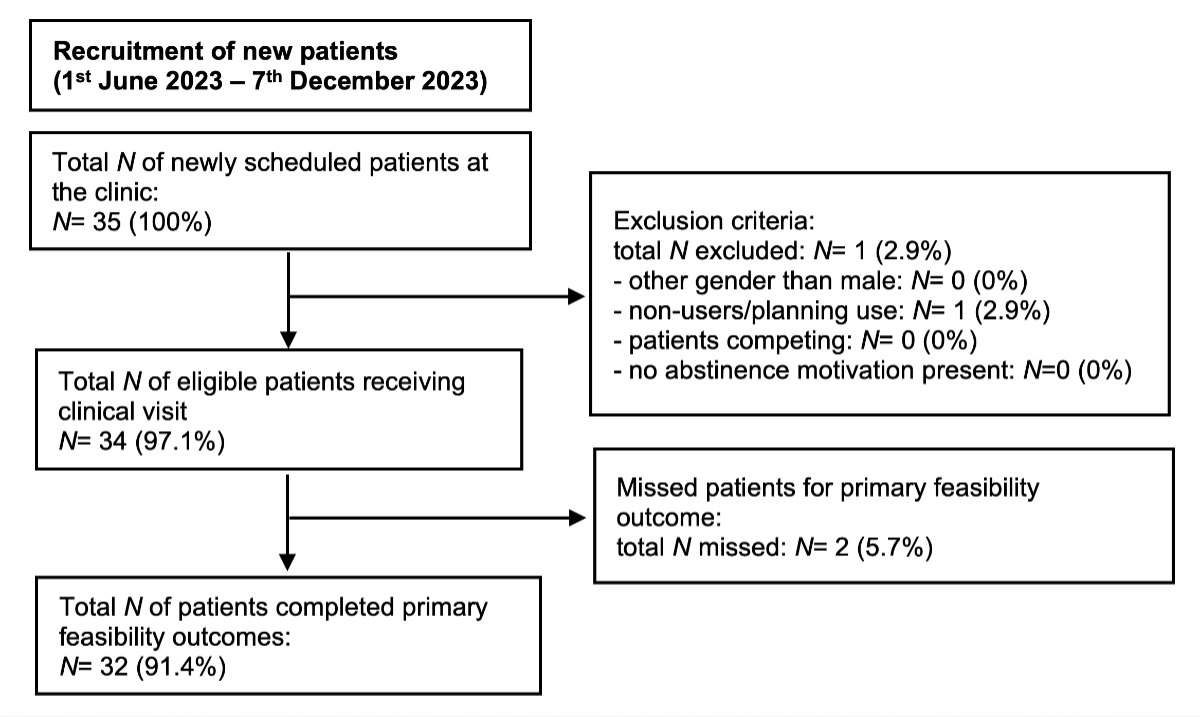
Figure 1Participant flow. Flow diagram of patients included in this quality assurance study.
DOI: https://doi.org/https://doi.org/10.57187/s.4225
The use of anabolic androgenic steroids, the most frequently used anabolic agents, as well as the use of other image- and performance-enhancing drugs to achieve personal image and sports performance goals represents one of the most recent global substance use disorders [1–5]. The global lifetime prevalence of anabolic androgenic steroids use is estimated as high as 1–5% in the general population and up to 30% among recreational gym users, with males being predominantly affected [6–8]. The problematic use of these substances should be considered a serious risk for public health, particularly as the popularity and prevalence of image- and performance-enhancing drugs appears to have increased in recent years [5]. Also, in Switzerland their use appears widespread with estimates of over 200,000 anabolic androgenic steroids users [8, 9]. Image- and performance-enhancing drugs in the context of sport or “doping” are banned by the World Anti-Doping Agency (WADA) and prohibited by regulatory agencies but can easily be acquired from different non-medical sources [10–12].
Anabolic androgenic steroids are often used in complex use patterns (so-called cycles) with additional extensive polypharmacy with other medications and possible concomitant substance use [3, 4]. The aim of these use patterns is to exploit synergistic effects on increasing fat-free muscle mass and strength as well as hypertrophic changes in muscle volume combined with strength training, but also to minimise side effects and speed up mental and physical recovery [3, 4]. Information on the use of these substances often originates from non-medical sources which often leads to major misinformation of users [4].
Acute and long-term side effects of image- and performance-enhancing drug use are various and complex, and may affect all levels of health – physical, mental and social wellbeing [4, 5, 13, 14]. Emerging evidence from cohort studies demonstrates that people who use these substances in recreational sports may have an increased mortality risk from both natural (i.e. cardiovascular-related deaths and cancer) as well as unnatural (accidents, violent crimes or suicide) causes [15]. Importantly, the development of substance dependence is common among anabolic androgenic steroids users [4, 16, 17]. Despite highly prevalent side effects, only a minority of anabolic androgenic steroids users will attend medical care or declare this substance use if they visit a physician [4, 18].
Most often anabolic androgenic steroids users self-medicate for anabolic androgenic steroids-related health problems and support is most often sought from non-medical sources (i.e. experienced users/peers or online forums) [19]. Although these services are commonly wanted by anabolic androgenic steroids users, several reasons for not accessing regular healthcare services are known. Most often these include fear of stigma or judgmental reactions from healthcare workers, as well as perceived lack of trust and lack of knowledge from healthcare professionals, but also the inability to obtain drugs wanted for treatment [19]. Doping policies in Switzerland can criminalise doctors who provide medical care to these patients under current legislation under certain circumstances. Doctors often face legal uncertainty when providing care for those patients, especially when it comes to harm-reducing measures [9]. Recently, a primary healthcare practice was established for anabolic androgenic steroids users in the Arud Centre for Addiction Medicine in Zurich, Switzerland. There are only a few medical services in Europe focused on helping patients with health problems related to anabolic agents [20], and to our knowledge no data of this kind is available in a Swiss context. The present quality assurance study aims to evaluate the feasibility of implementing current best clinical practice for anabolic androgenic steroids users within a Swiss primary care practice. Furthermore, routinely obtained data to capture current best practice are analysed and compared to existing best practice standards within the medical literature.
Quality assurance studies are investigations with the purpose of monitoring or improving the quality of service delivered by an institution. These studies comprise activities involving the systematic evaluation of healthcare practices to improve patient care by analysing routinely obtained data to capture current best practice compared to existing best practice standards. The methodology of feasibility studies was used for this study [21–23]. Understanding the feasibility of implementing best clinical practice will aid the development of future studies with the appropriate power for hypothesis testing.
