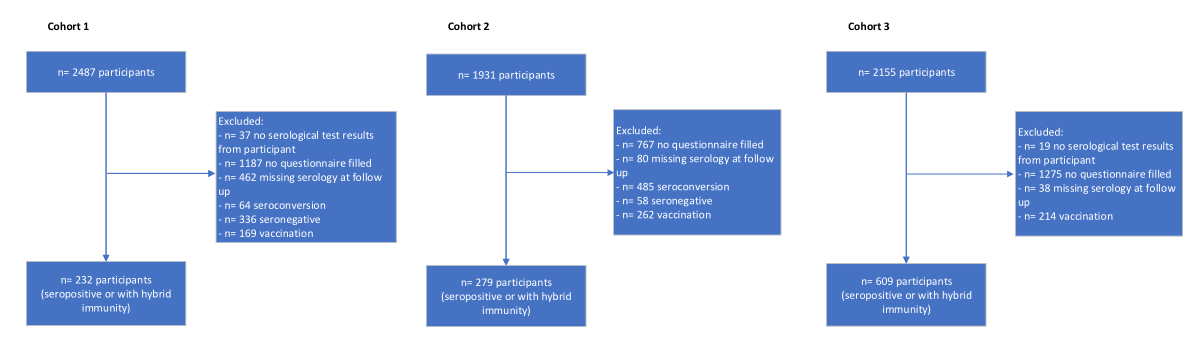
Figure 1Flowchart of participants for the three cross-sectional cohorts from June 2020 to July 2022. Cohort 1: March/April; Cohort 2: November/December; Cohort 3: June/July 2022.
DOI: https://doi.org/https://doi.org/10.57187/s.4337
Children and adolescents generally have a mild course of SARS-CoV-2 infection and rarely suffer from severe acute or long-term COVID-19 [1–4]. We recently reported a 2% prevalence of symptoms compatible with Long COVID in a population-based school cohort during the period when the wildtype virus variant was predominantly circulating [5]. The prevalence of Long COVID in children and adolescents ranges from 1% to 51%, depending on the population studied [4, 6–14]. This heterogeneity in prevalence estimates can be attributed to several factors: highly selected study populations (e.g. inpatients), and variable sample sizes, study designs and settings. Many of these studies rely on self- or proxy-reported data, lacking rigorous clinical assessments, introducing potential response and/or recall bias leading to an over- or underestimation of Long COVID prevalence [4, 11, 15–17]. The lack of a standardised approach to establishing a Long COVID diagnosis in children and adolescents complicates the accurate assessment of prevalence, risk factors and outcomes [4, 11, 15, 16]. Moreover, population-based studies often lack detailed context about the participants reporting Long COVID-compatible symptoms. To address these challenges and improve the accuracy of Long COVID symptom assessment, structured interviews may be used as a more reliable method of distinguishing children and adolescents affected by Long COVID from those who are not. Implementing such an approach would contribute substantially to our understanding of Long COVID in children and adolescents.
In this study, we followed up SARS-CoV-2 seropositive children and adolescents (hereinafter referred to as “infected” or having “hybrid immunity”) reporting symptoms compatible with Long COVID at three different time points during the pandemic. We interviewed such participants to gain a better understanding of the pattern, severity and timing of their symptoms. Based on these interviews, serology and vaccination history, an adjudication process involving three expert paediatricians not involved in the initial study was conducted to assess the probability of Long COVID.
The Ciao Corona study is part of Switzerland’s nationally coordinated Research Network “Corona Immunitas” [18]. The study protocol for Ciao Corona was registered before the start of the study (ClinicalTrials.gov identifier: NCT04448717) [19] and the study was approved by the Ethics Committee of the Canton of Zurich, Switzerland (2020-01336). We conducted the study in the Canton of Zurich, Switzerland, which comprises 1.5 million people. The region is characterised by linguistic and ethnic diversity, which includes both urban and rural areas, and accounts for 18% of the Swiss population. Informed consent was provided orally by children and in writing by parents or caregivers prior to study enrolment.
