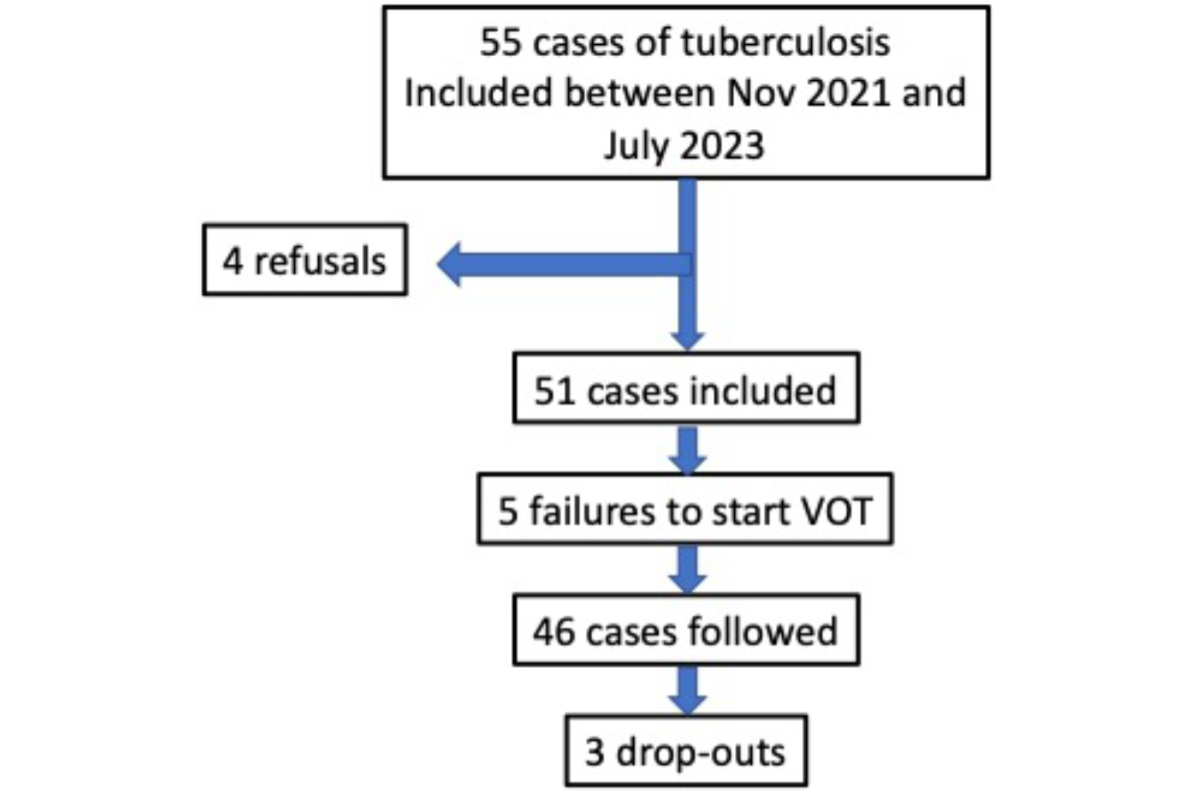
Figure 1Flowchart of the study. VOT: Video-Observed Therapy.
DOI: https://doi.org/https://doi.org/10.57187/s.4238
Directly Observed Therapy
Video-Observed Therapy
Treatment of tuberculosis (TB) remains too long, in spite of all efforts and availability of new drugs. Adherence to treatment is still a major challenge for controlling tuberculosis: non-adherence increases the risk of treatment failure and drug resistance [1]. Awareness of this problem led to the proposal of Directly Observed Therapy (DOT). Originally developed by Karel Styblo of the International Union Against Tuberculosis and Lung Disease in the 1980s, DOT was promoted in the early 1990s by WHO and is part of the Stop-tuberculosis strategy. Although DOT is probably efficient in enhancing adherence, this remains a matter of debate [2, 3]. DOT also raises several problems among which: who supervises that drugs are taken and where (tuberculosis centre, patients’ home, pharmacy …), stigma for patients and time spent for patients and healthcare workers.
In 2017, WHO endorsed Video-Observed Therapy (VOT) as an alternative to DOT for drug-susceptible and multidrug-resistant (MDR) tuberculosis. VOT has also been used for treating tuberculosis infection [4]. In recent years, several studies have documented the efficacy and feasibility of VOT in developing and a few industrialised countries [4–16]. The COVID pandemic further increased interest in VOT for managing patients with tuberculosis [17]. In 2019, a randomised controlled study performed by Story et al., including 226 patients from 22 clinics in Great Britain (incidence of tuberculosis: 7.7/100,000) was a major landmark for demonstrating the efficacy of VOT versus DOT in an industrialised country [18]. A recent systematic review of the literature also showed that VOT was comparable to DOT in terms of adherence, and that it was cost-effective, associated with improved patient satisfaction, and less time-consuming for healthcare workers [19].
Basically, VOT makes use of a smartphone and a simple app that allows the patient to send short videos to a secure platform. VOT can be used in a synchronous mode (both patient and healthcare worker are online) or asynchronous mode (when convenient to each party, the patient records and sends the video; then, the healthcare worker watches the video) [8, 20]. During the videos, the patient shows explicitly that he/she is swallowing his/her pills and can also mention any health issues to the managing team.
