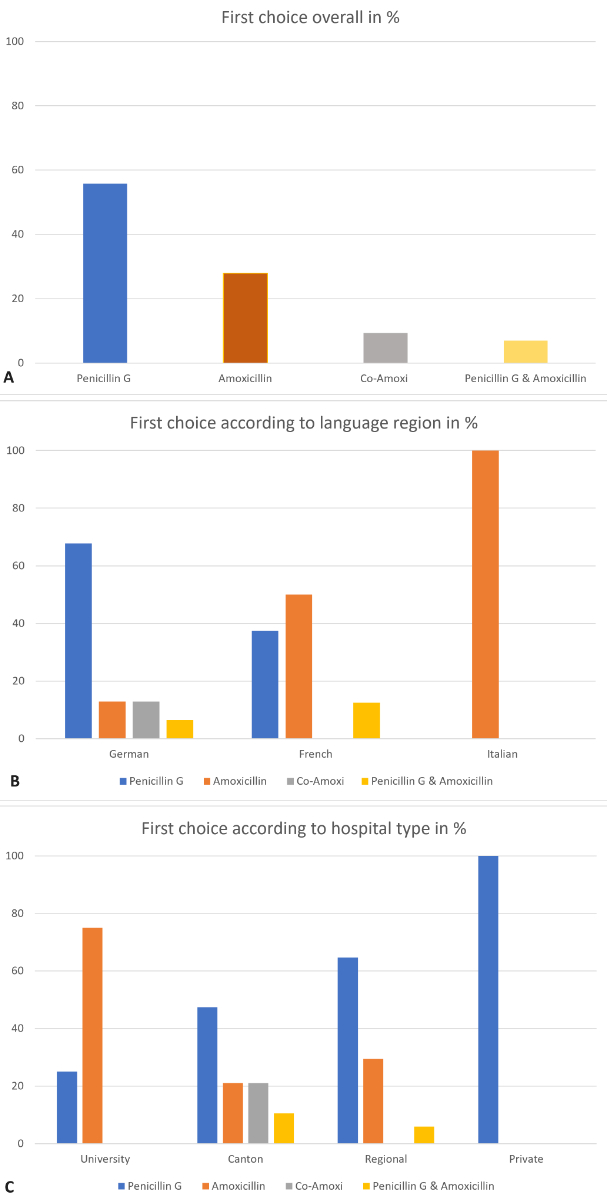
Figure 1Choice of antibiotics: (A) First-choice antibiotics in Switzerland overall; (B) First-choice antibiotics by language region; (C) First-choice antibiotics by hospital type. Data is expressed as percentages.
DOI: https://doi.org/https://doi.org/10.57187/s.4297
Association of the Scientific Medical Societies in Germany
Antimicrobial Stewardship Programme
National Institute for Health and Care Excellence
Paediatric Infectious Disease Group Switzerland
Royal College of Obstetricians and Gynaecologists
Swiss Society of Gynaecology and Obstetrics
Strategy on Antibiotic Resistance
Streptococcus agalactiae is a pathogen associated with maternal and neonatal sepsis, neonatal meningitis, intrauterine foetal death and preterm birth. In Western Europe, the prevalence of Streptococcus agalacticae-positive mothers is estimated at 19.5% (CI 13.9–25.1) [1]. A single-centre 2011 study in French-speaking Switzerland detected a 16.3 % prevalence of Streptococcus agalacticae-positive mothers [2].
In 2015, an estimated 90,000 infants aged under three months died from Streptococcus agalacticae worldwide, and another 10,000 were physically or mentally impaired. Up to 3.5 million preterm births globally are linked to Streptococcus agalacticae infection [3]. The World Health Organization (WHO) called for a surveillance and prevention programme for meningitis and its organic causes, such as Streptococcus agalacticae, in its 2020 strategy [4]. Despite guidelines from Swiss professional societies to reduce neonatal sepsis, there is no national surveillance system for peripartal Streptococcus agalacticae infections and therefore the current prevalence among mothers and neonates is unknown. One multicentre study in Switzerland conducted in ten tertiary hospitals prospectively investigated the incidence of sepsis among infants aged below 1 year between 2011 and 2015. Among the 539 diagnosed episodes of neonatal sepsis, 14% were caused by Streptococcus agalacticae and therefore this pathogen was the third most common pathogen causing neonatal sepsis. A third of cases of neonatal sepsis caused by Streptococcus agalacticae were early-onset sepsis. The incidence of Streptococcus agalacticae early-onset sepsis in Switzerland has been estimated at 0.10/1000 livebirths [5].
One measure to reduce the risk of neonatal infection is intrapartum antibiotic prophylaxis, administered to Streptococcus agalacticae-positive mothers during labour [6]. Various national treatment guidelines exist in Switzerland, developed by medical societies or within the hospital. The Swiss Society for Gynaecology and Obstetrics (SGGG) published expert recommendations for intrapartum antibiotic prophylaxis in 2012 [7], followed by a recommendation from the Swiss Society of Neonatology in collaboration with the Paediatric Infectious Disease Group Switzerland (PIGS) in 2013 [8]. Both societies renewed their recommendations in 2024 [9, 10]. Already in 2015, the Federal Council stated that these guidelines are not officially binding and there is no control mechanism over their implementation [11]. Giannoni et al. revealed in their 2016 study that only 48% of the Streptococcus agalacticae-positive mothers of infected neonates with early-onset sepsis or late-onset sepsis had received intrapartum antibiotic prophylaxis. For 12% of the neonates, the mother’s Streptococcus agalacticae status was unknown [5]. Although the number of included cases was small (74), the data underlines the non-binding nature of the guidelines. Nevertheless, the reason why only half of the Streptococcus agalacticae-positive mothers had received intrapartum antibiotic prophylaxis remains unknown. While there is data in high-income countries regarding the antibiotic exposure of neonates in early days of life [12], data on intrapartum antibiotic rates does not exist in Switzerland, or at least is not collected in a systematic way.
