Long-term impacts of Legionnaires’ disease on health and wellbeing: rationale, study
design and baseline findings of a matched cohort study (LongLEGIO)
DOI: https://doi.org/https://doi.org/10.57187/s.4333
Melina
Biglerab*,
Malina
Vaucherab*,
Manuel
Wiederkehrab,
Sophia
Brülisauerc,
Werner C. Albrichc,
Sarah
Drägerde,
Valentin
Gislerfg,
Isabel
Akersh,
Daniel
Mäusezahlab
a Swiss
Tropical and Public Health Institute, Basel, Switzerland
b University
of Basel, Basel, Switzerland
c Division
of Infectious Diseases, Infection Prevention and Travel Medicine, HOCH Health Ostschweiz,
Kantonsspital St. Gallen, St. Gallen, Switzerland
d Division
of Internal Medicine, University Hospital Basel, Basel, Switzerland
e Department
of Clinical Research, University of Basel, Basel, Switzerland
f Institute
for Laboratory Medicine, Cantonal Hospital Aarau, Aarau, Switzerland
g Department
for Infectious Diseases and Infection Prevention, Cantonal Hospital Aarau,
Aarau, Switzerland
h Division
of Internal Medicine, Spital Limmattal, Zurich-Schlieren, Switzerland
* Shared
first authorship
Summary
BACKGROUND
AND STUDY AIMS: Is there a post-acute infection
syndrome for Legionnaires’ disease? Legionnaires’ disease is a form of
primarily community-acquired pneumonia caused by Legionella spp. bacteria. Legionnaires’ disease and other forms of
bacterial community-acquired pneumonia may lead to persistent health and
wellbeing impairments. It remains unclear whether these are caused by the community-acquired
pneumonia-causing pathogen or the pneumonia itself. We present the rationale
and design of a matched cohort study to investigate the persistent health
impacts of Legionnaires’ disease and compare them with persistent
manifestations of other bacterial (Legionella
test-negative) community-acquired pneumonia. We also present baseline
characteristics of the study cohorts.
METHODS: Legionnaires’ disease patients and Legionella test-negative community-acquired
pneumonia patients with
confirmed or clinically suspected bacterial aetiology were recruited from
university and cantonal/regional hospitals and matched for sex, age, hospital
type and date of diagnosis. Questionnaire-based interviews are conducted at
baseline and 2, 6 and 12 months after the start of appropriate antibiotics. The
questionnaires focus on patient-reported outcome measures and cover long-term
symptoms, use of health services and health-related quality of life.
RESULTS: Between June 2023 and June 2024, 59 patients with Legionnaires’
disease (59.3% male, median age 69 years [interquartile range [IQR]: 57–80]) and
60 patients with other bacterial (Legionella test-negative) community-acquired
pneumonia (63.3% male, median age 69 years [IQR: 60–79]) were enrolled. Admission
to the intensive care unit was required for 13.6 % of Legionnaires’ disease
patients and 8.3 % of other bacterial community-acquired pneumonia patients. Chronic
kidney failure was more prevalent among Legionnaires’ disease patients (15.3% vs
10.0%), while chronic obstructive pulmonary disease (20.0% vs 11.9%),
malignancies (33.3% vs 13.6%) and an immunocompromised status (25.0% vs 13.6%)
were more common in Legionella
test-negative community-acquired pneumonia patients. Furthermore, Legionella test-negative
community-acquired pneumonia patients reported lower
baseline quality of life scores than Legionnaires’ disease patients. Differences
in pneumonia severity, comorbidities and self-reported quality of life scores
will be accounted for in future analyses.
CONCLUSIONS: The LongLEGIO
study will contribute to research on post-acute infection syndromes and provide
the data for a more holistic assessment of the disease burden of Legionnaires’
disease.
Introduction
Lower respiratory tract infections,
including pneumonia, remain a major cause of morbidity and mortality worldwide [1,
2] and pose a significant burden on healthcare systems [3]. Symptoms of
pneumonia and overall impaired wellbeing can persist for weeks to months after
the acute infection, leading to additional use of health services [4–6].
Persistent sequelae of pneumonia that are reported in the literature include
respiratory symptoms such as cough, dyspnoea and chest discomfort [7],
cardiovascular complications [8], cognitive impairment [9, 10], general fatigue
[7, 11, 12] and reduced quality of life [13]. Notably, extrapulmonary sequelae
often outlast the respiratory symptoms [6, 11]. In addition, up to 9% of patients
experience recurrent pneumonia within five years of an acute pneumonia episode [14].
Persistent manifestations of pneumonia –
particularly non-specific symptoms such as fatigue or cognitive impairment and
general reduced wellbeing – are difficult to capture in clinical practice and
are often not well documented in medical records [7, 15, 16]. As a result,
patient-reported outcome measures (PROMs) collected in prospective studies are
important endpoints for assessing recovery from pneumonia [7]. However, most
such prospective pneumonia studies do not differentiate between
pneumonia-causing pathogens in the analysis but instead stratify for Streptococcus pneumoniae as the most
commonly identified cause of community-acquired pneumonia (CAP) [11, 17] or
they report persistent symptoms for individual pathogens without comparing
findings to pneumonia of other aetiologies [18, 19]. The role of the infecting
organism in the persistence of pneumonia symptoms therefore remains poorly
understood.
Studying and comparing persistent symptoms
of Legionnaires’ disease (LD) with other bacterial pneumonia provides an
opportunity to investigate the role of the infection-causing pathogen in
persistent pneumonia sequelae. Legionnaires’ disease is caused by the
intracellular Gram-negative Legionella bacterium and accounts for about
4–7% of CAP cases in Europe [20]. Legionnaires’ disease often causes severe
pneumonia and, in addition to respiratory symptoms, is frequently associated
with extrapulmonary manifestations such as confusion, diarrhoea, headache and
acute kidney damage [21–23]. The underlying pathophysiological mechanisms of Legionella
infections are very similar to those of Coxiella burnetii infections
causing Q fever [24, 25]. The long-term health effects of Q fever, particularly
chronic fatigue, are well documented in the literature [15].