Swiss national guidelines for the management of tuberculosis recommend the use of DOT in patients with whom communication is impaired (e.g. a language barrier), those with a cognitive or mental disorder or in an unstable social situation, and those previously treated for tuberculosis [21]. In practice, these criteria apply to most patients treated in the Geneva area [22]. The decision to implement DOT is taken at the Canton level in agreement with the Cantonal Medical Officer, and thus varies from one Canton to another. In the Canton of Geneva, long-term local policy has so far been to encourage the use of DOT for all pulmonary cases of tuberculosis. DOT is however costly, and time-consuming for healthcare workers and patients, especially if patients must attend a dedicated centre. It also tends to emphasise stigma (for instance by imposing regular visits by a nurse or a visit to a healthcare centre) and may generate frustration. Alternative options, such as pharmacies for DOT closer to the place of work or home, also have their pitfalls. VOT appears as an efficient alternative.
The aims of this study, performed just after the SARS-CoV-2 pandemic in Switzerland, were (1) to determine whether VOT is feasible in a low-incidence high-income area, (2) to describe its acceptance rate among patients and healthcare professionals and (3) to analyse technical difficulties or pitfalls encountered.
All patients with a diagnosis of tuberculosis, irrespective of site(s) of infection (pulmonary tuberculosis or extrapulmonary tuberculosis), were invited to participate in this study.
In the Canton of Geneva, most cases of tuberculosis are identified either through the emergency department or referred to the outpatient clinic of the Division of Pulmonology of Geneva University Hospital. They are then usually hospitalised and subsequently managed as outpatients by our specialised tuberculosis nurses, a social worker and physicians of the Division of Pulmonology. The usual follow-up comprises an outpatient visit just after the hospital stay, then fortnightly visits in the first month, then monthly visits. Social worker consultations are adapted to individual needs (most patients are foreign-born, many undocumented, in the asylum process or often unaccepted asylum seekers). When DOT is implemented (default option before this study in Geneva), it is performed three times a week either at our outpatient clinic or at a local pharmacy. Our structure does not provide home visits for DOT.
According to the Swiss Public Health Office (https://www.bag.admin.ch), the annual number of cases in Geneva between 2020 and 2023 was on average 43 per year (range: 36–53). The incidence was 7–10.3/100,000 inhabitants compared to a national incidence of 3.99–4.72/100,000 inhabitants during the same period, with a transient decrease during the COVID pandemic.
Inclusion criteria for this study were as follows: patient aged over 16 years; provided informed consent (interpreters were used whenever necessary to ensure understanding of the study and the VOT procedure); has newly diagnosed tuberculosis, irrespective of site(s) of infection, confirmed by culture and/or polymerase chain reaction (PCR) (Xpert MTB/RIF Ultra, Cepheid®, USA, https://www.cepheid.com/en-GB/tests/tb-emerging-infectious-diseases/xpert-mtb-rif-ultra.html); and followed up by Geneva University Hospital. Because this was a feasibility study, VOT was proposed to all new patients between November 2021 and July 2023.
Specialised tuberculosis nurses were trained to use the VOT platform and provided the training with the help of interpreters whenever required. The app was installed on the patient’s smartphone, and a test video was sent to the platform. The app uses simple understandable pictograms and is thus not per se language-dependent. VOT was started as soon as possible after the initiation of tuberculosis treatment, either during a hospital stay or during the first outpatient follow-up visit at our outpatient clinic. Whenever necessary, a smartphone was provided to the patient, for the duration of the treatment, free of charge.
The SureAdhere® platform was used for VOT (https://dimagi.com/sureadhere/). For patient confidentiality reasons, a clone of the server storing the anonymised patient data, originally in the United States, was installed in Switzerland to comply with local legislation. The SureAdhere® team provided online training to our team and back-up for technical support whenever necessary. The SureAdhere® app is easy to use and pictogram-based. Once a video is recorded, it is sent automatically as soon as the smartphone has access to the internet. For each patient, the number of videos to be sent each week was specified (the most commonly selected option was five videos).
Patients were informed that non-urgent symptoms or treatment-related side effects could be mentioned during their videos but that there could be a delay of a few days between the video being sent and the video being watched by our specialised nurses (newly uploaded videos were systematically watched three times per week).
The following variables were recorded: age, sex, continent of origin, site of tuberculosis, type of bacteriological confirmation (microscopy, PCR, culture), resistance profile, treatment and adverse events when relevant, conditions of initiation of VOT, causes of failure of VOT, number of videos anticipated and sent (per month and total), symptoms mentioned during recordings, technical problems encountered, quality of videos (sound, image, brightness, framing). We also developed a questionnaire for the healthcare workers involved, concerning their evaluation of VOT and problems encountered. All data were recorded on REDCap® software (Vanderbilt University, Nashville, Tennessee, USA), version 10.06.28 (2021) to 14.00.30 (2024) (regular updating to the latest version performed).
The study outcomes were (1) to determine whether VOT is feasible in a low-incidence high-income area, (2) to describe its acceptance rate among patients and healthcare professionals, and implementation rate and (3) to report technical difficulties or pitfalls encountered.
Concerning the statistical analysis, because this was a descriptive non-randomised study, we did not perform a power calculation. We aimed to include a minimum of 50 patients over an 18-month period, taking into account the local tuberculosis incidence. Descriptive variables were reported as mean and standard deviation (SD), median and interquartile range (IQR) or percentage according to their distribution and nature. The ratio of observed/expected number of videos (%) was reported for total number of videos, and for individual patients (median %, IQR). We defined acceptance rate as the percentage of subjects who accepted to participate in the study, and implementation success rate as the percentage of patients who managed to start VOT among those who accepted. Acceptance rate and adherence to VOT were considered as surrogate markers of perception of VOT by patients.
To record the perceptions of nurses involved in the VOT process, all (n = 5) filled in an 18-item questionnaire with 5-point Likert scales. All analyses were performed using R Statistical Software (v4.21.2; R Core Team; 2022).
