Exacerbation of demyelinating polyneuropathy after adoptive cell therapy with tumour-infiltrating
lymphocytes by metastatic melanoma
DOI: https://doi.org/https://doi.org/10.57187/s.4221
Elisa Caninia,
Lorenza Pacchina*,
Ann Kristine Blackhamb,
Johannes Lorscheiderc,
Jakob Passwegde,
Alfred Zippeliusefg,
Heinz Läubliefg,
Markus R. Mutkea,
David Königefg
a Department of Internal Medicine, University Hospital Basel,
Basel, Switzerland
b Division of Radiology and Nuclear Medicine, University Hospital Basel,
Basel, Switzerland
c Department of Neurology,
University Hospital Basel, Basel, Switzerland
d Department of Haematology,
University Hospital Basel, Basel, Switzerland
e Innovation Focus Cell Therapies, University Hospital Basel,
Basel, Switzerland
f Division of Medical Oncology, University
Hospital Basel, Basel, Switzerland
g Department of Biomedicine, University of
Basel, Basel, Switzerland
* Equal contribution as first authors
Summary
Adoptive cell therapy (ACT) with tumour-infiltrating
lymphocytes (TIL) is an effective personalised immunotherapy for patients with
advanced pretreated melanoma. For TIL-ACT, tumour-specific T cells are expanded
from excised tumour samples and stimulated in cell culture with interleukin-2
(IL-2). The resulting autologous tumour-infiltrating lymphocytes are reinfused
to the patient after a non-myeloablative lymphodepleting chemotherapy with
cyclophosphamide and fludarabine. Thereafter, activation of tumour-infiltrating
lymphocytes in the patient is supported by the administration of high-dose
IL-2. Although effective, there is a need for enhancement of TIL-ACT in terms
of effectiveness and toxicity. Most of the toxicity in this multistep, complex
treatment regimen is due to the preparative chemotherapy and high-dose IL-2
treatment. At University Hospital Basel, we are currently evaluating an
experimental approach of TIL-ACT in which we replace high-dose IL-2 by in vivo tumour-infiltrating
lymphocyte activation with ANV419, a novel antibody-cytokine fusion protein
consisting of IL-2 fused to an anti-IL-2 monoclonal antibody, in an ongoing
phase I trial (BaseTIL-03M). The primary endpoint of the study is
safety.
We herein describe the case of a patient
included in the BaseTIL-03M trial with chronic inflammatory
demyelinating polyneuropathy who received TIL-ACT with ANV419 and developed an
acute polyneuropathy of Guillain-Barré syndrome.
Abbreviations
- ACT
-
adoptive cell therapy
- TIL
-
tumour-infiltrating lymphocyte
- TIL-ACT
-
tumour-infiltrating lymphocyte
adoptive cell therapy
Background
Adoptive cell therapy (ACT) with tumour-infiltrating lymphocytes (TIL) is a personalised
immunotherapy based on the infusion of autologous CD4+ and CD8+ T lymphocytes that
have been collected from tumour material and expanded ex vivo in the presence of interleukin-2
(IL-2). Preconditioning lymphodepleting chemotherapy is an integral part of current
tumour-infiltrating lymphocyte protocols. In vivo tumour-infiltrating lymphocyte activation
is generally supported by the administration of high-dose IL-2. TIL-ACT therapy was
developed and pioneered by Steven A. Rosenberg and colleagues at the National Cancer
Institute (Maryland, US) several years ago (1986) [1, 2]. Objective response rates
up to 72% were achieved with TIL-ACT in several consecutive clinical trials, including
10–20% complete remissions and 40% durable clinical responses [3]. Recently, a large
phase III clinical trial has demonstrated superiority of TIL-ACT in terms of progression-free
survival and overall response rate compared to the immune checkpoint inhibitor (ICI)
ipilimumab in pretreated melanoma patients [4]. Although TIL-ACT is an effective treatment,
there is still a substantial need to enhance the anti-tumour potential. Furthermore,
lymphodepletion and IL-2 treatment are associated with significant toxicities, restricting
TIL-ACT to medically fit patients only. New treatment combinations aim at increasing
the efficacy of TIL-ACT, while reducing toxicity. We therefore designed a phase I
clinical trial (BaseTIL-03M, NCT05869539) that investigates in vivo tumour-infiltrating
lymphocyte activation with the novel IL-2Rβγ binding agonist ANV419 in patients with
advanced melanoma. ANV419 is an antibody-cytokine fusion protein consisting of IL-2
fused to an anti-IL-2 monoclonal antibody that sterically hinders binding of IL-2
to IL-2Rα, retaining affinity for the receptor β- and γ-subunits and thus limiting
signalling through the α subunit on regulatory T cells. The safety of ANV419 has previously
been evaluated in a multicentre phase I clinical trial in patients with advanced solid
tumours [5]. The study intervention consists of multiple steps, starting with a surgical
intervention to collect tumour material for tumour-infiltrating lymphocyte expansion.
