Updated recommendations for diagnosis and treatment of multiple myeloma in Switzerland
DOI: https://doi.org/https://doi.org/10.57187/s.4252
Martina
Bertschingera,
Holger W. Aunerb,
Veronika Ballovac,
Carmen de
Ramon Ortizd,
Christoph
Driessene,
Sabine
Gerullf,
Dominik
Heimg,
Barbara Jekerh,
Erika Lerchi,
Rouven
Müllerj,
Thomas
Pabsth,
Panagiotis
Samarask,
Adrian
Schmidtl,
Christian
Tavernam,
Thilo Zandern,
Ulrich Meyo*,
Christoph
Rennerp*
a Clinics of Oncology and Haematology,
Cantonal Hospital, Winterthur, Switzerland
b Service and Central Laboratory of
Haematology, University Hospital of Lausanne, Lausanne, Switzerland
c Department of Medical Oncology and
Haematology Baden, Baden, Switzerland
d Division of Haematology, University
Hospital Geneva, Geneva, Switzerland
e Division of Oncology/Haematology,
Cantonal Hospital St. Gallen, St. Gallen, Switzerland
f Centre of Oncology/Haematology and
Transfusion Medicine, Cantonal Hospital Aarau, Aarau, Switzerland
g Department of Haematology, University
Hospital Basel, Basel, Switzerland
h Department of Medical Oncology, University
Hospital of Berne, Berne, Switzerland
i Oncology Institute of Southern
Switzerland (IOSI, EOC), Ospedale San Giovanni, Bellinzona, Switzerland
j Department of Medical Oncology and Haematology,
University Hospital Zurich, Zurich, Switzerland
k Centre of Oncology Zurich, Zurich, Switzerland
l Departement of Internal Medicine,
Clinic of Medical Oncology and Haematology, Municipal Hospital Zurich Triemli,
Zurich, Switzerland
m Oncology/Haematology, Cantonal Hospital
Münsterlingen, Münsterlingen, Switzerland
n Department of Medical Oncology,
Cantonal Hospital Lucerne, Lucerne, Switzerland
o Medical Oncology and Haematology,
Cantonal Hospital Graubuenden, Chur, Switzreland
p Medical Oncology and Haematology
Hirslanden Zurich, Zurich, Switzerland
* Equal contribution as last authors
Summary
Multiple myeloma is a malignant disease
characterised by the clonal proliferation of plasma cells. Since the last
update of the Swiss recommendations for the diagnosis and treatment of multiple
myeloma in 2019, the therapeutic landscape has evolved significantly, with the
development of new monoclonal antibodies, novel combination therapies, and the
introduction of T-cell-redirecting treatments such as bispecific antibodies and
CAR T-cell therapy. This article summarises the current diagnostic procedures
and therapeutic recommendations in Switzerland.
Introduction
Since the last update of the
recommendations for the diagnosis and treatment of multiple myeloma in
Switzerland in 2019 [1], therapies have advanced significantly. Key
developments include the use of highly active monoclonal antibodies in
first-line treatments and the introduction of immunotherapeutic strategies,
such as bispecific antibodies (bsAbs) and chimeric antigen receptor (CAR)
T-cell therapies, particularly at relapse or progression. This update provides
an overview of current diagnostic tests and highlights innovations in multiple
myeloma treatment.
Biology
Multiple myeloma is part of a broader group
of plasma cell disorders, including monoclonal gammopathy of undetermined significance
(MGUS), smouldering myeloma (SM), and other rarer conditions, such as solitary plasmacytoma,
monoclonal gammopathy of clinical significance (MGCS), POEMS syndrome, and light
chain (AL) amyloidosis. These disorders are characterised by the accumulation
of clonal plasma cells, primarily in the bone marrow, leading to the
overproduction of dysfunctional immunoglobulins. The malignant transformation
of plasma cells involves genetic changes, mostly early translocations of the
immunoglobulin heavy chain locus (IgH) on chromosome 14 or hyperdiploidy, followed
by secondary mutations in genes such as KRAS,
NRAS, BRAF, or TP53 [2].
Diagnosis
The diagnosis of multiple myeloma is based
on clinical findings, laboratory tests (especially the detection of monoclonal
protein in the urine or blood), imaging techniques, and bone marrow
examination. The diagnostic criteria have not changed since the last update in
2019. Multiple myeloma diagnosis requires ≥10% clonal plasma
cells on bone marrow examination or a biopsy-proven plasmacytoma plus the
presence of one or more myeloma-defining events or biomarkers of malignancy – the
so-called SLiM-CRAB criteria. The SLiM-CRAB criteria are as follows: ≥60%
clonal plasma cells in the bone marrow (S); an involved-to-uninvolved serum
free light chain (FLC) ratio of ≥100, provided the involved free light chain
level is ≥100 mg/l (Li); >1 focal lesion of ≥5 mm on MRI studies
(M); and the presence of hypercalcaemia (C), renal insufficiency (R), anaemia
(A), and ≥1 osteolytic lesions on skeletal radiography, CT, or FDG-PET/CT (B).
Treatment is indicated when multiple
myeloma is confirmed by the presence of at least one SLiM-CRAB criterion.
Imaging
Imaging techniques are essential for the diagnosis,
staging, and monitoring of disease progression in multiple myeloma. Whole-body
low-dose computed tomography (WBLD-CT) remains the preferred diagnostic method
for detecting osteolytic lesions in plasma cell disorders with high sensitivity
[3]. Magnetic resonance imaging (MRI) is highly sensitive for detecting bone
marrow infiltration by myeloma cells. A whole-body MRI in patients showing ≥10%
clonal bone marrow plasma cell infiltration in the absence of other myeloma-defining
criteria is mandatory to rule out active myeloma. MRI is the preferred
diagnostic tool for evaluating spinal or soft tissue compression and is
particularly useful in special cases, such as during pregnancy.
Fluorodeoxyglucose positron emission
tomography/computed tomography (FDG-PET/CT) is increasingly used in clinical
studies for staging and response assessment. If available, FDG-PET/CT can be
used in clinical practice, as it has a similar sensitivity to MRI in detecting
bone lesions [4] and is particularly effective at identifying active
extramedullary myeloma lesions. It may also play a key role in response
assessment and minimal residual disease (MRD) evaluation in the future.
However, as of 2024, FDG-PET/CT is not yet recommended for routine use in
Switzerland.
Genetic testing
Genetic testing of multiple myeloma cells
provides prognostic insights into newly diagnosed multiple myeloma. Common
methods include cytogenetics and fluorescence in situ hybridisation (FISH) to
detect chromosomal abnormalities. Additional methods, such as array-comparative
genomic hybridisation (aCGH) and next-generation sequencing, can identify
deletions, amplifications, and point mutations. As of 2024, the recommendations
in Switzerland [1] have not changed. Swiss recommendations advise that fluorescence
in situ hybridisation should be performed at diagnosis to identify high-risk
translocations, including t(4;14), del17p, t(14;16), t(14;20), 1q gain, and 1p
deletion [5]. Additionally, the presence of the t(11;14) translocation should
be determined, as it has predictive potential for the use of BCL-2 inhibitors such
as venetoclax [6]. Next-generation sequencing is recommended only in advanced
stages when standard treatment options are exhausted and targeted treatment approaches,
such as BRAF inhibition, are considered.
Supportive care
Because prolonged survival and repeated
therapies lead to severe immunosuppression, effective treatments for multiple
myeloma require adequate supportive measures. The following section discusses
the most important prophylactic approaches.
Antimicrobial prophylaxis
Patients with multiple myeloma are highly immunosuppressed
because of the disease itself and repeated anti-myeloma therapies, with the
highest risk of infection occurring within the first three months after
starting treatment. For most three- and four-drug combination therapies and T-cell-engaging
therapies, antibiotic prophylaxis with trimethoprim/sulfamethoxazole should be considered
to prevent Pneumocystis jirovecii pneumonia, especially when using
higher doses of corticosteroids [7]. Additional prophylactic use of
levofloxacin can help reduce severe infections and mortality during the first
three months of therapy [8]; however, the potential development of antibiotic
resistance should be considered. Antiviral prophylaxis is recommended for most combination
therapies, especially those involving proteasome inhibitors and monoclonal and
bispecific antibodies (bsAbs), to prevent herpes infections/reactivation (e.g. herpes
simplex and varicella zoster virus), with valacyclovir commonly used in
Switzerland.
Vaccinations are crucial in reducing
infection risk, particularly in immunocompromised patients. Early immunisation,
preferably before the initiation of therapy when clinically feasible, is
strongly recommended. According to the Swiss vaccination guidelines by the
Bundesamt für Gesundheit (BAG) and the Eidgenössische Kommission für Impffragen
(EKIF), patients with multiple myeloma should receive vaccinations against
pneumococcal disease, varicella zoster virus, seasonal influenza, and COVID-19 [9].
However, the effectiveness of vaccination during ongoing therapy and in
patients with hypogammaglobinaemia remains uncertain.
All patients with multiple myeloma should
be screened for immunoglobulin deficiency at diagnosis and throughout the
course of the disease. In IgG-secreting myeloma, diagnosing secondary
immunoglobulin deficiency can be challenging. Therefore, assessing the
polyclonal immunoglobulin component (by subtracting the M spike from the total
IgG level) or analysing immunoglobulin subclasses may be beneficial. With
advanced disease, especially after several multiple myeloma treatment lines, the
risk of developing secondary hypogammaglobinaemia increases. Generally, in
patients with hypogammaglobinaemia (IgG <4 g/l), intravenous immunoglobulin
substitution should only be initiated if recurrent severe infections occur
[10]. However, when using T-cell-engaging therapies in heavily pretreated
patients with multiple myeloma, the risk of hypogammaglobinaemia and lethal
infections is very high. It has been shown that severe infections can be
reduced by primary prophylactic administration of intravenous immunoglobulin
[11]. Thus, continuous monitoring of total IgG levels, and their substitution
if their count drops below 4 g/l, is recommended in patients treated with T-cell-engaging
therapies.
Thrombosis prophylaxis
The recommendations for thrombosis
prophylaxis remain unchanged from the previous update. Patients with active multiple
myeloma are at increased risk of venous thromboembolism due to both the disease
and its treatments, particularly when high-dose steroids, immunomodulators, and
chemotherapy (doxorubicin or multi-agent chemotherapy) are used [12]. Various
scores are available to assess the risk of thromboembolism, such as SAVED [13]
and IMPEDE [14]. Most patients can be effectively managed with low-dose
aspirin. Those at higher risk for venous thromboembolism may require low-molecular-weight
heparin or direct oral anticoagulants. However, the latter are off-label, and
insurance approval is required.