The study was reviewed and approved by the Cantonal Commission for Research Ethics (CCER) in Geneva, Switzerland (CCER 2021-01713), in agreement with the amended Declaration of Helsinki. Patients provided written informed consent to participate in this study. This study was registered at clinicaltrials.gov (NCT: 06574529).
Between 26 November 2021 and 18 July 2023, 55 patients were invited to participate in the present study.
Acceptance: 4 patients (7.3%) declined (reasons were not specified). The 51 remaining cases (acceptance rate: 92.7%) were included in our REDCap® database; their median age was 40.3 years (IQR: 33–45; range: 18–90) and 27 (53%) were female.
Implementation success rate (90.2%): 5 cases (9.8%; all with pulmonary tuberculosis) failed to start VOT (see below for details), thus leaving 46 patients followed by VOT. VOT began a median of 6.5 days (IQR: 2.0–14.0) after the start of antituberculosis therapy. Three cases (6.5%) dropped out between 144 and 180 days after starting their treatment (reasons not specified). The flowchart of the study is shown in figure 1.

Figure 1Flowchart of the study. VOT: Video-Observed Therapy.
Table 1 provides the origin of patients who started VOT (n = 46), site(s) of tuberculosis and treatment. Most patients were foreign-born; details as to social status were considered sensitive and are not reported. Table 2 provides bacteriological data (all cases were confirmed by culture and/or PCR, including resistance pattern (not performed in two cases). No cases of multidrug-resistant tuberculosis were documented during the study period.
Table 1Main characteristics of patients included in the study who started Video-Observed Therapy (VOT) (n = 46).
| Main characteristics | n | % | |
| Age in years (median and IQR) | 40 | 33–45 | |
| Female sex (n and %) | 27 | 59% | |
| Origin (n and %) | Europe | 3 | 6.5% |
| South America | 10 | 21.7% | |
| Asia | 13 | 28.2% | |
| Africa | 20 | 43.5% | |
| Site of tuberculosis (n and %)* | Pulmonary | 32 | 69.5% |
| Thoracic, non-pulmonary | 9 | 19.5% | |
| Extrathoracic | 15 | 32.6% | |
| Treatment (n and %) | 2RHZE 4HR** | 42 | 91.3% |
| Other | 4 | 8.7% | |
* Multiple answers possible.
** E: ethambutol; H: isoniazid; R: rifampicin; Z: pyrazinamide.
Table 2Details of bacteriological confirmation of included patients.
| Bacteriological confirmation | Result | n | % |
| PCR (Xpert MTB/RIF Ultra, Cepheid®, USA) | Positive | 37 | 72.5% |
| Trace | 2 | 3.9% | |
| Negative | 12 | 23.5% | |
| Mutation of rpoB gene | Positive | 0 | 0 |
| Negative | 49 | 96.1% | |
| Not performed | 2 | 3.9% | |
| Microscopy | Positive for acid-fast bacilli | 16 | 31.4% |
| Negative | 34 | 66.7% | |
| Not performed | 1 | 2.0% | |
| Culture | Positive | 37 | 73% |
| Negative | 14 | 27% | |
| Not performed | 0 | 0 | |
| Resistance to tuberculostatic drugs | None | 47 | 92.2% |
| INH | 1 | 2.0% | |
| No antibiogram | 2 | 3.9% | |
| Other | 1 | 2.0% |
INH: isonicotinic acid hydrazide; PCR: polymerase chain reaction.
When starting VOT, an interpreter or a French- or English-speaking family member was required in 15 (29.4%) cases. Teaching how to use the app (and thus how to use VOT) failed in 5 (9.8%) cases. Reasons were cognitive disorders (2), language barrier (1), lack of motivation (1) and Other (1). For 6 patients, a smartphone was provided for the duration of the study.
Treatment was 2 months of HRZE and 4 months of HR (H = isoniazid; R = rifampicin; Z = pyrazinamide; E = ethambutol) in 42 cases and Other (and thus longer) in 4 cases. Tuberculosis-related adverse events occurred in 6 cases, none of which were related to the study protocol or the app, and none of which were life-threatening, or led to hospitalisation or to discontinuation of the study.
VOT was pursued for a median of 178 days (IQR: 172–189). The specialised nurses chose the number of videos to be sent by the patient: the usual value was 5 per week; in 2 cases, daily videos were required. Thus a total of 6392 videos was expected, i.e. a median of 129 per patient (IQR: 124–163). The total number of videos received was 5744, i.e. a median of 121 per patient (IQR: 104–142). The ratio of the total number of videos received/videos expected (5744/6392) was 89.9%; the median individual ratio of videos received/videos expected was 96% (IQR: 73–100). The number of videos over the 6-month treatment period remained stable (figure 2).
