients without N2 disease, as well as those with unexpected pN2
disease. In definitive chemoradiotherapy, 89% of patients received concurrent
treatment, which is preferred over sequential chemoradiotherapy [16]. The rate
of patients receiving a radiotherapy dose of ≥60 Gy (71%) was lower than in
prospective clinical trials [17, 18]. However, this is explained by the less favourable
patient population, which included more elderly patients, and a higher rate of
stages IIIB–IIIC (72%), where lower doses were sometimes deemed appropriate. Furthermore,
the rate of patients receiving only palliative treatment or best supportive
care was 8% and 4%, respectively. This rate was 45% in stage IIIA and 60% in
stage IIIB in an analysis of patients treated in the United Kingdom in 2017 [19].
The reason for this discrepancy is likely multi-factorial, including variabilities
in clinical practice, which are known to be common in NSCLC [20].
Due to inherent differences, it is
difficult to compare treatment outcomes of different clinical trials, as well
as real-world cohorts. Similarly, retrospective analyses such as ours are
unable to account for all factors that will affect clinical outcomes, including
all-cause mortality. This is particularly relevant when comparing surgical approaches
and definitive chemoradiotherapy, for which randomised trials have shown
similar survival outcomes [21–23]. However, these trials were conducted prior
to significant advances in the field, such as the introduction of immune
checkpoint inhibitors in both surgical and non-surgical settings [6–9, 24]. In
Switzerland, treatment of resectable stage III NSCLC has been notably shaped by
clinical trials conducted by the Swiss Group for Clinical Cancer Research
(SAKK) [25]. A pooled analysis of four consecutive SAKK trials, which studied
different induction regimens in a pre-immunotherapy era, revealed a resection
rate of 79% and a median progression-free survival of 12.3 months in 437
patients treated between 1997 and 2016 [26]. Our data compare favourably to
these results, with a median progression-free survival of 16.2 months, despite
the inclusion of unresectable patients, and some patients receiving palliative
care. However, median progression-free survival was not reached in the more
recent SAKK 16/14 trial (induction immune checkpoint inhibition), which 7
of our patients participated in [24]. Furthermore, follow-up is generally less
rigid in a real-world setting, and underreporting of recurrences is possible in
some cases.
Our study has several limitations,
including the inherent limitations of any retrospective data analysis, which
can be affected by selection bias, unknown confounders and missing data. We
cannot exclude that some patients were not presented at the multidisciplinary tumour
board. Despite our best efforts, follow-up data was lacking in some patients,
and we did not contact external hospitals or caregivers, both due to
feasibility and ethical considerations. Our study period also covers a time of
significant evolution, and treatment patterns were heterogeneous particularly
with regards to systemic therapy. Since e.g. testing for predictive markers was
not routinely performed in all patients, we did not conduct additional subgroup
analyses at this stage. In the current era, molecular testing is more broadly recommended
in stage III NSCLC, as the presence of driver alterations will impact treatment
decisions (e.g. regarding consolidation immune checkpoint inhibitors or
adjuvant tyrosine kinase inhibitors) [27–29]. Finally, we recognise that our
report does not include information on treatment-related toxicities or
health-related quality of life. Patient-reported outcome measures are an
essential component of modern lung cancer care, and this is currently the
subject of other projects at our institution [30].
In conclusion, our long-term institutional
analysis in stage III NSCLC revealed that almost 90% of patients underwent
treatment with curative intent, including resection in a majority of cases.
Rates of treatment completion and survival outcomes were overall encouraging,
considering the challenges of lung cancer care in a real-world setting.
However, our experience also reflects the need for further improvements, as
half of our patients did eventually develop a recurrence. Multidisciplinary
care will remain the backbone of lung cancer treatment, which is a field of
rapidly increasing complexity. We
encourage others to review their clinical experience, as this may have
implications for local practice, and contribute to our general understanding of
lung cancer care in the real-world setting.
Data sharing statement
De-identified study data may be obtained
from the corresponding author upon reasonable request.
Acknowledgments
We thank our patients and their families for entrusting us with
their care, as well as all caregivers and healthcare professionals involved in
these treatments.
PD Dr. med.