Osteoprotection
Patients with multiple myeloma are prone to
bone loss and skeletal events due to bone marrow infiltration by plasma cells,
osteolytic bone changes, and high-dose steroid therapy. Zoledronic acid, a
bisphosphonate, is recommended for all patients with newly diagnosed multiple
myeloma, regardless of whether osteolytic lesions are present. Zoledronic acid
should be administered monthly for at least 12 months, with extended intervals recommended
after achieving a very good partial response [15]. A dental examination is
required before initiating zoledronic acid because of the increased risk of
osteonecrosis of the jaw. Additionally, renal function should be regularly
monitored, with dose adjustments made accordingly.
Denosumab, a nuclear factor-kappa B ligand
(RANKL) inhibitor, is equally effective in preventing skeletal events in patients
with multiple myeloma [16] and does not cause kidney damage, although it is
associated with an increased risk of hypocalcaemia. Abrupt discontinuation of
RANKL inhibitors can lead to a rebound phenomenon; therefore, a single dose of
zoledronic acid should be administered within six months of the last dose of
denosumab. However, denosumab is not approved for multiple myeloma treatment in
Switzerland and requires cost approval.
Treatment options for patients with multiple myeloma
The therapeutic landscape for multiple
myeloma has evolved substantially in recent years, both in initial and
later-line therapies. Various agents can be used alone or in combination. The
active substance classes include chemotherapy, proteasome inhibitors, immunomodulators,
monoclonal antibodies, CAR T cells, and bispecific antibodies. Additional
active substances, not yet approved/reimbursed in Switzerland, include BCL-2 and XPO1
inhibitors, the antibody-drug conjugate belantamab mafodotin, as well as Cereblon
E3 Ligase Modulatory Drugs (CelMods), which are likely to be incorporated into
clinical practice in the near future. Table 1 provides an overview of the
active substances used in multiple myeloma treatment.
Table 1Overview of active substances that can be used in the treatment of multiple
myeloma. The non-approved/reimbursed
drugs for multiple myeloma (so far) are marked with *.
| Cytotoxic
agents |
Adriblastin* |
| Bendamustin* |
| Carboplatin*, cisplatin* |
| Cyclophosphamide* |
| Etoposide* |
| Melphalan |
| Proteasome
inhibitors |
Bortezomib |
| Carfilzomib |
| Ixazomib |
| Immunomodulatory
drugs |
Thalidomide* |
| Lenalidomide |
| Pomalidomide |
| Iberdomide* |
| Mezigdomide* |
| Monoclonal antibodies |
Daratumumab |
| Isatuximab |
| Elotuzumab |
| CAR T |
Idecabtagene-vicleucel |
| Ciltacabtagene-autoleucel |
| Bispecific antibodies |
Teclistamab |
| Elranatamab |
| Talquetamab |
| Cevostamab* |
| Others |
Selinexor* |
| Venetoclax* |
| Belantamab mafodotin* |
| Panobinostat* |
At the time of diagnosis, patients are typically
classified as either young and fit (transplant-eligible) or not eligible for
transplant (transplant non-eligible). Eligibility for high-dose chemotherapy is not
standardised but is generally based on age (under 70 years), the absence of
significant comorbidities (e.g. heart dysfunction), and an Eastern Cooperative
Oncology Group (ECOG) performance status of 0–1. All patients under 70 years
should be evaluated for transplant eligibility, though biological fitness is
more important than chronological age. Additionally, the patient’s fitness
should be regularly reassessed during treatment to ensure that those with
disease-related impairments are not overlooked. However, kidney impairment per
se is not a contraindication for high-dose chemotherapy.
First-line treatment for transplant-eligible patients
Standard induction therapy typically includes
at least a triplet regimen. In Switzerland, the VRd regimen [17] – combining an
immunomodulator, a proteasome inhibitor, and dexamethasone – has been widely
used in recent years. However, recent advancements have introduced quadruplet
regimens that add an anti-CD38 antibody (daratumumab or isatuximab). These
regimens result in improved progression-free survival (PFS) and higher rates of
sustained MRD-negative complete remission (CR) compared to three-drug regimens [18–22],
albeit with slightly increased toxicity (e.g. neutropenia and infections) and
higher costs.
Two pivotal trials, the Phase II GRIFFIN
and Phase III PERSEUS trials, have demonstrated improved outcomes with the
addition of daratumumab to the VRd backbone, with significant differences in
their administration. The PERSEUS regimen administers lenalidomide on days 1–21
over a 4-week cycle, contrasting with the GRIFFIN regimen, which administers
the drug from days 1–14 over a 3-week cycle. In both trials, bortezomib follows,
as in the VRd regimen, the originally published and approved biweekly schedule
on days 1, 4, 8, and 11. Moreover, daratumumab is administered subcutaneously
in the PERSEUS trial, a method favoured in daily clinical practice for its
convenience. The PERSEUS protocol employs a significantly higher dose of
dexamethasone – a total calculated dose of 1920 mg over six cycles compared to
720 mg in the GRIFFIN trial. Because of concerns regarding the high steroid
dosage, most clinicians prefer lower dexamethasone doses, suggesting potential
dexamethasone modifications according to the GRIFFIN study.
In summary, the three-drug VRd regimen
remains a reasonable first-line option for patients with standard-risk disease.
However, due to prolonged progression-free survival and doubling of MRD-negative
complete remission rates with the addition of an anti-CD38 antibody, a quadruplet
regimen is highly recommended for patients with standard-risk disease. Because progression-free
survival and minimal residual disease benefits are observed across all
cytogenetic risk subgroups, including patients with revised high-risk disease and
those with gain(1q21) or amp(1q21), a four-drug regimen is also strongly recommended
for this high-risk patient population. A daratumumab-containing
quadruplet-based induction regimen has been approved by the FDA; however,
neither daratumumab nor isatuximab is approved for this indication in
Switzerland yet.
In all the landmark studies, bortezomib was
administered twice a week. This dosing schedule can lead to a quicker
therapeutic response but is also associated with a higher incidence of peripheral
neuropathy. When a rapid response is critical, initiating treatment with
biweekly dosing may help achieve faster reductions in paraprotein levels.
However, weekly dosing has become more common from the start of treatment because
it is convenient for patients and has a more favourable side effect profile.
Stem cell mobilisation and collection
Following 4–6 cycles of induction therapy,
high-dose chemotherapy with melphalan followed by autologous stem cell
transplant (HD-Mel-ASCT) remains the recommended consolidation therapy for
patients with newly diagnosed transplant-eligible multiple myeloma [18, 23–25].
Mobilisation of peripheral blood stem cells is crucial and can be achieved
using granulocyte-colony stimulating factor (G-CSF) alone or in combination
with other agents, such as plerixafor or cyclophosphamide. In Switzerland,
mobilisation chemotherapy with vinorelbine and G-CSF is commonly used [26, 27],
though alternatives such as gemcitabine may be considered in cases of
pre-existing polyneuropathy [28, 29]. The target number of CD34+ cells during
the stem cell collection depends on the patient’s condition and treatment plan.
In general, the goal is to collect at least 4.0 x 10^6 per kg body weight CD34+
cells (i.e. enough stem cells for two transplants), and this can typically be
achieved using G-CSF and plerixafor. Induction regimens containing anti-CD38
antibodies decrease CD34+ cell yield [30]. Therefore, when using an anti-CD38
antibody-containing regimen in induction, early stem cell collection (e.g. after
three cycles) is recommended.
ASCT consolidation
With each advancement in induction therapy,
the value of HD-Mel-ASCT as consolidation therapy has been challenged.
Nevertheless, randomised trials have continued to show a benefit for the
transplant arm. HD-Mel-ASCT resulted in a significant benefit in progression-free
survival in the IFM2009 [31] and DETERMINATION [17] trials, which used VRd
induction and consolidation followed by lenalidomide maintenance. However,
HD-Mel-ASCT was not linked to an overall survival (OS) benefit. Recent pivotal
first-line clinical trials incorporated HD-Mel-ASCT in their standard and
experimental arms [18, 32, 33]. Trials challenging the role of HD-Mel-ASCT in
the context of quadruplet induction, or comparing it with the early application
of CAR T-cell therapy, are currently recruiting.
As of today, and awaiting the readout of the
above-mentioned and other ongoing trials, HD-Mel-ASCT after triplet or quadruplet
induction is still considered the standard of care for transplant-eligible patients
with newly diagnosed multiple myeloma in Switzerland.
Role of tandem transplant
Tandem HD-Mel-ASCT has been incorporated into
first-line treatment for patients with high-risk disease based on data from the
EMN02/HOVON95 Phase III trial, which showed a progression-free survival and overall
survival benefit in the whole study population, mainly driven by patients with high-risk
disease, especially those with del17p [34]. This strategy has been challenged
by the results of the Phase III BMT CTN 0702 trial using primarily a VRd-based
induction regimen for up to 12 months, followed by randomisation to tandem
HD-Mel-ASCT, single HD-Mel-ASCT combined with VRd consolidation, or HD-Mel-ASCT
with a lenalidomide consolidation. No significant difference was observed in progression-free
survival between the three consolidation arms in the primary intention-to-treat
analysis [35]. However, a post hoc per-protocol analysis – conducted because a substantial
proportion of patients did not receive the assigned second transplant – showed
a progression-free survival benefit for a second HD-Mel-ASCT, which was again
mainly driven by patients with high-risk disease [36]. It should be noted that
in patients receiving modern quadruplet induction therapy, the value of tandem
transplantation is even less clear.
In summary, tandem-HD-Mel-ASCT is not
mandatory but can be considered for patients with high-risk or ultra-high-risk disease
on an individualised, case-by-case basis. Key factors that should be considered
include cytogenetic risk, suboptimal response to induction therapy, deepening
of response after the first HD-Mel-ASCT course, excellent patient fitness, and
good tolerability of the initial HD-Mel-ASCT course. However, the definition of
high-risk multiple myeloma is evolving, and an international consensus is
awaited to clarify which patients should be classified as functionally high-risk
in the context of current highly active multiple myeloma treatments.
Maintenance therapy
Lenalidomide remains the standard-of-care
maintenance therapy and is typically recommended until disease progression or
unacceptable toxicity occurs [17, 37].
Both the GRIFFIN and PERSEUS trials have
incorporated daratumumab alongside lenalidomide for maintenance over at least
two years. In the PERSEUS study, patients in the daratumumab-containing
treatment arm who achieved sustained MRD negativity for at least one year were
allowed to discontinue daratumumab after at least 24 months of maintenance
treatment, whereas the drug was continued until disease progression in patients
who did not achieve sustained MRD negativity.