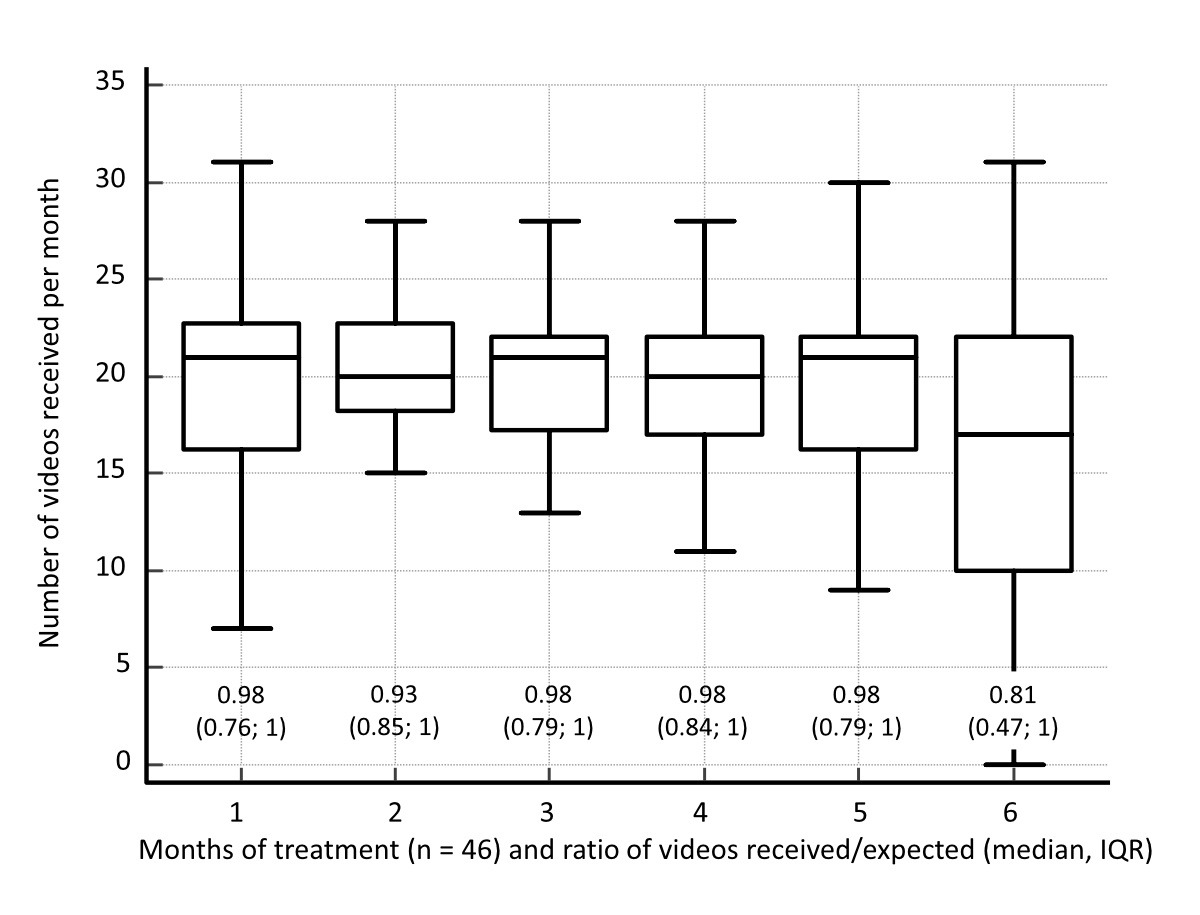
Figure 2Number of videos sent during each month of treatment after starting antituberculosis medication for 6 months, for the 46 patients starting Video-Observed Therapy (VOT). The ratio of videos received/expected is provided above the x-axis (median, interquartile range [IQR]). The numbers of videos are shown as box plots: the horizontal line depicts the median, the box denotes the interquartile range and the whiskers show the minimal and maximal values. At 6 months, 2 cases had dropped out (not deleted from figure).
A total of 12 requests for consultations was sent to the nurses via videos (median per case: 0, IQR: 0–3). Symptoms mentioned during the videos were: None (37 or 80.4%), gastrointestinal (5 or 10.9%) and Other (2 or 4.3%) (missing data: 2).
No technical problems were encountered in 35 (76%) cases. Problems noted were: the app failed to send videos (3 or 6.5%); the app didn’t work (3 or 6.5%); the videos were sent but not received on the platform (2 or 4.4%); the app was uninstalled (1 or 2.2%); and Other (7 or 15.2%: recording too long or too short; no connection to the internet; phone broken; difficulty understanding the app; videos unfinished).
Quality of videos received: of the 5744 videos, 4 (0.1%) had poor sound quality and 150 (2.6%) had poor image quality. Ten (0.2%) were out of focus, 79 (1.3%) were too dark and framing was inappropriate in 44 (0.7%) of cases. Other problems were noted in 35 (0.6%) of videos sent, among which subjects not opening their mouth to show that they had taken their treatment.
VOT was well accepted by our specialised tuberculosis nurses: results of the 18-item questionnaire are provided in figure 3.