From 1 June 2023 onwards, patients presenting at the primary care practice at the Arud centre who fulfilled eligibility criteria were accepted. They could be self-referred (low-threshold access) or referred from medical specialists. The recruitment strategy was word-of-mouth advertising among anabolic androgenic steroids users, flyers about the healthcare service in a gym, as well as an associated “queer health” clinic. Eligibility criteria were selected mostly based on the established antidoping regulations on providing medical care for anabolic androgenic steroids users in Switzerland.
Inclusion criteria:
Exclusion criteria:
A primary healthcare practice for current or past anabolic androgenic steroids users was established at the Arud Centre for Addiction Medicine in Zurich to improve the provision of medical care for anabolic androgenic steroids users as well as to gain more insights into the characteristics of users, methods of use and health risks associated with use in Switzerland. The healthcare provided is covered by mandatory healthcare insurance besides the deductible and co-payment. Medically indicated off-label medication was given directly at the practice and was not covered by the health insurance. Arud is located in central Zurich. The service for anabolic androgenic steroids users has been running since 1 June 2023. The Arud centre provides integrated medical care for patients with problematic substance use or substance use disorders.
The baseline assessment was implemented based on current best practice guidance from the published literature [3, 7, 20, 24–31]. During the first clinical appointment, a focused patient history from eligible patients was assessed, and they received a physical, an instrumental (i.e. ECG) and a laboratory examination (i.e. blood and urine), as well as a psychometric evaluation (i.e. screening questionnaires for muscle dysmorphia and anabolic steroid addiction [32, 33]). Health problems regarding use of these substances were asked based on a preselected list of items (quantitative) in a patient history form, leaving space for qualitative information [4, 34]. For mental health problems, no validated psychometric tools were used; disease symptoms were asked during the consultation. A conclusive list of items that were assessed during the first clinical visit of each patient can be found in table S1 in the appendix.
With this quality assurance study, we aim:
We hypothesise that integration of medical services for anabolic androgenic steroids users into primary care practice is feasible and accepted by patients in a Swiss primary care context.
Customer experience metrics have been successfully used in the medical field to measure patient experiences. Possible metrics to measure patient experiences are the single‐item net promoter score as well as the customer satisfaction score [35–37]. These metrics have been used in a variety of industries around the world, including banking, insurance and technology and have been adopted into healthcare settings, frequently for the purpose of system‐level benchmarking. Primary feasibility outcomes for this study were:
Secondary feasibility outcomes were:
Based on the feasibility outcomes, the main targets determining the success of feasibility were:
Net promoter score: Respondents must answer the question “How likely is it that you would recommend the service to a friend or colleague/peer?” on a 10-point scale from 0 (Not likely) to 10 (Very likely). Responses are placed into one of three groups depending on the rating: Detractors (0–6), Passives (7 or 8) and Promoters (9 or 10). The overall score is calculated by subtracting the percentage of Detractors from the percentage of Promoters; therefore the net promoter score can range from −100 (i.e. all Detractors) to +100 (i.e. all Promoters) with the final result displayed as an integer. Interpretation is suggested as: -100–0: needs improvement; 0–30: good; 30–70: great; 70–100: excellent; with a score ≥30 commonly associated with positive word-of-mouth advertising (return as well as referral).
Customer satisfaction score: Respondents must answer the question “How satisfied were you with the service provided?” on a 5-point scale from 1 (Very dissatisfied) to 5 (Very satisfied). The overall score is calculated as the percentage of satisfied customers (a rating of 4 [Satisfied] or 5) divided by the total number of responses, then multiplied by 100 to obtain a customer satisfaction percentage. Interpretation is suggested as: 0–50%: needs improvement; 50–70%: fair; 70–90%: good; 90–100%: excellent; with a score ≥70% commonly associated with great and excellent satisfaction with the delivered services.
Recruitment rate: defined as the proportion of eligible patients recruited per consultation day. The proportion is calculated as the percentage of eligible patients presenting at the practice divided by the total number of consultation days. For cost-effective implementation, ≥1 new recruited patient(s) per consultation day was estimated.
Consent rate: defined as the proportion of eligible participants consenting to research. The proportion is calculated as the percentage of eligible patients presenting at the practice and consenting to research divided by the total number of eligible patients. No clear cut-off was defined, and it is informative for the future development of experimental studies with hypothesis testing (e.g. sample size calculation).
Simple descriptive statistics were used to display quantitative data.
An appropriate sample size needs to be determined, not for providing appropriate power for hypothesis testing, but to understand the feasibility of participant recruitment or study design. A sample size of ≥30 patients was determined for this evaluation based on Junyong et al. [22].
Routinely collected data from patient history, clinical information, laboratory analysis, psychometric screening instruments, as well as instrumental examination from clinical appointments were captured on paper (hard copy) and were transferred to an Excel spreadsheet saved on a local password-protected server. All relevant patient data and consent forms were entered into an electronic medical record system (Triamed). Documentation was filled in by two clinicians (RM/KK). Questionnaires for primary feasibility outcomes (self-reported and anonymous) were captured in RedCap through a QR code with final scores saved on a server at the University of Zurich. At the end of the evaluation all case files were reviewed for data analysis. The data extraction for this study was done at the end of the study and was stored in a secure cloud system (RM/KK).
Simple descriptive statistics were used to display quantitative data. For continuous variables, mean and standard deviation were calculated. For categorical variables, proportions were calculated.
In case of missing data, missing variables were removed and datasets for each item were analysed with a reduced sample size.
The present quality assurance study was reviewed by the Cantonal Ethics Committee Zurich, Switzerland (BASEC-Nr.: Req-2024-00586) and did not fall within the scope of the Human Research Act (HRA).
Each patient attending the practice was informed about the possibility of future research with routinely collected data and biological samples. A general research consent by the Arud centre was used. The general consent was approved through the Ethics Commission of the canton of Zurich (19 August 2015). Patients were asked to sign an informed research consent during the first consultation. Patients could either accept or reject the informed research consent. Patients who did not wish to consent received the same standard of care, clinical workup and treatment as patients who consented.
Data generalisation was used as the data anonymisation technique. Data was completely anonymised; all individual or identifiable data was deleted. Strategies included mapping several values into a single value or range. All relevant health-related personal data and biological samples were entered into an electronic medical record system. Non-anonymised data was only accessible by healthcare specialists involved in the patients’ treatment. The involved healthcare specialists conducted the anonymisation so that study team members were not able to identify any participants. All medical information obtained within this study will be considered confidential.
The medical care of athletes is subject to strict legal requirements, which currently limits holistic medical care by doctors under criminal law. In Switzerland, the provision of medical care is based on the medicinal products law (HMG, SR 812.21.; VAM, SR 812.212.21.), the provisions of the doping and narcotics (BetmG) legislation [38], as well as the Medical Professions Act (MedBG). In addition to these legally binding standards, the professional ethics rules of the Swiss Medical Association (FMH) [39] also serve as an important basis. A legal opinion ([40], available in German only) supporting the implementation of best current practice was conducted prior to implementation and is publicly available.