Despite its potential to cause persistent
symptoms and health impairments that might be even more pronounced than for
other types of bacterial pneumonia, the chronic impact of Legionnaires’ disease
remains little studied. Previous studies have reported persistent fatigue,
neurological symptoms such as concentration difficulties and memory loss,
muscle weakness and reduced quality of life for up to 17 months after an acute Legionella
infection [18, 26]. However,
the studies were not specifically designed to compare these symptoms with the
persistent manifestations of other types of pneumonia. It therefore remains
unclear whether the observed persistent sequelae were due to the underlying Legionella
infection or more generally
due to the severity of the pneumonia per se. In addition, no study has
systematically assessed the use of health services by Legionnaires’ disease
patients beyond the acute phase of infection.
Here, we describe the rationale and design
of the LongLEGIO study. This prospective study explores the persisting impacts
of community-acquired Legionnaires’ disease (CALD) on patients’ health,
wellbeing and health service utilisation and compares CALD patients with
bacterial community-acquired pneumonia patients who tested negative for Legionella.
We will
further present the baseline characteristics of the two study cohorts.
Methods
Study design and objectives
The LongLEGIO study is a matched cohort
study with the following objectives: (a) To explore CALD patients’ persistent
symptoms and general wellbeing and compare them to those of Legionella test-negative
bacterial community-acquired
pneumonia patients (non-LD CAP), and (b) To describe and compare the care needs
and health service utilisation of CALD and non-LD CAP patients during their
recovery.
Recruitment and data collection
The LongLEGIO study enrolled CALD and
non-LD CAP patients from university and cantonal/regional hospitals in
Switzerland. For the recruitment of CALD patients and the collection of
baseline data on these patients, the LongLEGIO
study builds on a national case-control and molecular source attribution study
investigating risk factors and sources of infection for CALD in Switzerland (SwissLEGIO) (figure 1) [27].
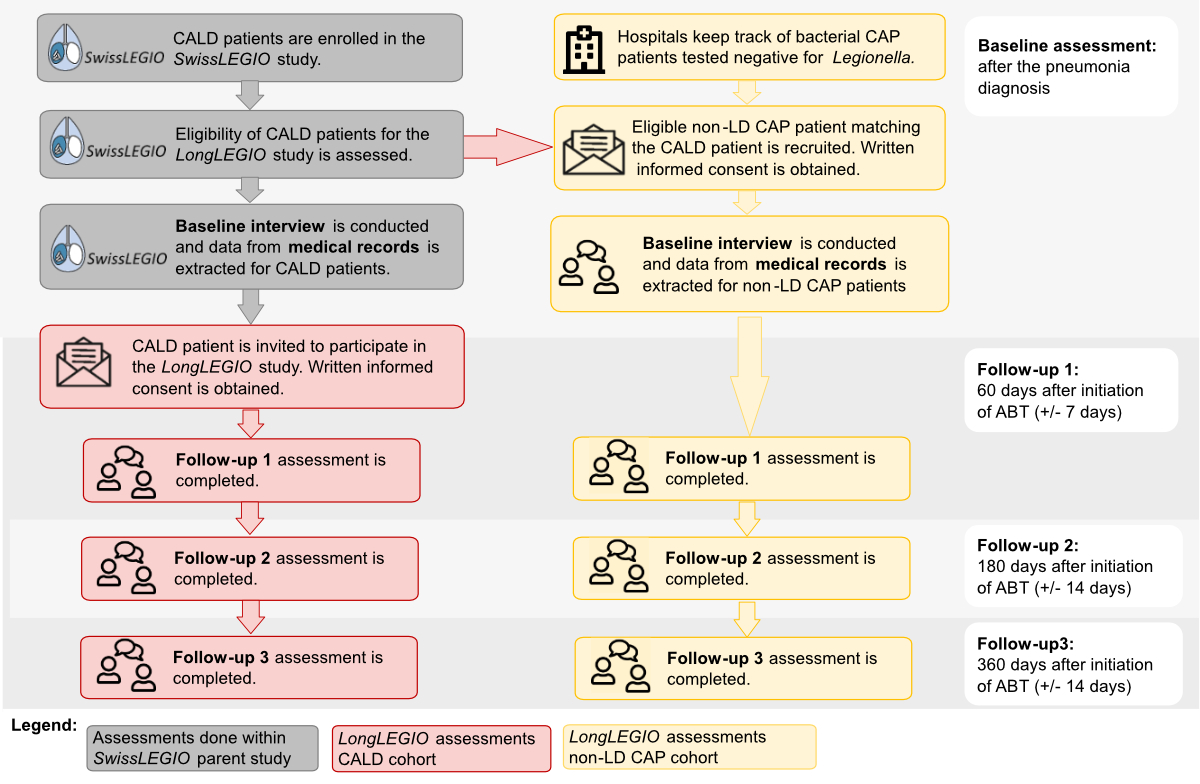
Figure 1Recruitment, enrolment and data
collection for the community-acquired Legionnaires’ disease (CALD) and Legionella
test-negative bacterial community-acquired pneumonia (non-LD CAP) cohorts. Grey:
Baseline interviews and data extraction from electronic medical records for
CALD patients were done within the context of the SwissLEGIO parent study. Red:
LongLEGIO recruitment and data collection for the CALD cohort. Patients were
recruited two months after the acute infection from a pool of CALD patients
previously included in the SwissLEGIO parent study. Yellow: LongLEGIO recruitment
and data collection for the non-LD CAP cohort. Non-LD CAP patients were
enrolled from four university and cantonal/regional hospitals shortly after the
pneumonia diagnosis. ABT: appropriate antibiotic therapy; CAP:
community-acquired pneumonia; LD: Legionnaires’ disease.
Recruitment of community-acquired Legionnaires’
disease patients
CALD patients were recruited from a
representative pool of Legionnaires’ disease patients who participated in the SwissLEGIO study [27]. The SwissLEGIO
study recruited 204 CALD patients between August 2021 and March 2024 from 20
university and cantonal hospitals across Switzerland, which jointly reported
about 45% of all CALD cases notified to the Swiss health authorities during
this period [27]. CALD patients, who previously participated in the SwissLEGIO study, were eligible to
participate in the LongLEGIO
study if they fulfilled the study criteria summarised in table 1. CALD patients
were invited to participate in the LongLEGIO
study by post or e-mail. Written informed consent was obtained from all CALD
patients prior to the two-month follow-up interview (figure 1).