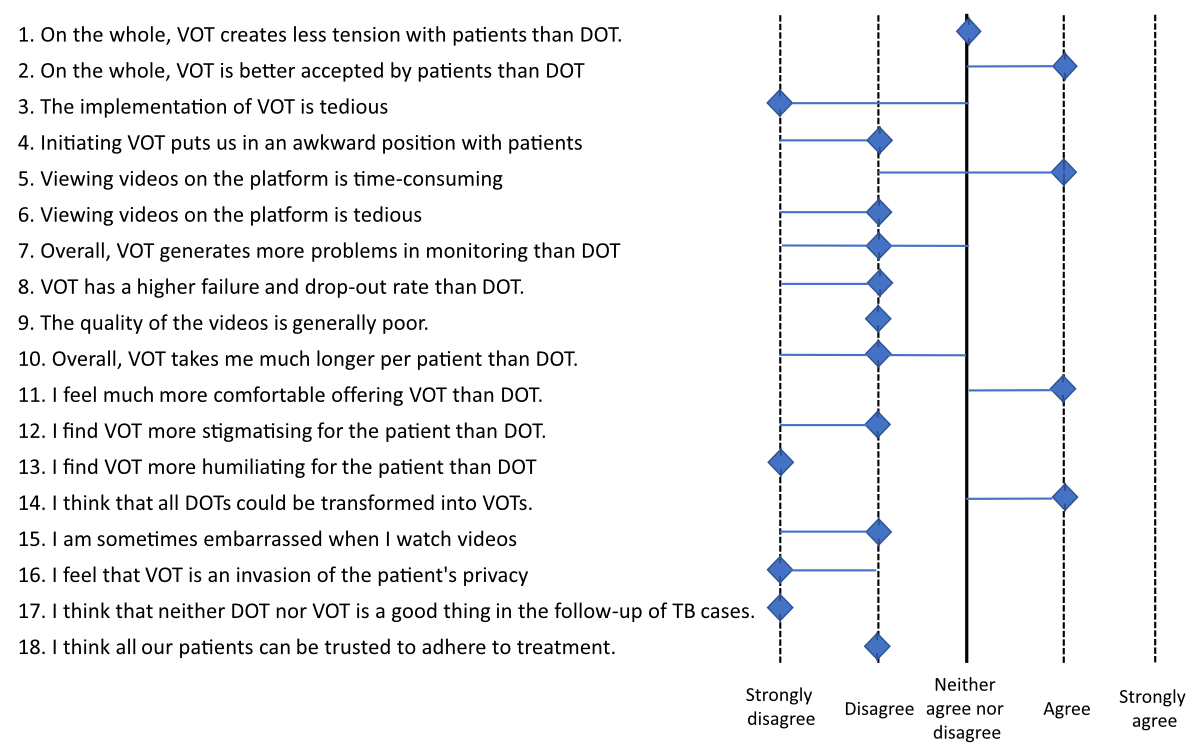
Figure 3Eighteen-item questionnaires (5-point Likert scales) given to specialised nurses involved in the study (n = 5) and completed at the end of the study period. Scales range from 1 (strongly disagree) to 5 (strongly agree). Values shown are median values for all nurses. Blue lines are interquartile ranges. DOT: Directly Observed Therapy; TB: Tuberculosis; VOT: Video-Observed Therapy.
This study was performed to document the feasibility and acceptability by patients and healthcare workers of Video-Observed Therapy (VOT) for follow-up of tuberculosis patients in a high-income low-incidence area. All tuberculosis patients were considered as potential candidates to participate in the study: 7.2% declined to participate, 9.8% of the remaining cases failed to implement VOT and 3 other cases were late dropouts. The initial acceptance rate was thus 92.7% and the implementation success rate was 90.2%. The ratio of videos sent/expected was high, 89.9%, reflecting good adherence to treatment, and the number of videos sent remained stable during the treatment period (figure 2). Technical problems encountered were minor and did not require interruption of VOT. Minor, infrequent issues were noted with video quality. For healthcare workers, perception of VOT was overall favourable (figure 3).
Therefore VOT appears to be an alternative to Directly Observed Therapy (DOT) for enhancing adherence [9, 13, 16, 18, 23–25]. In fact, the US Centers for Disease Control has recently stated that VOT was at least equivalent and probably superior to DOT in terms of impact on adherence [19]. Percentages of videos sent / expected, considered to be acceptable surrogates of adherence to treatment, have been repeatedly reported as higher than adherence to DOT, in emerging or industrialised countries, with rates of 68% to 88% [5, 7, 11, 12, 14, 23, 26].
Although dependent on local health care systems [27], and more specifically on local management of tuberculosis, VOT-associated costs are most often reported as substantially lower than clinic or field DOT [5–7, 10, 13, 18, 28]. Asynchronous VOT as performed in this study does not require the physical presence of healthcare workers and receipt and analysis of videos can be handled with greater flexibility according to the healthcare workers’ schedules. From the patient’s perspective, VOT is less stigmatising and time-consuming than any other form of DOT [29]. It avoids unnecessary trips to the tuberculosis centre while allowing patients to send messages to the managing team regarding health status, symptoms and side effects of treatment, most often in a confidential environment. Because VOT replaced DOT during the study period, this allowed a reduction in the presence of specialised tuberculosis nurses at our tuberculosis centre, and thus a financial saving.
Evaluation of VOT by our specialised nurses was overall favourable. Nurses’ perception was that VOT was better accepted than DOT, led to fewer adherence problems and was less stigmatising or potentially humiliating than DOT. Also, video images allowed the nurses to check that the prescription was followed appropriately (figure 4). Negative points were the time spent watching videos, which was also considered tedious (figure 3).