Recently, long-term follow-up of the
CASSIOPEIA trial demonstrated a progression-free survival benefit for
daratumumab in the maintenance setting, even in patients who had received
daratumumab (dara) during induction (Dara-VTd + daratumumab vs Dara-VTd +
observation) [38]. The Phase 3 AURIGA trial showed a deepening of remission and
a significant prolongation of progression-free survival with the addition of daratumumab
during maintenance [39]. Thus, considering the maturing data from the PERSEUS
trial showing relevant deepening of response under daratumumab-containing
maintenance [40], the incorporation of an anti-CD38 antibody as part of
maintenance is currently being studied further in prospective trials (e.g. the GMMG-HD7
(NCT0361773) and S1803 DRAMMATIC (NCT04071457) trials).
Particularly for patients with high-risk disease,
attempts have been made to incorporate a proteasome inhibitor in maintenance
treatment based on the class-inherent favourable impact on certain high-risk
cytogenetic features. Bortezomib has a role as a single agent, especially in
high-risk patients, based on the HOVON65/GMMG-HD4 trial [41]. Lenalidomide as
monotherapy has shown benefit in patients with high-risk disease in the UK Myeloma
XI trial, depending on the type and co-occurrence of high-risk cytogenetic
markers [42]. No prospective randomised trial data are currently available regarding
the additional value of bortezomib to the lenalidomide backbone. However,
retrospective data on a risk-adapted intensified maintenance approach after VRd
induction and HD-Mel-ASCT showed a higher-than-expected progression-free
survival of 40.3 months for a subgroup of patients with high-risk disease treated
with combined immunomodulator and proteasome inhibitor maintenance,
predominantly with VRd, comparing favourably with historic controls [43].
For the second-generation proteasome
inhibitor carfilzomib, data from the FORTE trial showed a progression-free
survival benefit for carfilzomib + lenalidomide vs lenalidomide alone [44].
Likewise, an unplanned interim analysis of the ATLAS trial showed a progression-free
survival benefit after 33.8 months of follow-up for intensified maintenance
treatment with carfilzomib, lenalidomide, and dexamethasone versus lenalidomide
alone [45]. The progression-free survival benefit was significantly longer in
both trials for the whole study population, whereas it did not reach
statistical significance when focusing on the high-risk group, mainly due to the
small sample size.
In summary, lenalidomide maintenance
treatment remains the approved standard-of-care maintenance treatment, and a proteasome
inhibitor can be used in high-risk situations. However, proteasome inhibitor
maintenance therapy has not been approved in Switzerland. The role of anti-CD38
antibodies in maintenance therapy, including optimal duration, remains
uncertain, and the results of ongoing studies are awaited. Since sustained
MRD-negative complete remission is a strong predictor of improved outcomes,
MRD-informed treatment decisions may provide a strategy for ending maintenance
in certain subsets of patients with multiple myeloma patients in the future.
Treatment of non-transplant-eligible patients
Frailty assessment
All non-transplant-eligible patients should
undergo a frailty assessment at diagnosis and before each line of therapy to
tailor treatment strategies, minimise the risk of toxicities, and reduce the
likelihood of early treatment discontinuation. Several scoring systems have
been proposed for assessing frailty in non-transplant-eligible patients; the
most commonly used tool is the International Myeloma Working Group (IMWG)
frailty score, which predicts the risk of toxicity and mortality following
first-line treatment. An online calculator for this score is available at
http://www.myelomafrailtyscorecalculator.net. A simplified version, known as
the simplified IMWG frailty score, incorporates age, the Charlson comorbidity
index, and ECOG status to provide an easier assessment. Additionally, cognitive
function (e.g. the Mini-Cog test), physical function (e.g. the Up and Go test),
and nutritional status should be assessed to facilitate early interventions
aimed at improving overall health, such as home support, physical therapy, and
nutritional counselling.
First-line therapy in newly diagnosed non-transplant-eligible
patients
For upfront therapy in non-transplant-eligible
patients, the most effective regimen should be used, as approximately 60% of
these patients may not receive subsequent lines of therapy. Additionally, the
first-line treatment offers the best opportunity to achieve durable disease
control and improve survival [46]. Currently, first-line therapy is continued
until disease progression or intolerable toxicity. However, particularly in
older patients, in whom maintaining quality of life is a key treatment goal, treatment
should be individualised. This may include de-escalating treatment through dose
reduction, omitting certain agents, extending intervals, implementing treatment
breaks, or even discontinuing therapy when appropriate.
Several Phase III clinical trials have
evaluated drug combinations for newly diagnosed non-transplant-eligible
patients. The most relevant regimens for the Swiss landscape are discussed
below.
The most commonly used first-line regimen
for non-transplant-eligible patients is the triple combination of daratumumab,
lenalidomide, and dexamethasone (Dara-Rd), based on the Phase III MAIA study [47].
This study compared Dara-Rd with Rd alone, demonstrating improved progression-free
survival at 30 months (70.6% vs 55.6%) and a higher complete response (CR) rate (47.6%
vs 24.9%). After a median follow-up of 89.3 months, an overall
survival benefit was shown for Dara-Rd (90.3 months compared to 64.1 months) [48].
Regarding toxicity, the triple combination is associated with increased rates
of pneumonia and neutropenia [47]. The combination Dara-Rd is administered
until progression and is approved in Switzerland.
The results of the SWOG-S0777 trial led to
the approval of the VRd combination [49] . This randomised Phase III trial compared
VRd with Rd and demonstrated a survival advantage for VRd (median overall
survival not reached vs 69 months). Unlike the MAIA trial, the overall survival
benefit was not statistically significant in the subgroup of patients aged ≥65
years [50]. For patients classified as frail, a dose-reduced regimen known as
VRd-lite is available, providing a feasible alternative [51].
In cases of lenalidomide intolerance, the
combination of daratumumab with bortezomib, melphalan, and prednisone (Dara-VMP)
is another effective regimen. The Phase III ALCYONE study demonstrated a progression-free
survival benefit for Dara-VMP at 18 months (71.6% vs 50.2%) and a significant overall
survival difference after 74.4 months of follow-up (82.7 months vs 53.6 months)
[52]. Notably, patients given Dara-VMP received continuous maintenance with
daratumumab, whereas those in the VMP arm had time-limited therapy.
Post hoc frailty sub-analyses of the MAIA
and ALCYONE trials showed that patients classified as frail experienced higher
toxicity rates and shorter survival compared to non-frail patients. However,
the addition of daratumumab improved quality of life and led to similar or
lower discontinuation rates compared to the control groups [53]. As a result,
triplet regimens, particularly those including an anti-CD38 antibody, are
recommended over doublets, even for patients with frailty.
Recent data from the Phase III IMROZ, BENEFIT,
and CEPHEUS trials indicate that quadruplet combinations (Isa-VRd or Dara-VRd)
are feasible and more effective than triplet combinations in non-transplant-eligible
patients. In the IMROZ trial, which compared Isa-VRd to VRd, the estimated progression-free
survival at 60 months was 63.2% in the Isa-VRd group versus 45.2% in the VRd
group [54]. Similarly, the
BENEFIT trial showed that adding weekly bortezomib to the Isa-Rd combination
significantly improved 18-month MRD negativity rates (53% vs 26%) [55]. The
CEPHEUS trial, which compared Dara-VRd to VRd, demonstrated a higher
MRD-negativity rate for the quadruplet regimen (60.9% vs 39.4%) after a median
follow-up of 58.7 months. Additionally, the estimated 54-month progression-free
survival, a secondary endpoint, was significantly improved with the addition of
the anti-CD38 antibody, consistent with findings from isatuximab studies (86.1%
vs 49.5%) [56].
Ongoing trials are still evaluating
quadruplet regimens in non-transplant-eligible patients, and these combinations
will become part of first-line therapies in both transplant-eligible and
non-transplant-eligible patients. However, clinical trials for newly diagnosed non-transplant-eligible
patients often enrol fitter individuals than those typically seen in real-world
settings. Additionally, quadruplet regimens are more toxic, especially in older
or frailer patients. As a result, it is not yet clear which non-transplant-eligible
patients should be treated with the quadruplet regimen. Consequently,
recommendations for patients classified as frail are largely based on expert
opinions and extrapolations. Several approaches have been suggested to tailor
first-line therapy according to the IMWG Frailty Score at diagnosis (Table 2). Dose
adaptations for patients
classified as intermediate-fit and frail are recommended to minimise early
discontinuation and mortality, with the possibility of dose escalation if
treatment is well tolerated or if patient fitness improves.
Table 2Expert consensus recommendations for dose
adaption for non-transplant-eligible patients.
| IMWG Frailty
score |
Fit patients |
Intermediate-fit
patients |
Patients
with frailty |
| Score |
Score 0 |
Score 1 |
Score ≥2 |
| Aim |
Reduction of
multiple myeloma clone |
Balance
efficacy and safety |
Quality of
life |
| First-line
treatment |
Dara-Rd or
Isa-VRd* |
Dara-Rd |
Dara-Rd |
| VRd |
VRd-lite |
VRd-lite |
| Dara-VMP |
Dara-VMP |
Rd |
| Dara
monotherapy* |
| Dose reduction |
Bortezomib |
1.3 mg/m2
weekly |
1 mg/m2
weekly |
1 mg/m2
weekly |
| Lenalidomide |
25 mg |
15 mg |
10 mg |
| Dexamethasone |
20 mg weekly |
20 mg weekly |
8–10 mg weekly |
Use of steroids in older patients
Most multiple myeloma clinical trials
recommend reducing corticosteroid dosing by half (20 mg instead of 40 mg per
week) for older patients. However, this population remains particularly
vulnerable to complications from steroids, including infections, diabetes
mellitus, agitation/delirium, and osteopenia. Recent studies suggest that
shortening dexamethasone exposure in patients with frailty maintains similar
efficacy while offering a better safety profile [57]. For patients classified
as intermediate-fit and frail, early discontinuation of dexamethasone may be a
good strategy. This approach is currently under investigation in the French IFM
2017-03 trial, which has shown promising preliminary results [58].
Relapsed/refractory multiple myeloma (rrMM)
Relapse criteria
Relapsed multiple myeloma refers to disease
progression following an initial response to previous therapy. Biochemical relapse
typically occurs before clinical progression. The definition of progressive
disease remains unchanged [59]. Refractoriness to a drug is defined as disease
progression observed during or within 60 days of stopping systemic anti-myeloma
treatment [59].