Recruitment of Legionella test-negative bacterial community-acquired pneumonia patients
Non-LD
CAP patients were recruited through one university and
three cantonal hospitals. All four hospitals also participated in the SwissLEGIO parent study. During the
recruitment period, the hospitals kept track of all community-acquired
pneumonia patients with pneumonia of confirmed or clinically suspected
bacterial aetiology and who tested negative for Legionella spp. and COVID-19. The recruitment of the non-LD CAP patients
occurred as soon as possible after the patients were diagnosed with pneumonia (figure
1).
To ensure comparability, non-LD CAP patients
were matched to CALD patients based on sex, age (±5 years), hospital level
(university or cantonal hospital) and date of diagnosis (up to +60 days). The
recruitment of the non-LD CAP patients was triggered by the enrolment of a CALD
patient – eligible for the LongLEGIO
study – into the SwissLEGIO
parent study. In short, a recruitment request with the match criteria was sent
to the four partner hospitals. The eligible non-LD CAP patient with the closest
matching diagnosis date was recruited. Eligibility criteria for non-LD CAP
patients are summarised in table 1. Written informed consent was obtained
prior to the baseline interview.
Table 1Eligibility criteria for
participation in the LongLEGIO study for community-acquired Legionnaires’ disease
patients and Legionella
test-negative bacterial community-acquired pneumonia patients.
|
Inclusion criteria |
Exclusion criteria |
| Community-acquired Legionnaires’ disease |
Lives in
Switzerland and speaks German, French, Italian or English |
Suspected
hospital-acquired pneumonia |
| Aged ≥18
years |
Suspected
travel-associated pneumonia |
| Health status
permits provision of informed consent and participation in the study |
| No COVID-19
laboratory test was performed** |
| Laboratory-confirmed
COVID-19 infection at hospital admission |
| Clinical
signs and symptoms are suggestive of Legionnaires’ disease (confirmed
pneumonia*) |
| Laboratory-confirmed
Legionella spp. infection (urinary antigen
test, PCR or culture on sputum/ tracheobronchial aspirates)** |
| Non-Legionnaires’ disease community-acquired
pneumonia |
Lives in
Switzerland and speaks German, French, Italian or English |
Suspected
hospital-acquired pneumonia |
| Aged ≥18
years |
Suspected
travel-associated pneumonia |
| Health status
permits provision of informed consent and participation in the study |
| No COVID-19
laboratory test was performed** |
| Laboratory-confirmed
COVID-19 infection at hospital admission |
| Confirmed
pneumonia* |
| Confirmed or
suspected bacterial origin of pneumonia*** |
| Laboratory test
for Legionella spp. (urinary antigen
test, PCR or culture on sputum/ tracheobronchial aspirates) performed and
negative** |
Data collection
For the LongLEGIO study, the two cohorts of CALD patients and non-LD CAP
patients are followed up over 12 months. Assessments are done at four different
time points (figure 1). As soon as possible after the pneumonia diagnosis, a questionnaire-based
baseline interview is conducted and additional data are extracted from
electronic medical records for the current hospitalisation. Follow-up
assessments are conducted at 2, 6 and 12 months after patients have received
appropriate antibiotic therapy. All interviews are conducted in person or by a phone/video
call.
At baseline, the questionnaire consists of
closed and open questions related to patients’ pre-existing comorbidities,
their acute illness experience, the perceived disease severity and patients’
health-seeking (table 2). In
addition to the baseline interview, information on the patient’s medical
history, disease severity, the pneumonia-causing pathogen and the treatment was
extracted from electronic medical records.
The follow-up questionnaire focuses on patient-reported
outcome measures used in previous studies assessing recovery from pneumonia [7,
13, 33–35] and in studies on Long COVID [36–38]. The questionnaire consists of
closed and open questions to investigate (a) the patient’s perceived health,
recovery and health-related quality of life (HRQoL) after the acute pneumonia
episode, (b) the presence of persistent pneumonia-related symptoms, (c)
patients’ (potentially extended) health service utilisation and health-seeking
in the informal health sector after hospital discharge, and (d) potential
impacts of the infection on patients’ social and work life (table 2).
Table 2Content of the LongLEGIO
questionnaire.
| Baseline questionnaire |
| Patient characteristics |
Demographic information (e.g.
sex, age, education, income) |
| Illness experience and
health-seeking for acute pneumonia |
Pneumonia manifestation |
| Disease perception and
perceived severity of the pneumonia |
| Health-seeking prior to hospital
admission |
| Comorbidities and
health-related quality of life |
Comorbidities |
| Health-related quality of life
prior to pneumonia (EQ-5D-5L) [29] |
| Follow-up questionnaire |
| Perceived recovery from
pneumonia and health-related quality of life |
Subjective judgement on
overall health |
| Subjective assessment of the
degree of recovery from pneumonia |
| 5-item World Health
Organisation Well-being Index (WHO-5) [30] |
| Health-related quality of life
at the time of follow-up interview (EQ-5D-5L) [29] |
| Persistent symptoms |
Presence and perceived
severity of symptoms (extensive list of pneumonia symptoms reported in the
literature is provided). Patients are asked to judge whether symptoms are
related to the pneumonia. |
| Quantification of dyspnoea
(modified Medical Research Council [mMCR]) [31] |
| Fatigue assessment scale (FAS)
[32] |
| Worsening of pre-existing
comorbidities or newly diagnosed medical conditions |
| Healthcare utilisation and
patients’ health-seeking behaviour |
Quantification of formal
healthcare utilisation since the last assessment |
| Quantification of
health-seeking outside the formal health sector since the last assessment |
| Changes in prescribed / newly
prescribed medications |
| Patient experiences in
navigating the health system and perceived challenges |
| Social life and work
participation |
If persisting symptoms: impact
on work participation and social life |
Statistical analysis
Considering sample size, we assumed a 15%
prevalence of symptoms such as fatigue, weakness and a general reduction in
quality of life in the non-LD CAP group two months after the acute pneumonia
episode. We therefore aimed to recruit and complete the first follow-up
interview for 80 CALD patients and 80 non-LD CAP patients to detect a 20%
difference between our two cohorts with 80% power and alpha = 0.05. The
achieved sample size (59 CALD patients and 60 non-LD CAP patients) is
sufficient to provide a power of 70% with alpha = 0.05.