Frequent and improper use of antibiotics promotes the development of antimicrobial resistance. Resistant microorganisms can spread between humans, animals and the environment through direct contact, contaminated food and environmental sources. Globalisation has expanded transmission routes worldwide [13]. Streptococcus agalacticae poses a risk not only to pregnant women and neonates but also increasingly to older individuals. Antibiotic resistance limits treatment options for everyone [14]. In 2019 it was estimated that antimicrobial resistance was associated with 4.95 million deaths globally. Escherichia coli is responsible for most antimicrobial resistance-related deaths, and it also plays a role in neonatal sepsis. Streptococcus agalacticae accounts for 100,000–250,000 antimicrobial resistance-related deaths globally, ranking 10th among pathogens associated with antimicrobial resistance mortality [15]. The WHO listed penicillin-resistant group B streptococcus as a medium-priority pathogen in their WHO Bacterial Priority Pathogens List [16]. Although especially risky for low-resource settings, other settings must keep it in mind in their policy making.
If antimicrobial resistance rates continue to rise, treatment costs for resistant infections in OECD and EU/EFTA countries could reach USD 28.9 billion annually by 2050. Switzerland is expected to bear one of the highest shares of these costs if strategies are not optimised. Countries in the OECD and EU/EFTA could face severe socioeconomic consequences, with an estimated loss of over 734,000 full-time jobs annually due to mortality and morbidity caused by antimicrobial resistance [17]. The United Nations General Assembly once again highlighted the need for joint action against antimicrobial resistance at their High-Level Meeting on antimicrobial resistance in September 2024 [18]. The need for changes in antibiotic use in Switzerland is not given the attention it deserves by the target group in human medicine. Often, resources such as personnel, finances and technology to implement measures like Antimicrobial Stewardship Programmes (ASP) are lacking [19]. Switzerland has started a programme to enforce ASP in hospital settings within its Strategy on Antibiotic Resistance (StAR) [20].
Taking the above considerations into account, a national strategy for intrapartum antibiotic prophylaxis in obstetrics is sensible. The aim of this study was to answer the following questions: “What do the internal hospital protocols regarding the intrapartum antibiotic prophylaxis management of Streptococcus agalacticae-positive mothers in Switzerland recommend? What literature are they based on, and how are they established?” These questions specifically address the choice of antibiotic, the referenced literature and the professions involved in the development of the internal hospital protocols. The aim is to provide an initial overview of the nationwide practice, which can serve as guidance for healthcare professionals and policy makers.
We conducted a cross-sectional study, comparing internal hospital protocols in obstetric wards in Switzerland to investigate and describe the frequency of antibiotic types used to prevent neonatal sepsis, the referenced literature supporting their protocols and the professionals involved in their development. Information was extracted from the submitted protocols, and results were presented quantitively.
All obstetric wards in Swiss hospitals were identified via the website “Geburtsspitäler und Kliniken in der Schweiz (n.d.)” and updated manually and listed in an Excel sheet. The classification of hospitals was based on Federal Office of Public Health criteria [22]. In case of uncertainties, the hospital’s website was consulted.
From March to April 2024, obstetric wards were contacted via email with a standardised letter (see appendix) in three national languages requesting their currently valid internal protocols on intrapartum antibiotic prophylaxis for neonatal sepsis. These protocols were subsequently screened for the following primary outcome:
and the following secondary outcomes:
If no email address was available or no response was received, departments were contacted by phone. The researchers also used personal contacts and their professional network.
Participation was voluntary, and submission of protocols implied consent. Anonymity was preserved, and data was protected from unauthorised access. No additional, uncommunicated analyses were conducted. Since no direct personal data was collected, ethical approval was not required.
Categorisation within the outcomes was done deductively and adapted inductively while reviewing the received protocols. For the “Choice of antibiotics” outcome, categorisation was based on antibiotics recommended as 1st and 2nd line alternatives by (inter)national guidelines for intrapartum antibiotic prophylaxis of neonatal sepsis, such as those by SGGG [7], Swiss Neonatology and PIGS [8] and the National Institute for Health and Care Excellence (NICE) [23]. For the “Referenced literature” outcome, categorisation was first defined deductively by the researchers based on guidelines of professional societies such as Collège national des gynécologues et obstétriciens français (CNOFG), NICE, Expert Letter by the SGGG, Swiss Neonatology and PIGS. While extracting data from received protocols, the list was extended inductively with guidelines of the following societies: the American College of Obstetricians and Gynecologists (ACOG), the Association of the Scientific Medical Societies in Germany (AWMF) and the Royal College of Obstetricians & Gynaecologists (RCOG). “Referencing other hospitals” and “Other literature” were added for further completion. Other literature was defined as inclusion of reviews, meta-analysis or other studies. For the categorisation of professions involved, we followed the recommendations of the Swiss ASP working group, namely gynaecologists, midwives, nurses, neonatologists, pharmacists and infectious disease specialists [24]. These categories were broken down by hospital type (university, cantonal, regional, private) and language region (German, French, Italian).
Data was extracted from the internal protocols and entered into an Excel sheet, both numerically and binary: First it was determined whether they included information on the investigated outcome or not (category present or not). Further analysis of the outcome proceeded only with the protocols that included this information and frequency was analysed in relation to the total number of protocols that contained information on that outcome. Regarding the secondary outcomes, where multiple answers per participating wards are possible, the total count of each possible answer was divided by the total number of protocols with the respective outcome, resulting in the proportion of each answer relative to the total number of protocols stating it. Another researcher double-checked data entry and calculation. Inconsistencies were subsequently discussed and resolved.