Tumour-infiltrating lymphocyte expansion is performed at University Hospital Basel’s
Good Manufacturing Practice (GMP) Facility for Advanced Therapies (duration: approximately
26 days). Patients undergo preparative lymphodepleting chemotherapy with cyclophosphamide
(dose: 60 mg/kg i.v., 1× daily, for two consecutive days) and fludarabine (dose: 25
mg/m2 body surface area i.v., max. dose of 50 mg, 1× daily, for five consecutive days)
before receiving the expanded tumour-infiltrating lymphocytes (variable cell number
between 5 × 109 and 2 × 1011 tumour-infiltrating lymphocytes) in a single infusion.
For in vivo tumour-infiltrating lymphocyte stimulation, patients receive two doses
of ANV419 (dose: 243 µg/kg i.v.): the first dose directly after and the second dose
two weeks after tumour-infiltrating lymphocyte transfer. Here we report the case of
an adult patient with chronic inflammatory demyelinating polyneuropathy who developed
a severe exacerbation of chronic inflammatory demyelinating polyneuropathy following
TIL-ACT with ANV419.
Case presentation
An adult patient with secondary metastatic cutaneous
melanoma experienced disease progression during his 4th line of treatment
and was enrolled in the BaseTIL-03M trial. The patient was diagnosed with UICC
stage IIIC melanoma localised on the back in 2008. He then underwent resection
of the melanoma including sentinel lymph node biopsy followed by adjuvant
treatment with interferon. The diagnosis of chronic inflammatory demyelinating
polyneuropathy was made in November 2020 concomitantly with the diagnosis of
axillary lymph node metastases (October 2020). The patient experienced
progressive neurological symptoms three months before the diagnosis of melanoma
recurrence. Ganglioside antibodies were negative. However, magnetic resonance
imaging revealed a pathological contrast finding suggestive of chronic
inflammatory demyelinating polyneuropathy at the level of the cauda equina.
Further investigations using electroneurography and somatosensory evoked
potential confirmed the presence of a demyelinating polyneuropathy, as
well as cyto-albumin dissociation in the spinal fluid. In the light of the
results of the various diagnostic investigations, the diagnosis of chronic
inflammatory demyelinating polyneuropathy was confirmed. The melanoma
recurrence was treated with right axillary lymphadenectomy. No additional
immunotherapy (immune checkpoint inhibitor) was given due to chronic
inflammatory demyelinating polyneuropathy. For chronic inflammatory
demyelinating polyneuropathy, a full-dose treatment with intravenous
immunoglobulin was administered between December 2020 and January 2021,
resulting in marked improvement of chronic inflammatory demyelinating
polyneuropathy-related symptoms. Due to simultaneous occurrence of melanoma
recurrence and symptoms of chronic inflammatory demyelinating polyneuropathy,
the absence of other possible exacerbating triggers and data from literature
where chronic inflammatory demyelinating polyneuropathy occurred prior to the
diagnosis of melanoma [6–8], chronic inflammatory demyelinating polyneuropathy
was interpreted as melanoma-related. Adjuvant radiotherapy of the right
axillary region was started in the beginning of 2021 but discontinued
prematurely due to the occurrence of a new cervical lymph node metastasis. Subsequently,
three cycles of combined
immunotherapy with the CTLA-4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab
were administered. The treatment was discontinued due to the emergence of immune-related
hepatitis. Interestingly, no exacerbations of chronic inflammatory
demyelinating polyneuropathy or other neurological symptoms were observed. The
patient experienced disease progression in June 2021 and received experimental
treatment with nivolumab and an anti-IL-8 monoclonal antibody from October 2021
to June 2022. Upon further progression, the patient received an experimental
study treatment including thermal ablation of (cervical) lymph node metastases and
a novel (systemic) immunotherapy (IP-001). Bridging therapy for the planned
inclusion in the BaseTIL-03M study included chemotherapy with paclitaxel
and carboplatin from May to June 2023.