The present manuscript was prepared according to the STROBE guideline [20].
The primary aims of the study were to assess the probability of Long COVID through questionnaire-based self-reporting of persisting symptoms and to examine whether Long COVID may be misclassified by other non-infection-related reasons, using a combination of self- or proxy-reported standardised interviews and an adjudication process.
Primary schools across the Canton of Zurich were randomly selected to assess the spread and evolution of SARS-CoV-2 infections repeatedly from June 2020 to 2022. Subsequently, we extended invitations to the geographically closest secondary school for each primary school selected. The number of schools invited in each district was proportional to the population size of the 12 districts within the Canton of Zurich. Among the 156 schools invited, including both public and private (around 10%) institutions, 55 agreed to participate. Classes were randomly selected, stratified by school level: grades 1–2 (6- to 8-year-old children) of lower school level, grades 4–5 (9- to 11-year-old children) of middle school level and grades 7–9 (12- to 14-year-old adolescents) of higher school level. All children and adolescents of the randomly selected classes were eligible to participate in any testing round, regardless of their involvement at baseline.
At three different time points during the Ciao Corona study (March/April 2021, November/December 2021 and June/July 2022; see table 1), children and adolescents were included if they had undergone serology testing and completed several questionnaires over time. We only included children and adolescents who were either infected (i.e. seropositive for anti-spike IgG) or had hybrid immunity (i.e. seropositive for anti-nucleocapsid IgG and vaccinated), as shown in the study participant flowchart in figure 1. Cohort 1 tested seropositive in March/April 2021 and was followed up until November/December 2021; Cohort 2 tested seropositive in November/December 2021 and was followed up until June/July 2022; and Cohort 3 tested seropositive in June/July 2022 and was followed up until November/December 2022 (table 1). Within 6 to 9 months post-serology testing, each cohort completed online questionnaires to assess symptoms persisting for more than 12 weeks. The list of symptoms provided in the questionnaire was prepared in accordance with the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) questionnaire [21]. Of note, we intentionally avoided using the term “Long COVID” in any questionnaire. We contacted participants with persisting symptoms for a structured interview in the presence of a parent to assess more detailed information of the reported symptoms compatible with Long COVID. The structured interview comprised four categories, each containing a combination of open and closed questions: physical symptoms, psychological symptoms, impact of symptoms on everyday life, and overall health status (further details are given in appendix table S1). The interviews were conducted within 3 months following the questionnaire assessment.
The summarized interviews of each participant are presented in a supplementary Excel file (appendix table S2, downloadable as a separate file at https://doi.org/10.57187/s.4337). This table presents the summarized interviews of children and adolescents along with their proxies. Each row represents an individual interview with a participant. The initial columns contain data from questionnaires and serology testing, followed by the questions asked during the interview (for more details, see Table S1). All interviews were pseudonymized and summarized to ensure individuals cannot be identified.
After all interviews were completed, we conducted an adjudication process involving three external expert paediatricians (one infectious disease specialist, two paediatric pulmonologists) with clinical experience in the evaluation of children and adolescents potentially affected by Long COVID. Following detailed instructions by the research team, each member of the adjudication committee evaluated children and adolescents independently based on the table summarising transcribed interviews, baseline questionnaire data and serology status. The goal was to use evaluation criteria, based on a modified version of the Delphi process by Stephenson et al. [22] and the WHO Long COVID definition [23], to categorise children and adolescents into “unlikely”, “possible” or “likely” probability of Long COVID (appendix table S2). We set up an online consensus meeting to discuss and clarify any discrepancies between the members of the adjudication committee regarding individual patient cases. Consensus was reached when all members agreed on the probability of Long COVID (unlikely, possible, likely); however, the adjudication committee members did not have to agree on each of the individual criteria.
