The impact of the COVID-19 pandemic on cancer incidence, stage distribution and survival
in Switzerland: a register-based cohort study
DOI: https://doi.org/https://doi.org/10.57187/s.4354
Luzius Madera,
Lea Wildisenbc,
Dominik Mengesde,
Flurina Suterdf,
Gautier Defossezg,
Jean-Luc Bulliardgh,
Sabine Rohrmanndf,
Katharina Staehelinbc
a Cancer Registry Bern Solothurn, University of
Bern, Bern, Switzerland
b National Agency for Cancer Registration
(NACR), Zurich, Switzerland
c National Institute for Cancer Epidemiology
and Registration (NICER), Zurich, Switzerland
d Division of Chronic Disease Epidemiology, Epidemiology,
Biostatistics and Prevention Institute (EBPI), University of Zurich (UZH), Zurich,
Switzerland
e Department
of Medical Epidemiology and Biostatistics (MEB), Karolinska Institutet (KI),
Stockholm, Sweden
f Cancer Registry Zurich, Zug, Schaffhausen and
Schwyz, Institute of Pathology and Molecular Pathology, University Hospital
Zurich, Zurich, Switzerland
g Vaud Cancer Registry, Centre for Primary Care
and Public Health (Unisanté), University of Lausanne, Lausanne, Switzerland
h Cancer Registry Neuchâtel and Jura,
Neuchâtel, Switzerland
Summary
BACKGROUND: The COVID-19 pandemic disrupted
healthcare systems worldwide. This raised concerns about delays in cancer
diagnosis and treatment, with potentially worse patient outcomes. The aim of
this nationwide, population-based cohort study was to investigate the impact of
the COVID-19 pandemic on cancer incidence, stage distribution and one-year
survival in Switzerland.
METHODS: We used national cancer registry data
for the period 2017–2021 from the National Agency for Cancer Registration in
Switzerland, covering all except three cantons. We estimated national cancer
incidence counts and calculated age-standardised incidence rates for all
cancers and separately for female breast cancer, colorectal cancer, lung
cancer, melanoma and prostate cancer. We calculated proportional stage
distributions for cancer types and estimated observed and relative one-year
survival for all cancers and cancer types based on Swiss population life tables.
Results were analysed descriptively.
RESULTS: We included 218,736 cancer cases diagnosed
between 2017 and 2021. Annual incidence counts of all cancer cases increased in
2020 (2.1%) and 2021 (7.3%) compared to the mean of 2017–2019. When evaluating
monthly incidence counts, we observed a substantial decrease during the
COVID-19 lockdown period, which was largest in April 2020 (−19.9%
for all cancers). This decrease was most pronounced for female breast cancer (−39.9%),
followed by prostate cancer (−29.0%), colorectal cancer (−28.7%) and melanoma (−26.9%).
An increase in incidence counts for all cancers was observed in March 2021
(18.8%). We observed no clear shift in stage distributions across 2017–2021. The
observed and relative one-year survival for all cancers and individual cancer
types was similar in 2020 and slightly higher in 2021 compared to 2017–2019.
CONCLUSIONS: This nationwide study suggests
that the pandemic had no major effect on short-term cancer patient outcomes.
These findings are of importance for policymakers and the public health system regarding
future pandemics.
Introduction
The COVID-19 pandemic caused an
unprecedented burden on healthcare systems worldwide with wide-ranging
implications. Pandemic measures including mandated lockdowns, restricted and
disrupted access to healthcare services as well as altered health-seeking behaviour
have been shown to affect a broad range of health conditions including cancer [1].
Several studies in European countries have shown a decrease in the number of
new cancer diagnoses [2–8], a shift towards higher stages at diagnosis [9–11]
and a decrease in one-year survival [12] during the pandemic. However, a recent
systematic review on the impact of the COVID-19 pandemic on cancer diagnoses
worldwide reported considerable heterogeneity in estimates across countries [13].
This may be explained by the extent of how the respective countries were
affected by COVID-19, their lockdown measures and the response of their
healthcare system to the pandemic. In Switzerland, the first COVID-19 cases
were registered in February 2020. The government enforced a general lockdown
from 17 March to 26 April 2020 [14]. During this period, all non-urgent
examinations and treatments were federally prohibited throughout Switzerland.
After this period, hospitals were repeatedly asked to reserve inpatient capacity
for potential increases in COVID-19 cases. Indeed, a substantial overall
reduction in hospital admissions was reported during 2020 in Switzerland,
particularly in elective procedures [15].
Although cancer remains a leading cause of
death, and early diagnosis and treatment benefit patient outcomes [16], little
is known about the impact of the COVID-19 pandemic on cancer outcomes in
Switzerland. The Swiss Federal Statistical Office found almost 3900 fewer
hospitalisations due to cancer (−16%) between 16 March and 24 May 2020 [14].
Additionally, organised screening programmes (breast cancer, colorectal cancer)
were interrupted and the number of mammographies and colonoscopies decreased
substantially during the lockdown period [17]. One Swiss study revealed a shift
in newly diagnosed melanomas towards stage IV during the lockdown [18]. Last,
data from the cantons of Zurich and Zug from 2018 to 2021 have shown a decrease
in registered cancer cases during the first year of the COVID-19 pandemic,
which was particularly pronounced during the lockdown [19]. However, a
comprehensive nationwide overview including the most common cancer types is
lacking.
In this population-based study, we aimed to
investigate the impact of the COVID-19 pandemic on cancer incidence, stage
distribution at diagnosis and one-year survival in Switzerland by comparing data
from pre-pandemic years (2017–2019) and pandemic years (2020, 2021).
Materials and methods
Study population and inclusion criteria
In Switzerland, registration of adult cancer
cases is organised on a cantonal level and cases are reported to the
responsible cantonal cancer registry. Cancer cases of patients aged below 20
years are reported to the national childhood cancer registry. Cancer registry
data are anonymously centralised and evaluated at the national level by the
National Agency for Cancer Registration (NACR). Since 2020, cancer registration
is regulated by the Cancer Registration Act [20]. The law obliges private and
public medical institutions to report all cancer cases to the cancer registry
in charge [20]. Due to the lack of complete data on pre-pandemic years, three
cantons (Schaffhausen, Schwyz, Solothurn), which started cancer registration in
2019 or 2020, were excluded from the analyses.
In Switzerland, cancer cases are defined
according to the tenth revision of the international classification of diseases
(ICD-10) [21]. In this study, we included all cases with a malignant primary
cancer diagnosis (all cancers; ICD-10 C00 to C97, excluding C44
non-melanoma skin cancer) diagnosed between 2017 and 2021. We separately
examined the five most common cancer types in Switzerland: female breast
cancer (ICD-10 C50), colorectal cancer (ICD-10 C18–C20), lung
cancer (ICD-10 C33–C34), melanoma (ICD-10 C43) and prostate
cancer (ICD-10 C61).