The patient was enrolled into the BaseTIL-03M
trial in July 2023 (figure 1).
He received the study treatment as planned, including the scheduled preparative
lymphodepletion with cyclophosphamide and fludarabine, followed by the transfer
of the expanded tumour-infiltrating
lymphocytes (cell number: 59.25 × 109 tumour-infiltrating lymphocytes) and received the first dose of
ANV419 (243 μg/kg, absolute dose: 19 mg). Treatment-associated haematological
toxicities included grade 3 (G3) anaemia, G4 lymphopenia, G4 neutropenia
including febrile neutropenia and G4 thrombocytopenia,
expected due to the preparative chemotherapy. No erythrocyte or thrombocyte transfusions
were required. For the treatment of neutropenia, filgrastim 30 million
international units (MIU) was administered according to the study schedule
(G-CSF filgrastim at a dose of 30 MIU equivalent to 300 micrograms for a body
weight <100 kg; or 48 MIU equivalent to 480 μg for a body weight ≥100 kg,
administered subcutaneously, starting on day +1, until neutrophil count >1.0
× 109/l), starting after tumour-infiltrating lymphocyte
transfer. The patient experienced fever already before the infusion of the tumour-infiltrating
lymphocyte product and received empirical broad-spectrum antibiotic therapy,
which was continued during the subsequent phase of neutropenia, including the
administration of tumour-infiltrating lymphocytes and the first dose of ANV419.
During this period, different episodes of fever were recorded, and antibiotic
treatment was changed multiple times (cefepime, piperacillin/tazobactam,
meropenem). In addition, the patient received the standard antimicrobial
treatment with trimethoprim/sulfamethoxazole, valaciclovir and fungal
prophylaxis with fluconazole. Recurrent sampling did not evidence any
infectious cause. Imaging findings suggested possible pulmonary infection
focus. An additional non-haematological side effect of G2 or higher was an increase
in INR.
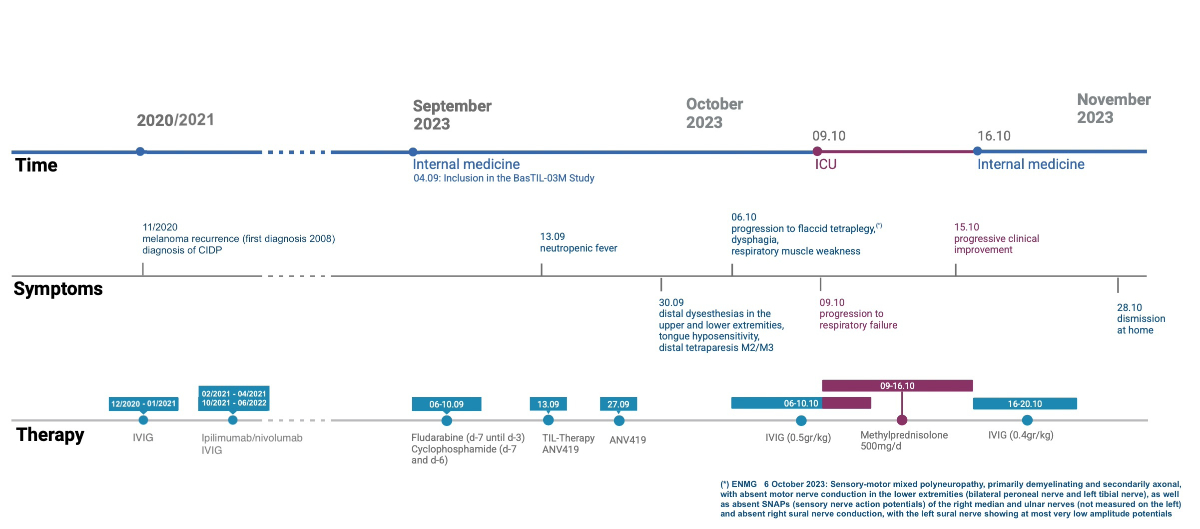
Figure 1Timeline of events. CIDP: chronic inflammatory demyelinating
polyneuropathy; IVIG: intravenous immunoglobulin; TIL: tumour-infiltrating
lymphocyte. (*) ENMG 6 October 2023: Sensorimotor mixed polyneuropathy, primarily
demyelinating and secondarily axonal, with absent motor nerve conduction in the
lower extremities (bilateral peroneal nerve and left tibial nerve), as well as
absent sensory nerve action potentials of the right median and ulnar
nerves (not measured on the left) and absent right sural nerve conduction, with
the left sural nerve showing at most very low amplitude potentials.