Table 1Characteristics of the three distinct cohorts. This table shows the baseline characteristics of participants per cohort. We stratified the participants by serology into infected or hybrid immunity. The “overall” cohort includes all those who underwent serology testing and completed a questionnaire, while the “interviewed” cohort includes only those participants who were interviewed.
| Cohort 1: Mar/Apr 2021 | Cohort 2: Nov/Dec 2021 | Cohort 3: Jun/Jul 2022 | ||||
| Infected | Hybrid immunity | Infected | Hybrid immunity | Infected | Hybrid immunity | |
| Overall children and adolescents cohort (n = 1120) | ||||||
| No of participants | 232 | – | 264 | 15 | 416 | 193 |
| …Age, median (IQR) | 11 (9–12) | – | 10 (9–12) | 13 (12–15) | 10 (9–12) | 13 (10–14) |
| …Female sex, n (%) | 117 (50.4) | – | 129 (48.9) | 7 (46.7) | 213 (51.2) | 113 (58.5) |
| …Chronic condition, n (%) * | 59 (25.4) | – | 51 (19.3) | 5 (33.3) | 83 (20.0) | 51 (26.4) |
| ≥1 symptom ≥12 weeks, n (%) | 8 (3.4) | – | 14 (5.3) | 1 (6.7) | 8 (1.9) | 8 (4.1) |
| …Tiredness, n (%) | 5 (2.2) | – | 7 (2.7) | 1 (6.7) | 4 (1.0) | 3 (1.6) |
| …Headache, n (%) | 3 (1.3) | – | 4 (1.5) | 1 (6.7) | 2 (0.5) | 1 (0.5) |
| …Stomach ache, n (%) | 3 (1.3) | – | 2 (1.0) | 0 (0) | 1 (0.5) | 1 (0.5) |
| …Difficulty concentrating, n (%) | 0 (0) | – | 3 (1.1) | 0 (0) | 0 (0) | 2 (0.5) |
| Interviewed children and adolescents (n = 20) | ||||||
| ≥1 symptom ≥12 weeks, n / Total n ** | 5/8 | – | 11/14 | – | 4/8 | 4/8 |
| …Age, median (IQR) | 11 (8–15) | – | 10 (7–15) | – | 12 (10–15) | 12 (9–15) |
| …Female sex, n | 2 | – | 5 | – | 2 | 2 |
| …Chronic condition, n | 2 | – | 4 | – | 1 | 3 |
IQR: interquartile range.
* Chronic conditions reported by parents in the questionnaire: asthma, hay fever, coeliac disease, lactose intolerance, allergies (other than hay fever), neurodermitis, diabetes mellitus, inflammatory bowel disease, hypertension, arthritis, other chronic diseases potentially affecting the immune response (neutropenia; periodic fever with aphthous stomatitis, pharyngitis and adenitis [PFAPA] syndrome; renal failure; cystic fibrosis; bronchitis).
** Children and adolescents with persisting symptoms (≥12 weeks) who participated in the interviews. The denominator is the number of children and adolescents from the entire cohort (n = 1120) who reported persisting symptoms (≥12 weeks).

Figure 1Flowchart of participants for the three cross-sectional cohorts from June 2020 to July 2022. Cohort 1: March/April; Cohort 2: November/December; Cohort 3: June/July 2022.
We performed descriptive analyses for participants’ characteristics. The analyses were performed with the R programming language (v4.2.1), using the tidyverse (v1.3.2), openxlsx (v4.2.5), tableone (v0.13.2), stringr (v1.5.1), viridisLite (v0.4.1), lubridate (v1.9.3), knitr (v1.47), kableExtra (v1.3.4), gtsummary (v1.6.2) and flextable (v0.8.2) packages. Results were visualised using the ggplot2 (v3.3.6) and RColorBrewer (v1.1-2) packages [24].
During the conduct of the study, Long COVID emerged as a new clinical condition. We decided to amend the study protocol to capture persisting symptoms in children and adolescents but did not perform an a priori sample size calculation due to the lack of available data on which to base sample size calculations.