A total of 87 obstetric wards in hospitals across Switzerland were identified and contacted. By mid-April 2024, 49% (n = 43) of these wards had submitted their internal protocols for analysis. Of these, 72% (n = 31) were German-speaking, 19% (n = 8) were French-speaking and 9% (n = 4) were Italian-speaking (see table 1). Most of the protocols were sent by cantonal and regional hospitals (44%, n = 19, and 40%, n = 17, respectively). The internal protocols submitted by 7 obstetric wards were undergoing revision at the time of analysis. For those wards, the internal protocols valid at the time of protocol submission were included.
Compared to all identified and invited obstetric wards, the study population has a similar distribution of language region. Regarding the hospital type, cantonal hospitals are overrepresented with 44% vs 22% of the distribution, and private clinics and regional hospitals are underrepresented with 7% vs 22% and 40% vs 51%, respectively. With 19 of 19 cantonal wards, this hospital type has been fully covered. With the included hospitals, we covered approximately 65% of all births in the clinical setting in Switzerland.
Table 1Characteristics of obstetric wards. Reference birth statistics from: BAG, B. für G. (n.d.). Qualitätsindikatoren Fallzahl. Retrieved on 28 January 2025, from https://www.bag.admin.ch/bag/de/home/zahlen-und-statistiken/zahlen-fakten-zu-spitaelern/qualitaetsindikatoren-der-schweizer-akutspitaeler/qualitaetsindikatoren-fallzahl.html.
| Total identified | Total included | |||||
| # | % | # | % | |||
| Distribution of obstetric wards | 87 | 100% | 43 | 100% | ||
| Language region | German | 62 | 71% | 31 | 72% | |
| French | 20 | 23% | 8 | 19% | ||
| Italian | 5 | 6% | 4 | 9% | ||
| Hospital type | University | 5 | 6% | 4 | 9% | |
| Cantonal | 19 | 22% | 19 | 44% | ||
| Regional | 44 | 51% | 17 | 40% | ||
| Private | 19 | 22% | 3 | 7% | ||
| Distribution of births | Total clinical births* | 77850 | 100% | |||
| Total births covered by study** | 50680 | 65% | ||||
* Births taking place in the obstetric wards in Switzerland still operating in 2024 (contacted for participation), based on data from 2022.
** Births covered by obstetric wards in Switzerland participating in the study, based on data from 2022.
All submitted protocols (n = 43) offer at least two different antibiotics as options. Three β-lactam antibiotics were identified as the first choice: penicillin G (56%, n = 24), amoxicillin (28 %, n = 12) and co-amoxicillin (9%, n = 4). In 3 cases (7%), both penicillin G and amoxicillin were listed as the first choice, depending on availability (see figure 1A). In predominantly German-speaking obstetric wards, penicillin G is mostly used (68% of n = 31), while in Italian-speaking departments, amoxicillin is dominant (100% of n = 4). In French-speaking departments, penicillin G and amoxicillin are equally used (n = 4 amoxicillin, n = 3 penicillin G, n = 1 either amoxicillin or penicillin G) (see figure 1B). Regional, cantonal and private hospitals mainly use penicillin G as the first choice (65% of n = 17, 47% of n = 19 and 100% of n = 3, respectively), while university hospitals prefer amoxicillin (75% of n = 3). Co-amoxicillin is used in 4 German-speaking cantonal hospitals (see figure 1C).

Figure 1Choice of antibiotics: (A) First-choice antibiotics in Switzerland overall; (B) First-choice antibiotics by language region; (C) First-choice antibiotics by hospital type. Data is expressed as percentages.
Second-choice antibiotics were identified for use in cases of known or suspected allergies to the first-choice antibiotics. In 19 % of the protocols (n = 8), there was no distinction between mild or severe allergies to penicillin derivatives; all these protocols belong to German- and French-speaking obstetric wards, with the majority (n = 5) from regional hospitals. In this group (n = 8), 75% (n = 6) administered clindamycin as the second choice, with 50% (n = 3) offering vancomycin as an alternative in cases of known clindamycin resistance or allergy.
In the group with allergy severity classification (n = 35), first- and second-generation cephalosporins were preferred for mild penicillin allergy (see figure 2): 71% (n = 25) used cefazolin, 26% (n = 9) used cefuroxime and erythromycin was mentioned by n = 1. The distribution of the total sample was consistent across subcategories of language regions and hospital ownership. 80% (n = 28) used clindamycin for severe penicillin allergy, with 86% (n = 24 of 28) switching to vancomycin if clindamycin resistance was known. 11% (n = 4) used vancomycin directly, and 6% (n = 2) listed both clindamycin and vancomycin as second-choice antibiotics for severe allergies without further differentiation. In French- and Italian-speaking obstetric wards, only clindamycin was used (n = 5 and n = 4, respectively), with all switching to vancomycin in cases of known clindamycin resistance (n = 5 and n = 4, respectively).