The second dose of ANV419 was given as
planned and at the same dose two weeks after tumour-infiltrating lymphocyte
transfer. Subsequently, the patient reported an increasing worsening of the
previously present sensory polyneuropathy of the hands and feet, as well as new
distal muscle weakness. Muscle weakening was measurable as a new distal paresis
of both feet: foot elevation: 3/5, foot drop: 4/5, big toe elevation: 3/5, toe
elevation: 3/5, according to the Medical Research Council (MRC) Scale (Grade 5:
normal, Grade 4: movement against gravity and resistance, Grade 3: movement
against gravity over the full range, Grade 2: movement of the limb but not
against gravity, Grade 1: visible contraction without movement of the limb,
Grade 0: no visible contraction). The patient developed an increasingly ataxic
gait. Heel and toe gait was not possible. Both feet showed pall-hypoaesthesia,
tested with 128 Hz-Diapason with result 4/8 (scale for assessment of disorders
of vibratory sensitivity, range 0 to 8, with 8 being intact vibratory
sensitivity). Furthermore, the muscle reflexes were absent bilaterally. A brain
and spine MRI excluded the presence of central nervous system metastases but
revealed a diffuse pathological contrast enhancement of the intraspinal nerves from
cervical to sacrum including the cauda equina as well as a symmetrical
pathological contrast enhancement of several cranial nerves on both sides. This
finding was primarily compatible with the established diagnosis of chronic
inflammatory demyelinating polyneuropathy (figures 2 and 3).
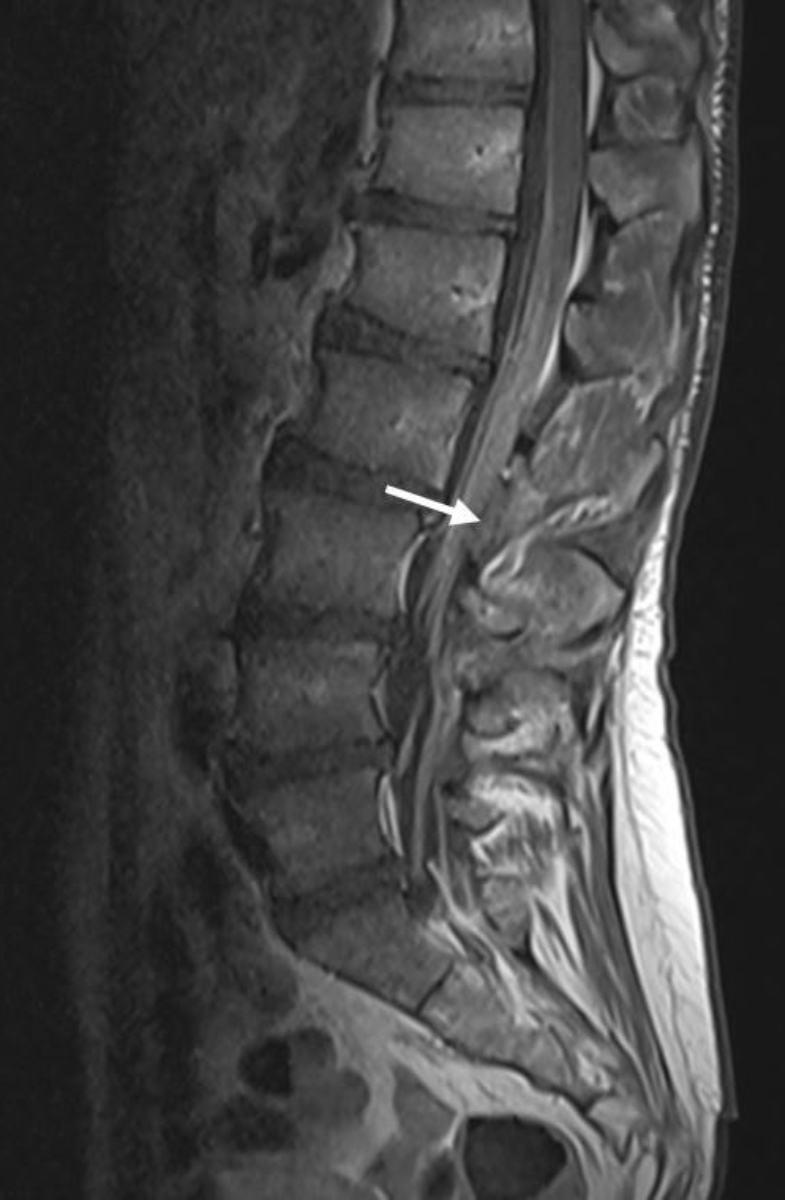
Figure 2Spine MRI (T1 TSE with gadolinium): smooth, non-nodular and diffuse pathological contrast
enhancement of the intraspinal nerves and the entire cauda equina primarily compatible
with the diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP).