All data generated or analysed during this study is included in this published article and its supplementary information files.
The flow diagram of patients included in this study is shown in figure 1. During the recruitment period, we included 34 patients seen at the practice. One patient did not fulfil eligibility criteria as he was a non-user planning to use these substances, thus was outside the legal scope and was not received. Two patients failed to complete the feasibility questionnaire (primary feasibility outcome).

Figure 1Participant flow. Flow diagram of patients included in this quality assurance study.
From 1 June to 7 December 2023, patients who fulfilled eligibility criteria were seen at the practice. Consultation days occurred once weekly from 1 June to 31 August 2023, and twice per week from 1 September 2023 to the end of recruitment. Patients were included for data analysis if they provided informed consent until the necessary sample size was achieved.
All sociodemographic data of male participating patients are summarised in table S2 in the appendix. Most patients were self-referrals (82%) seeking medical laboratory screening while using anabolic agents (91%), but also seeking support with anabolic steroid cessation (47%) or advice and information about the problematic use of these substances (21%). The mean age of patients was 38.5 years (standard deviation [SD]: 8) with a range from 21 to 61 years of age displayed in figure 2. Patients were mostly homosexual (68%) or heterosexual (29%) males; either in a relationship (56%) or single (35%); were currently working (88%); had an educational level beyond compulsory schooling (94%); many had an academic career (41%). Patients were most frequently working out over 3 days per week and had multiple years of experience at the gym. Patients had diverse professional backgrounds (qualitative data), including directors/CEOs, managers, IT specialists, engineers, insurance specialists, as well as a consultant, lawyer, police officer, school principal, hairdresser, photographer, banker, medical aesthetician, sex worker, with some being unemployed and on social welfare.
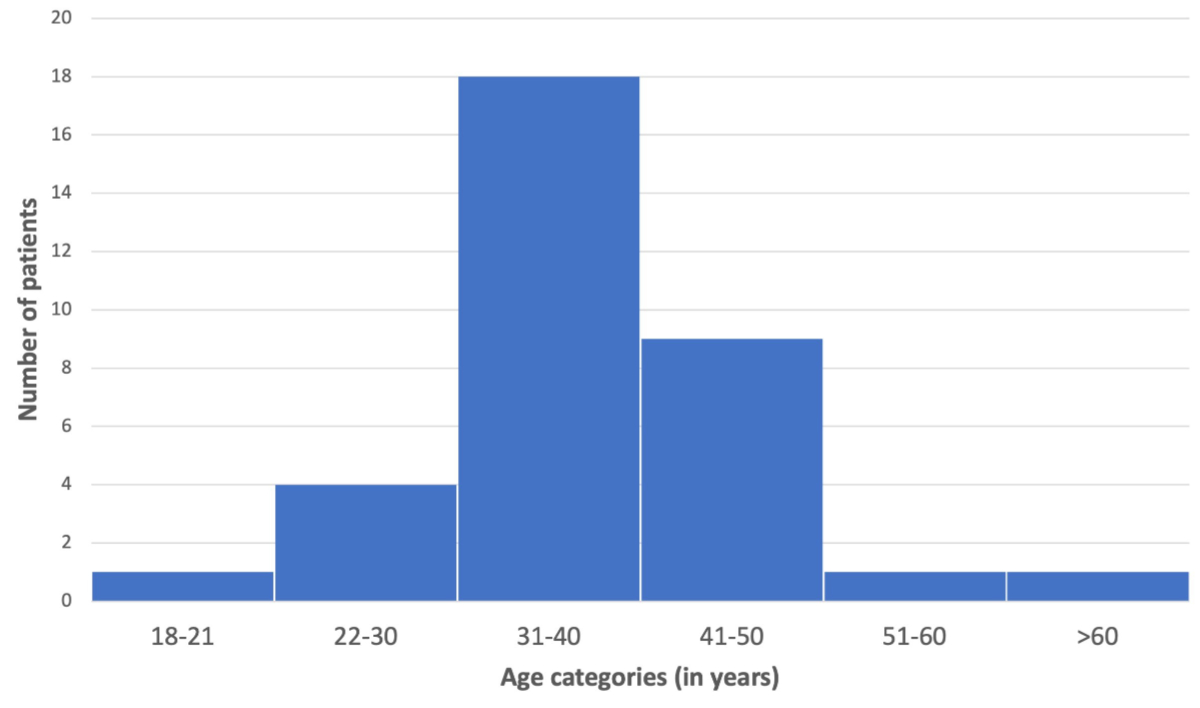
Figure 2Age distribution of patients using anabolic androgenic steroids presenting at the primary care practice during the first clinical assessment in Zurich grouped by age categories.
Feasibility outcomes are displayed in table 1. Patients were asked anonymously how likely they would recommend the provided medical services for anabolic androgenic steroids to a friend or peer/friend (net promoter score) as well as to rate their experience with the provided service (customer satisfaction score) – 32 patients answered the primary feasibility outcomes. All (100%) were Promoters of the service and were satisfied with the service they received. The overall calculated net promoter score was 100 (integer) which refers to excellent overall results of the medical services provided for anabolic androgenic steroids users. The customer satisfaction score was 100% which refers to excellent overall patient satisfaction. Secondary feasibility outcomes demonstrate that the recruitment rate was lower than target with 0.92 clients recruited per consultation day. In this evaluation all patients consented to research (100%).
Table 1Feasibility outcomes.
| Primary feasibility outcomes | Net promoter score | n | % |
| Promoters (rating of 9 or 10) | 32/32# | 100% | |
| Passives (7 or 8) | 0 | 0% | |
| Detractors (0 to 6) | 0 | 0% | |
| Achieved net promoter score | Feasibility outcome target | ||
| Final net promoter score (integer)* | 100 | >30 | |
| Customer satisfaction score | n | % | |
| Satisfied (rating of 4 or 5) | 32/32# | 100% | |
| Unsatisfied/Indifferent (rating of 1 to 3) | 0 | 0% | |
| Achieved customer satisfaction score | Feasibility outcome target | ||
| Final customer satisfaction score (%)** | 100% | >70% | |
| Secondary feasibility outcomes | Achieved outcome target | Feasibility outcome target | |
| Recruitment rate*** | 0.92 | ≥1 | |
| Consent rate to research**** | 100% | Not defined |
* Net promoter score interpretation: -100–0: needs improvement; 0–30: good; 30–70: great; 70–100: excellent.