According to the 2021 International Myeloma
Working Group (IMWG) recommendations, initiating the next line of treatment before
the
development of new myeloma-related organ dysfunction, specifically before any CRAB
criteria are met, is advised. However, this does not mandate immediate changes
to therapy in cases of biochemical relapse only. The initiation of re-treatment
is recommended in cases of either clinical relapse or relevant biochemical
relapse, as defined in Table 3, according to the recommendations of an
International Myeloma Workshop Consensus Panel [59].
Table 3Suggested criteria to initiate or modify multiple myeloma therapy. Relevant
biochemical relapse and clinical relapse are distinguished. Relevant
biochemical relapse is sufficient to warrant a change in therapy (no clinical
criteria are required). The clinical relapse criteria only apply if they can be
attributed to the myeloma.
| Clinical
relapse/direct indicators of end-organ damage |
New soft tissue plasmacytoma
or bone lesion |
| Definite increase in
the size of existing plasmacytomas or bone lesions* |
| Hypercalcaemia
>2.875 mmol/l |
| Decrease in haemoglobin
of more than 20 g/l or to less than 100 g/l |
| Rise in serum
creatinine by ≥177 mmol/l |
| Hyperviscosity |
| Relevant paraprotein
relapse |
Doubling of serum
M-protein (2 measurements ≤2 months) |
| Absolute serum
M-Protein ≥10 g/l |
| Increase in urine
M-protein ≥500 mg/24 hours |
| Increase of involved
FLC level ≥200 mg/l with an abnormal FLC ratio (2 measurements ≤2 months) |
Before starting a new therapy, a re-evaluation
of the bone marrow is recommended, particularly in patients initially diagnosed
with standard-risk disease, to identify any new high-risk cytogenetic features.
If not assessed at initial diagnosis, predictive molecular markers such as the
t(11;14) translocation should be tested at relapse, as venetoclax may be a
potential therapeutic option.
Treatment strategies at first relapse
The selection of the next line of treatment
depends mainly on prior drug exposure and refractoriness. Key considerations
include the duration and effectiveness of previous therapy, the kinetics of the
first relapse, patient-specific factors such as side effects (e.g.
polyneuropathy), comorbidities, and patient preferences. The general rule for
second-line treatment is to use the most potent available regimen and select
drug classes not previously used. Triple-drug regimens are superior to doublets,
and the new T-cell-engaging therapies outperform even triplet regimens in the
relapsed/refractory setting. Treatment decisions are influenced by the
patient’s risk classification, which may differ between those with high-risk aggressive
disease and those with standard-risk disease. Achieving MRD-negativity strongly
correlates with better outcomes in both previously untreated patients and those
with relapsed/refractory multiple myeloma [60]. Due to the current trend of
intensive induction therapy using quadruplet regimens in frontline therapy, most
patients will be exposed to three drug classes and be single- or double-class
refractory at first relapse. This will influence future treatment strategies.
Almost all transplant-eligible patients will
have received maintenance therapy with lenalidomide after HD-Mel-ASCT. As a
result, lenalidomide refractoriness is very common at first relapse. In the
future, an increasing number of patients may also be double-refractory to
lenalidomide and anti-CD38 antibodies. The decision algorithm shown in Figure 1
is mainly based on lenalidomide and anti-CD38 refractoriness.
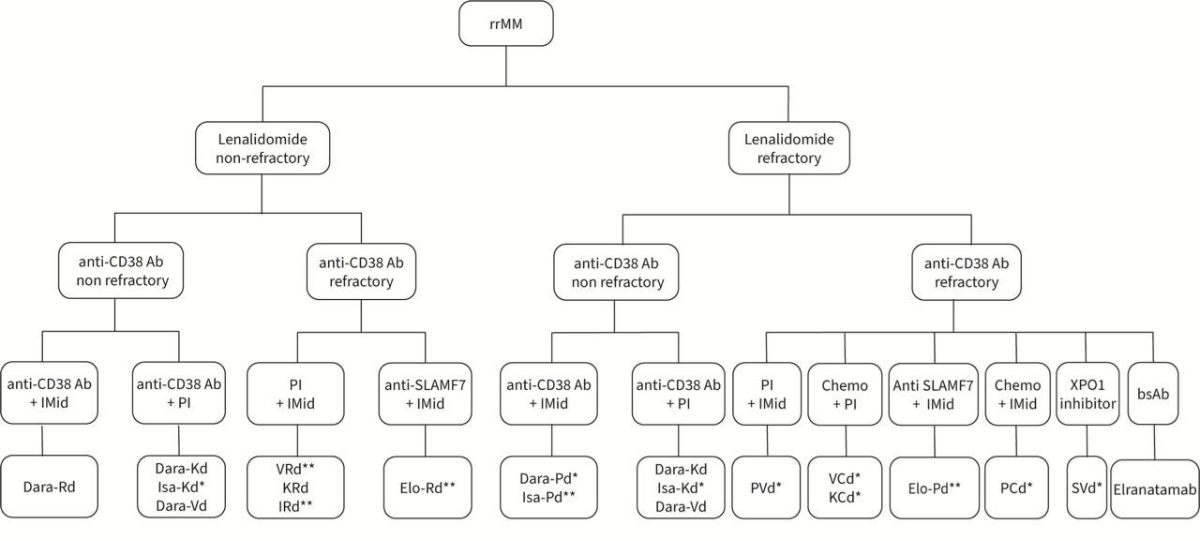
Figure 1Treatment options after first relapse. The primary decision criterion is based
on lenalidomide or anti-CD38 refractoriness. * Not approved in Switzerland. ** Approved
in Switzerland, but not for use in second-line therapy. Ab: antibody; bsAB: bispecific
antibody; C: cyclophosphamide;
Dara: daratumumab; d: dexamethasone; Elo: elotuzumab; IMiD: immunomodulatory
agent; Isa: isatuximab. I: ixazomib; K: carfilzomib; PI:
proteasome inhibitor; P: pomalidomide; R: lenalidomide; rrMM: relapsed/refractory
multiple myeloma; S: Selinexor; SLAMF7: Signalling Lymphocyte Activation
Molecule F7; V: bortezomib; XPO1: exportin 1.
Anti-CD38-sensitive disease
For patients who are refractory to
lenalidomide but sensitive to anti-CD38 antibodies, an anti-CD38 antibody
should be included in second-line treatment. In the now rare cases in which the
disease is sensitive to both lenalidomide and anti-CD38 antibodies, the
preferred option is Dara-Rd, which achieves an mPFS of 55 months [61]. For patients
with lenalidomide-refractory disease, various combinations of anti-CD38
antibodies with proteasome inhibitors (such as Dara-Vd, Dara-Kd, or Isa-Kd) and
the second-generation immunomodulator pomalidomide (Dara-Pd or Isa-Pd) are
approved.
In the CASTOR study, the Dara-Vd regimen
(daratumumab, bortezomib, and dexamethasone) was compared to Vd alone. In the
experimental arm, daratumumab maintenance was continued until disease
progression after eight cycles of Dara-Vd, unlike in the control arm, in which no
maintenance was administered. The addition of the anti-CD38 antibody led to a
significant overall survival benefit with longer follow-up (49.6 months vs 38.5
months) [62], although the progression-free survival observed with Dara-Vd in
this population is relatively short [63].
The effects of the second-generation proteasome
inhibitor carfilzomib combined with an anti-CD38 antibody were examined in the
CANDOR (daratumumab) [64] and IKEMA (isatuximab) [65] studies. These regimens
demonstrated significant progression-free survival benefits of 28.6 and 35.7
months, respectively. Administering carfilzomib weekly instead of biweekly
improves convenience for patients while maintaining or even enhancing its effectiveness
[66]. However, patients must be selected carefully because of the potential
cardiac toxicities of carfilzomib, and it is important to note that Isa-Kd, unlike
Dara-Kd, has not yet been approved.
The combination of pomalidomide with
anti-CD38 antibodies was evaluated in the APOLLO (daratumumab) [67] and ICARIA
(isatuximab) [68] trials. These regimens are generally well tolerated and are
viable options for patients with lenalidomide-refractory disease, although the progression-free
survival of 18 months is lower than that of the carfilzomib-containing regimens
in cross-trial comparisons, likely due to a higher percentage of patients with
lenalidomide-refractory disease accrued in the later trials compared to the anti-CD38-containing
regimens.
Lenalidomide-sensitive, anti-CD38-refractory disease
For patients who are sensitive to
lenalidomide but refractory to anti-CD38 antibodies, the combination of
carfilzomib, lenalidomide, and dexamethasone (KRd) is an approved option. The Phase
III ASPIRE trial demonstrated an mPFS of 26.3 months with this regimen [69].
Double-refractory disease to lenalidomide and
anti-CD38 antibody
In patients refractory to both lenalidomide
and anti-CD38 antibodies, several triple combinations – including pomalidomide
with the Signalling Lymphocyte Activation Molecule F7 (SLAMF7) antibody
elotuzumab or first- or second-generation proteasome inhibitors (bortezomib or
carfilzomib) – have been tested.
The Phase III ELOQUENT-3 trial demonstrated
a benefit of adding elotuzumab to pomalidomide and dexamethasone (EPd) [70]. This
combination is preferably used after an interval from prior anti-CD38 antibody
therapy to restore NK cell activity and is approved after at least two lines of
therapy, including lenalidomide and at least one proteasome inhibitor.
In the Phase III OPTIMISMM trial,
pomalidomide combined with bortezomib and dexamethasone (PVd) was compared to
bortezomib and dexamethasone alone. Treatment with PVd resulted in a
significant improvement in progression-free survival (11.2 vs 7.1 months) [71].
In patients who have not received HD-Mel-ASCT
as frontline therapy or who have achieved a very long remission after
HD-Mel-ASCT, HD-Mel-ASCT may be an option, although supporting data are limited
and somewhat contradictory [72, 73].
T-cell-engaging therapies
Despite significant advancements in
treatment options, multiple myeloma remains an incurable disease, with most
patients experiencing relapses. The need for novel therapeutic approaches has
led to the development of T-cell-engaging therapies, which harness the patient’s
immune system to target and eliminate malignant plasma cells.
Among the most promising T-cell-engaging
strategies are CAR T-cell therapy and bispecific antibodies. CAR T-cell therapy
involves the genetic modification of a patient’s T cells to express a CAR, which
recognises specific antigens on multiple myeloma cells, such as BCMA. Once
reinfused, these CAR T cells bind to their target antigen, leading to T-cell
activation and proliferation and the subsequent destruction of malignant cells.