Statistical analysis
We first descriptively summarised baseline
characteristics of registered cancer cases stratified by cancer type. We then
compared our main outcomes – cancer incidence, stage distribution at diagnosis
and one-year survival – between pre-pandemic years (2017–2019) and pandemic
years (2020, 2021). Cancer
cases diagnosed between 2017 and 2019 were averaged into a pre-pandemic period
to reduce random annual fluctuation. We evaluated the incidence years 2020 and
2021 separately to account for different governmental COVID-19-related measures
during these years. All analyses for all cancers and separately for the five
most common cancer types were carried out using the software R (version 4.3.3) [22].
Cancer incidence
We
estimated the total number of cancer cases (incidence counts) in the Swiss
population by extrapolating observed cases from cantons included in the study.
This extrapolation assumed that the cancer incidence rates in included cantons
is equivalent to those not included, while adjusting for language region, age,
sex and year of incidence [23]. We additionally calculated directly age-standardised
cancer incidence rates based on observed cases from
the cantons included in the study. Incidence rates were first age-standardised
using the 1976 European Standard Population. These rates were additionally
weighted to reflect the demographic structure of the Swiss population,
accounting for sex, age and language region [23]. We calculated 95% confidence
intervals (CIs) for standardised incidence rates using the method by Fay and
Feuer [24].
The
estimated counts and age-standardised rates were calculated annually and
monthly for the incidence periods (2017–2019, 2020, 2021), for all cancers and
stratified by cancer type. We then calculated absolute
and relative differences in annual and monthly incidence counts between the
pre-pandemic period (2017–2019) and the pandemic years 2020 and 2021,
respectively.
Stage distribution at diagnosis
We
calculated the stage distribution at diagnosis following the rules outlined in
the Union of International Cancer Control’s (UICC) TNM classification of
malignant tumours, 8th edition [25]. TNM staging required at least
some recorded information on T (tumour size), N (node involvement) and M
(metastasis) categories, either clinically or pathologically. For the analysis
of stage distribution, we excluded data from two cantons (Vaud, Aargau) due to
insufficient completeness of TNM data. To determine the stage, we prioritised
pathological TNM data. In cases where neoadjuvant therapy was administered (6.6%
of all cases) or if pathological information was unavailable, clinical TNM data
were used. Stage distributions (stages I–IV, unknown) were compared descriptively
between pre-pandemic years (2017–2019) and pandemic years (2020, 2021).
Survival
Survival
time was calculated from the date of incidence to the date of death or the last
known date alive. We evaluated one-year survival using
two measures. Observed survival represents the time from incidence date to date
of death from any cause, while relative survival incorporates general
population expected mortality rates to account for mortality from causes
unrelated to cancer. The analysis was age-adjusted using weights from the
International Cancer Survival Standard (ICSS) to ensure comparability across time
periods [26]. Both age-adjusted observed and relative one-year survival rates
were calculated for the periods 2017–2019, 2020 and 2021. Relative survival was
estimated with the Ederer II method [27], using life tables obtained from the Swiss
Federal Statistical Office (FSO). Survival analyses were performed using the
popEpi package in R [28].
Results
Characteristics of the study population
We included 218,736 cancer cases diagnosed
between 2017 and 2021 (table 1).
The annual number of all cancer cases was higher in 2021 (46,051) than in 2020
(43,830) and 2017–2019 (annual average: 42,952). This increase was mainly
observed for melanoma and prostate cancer whereas the annual distribution of
female breast cancer, colorectal cancer and lung cancer was similar across
incidence years. Of all cancer cases, 67.4% were from the German-speaking
language region. The median age at incidence of all cancers was 69.7 years
(interquartile range [IQR] 18.2). The median age at incidence was comparable across
cancer types, except for female breast cancer with a lower median age at
incidence of 64.3 years (IQR 22.8). For all cancers overall and also colorectal
cancer, lung cancer and melanoma, more males (54.8%, 55.7%, 56.9% and 54.0%,
respectively) than females were affected.
Table 1Characteristics of included cancer cases in Switzerland in the
period 2017–2021 (based on observed cases from Swiss cantons included in the
study).
|
All cancers* |
Female breast cancer* |
Colorectal cancer* |
Lung cancer* |
Melanoma* |
Prostate cancer* |
| Total number of cases |
218,736 |
31,074 |
21,027 |
23,264 |
15,480 |
36,588 |
| Sex, n (%) |
Female |
98,940 (45.2%) |
31,074 (100.0%) |
9319
(44.3%) |
10,031 (43.1%) |
7118 (46.0%) |
– |
| Male |
119,796 (54.8%) |
– |
11,708 (55.7%) |
13,233 (56.9%) |
8362 (54.0%) |
36,588 (100.0%) |
| Age at incidence, n (%) |
0–49 years |
23,442 (10.7%) |
5986 (19.3%) |
1717 (8.2%) |
626 (2.7%) |
2882 (18.6%) |
280 (0.8%) |
| 50–74 years |
122,334 (55.9%) |
17,258 (55.5%) |
10,650 (50.6%) |
14,482 (62.3%) |
7858 (50.8%) |
25,067 (68.5%) |
| ≥75 years |
72,960 (33.4%) |
7830 (25.2%) |
8660 (41.2%) |
8156 (35.1%) |
4740 (30.6%) |
11,241 (30.7%) |
| Median (IQR) |
69.7 (18.2) |
64.3 (22.8) |
72.0 (18.9) |
71.2 (14.2) |
67.1 (23.2) |
70.7 (12.1) |
| Incidence year, n (%) |
2017 |
42,168 (19.3%) |
6042 (19.4%) |
4204 (20.0%) |
4573 (19.7%) |
2888 (18.7%) |
6799 (18.6%) |
| 2018 |
42,943 (19.6%) |
6097 (19.6%) |
4285 (20.4%) |
4617 (19.8%) |
3060 (19.8%) |
6893 (18.8%) |
| 2019 |
43,744 (20.0%) |
6384 (20.5%) |
4218 (20.1%) |
4674 (20.1%) |
3060 (19.8%) |
7230 (19.8%) |
| 2020 |
43,830 (20.0%) |
6081 (19.6%) |
4132 (19.7%) |
4687 (20.1%) |
3123 (20.2%) |
7393 (20.2%) |
| 2021 |
46,051 (21.1%) |
6470 (20.8%) |
4188 (19.9%) |
4713 (20.3%) |
3349 (21.6%) |
8273 (22.6%) |
| Language region, n (%) |
German-speaking |
147,478 (67.4%) |
20,506 (66.0%) |
14,196 (67.5%) |
15,187 (65.3%) |
11,145 (72.0%) |
25,423 (69.5%) |
| French-/Italian-speaking |
71,258 (32.6%) |
10,568 (34.0%) |
6831 (32.5%) |
8077 (34.7%) |
4335 (28.0%) |
11,165 (30.5%) |
Cancer incidence
The annual incidence counts of all cancer
cases increased in 2020 and 2021 compared to 2017–2019 (tables 2–7; figure 1). This
increase was more pronounced in 2021
(7.3%) than in 2020 (2.1%) and mainly observed for melanoma (2020: 4.3%; 2021:
11.9%) and prostate cancer (2020: 6.1%; 2021: 18.7%). For female breast cancer,
the annual incidence counts slightly decreased in 2020 compared to 2017–2019 (−1.3%)
followed by an increase in 2021 (5.1%). For colorectal cancer and lung cancer,
relatively small differences in annual incidence counts were observed across
incidence periods.