** Customer satisfaction score interpretation: 0–50: needs improvement; 50–70: fair; 70–90: good; 90–100: excellent.
*** Defined as the proportion of eligible patients recruited per consultation day.
**** Defined as the proportion of eligible participants consenting to research.
# Missing data n = 2
Patient-reported medical problems from use of anabolic agents at patient presentation are summarised in table S3 in the appendix. Most patients (97%) presented with medical problems, physical (97%) or signs of mental health problems (68%).
Current and past medication history (patient-reported and/or based on medical record), as well as concomitant substance use (self-reported) are summarised in table S4 in the appendix. During presentation at the practice, the use of anabolic steroids (91%) was most prevalent, with other anabolic agents (18%), antioestrogens (15%) as well as other image- and performance-enhancing drugs or methods (e.g. synthol injections) (15%) also being used, although patients had exposure to these substances more frequently in the past with 97%, 62%, 64%, 47%, respectively. Other medications (e.g. antiretroviral therapy, psychotherapeutics) were frequent. Concomitant illicit substance use (65%) among patients was frequently reported in the past year (excluding recreational alcohol use [59%] and nicotine i.e. tobacco, smoking and/or vaping [47%]).
Consumption characteristics of anabolic androgenic steroids can be seen in table S5 in the appendix. The mean age at which anabolic steroid use was initiated was 30.6 years (SD: 9.53) and they had been used over a mean duration of 4.91 years (SD: 3.58). Overall, consumption patterns were very complex. Most often more than one anabolic androgenic steroid was used during one application (“stacking”) (71%) with changes in dosages (“pyramiding”) (59%), as well as weekly dosages of testosterone compounds up to 2000 mg per week that were administered. Most patients were using anabolic androgenic steroids in cyclic consumption patterns (73%) and/or had been using a continuous consumption pattern (29%). First-time use during consultation was reported in six patients (18%). Most patients presented anywhere after 1 to >15 cyclic applications with anabolic androgenic steroids most often used over 3–4 months per application (52%). Most patients followed safer-use practices when using anabolic steroids (97%). Substances for use were mostly acquired from non-medical sources (figure 3). Of the many motivations for using these substances, the most frequent was to improve physical appearance and increase muscle mass (figure S1 in the appendix).
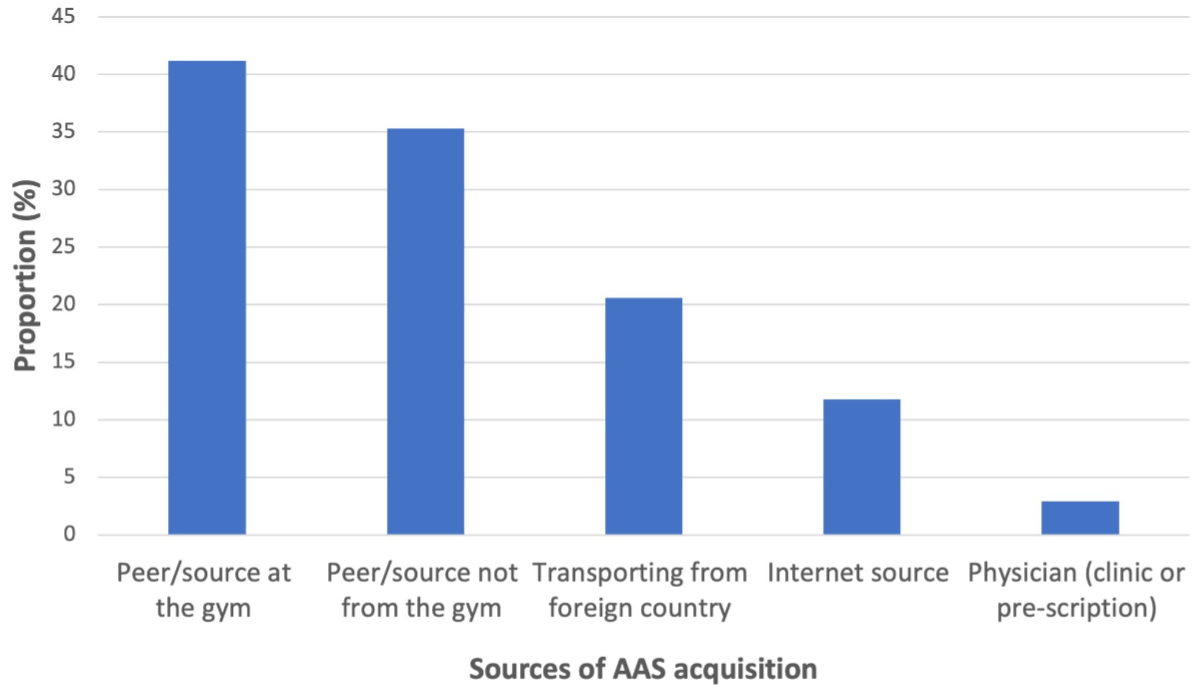
Figure 3Sources of anabolic androgenic steroid (ASS) acquisition among patients using anabolic androgenic steroids (proportion in %) presenting at the primary care practice during the first clinical assessment in Zurich, grouped by sources.
Sexual and reproductive medical history (patient-reported) can be seen in table S6 in the appendix. The 12-month prevalence of different sexual partners in this study was high: most patients reported ≥5 different sexual partners (62%), and over 50 different sexual partners was frequent (35%). Most patients reported having STI tests within the 12 months before consultation (67.6%). Few patients had children (9%), and a minority had a wish for children in the future (38%).
Past medical history (patient-reported and/or based on medical record) can be seen in table S7 in the appendix. Most patients (79%) reported pre-existing medical conditions. Notably, the most frequent physical medical conditions concerned cardiovascular disease (hypertension [15%], dyslipidaemia [9%], obstructive sleep apnoea [12%]), musculoskeletal problems (muscle tendon rupture [9%]), cancer (9%), infectious diseases (HIV [18%]; hepatitis B [3%], assessed by medical history; hepatitis C (3%), assessed by residual antibodies), as well as mental health conditions (ADHD [21%] and depression/anxiety [9%]).
A physical patient examination was conducted in all patients. Abnormal clinical findings among anabolic androgenic steroids users were common, particularly regarding blood pressure measurements (35%) and anthropometry (77%), hair (38%) and skin (53%), cardiovascular (44%), breast examination (41%) and urogenital examination (29%), and less frequent for abdominal (3%), musculoskeletal (3%), neurological (3%), pulmonary (0%) or thyroid (0%) examinations. An overview of abnormal findings in patient examinations is displayed in table S8 in the appendix.