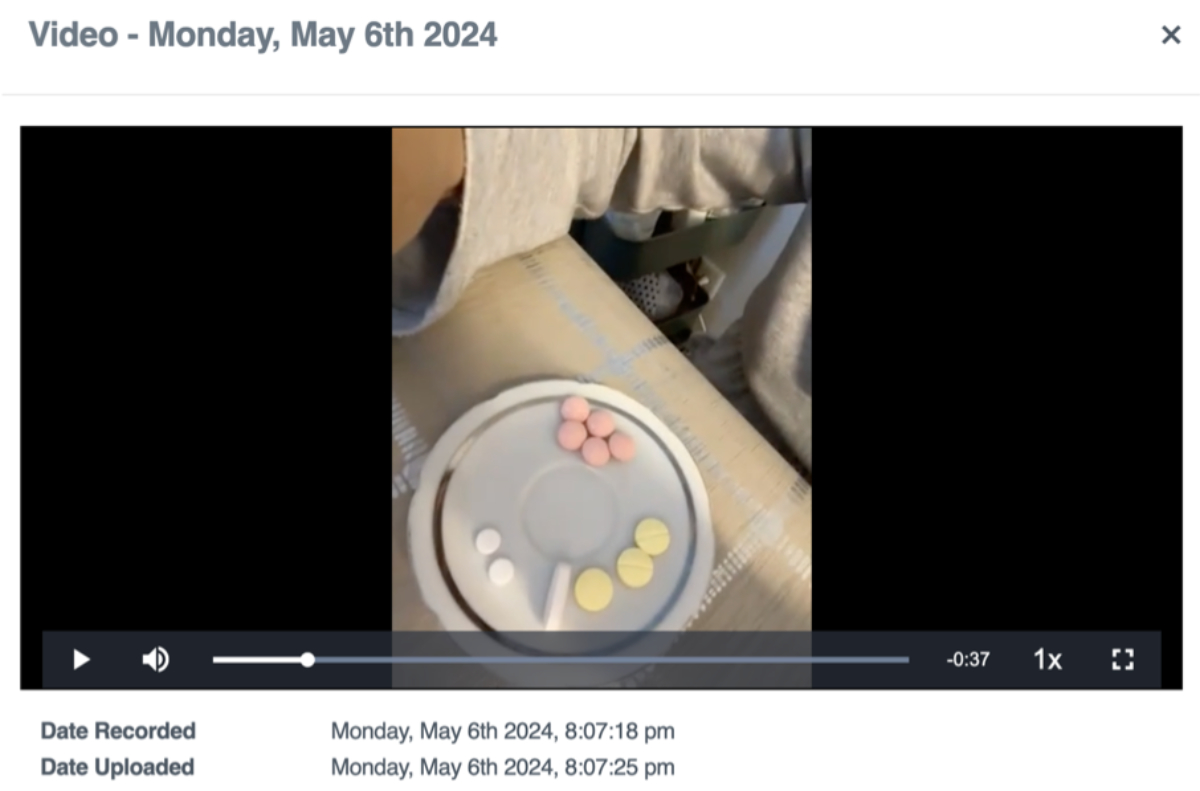
Figure 4Partial image of the SureAdhere® platform in which the patient shows the detail of his treatment before taking it.
The VOT platform has a cost. During this study, a non-profit local healthcare provider (Ligue Pulmonaire Genevoise), an official partner of our tuberculosis clinic, covered the costs related to the server and its maintenance. In six cases, smartphones were made available to patients who could not afford them with a minimal subscription. This was part of the support for the study. In some settings, for patients in a precarious financial situation and/or in emerging countries, availability of smartphones, smartphone network coverage, unstable electricity or access to internet can all be limiting factors to widespread adoption of VOT.
The patients’ perception of VOT was not formally assessed, the main reasons being the language barrier which would have rendered impossible or very costly any formal evaluation, and time constraints because the study was performed in “real-life” conditions. The high adherence and acceptance levels of VOT in this study were considered surrogate markers of positive perception by our patients, as in previous studies. Several studies have reported that patients preferred VOT over DOT because of increased convenience, privacy and perception of a more patient-centred and flexible approach, although privacy and stigma issues do not disappear with VOT [13, 30, 31]. In fact, mixed perceptions about impact on privacy and confidentiality have been reported in Uganda [32]. To our knowledge, only one study (performed in Cambodia) reported a more negative perception of VOT than at-home DOT, because of the lack of direct support provided by the healthcare workers during home visits [33].
This study has some limitations. In this proof-of-concept study, our aim was to expose as many patients as possible to VOT and to assess their acceptance and understanding of the technique in our setting. Including all tuberculosis patients irrespective of their risk of transmission is debatable. Indeed, from a community point of view, tuberculosis patients who do not have smear-positive pulmonary tuberculosis do not put their family, friends or co-workers at short-term risk in case of non-adherence to their treatment. However, in case of poor response to treatment or paradoxical evolution, VOT provides the clinician with the precious information that non-adherence can be reasonably excluded. Also, this study does not provide a formal comparison between DOT and VOT: this has been convincingly shown by others, including the important randomised controlled study performed in the UK by Story et al. and the systematic literature review by Areas Lisboa Netto et al. [18, 19]. We documented acceptance rate and ratio of videos sent/expected as surrogates of adherence, and both were high.
The numbers of patients who declined to participate, failed to start VOT or dropped out were too small to draw conclusions as to their profile: cognitive disorders, language barriers and lack of motivation were the main causes identified.
Finally, multidrug-resistant tuberculosis cases were not included: our area (locally and nationally) has been spared of multidrug-resistant tuberculosis and extensively drug-resistant (XDR) tuberculosis with a remarkably low number of cases over recent years. None occurred in Geneva during the study period.
In summary, in an 18-month observational study, including most of our tuberculosis cases, VOT was shown to be feasible in a high-income low-incidence area and to provide an alternative to DOT with high acceptance and adherence rates. VOT allows personalised supervision of adherence to treatment and seems to be less stigmatising than DOT. In our area, tuberculosis affects patients who are often in unstable social/financial/personal situations (unaccepted asylum seekers, undocumented migrants, lower economic status), and VOT provides a simple way to keep an eye on adherence and stay in touch with these patients.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The authors wish to express their gratitude to the Ligue Pulmonaire Genevoise, (https://www.lpge.ch/), a non-profit organisation involved in the care of patients with tuberculosis, chronic respiratory failure and sleep-disordered breathing, and to the Lancardis Foundation for their financial support.
We wish to thank Cyril Jaksic (Center for Clinical Research & Division of Clinical Epidemiology, Department of Health and Community Medicine, Geneva University Hospitals) for his support in the statistical and methodological aspects of this study.
We also wish to express our gratitude to Kelly Collins, Mckenna Cunniff and Patricia Waterhouse and their team at Dimagi (https://dimagi.com/sureadhere/) who provided training to our specialised nurses on the SureAdhere® platform, provided the logistics for transferring a clone of the United States SureAdhere® platform to Switzerland in compliance with local legislation and also provided technical and logistical support throughout the development of VOT at our centre and the study period.
Neither the Ligue Pulmonaire Genevoise, the Lancardis Foundation nor Dimagi participated in patient inclusion, data recording, analysis or manuscript preparation and revision.