The study populationcomprised three cross-sectional cohorts among seropositive children and adolescents (i.e. infected or hybrid immunity) from three different time periods throughout the pandemic (figure 2). In total, we included 1120 children and adolescents (table 1) of whom 39 (3.5%) reported having persisting symptoms compatible with Long COVID (figure 1). Overall, the most frequently reported symptoms lasting more than 12 weeks were headache, tiredness and stomach ache (table 1). Other reported symptoms (i.e. mentioned more than once) were difficulties concentrating, nasal congestion and/or runny nose. Across the three cohorts, 24 interviews were conducted with 20 (51%) children and adolescents (table 1) reporting symptoms that persisted for more than 12 weeks. All interviews are summarised in the supplementary Excel file (downloadable as a separate file at https://doi.org/10.57187/s.4337). The overall and individual ratings of the external adjudication committee are displayed in table 2 (see table S3). Individual expert ratings with respect to the probability of Long COVID were heterogeneous (see table 2 and table S3). Alternative diagnoses and/or other non-SARS-CoV-2-related reasons for persisting symptoms were, among others, lack of temporal relationship (e.g. symptoms were present before the pandemic), psychosocial stress from school and family environments (e.g. bullying at school, burnout of a parent, learning difficulties at school) and individual psychological conditions (e.g. attention deficit hyperactivity disorder [ADHD], anxiety, depression) (see supplementary file). Finally, the adjudication committee agreed that among the 20 interviewed children and adolescents, none of them was likely to have Long COVID, seven (35%) had possible Long COVID and 13 (65%) were unlikely to have Long COVID.
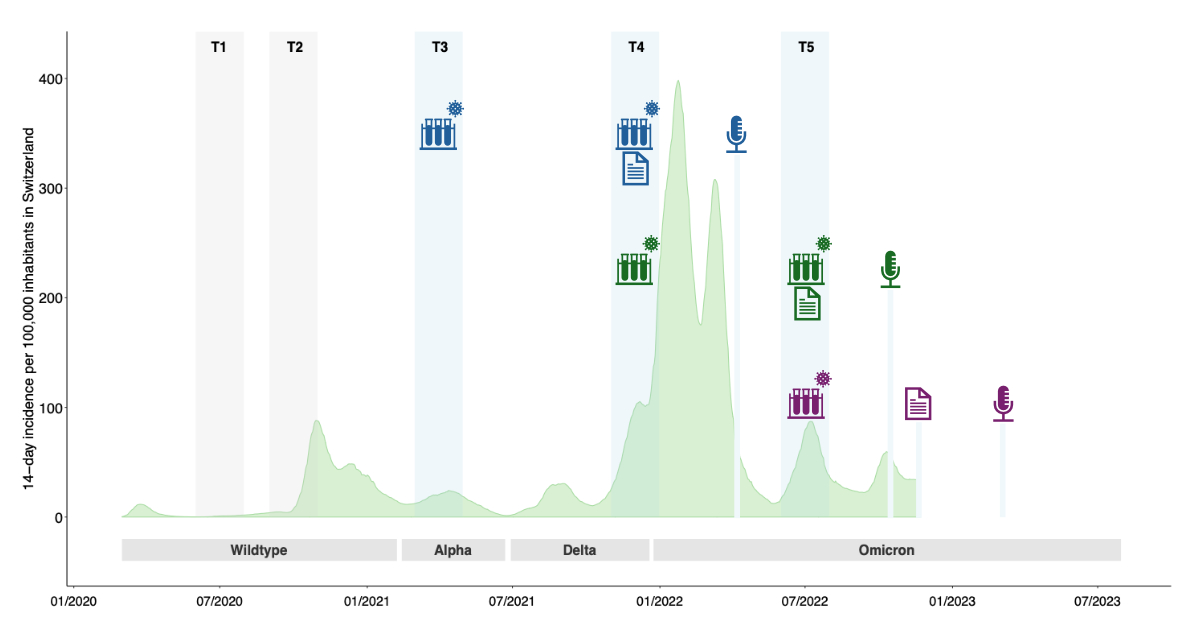
Figure 2Overview of the Ciao Corona timeline (2020–2023) and the three study cohorts comprising the interviewed children and adolescents. This figure illustrates the timeline of the Ciao Corona study for the three cohorts, highlighting the time points for serology, questionnaire assessments and interviews. Each cohort is represented by a colour: blue for Cohort 1, green for Cohort 2 and violet for Cohort 3. T1: first study round in June/July 2020; T2: second study round in October/November 2020; T3: third study round in March/April 2021 (Cohort 1); T4: fourth study round in November/December 2021 (Cohort 2); T5: fifth study round in June/July 2022 (Cohort 3). The icon meanings are: Test tube with virus: serology testing; Written document: questionnaire data assessment; Microphone: interviews. The light green area depicts the evolution of the incidence of diagnosed SARS-CoV-2 infections in Switzerland, shown as the 14-day incidence per 100,000 inhabitants. Grey: the predominant variant of concern in Switzerland (>50% of variants circulating).