An overview of abnormal findings in laboratory as well as ECG examinations is displayed in table S9 in the appendix. A laboratory examination was conducted in all patients. Abnormal clinical findings among anabolic androgenic steroids users were common, particularly regarding the standard hormone panel (97%), creatine kinase test (77%), lipid panel (65%), liver function tests (53%), haematology (44%), prostate-specific antigen (PSA) test (29%), kidney function tests (21%), iron profile (18%), urine analysis (9%) and electrolyte panel (12%). All glucose tests were normal. Furthermore, all patients received an ECG screen, whereas 59% demonstrated abnormal findings – approximately one quarter (21–29%) of all were screened positive for left ventricular hypertrophy with different ECG indices (Sokolov-Lyon criteria, Romhilt-Estes criteria). Importantly, regarding PSA levels, two patients screened were found to have moderate to high levels of PSA; one patient was diagnosed with a metastatic prostate cancer.
Results from a brief psychiatric evaluation are shown intable S10 in the appendix. When asked, half of the patients reported problems with self-worth and body image (50%) with a great proportion (38%) screened at risk of body dysmorphic disorder. Over a quarter of patients (27%) reported problems with drug and substance use, as well as having problems with sexuality and relationships (29%) – a majority were positive for being at risk of substance dependence for androgens (64%). Patients were asked if they would like to talk to a psychiatrist/psychotherapist – notably, approximately a third (32%) wanted to further discuss mental health problems with a specialist or were already in psychiatric care.
In this quality assurance study, feasibility was measured by the net promoter score and customer satisfaction score to determine how likely respondents would either recommend the received services for anabolic androgenic steroids to a friend or peer (loyalty) or to rate their experience with the service (satisfaction), respectively; both outcomes were fully met. Offering best clinical practice by a primary health care practice for anabolic androgenic steroids users was feasible and highly accepted, and results indicate that they would most likely return to use the services and promote positive word-of-mouth propaganda and referral of the service. The current implementation phase was interpreted as successful, and the intervention will be continued and further evaluated for roll-out at a bigger scale. Importantly, consenting to research among anabolic androgenic steroids users was very high (100%). A future subsequent larger study, with the appropriate methodology, power and sample size for hypothesis testing should be feasible to further investigate and confirm these initial study results among patients attending the primary care practice. The recruitment rate was slightly below expectations. Future research will focus on how to appropriately access this hard-to-reach population and how to establish trust in the provided primary healthcare services among users, as our data suggests a level of mistrust in medical care offerings – a finding consistent with the literature [19].
The methodology of quality assurance aims to determine the feasibility of implementation, not for providing appropriate power for hypothesis testing – this may have led to a risk of bias, particularly a selection bias, sampling bias (small sample size), as well as detection bias. The recruitment strategy of this current study was word-of-mouth propaganda among patients and advertisements of this service in a gym, as well as the associated “queer health” practice of the Arud centre. Furthermore, the Arud centre is specialised in healthcare for substance use disorders and infectious diseases (i.e. HIV, hepatitis C) – likely leading to a selection bias. Furthermore, patients were interviewed for data collection during the first clinical visit that may have led to different bias(es) in this research, such as an information bias (i.e. recall bias), interviewer bias, question-order bias or response bias. These bias(es) may have impacted the results and conclusions, as well as generalisability of this quality assurance study; thus, these results must be interpreted with caution. Also, little external validity exists with only few studies that assessed the implementation of such healthcare services within the published medical literature, leading to limited comparability of current studies [20].
The results of this quality assurance study are interpreted with the contemporary knowledge and recent understanding of the non-medical use of anabolic steroids. The current evidence most often consists of case-control studies, user surveys, retrospective reviews and case series. The lack of randomised controlled and prospective data are limitations. More trials are needed with solid methodologies to further validate and build upon these initial results. The recruitment strategy needs to be adapted for future studies to provide a more representative patient cohort. Previous research demonstrated that peers, rooted in lived experiences, play an important role in mitigating potential risks associated with anabolic steroid and image- and performance-enhancing drug use [4]. This highlights that incorporating peers in developing comprehensive and effective harm-reduction strategies for anabolic androgenic steroids users may be crucial for the future success of the implementation.
Characteristics of anabolic androgenic steroids users presenting in a primary care setting within this Swiss sample broadly reflects data from previously published literature [4, 20]. The typical anabolic androgenic steroids users who accessed medical services were young male professionals with a higher education. Importantly, the use of these substances was also prevalent among young adults as well as older males. Furthermore, this sample demonstrates a high proportion of men that have sex with men (MSM) using these substances. These patient demographics appear to be of particular interest for healthcare regarding prevalence, negative health outcomes, accessing healthcare and/or risk-taking behaviour [41–45]. Overall, the main motivations, among many, for using these substances in this sample were to improve physical appearance. The consumption patterns demonstrate that many different anabolic agents have been used by patients in the past, with anabolic androgenic steroids commonly used in complex use patterns with supraphysiological testosterone dosages, extensive polypharmacy, as well as concomitant illicit substance use. Knowledge of these use characteristics will help in developing tailored harm-reduction services (i.e. adolescents, women, MSM) and health messaging which will be further explored in future studies.
In addition to evaluating the feasibility of current best clinical practice for anabolic androgenic steroids users, this quality assurance study has identified various health problems among anabolic androgenic steroids users based on clinical, psychometric, instrumental and laboratory evaluations and findings – they are complex, multifactorial and many are not comprehensively understood. Although this quality assurance study gives valuable insight into the health status of anabolic androgenic steroids users, the goal of this study was to assess the feasibility of implementation, not health outcomes among participants. Results from this initial patient evaluation, most often based on results from a single-point screening and based on patient-reported problems and conditions, would need to be further objectified and confirmed in follow-up visits, thus need to be interpreted with caution. The longitudinal observation and outcome assessments were outside of the scope of this study but will be systematically assessed in future studies. Most patients experienced complications from using these substances affecting both physical and mental health, most well-established and consistent with the published literature [4, 5, 13, 14]. Complications may arise through high-risk behavioural aspects, such as engagement in strict workout routines which may have led to musculoskeletal complications or the extensive concomitant polypharmacy, which can also cause many complications as well as drug-drug interactions. The use of possibly counterfeit substances from unregulated underground pharmacies may additionally lead to unforeseeable adverse events and complications [11]. Furthermore, this study demonstrated that anabolic androgenic steroids users may partake in high-risk injection practices, as well as appear to engage in high-risk sexual behaviours [5, 46], possibly leading to higher prevalence of blood-borne viruses (i.e. HIV infection [46]). Discussing individual experiences in a non-stigmatising way appears to be crucial in establishing a trustful doctor-patient relationship with these patients. Behavioural risk factors, particularly safer sex behaviours and safer use practices should be discussed and reinforced with anabolic androgenic steroids users.