Table 2Difference in annual and monthly incidence counts in 2017–2019,
2020, 2021 in Switzerland for all cancers (based on estimated number of cancer
cases in the Swiss population by extrapolating observed cases from Swiss
cantons included in the study). Cancer cases were defined according to the
tenth revision of the international classification of diseases (ICD-10): All
cancers: all primary invasive cancers (C00 to C97, excluding C44
non-melanoma skin cancer). Absolute and
relative differences were calculated between the average of the years 2017–2019
and the years 2020 and 2021, respectively.
| |
2017–2019 (average) |
2020 |
2021 |
Difference (%) 2020 vs 2017–2019 |
Difference (%) 2021 vs 2017–2019 |
| Annual
incidence |
45,785 |
46,760 |
49,117 |
975
(2.1%) |
3332
(7.3%) |
| Monthly
incidence |
January |
3935 |
4233 |
3910 |
298
(7.6%) |
−25
(−0.6%) |
| February |
3656 |
3983 |
3967 |
327
(8.9%) |
311
(8.5%) |
| March |
4031 |
3906 |
4790 |
−125
(−3.1%) |
759
(18.8%) |
| April |
3548 |
2841 |
4069 |
−707
(−19.9%) |
521
(14.7%) |
| May |
4093 |
3735 |
3906 |
−358
(−8.7%) |
−187
(−4.6%) |
| June |
3916 |
4145 |
4379 |
229
(5.8%) |
463
(11.8%) |
| July |
3756 |
4140 |
3943 |
384
(10.2%) |
187
(5.0%) |
| August |
3572 |
3815 |
3937 |
243
(6.8%) |
365
(10.2%) |
| September |
3624 |
4097 |
4205 |
473
(13.1%) |
581
(16.0%) |
| October |
3967 |
3998 |
3818 |
31 (0.8%) |
−149
(−3.8%) |
| November |
4144 |
4045 |
4340 |
−99
(−2.4%) |
196
(4.7%) |
| December |
3544 |
3823 |
3852 |
279
(7.9%) |
308
(8.7%) |
Table 3Difference in annual and monthly incidence counts in 2017–2019,
2020, 2021 in Switzerland for female breast cancer (ICD-10: C50; based on estimated number
of cancer cases in the Swiss population by extrapolating observed cases from
Swiss cantons included in the study). Absolute and relative differences were calculated
between the average of the years 2017–2019 and the years 2020 and 2021,
respectively.
| |
2017–2019 (average) |
2020 |
2021 |
Difference (%) 2020 vs 2017–2019 |
Difference (%) 2021 vs 2017–2019 |
| Annual
incidence |
6613 |
6528 |
6952 |
−85
(−1.3%) |
339
(5.1%) |
| Monthly
incidence |
January |
560 |
576 |
533 |
16
(2.9%) |
−27
(−4.8%) |
| February |
505 |
585 |
573 |
80
(15.8%) |
68
(13.5%) |
| March |
595 |
519 |
689 |
−76
(−12.8%) |
94
(15.8%) |
| April |
499 |
300 |
559 |
−199
(−39.9%) |
60
(12.0%) |
| May |
608 |
462 |
539 |
−146
(−24.0%) |
−69
(−11.3%) |
| June |
547 |
570 |
627 |
23
(4.2%) |
80
(14.6%) |
| July |
513 |
632 |
563 |
119
(23.2%) |
50
(9.7%) |
| August |
456 |
507 |
480 |
51
(11.2%) |
24
(5.3%) |
| September |
529 |
574 |
609 |
45
(8.5%) |
80
(15.1%) |
| October |
605 |
603 |
555 |
−2
(−0.3%) |
−50
(−8.3%) |
| November |
665 |
618 |
686 |
−47
(−7.1%) |
21
(3.2%) |
| December |
530 |
583 |
538 |
53
(10.0%) |
8
(1.5%) |
Table 4Difference in annual and monthly incidence
counts in 2017–2019, 2020, 2021 in Switzerland for colorectal cancer (ICD-10: C18–C20; based on
estimated number of cancer cases in the Swiss population by extrapolating
observed cases from Swiss cantons included in the study). Absolute and relative differences
were calculated between
the average of the years 2017–2019 and the years 2020 and 2021, respectively.
| |
2017–2019 (average) |
2020 |
2021 |
Difference (%) 2020 vs 2017–2019 |
Difference (%) 2021 vs 2017–2019 |
| Annual
incidence |
4517 |
4400 |
4467 |
−117
(−2.6%) |
−50
(−1.1%) |
| Monthly
incidence |
January |
396 |
389 |
373 |
−7
(−1.8%) |
−23
(−5.8%) |
| February |
351 |
423 |
344 |
72
(20.5%) |
−7
(−2.0%) |
| March |
411 |
354 |
448 |
−57
(−13.9%) |
37
(9.0%) |
| April |
339 |
242 |
373 |
−97
(−28.6%) |
34
(10.0%) |
| May |
385 |
346 |
327 |
−39
(−10.1%) |
−58
(−15.1%) |
| June |
399 |
394 |
424 |
−5
(−1.3%) |
25
(6.3%) |
| July |
376 |
405 |
377 |
29
(7.7%) |
1 (0.3%) |
| August |
377 |
376 |
354 |
−1
(−0.3%) |
−23
(−6.1%) |
| September |
363 |
403 |
392 |
40
(11.0%) |
29
(8.0%) |
| October |
387 |
377 |
326 |
−10
(−2.6%) |
−61
(−15.8%) |
| November |
381 |
339 |
382 |
−42
(−11.0%) |
1
(0.3%) |
| December |
353 |
352 |
347 |
−1
(−0.3%) |
−6
(−1.7%) |
Table 5Difference in annual and monthly incidence
counts in 2017–2019, 2020, 2021 in Switzerland for lung cancer (ICD-10: C33–C34; based on
estimated number of cancer cases in the Swiss population by extrapolating
observed cases from Swiss cantons included in the study). Absolute and relative differences
were calculated between
the average of the years 2017–2019 and the years 2020 and 2021, respectively.
| |
2017–2019 (average) |
2020 |
2021 |
Difference (%) 2020 vs 2017–2019 |
Difference (%) 2021 vs 2017–2019 |
| Annual
incidence |
4918 |
4996 |
5017 |
78
(1.6%) |
99
(2.0%) |
| Monthly
incidence |
January |
404 |
451 |
381 |
47
(11.6%) |
−23
(−5.7%) |
| February |
380 |
416 |
399 |
36
(9.5%) |
19
(5.0%) |
| March |
440 |
426 |
461 |
−14
(−3.2%) |
21
(4.8%) |
| April |
394 |
367 |
402 |
−27
(−6.9%) |
8
(2.0%) |
| May |
449 |
414 |
407 |
−35
(−7.8%) |
−42
(−9.4%) |
| June |
409 |
428 |
480 |
19
(4.6%) |
71
(17.4%) |
| July |
421 |
429 |
405 |
8
(1.9%) |
−16
(−3.8%) |
| August |
389 |
432 |
453 |
43
(11.1%) |
64
(16.5%) |
| September |
384 |
418 |
404 |
34
(8.9%) |
20
(5.2%) |
| October |
431 |
436 |
407 |
5
(1.2%) |
−24
(−5.6%) |
| November |
430 |
407 |
425 |
−23
(−5.3%) |
−5
(−1.2%) |
| December |
386 |
373 |
396 |
−13
(−3.4%) |
10
(2.6%) |
Table 6Difference in annual and monthly
incidence counts in 2017–2019, 2020, 2021 in Switzerland for melanoma (ICD-10: C43; based on
estimated number of cancer cases in the Swiss population by extrapolating
observed cases from Swiss cantons included in the study). Absolute and relative differences
were calculated between
the average of the years 2017–2019 and the years 2020 and 2021, respectively.