Table 2The individual ratings of the 20 interviewed children and adolescents by the external adjudication committee. This table presents the five criteria used by the adjudication committee to rate each interviewed participant according to a modified version of the Delphi process by Stephenson et al. [22] and the WHO Long COVID definition [23].
| Criteria | Expert | No | Yes | No categorisation possible |
| Start of symptoms: Symptoms present since acute SARS-CoV-2 infection or developed thereafter | Expert 1 | 5 | 13 | 2 |
| Expert 2 | 8 | 12 | 0 | |
| Expert 3 | 7 | 13 | 0 | |
| Testing: At least one positive COVID-19 test | Expert 1 | 0 | 18 | 2 |
| Expert 2 | 0 | 20 | 0 | |
| Expert 3 | 1 | 19 | 0 | |
| Burden of symptoms: The young person had symptoms that continued or developed after SARS-CoV-2 which impacted their physical, mental or social wellbeing | Expert 1 | 4 | 12 | 4 |
| Expert 2 | 0 | 19 | 1 | |
| Expert 3 | 6 | 12 | 2 | |
| Burden of symptoms: The young person had symptoms that were interfering with some aspect of daily living (e.g. school, work, home, relationships) | Expert 1 | 7 | 10 | 3 |
| Expert 2 | 4 | 15 | 1 | |
| Expert 3 | 7 | 12 | 1 | |
| Duration: Symptoms persisted for a minimum duration of 12 weeks after testing | Expert 1 | 0 | 17 | 3 |
| Expert 2 | 0 | 20 | 0 | |
| Expert 3 | 4 | 16 | 0 |
In this large school-based study, we demonstrate that conducting interviews with children and adolescents and/or their proxies can provide important contextual information to self- or proxy-reported data in the assessment of Long COVID. Overall, we found that none of the children and adolescents were likely to have Long COVID, seven (35%) possibly had Long COVID and 13 (65%) were unlikely to have Long COVID over a timeframe of two years. Thus, the probability of the questionnaire-based assessment of Long COVID was reduced following evaluation of self- or proxy-reported symptoms and additional information from interviews by an adjudication committee.
Most studies on Long COVID in children and adolescents rely on self- or proxy-reported data, with prevalence estimates varying from 1% to 51% for the same clinical condition [4–14]. However, this self- or proxy-reported data presents challenges, potentially impacting its reliability and validity, as shown by different studies and reports [4, 11, 15, 16]. Relying solely on self- or proxy-reported data can pose difficulties due to potential recall bias [25, 26], subjective interpretation of symptoms [27–29] and the risk of response bias [30]. Although the number of participants reporting persisting symptoms in our study was low, evaluating the probability of Long COVID solely based on self- or proxy-reported symptoms without contextual information is likely to be similar in larger studies. Therefore, we decided to integrate standardised interviews, aiming to gain more additional contextual information regarding the symptoms possibly related to Long COVID. These individual interviews offered insights into the pattern, severity and timing of symptoms, as well as their effects on the participant’s environment, social interactions and school performance. Furthermore, the interviews helped establish a timeline with respect to the occurrence of SARS-CoV-2 infection and the first presentation of symptoms possibly related to Long COVID. All this information seems crucial for the evaluation of the plausibility of Long COVID in the absence of clear clinical diagnostic criteria.