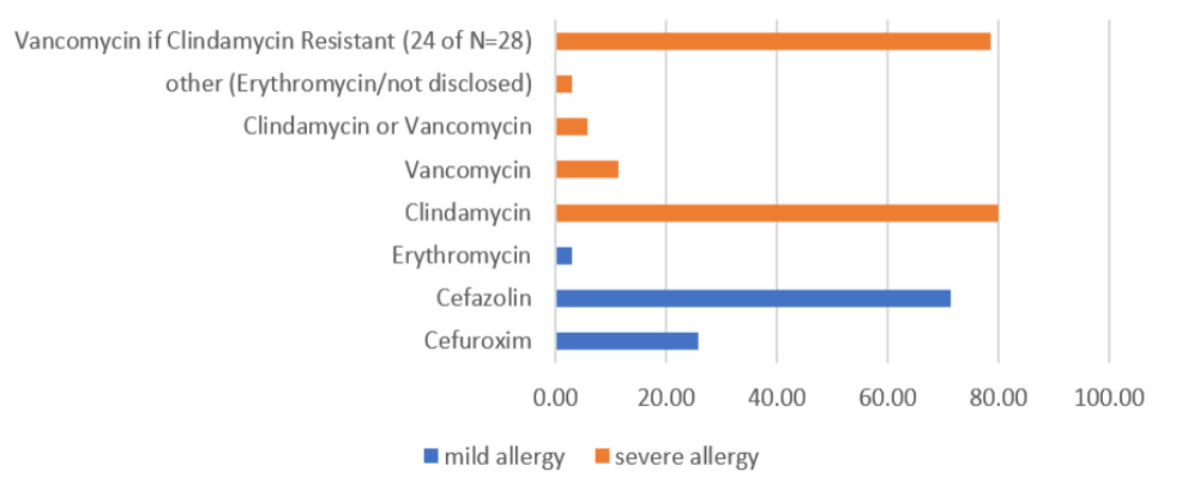
Figure 2Second-choice antibiotics with allergy differentiation (n = 35). Differentiation is expressed as percentages.
Of 43 protocols, 23% (n = 10) did not state any references. Of the remaining 33, the majority cited guidelines of national or international professional societies, with SGGG Expert Letter No. 19 [7] being the most frequently cited (64%, n = 21) (see figure 3). This was followed by the guidelines of the Swiss Society of Neonatology in collaboration with the Paediatric Infectious Disease Group Switzerland [8] (30%, n = 10) and AWMF guidelines [25] (27%, n = 9). Over a half (58%, n = 19) cited additional literature (systematic reviews, scientific articles, etc).
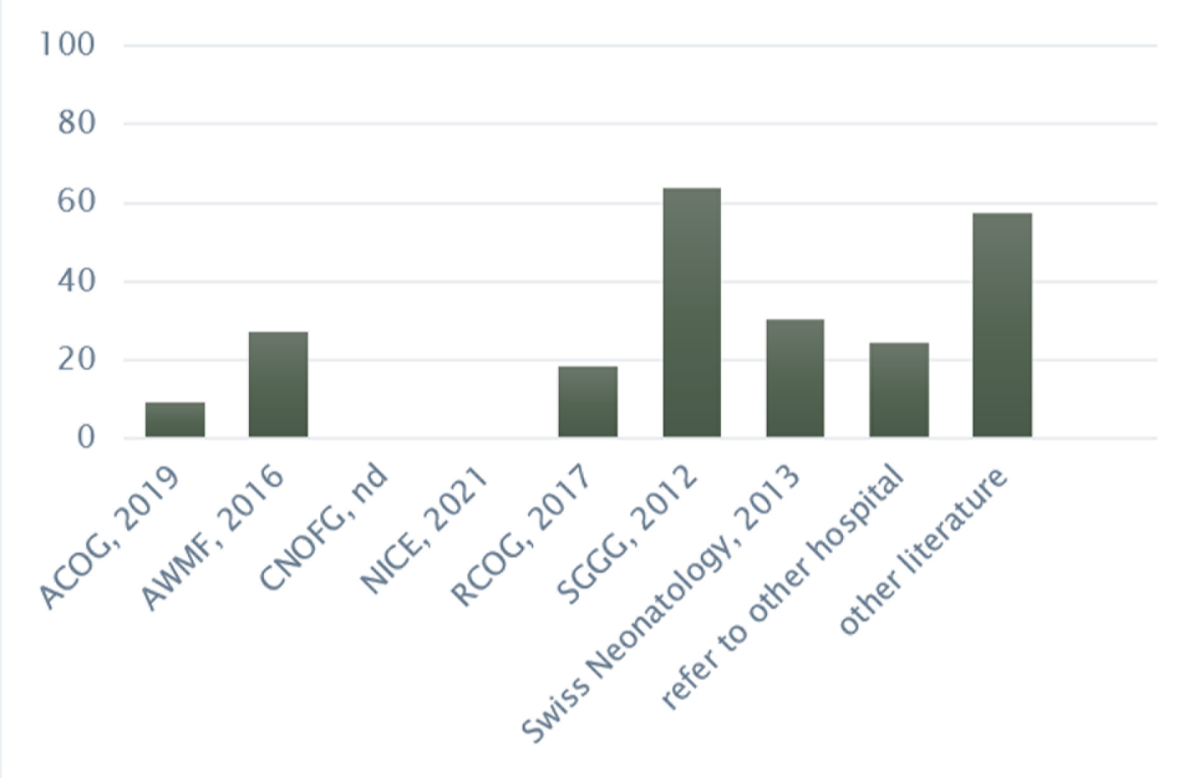
Figure 3Literature referenced (n = 33). Data is expressed as percentages. ACOG:The American College of Obstetricians and Gynecologists (2019) Prevention of Group B Streptococcal Early-Onset Disease in Newborns. Committee opinion No 797. AWMF:Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (2016) Prophylaxe der Neugeborenensepsis – frühe Form – durch Streptokokken der Gruppe B. S2k-Leitline AWMF-Register Nr. 024/20 (in Revision). CNOFG: Collège national des gynécologues et obstétriciens (n.d.) Rupture des membranes à terme avant travail. Recommendations pour la pratique clinique. NICE: National Institute for Health and Care Excellence (2021) Neonatal infection: antibiotics for prevention and treatment. RCOG: Royal College of Obstetricians & Gynaecologists (2017) Prevention of Early-onset Neonatal Group B Streptococcal Disease. SGGG: Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe (2012) Prophylaxe der Early-onset-Neugeborenensepsis durch Streptokokken der Gruppe B. Expertenbrief. Swiss Neonatology (2013) Empfehlungen zur Prävention und Therapie von Termin- und knapp frühgeborenen Kindern (>34 SSW) mit erhöhtem Risiko einer perinatalen bakteriellen Infektion (early-onset Sepsis). Paediatrica (24/1)
Grouped by language region, the recommendation by SGGG was also the most frequently cited (German 61%, French 50%, Italian 100%) followed by other literature (German 52%, French 50%, Italian 100%). The AWMF guidelines were only used in German-speaking obstetric wards (n = 9). 100% (n = 3 of 3) and 80% (n = 12 of 15) of private and cantonal hospitals, respectively, cited the SGGG recommendations the most. The use of other literature varied by hospital type, ranging from 47% (cantonal) to 100% (university).