Bispecific antibodies are engineered to
simultaneously bind to a T-cell receptor, typically CD3, and a tumour-specific
antigen, such as BCMA or GPRC5D, on multiple myeloma cells. This dual binding
brings T cells into close proximity to cancer cells, facilitating their
activation and targeted killing. Unlike CAR T-cell therapy, which requires ex
vivo manipulation, bispecific antibodies can be administered directly to
the patient, offering a more readily accessible therapeutic option. Both CAR
T-cell therapy and bispecific antibodies have shown remarkable efficacy in
clinical trials, particularly in patients with relapsed/refractory multiple
myeloma in whom other treatment options have been exhausted. However, these
therapies are not without challenges; cytokine release syndrome (CRS), immune
effector cell-associated neurotoxicity syndrome (ICANS), and infectious
complications are the most common adverse effects.
BCMA-directed bispecific antibodies
The two BCMA-directed bispecific antibodies,
elranatamab (MagnestiMM-3) [74] and teclistamab (MajesTEC-1) [75], are approved
based on early Phase II data, with an overall response rate (ORR) of around
60%. Both agents have the same target antigen (BCMA), route of administration,
and safety profiles, and they show similar efficacy. However, dosing protocols
and patient demographics vary in their registration trials. Notably, MagnetisMM-3
included a higher proportion of patients with triple-refractory (97%) and
penta-refractory (42%) disease, achieving a mPFS of 17.2 months [74], compared
to 11.4 months for teclistamab in the MajesTEC-1 study [75]. In Switzerland,
teclistamab is approved and reimbursed for triple-class-exposed relapsed/refractory
multiple myeloma after at least three lines of prior therapy, whereas elranatamab
has approval and reimbursement for triple-class relapsed/refractory multiple
myeloma irrespective of the number of prior lines of therapy.
Cytokine release syndrome is common, with
most patients receiving elranatamab or teclistamab experiencing grade 1-2 cytokine
release syndrome. Inpatient step-up dosing is recommended for both, with elranatamab
requiring inpatient monitoring during the first two dose levels and teclistamab
during the first three, which may affect treatment logistics due to hospital
bed availability constraints.
Infection is a major complication,
occurring in approximately 60–70% of patients treated with BCMA-directed bispecific
antibodies, with 40% experiencing severe (grade 3 or 4) infections. This is
attributed to prolonged B-cell immunodeficiency, hypogammaglobinaemia,
neutropenia, and cumulative immune system damage from prior therapies.
Besides intravenous immunoglobulin
substitution, prolonging the dosing interval of bispecific antibodies is an important
strategy to manage infection risk. The MajesTEC-1 study allowed responding
patients to switch from weekly to fortnightly dosing. Most patients who
switched (68.7%) continued with responses of two or more years. Notably,
spacing out the dosing reduced the rate of grade ≥3 infections (15.6% vs 33.3%)
[75].
GPRC5D-directed bispecific antibody talquetamab
Talquetamab is approved in Switzerland and is
a valuable therapeutic option for patients with triple-class-exposed relapsed/refractory
multiple myeloma after three lines of therapy, possibly capable of bypassing
resistance mechanisms developed against BCMA-directed therapies. Talquetamab showed
a 71% overall response rate in patients previously treated with CAR T-cell
therapy or antibody-drug conjugates [76]. While talquetamab is associated with
lower infection rates compared to BCMA-directed bispecific antibodies in cross-trial
comparisons, a consistent pattern of dermatological and oral adverse events,
including skin and nail disorders, xerostomia, dysgeusia, and significant
weight loss, has been reported [76]. Because of its distinct antigen target,
talquetamab is occasionally used in clinical practice as a bridging therapy before
BCMA-directed CAR T-cell therapy, though randomised data are lacking. In light
of the potentially burdensome side effects, we generally recommend the use of
talquetamab after BCMA-directed therapy in patients who are not intended to
undergo subsequent BCMA-directed CAR T-cell treatment.
CAR T-cell therapy
Currently, two BCMA-directed CAR T-cell
products are approved in Switzerland: idecabtagene vicleucel (ide-cel) since
2021 and ciltacabtagene autoleucel (cilta-cel) since 2022. Ide-cel is approved
for third- and fourth-line treatment based on the KarMMa-3 study [77], whereas cilta-cel
is approved for fourth-line treatment based on the CARTITUDE-1 study [78] and as
third-line therapy in patients with lenalidomide-refractory triple-class-exposed
disease, based on the data from the CARTITUDE-4 study (s. below) [79].
Indirect comparisons between these two
products must be made cautiously because of differences in patient populations
and study design. However, the data suggest that cilta-cel offers a higher overall
response rate, improved progression-free survival, and better overall survival,
with similar rates of cytokine release syndrome but with a later onset. In
addition, a significant safety difference for patients treated with cilta-cel
is the occurrence of late-onset neurotoxicity, particularly movement and
neurocognitive treatment-emergent adverse events, which have been reduced
following preventive measures. Recommended strategies include reducing tumour
burden using bridging therapy, aggressive treatment of cytokine release
syndrome and immune effector cell-associated neurotoxicity syndrome, and
extended monitoring beyond day 100.
The Phase III CARTITUDE-4 trial compared cilta-cel
to conventional triplets (Dara-Pd or PVd) in patients with
lenalidomide-refractory multiple myeloma who had received only one to three
prior lines of therapy [79]. After a median follow-up of 15.9 months, cilta-cel
showed superior response and progression-free survival at 12 months (75.9% vs
48.6%), and in an updated analysis, it even showed an improved 34-month overall
survival (76.4% vs 63.8%) [80], leading to expanded approval for use in relapsed/refractory
multiple myeloma.
Sequencing of bispecific antibodies and CAR T-cell
therapies
Determining the optimal sequence of bispecific
antibodies and CAR T cells is an ongoing challenge. Preliminary data suggest
that using CAR T-cell therapy first and reserving bispecific antibodies for
later lines of therapy may be more effective, as response rates decline more
when CAR T cells are administered after bispecific antibodies rather than vice
versa. For example, in cohort C of the CARTITUDE-2 study, only 60% of patients
previously treated with bispecific antibodies responded to cilta-cel [81], compared
to 97% in the initial cohort [78]. In contrast, in a pooled analysis of the
MagnetisMM studies, elranatamab following CAR T-cell therapy showed an overall
response rate of 52.8% [82], compared to 61% in the MagnetisMM-3 study [74]. Real-world
data indicate similar response rates for ide-cel following bispecific
antibodies compared to no prior bispecific antibodies treatment (86% and 88%,
respectively), although the patient numbers in this study were low [83]. Future
studies analysing the sequencing of bispecific antibodies and CAR T-cell
therapies targeting different antigens will provide further insights into the
optimal sequencing of these T-cell-activating therapies.
In summary, T-cell-engaging therapies are highly
effective new therapeutic options for relapsed/refractory multiple myeloma.
However, although generally well-tolerated, specific side effects such as cytokine
release syndrome, immune effector cell-associated neurotoxicity syndrome, and
long-lasting immunosuppression must be considered.
Other active agents: venetoclax, selinexor, and belantamab
mafodotin
Venetoclax, a BCL-2 inhibitor, was
evaluated in combination with bortezomib and dexamethasone (VenVd) or daratumumab
and dexamethasone and resulted in a high overall response rate. However, venetoclax
combination regimens should be used exclusively in multiple myeloma patients harbouring
the t(11;14) translocation [6, 84].
Selinexor is a selective inhibitor of
nuclear export factor 1 (XPO1). In the single-arm STORM trial, selinexor
combined with dexamethasone showed moderate anti-myeloma activity in a heavily
pretreated patient population (median of seven previous lines of therapy),
achieving a partial response rate of 26% and an mPFS of 4.4 months [85]. In the
randomised BOSTON trial, adding selinexor to bortezomib and dexamethasone
resulted in a significant progression-free survival benefit compared to
bortezomib and dexamethasone alone (13.9 months vs 9.5 months) [86]. Selinexor is
an effective treatment option in specific situations; however, particular
attention should be given to gastrointestinal toxicities, especially nausea and
vomiting. Selinexor was recently approved after four lines of therapy.
Belantamab mafodotin is the first and
currently the only antibody-drug conjugate for multiple myeloma treatment. It
consists of a humanised monoclonal IgG1 antibody against BCMA, conjugated to
the microtubule inhibitor monomethyl auristatin F [87]. Disappointing results
from the DREAMM-3 study, in which single-agent belantamab mafodotin failed to
show superior activity over pomalidomide plus dexamethasone (Pd) [88], dampened
enthusiasm for the drug and led to US and Swiss market withdrawal. However, two
recently published Phase III trials have renewed interest in the drug. In the
DREAMM-7 trial, patients with relapsed multiple myeloma, after receiving at
least one line of therapy, experienced significantly better mPFS and overall
survival with belantamab mafodotin combined with bortezomib and dexamethasone
(BVd) compared to Dara-Vd (36.6 months vs 13.4 months) [89]. In the DREAMM-8
study, belantamab mafodotin combined with Pd (BPd) was superior to bortezomib
plus Pd, with a 12-month estimated progression-free survival of 71% versus 51%
in lenalidomide-exposed patients with at least one prior line of therapy [90].
While ocular toxicity remains a common issue, dose modifications, treatment
interval prolongation, and supportive care measures may help mitigate this
limitation. Belantamab mafodotin is currently not approved in Switzerland, but
an EAP is available. The different treatment options after second line are summarised
in figure 2. Due to the lack of data, the optimal sequencing of the available drugs
is unknown.