| |
2017–2019 (average) |
2020 |
2021 |
Difference (%) 2020 vs 2017–2019 |
Difference (%) 2021 vs 2017–2019 |
| Annual
incidence |
3209 |
3347 |
3592 |
138
(4.3%) |
383
(11.9%) |
| Monthly
incidence |
January |
248 |
298 |
264 |
50
(20.2%) |
16
(6.5%) |
| February |
259 |
276 |
242 |
17
(6.6%) |
−17
(−6.6%) |
| March |
275 |
274 |
331 |
−1
(−0.4%) |
56
(20.4%) |
| April |
223 |
163 |
278 |
−60
(−26.9%) |
55
(24.7%) |
| May |
284 |
279 |
266 |
−5
(−1.8%) |
−18
(−6.3%) |
| June |
303 |
340 |
337 |
37
(12.2%) |
34
(11.2%) |
| July |
266 |
284 |
288 |
18 (6.8%) |
22
(8.3%) |
| August |
264 |
314 |
290 |
50
(18.9%) |
26
(9.8%) |
| September |
283 |
283 |
363 |
0
(0.0%) |
80
(28.3%) |
| October |
274 |
267 |
285 |
−7
(−2.4%) |
11
(4.0%) |
| November |
295 |
302 |
362 |
7
(2.4%) |
67
(22.7%) |
| December |
234 |
269 |
287 |
35
(15.0%) |
53
(22.6%) |
Table 7Difference in annual and monthly
incidence counts in 2017–2019, 2020, 2021 in Switzerland for prostate cancer
(ICD-10: C61; based on estimated number of cancer cases in the Swiss population by
extrapolating observed cases from Swiss cantons included in the study). Absolute and
relative differences were
calculated between the average of the years 2017–2019 and the years 2020 and
2021, respectively.
| |
2017–2019 (average) |
2020 |
2021 |
Difference (%) 2020 vs 2017–2019 |
Difference (%) 2021 vs 2017–2019 |
| Annual
incidence |
7463 |
7916 |
8859 |
453
(6.1%) |
1396
(18.7%) |
| Monthly
incidence |
January |
717 |
812 |
788 |
95
(13.2%) |
71
(9.9%) |
| February |
631 |
705 |
768 |
74
(11.7%) |
137
(21.7%) |
| March |
682 |
678 |
885 |
−4
(−0.6%) |
203
(29.8%) |
| April |
585 |
415 |
735 |
−170
(−29.1%) |
150
(25.6%) |
| May |
661 |
664 |
706 |
3
(0.5%) |
45
(6.8%) |
| June |
649 |
685 |
761 |
36
(5.5%) |
112
(17.3%) |
| July |
547 |
600 |
629 |
53
(9.7%) |
82
(15.0%) |
| August |
578 |
647 |
721 |
69
(11.9%) |
143
(24.7%) |
| September |
556 |
676 |
714 |
120
(21.6%) |
158
(28.4%) |
| October |
617 |
689 |
661 |
72
(11.7%) |
44
(7.1%) |
| November |
700 |
742 |
803 |
42
(6.0%) |
103
(14.7%) |
| December |
541 |
604 |
688 |
63
(11.6%) |
147
(27.2%) |
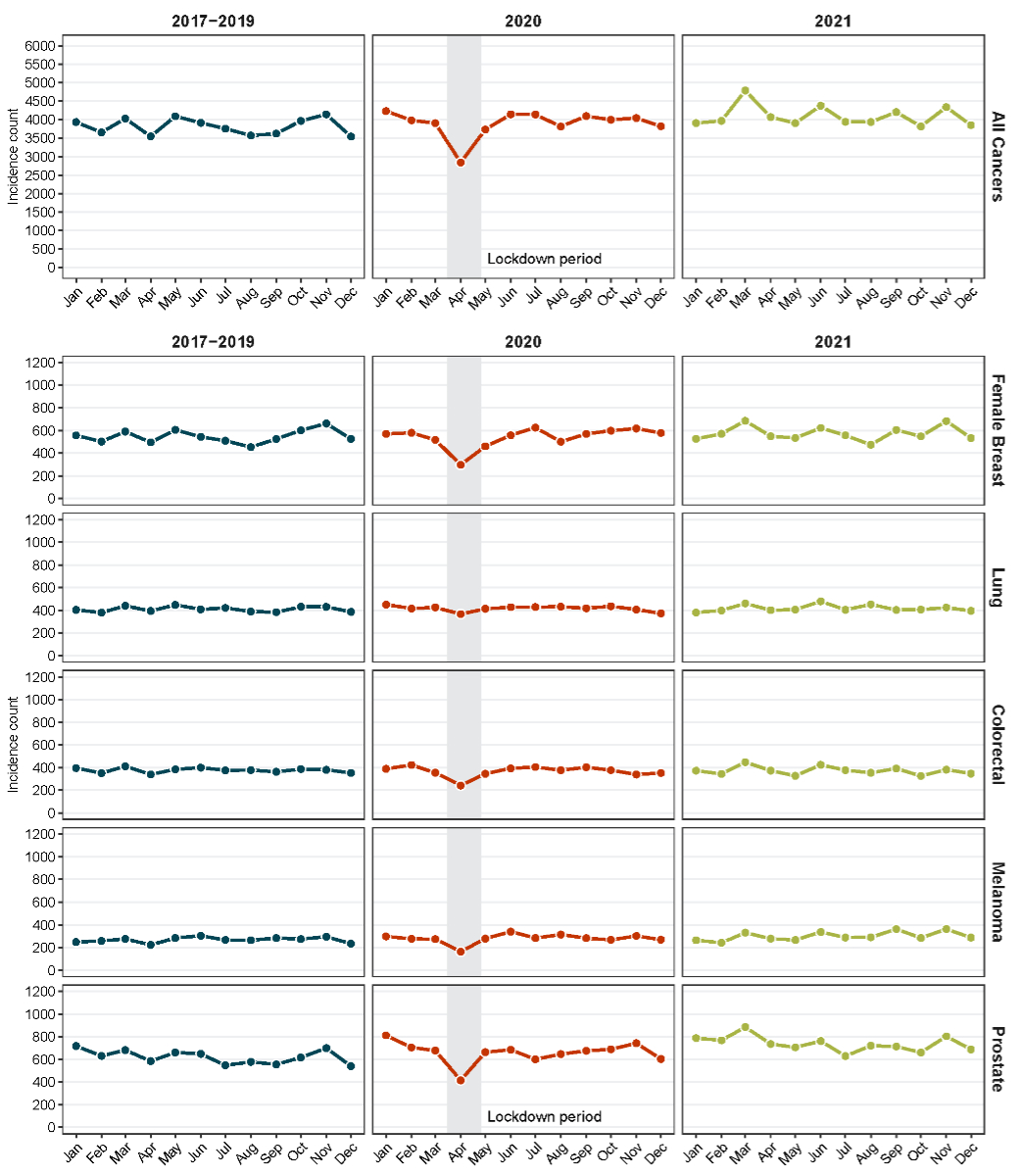
Figure 1The absolute number of monthly cancer cases for all cancers, female
breast cancer, colorectal cancer, lung cancer, melanoma and prostate cancer in
Switzerland for the incidence years 2017–2019, 2020 and 2021 stratified by
incidence month (based on estimated number of cancer cases in
the Swiss population by extrapolating observed cases from Swiss cantons
included in the study).