Of 43 protocols, 28% (n = 12) did not clearly state the authors or professions involved. In the remaining 31 protocols, the healthcare professionals most involved were gynaecologists, at 90% (n = 28), while midwives or nurses were involved in 23% (n = 7) of protocols and infectious disease specialists in 7% (n = 2).
Involvement of midwives/nurses was highest in regional hospitals (39%, n = 5 of 13) compared to other hospital types (25%, n = 1 in university hospitals; 8%, n = 1 in cantonal hospitals; 0%, n = 0 in private clinics). Medical professionals specialising in neonatology or pharmacy were not involved in any of the submitted protocols (n = 0).
This study of Swiss hospital protocols regarding intrapartum antibiotic treatment of Streptococcus agalacticae-positive mothers provides an overview of antibiotics recommended, literature referenced and professionals involved in guideline development.
All participating hospitals use β-lactam antibiotics as the first choice, as recommended by professional societies internationally and in the literature. The preference for penicillin G is clear [14, 23, 26, 27]; however, a recent systematic literature review highlights the knowledge gap regarding intrapartum antibiotics in general with only few studies available [28]. In the present survey, a large proportion of the internal protocols still propose the use of amoxicillin (28%), which is recommended as an alternative to penicillin G by some sources [8, 25–27], and a smaller proportion proposes co-amoxicillin (9%). The Swiss Society of Gynaecology and Obstetrics (SGGG) recommends penicillin G or amoxicillin as first-line antibiotics [9]. Another common pathogen of neonatal sepsis, Escherichia coli, is only 58% sensitive to amoxicillin [29] and 83% to co-amoxicillin [30]. Additionally, co-amoxicillin has been associated with an increased risk of necrotising enterocolitis in preterm birth [31]. However, this association was not confirmed in recent, larger studies; hence robust evidence remains unclear and is particularly lacking for term births. Nevertheless, the use of amoxicillin as intrapartum antibiotic prophylaxis should be reevaluated considering the low sensitivity towards another top cause of early-onset sepsis in neonates.
Correct identification, verification and differentiation of severity in case of a penicillin allergy are essential not only for patient safety but also as part of a strategy against antibiotic resistance and healthcare costs. Also, selection of antibiotics should be based on allergy severity. Although some individuals report a penicillin allergy, few show clinically significant severe type with hypersensitivity to an immunoglobulin E reaction [32]. Despite its importance, not all investigated protocols differentiate between allergy severity and the corresponding choice of antibiotics are handled differently. Most professional societies recommend cephalosporins in case of mild allergies and clindamycin in case of severe allergies instead of penicillin derivates. Vancomycin is an alternative in case of clindamycin resistance. However, some internal hospital protocols directly administer clindamycin, ignoring allergy severity, while others directly use vancomycin for severe allergies. This can be explained by the different recommendations in the literature. For example, the Royal College of Obstetricians and Gynaecologists (RCOG) and National Institute for Health and Care Excellence (NICE) no longer recommend clindamycin but directly suggest vancomycin for severe allergies. This may be explained by their approach to Streptococcus agalacticae risk assessment during labour, which does not allow enough time for sensitivity testing, rather than standardised screening before labour [23, 26]. The use of clindamycin should be critically evaluated given a resistance rate of approximately 23% for Streptococcus agalacticae [30]. Ideally, a resistance test against clindamycin would be carried out in the case of penicillin allergy to select the most effective antibiotic as recommended by SGGG [9].
In September 2023, an interdisciplinary Swiss working group launched a follow-up project to StAR, a national governmental strategy against antimicrobic resistance, called “StAR-3: Implementing Antimicrobial Stewardship”, with the goal of developing a handbook to support Swiss acute care hospitals in implementing an Antimicrobial Stewardship Programme (ASP) [33]. This handbook was published in October 2024 and suggests to the hospitals to engage with the ANRESIS platform to systematically monitor the use of antibiotics in Switzerland [20]. The data should not only be differentiated by hospital and region, but also by medical discipline to guide targeted measures.
National and international guidelines are usually authored by specific professional societies. It often remains unclear whether (informal) consultations with other relevant experts occurred. The recommendation by Swiss Neonatology and PIGS represents an interdisciplinary team of neonatology and infectious paediatrics [8, 10]. The Association of the Scientific Medical Societies in Germany (AWMF) guideline is also interdisciplinary [25]. For Switzerland, it would be desirable to have one joint guideline developed by professional societies of gynaecologists, neonatologists, midwives/nurses, infectiologists and pharmacists according to ASP.