By implementing the adjudication process, i.e. a structured evaluation by an independent adjudication committee, we were able to carefully assess each participant’s data against evaluation criteria for Long COVID. Taking this process into consideration, the number of children and adolescents with symptoms possibly related to Long COVID decreased substantially compared to only self- or proxy-reported data. The adjudication process is particularly valuable in the context of diseases like Long COVID, where symptoms are heterogeneous and diagnostic criteria are continuously evolving. As reported elsewhere [31], the adjudication process can significantly enhance the validity of self- or proxy-reported data and improve its accuracy.
The strengths of our study include its large, randomly selected and longitudinal school cohort, enabling us to capture the different waves of the SARS-CoV-2 pandemic from 2020 to 2022. Further, the standardised interviews and subsequent adjudication process allowed us to weigh the probability of Long COVID in children and adolescents with symptoms attributable to Long COVID.
Some limitations need to be considered when interpreting the findings of this study. First, we were only able to follow up children and adolescents who filled out the questionnaires by themselves or by their proxy, potentially introducing a selection and response bias. Second, the exact timing of SARS-CoV-2 infections in children and adolescents is not known as we did not perform PCR or antigen tests – although we did enquire during interviews whether the participants had experienced an infection and when. Third, we did not conduct clinical assessments, which may have been useful for excluding underlying diseases or conditions related to self- or proxy-reported symptoms. Fourth, the study population overrepresented individuals from higher socioeconomic backgrounds, indicating a clear dominance of responses from educated proxies [32]. Finally, it is possible that some participants may have been missed due to their failure to complete the questionnaires, lack of understanding of the study purpose, or language barriers preventing them from participating in the interviews or the study in general.
Our study highlights the usefulness of implementing standardised interviews and an adjudication process in addition to self- or proxy-reported data to better understand the likelihood that self- or proxy-reported persisting symptoms are related to a SARS-CoV-2 infection. Thus, relying solely on self- or proxy-reported data, without more detailed contextual information, likely overestimates symptoms compatible with Long COVID in children and adolescents.
For confidentiality reasons (e.g. potential identifiable information from individual interviews), we would like to abstain from sharing individual participant data. The code used to support the findings of this study is available from the corresponding author upon reasonable request.
Author contributions: SK and MAP conceived the study. TR, SK and MAP developed the preliminary design. TR, SK, MAP, AR, SR, SRH and AU established the study design and methodology. TR, SK, AU, AR, SRH and SR recruited participants. TR, SK, AU, AR, SRH, SR, PZ, NR, CB and MAP performed data acquisition, management and interpretation. AR, SR, SRH and AU conducted statistical analysis. AR wrote the first draft of the manuscript. All authors were involved in the interpretation of the findings, the review and authorisation of the manuscript for intellectual accuracy. TR is the corresponding author and guarantor, assuming complete accountability for the conducted research. Furthermore, TR had full access to the data and made the final decision to publish. The corresponding author (TR) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All contributing authors approved the submitted manuscript.