The statistical analyses are being conducted
according to the predefined Statistical Analysis Plan (SAP), available in the appendix. In summary, CALD and non-LD CAP patients were characterised at
baseline in terms of demographics, illness experience, help-seeking,
comorbidities and health-related quality of life. Continuous variables are
presented as medians with interquartile ranges (IQRs) and categorical variables
as n (%).
To assess long-term effects, we will
tabulate the prevalence of symptoms and impairments in the EQ-5D-5L dimensions
(mobility, self-care, usual activities, pain/discomfort and anxiety/depression)
as n (%). EQ-VAS scores will be reported as medians with IQRs at baseline (0
months), 2, 6 and 12 months. We will use bar charts and histograms to present the
distribution of symptoms and health-related quality of life measures over time
and to visualise differences between cohorts.
To estimate differences in the perceived
overall recovery and in the recovery of selected symptoms between CALD and
non-LD CAP, we will use non-parametric survival models. Differences in time to
recovery between groups will be compared using log-rank tests with survival
curves visualised using Kaplan-Meier estimates. Confounders such as selected
comorbidities and working status will be accounted for using inverse
probability weighting (IPW). Changes in health-related quality of life measures
are estimated using a linear or logistic mixed model with a random effect on
the individual patient. We will again correct for confounders.
Healthcare utilisation – including visits
to general practitioners, specialist physicians, emergency departments and
rehospitalisations – will be analysed across the entire follow-up period and
within each follow-up interval. We will assess both the proportion of patients
consulting healthcare services at least once for any reason and the proportion
of patients consulting healthcare services specifically due to pneumonia after
hospital discharge. Additionally, we will report the absolute number of
consultations.
Only participants who completed at least
the first follow-up interview will be included in the analysis. Missing data
will be imputed using logical rules. No further imputation for missing values
is foreseen.
All analyses will be conducted using R (version
4.4.1; R Core Team, Vienna, Austria).
Data management
Data are collected on standardised
electronic Case Report Forms (eCRF) using the data collection software Open
Data Kit (ODK, getodk.org). Forms are encrypted and all patient-identifying
information is removed. Individual forms are linked through unique subject
identification. Automated validations are implemented in the eCRF to check for
data completeness and plausibility and submitted forms are continuously checked
for plausibility and accuracy. Data are stored on a secured network drive
accessible only to authorised study personnel.
Ethical considerations
Ethical approval for the study was obtained
from the Ethics Commission of Northwestern and Central Switzerland (EKNZ
2023-00639) and the boards of the participating hospitals. This study is
conducted in accordance with the principles of Good Epidemiological Practice
and the Declaration of Helsinki. Data are stored in concordance with Swiss data
protection laws.
Study population
Enrolment
The recruitment timelines and the sample
size for the LongLEGIO study
were bound to the enrolment of CALD patients in the SwissLEGIO parent study. Overall, 86 CALD patients and 110
non-LD CAP patients were invited to participate in the LongLEGIO study between June
2023 and June 2024. In total, 59
CALD patients (enrolment rate of 69%) and 60 non-LD CAP patients (enrolment
rate of 55%) agreed to participate. There were no significant differences in age,
sex and
ICU admission rates between participants and non-participants among CALD
patients, and in sex and ICU admission rates among the patients with non-LD CAP
(appendix table S1). There was a difference in age between participants and
non-participants among the non-LD CAP patients (69 years vs 77 years).
Baseline characteristics
The baseline characteristics are summarised
in table 3. The median age for
both cohorts is 69 years; males comprised 59.3% of CALD and 63.3% of non-LD CAP
patients. Both cohorts have a similar comorbidity burden as measured by the
Charlson Comorbidity Index. Non-LD CAP patients, however, were more likely to have
chronic obstructive pulmonary disease (COPD) (20.0% vs 11.9%), malignancies
(33.3% vs 13.6%) and were more likely immunocompromised (25.0% vs 13.6%). In
contrast, more CALD than non-LD CAP patients had chronic kidney failure (15.3%
vs 10.0%). Patients in both cohorts exhibited similar health-seeking behaviour
prior to the hospital visit with the majority of patients initially consulting
a general practitioner. CALD patients were more frequently pre-treated with
antibiotics (30.4% vs 15.3%).
Table 3Baseline characteristics of 119
Legionnaires’ disease patients and non-Legionnaires’ disease bacterial community-acquired
pneumonia patients.