Tobias Finazzi
Clinic of
Radiotherapy and Radiation Oncology
University
Hospital Basel
CH-4031 Basel
tobias.finazzi[at]ksb.ch
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global
cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. doi: https://doi.org/10.3322/caac.21834
2. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The Effect
of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med. 2020 Aug;383(7):640–9.
doi: https://doi.org/10.1056/NEJMoa1916623
3. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al.; International
Association for the Study of Lung Cancer International Staging Committee; Participating
Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification
of malignant tumours. J Thorac Oncol. 2007 Aug;2(8):706–14. doi: https://doi.org/10.1097/JTO.0b013e31812f3c1a
4. Eberhardt WE, De Ruysscher D, Weder W, Le Péchoux C, De Leyn P, Hoffmann H, et al.;
Panel Members. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage
III non-small-cell lung cancer. Ann Oncol. 2015 Aug;26(8):1573–88. doi: https://doi.org/10.1093/annonc/mdv187
5. Finazzi T, Schneiders FL, Senan S. Developments in radiation techniques for thoracic
malignancies. Eur Respir Rev. 2021 May;30(160):200224. doi: https://doi.org/10.1183/16000617.0224-2020
6. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-Year
Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage
III Non-Small-Cell Lung Cancer. J Clin Oncol. 2022 Apr;40(12):1301–11. doi: https://doi.org/10.1200/JCO.21.01308
7. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al.; CheckMate 816
Investigators. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer.
N Engl J Med. 2022 May;386(21):1973–85. doi: https://doi.org/10.1056/NEJMoa2202170
8. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al.; KEYNOTE-671 Investigators.
Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med.
2023 Aug;389(6):491–503. doi: https://doi.org/10.1056/NEJMoa2302983
9. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al.; AEGEAN
Investigators. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer.
N Engl J Med. 2023 Nov;389(18):1672–84. doi: https://doi.org/10.1056/NEJMoa2304875
10. Adizie JB, Khakwani A, Beckett P, Navani N, West D, Woolhouse I, et al. Stage III
Non-small Cell Lung Cancer Management in England. Clin Oncol (R Coll Radiol). 2019 Oct;31(10):688–96.
doi: https://doi.org/10.1016/j.clon.2019.07.020
11. Evison M, Edwards J, McDonald F, Popat S. Stage III Non-small Cell Lung Cancer: A
UK National Survey of Practice. Clin Oncol (R Coll Radiol). 2020 Aug;32(8):527–36.
doi: https://doi.org/10.1016/j.clon.2020.03.001
12. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al.; ESMO
Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC):
ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
2017 Jul;28(January suppl_4):iv1–21. doi: https://doi.org/10.1093/annonc/mdx222
13. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al.;
International Association for the Study of Lung Cancer Staging and Prognostic Factors
Committee, Advisory Boards, and Participating Institutions; International Association
for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards
and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for
Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM
Classification for Lung Cancer. J Thorac Oncol. 2016 Jan;11(1):39–51. doi: https://doi.org/10.1016/j.jtho.2015.09.009
14. Provencio M, Carcereny E, López Castro R, Calvo V, Rodríguez Abreu D, Cobo M, et al. Real-world
treatment patterns and survival outcomes for patients with stage III non-small cell
lung cancer in Spain: a nationwide cohort study. Transl Lung Cancer Res. 2023 Oct;12(10):2113–28.
doi: https://doi.org/10.21037/tlcr-23-176
15. Jazieh AR, Onal HC, Tan DS, Soo RA, Prabhash K, Kumar A, et al. Real-World Treatment
Patterns and Clinical Outcomes in Patients With Stage III NSCLC: Results of KINDLE,
a Multicountry Observational Study. J Thorac Oncol. 2021 Oct;16(10):1733–44. doi: https://doi.org/10.1016/j.jtho.2021.05.003
16. Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs.
concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase
III trial RTOG 9410. J Natl Cancer Inst. 2011 Oct;103(19):1452–60. doi: https://doi.org/10.1093/jnci/djr325
17. Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose
versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin
plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell
lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet
Oncol. 2015 Feb;16(2):187–99. doi: https://doi.org/10.1016/S1470-2045(14)71207-0
18. Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: randomized
Phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation
therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell
lung cancer. J Clin Oncol. 2016 Mar;34(9):953–62. doi: https://doi.org/10.1200/JCO.2015.64.8824
19. Evison M; AstraZeneca UK Limited. The current treatment landscape in the UK for stage
III NSCLC. Br J Cancer. 2020 Dec;123(S1 Suppl 1):3–9. doi: https://doi.org/10.1038/s41416-020-01069-z
20. Carrato A, Vergnenègre A, Thomas M, McBride K, Medina J, Cruciani G. Clinical management
patterns and treatment outcomes in patients with non-small cell lung cancer (NSCLC)
across Europe: EPICLIN-Lung study. Curr Med Res Opin. 2014 Mar;30(3):447–61. doi: https://doi.org/10.1185/03007995.2013.860372
21. Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, et al. Radiotherapy
plus chemotherapy with or without surgical resection for stage III non-small-cell
lung cancer: a phase III randomised controlled trial. Lancet. 2009 Aug;374(9687):379–86.
doi: https://doi.org/10.1016/S0140-6736(09)60737-6
22. van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, et al.;
European Organisation for Research and Treatment of Cancer-Lung Cancer Group. Randomized
controlled trial of resection versus radiotherapy after induction chemotherapy in
stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007 Mar;99(6):442–50.
doi: https://doi.org/10.1093/jnci/djk093
23. Eberhardt WE, Pöttgen C, Gauler TC, Friedel G, Veit S, Heinrich V, et al. Phase III
study of surgery versus definitive concurrent chemoradiotherapy boost in patients
with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after
induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol. 2015 Dec;33(35):4194–201.
doi: https://doi.org/10.1200/JCO.2015.62.6812
24. Rothschild SI, Zippelius A, Eboulet EI, Savic Prince S, Betticher D, Bettini A, et
al.; Swiss Group for Clinical Cancer Research (SAKK). SAKK 16/14: Durvalumab in Addition
to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A
Multicenter Single-Arm Phase II Trial. J Clin Oncol. 2021 Sep;39(26):2872–80. doi: https://doi.org/10.1200/JCO.21.00276
25. Werner RS, Curioni-Fontecedro A, Mauti LA, Addeo A, Peters S, Frauenfelder T, et al.;
SAKK. Lung Cancer in Switzerland. J Thorac Oncol. 2024 Mar;19(3):385–94. doi: https://doi.org/10.1016/j.jtho.2023.12.005
26. König D, Schär S, Vuong D, Guckenberger M, Furrer K, Opitz I, et al. Long-term outcomes
of operable stage III NSCLC in the pre-immunotherapy era: results from a pooled analysis
of the SAKK 16/96, SAKK 16/00, SAKK 16/01, and SAKK 16/08 trials. ESMO Open. 2022 Apr;7(2):100455.
doi: https://doi.org/10.1016/j.esmoop.2022.100455
27. Naidoo J, Antonia S, Wu YL, Cho BC, Thiyagarajah P, Mann H, et al. Brief Report: Durvalumab
After Chemoradiotherapy in Unresectable Stage III EGFR-Mutant NSCLC: A Post Hoc Subgroup
Analysis From PACIFIC. J Thorac Oncol. 2023 May;18(5):657–63. doi: https://doi.org/10.1016/j.jtho.2023.02.009
28. Lu S, Kato T, Dong X, Ahn MJ, Quang LV, Soparattanapaisarn N, et al.; LAURA Trial
Investigators. Osimertinib after Chemoradiotherapy in Stage III EGFR-Mutated NSCLC. N Engl J Med. 2024 Aug;391(7):585–97. doi: https://doi.org/10.1056/NEJMoa2402614
29. Wu YL, Dziadziuszko R, Ahn JS, Barlesi F, Nishio M, Lee DH, et al.; ALINA Investigators.
Alectinib in Resected ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2024 Apr;390(14):1265–76. doi: https://doi.org/10.1056/NEJMoa2310532
30. Bouazza YB, Chiairi I, El Kharbouchi O, De Backer L, Vanhoutte G, Janssens A, et al. Patient-reported
outcome measures (PROMs) in the management of lung cancer: A systematic review. Lung
Cancer. 2017 Nov;113:140–51. doi: https://doi.org/10.1016/j.lungcan.2017.09.011
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4522.