Not all internal protocols indicated referenced literature, and for those, it is not possible to know which sources the recommendations are based on. In terms of transparency and best practice, it is advisable to have referenced literature indicated in internal protocols. Although most hospitals in Switzerland refer to the guidelines of Swiss professional groups, it must be noted that both Swiss guidelines were more than 10 years old at the time of the survey. Therefore, the inclusion of additional literature is indispensable. This additional literature can vary from hospital to hospital, broadening the range of treatment management in hospital internal protocols. So far, in the new SGGG recommendations published in Summer 2024, there are no major changes regarding the choice of antibiotics as compared to the 2012 guideline, except for the clear statement against the use of erythromycin. The updated recommendations are as follows: 1st line treatment with penicillin or amoxicillin, 2nd line with cephalosporin in mild allergy, and clindamycin in severe allergy with caution that sensitivity testing towards clindamycin is mandatory. In clindamycin-insensitive Streptococcus agalacticae, vancomycin is the antibiotic of choice. The Swiss Society of Neonatology and PIGS updated their national guideline on the management of neonates at risk of early-onset sepsis in October 2024, and do not state any recommendations for the choice of antibiotics for intrapartum antibiotic prophylaxis anymore, but rather towards the management, when the neonate is born [10]. It remains to be seen whether the new recommendations will lead to harmonisation in the landscape of internal protocols. Heterogeneity among the three language regions regarding referenced literature is evident. The German region is more orientated towards the German-speaking neighbouring countries.
In a 2017 survey in general medicine, 90% of respondents supported the need for national guidelines on antibiotic use [34]. Although not focused on obstetrics, this finding supports the conclusion of this evaluation report that national treatment guidelines for infectious diseases are needed in Switzerland. With the recently published One Health Action Plan 2024–2027, the federal government aims to create national treatment guidelines and increase their binding nature [35]. It is hoped that the implementation of the “prescription guidelines” measure of the StAR programme will also create recommendations for antibiotic use in obstetrics. A first step has been taken with the new recommendations on the ANRESIS website [30]. This is not only interesting from the perspective of combating antibiotic resistance but also for potential economic changes, given the presence of clear national guidelines instead of multiple different internal protocols developed by various individuals.
Professional groups were recorded based on the authors listed in the hospital internal protocols. It is not clear whether consultations with other professional groups took place. Some hospitals indicated that they plan to involve more professional groups in their current revision. We would expect university hospitals to have interdisciplinary personnel resources available to develop interdisciplinary internal protocols compared to smaller hospitals like regional or private hospitals.
Referring to the study by Osthoff et al. [34], it may seem surprising that pharmacists and experts in infectious diseases were scarcely represented in the current survey. In their 2017 study, those professional groups were included in the improvement of antibiotic use in 13% and 71% of the hospitals, respectively. By then, only 21% of the surveyed hospitals had ASP teams [34]. It raises the question of whether the inclusion of potential ASP teams is less utilised in obstetrics.
Staff shortages (70%) and lack of financial resources (42%) were the most cited reasons hindering the implementation of an ASP. The One Health Action Plan 2024–2027 of the Swiss government aims to assess the implementation of ASPs in Swiss hospitals. Additionally, the federal government intends to create tools to support the implementation of stewardship programmes [35]. Therefore, the development of ASP teams within hospital systems should improve within the next years through further support of the Swiss government.
This survey is based on a snapshot taken in March/April 2024 in Switzerland and the first of its kind, focusing on antibiotic management during labour in obstetric wards. Being a federalist country with different language and cultural regions, a strength of this study is the inclusion of all language regions with a similar distribution like all identified obstetric wards. The goal of the current survey was to create an overview of the current situation and uncover possible differences.
Nevertheless, private clinics and regional hospitals were underrepresented. Private clinics often operate under an attending doctor system, which significantly differs from the structures of university or regional hospitals. Future analyses should aim to reflect this aspect of the healthcare system as well.
The chosen method of descriptive analysis of existing protocols regarding the three predetermined outcomes provides a good overview without explaining the reasons behind the corresponding results. Future studies could employ a qualitative or mixed-method approach to understand the motivation for certain choices regarding antibiotics, literature and involved professions.
The analysis of the received protocols poses a risk of selection biases. First, we did not receive more than 50% of internal protocols and second, not all information we wanted to extract was available in the protocols. For example, not all internal protocols included the names and functions of the individuals involved or the referenced literature. The reason why some obstetric wards refrained from participating in this study remains unknown. Further investigation is needed to determine if the reasons are lack of existing internal protocols, lack of time or resources, point of view towards the need of participation in research or other. However, we covered more than half of the births in clinical settings in Switzerland with the obstetric wards involved in the study. In future studies, more characteristics of the hospitals should be asked to have more insights of the impact of the results and their generalisability.
Furthermore, the survey did not investigate whether the protocols explicitly require an antibiogram in cases of suspected penicillin allergy, especially in clinics that do not suggest alternatives to clindamycin. Further investigations for the correct implementation of the guidelines in hospitals, including indications for intrapartum antibiotic prophylaxis and administration schedule for intrapartum antibiotic prophylaxis are advisable.
There is room for improvement in the interdisciplinary creation of guidelines; the inclusion of ASP teams is recommended. Expertise in gynaecology, neonatology, infectious diseases, pharmacy and midwifery/nursing is needed to provide comprehensive care in obstetric antibiotic use; both on the wards and in joint national guideline development. On a national level, a collaboration particularly between ANRESIS, SGGG, the Swiss midwifery association and the Swiss Society of Neonatology is advisable.
We would recommend monitoring and reporting results of the antenatal screening, and cases of newborns with Streptococcus agalacticae early-onset and late-onset sepsis, via a national reporting system. This reporting system should also record the use of antibiotics and potential resistance developments. The StAR One Health Action Plan 2024–2027 suggests important measures, but they also need to be introduced in obstetrics. Streptococcus agalacticae and intrapartum antibiotics need to be put on the agenda of ASPs and should not be neglected.
In summary, more efforts are needed to unify interdisciplinary collaboration from pregnancy to the discharge of a healthy parent-child triad from the postpartum period. A joint approach in research, education and practice is needed.