The Ciao Corona study is part of Switzerland’s nationally coordinated Research Network “Corona Immunitas” and the Swiss School of Public Health (SSPH+). The Ciao Corona study was funded by fundraising of SSPH+, including funds of the Swiss Federal Office of Public Health and private funders (considering the SSPH+ ethical guidelines for funding), by Swiss Cantons (Vaud, Zurich and Basel), by institutional funds of the Universities and by the EU-grant CoVICIS (HORIZON-HLTH-2021-CORONA-0, grant No 101046041). The University of Zurich Foundation provided funding specific to the Ciao Corona study. The funders were not involved in the planning or implementation of the study; the collection, management, analysis or interpretation of the data; the writing, review or approval of the manuscript; or the decision to submit the manuscript for publication. All authors had full access to the data analysis results and take responsibility for the integrity and accuracy of the data.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Zimmermann P, Curtis N. COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr Infect Dis J. 2020 Jun;39(6):469–77. doi: https://doi.org/10.1097/INF.0000000000002700
2. Bialek S, Boundy E, Bowen V, Chow N, Cohn A, Dowling N, et al.; CDC COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020 Mar;69(12):343–6. doi: https://doi.org/10.15585/mmwr.mm6912e2
3. Neeland MR, Bannister S, Clifford V, Dohle K, Mulholland K, Sutton P, et al. Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children. Nat Commun. 2021 Feb;12(1):1084. doi: https://doi.org/10.1038/s41467-021-21414-x
4. Zimmermann P, Pittet LF, Curtis N. How Common is Long COVID in Children and Adolescents? Pediatr Infect Dis J. 2021 Dec;40(12):e482–7. doi: https://doi.org/10.1097/INF.0000000000003328
5. Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term Symptoms After SARS-CoV-2 Infection in Children and Adolescents. JAMA. 2021 Jul;326(9):869–71. doi: https://doi.org/10.1001/jama.2021.11880
6. Buonsenso D, Di Gennaro L, De Rose C, Morello R, D’Ilario F, Zampino G, et al. Long-term outcomes of pediatric infections: from traditional infectious diseases to long Covid. Future Microbiol. 2022 May;17(7):551–71. doi: https://doi.org/10.2217/fmb-2022-0031
7. Smane L, Roge I, Pucuka Z, Pavare J. Clinical features of pediatric post-acute COVID-19: a descriptive retrospective follow-up study. Ital J Pediatr. 2021 Aug;47(1):177. doi: https://doi.org/10.1186/s13052-021-01127-z
8. Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur J Pediatr. 2022 Apr;181(4):1597–607. doi: https://doi.org/10.1007/s00431-021-04345-z
9. Stephenson T, Pinto Pereira SM, Shafran R, de Stavola BL, Rojas N, McOwat K, et al.; CLoCk Consortium. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. 2022 Apr;6(4):230–9. doi: https://doi.org/10.1016/S2352-4642(22)00022-0
10. Kikkenborg Berg S, Palm P, Nygaard U, Bundgaard H, Petersen MN, Rosenkilde S, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. 2022 Sep;6(9):614–23. doi: https://doi.org/10.1016/S2352-4642(22)00154-7
11. Woodrow M, Carey C, Ziauddeen N, Thomas R, Akrami A, Lutje V, et al. Systematic Review of the Prevalence of Long COVID. Open Forum Infect Dis. 2023 May;10(7):ofad233. doi: https://doi.org/10.1093/ofid/ofad233
12. Twohig H, Bajpai R, Corp N, Faux-Nightingale A, Mallen C, Robinson T, et al. Long-term outcomes of COVID-19 infection in children and young people: a systematic review and meta-analysis. NIHR Open Res. 2024 Apr;4:22. doi: https://doi.org/10.3310/nihropenres.13549.1
13. Hahn LM, Manny E, Mamede F, Dhaliwal G, Chikuma J, Robinson JL, et al. Post-COVID-19 Condition in Children. JAMA Pediatr. 2023 Nov;177(11):1226–8. doi: https://doi.org/10.1001/jamapediatrics.2023.3239
14. Roessler M, Tesch F, Batram M, Jacob J, Loser F, Weidinger O, et al. Post-COVID-19-associated morbidity in children, adolescents, and adults: A matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med. 2022 Nov;19(11):e1004122. doi: https://doi.org/10.1371/journal.pmed.1004122