Stratification by incidence month revealed a
substantial decrease in incidence counts for all cancers from March to May 2020
including the COVID-19 lockdown period (tables
2–7; figure 1; figure S1 in the appendix). This pattern was most pronounced in
April 2020 with a change of −19.9% compared to April 2017–2019. The
extent of the decrease varied by cancer type and ranged from −6.9% (lung
cancer) to −39.9% (breast cancer).
Stratification by age revealed that the
decrease in April 2020 was most prominent for patients aged 50–74 years at diagnosis,
particularly for female breast cancer and prostate cancer (figure S2).
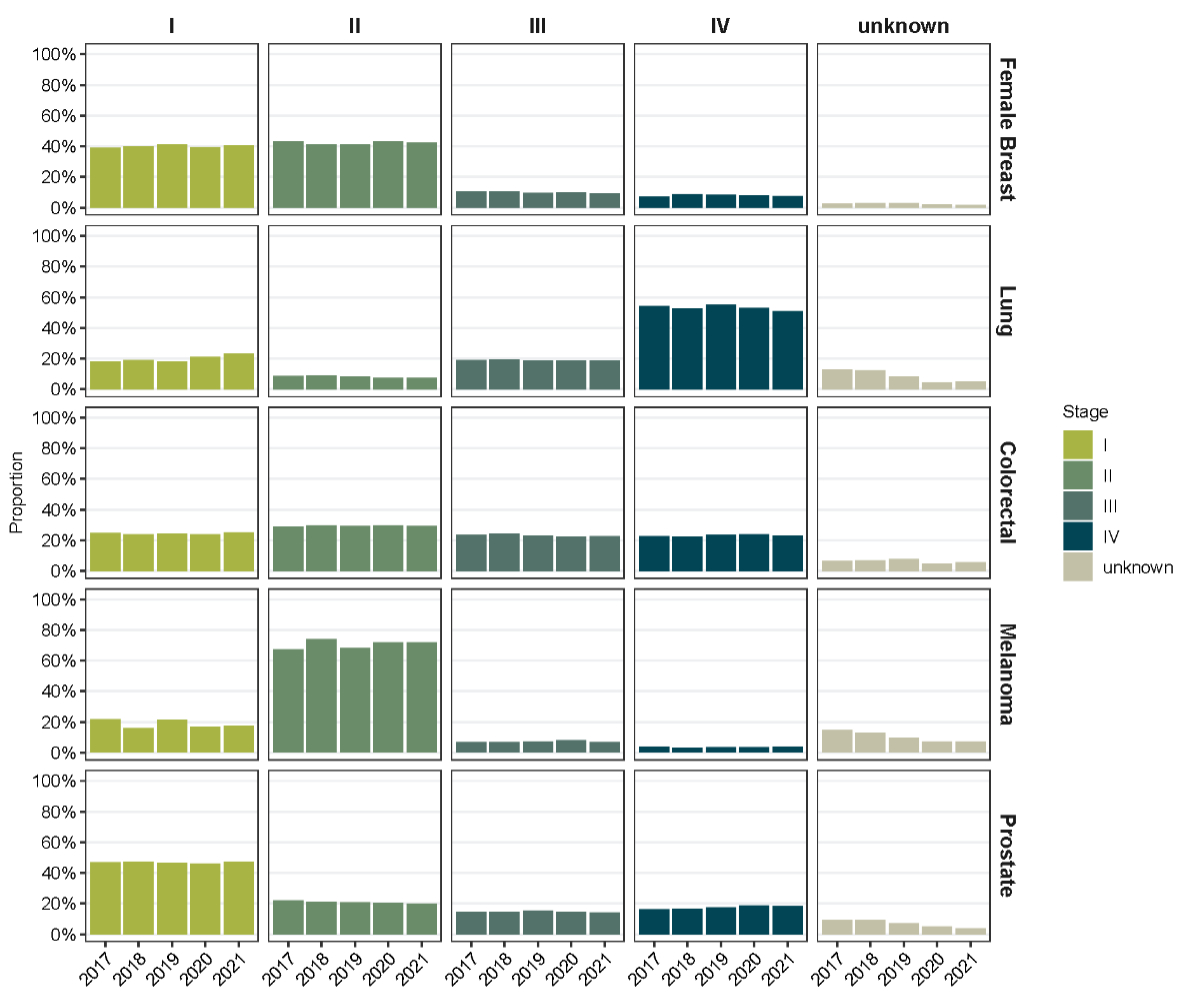
Figure 2Stage distribution based on TNM classification of malignant tumours
for female breast cancer, colorectal cancer, lung cancer, melanoma and prostate
cancer in Switzerland for the incidence years 2017–2019, 2020 and 2021. Percentages
for stages I–IV sum to 100%. The
percentage of unknown cases refers to the total number of cases per cancer type
and incidence year.
A comparatively large increase in incidence
counts was observed in March and April 2021 (tables 2–7; figure S1). This increase
was most pronounced in March
2021, with an increase of 18.8% for all cancers compared to March 2017–2019. Cancers
with the largest relative monthly increase in 2021 were prostate cancer (March:
29.8%; April: 25.6%) and melanoma (March: 20.4%; April: 24.7%), while monthly
variations below 20% were observed for female breast cancer, colorectal cancer
and lung cancer. Similarly, increases of 28.3% and 28.4% were also observed in
September 2021 for melanoma and prostate cancer, respectively. For other
months, we observed variations in incidence counts for all cancers and
individual cancer types, but with no consistent patterns. Overall similar findings
were observed using annual and monthly age-standardised incidence rates (appendix
table S1; figure S3).