|
Community-acquired Legionnaires’ disease (n = 59) |
Non-Legionnaires’ disease community-acquired
pneumonia (n = 60) |
| Patient characteristics |
Male |
35 (59.3%) |
38 (63.3%) |
| Age, median [IQR] |
69 [57–80] |
69 [60–79] |
| Annual household
income |
<60,000 CHF |
15 (36.6%) |
21 (39.6%) |
| 60,000–100,000
CHF |
21 (51.2%) |
22 (41.5%) |
| >100,000 CHF |
5 (12.2%) |
10 (18.9%) |
| Body mass index in
kg/m2, median [IQR] |
24.0 [22.0–28.0] |
25.0 [21.0–28.0] |
| Medical conditions |
Comorbidities* |
Heart
disease |
21 (35.6%) |
25 (41.7%) |
| Heart
failure |
7 (11.9%) |
8 (13.3%) |
| Diabetes
mellitus |
7 (11.9%) |
10 (16.7%) |
| Chronic
obstructive pulmonary disease |
7 (11.9%) |
12 (20.0%) |
| Chronic
kidney failure |
9 (15.3%) |
6 (10.0%) |
| Cerebrovascular
disease |
5 (8.5%) |
6 (10.0%) |
| Malignancy
(haematological/solid organ) |
8 (13.6%) |
20 (33.3%) |
| Immunosuppression** |
8 (13.6%) |
15 (25.0%) |
| Charlson
Comorbidity Index, median [IQR] |
1 [0–2] |
2 [0–3] |
| Active smokers
(self-reported) |
20 (33.9%) |
13 (22.4%) |
| Health-seeking prior to hospital visit |
Medical advice
prior to admission |
None |
20 (34.5%) |
18 (30.5%) |
| General practitioner |
28 (48.3%) |
26 (44.1%) |
| Hospital/emergency
department |
2 (3.4%) |
6 (10.2%) |
| Specialist doctor |
4 (6.9%) |
5 (8.5%) |
| Caregiver/family
members/close friends |
7 (12.1%) |
6 (10.2%) |
| Antibiotics
prescribed prior to current pneumonia/ hospital admission |
None |
39 (66.1%) |
50 (83.3%) |
| Amoxicillin/clavulanic
acid |
15 (25.4%) |
6 (10.0%) |
| Cephalosporin |
1 (1.7%) |
0 (0.0%) |
| Combination
therapies*** |
1 (1.7%) |
1 (1.7%) |
| Prior antibiotic
treatment with unknown agent |
0 (0.0%) |
2 (3.3%) |
| Unknown if prior antibiotics |
3 (5.1%) |
1 (1.7%) |
| Time from symptom
onset to hospital admission in days, median [IQR] |
4 [2–6] |
5 [3–10] |
| Self-reported symptoms during the acute phase
of illness |
Fatigue/weakness |
55 (94.8%) |
49 (83.1%) |
| Fever |
50 (89.3%) |
45 (76.3%) |
| Cough |
38 (66.7%) |
48 (80.0%) |
| Shortness of
breath |
37 (66.1%) |
46 (78.0%) |
| Muscle aches |
29 (51.8%) |
15 (25.9%) |
| Headache |
26 (47.3%) |
16 (28.1%) |
| Chest pain |
20 (35.1%) |
28 (49.1%) |
| Confusion |
19 (33.3%) |
20 (35.1%) |
| Diarrhoea |
15 (26.3%) |
17 (28.8%) |
| Nausea/emesis |
7 (11.9%) |
2 (3.3%) |
| Loss of appetite |
5 (8.5%) |
4 (6.7%) |
| Self-rated
severity#, median [IQR] |
8 [7–9] |
7 [5–8] |
| Progression of pneumonia and severity |
Time from
hospital admission to start of adequate antibiotic treatment in days, median [IQR] |
0 [0–1] |
0 [0–0] |
| Length of
hospital stay in days, median [IQR] |
6 [4–9] |
6 [4–7] |
| Intensive care
unit admission |
8 (13.6%) |
5 (8.3%) |
| Non-invasive
ventilation |
7 (11.9%) |
12 (28.6%) |
| Invasive
ventilation |
4 (6.8%) |
1 (1.8%) |
| Discharge
followed by |
Outpatient care |
47 (81.0%) |
52 (86.7%) |
| Referral to
another hospital |
5 (8.6%) |
0 (0.0%) |
| Referral to a
rehabilitation centre |
6 (10.3%) |
8 (13.3%) |
| Quality of life (EQ-5D-5L)## |
Mobility |
14 (23.7%) |
22 (36.7%) |
| Self-care |
2 (3.4%) |
3 (5.0%) |
| Usual activities |
13 (22.0%) |
20 (33.3%) |
| Pain/discomfort |
20 (34.5%) |
26 (43.3%) |
| Anxiety/depression |
20 (35.1%) |
20 (33.3%) |
| EQ-VAS score, median [IQR] |
85 [75–90] |
70 [50–85] |
During the acute phase of their pneumonia,
non-LD CAP patients more frequently reported respiratory symptoms including
cough (80.0% vs 66.7%), shortness of breath (78.0% vs 66.1%) and chest pain
(49.1% vs 35.1%). On the contrary, CALD patients reported more frequent
extrapulmonary symptoms such as fever (89.3% vs 76.3%), muscle aches (51.8% vs
25.9%), headaches (47.3% vs 28.1%), and nausea or emesis (11.9% vs 3.3%). The
ICU admission rate and the proportion of patients who needed invasive
ventilation were higher for CALD than for non-LD CAP patients (13.6% vs 8.3%
and 6.8% vs 1.8%, respectively).
Finally, participants were asked to report
on their perceived health-related quality of life prior to the onset of
pneumonia symptoms. We used the standardised EQ-5D-5L questionnaire which
measures health-related quality of life across five dimensions (mobility,
self-care, usual activities, pain/discomfort and anxiety/depression). In
addition, patients are also asked to rate their overall health on a scale from
0 (lowest possible score) to 100 (EQ-VAS score) [29]. In general, non-LD CAP
patients reported lower EQ-VAS scores compared to CALD patients. Differences in
the reported health-related quality of life were observed for the dimensions
mobility, daily activity and pain/discomfort (table 3, appendix table S2).
For non-LD CAP patients, Streptococcus pneumoniae and Haemophilus influenzae were each
identified as the causative pathogen in 10.0% of the patients. Mycoplasma pneumoniae was the causative pathogen in 5.0% of
patients. Viral-bacterial pneumonia with bacterial superinfection was recorded
for 3.3% of non-LD CAP patients. No pathogen was detected in 73.3% of non-LD
CAP patients (appendix table S2).
Discussion
Acute infections can cause persistent health
problems. Although post-acute infection syndromes have been recognised for some
time, they have often been neglected and underreported, with few exceptions
like Q fever [15]. The COVID-19 pandemic, however, brought renewed attention to
this issue, when long-term health and wellbeing impairments were observed in a
significant subset of patients months or even years after the acute SARS-CoV-2
infection [36–38]. Similarly, persistent health impairments have been reported
for community-acquired pneumonia [8–13, 17]. However, the role of the
infection-causing pathogen in the persistence of community-acquired pneumonia
symptoms and sequelae in general remains unclear, and data on the long-term
health effects of Legionnaires’ disease are scarce. Here, we described the
rationale and design of a matched cohort study to investigate persistent
sequelae of community-acquired Legionnaires’ disease (CALD) and compare them
with manifestations of persistent pneumonia in non-LD CAP patients. We also
presented the baseline characteristics of our two study cohorts, which are
representative of their respective patient populations.