15. Stephenson T, Shafran R, Ladhani SN. Long COVID in children and adolescents. Curr Opin Infect Dis. 2022 Oct;35(5):461–7. doi: https://doi.org/10.1097/QCO.0000000000000854
16. Pellegrino R, Chiappini E, Licari A, Galli L, Marseglia GL. Prevalence and clinical presentation of long COVID in children: a systematic review. Eur J Pediatr. 2022 Dec;181(12):3995–4009. doi: https://doi.org/10.1007/s00431-022-04600-x
17. Azzam A, Khaled H, Refaey N, Mohsen S, El-Emam OA, Dawood N, et al. The burden of persistent symptoms after COVID-19 (long COVID): a meta-analysis of controlled studies in children and adults. Virol J. 2024 Jan;21(1):16. doi: https://doi.org/10.1186/s12985-024-02284-3
18. West EA, Anker D, Amati R, Richard A, Wisniak A, Butty A, et al.; Corona Immunitas Research Group. Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int J Public Health. 2020 Dec;65(9):1529–48. doi: https://doi.org/10.1007/s00038-020-01494-0
19. Ulyte A, Radtke T, Abela IA, Haile SR, Braun J, Jung R, et al. Seroprevalence and immunity of SARS-CoV-2 infection in children and adolescents in schools in Switzerland: design for a longitudinal, school-based prospective cohort study. Int J Public Health. 2020 Dec;65(9):1549–57. doi: https://doi.org/10.1007/s00038-020-01495-z
20. EQUATOR network. Enhancing the QUAlity and Transparency Of health Research [Internet]. [cited 2025 May 21]. Available from: https://www.equator-network.org/reporting-guidelines/
21. ISARIC. COVID-19 [Internet]. ISARIC.org. [cited 2024 Jul 8]. Available from: https://isaric.org/research/covid-19-clinical-research-resources/
22. Stephenson T, Allin B, Nugawela MD, Rojas N, Dalrymple E, Pinto Pereira S, et al.; CLoCk Consortium. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child. 2022 Jul;107(7):674–80. doi: https://doi.org/10.1136/archdischild-2021-323624
23. WHO. WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. WHO; 2021.
24. R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2022 [cited 2023 Mar 13]. Available from: https://www.R-project.org/
25. Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43(1):87–91. doi: https://doi.org/10.1016/0895-4356(90)90060-3
26. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016 May;9(May):211–7. doi: https://doi.org/10.2147/JMDH.S104807
27. Mack JW, McFatrich M, Withycombe JS, Maurer SH, Jacobs SS, Lin L, et al. Agreement Between Child Self-report and Caregiver-Proxy Report for Symptoms and Functioning of Children Undergoing Cancer Treatment. JAMA Pediatr. 2020 Nov;174(11):e202861. doi: https://doi.org/10.1001/jamapediatrics.2020.2861
28. Mockler GL, Novotny SP, Hou W, Liu Y, Schoenfeld ER. Patient Self-Report Superior to Electronic Medical Record Abstraction for Identifying Positive COVID-19 Symptoms at Illness Onset. AJPM Focus. 2022 Sep;1(1):100005. doi: https://doi.org/10.1016/j.focus.2022.100005
29. Barbara AM, Loeb M, Dolovich L, Brazil K, Russell M. Agreement between self-report and medical records on signs and symptoms of respiratory illness. Prim Care Respir J. 2012 Jun;21(2):145–52. doi: https://doi.org/10.4104/pcrj.2011.00098
30. Rosenman R, Tennekoon V, Hill LG. Measuring bias in self-reported data. Int J Behav Healthc Res. 2011 Oct;2(4):320–32. doi: https://doi.org/10.1504/IJBHR.2011.043414
31. Frei A, Siebeling L, Wolters C, Held L, Muggensturm P, Strassmann A, et al. The Inaccuracy of Patient Recall for COPD Exacerbation Rate Estimation and Its Implications: Results from Central Adjudication. Chest. 2016 Oct;150(4):860–8. doi: https://doi.org/10.1016/j.chest.2016.06.031
32. Raineri A, Radtke T, Rueegg S, Haile SR, Menges D, Ballouz T, et al. Persistent humoral immune response in youth throughout the COVID-19 pandemic: prospective school-based cohort study. Nat Commun. 2023 Nov;14(1):7764. doi: https://doi.org/10.1038/s41467-023-43330-y
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4337.
The appendix table S2 is available for download as a separate Excel file at https://doi.org/10.57187/s.4337.