Author contributions: Chloé Cantero, Christelle Lhonneux, Amélie Vaudaux, Sabrina Gabrielli, Anne Bergeron and Jean-Paul Janssens all participated in acquisition, analysis and interpretation of the data of this study, and in drafting and reviewing the manuscript. They all approved the final version submitted for publication and agree to be accountable for all aspects of the work, its accuracy and its integrity.
Funding was provided by the Ligue Pulmonaire Genevoise and the Lancardis Foundation.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994 Apr;330(17):1179–84. doi: https://doi.org/10.1056/NEJM199404283301702
2. Alipanah N, Jarlsberg L, Miller C, Linh NN, Falzon D, Jaramillo E, et al. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018 Jul;15(7):e1002595. doi: https://doi.org/10.1371/journal.pmed.1002595
3. Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2015 May;2015(5):CD003343. doi: https://doi.org/10.1002/14651858.CD003343.pub4
4. Holzschuh EL, Province S, Johnson K, Walls C, Shemwell C, Martin G, et al. Use of Video Directly Observed Therapy for Treatment of Latent Tuberculosis Infection - Johnson County, Kansas, 2015. MMWR Morb Mortal Wkly Rep. 2017 Apr;66(14):387–9. doi: https://doi.org/10.15585/mmwr.mm6614a3
5. Perry A, Chitnis A, Chin A, Hoffmann C, Chang L, Robinson M, et al. Real-world implementation of video-observed therapy in an urban TB program in the United States. Int J Tuberc Lung Dis. 2021 Aug;25(8):655–61. doi: https://doi.org/10.5588/ijtld.21.0170
6. Bendiksen R, Ovesen T, Asfeldt AM, Halvorsen DS, Gravningen K. Use of video directly observed treatment for tuberculosis in Northern Norway [Bruk av videosamtale i behandling av tuberkulose-sykdom i Nord-Norge]. Tidsskr Nor Laegeforen. 2020 Jan;140(1).
7. Beeler Asay GR, Lam CK, Stewart B, Mangan JM, Romo L, Marks SM, et al. Cost of Tuberculosis Therapy Directly Observed on Video for Health Departments and Patients in New York City; San Francisco, California; and Rhode Island (2017-2018). Am J Public Health. 2020 Nov;110(11):1696–703. doi: https://doi.org/10.2105/AJPH.2020.305877
8. Garfein RS, Doshi RP. Synchronous and asynchronous video observed therapy (VOT) for tuberculosis treatment adherence monitoring and support. J Clin Tuberc Other Mycobact Dis. 2019 Apr;17:100098. doi: https://doi.org/10.1016/j.jctube.2019.100098
9. Guo X, Yang Y, Takiff HE, Zhu M, Ma J, Zhong T, et al. A Comprehensive App That Improves Tuberculosis Treatment Management Through Video-Observed Therapy: usability Study. JMIR Mhealth Uhealth. 2020 Jul;8(7):e17658. doi: https://doi.org/10.2196/17658
10. Lam CK, Fluegge K, Macaraig M, Burzynski J. Cost savings associated with video directly observed therapy for treatment of tuberculosis. Int J Tuberc Lung Dis. 2019 Nov;23(11):1149–54. doi: https://doi.org/10.5588/ijtld.18.0625
11. Nguyen TA, Pham MT, Nguyen TL, Nguyen VN, Pham DC, Nguyen BH, et al. Video Directly Observed Therapy to support adherence with treatment for tuberculosis in Vietnam: A prospective cohort study. Int J Infect Dis. 2017 Dec;65:85–9. doi: https://doi.org/10.1016/j.ijid.2017.09.029
12. Olano-Soler H, Thomas D, Joglar O, Rios K, Torres-Rodríguez M, Duran-Guzman G, et al. Notes from the Field: Use of Asynchronous Video Directly Observed Therapy for Treatment of Tuberculosis and Latent Tuberculosis Infection in a Long-Term-Care Facility - Puerto Rico, 2016-2017. MMWR Morb Mortal Wkly Rep. 2017 Dec;66(50):1386–7. doi: https://doi.org/10.15585/mmwr.mm6650a5
13. Ravenscroft L, Kettle S, Persian R, Ruda S, Severin L, Doltu S, et al. Video-observed therapy and medication adherence for tuberculosis patients: randomised controlled trial in Moldova. Eur Respir J. 2020 Aug;56(2):2000493. doi: https://doi.org/10.1183/13993003.00493-2020
14. Rodrigues R, Varghese SS, Mahrous M, Ananthaneni Kumar A, Ahmed MN, D’Souza G. Feasibility and acceptability pilot of video-based direct observed treatment (vDOT) for supporting antitubercular treatment in South India: a cohort study. BMJ Open. 2023 May;13(5):e065878. doi: https://doi.org/10.1136/bmjopen-2022-065878
15. Sinkou H, Hurevich H, Rusovich V, Zhylevich L, Falzon D, de Colombani P, et al. Video-observed treatment for tuberculosis patients in Belarus: findings from the first programmatic experience. Eur Respir J. 2017 Mar;49(3):1602049. doi: https://doi.org/10.1183/13993003.02049-2016
16. Chuck C, Robinson E, Macaraig M, Alexander M, Burzynski J. Enhancing management of tuberculosis treatment with video directly observed therapy in New York City. Int J Tuberc Lung Dis. 2016 May;20(5):588–93. doi: https://doi.org/10.5588/ijtld.15.0738