Previous studies on persistent sequelae of community-acquired
pneumonia have rarely stratified analyses by the different pneumonia-causing
pathogens [8–10, 12, 13]. Of those that have, the focus has been on Streptococcus pneumoniae [11, 17]. This limited stratification
was mainly due to sample size limitations and a lack of information on the community-acquired
pneumonia-causing pathogens. In contrast, studies examining persistent sequelae of
Legionella infections did not include
pneumonia patients as a comparison group [18, 26]. To our knowledge, the LongLEGIO
study is the first study that systematically compares the
persistent health effects of CALD with the persistent manifestations of non-LD
CAP, going beyond a survival analysis based on mortality data [39]. Such a comparison
is crucial to contextualise the frequency of
observed health and wellbeing impairments in CALD patients against the
background occurrence in a suitable control group. Moreover, this approach will
allow us to explore how the persistence of community-acquired pneumonia-related
health impairments is linked to the pathogenic mechanisms of the causative
bacteria. Ultimately, this may improve our understanding of the underlying
pathogenesis of persistent pneumonia manifestations and post-acute infection
syndromes [15, 16].
The LongLEGIO
study prioritised patient-reported outcome measures, rather than focusing on
mortality or hospital readmission rates which have been the primary outcomes in
previous large studies on Legionella
that relied on medical record data [39, 40]. Such patient-reported outcome
measures provide robust measures of persistent symptoms and functional
impairments that patients experience [7]. Persistent symptoms and functional
impairments, as measured by patient-reported outcome measures, are also one of
the main reasons for additional general practitioner consultations and
emergency department visits after the acute phase of pneumonia [4, 5]. Patient-reported
outcome measures, however, are usually not well documented in medical records
and hence can only be adequately captured by applying prospective study designs
[15, 16].
The LongLEGIO
study is also the first study to assess healthcare utilisation among
CALD patients beyond the acute infection and its association with persistent
sequelae of CALD. As demonstrated by the COVID-19 pandemic and the rise of Long
COVID, post-acute infection syndromes can significantly contribute to the
burden of infectious diseases [16, 37]. However, current estimates of the
burden of Legionnaires’ disease do not account for persistent health impairment
or the resulting increased medical costs [41, 42]. By examining both persistent
sequelae of CALD and additional healthcare utilisation after the acute
infection, the LongLEGIO study
will provide data for a better disease burden estimate for Legionnaires’
disease in the future. This, in turn, may help public health specialists and
physicians to plan and anticipate the health care resources needed for the
holistic treatment of CALD beyond the acute phase of the disease.
A total of 59 CALD patients and 60 non-LD
CAP patients were enrolled between June 2023 and June 2024. Our non-LD CAP and
CALD patient cohorts appear representative of the two patient populations. Similarly
to what is reported in previous studies, Legionnaires’ disease patients were
more likely to suffer from extrapulmonary symptoms, were more frequently
pre-treated with antibiotics before hospital admission and were more often
admitted to the ICU [21–23, 43]. Non-LD CAP patients, on the other hand, were
more likely to suffer from chronic obstructive pulmonary disease,
immunosuppression and malignancies, which is also consistent with findings from
previous research [23, 43]. Finally, we also observed differences in the
self-reported quality of life scores (lower scores were reported by non-LD CAP
patients). Both comorbidities and pneumonia severity may influence the
long-term health outcomes after pneumonia [4, 6, 11]. We will therefore correct
for these differences in our future analyses of long-term outcomes. For the
self-reported quality of life score, each patient will serve as their control
and relative rather than absolute changes in reported scores from the
pre-pneumonia baseline score will be assessed and compared between groups.
The LongLEGIO
study has limitations. First, the study may be subject to recall bias,
especially between the 6- and 12-month follow-up points. We try to mitigate
this recall bias by specifically probing for both intermittent and current
symptoms. In addition, interviewers are instructed to systematically probe for
all the symptoms and health service consultations that were reported by the patient
in previous interviews. Second, despite the integration of the LongLEGIO study into
the national SwissLEGIO parent study, our sample
size for CALD remains relatively small. It is therefore possible that we
underestimate functional impairments that are relatively rare although we are
confident of fully capturing the common persistent sequelae of CALD. Finally,
the study compared only patients surviving hospitalisation, restricting the
population of CALD and non-LD CAP.
In summary, the LongLEGIO study explores and compares
persistent health and wellbeing impacts of CALD and non-LD CAP in two
representative cohorts. The study aims to contribute to ongoing research on
post-acute infection syndromes, a phenomenon long recognised and
well-documented for conditions like Q fever but often challenging to capture in
daily medical practice [15]. Here, we presented the rationale and study design of
the LongLEGIO study and characterised the
two study cohorts. Results of the long-term follow-up
of these two cohorts will be reported in subsequent publications.
Availability of data and material
The datasets used and/or analysed during
the current study are available from the corresponding author on reasonable
request.
Acknowledgments
We thank Jan Hattendorf for his advice on the
design and development of the study protocol. At the Federal Office of Public
Health, we would like to thank Ornella Luminati and Monica Wymann for the
exchanges surrounding this study. We thank Katia Sidler and Sereina Livia
Müller for their assistance with patient recruitment at the University Hospital
of Basel. We thank Chiara Strozzi, Florian Zacher, Helena Greter, Cornelia
Speich and Céline Fürer for their support in the data collection.