Stage distribution at diagnosis
We observed no clear shifts in stage
distribution between 2017–2019, 2020 and 2021 (figure 2). However, we observed
a decrease in the proportion of cases with unknown stage for all cancer types
from 2017 to 2021. This decrease was most pronounced for melanoma (table S2) (14.6%
in 2017 vs 3.8% in
2021) and lung cancer (12.8% vs 3.5%). Stage I and II disease at diagnosis was
most common for female breast cancer (range 2017–2021: 39.0–41.0% and 40.9–43.2%)
and colorectal cancer (range 2017–2021: 23.8–25.2% and 29.0–29.8%). Most melanoma
cases were diagnosed at stage II (range 2017–2021: 67.5–74.0%), most lung
cancer cases at stage IV (range 2017–2021: 50.8–55.1%) and most prostate cancer
cases at stage I (range 2017–2021: 46.2–47.5%).
Survival
Observed and relative one-year survival
estimates were highest for melanoma followed by female breast cancer and
prostate cancer across all incidence periods (table 8). Lowest one-year
survival estimates were observed for lung cancer. The observed and relative
one-year survival for all cancers was similar between 2017–2019 and 2020 and
increased in 2021. The relative one-year survival for all cancers was 85.4%
(95% CI: 85.0–85.7%) in 2021 compared to 84.6% (84.3–85.0%) in 2020 and 84.1%
(83.9–84.3%) in 2017–2019. Stratifying by cancer type, differences in one-year
survival across incidence periods were only observed for lung and prostate
cancer. The relative one-year survival for lung cancer was 62.6% (61.1–64.3%) in 2021
compared to 62.3% (60.7–64.0%) in 2020 and 59.9% (59.0–60.9%)
in 2017–2019. For prostate cancer, the relative one-year survival was 97.4% (97.1–97.8%)
in 2021, 94.9% (94.5–95.4%) in 2020 and 96.4% (95.6–97.3%) in 2017–2019.
Table 8Observed and relative one-year survival for patients with cancer
diagnosed in 2017–2019, 2020, 2021 in Switzerland for all cancers, female
breast cancer, colorectal cancer, lung cancer, melanoma and prostate cancer. Cancer
cases were defined according to
the tenth revision of the international classification of diseases (ICD-10): All
cancers: all primary invasive cancers (C00 to C97, excluding C44
non-melanoma skin cancer); female breast cancer: C50; colorectal
cancer: C18–C20; lung cancer: C33–C34; melanoma: C43;
prostate cancer: C61.
|
Observed one-year survival* |
Relative one-year survival** |
| 2017–2019 |
2020 |
2021 |
2017–2019 |
2020 |
2021 |
| % (95% CI) |
% (95% CI) |
% (95% CI) |
% (95% CI) |
% (95% CI) |
% (95% CI) |
| All cancers |
82.6% (82.4–82.8) |
83.0% (82.7–83.4) |
83.8% (83.5–84.2) |
84.1% (83.9–84.3) |
84.6% (84.3–85.0) |
85.4% (85.0–85.7) |
| Female breast cancer |
96.0% (95.8–96.3) |
95.7% (95.2–96.2) |
95.9% (95.5–96.4) |
97.6% (97.3–97.9) |
97.4% (96.8–97.9) |
97.5% (97.0–98.0) |
| Colorectal cancer |
86.0% (85.4–86.6) |
85.9% (84.9–86.9) |
86.7% (85.7–87.7) |
87.6% (87.0–88.2) |
87.6% (86.5–88.7) |
88.3% (87.3–89.4) |
| Lung cancer |
59.0% (58.1–59.9) |
61.3% (59.7–62.9) |
61.7% (60.1–63.3) |
59.9% (59.0–60.9) |
62.3% (60.7–64.0) |
62.6% (61.1–64.3) |
| Melanoma |
96.3% (95.9–96.7) |
96.9% (96.2–97.5) |
96.2% (95.6–96.9) |
98.3% (97.9–98.7) |
99.1% (98.5–99.7) |
98.3% (97.6–98.9) |
| Prostate cancer |
94.2% (93.4–95.1) |
92.6% (92.2–93.0) |
95.2% (94.9–95.6) |
96.4% (95.6–97.3) |
94.9% (94.5–95.4) |
97.4% (97.1–97.8) |
Discussion
This
nationwide population-based study showed a marked
decrease in cancer diagnoses during the COVID-19 lockdown in March–May 2020,
but no overall annual decrease in 2020 and 2021 compared to pre-pandemic years.
The decrease during the lockdown period was most pronounced for female breast
cancer, prostate cancer and colorectal cancer and was most evident among cases
aged 50–74 years at diagnosis. An increase in cancer diagnoses was observed in
March–April 2021 across all cancer types. No clear shifts in stage
distributions or one-year survival were observed.
Our study revealed similar incidence counts
of all cancer cases in 2020 compared to 2017–2019. This is in contrast to
findings from a recent meta-analysis that showed an overall decrease of 27%
from January to October 2020 compared to the pre-pandemic period with, however,
considerable heterogeneity across geographical regions [13]. In line with our
findings, a Swiss study using data from the cantons of Zurich and Zug found
only a slight decrease in all cancer cases in 2020, suggesting a less severe
impact of the COVID-19 pandemic in Switzerland compared to other countries [19].
In 2021, we observed an overall increase of 7% of all cancer cases compared to
the pre-pandemic period. Given the simultaneous implementation of the Cancer
Registration Act in 2020 in Switzerland enforcing the reporting of cancer cases
to cantonal cancer registries [20], we can only speculate to what extent this
may have counteracted any decrease in cancer incidence in 2020 and led to an
observed increase in 2021. Meanwhile, continuous population growth and ageing may
have had similar effects on incidence counts, although trends for age-standardised
incidence rates were similar.
Monthly incidence counts for all cancers
revealed a substantial decrease during the COVID-19 lockdown period of up to −20%
in April 2020 compared to the pre-pandemic period. This decrease coincided with
governmental COVID-19 measures and is in line with previous regional or
cancer-specific studies in Switzerland [18, 19] and other European countries [6,
16, 29]. This decrease may be related to restricted access to healthcare
services, limited capacities of the healthcare system and the advice,
especially to elderly people, to stay at home during this period [14]. We found
the most pronounced decreases for female breast cancer (−40%) followed by
prostate cancer (−29%), colorectal cancer (−29%) and melanoma (−27%). The
particularly large reduction for female breast and colorectal cancer may be
mainly explained by the COVID-19-related interruption of organised cancer
screening programmes that most Swiss cantons have implemented [17]. Indeed, we
observed the largest decrease among those aged 50–74 years, which corresponds
to the age range covered by screening programmes. However, our findings suggest
that also cancer types subject to opportunistic screening, such as PSA testing
for prostate cancer or regular skin examinations for melanoma, were affected.
The sudden decrease in incidence counts in
2020 compared to the corresponding months in the pre-pandemic period
disappeared from June 2020 onwards. The lack of differences in annual incidence
counts and age-standardised incidence rates between 2020 and 2017–2019 further
suggests that the observed decrease in spring was recouped by the end of 2020. However,
in March and April 2021 we observed
substantial increases in incidence counts compared to the pre-pandemic period,
particularly for prostate cancer and melanoma. This coincided with the end of
the second COVID-19 wave in Switzerland [30] and may in part explain the higher
overall case numbers in 2021 compared to 2017–2019. However, it remains unclear
whether this is a rebound effect after the decrease observed during the
lockdown period. Suter et al. observed similar trends for prostate cancer in
the cantons of Zurich and Zug and hypothesised that adaptations of screening
recommendations, steady increases in age and life expectancy and the relatively
high immigration rate in Switzerland may also play a role [19].