Individual data that underlies the results reported in this article can be shared by the corresponding author upon reasonable request, after deidentification (text, tables, figures, and appendices).
We thank all hospitals that entrusted us with their internal protocols for this evaluation. Thanks to Julia Moor, PhD Biomedical Science, for her support in data collection and analysis, and Béatrice Rouiller, MSc Midwifery, for her support in translating the French article.
AI tools (CoPilot and Grammarly August 2024) were used for translation corrections (whole text). For referencing, the referencing program Zotero 7.0.3. (64-bit) was used.
No external funding was received for this study.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
Presented as poster at the global conference of the Society of Maternal Fetal Medicine (SMFM), which took place 25–28 September 2024 in Rome, Italy. Poster available online: Genier SA, Moor JA, Trivelli G, Mitter V. Intrapartum antibiotic prophylaxis for streptococcal B colonization: A survey of hospital internal guidelines in Switzerland. SMFM Global Congress 2024. https://doi.org/10.24451/arbor.22592
1. Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, et al.; GBS Maternal Colonization Investigator Group. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017 Nov;65 suppl_2:S100–11. doi: https://doi.org/10.1093/cid/cix658
2. Capanna F, Emonet SP, Cherkaoui A, Irion O, Schrenzel J, Martinez de Tejada B. Antibiotic resistance patterns among group B Streptococcus isolates: implications for antibiotic prophylaxis for early-onset neonatal sepsis. Swiss Med Wkly. 2013 Mar;143(1314):w13778. doi: https://doi.org/10.4414/smw.2013.13778
3. Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin Infect Dis. 2017 Nov;65 suppl_2:S200–19. doi: https://doi.org/10.1093/cid/cix664
4. World Health Organization. Defeating Meningitis by 2030. A Global Road MAP. 2021 Jun; Available from: https://iris.who.int/bitstream/handle/10665/342010/9789240026407-eng.pdf?sequence=1
5. Giannoni E, Berger C, Stocker M, Agyeman P, Posfay-Barbe KM, Heininger U, et al.; Swiss Pediatric Sepsis Study Group. Incidence and Outcome of Group B Streptococcal Sepsis in Infants in Switzerland. Pediatr Infect Dis J. 2016 Feb;35(2):222–4. doi: https://doi.org/10.1097/INF.0000000000000974
6. Paul P, Gonçalves BP, Le Doare K, Lawn JE. 20 million pregnant women with group B streptococcus carriage: consequences, challenges, and opportunities for prevention. Curr Opin Pediatr. 2023 Apr;35(2):223–30. doi: https://doi.org/10.1097/MOP.0000000000001223
7. Surbek D, Henle-Gross A, Seydoux J, Honegger C, Irion O, Drack G. Prophylaxe der Early-onset-Neugeborenensepsis durch Streptokokken der Gruppe B (aktualisierte Version, 19.7.2012). Available from: https://www.sggg.ch/fileadmin/user_upload/Dokumente/3_Fachinformationen/1_Expertenbriefe/De/19_Prophylaxe_Neugeborenensepsis_Streptokokken_2012.pdf
8. Berger C, Giannoni E, McDougall J, Stocker M. Empfehlungen zur Prävention und Therapie von Termin- und knapp frühgeborenen Kindern (>34 SSW) mit erhöhtem Risiko einer perinatalen bakteriellen Infektion (early-onset Sepsis). Revidierte Empfehlungen der Schweizerischen Gesellschaft für Neonatologie in Zusammenarbeit mit der Pädiatrischen Infektiologiegruppe (PIGS). Paediatrica. 2013;24(1):11–3. Available from: https://www.paediatrieschweiz.ch/empfehlungen-zur-pravention-und-therapie-von-termin-und-knapp-fruh-geborenen-kindern-34-ssw-mit-erhohtem-risiko-einer-perinatalen-bakteriellen-infektion-early-onset-sepsis/
9. Amylidi-Mohr S, Ardabili S, Hamza A, Honegger Ch, Kidszun A, Martinez de Tejada B, Stahel M, Surbek D. Clinical Practice Recommendations No 86 - Prophylaxis of early-onset neonatal sepsis caused by groub B streptococci. Swiss Academy for Maternal Fetal Medicine (AFMM); 2024 May. Available from: Amylidi-Mohr S, Ardabili S, Hamza A, Honegger Ch, Kidszun A, Martinez de Tejada B, Stahel M, Surbek D. Clinical Practice Recommendations No 86 - Prophylaxis of early-onset neonatal sepsis caused by groub B streptococci. Swiss Academy for Maternal Fetal Medicine (AFMM); 2024 May.