Prof. Dr. Daniel
Mäusezahl
Department
of Epidemiology and Public Health
Swiss Tropical and Public Health Institute
Kreuzstrasse
2
CH-4123
Allschwil
daniel.maeusezahl[at]unibas.ch
References
1. Bender RG, Sirota SB, Swetschinski LR, et al.; GBD 2021 Lower Respiratory Infections
and Antimicrobial Resistance Collaborators. Global, regional, and national incidence
and mortality burden of non-COVID-19 lower respiratory infections and aetiologies,
1990-2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet
Infect Dis. 2024 Sep;24(9):974–1002. doi: https://doi.org/10.1016/S1473-3099(24)00176-2
2. World Health Organization. Global Health Estimates. Available from: https://www.who.int/data/global-health-estimates
3. Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired
pneumonia among adults in Europe. Thorax. 2012 Jan;67(1):71–9. doi: https://doi.org/10.1136/thx.2009.129502
4. Adamuz J, Viasus D, Campreciós-Rodríguez P, Cañavate-Jurado O, Jiménez-Martínez E,
Isla P, et al. A prospective cohort study of healthcare visits and rehospitalizations
after discharge of patients with community-acquired pneumonia. Respirology. 2011 Oct;16(7):1119–26.
doi: https://doi.org/10.1111/j.1440-1843.2011.02017.x
5. Daniel P, Bewick T, McKeever TM, Roberts M, Ashton D, Smith D, et al. Healthcare reconsultation
in working-age adults following hospitalisation for community-acquired pneumonia.
Clin Med (Lond). 2018 Feb;18(1):41–6. doi: https://doi.org/10.7861/clinmedicine.18-1-41
6. Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI. Community-acquired pneumonia.
Lancet. 2021 Sep;398(10303):906–19. doi: https://doi.org/10.1016/S0140-6736(21)00630-9
7. Pick HJ, Bolton CE, Lim WS, McKeever TM. Patient-reported outcome measures in the
recovery of adults hospitalised with community-acquired pneumonia: a systematic review.
Eur Respir J. 2019 Mar;53(3):1802165. doi: https://doi.org/10.1183/13993003.02165-2018
8. Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, et
al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular
disease. JAMA. 2015 Jan;313(3):264–74. doi: https://doi.org/10.1001/jama.2014.18229
9. Davydow DS, Hough CL, Levine DA, Langa KM, Iwashyna TJ. Functional disability, cognitive
impairment, and depression after hospitalization for pneumonia. Am J Med. 2013 Jul;126(7):615–24.e5.
doi: https://doi.org/10.1016/j.amjmed.2012.12.006
10. Chalitsios CV, Baskaran V, Harwood RH, Lim WS, McKeever TM. Incidence of cognitive
impairment and dementia after hospitalisation for pneumonia: a UK population-based
matched cohort study. ERJ Open Res. 2023 May;9(3):00328–02022. doi: https://doi.org/10.1183/23120541.00328-2022
11. El Moussaoui R, Opmeer BC, de Borgie CA, Nieuwkerk P, Bossuyt PM, Speelman P, et al. Long-term
symptom recovery and health-related quality of life in patients with mild-to-moderate-severe
community-acquired pneumonia. Chest. 2006 Oct;130(4):1165–72. doi: https://doi.org/10.1378/chest.130.4.1165
12. Metlay JP, Fine MJ, Schulz R, Marrie TJ, Coley CM, Kapoor WN, et al. Measuring symptomatic
and functional recovery in patients with community-acquired pneumonia. J Gen Intern
Med. 1997 Jul;12(7):423–30. doi: https://doi.org/10.1046/j.1525-1497.1997.00074.x
13. Glick HA, Miyazaki T, Hirano K, Gonzalez E, Jodar L, Gessner BD, et al. One-Year Quality
of Life Post-Pneumonia Diagnosis in Japanese Adults. Clin Infect Dis. 2021 Jul;73(2):283–90.
doi: https://doi.org/10.1093/cid/ciaa595
14. Dang TT, Eurich DT, Weir DL, Marrie TJ, Majumdar SR. Rates and risk factors for recurrent
pneumonia in patients hospitalized with community-acquired pneumonia: population-based
prospective cohort study with 5 years of follow-up. Clin Infect Dis. 2014 Jul;59(1):74–80.
doi: https://doi.org/10.1093/cid/ciu247
15. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes.
Nat Med. 2022 May;28(5):911–23. doi: https://doi.org/10.1038/s41591-022-01810-6
16. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms
and recommendations. Nat Rev Microbiol. 2023 Mar;21(3):133–46. doi: https://doi.org/10.1038/s41579-022-00846-2
17. Kruckow KL, Zhao K, Bowdish DM, Orihuela CJ. Acute organ injury and long-term sequelae
of severe pneumococcal infections. Pneumonia. 2023 Mar;15(1):5. doi: https://doi.org/10.1186/s41479-023-00110-y
18. Lettinga KD, Verbon A, Nieuwkerk PT, Jonkers RE, Gersons BP, Prins JM, et al. Health-related
quality of life and posttraumatic stress disorder among survivors of an outbreak of
Legionnaires disease. Clin Infect Dis. 2002 Jul;35(1):11–7. doi: https://doi.org/10.1086/340738
19. Marrie TJ, Beecroft M, Herman-Gnjidic Z, Poulin-Costello M. Symptom resolution in
patients with Mycoplasma pneumoniae pneumonia. Can Respir J. 2004;11(8):573–7. doi: https://doi.org/10.1155/2004/659187
20. Graham FF, Finn N, White P, Hales S, Baker MG. Global Perspective of Legionella Infection in Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis
of Observational Studies. Int J Environ Res Public Health. 2022 Feb;19(3):1907. doi: https://doi.org/10.3390/ijerph19031907
21. Roed T, Schønheyder HC, Nielsen H. Predictors of positive or negative legionella urinary
antigen test in community-acquired pneumonia. Infect Dis (Lond). 2015 Jul;47(7):484–90.
doi: https://doi.org/10.3109/23744235.2015.1021830
22. Viasus D, Di Yacovo S, Garcia-Vidal C, Verdaguer R, Manresa F, Dorca J, et al. Community-acquired
Legionella pneumophila pneumonia: a single-center experience with 214 hospitalized
sporadic cases over 15 years. Medicine (Baltimore). 2013 Jan;92(1):51–60. doi: https://doi.org/10.1097/MD.0b013e31827f6104
23. Allgaier J, Lagu T, Haessler S, Imrey PB, Deshpande A, Guo N, et al. Risk Factors,
Management, and Outcomes of Legionella Pneumonia in a Large, Nationally Representative
Sample. Chest. 2021 May;159(5):1782–92. doi: https://doi.org/10.1016/j.chest.2020.12.013
24. Cunha BA, Burillo A, Bouza E. Legionnaires’ disease. Lancet. 2016 Jan;387(10016):376–85.
doi: https://doi.org/10.1016/S0140-6736(15)60078-2
25. Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet
Infect Dis. 2005 Apr;5(4):219–26. doi: https://doi.org/10.1016/S1473-3099(05)70052-9
26. van Loenhout JA, van Tiel HH, van den Heuvel J, Vercoulen JH, Bor H, van der Velden K,
et al. Serious long-term health consequences of Q-fever and Legionnaires’ disease.