17. Lippincott CK, Perry A, Munk E, Maltas G, Shah M. Tuberculosis treatment adherence in the era of COVID-19. BMC Infect Dis. 2022 Oct;22(1):800. doi: https://doi.org/10.1186/s12879-022-07787-4
18. Story A, Aldridge RW, Smith CM, Garber E, Hall J, Ferenando G, et al. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet. 2019 Mar;393(10177):1216–24. doi: https://doi.org/10.1016/S0140-6736(18)32993-3
19. Areas Lisboa Netto T, Diniz BD, Odutola P, Dantas CR, de Freitas MC, Hefford PM, et al. Video-observed therapy (VOT) vs directly observed therapy (DOT) for tuberculosis treatment: A systematic review on adherence, cost of treatment observation, time spent observing treatment and patient satisfaction. PLoS Negl Trop Dis. 2024 Oct;18(10):e0012565. doi: https://doi.org/10.1371/journal.pntd.0012565
20. Burzynski J, Mangan JM, Lam CK, Macaraig M, Salerno MM, deCastro BR, et al.; eDOT Study Team. In-Person vs Electronic Directly Observed Therapy for Tuberculosis Treatment Adherence: A Randomized Noninferiority Trial. JAMA Netw Open. 2022 Jan;5(1):e2144210. doi: https://doi.org/10.1001/jamanetworkopen.2021.44210
21. Schoch OD, Barben J, Berger C, Böttger EC, Egger JM, Fenner L, et al. Tuberculosis in Switzerland. Guidance for healthcare professionals Bern, Switzerland; 2021. Available from: https://www.liguepulmonaire.ch/centre-de-competence-tuberculose/directives
22. Kherad O, Herrmann FR, Zellweger JP, Rochat T, Janssens JP. Clinical presentation, demographics and outcome of tuberculosis (TB) in a low incidence area: a 4-year study in Geneva, Switzerland. BMC Infect Dis. 2009 Dec;9(1):217. doi: https://doi.org/10.1186/1471-2334-9-217
23. Bachina P, Lippincott CK, Perry A, Munk E, Maltas G, Bohr R, et al. Programmatic Adoption and Implementation of Video-Observed Therapy in Minnesota: Prospective Observational Cohort Study. JMIR Form Res. 2022 Aug;6(8):e38247. doi: https://doi.org/10.2196/38247
24. Holzman SB, Atre S, Sahasrabudhe T, Ambike S, Jagtap D, Sayyad Y, et al. Use of Smartphone-Based Video Directly Observed Therapy (vDOT) in Tuberculosis Care: Single-Arm, Prospective Feasibility Study. JMIR Form Res. 2019 Aug;3(3):e13411. doi: https://doi.org/10.2196/13411
25. Holzman SB, Zenilman A, Shah M. Advancing Patient-Centered Care in Tuberculosis Management: A Mixed-Methods Appraisal of Video Directly Observed Therapy. Open Forum Infect Dis. 2018 Apr;5(4):ofy046. doi: https://doi.org/10.1093/ofid/ofy046
26. Sekandi JN, Buregyeya E, Zalwango S, Dobbin KK, Atuyambe L, Nakkonde D, et al. Video directly observed therapy for supporting and monitoring adherence to tuberculosis treatment in Uganda: a pilot cohort study. ERJ Open Res. 2020 Apr;6(1):00175–02019. doi: https://doi.org/10.1183/23120541.00175-2019
27. Nsengiyumva NP, Khan A, Gler MM, Tonquin ML, Marcelo D, Andrews MC, et al. Costs of Digital Adherence Technologies for Tuberculosis Treatment Support, 2018-2021. Emerg Infect Dis. 2024 Jan;30(1):79–88. doi: https://doi.org/10.3201/eid3001.230427
28. Garfein RS, Liu L, Cuevas-Mota J, Collins K, Muñoz F, Catanzaro DG, et al. Tuberculosis Treatment Monitoring by Video Directly Observed Therapy in 5 Health Districts, California, USA. Emerg Infect Dis. 2018 Oct;24(10):1806–15. doi: https://doi.org/10.3201/eid2410.180459
29. Ting NC, El-Turk N, Chou MS, Dobler CC. Patient-perceived treatment burden of tuberculosis treatment. PLoS One. 2020 Oct;15(10):e0241124. doi: https://doi.org/10.1371/journal.pone.0241124
30. Chen EC, Owaisi R, Goldschmidt L, Maimets IK, Daftary A. Patient perceptions of video directly observed therapy for tuberculosis: a systematic review. J Clin Tuberc Other Mycobact Dis. 2023 Nov;35:100406. doi: https://doi.org/10.1016/j.jctube.2023.100406
31. Sekandi JN, McDonald A, Nakkonde D, Zalwango S, Kasiita V, Kaggwa P, et al. Acceptability, Usefulness, and Ease of Use of an Enhanced Video Directly Observed Treatment System for Supporting Patients With Tuberculosis in Kampala, Uganda: Explanatory Qualitative Study. JMIR Form Res. 2023 Nov;7:e46203. doi: https://doi.org/10.2196/46203
32. Sekandi JN, Kasiita V, Onuoha NA, Zalwango S, Nakkonde D, Kaawa-Mafigiri D, et al. Stakeholders’ Perceptions of Benefits of and Barriers to Using Video-Observed Treatment for Monitoring Patients With Tuberculosis in Uganda: Exploratory Qualitative Study. JMIR Mhealth Uhealth. 2021 Oct;9(10):e27131. doi: https://doi.org/10.2196/27131
33. Rabinovich L, Molton JS, Ooi WT, Paton NI, Batra S, Yoong J. Perceptions and Acceptability of Digital Interventions Among Tuberculosis Patients in Cambodia: Qualitative Study of Video-Based Directly Observed Therapy. J Med Internet Res. 2020 Jul;22(7):e16856. doi: https://doi.org/10.2196/16856