10.Stocker M, Rosa-Mangeret F, Agyeman PK, McDougall J, Berger C, Giannoni E. Management of neonates at risk of early onset sepsis: a probability-based approach and recent literature appraisal : Update of the Swiss national guideline of the Swiss Society of Neonatology and the Pediatric Infectious Disease Group Switzerland. Eur J Pediatr. 2024 Dec;183(12):5517–29. doi: https://doi.org/10.1007/s00431-024-05811-0
11. Eidgenossenschaft S. Strategie Antibiotikaresistenzen Schweiz [Internet]. 2015. Available from: https://www.star.admin.ch/dam/star/de/dokumente/strategiebericht-star.pdf.download.pdf/strategiebericht-star-de.pdf
12. Giannoni E, Dimopoulou V, Klingenberg C, Navér L, Nordberg V, Berardi A, et al. Analysis of Antibiotic Exposure and Early-Onset Neonatal Sepsis in Europe, North America, and Australia. 2022 Nov 1; Available from: https://boris-portal.unibe.ch/handle/20.500.12422/115558
13. BAG B für G. Strategie Antibiotikaresistenzen Bereich Mensch. Available from: https://www.bag.admin.ch/bag/de/home/strategie-und-politik/nationale-gesundheitsstrategien/strategie-antibiotikaresistenzen-schweiz.html
14. Raabe VN, Shane AL. Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr. 2019 Mar;7(2):7.2.17. doi: https://doi.org/10.1128/microbiolspec.GPP3-0007-2018
15. Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al.; Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022 Feb;399(10325):629–55. doi: https://doi.org/10.1016/S0140-6736(21)02724-0
16. WHO Bacterial Priority Pathogens List 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance. 1st ed. Geneva: World Health Organization; 2024. 1 p. Available from: https://www.who.int/publications/i/item/9789240093461
17. OECD. Embracing a One Health Framework to Fight Antimicrobial Resistance. OECD; 2023. (OECD Health Policy Studies). Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/embracing-a-one-health-framework-to-fight-antimicrobial-resistance_ce44c755-en
18. World leaders commit to decisive action on antimicrobial resistance. Available from: https://www.who.int/news-room/26-09-2024-world-leaders-commit-to-decisive-action-on-antimicrobial-resistance
19. Rüefli C. Formative Evaluation der Umsetzung der Strategie Antibiotikaresistenzen Schweiz (StAR). Zusammenfassung. Bern: Bundesamt für Gesundheit; 2023., Available from https://www.bag.admin.ch/dam/bag/de/dokumente/e-f/evalber-mt/2023-formative-evaluation-umsetzung-star-zusammenfassung.pdf.download.pdf/2023-zusammenfassung-formative-evaluation-umsetzung-star-d.pdf
20. Projek-Partnerschaft StAR-3. Leitfaden für die Initiierung eines Antimicrobial Stewardship Programms (ASP). 2024 Available from: https://swissnoso.ch/fileadmin/swissnoso/Dokumente/5_Forschung_und_Entwicklung/3_Umsetzung_StAR/StAR_3/Handbook_Deutsch/ASP_Starting_Shortguide_Deutsch.pdf
21. Geburtsspitäler und Kliniken in der Schweiz. Available from: https://www.geburtsspital.ch/
22. BAG B für G. Spital suchen. Available from: https://www.bag.admin.ch/bag/de/home/zahlen-und-statistiken/zahlen-fakten-zu-spitaelern/spital-suchen.html
23. National institute for Health and Care Excellence [NICE]. Neonatal infection: antibiotics for prevention and treatment - NICE guieline 195. 2021; Available from: https://www.nice.org.uk/guidance/ng195
24. Bielicki J, Berger C, Bornand D, Huttner B, Kuster SP, Senn L, et al. SwissASP - framework conditions. Swissnoso SwissASP working group; 2020. Available from: https://www.swissnoso.ch/fileadmin/swissnoso/Dokumente/5_Forschung_und_Entwicklung/9_AB_Stewardship/201119_SwissASP_framework_conditions_en.pdf
25. Herting E, Härtel C, Franz A, Kehl S, Gille C, Doubek K, et al. S2k-Leitlinie 024/20 Prophylaxe der Neugeborenensepsis (frühe Form) durch Streptokokken der Gruppe B. AWMF Online. 2016; Available from: https://register.awmf.org/assets/guidelines/024-020l_S2k_Prophylaxe_Neugeborenensepsis_Streptokokken_2016-04-abgelaufen.pdf
26. Royal College of Obstetricians and Gynaecologists. Prevention of Early‐onset Neonatal Group B Streptococcal Disease: Green‐top Guideline No. 36. BJOG Int J Obstet Gynaecol. 2017 Nov;124(12). Available from: https://obgyn.onlinelibrary.wiley.com/doi/10.1111/1471-0528.14821
27. The Amercian college of Obstetricians and Gynecologists. Committee Opinion Early-Onset Group B Streptococcal Disease. Nr 797. 2020; Available from: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/02/prevention-of-group-b-streptococcal-early-onset-disease-in-newborns
28. Winteler C, Ardabili S, Hodel M, Stocker M. A systematic review of Perinatal Antibiotic Stewardship - where we are, where to go? J Perinatol Off J Calif Perinat Assoc. 2025 Jan 20;
29. Miselli F, Cuoghi Costantini R, Creti R, Sforza F, Fanaro S, Ciccia M, et al. Escherichia coli Is Overtaking Group B Streptococcus in Early-Onset Neonatal Sepsis. Microorganisms. 2022 Sep;10(10):1878. doi: https://doi.org/10.3390/microorganisms10101878
30. Guide A. Available from: https://guide.anresis.ch/human-bacteria?filters=[%22filter-gramStain-2%22,%22mo-17%22]
31. Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2013 Dec;2013(12):CD001058. doi: https://doi.org/10.1002/14651858.CD001058.pub3
32. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and Management of Penicillin Allergy: A Review. JAMA. 2019 Jan;321(2):188–99. doi: https://doi.org/10.1001/jama.2018.19283
33. StAR-3. Available from: https://swissnoso.ch/forschung-entwicklung/umsetzung-star/star-3-antimicrobial-stewardship-programs-for-swiss-hospitals
34. Osthoff M, Bielicki J, Widmer AF, For Swissnoso. Evaluation of existing and desired antimicrobial stewardship activities and strategies in Swiss hospitals. Swiss Med Wkly. 2017 Oct;147(4142):w14512.
35. One Health Aktionsplan 2024-2027. Bundesamt für Gesundheit BAG, Bundesamt für Lebenmittelsicherheit und Veterinärwesen BLV, Bundesamt für Landwirtschaft BLW, Bundesamt für Umwelt BAFU; 2024. Available from: https://www.star.admin.ch/dam/star/de/dokumente/one-health-aktionsplan-star.pdf.download.pdf/One-Health-Aktionsplan-StAR-de-v7.pdf