Most anabolic androgenic steroids users presenting in the primary care practice were familiar with common experienced side effects and complications from anabolic androgenic steroid use (e.g. male gynaecomastia, male-pattern hair loss, testicular volume loss, sexual dysfunction, subfertility, sleep disorders) which were often described as mild or temporary and/or accepted with respect to the goal of improvement in body image. Importantly, some potentially severe side effects may go unnoticed by patients, such as complications regarding cardiovascular disease and anabolic steroid-induced cardiomyopathy [27, 47, 48], liver injury [49], kidney disease [50–53], endocrine and metabolic disorders [25, 33, 54, 55] and cancer development [13, 49, 56]. Furthermore, mental health problems and concomitant substance use disorders were prevalent.
With this evaluation, we demonstrated that anabolic androgenic steroid use may be associated with a wide range of chronic disorders – either communicable (e.g. HIV, hepatitis B and C) and non-communicable (e.g. physical health and mental health disorders, as well as substance use disorders). The findings of this study demonstrate that anabolic androgenic steroids users likely benefit from integrated care in a primary health care setting. Healthcare systems, which traditionally focus on providing acute care and/or providing specialised medical care, are not set up to address the challenge of providing care for people with multiple medical problems, thus, integrated care approaches are needed to reduce the disease burden in these patients [57]. In primary care settings, patients benefit from low-threshold healthcare access, holistic and adequate long-term care, as well as coordination within the healthcare sector when dealing with multiple complications from this substance use disorder and addiction problems for this hard-to-reach user population. Although many knowledge gaps regarding the provision of care for this population remain, our initial findings support the recommendations regarding delivery of current best clinical practice among anabolic androgenic steroids users for a Swiss context. Future studies should focus on optimising best practice guidance for optimal care provision to anabolic androgenic steroids users in recreational sports in primary care settings.
Limitations in providing medical care to anabolic androgenic steroids users are the existing antidoping regulations. These policies should be critically reviewed as simple medical care, as well as the evidence-based treatment for non-athlete anabolic androgenic steroids users, is criminalised under current legislation, leaving patients with inadequate care as well as instilling fear in doctors who aim to provide medical care to these patients [9]. Recent survey data among general practitioners in Australia demonstrated that they most often feel inadequately prepared to provide services to anabolic androgenic steroids users, particularly due to specific challenges regarding professional ethics and legality [58]. Furthermore, international data demonstrates that anabolic androgenic steroids users express challenges in seeking support from medical professionals due to their fear of the illegal nature of these substances and criminalisation of use with the potential for legal consequences that hinders an open discussion and engagement with healthcare providers [59]. Anabolic androgenic steroids users have the right to careful medical diagnosis, advice and treatment as part of the law; not providing these medical services may be a breach of duty of care from a healthcare perspective. The antidoping policies should further clearly distinguish between the use of these substances in competitive sports and use outside competitions, as medical care outside competitive sports is aimed at treating addiction and not aimed at supporting doping. The Swiss drug policy aims to reduce drug use and its negative consequences for anabolic androgenic steroids users and society with a four-pillar drug policy law [60], comprising prevention, harm reduction, therapy and repression. Patients that do not partake in competitive sports should be able to receive adequate harm reduction services as well as treatment without putting the treating physician at legal risk [6, 9].
With this first quality assurance study, we demonstrate that anabolic androgenic steroids users may engage in high-risk behaviours and possibly suffer from a high prevalence of comorbid medical conditions in a Swiss primary care practice. The integration of current best clinical practice within a primary care context for patients who consume these substances and engage in recreational sports appears to be feasible with a high acceptance in Switzerland. This study suggests that anabolic androgenic steroids users likely benefit from integrated medical care provided and coordinated in a primary health care setting; thus, upon these initial study results these services were continued on a larger scale to further assess as well as mitigate health risks among this user population. The delivery of medical care to anabolic androgenic steroids users comprises legal aspects that need to be considered and must be addressed in the future to close the current treatment gap within this population and avert this growing public health threat.
This research received no specific grant from any funding agency.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Kanayama G, Pope HG Jr. History and epidemiology of anabolic androgens in athletes and non-athletes. Mol Cell Endocrinol. 2018 Mar;464:4–13. doi: https://doi.org/10.1016/j.mce.2017.02.039
2. Ip EJ, Lu DH, Barnett MJ, Tenerowicz MJ, Vo JC, Perry PJ. Psychological and physical impact of anabolic-androgenic steroid dependence. Pharmacotherapy. 2012 Oct;32(10):910–9. doi: https://doi.org/10.1002/j.1875-9114.2012.01123
3. Bonnecaze AK, O’Connor T, Burns CA. Harm Reduction in Male Patients Actively Using Anabolic Androgenic Steroids (AAS) and Performance-Enhancing Drugs (PEDs): a Review. J Gen Intern Med. 2021 Jul;36(7):2055–64. doi: https://doi.org/10.1007/s11606-021-06751-3
4. Bonnecaze AK, O’Connor T, Aloi JA. Characteristics and Attitudes of Men Using Anabolic Androgenic Steroids (AAS): A Survey of 2385 Men. Am J Mens Health. 2020;14(6):1557988320966536. doi: https://doi.org/10.1177/1557988320966536