We observed no clear shifts in stage
distribution between pandemic and pre-pandemic years in Switzerland. This is in
contrast to a study from Northern Ireland that revealed shifts from early- to late-stage
tumours for lung, prostate and kidney cancer [16]. Furthermore, a study from
Belgium showed a stage upshifting for cervical, prostate, bladder, ovarian and
fallopian tube tumours [12]. These studies hypothesised that in 2020 advanced
tumours were more likely to be diagnosed because of the symptom severity,
particularly for lung cancer, and examinations for early-stage tumours with
milder symptoms and screening for asymptomatic tumours may have been postponed
or interrupted [12, 16].
Our study further showed comparable
one-year survival rates between 2020 and the pre-pandemic period 2017–2019
followed by a modest increase in 2021. This finding supports the robustness of
the Swiss health care system and the limited effect of COVID-19 on short-term
cancer outcomes in Switzerland. Similarly, a Belgian study showed no overall decline
in one-year relative survival in 2020 [10]. However, a study from Northern
Ireland revealed reductions in survival for tumours of the lung, head and neck,
oesophagus, uterus, and for lymphomas [16]. Especially for lung cancer, the
overlap of symptoms with COVID-19 may have potentially led to delayed diagnosis
[16]. As the detection of cancer at a later stage often results in a poorer
prognosis, the lack of an effect on one-year survival in Switzerland could be related
to the absence of a stage shift at diagnosis. However, changes in treatment type,
timing or guidelines may also affect survival. Longer follow-up is needed to
investigate such effects. We further hypothesise that the modest increase in
one-year survival in 2021 may rather reflect generally increasing cancer
survival rates over time than an effect of the pandemic [31].
Our
study has some limitations. The concomitant occurrence of the COVID-19 pandemic
and the implementation of the Cancer Registration Act
made it more difficult to interpret the effect of the pandemic. Another limitation
is the
descriptive nature of our study. Due to the multifactorial impact of COVID-19
including societal and governmental restrictions, disrupted access to
healthcare services, altered health-seeking behaviour and potentially other
confounding factors, our study cannot establish causal inferences on the impact
of COVID-19 on cancer outcomes. Finally, we lacked data on survival beyond one
year after diagnosis and information on co-morbidities that may have affected
survival. Further research and long-term monitoring are necessary to address
these knowledge gaps.
A major
strength of our study is the use of high-quality population-based cancer
registry data and the nationwide coverage by extrapolating the included cancer
cases to the entire population of Switzerland. Another strength is the
inclusion of three pre-pandemic years (2017–2019) and two pandemic years (2020,
2021) that allowed us to adequately account for random fluctuations before the
pandemic and to investigate beyond the initial effects of the pandemic.
Finally, our study covered a broad range of cancer outcomes and provided
separate information for the five most common cancer types in Switzerland.
Conclusion
In this nationwide study, we found a marked
decrease in cancer diagnoses during the COVID-19 lockdown in March–May 2020,
but no overall annual decrease in cancer diagnoses during 2020 and 2021
compared to pre-pandemic years. Given the relatively stable stage distributions
and one-year survival estimations across cancer types, the pandemic may have
had no major effect on cancer detection and short-term patient outcomes in
Switzerland. These findings are of importance for policymakers and the public
health system regarding future pandemics.
Data sharing statement
The
datasets used in this work are held by the National Agency for Cancer
Registration (NACR) and the Swiss Federal Statistical Office (FSO). The NACR
data are not publicly available but can be obtained upon reasonable request.
Population data and life tables from the FSO are publicly available.
Acknowledgments
We would like to thank
the Cancer Registries (CR) for the collection of the data used in this study.
Namely: Bergeron Y (CR-FR); Bordoni A (CR-TI); Curjuric I, Adam M (CR-AG);
Defossez G, Bulliard JL (CR-VD); Diebold J (CR-LU/UR/OW/NW); Erny S (CR-BS/BL);
Konzelmann I (CR-VS); Kuehni C (Childhood CR); Maspoli M, Bulliard JL
(CR-NE/JU); Mousavi M (CR-SG/TG/AI/AR); Perren A (CR-BE/SO); Rapiti E (CR-GE);
Rohrmann S (CR-ZH/ZG/SH/SZ); von Moos R (CR-GR/GL). We also acknowledge the
National Agency for Cancer Registration (NACR) for merging the cantonal data
and providing the national data, which enabled the national analysis.
Katharina
Staehelin
National
Agency for Cancer Registration (NACR)
Hirschengraben
82
CH-8001
Zurich
katharina.staehelin[at]nkrs.ch
References
1. Moynihan R, Sanders S, Michaleff ZA, Scott AM, Clark J, To EJ, et al. Impact of COVID-19
pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021 Mar;11(3):e045343.
doi: https://doi.org/10.1136/bmjopen-2020-045343
2. Greene GJ, Thomson CS, Donnelly D, Chung D, Bhatti L, Gavin AT, et al. Whole-population
trends in pathology-confirmed cancer incidence in Northern Ireland, Scotland and Wales
during the SARS-CoV-2 pandemic: A retrospective observational study. Cancer Epidemiol.
2023 Jun;84:102367. doi: https://doi.org/10.1016/j.canep.2023.102367
3. Johansson AL, Larønningen S, Skovlund CW, Kristiansen MF, Mørch LS, Friis S, et al. The
impact of the COVID-19 pandemic on cancer diagnosis based on pathology notifications:
A comparison across the Nordic countries during 2020. Int J Cancer. 2022 Aug;151(3):381–95.
doi: https://doi.org/10.1002/ijc.34029
4. Mitchell H, Mclean J, Gavin AT, Visser O, Millar E, Luff T, et al. Impact of COVID-19
control on lung, breast, and colorectal pathological cancer diagnoses. A comparison
between the Netherlands, Aotearoa New Zealand, and Northern Ireland. BMC Cancer. 2023 Jul;23(1):700.
doi: https://doi.org/10.1186/s12885-023-11216-3
5. Negoita S, Chen HS, Sanchez PV, Sherman RL, Henley SJ, Siegel RL, et al. Annual Report
to the Nation on the Status of Cancer, part 2: early assessment of the COVID-19 pandemic’s
impact on cancer diagnosis. Cancer. 2024 Jan;130(1):117–27. doi: https://doi.org/10.1002/cncr.35026
6. Peacock HM, Tambuyzer T, Verdoodt F, Calay F, Poirel HA, De Schutter H, et al. Decline
and incomplete recovery in cancer diagnoses during the COVID-19 pandemic in Belgium:
a year-long, population-level analysis. ESMO Open. 2021 Aug;6(4):100197. doi: https://doi.org/10.1016/j.esmoop.2021.100197
7. Trojanowski M, Radomyski P, Kycler W, Michalek IM. Decrease in the number of new cancer
diagnoses during the first year of the COVID-19 pandemic - cohort study of 3.5 million
individuals in western Poland. Front Oncol. 2023 Dec;13:1230289. doi: https://doi.org/10.3389/fonc.2023.1230289
8. Zagar T, Tomsic S, Zadnik V, Bric N, Birk M, Vurzer B, et al. Impact of the COVID-19
epidemic on cancer burden and cancer care in Slovenia: a follow-up study. Radiol Oncol.