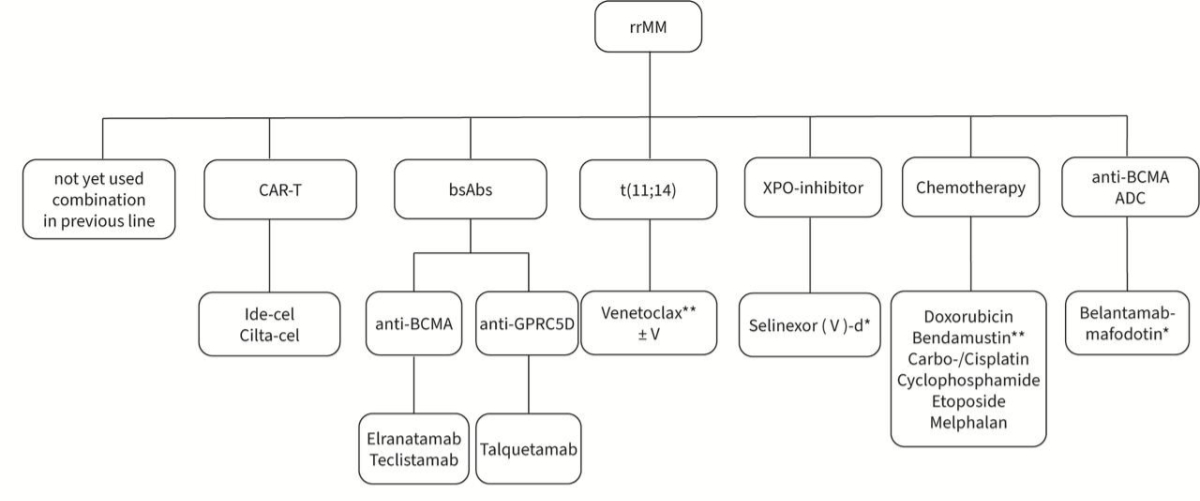
Figure 2Treatment options after second line. * Not approved or reimbursed in
Switzerland. ** Off-label indication. ADC: antibody-drug conjugate; BCMA: B-cell maturation
antigen; bsAbs: bispecific
antibodies; CAR-T: chimeric antigen receptor T-cell
therapy; cilta-cel: ciltacabtagene
autoleucel; d: dexamethasone; GPRC5D: G protein-coupled receptor class C group 5 member
D; ide-cel: idecabtagene
vicleucel; rrMM: relapsed/refractory
multiple myeloma; t(11;14): translocation 11;14; V: bortezomib; XPO1: exportin 1.
Take-home messages
Overview
- Although multiple myeloma
remains incurable for most patients, survival rates have significantly improved
in recent years. Achieving optimal disease control typically requires long-term,
continuous treatment with appropriate sequencing of effective drugs.
First-line treatment options
- For transplant-eligible
patients, the quadruple combination Dara-VRd is considered the preferred
first-line regimen.
- In fit, non-transplant-eligible
patients, Dara-Rd or Isa-VRd (if available / approved) is the preferred option.
High-dose chemotherapy and stem cell transplantation
- HD-Mel-ASCT can still be
considered standard of care for transplant-eligible patients in the first-line
setting, as it can achieve deeper responses and prolonged progression-free
survival.
- Tandem HD-Mel-ASCT should be
considered only in patients with high-risk and ultra-high-risk disease.
Maintenance therapy
- Lenalidomide maintenance until
progression or intolerance is the standard of care for most patients following HD-Mel-ASCT.
Preliminary data suggest that maintenance with an additional anti-CD38 antibody
may further prolong progression-free survival.
Second-line and later treatments
- Numerous new treatment options
are available for second-line or later lines of therapy. The choice of the
optimal regimen should consider prior treatments, toxicity profiles,
comorbidities, and patient preferences.
- Key decision criteria include
assessing refractoriness to lenalidomide and/or anti-CD38 antibodies.
T-cell-engaging therapies
- T-cell-engaging therapies,
including CAR T cells and bispecific antibodies, have revolutionised the
treatment landscape for multiple myeloma.
Prof. Christoph Renner, MD
Medical Oncology and Haematology
Hirslanden Zurich
Witellikerstrasse 40
CH-8032 Zurich
christoph.renner[at]hirslanden.ch
References
1. Samaras, P., et al., Updated recommendations for diagnosis and treatment of plasma
cell myeloma in Switzerland. Swiss Med Wkly, 2019.149(1314):w20031. doi: https://doi.org/10.4414/smw.2019.20031
2. Bergsagel PL, Chesi MV. Molecular classification and risk stratification of myeloma.
Hematol Oncol, 2013 Jun;31 Suppl 1(0 1):38-41. 10.1002/hon.2065
3. Moulopoulos LA, Koutoulidis V, Hillengass J, Zamagni E, Aquerreta JD, Roche CL, et
al. Recommendations for acquisition, interpretation and reporting of whole body low
dose CT in patients with multiple myeloma and other plasma cell disorders: a report
of the IMWG Bone Working Group. Blood Cancer J. 2018 Oct;8(10):95. doi: https://doi.org/10.1038/s41408-018-0124-1
4. Moreau P, Attal M, Caillot D, Macro M, Karlin L, Garderet L, et al. Prospective Evaluation
of Magnetic Resonance Imaging and [18F]Fluorodeoxyglucose Positron Emission Tomography-Computed
Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients With
Multiple Myeloma Included in the IFM/DFCI 2009 Trial: results of the IMAJEM Study.
J Clin Oncol. 2017 Sep;35(25):2911–8. doi: https://doi.org/10.1200/JCO.2017.72.2975
5. Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment
of multiple myeloma with high-risk cytogenetics: a consensus of the International
Myeloma Working Group. Blood. 2016 Jun;127(24):2955–62. doi: https://doi.org/10.1182/blood-2016-01-631200
6. Kumar SK, Harrison SJ, Cavo M, de la Rubia J, Popat R, Gasparetto C, et al. Venetoclax
or placebo in combination with bortezomib and dexamethasone in patients with relapsed
or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre,
phase 3 trial. Lancet Oncol. 2020 Dec;21(12):1630–42. doi: https://doi.org/10.1016/S1470-2045(20)30525-8
7. Raje NS, Anaissie E, Kumar SK, Lonial S, Martin T, Gertz MA, et al. Consensus guidelines
and recommendations for infection prevention in multiple myeloma: a report from the
International Myeloma Working Group. Lancet Haematol. 2022 Feb;9(2):e143–61. doi: https://doi.org/10.1016/S2352-3026(21)00283-0
8. Drayson MT, Bowcock S, Planche T, Iqbal G, Pratt G, Yong K, et al.; TEAMM Trial Management
Group and Trial Investigators. Levofloxacin prophylaxis in patients with newly diagnosed
myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase
3 trial. Lancet Oncol. 2019 Dec;20(12):1760–72. doi: https://doi.org/10.1016/S1470-2045(19)30506-6
9. Bundesamt für Gesudheit (BAG), Eidgnössische Kommission für Impffragen (EKIF). Empfehlung
zur Impfung von Personen mit malignen Erkrankungen und deren Haushaltskontakte. BAG-Bulletin.
2022;20:20-30. Internet: https://www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/richtlinien-empfehlungen-impfungen-prophylaxe.html
10. Goede JS, Baumann CK, Cathomas R, Khanna N, Lambert JF, Lehmann T, et al. Rational
use of immunoglobulins (IVIgs and SCIgs) in secondary antibody deficiencies. Swiss
Med Wkly. 2024 Sep;154(9):3559. doi: https://doi.org/10.57187/s.3559
11. Lancman G, Parsa K, Kotlarz K, Avery L, Lurie A, Lieberman-Cribbin A, et al. IVIg
Use Associated with Ten-Fold Reduction of Serious Infections in Multiple Myeloma Patients
Treated with Anti-BCMA Bispecific Antibodies. Blood Cancer Discov. 2023 Nov;4(6):440–51.
doi: https://doi.org/10.1158/2643-3230.BCD-23-0049
12. Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et
al.; International Myeloma Working Group. Prevention of thalidomide- and lenalidomide-associated
thrombosis in myeloma. Leukemia. 2008 Feb;22(2):414–23. doi: https://doi.org/10.1038/sj.leu.2405062
13. Li A, Wu Q, Luo S, Warnick GS, Zakai NA, Libby EN, et al. Derivation and Validation
of a Risk Assessment Model for Immunomodulatory Drug-Associated Thrombosis Among Patients
With Multiple Myeloma. J Natl Compr Canc Netw. 2019 Jul;17(7):840–7. doi: https://doi.org/10.6004/jnccn.2018.7273
14. Sanfilippo KM, Luo S, Wang TF, Fiala M, Schoen M, Wildes TM, et al. Predicting venous
thromboembolism in multiple myeloma: development and validation of the IMPEDE VTE
score. Am J Hematol. 2019 Nov;94(11):1176–84. doi: https://doi.org/10.1002/ajh.25603
15. Terpos E, Zamagni E, Lentzsch S, Drake MT, García-Sanz R, Abildgaard N, et al.; Bone
Working Group of the International Myeloma Working Group. Treatment of multiple myeloma-related
bone disease: recommendations from the Bone Working Group of the International Myeloma
Working Group. Lancet Oncol. 2021 Mar;22(3):e119–30. doi: https://doi.org/10.1016/S1470-2045(20)30559-3
16. Raje N, Terpos E, Willenbacher W, Shimizu K, García-Sanz R, Durie B, et al. Denosumab
versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma:
an international, double-blind, double-dummy, randomised, controlled, phase 3 study.
Lancet Oncol. 2018 Mar;19(3):370–81. doi: https://doi.org/10.1016/S1470-2045(18)30072-X
17. Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al.; DETERMINATION
Investigators. Triplet Therapy, Transplantation, and Maintenance until Progression
in Myeloma. N Engl J Med. 2022 Jul;387(2):132–47. doi: https://doi.org/10.1056/NEJMoa2204925
18. Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al.; PERSEUS
Trial Investigators. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for
Multiple Myeloma. N Engl J Med. 2024 Jan;390(4):301–13. doi: https://doi.org/10.1056/NEJMoa2312054
19. Voorhees PM, Sborov DW, Laubach J, Kaufman JL, Reeves B, Rodriguez C, et al. Addition
of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible
patients with newly diagnosed multiple myeloma (GRIFFIN): final analysis of an open-label,
randomised, phase 2 trial. Lancet Haematol. 2023 Oct;10(10):e825–37. doi: https://doi.org/10.1016/S2352-3026(23)00217-X
20. Leypoldt LB, Tichy D, Besemer B, Hänel M, Raab MS, Mann C, et al. Isatuximab, Carfilzomib,
Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple
Myeloma. J Clin Oncol. 2024 Jan;42(1):26–37. doi: https://doi.org/10.1200/JCO.23.01696
21. Goldschmidt H, Mai EK, Bertsch U, Fenk R, Nievergall E, Tichy D, et al.; German-Speaking
Myeloma Multicenter Group (GMMG) HD7 investigators. Addition of isatuximab to lenalidomide,
bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible
patients with multiple myeloma (GMMG-HD7): part 1 of an open-label, multicentre, randomised,
active-controlled, phase 3 trial. Lancet Haematol. 2022 Nov;9(11):e810–21. doi: https://doi.org/10.1016/S2352-3026(22)00263-0
22. Broijl A. Results of the Phase III Randomized Iskia Trial: Isatuximab-Carfilzomib-Lenalidomide-Dexamethasone
Vs Carfilzomib-Lenalidomide-Dexamethasone As Pre-Transplant Induction and Post-Transplant
Consolidation in Newly Diagnosed Multiple Myeloma Patients: Results of the phase III
randomized EMN24 Iskia trial. EMN; 2024.
23. Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective,
randomized trial of autologous bone marrow transplantation and chemotherapy in multiple
myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996 Jul;335(2):91–7. doi: https://doi.org/10.1056/NEJM199607113350204
24. Cavo M, Rajkumar SV, Palumbo A, Moreau P, Orlowski R, Bladé J, et al.; International
Myeloma Working Group. International Myeloma Working Group consensus approach to the
treatment of multiple myeloma patients who are candidates for autologous stem cell
transplantation. Blood. 2011 Jun;117(23):6063–73. doi: https://doi.org/10.1182/blood-2011-02-297325
25. Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab,
lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed
multiple myeloma: the GRIFFIN trial. Blood. 2020 Aug;136(8):936–45. doi: https://doi.org/10.1182/blood.2020005288
26. Samaras P, Pfrommer S, Seifert B, Petrausch U, Mischo A, Schmidt A, et al. Efficacy
of vinorelbine plus granulocyte colony-stimulation factor for CD34+ hematopoietic
progenitor cell mobilization in patients with multiple myeloma. Biol Blood Marrow
Transplant. 2015 Jan;21(1):74–80. doi: https://doi.org/10.1016/j.bbmt.2014.09.020
27. Samaras P, Rütti MF, Seifert B, Bachmann H, Schanz U, Eisenring M, et al. Mobilization
of Hematopoietic Progenitor Cells with Standard- or Reduced-Dose Filgrastim after
Vinorelbine in Multiple Myeloma Patients: A Randomized Prospective Single-Center Phase
II Study. Biol Blood Marrow Transplant. 2018 Apr;24(4):694–9. doi: https://doi.org/10.1016/j.bbmt.2017.12.775
28. Mueller BU, Keller S, Seipel K, Mansouri Taleghani B, Rauch D, Betticher D, et al. Stem
cell mobilization chemotherapy with gemcitabine is effective and safe in myeloma patients
with bortezomib-induced neurotoxicity. Leuk Lymphoma. 2016 May;57(5):1122–9. doi: https://doi.org/10.3109/10428194.2015.1079315
29. Jeker B, Farag S, Taleghani BM, Novak U, Mueller BU, Li Q, et al. A randomized evaluation
of vinorelbine versus gemcitabine chemotherapy mobilization of stem cells in myeloma
patients. Bone Marrow Transplant. 2020 Oct;55(10):2047–51. doi: https://doi.org/10.1038/s41409-020-0875-8
30. Chhabra, S., et al., Stem Cell Mobilization Yields with Daratumumab- and Lenalidomide-Containing
Quadruplet Induction Therapy in Newly Diagnosed Multiple Myeloma: Findings from the
MASTER and GRIFFIN Trials. Transplant Cell Ther, 2023. 29(3): p. 174 e1-174 e10.
31. Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al.; IFM 2009
Study. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma.
N Engl J Med. 2017 Apr;376(14):1311–20. doi: https://doi.org/10.1056/NEJMoa1611750
32. Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib,
thalidomide, and dexamethasone with or without daratumumab before and after autologous
stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised,
open-label, phase 3 study. Lancet. 2019 Jul;394(10192):29–38. doi: https://doi.org/10.1016/S0140-6736(19)31240-1
33. Gay F, Roeloffzen W, Dimopoulos MA, Rosiñol L, van der Klift M, Mina R, et al. Results
of the Phase III Randomized Iskia Trial: Isatuximab-Carfilzomib-Lenalidomide-Dexamethasone
Vs Carfilzomib-Lenalidomide-Dexamethasone As Pre-Transplant Induction and Post-Transplant
Consolidation in Newly Diagnosed Multiple Myeloma Patients. Blood. 2023;142 Supplement
1:4. doi: https://doi.org/10.1182/blood-2023-177546
34. Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous
haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with
or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide
maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised,
open-label, phase 3 study. Lancet Haematol. 2020 Jun;7(6):e456–68. doi: https://doi.org/10.1016/S2352-3026(20)30099-5
35. Stadtmauer EA, Pasquini MC, Blackwell B, Hari P, Bashey A, Devine S, et al. Autologous
Transplantation, Consolidation, and Maintenance Therapy in Multiple Myeloma: results
of the BMT CTN 0702 Trial. J Clin Oncol. 2019 Mar;37(7):589–97. doi: https://doi.org/10.1200/JCO.18.00685
36. Parameswaran H, et al. Long-term follow-up of BMT CTN 0702 (STaMINA) of postautologous
hematopoietic cell transplantation (autoHCT) strategies in the upfront treatment of
multiple myeloma (MM). J Clin Oncol. 2020;38(15 _suppl.).
37. McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide
Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple
Myeloma: A Meta-Analysis. J Clin Oncol. 2017 Oct;35(29):3279–89. doi: https://doi.org/10.1200/JCO.2017.72.6679
38. Moreau P, Hulin C, Perrot A, Arnulf B, Belhadj K, Benboubker L, et al.; Intergroupe
Francophone du Myélome, the Dutch-Belgian Cooperative Trial Group for Hematology Oncology
and the CASSIOPEIA Investigators. Bortezomib, thalidomide, and dexamethasone with
or without daratumumab and followed by daratumumab maintenance or observation in transplant-eligible
newly diagnosed multiple myeloma: long-term follow-up of the CASSIOPEIA randomised
controlled phase 3 trial. Lancet Oncol. 2024 Aug;25(8):1003–14. doi: https://doi.org/10.1016/S1470-2045(24)00282-1
39. Badros A, Foster L, Anderson LD Jr, Chaulagain CP, Pettijohn E, Cowan AJ, et al. Daratumumab
with lenalidomide as maintenance after transplant in newly diagnosed multiple myeloma:
the AURIGA study. Blood. 2025 Jan;145(3):300–10. doi: https://doi.org/10.1182/blood.2024025746
40. Sonneveld, P., et al., Daratumumab + bortezomib/lenalidomide/dexamethasone in transplant-eligible
patients with newly diagnosed multiple myeloma: analysis of minimal residual disease
in the PERSEUS trial. EHA Library 2024, Abstract S201.
41. Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H,
et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed
multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J
Clin Oncol. 2012 Aug;30(24):2946–55. doi: https://doi.org/10.1200/JCO.2011.39.6820
42. Panopoulou A, Cairns DA, Holroyd A, Nichols I, Cray N, Pawlyn C, et al. Optimizing
the value of lenalidomide maintenance by extended genetic profiling: an analysis of
556 patients in the Myeloma XI trial. Blood. 2023 Apr;141(14):1666–74. doi: https://doi.org/10.1182/blood.2022018339
43. Joseph NS, Kaufman JL, Dhodapkar MV, Hofmeister CC, Almaula DK, Heffner LT, et al. Long-Term
Follow-Up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy
and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma. J Clin
Oncol. 2020 Jun;38(17):1928–37. doi: https://doi.org/10.1200/JCO.19.02515
44. Gay F, Musto P, Rota-Scalabrini D, Bertamini L, Belotti A, Galli M, et al. Carfilzomib
with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous
transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance
with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed
multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. 2021 Dec;22(12):1705–20.
doi: https://doi.org/10.1016/S1470-2045(21)00535-0
45. Dytfeld D, Wróbel T, Jamroziak K, Kubicki T, Robak P, Walter-Croneck A, et al. Carfilzomib,
lenalidomide, and dexamethasone or lenalidomide alone as maintenance therapy after
autologous stem-cell transplantation in patients with multiple myeloma (ATLAS): interim
analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023 Feb;24(2):139–50.
doi: https://doi.org/10.1016/S1470-2045(22)00738-0
46. Fonseca R, Usmani SZ, Mehra M, Slavcev M, He J, Cote S, et al. Frontline treatment
patterns and attrition rates by subsequent lines of therapy in patients with newly
diagnosed multiple myeloma. BMC Cancer. 2020 Nov;20(1):1087. doi: https://doi.org/10.1186/s12885-020-07503-y
47. Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al.; MAIA Trial Investigators.
Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med.
2019 May;380(22):2104–15. doi: https://doi.org/10.1056/NEJMoa1817249
48. Facon, T., Final survival analysis of daratumumab plus lenalidomide and dexamethasone
versus lenalidomide and dexamethasone in transplant-ineligible patients with newly
diagnose multiple myeloma: MAIA study. EHA 2024, Abstract P968.
49. Durie BG, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib
with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in
patients with newly diagnosed myeloma without intent for immediate autologous stem-cell
transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017 Feb;389(10068):519–27.
doi: https://doi.org/10.1016/S0140-6736(16)31594-X
50. Durie BG, Hoering A, Sexton R, Abidi MH, Epstein J, Rajkumar SV, et al. Longer term
follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and
dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously
untreated multiple myeloma without an intent for immediate autologous stem cell transplant
(ASCT). Blood Cancer J. 2020 May;10(5):53. doi: https://doi.org/10.1038/s41408-020-0311-8
51. O’Donnell EK, Laubach JP, Yee AJ, Chen T, Huff CA, Basile FG, et al. A phase 2 study
of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple
myeloma. Br J Haematol. 2018 Jul;182(2):222–30. doi: https://doi.org/10.1111/bjh.15261
52. Mateos MV, San-Miguel J, Cavo M, Bladé Creixenti J, Suzuki K, Jakubowiak A, et al. Daratumumab
Plus Bortezomib, Melphalan, and Prednisone (D-VMP) Versus Bortezomib, Melphalan, and
Prednisone (VMP) Alone in Transplant-Ineligible Patients with Newly Diagnosed Multiple
Myeloma (NDMM): Updated Analysis of the Phase 3 Alcyone Study. Blood. 2022;140 Supplement
1:10157–9. doi: https://doi.org/10.1182/blood-2022-163347
53. Perrot A, Facon T, Plesner T, Usmani SZ, Kumar S, Bahlis NJ, et al. Health-Related
Quality of Life in Transplant-Ineligible Patients With Newly Diagnosed Multiple Myeloma:
Findings From the Phase III MAIA Trial. J Clin Oncol. 2021 Jan;39(3):227–37. doi: https://doi.org/10.1200/JCO.20.01370
54. Facon T, Dimopoulos MA, Leleu XP, Beksac M, Pour L, Hájek R, et al.; IMROZ Study Group.
Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl
J Med. 2024 Oct;391(17):1597–609. doi: https://doi.org/10.1056/NEJMoa2400712
55. Leleu X, Hulin C, Lambert J, Bobin A, Perrot A, Karlin L, et al. Isatuximab, lenalidomide,
dexamethasone and bortezomib in transplant-ineligible multiple myeloma: the randomized
phase 3 BENEFIT trial. Nat Med. 2024 Aug;30(8):2235–41. doi: https://doi.org/10.1038/s41591-024-03050-2
56. Usmani SZ, Facon T, Hungria V, Bahlis NJ, Venner CP, Braunstein M, et al. Daratumumab
plus bortezomib, lenalidomide and dexamethasone for transplant-ineligible or transplant-deferred
newly diagnosed multiple myeloma: the randomized phase 3 CEPHEUS trial. Nat Med. 2025 Apr;31(4):1195–202.
doi: https://doi.org/10.1038/s41591-024-03485-7
57. Larocca A, Bonello F, Gaidano G, D’Agostino M, Offidani M, Cascavilla N, et al. Dose/schedule-adjusted
Rd-R vs continuous Rd for elderly, intermediate-fit patients with newly diagnosed
multiple myeloma. Blood. 2021 Jun;137(22):3027–36. doi: https://doi.org/10.1182/blood.2020009507
58. Manier S, Corre J, Hulin C, Laribi K, Araujo C, Pica GM, et al. A Dexamethasone Sparing-Regimen
with Daratumumab and Lenalidomide in Frail Patients with Newly-Diagnosed Multiple
Myeloma: Efficacy and Safety Analysis of the Phase 3 IFM2017-03 Trial. Blood. 2022;140 Supplement
1:1369–70. doi: https://doi.org/10.1182/blood-2022-159933
59. Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al.; International
Myeloma Workshop Consensus Panel 1. Consensus recommendations for the uniform reporting
of clinical trials: report of the International Myeloma Workshop Consensus Panel 1.