J Infect. 2014 Jun;68(6):527–33. doi: https://doi.org/10.1016/j.jinf.2014.01.004
27. Fischer FB, Bigler M, Mäusezahl D, Hattendorf J, Egli A, Julian TR, et al.; SwissLEGIO
Hospital Network. Legionnaires’ disease in Switzerland: rationale and study protocol
of a prospective national case-control and molecular source attribution study (SwissLEGIO).
Infection. 2023 Oct;51(5):1467–79. doi: https://doi.org/10.1007/s15010-023-02014-x
28. Schweizerische Gesellschaft für Infektiologie. SGInf-Guidelines: Ambulant-erworbene
Pneumonie CAP. Available from: https://ssi.guidelines.ch/guideline/3007/de
29. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary
testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011 Dec;20(10):1727–36.
doi: https://doi.org/10.1007/s11136-011-9903-x
30. World Health Organization. Regional Office for Europe. Wellbeing measures in primary
health care/the DepCare Project: report on a WHO meeting: Stockholm, Sweden, 12–13 February 1998.
Copenhagen: World Health Organization. Regional Office for Europe 1998. Contract No.:
WHO/EURO:1998-4234-43993-62027. Available from: https://iris.who.int/handle/10665/349766
31. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988 Mar;93(3):580–6.
doi: https://doi.org/10.1378/chest.93.3.580
32. Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated
fatigue measure: The Fatigue Assessment Scale. J Psychosom Res. 2003 Apr;54(4):345–52.
doi: https://doi.org/10.1016/S0022-3999(02)00392-6
33. El Moussaoui R, Opmeer BC, Bossuyt PM, Speelman P, de Borgie CA, Prins JM. Development
and validation of a short questionnaire in community acquired pneumonia. Thorax. 2004 Jul;59(7):591–5.
doi: https://doi.org/10.1136/thx.2003.015107
34. Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden
and time for recovery from community-acquired pneumonia reported by older adults surveyed
nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas.
2015 Jul;6:215–23.
35. Lamping DL, Schroter S, Marquis P, Marrel A, Duprat-Lomon I, Sagnier PP. The community-acquired
pneumonia symptom questionnaire: a new, patient-based outcome measure to evaluate
symptoms in patients with community-acquired pneumonia. Chest. 2002 Sep;122(3):920–9.
doi: https://doi.org/10.1378/chest.122.3.920
36. Horn A, Krist L, Lieb W, Montellano FA, Kohls M, Haas K, et al. Long-term health sequelae
and quality of life at least 6 months after infection with SARS-CoV-2: design and
rationale of the COVIDOM-study as part of the NAPKON population-based cohort platform
(POP). Infection. 2021 Dec;49(6):1277–87. doi: https://doi.org/10.1007/s15010-021-01707-5
37. Menges D, Ballouz T, Anagnostopoulos A, Aschmann HE, Domenghino A, Fehr JS, et al. Burden
of post-COVID-19 syndrome and implications for healthcare service planning: A population-based
cohort study. PLoS One. 2021 Jul;16(7):e0254523. doi: https://doi.org/10.1371/journal.pone.0254523
38. Zhang H, Huang C, Gu X, Wang Y, Li X, Liu M, et al. 3-year outcomes of discharged
survivors of COVID-19 following the SARS-CoV-2 omicron (B.1.1.529) wave in 2022 in
China: a longitudinal cohort study. Lancet Respir Med. 2024 Jan;12(1):55–66. doi: https://doi.org/10.1016/S2213-2600(23)00387-9
39. Serrano L, Ruiz LA, Perez-Fernandez S, España PP, Gomez A, Gonzalez B, et al. Short-
and long-term prognosis of patients with community-acquired Legionella or pneumococcal
pneumonia diagnosed by urinary antigen testing. Int J Infect Dis. 2023 Sep;134:106–13.
doi: https://doi.org/10.1016/j.ijid.2023.05.065
40. Gamage SD, Ross N, Kralovic SM, Simbartl LA, Roselle GA, Berkelman RL, et al. Health
after Legionnaires’ disease: A description of hospitalizations up to 5 years after
Legionella pneumonia. PLoS One. 2021 Jan;16(1):e0245262. doi: https://doi.org/10.1371/journal.pone.0245262
41. Cassini A, Colzani E, Pini A, Mangen MJ, Plass D, McDonald SA, et al.; BCoDE consortium.
Impact of infectious diseases on population health using incidence-based disability-adjusted
life years (DALYs): results from the Burden of Communicable Diseases in Europe study,
European Union and European Economic Area countries, 2009 to 2013. Euro Surveill.
2018 Apr;23(16):17–00454. doi: https://doi.org/10.2807/1560-7917.ES.2018.23.16.17-00454
42. Collier SA, Deng L, Adam EA, Benedict KM, Beshearse EM, Blackstock AJ, et al. Estimate
of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United
States. Emerg Infect Dis. 2021 Jan;27(1):140–9. doi: https://doi.org/10.3201/eid2701.190676
43. Bolliger R, Neeser O, Merker M, Vukajlovic T, Felder L, Fiumefreddo R, et al. Validation
of a Prediction Rule for Legionella Pneumonia in Emergency Department Patients. Open Forum Infect Dis. 2019 Jun;6(7):ofz268.
doi: https://doi.org/10.1093/ofid/ofz268
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4333.