2022 Dec;56(4):488–500. doi: https://doi.org/10.2478/raon-2022-0050
9. Greene G, Griffiths R, Han J, Akbari A, Jones M, Lyons J, et al. Impact of the SARS-CoV-2
pandemic on female breast, colorectal and non-small cell lung cancer incidence, stage
and healthcare pathway to diagnosis during 2020 in Wales, UK, using a national cancer
clinical record system. Br J Cancer. 2022 Aug;127(3):558–68. doi: https://doi.org/10.1038/s41416-022-01830-6
10. Peacock HM, De Gendt C, Silversmit G, Nuyts S, Casselman J, Machiels JP, et al. Stage
shift and relative survival for head and neck cancer during the 2020 COVID-19 pandemic:
a population-based study of temporal trends. Front Oncol. 2023 Sep;13:1253968. doi: https://doi.org/10.3389/fonc.2023.1253968
11. Reinwald F, Justenhoven C. Impact of the COVID-19 pandemic on number and stages of
tumors - data of a German cancer registry. Acta Oncol. 2023 Mar;62(3):315–7. doi: https://doi.org/10.1080/0284186X.2023.2187259
12. Peacock HM, Van Meensel M, Van Gool B, Silversmit G, Dekoninck K, Brierley JD, et
al. Cancer incidence, stage shift and survival during the 2020 COVID-19 pandemic:
A population-based study in Belgium. Int J Cancer. 2024 Oct;155(7):1212–24. doi: https://doi.org/10.1002/ijc.35001
13. Angelini M, Teglia F, Astolfi L, Casolari G, Boffetta P. Decrease of cancer diagnosis
during COVID-19 pandemic: a systematic review and meta-analysis. Eur J Epidemiol.
2023 Jan;38(1):31–8. doi: https://doi.org/10.1007/s10654-022-00946-6
14. Bundesamt für Statistik. Auswirkungen der Covid-19-Pandemie auf die Gesundheitsversorgung
im Jahr 2020. Neuchâtel; 2021. Available from: https://www.bfs.admin.ch/news/de/2021-0247
15. Wirth B, Stucki M, Joerg R, Thommen C, Höglinger M. Impact of the Covid-19 pandemic
on inpatient health care in Switzerland 2020-2021-A descriptive retrospective study
using admission data of all Swiss hospitals. PLoS One. 2024 Jul;19(7):e0306791. doi: https://doi.org/10.1371/journal.pone.0306791
16. Bennett D, Murray I, Mitchell H, Gavin A, Donnelly D. Impact of COVID-19 on cancer
incidence, presentation, diagnosis, treatment and survival in Northern Ireland. Int
J Cancer. 2024 May;154(10):1731–44. doi: https://doi.org/10.1002/ijc.34847
17. Puricelli Perin DM, Elfström KM, Bulliard JL, Burón A, Campbell C, Flugelman AA, et
al.; International Cancer Screening Network. Early assessment of the first wave of
the COVID-19 pandemic on cancer screening services: The International Cancer Screening
Network COVID-19 survey. Prev Med. 2021 Oct;151:106642. doi: https://doi.org/10.1016/j.ypmed.2021.106642
18. Kostner L, Cerminara SE, Pamplona GS, Maul JT, Dummer R, Ramelyte E, et al. Effects
of COVID-19 Lockdown on Melanoma Diagnosis in Switzerland: Increased Tumor Thickness
in Elderly Females and Shift towards Stage IV Melanoma during Lockdown. Cancers (Basel).
2022 May;14(10):2360. 10.3390/cancers14102360
19. Suter F, Wanner M, Menges D, Wicki A, Korol D, Rohrmann S. Impact of the COVID-19
Pandemic and Lockdown on Cancer Diagnoses Using Swiss Cantonal Cancer Registry Data.
Cancers (Basel). 2024 Oct;16(19):3381. doi: https://doi.org/10.3390/cancers16193381
20. Federal Office of Public Health (FOPH). Gesetzgebung Krebsregistrierung. 2025. Available
from: https://www.bag.admin.ch/de/gesetzgebung-krebsregistrierung-datenbearbeitung-und-datennutzung
21. World Health Organization. ICD-10 Version: 2019 – International Statistical Classification
of Diseases and Related Health Problems 10th Revision. Available from: https://icd.who.int/browse10/2019/en
22. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria:
R Foundation for Statistical Computing; 2024. Internet: https://www.R-project.org/
23. Abawi K, Feller A, Lorez M, Michalopoulou E, Roy E, Spycher B, et al. Statistical
Methods for Cancer Reporting in Switzerland. Zürich, Bern, Neuchâtel. National Agency
for Cancer Registration (NACR), Childhood Cancer Registry (ChCR), Federal Statistical
Office (FSO). 2022. Available from: https://www.kinderkrebsregister.ch/wp-content/uploads/sites/2/2022/06/Methodenbericht_final_20220617.pdf
24. Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based
on the gamma distribution. Stat Med. 1997 Apr;16(7):791–801. doi: https://doi.org/10.1002/(SICI)1097-0258(19970415)16:7<791::AID-SIM500>3.0.CO;2-#
25. Union for International Cancer Control (UICC). TNM Classification of Malignant Tumours,
8th Edition: Wiley-Blackwell; 2017. Internet: https://www.uicc.org/what-we-do/sharing-knowledge/tnm
26. Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising
survival ratios. Eur J Cancer. 2004 Oct;40(15):2307–16. doi: https://doi.org/10.1016/j.ejca.2004.07.002
27. Ederer F, Hernan H. Instructions to IBM 650 programmers in processing survival computations.
No. 10. Methodological note. End Results Evaluation Section. Bethesda (MD): National
Cancer Institute; 1959.
28. Miettinen J, Rantanen M. popEpi: Functions for Epidemiological Analysis using Population
Data. R package version 0.4.12. 2024. Internet: https://CRAN.R-project.org/package=popEpi
29. Voigtländer S, Hakimhashemi A, Grundmann N, Radespiel-Tröger M, Inwald EC, Ortmann O,
et al. Impact of the COVID-19 pandemic on reported cancer diagnoses in Bavaria, Germany.
J Cancer Res Clin Oncol. 2023 Aug;149(10):7493–503. doi: https://doi.org/10.1007/s00432-023-04707-0
30. Bundesamt für Statistik. Hospitalisierungen mit Covid-19-Diagnose 2020 und 2021. Neuchâtel;
2022. Available from: https://www.bfs.admin.ch/bfs/de/home/aktuell/neue-veroeffentlichungen.assetdetail.23771995.html
31. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al.; CONCORD Working
Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis
of individual records for 37 513 025 patients diagnosed with one of 18 cancers from
322 population-based registries in 71 countries. Lancet. 2018 Mar;391(10125):1023–75.
doi: https://doi.org/10.1016/S0140-6736(17)33326-3
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4354.