Blood. 2011 May;117(18):4691–5. doi: https://doi.org/10.1182/blood-2010-10-299487
60. Munshi NC, Avet-Loiseau H, Anderson KC, Neri P, Paiva B, Samur M, et al. A large meta-analysis
establishes the role of MRD negativity in long-term survival outcomes in patients
with multiple myeloma. Blood Adv. 2020 Dec;4(23):5988–99. doi: https://doi.org/10.1182/bloodadvances.2020002827
61. Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Overall
Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple
Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial. J Clin Oncol. 2023 Mar;41(8):1590–9.
doi: https://doi.org/10.1200/JCO.22.00940
62. Sonneveld P, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Overall
Survival With Daratumumab, Bortezomib, and Dexamethasone in Previously Treated Multiple
Myeloma (CASTOR): A Randomized, Open-Label, Phase III Trial. J Clin Oncol. 2023 Mar;41(8):1600–9.
doi: https://doi.org/10.1200/JCO.21.02734
63. Mateos MV, Sonneveld P, Hungria V, Nooka AK, Estell JA, Barreto W, et al. Daratumumab,
Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients With
Previously Treated Multiple Myeloma: three-year Follow-up of CASTOR. Clin Lymphoma
Myeloma Leuk. 2020 Aug;20(8):509–18. doi: https://doi.org/10.1016/j.clml.2019.09.623
64. Usmani SZ, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Carfilzomib,
dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with
relapsed or refractory multiple myeloma (CANDOR): updated outcomes from a randomised,
multicentre, open-label, phase 3 study. Lancet Oncol. 2022 Jan;23(1):65–76. doi: https://doi.org/10.1016/S1470-2045(21)00579-9
65. Martin T, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, et al. Isatuximab, carfilzomib,
and dexamethasone in patients with relapsed multiple myeloma: updated results from
IKEMA, a randomized Phase 3 study. Blood Cancer J. 2023 May;13(1):72. doi: https://doi.org/10.1038/s41408-023-00797-8
66. Moreau P, Mateos MV, Berenson JR, Weisel K, Lazzaro A, Song K, et al. Once weekly
versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple
myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet
Oncol. 2018 Jul;19(7):953–64. doi: https://doi.org/10.1016/S1470-2045(18)30354-1
67. Dimopoulos MA, Terpos E, Boccadoro M, Delimpasi S, Beksac M, Katodritou E, et al.;
APOLLO Trial Investigators. Daratumumab plus pomalidomide and dexamethasone versus
pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO):
an open-label, randomised, phase 3 trial. Lancet Oncol. 2021 Jun;22(6):801–12. doi: https://doi.org/10.1016/S1470-2045(21)00128-5
68. Richardson PG, Perrot A, San-Miguel J, Beksac M, Spicka I, Leleu X, et al. Isatuximab
plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone
in patients with relapsed and refractory multiple myeloma (ICARIA-MM): follow-up analysis
of a randomised, phase 3 study. Lancet Oncol. 2022 Mar;23(3):416–27. doi: https://doi.org/10.1016/S1470-2045(22)00019-5
69. Dimopoulos M, Wang M, Maisnar V, Minarik J, Bensinger W, Mateos MV, et al. Response
and progression-free survival according to planned treatment duration in patients
with relapsed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone
(KRd) versus lenalidomide and dexamethasone (Rd) in the phase III ASPIRE study. J
Hematol Oncol. 2018 Apr;11(1):49. doi: https://doi.org/10.1186/s13045-018-0583-7
70. Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab
plus Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med. 2018 Nov;379(19):1811–22.
doi: https://doi.org/10.1056/NEJMoa1805762
71. Dimopoulos M, Weisel K, Moreau P, Anderson LD Jr, White D, San-Miguel J, et al. Pomalidomide,
bortezomib, and dexamethasone for multiple myeloma previously treated with lenalidomide
(OPTIMISMM): outcomes by prior treatment at first relapse. Leukemia. 2021 Jun;35(6):1722–31.
doi: https://doi.org/10.1038/s41375-020-01021-3
72. Garderet L, Kuhnowski F, Berge B, Roussel M, Devlamynck L, Petillon MO, et al. Pomalidomide
and dexamethasone until progression after first salvage therapy in multiple myeloma.
Br J Haematol. 2023 Jun;201(6):1103–15. doi: https://doi.org/10.1111/bjh.18772
73. Goldschmidt H, Baertsch MA, Schlenzka J, Becker N, Habermehl C, Hielscher T, et al.;
German Myeloma Multicenter Group (GMMG). Salvage autologous transplant and lenalidomide
maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized
GMMG phase III trial ReLApsE. Leukemia. 2021 Apr;35(4):1134–44. doi: https://doi.org/10.1038/s41375-020-0948-0
74. Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, et al. Elranatamab
in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat
Med. 2023 Sep;29(9):2259–67. doi: https://doi.org/10.1038/s41591-023-02528-9
75. Moreau P, Garfall AL, van de Donk NW, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab
in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2022 Aug;387(6):495–505.
doi: https://doi.org/10.1056/NEJMoa2203478
76. Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk NW, Rodríguez-Otero P, et al. Talquetamab,
a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N Engl J Med.
2022 Dec;387(24):2232–44. doi: https://doi.org/10.1056/NEJMoa2204591
77. Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel
or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2023 Mar;388(11):1002–14.
doi: https://doi.org/10.1056/NEJMoa2213614
78. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene
autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell
therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a
phase 1b/2 open-label study. Lancet. 2021 Jul;398(10297):314–24. doi: https://doi.org/10.1016/S0140-6736(21)00933-8
79. San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel
or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J Med. 2023 Jul;389(4):335–47.
doi: https://doi.org/10.1056/NEJMoa2303379
80. Mateos MV. Overall Survival With Ciltacabtagene Autoleucel Versus Standard of Care
in Lenalidomide-Refractory Multiple Myeloma: Phase 3 CARTITUDE-4 Study Update. IMS;
2024.
81. Cohen AD, Mateos MV, Cohen YC, Rodriguez-Otero P, Paiva B, van de Donk NW, et al. Efficacy
and safety of cilta-cel in patients with progressive multiple myeloma after exposure
to other BCMA-targeting agents. Blood. 2023 Jan;141(3):219–30. doi: https://doi.org/10.1182/blood.2022015526
82. Nooka A, et al. Efficacy and safety of elranatamab in patients with relapsed/refractory
multiple myeloma (RRMM) and prior B-cell maturation antigen (BCMA)-directed therapies:
A pooled analysis from MagnetisMM studies. J Clin Oncol. 2023;41(16 _suppl.).
83. Ferreri CJ, Hildebrandt MA, Hashmi H, Shune LO, McGuirk JP, Sborov DW, et al. Real-world
experience of patients with multiple myeloma receiving ide-cel after a prior BCMA-targeted
therapy. Blood Cancer J. 2023 Aug;13(1):117. doi: https://doi.org/10.1038/s41408-023-00886-8
84. Bahlis NJ, Baz R, Harrison SJ, Quach H, Ho SJ, Vangsted AJ, et al. Phase I Study of
Venetoclax Plus Daratumumab and Dexamethasone, With or Without Bortezomib, in Patients
With Relapsed or Refractory Multiple Myeloma With and Without t(11;14). J Clin Oncol.
2021 Nov;39(32):3602–12. doi: https://doi.org/10.1200/JCO.21.00443
85. Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, et al. Oral Selinexor-Dexamethasone
for Triple-Class Refractory Multiple Myeloma. N Engl J Med. 2019 Aug;381(8):727–38.
doi: https://doi.org/10.1056/NEJMoa1903455
86. Grosicki S, Simonova M, Spicka I, Pour L, Kriachok I, Gavriatopoulou M, et al. Once-per-week
selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone
in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial.
Lancet. 2020 Nov;396(10262):1563–73. doi: https://doi.org/10.1016/S0140-6736(20)32292-3
87. Leong S, Lam HP, Kirkham Z, Popat R. Antibody drug conjugates for the treatment of
multiple myeloma. Am J Hematol. 2023 Mar;98(S2 Suppl 2):S22–34. doi: https://doi.org/10.1002/ajh.26750
88. Dimopoulos MA, Hungria VT, Radinoff A, Delimpasi S, Mikala G, Masszi T, et al. Efficacy
and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose
dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3):
a phase 3, open-label, randomised study. Lancet Haematol. 2023 Oct;10(10):e801–12.
doi: https://doi.org/10.1016/S2352-3026(23)00243-0
89. Hungria V, Robak P, Hus M, Zherebtsova V, Ward C, Ho PJ, et al.; DREAMM-7 Investigators.
Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J
Med. 2024 Aug;391(5):393–407. doi: https://doi.org/10.1056/NEJMoa2405090
90. Dimopoulos MA, Beksac M, Pour L, Delimpasi S, Vorobyev V, Quach H, et al.; DREAMM-8
Investigators. Belantamab Mafodotin, Pomalidomide, and Dexamethasone in Multiple Myeloma.
N Engl J Med. 2024 Aug;391(5):408–21. doi: https://doi.org/10.1056/NEJMoa2403407