5. Mullen C, Whalley BJ, Schifano F, Baker JS. Anabolic androgenic steroid abuse in the United Kingdom: an update. Br J Pharmacol. 2020 May;177(10):2180–98. doi: https://doi.org/10.1111/bph.14995
6. Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014 May;24(5):383–98. doi: https://doi.org/10.1016/j.annepidem.2014.01.009
7. Anawalt BD. Diagnosis and Management of Anabolic Androgenic Steroid Use. J Clin Endocrinol Metab. 2019 Jul;104(7):2490–500. doi: https://doi.org/10.1210/jc.2018-01882
8. Iff S, Butzke I, Quednow B, Gupta R, Imboden C, Claussen M. «Image and performance enhancing drugs» im Freizeitsport. Swiss Medical Forum 2021;21:843-47. Available from: https://pdfs.semanticscholar.org/0a4b/235bbb38cb2885662831e2954db8906f7d01.pdf
9. Kruijver M, Bruggmann P, Magnolini R. Evidence of use and users of image- and performance-enhancing drugs in sports in Switzerland: a scoping literature review and implications for Swiss drug policy. Swiss Med Wkly. 2023 May;153(5):40080. doi: https://doi.org/10.57187/smw.2023.40080
10. World Anti-Doping Agency (WADA). Prohibited List 2021. Available from: https://www.wada-ama.org/sites/default/files/resources/files/2021list_en.pdf
11. Magnolini R, Falcato L, Cremonesi A, Schori D, Bruggmann P. Fake anabolic androgenic steroids on the black market - a systematic review and meta-analysis on qualitative and quantitative analytical results found within the literature. BMC Public Health. 2022 Jul;22(1):1371. doi: https://doi.org/10.1186/s12889-022-13734-4
12. McBride JA, Carson CC 3rd, Coward RM. The Availability and Acquisition of Illicit Anabolic Androgenic Steroids and Testosterone Preparations on the Internet. Am J Mens Health. 2018 Sep;12(5):1352–7. doi: https://doi.org/10.1177/1557988316648704
13. Nieschlag E, Vorona E. Doping with anabolic androgenic steroids (AAS): adverse effects on non-reproductive organs and functions. Rev Endocr Metab Disord. 2015 Sep;16(3):199–211. doi: https://doi.org/10.1007/s11154-015-9320-5
14. Christou MA, Christou PA, Markozannes G, Tsatsoulis A, Mastorakos G, Tigas S. Effects of Anabolic Androgenic Steroids on the Reproductive System of Athletes and Recreational Users: A Systematic Review and Meta-Analysis. Sports Med. 2017 Sep;47(9):1869–83. doi: https://doi.org/10.1007/s40279-017-0709-z
15. Windfeld-Mathiasen J, Heerfordt IM, Dalhoff KP, Andersen JT, Horwitz H. Mortality Among Users of Anabolic Steroids. JAMA. 2024 Apr;331(14):1229–30. doi: https://doi.org/10.1001/jama.2024.3180
16. Pope HG Jr, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. Am J Addict. 2014;23(4):371–7. doi: https://doi.org/10.1111/j.1521-0391.2013.12118.x
17. Skauen JE, Pallesen S, Bjørnebekk A, Chegeni R, Syvertsen A, Petróczi A, et al. Prevalence and correlates of androgen dependence: a meta-analysis, meta-regression analysis and qualitative synthesis. Curr Opin Endocrinol Diabetes Obes. 2023 Dec;30(6):309–23. doi: https://doi.org/10.1097/MED.0000000000000822
18. Amaral JM, Kimergård A, Deluca P. Prevalence of anabolic steroid users seeking support from physicians: a systematic review and meta-analysis. BMJ Open. 2022 Jul;12(7):e056445. doi: https://doi.org/10.1136/bmjopen-2021-056445
19. Harvey O, Keen S, Parrish M, van Teijlingen E. Support for people who use Anabolic Androgenic Steroids: A Systematic Scoping Review into what they want and what they access. BMC Public Health. 2019 Jul;19(1):1024. doi: https://doi.org/10.1186/s12889-019-7288-x
20. Smit DL, de Ronde W. Outpatient clinic for users of anabolic androgenic steroids: an overview. Neth J Med. 2018 May;76(4):167.
21. Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010 Jan;10(1):1. doi: https://doi.org/10.1186/1471-2288-10-1
22. In J. Introduction of a pilot study. Korean J Anesthesiol. 2017 Dec;70(6):601–5. doi: https://doi.org/10.4097/kjae.2017.70.6.601
23. Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010 Jul;10(1):67. doi: https://doi.org/10.1186/1471-2288-10-67
24. Butzke, I., et al., Interdisciplinary and Psychiatric Treatment of Anabolic Androgenic Steroids Users. Praxis (Bern 1994), 2022 Apr;111(6):e339-e344. doi: .
25. Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014 May;101(5):1271–9. doi: https://doi.org/10.1016/j.fertnstert.2014.02.002
26. Bond P, Smit DL, de Ronde W. Anabolic-androgenic steroids: how do they work and what are the risks? Front Endocrinol (Lausanne). 2022 Dec;13:1059473. doi: https://doi.org/10.3389/fendo.2022.1059473
27. Smit DL, Voogel AJ, den Heijer M, de Ronde W. Anabolic Androgenic Steroids Induce Reversible Left Ventricular Hypertrophy and Cardiac Dysfunction. Echocardiography Results of the HAARLEM Study. Front Reprod Health. 2021 Sep;3:732318. doi: https://doi.org/10.3389/frph.2021.732318
28. de Ronde W, Smit DL. Anabolic androgenic steroid abuse in young males. Endocr Connect. 2020 Apr;9(4):R102–11. doi: https://doi.org/10.1530/EC-19-0557
29. Camilleri E, Smit DL, van Rein N, Le Cessie S, de Hon O, den Heijer M, et al. Coagulation profiles during and after anabolic androgenic steroid use: data from the HAARLEM study. Res Pract Thromb Haemost. 2023 Oct;7(7):102215. doi: https://doi.org/10.1016/j.rpth.2023.102215
30. Smit DL, Buijs MM, de Hon O, den Heijer M, de Ronde W. Positive and negative side effects of androgen abuse. The HAARLEM study: A one-year prospective cohort study in 100 men. Scand J Med Sci Sports. 2021 Feb;31(2):427–38. doi: https://doi.org/10.1111/sms.13843
31. Smit DL, Buijs MM, de Hon O, den Heijer M, de Ronde W. Disruption and recovery of testicular function during and after androgen abuse: the HAARLEM study. Hum Reprod. 2021 Mar;36(4):880–90. doi: https://doi.org/10.1093/humrep/deaa366
32. Zeeck A, Welter V, Alatas H, Hildebrandt T, Lahmann C, Hartmann A. Muscle Dysmorphic Disorder Inventory (MDDI): validation of a German version with a focus on gender. PLoS One. 2018 Nov;13(11):e0207535. doi: https://doi.org/10.1371/journal.pone.0207535
33. Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG Jr. Issues for DSM-V: clarifying the diagnostic criteria for anabolic-androgenic steroid dependence. Am J Psychiatry. 2009 Jun;166(6):642–5. doi: https://doi.org/10.1176/appi.ajp.2009.08111699
34. Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ. The Anabolic 500 survey: characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy. 2011 Aug;31(8):757–66. doi: https://doi.org/10.1592/phco.31.8.757
35. Adams C, Walpola R, Schembri AM, Harrison R. The ultimate question? Evaluating the use of Net Promoter Score in healthcare: A systematic review. Health Expect. 2022 Oct;25(5):2328–39. doi: https://doi.org/10.1111/hex.13577
36. Koladycz R, Fernandez G, Gray K, Marriott H. The Net Promoter Score (NPS) for Insight Into Client Experiences in Sexual and Reproductive Health Clinics. Glob Health Sci Pract. 2018 Oct;6(3):413–24. doi: https://doi.org/10.9745/GHSP-D-18-00068
37. Ämmälä AJ, Taimela S. Association Between Patient-Reported Enablement and Customer Satisfaction in 140055 Primary Care Patients After Doctor Appointment. Association Between Patient-Reported Enablement and Customer Satisfaction in 140055 Primary Care Patients After Doctor Appointment. J Patient Exp. 2024 Oct;11:23743735241293631. doi: https://doi.org/10.1177/23743735241293631
38. Swiss Federal Law. Federal Act on the Promotion of Sport and Exercise. https://www.fedlex.admin.ch/eli/cc/2012/460/en
39. Anhang 5 zur Standesordnung FMH: Richtlinien für die ärztliche Betreuung von Sporttreibenden. https://www.fmh.ch/ueber-die-fmh/organisation/die-organe-der-fmh/standeskommission.cfm
40. Wyss W. Betreuung und Behandlung von Patienten mit problematischem Anabolikakonsum und medizinischer Komplikationen ausserhalb des Wettkampfsports. https://zenodo.org/records/13318794
41. Mulcahey MK, Schiller JR, Hulstyn MJ. Anabolic steroid use in adolescents: identification of those at risk and strategies for prevention. Phys Sportsmed. 2010 Oct;38(3):105–13. doi: https://doi.org/10.3810/psm.2010.10.1815
42. Hearne E, Atkinson A, Boardley I, McVeigh J, Van Hout MC. ‘Sustaining masculinity’: a scoping review of anabolic androgenic steroid use by older males. Drugs Educ Prev Policy. 2022;31(1):27–53. doi: https://doi.org/10.1080/09687637.2022.2132135
43. Guerras JM, Hoyos J, de la Fuente L, Román F, Ayerdi O, García-Pérez JN, et al.; The Methysos Project Group. Injection of Anabolic Steroids in Men Who Had Sex with Men in Madrid and Barcelona: Prevalence Correlates and Role as a Risk Factor for Transmitted Infections. Int J Environ Res Public Health. 2021 Aug;18(16):8289. doi: https://doi.org/10.3390/ijerph18168289
44. Ip EJ, Doroudgar S, Shah-Manek B, Barnett MJ, Tenerowicz MJ, Ortanez M, et al. The CASTRO study: unsafe sexual behaviors and illicit drug use among gay and bisexual men who use anabolic steroids. Am J Addict. 2019 Feb;28(2):101–10. doi: https://doi.org/10.1111/ajad.12865
45. Amaral JM, Kimergård A, Deluca P. Preventing and treating the adverse health conditions of androgenic-anabolic steroids: an online survey with 883 users in the United Kingdom. Perform Enhanc Health. 2023;11(4):100267. doi: https://doi.org/10.1016/j.peh.2023.100267
46. Ip EJ, Yadao MA, Shah BM, Lau B. Infectious disease, injection practices, and risky sexual behavior among anabolic steroid users. AIDS Care. 2016;28(3):294–9. doi: https://doi.org/10.1080/09540121.2015.1090539
47. Fadah K, Gopi G, Lingireddy A, Blumer V, Dewald T, Mentz RJ. Anabolic androgenic steroids and cardiomyopathy: an update. Front Cardiovasc Med. 2023 Jul;10:1214374. doi: https://doi.org/10.3389/fcvm.2023.1214374
48. Doleeb S, Kratz A, Salter M, Thohan V. Strong muscles, weak heart: testosterone-induced cardiomyopathy. ESC Heart Fail. 2019 Oct;6(5):1000–4. doi: https://doi.org/10.1002/ehf2.12494
49. Petrovic A, Vukadin S, Sikora R, Bojanic K, Smolic R, Plavec D, et al. Anabolic androgenic steroid-induced liver injury: an update. World J Gastroenterol. 2022 Jul;28(26):3071–80. doi: https://doi.org/10.3748/wjg.v28.i26.3071
50. Parente Filho SL, Gomes PE, Forte GA, Lima LL, Silva Júnior GB, Meneses GC, et al. Kidney disease associated with androgenic-anabolic steroids and vitamin supplements abuse: be aware! Nefrologia (Engl Ed). 2020;40(1):26–31. doi: https://doi.org/10.1016/j.nefroe.2019.06.005
51. Ko GJ, Rhee CM, Kalantar-Zadeh K, Joshi S. The Effects of High-Protein Diets on Kidney Health and Longevity. J Am Soc Nephrol. 2020 Aug;31(8):1667–79. doi: https://doi.org/10.1681/ASN.2020010028
52. Albano GD, Amico F, Cocimano G, Liberto A, Maglietta F, Esposito M, et al. Adverse Effects of Anabolic-Androgenic Steroids: A Literature Review. Healthcare (Basel). 2021 Jan;9(1):97. doi: https://doi.org/10.3390/healthcare9010097
53. Gupta A, Thorson P, Penmatsa KR, Gupta P. Rhabdomyolysis: revisited. Ulster Med J. 2021 May;90(2):61–9.
54. Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG Jr. Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009 Dec;104(12):1966–78. doi: https://doi.org/10.1111/j.1360-0443.2009.02734.x
55. Grant B, Kean J, Vali N, Campbell J, Maden L, Bijral P, et al. The use of post-cycle therapy is associated with reduced withdrawal symptoms from anabolic-androgenic steroid use: a survey of 470 men. Subst Abuse Treat Prev Policy. 2023 Nov;18(1):66. doi: https://doi.org/10.1186/s13011-023-00573-8
56. Strauss RH, Yesalis CE. Anabolic steroids in the athlete. Annu Rev Med. 1991;42(1):449–57. doi: https://doi.org/10.1146/annurev.me.42.020191.002313
57. Thornicroft G, Ahuja S, Barber S, Chisholm D, Collins PY, Docrat S, et al. Integrated care for people with long-term mental and physical health conditions in low-income and middle-income countries. Lancet Psychiatry. 2019 Feb;6(2):174–86. doi: https://doi.org/10.1016/S2215-0366(18)30298-0
58. Dunn M, Piatkowski T, Whiteside B, Eu B. Exploring the experiences of general practitioners working with patients who use performance and image enhancing drugs. Perform Enhanc Health. 2023 Jun;11(2):100247. doi: https://doi.org/10.1016/j.peh.2023.100247
59. Piatkowski T, Gibbs N, Dunn M. Beyond the law: exploring the impact of criminalising anabolic–androgenic steroid use on help-seeking and health outcomes in Australia. J Criminol. 2024;57(1):62–82. doi: https://doi.org/10.1177/26338076231209044
60. Federal Office of Public Health (FOPH). National Strategy on Addiction. Available from: https://www.bag.admin.ch/bag/en/home/strategie-und-politik/nationale-gesundheitsstrategien/strategie-sucht.html
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4225.