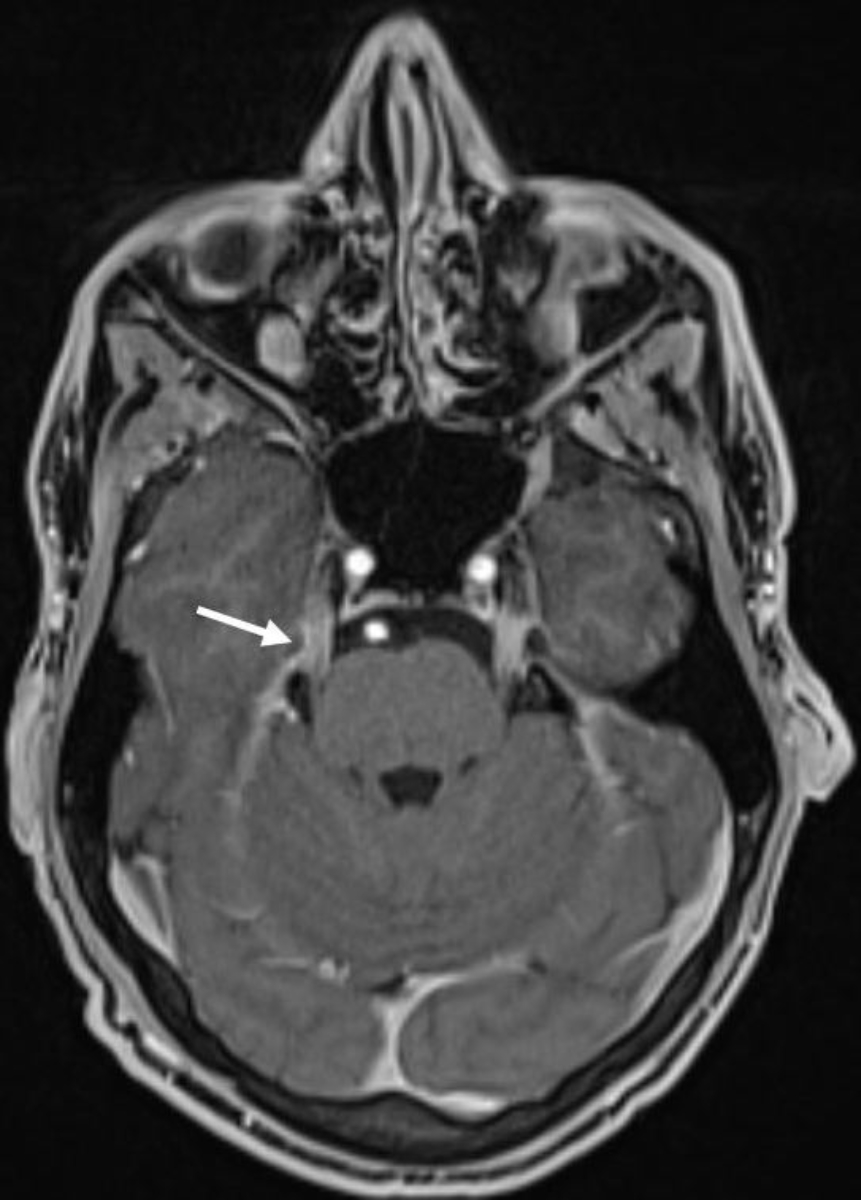
Figure 3Brain MRI (T1 VIBE with gadolinium): symmetrical pathological contrast enhancement
of several cranial nerves on both sides (N. trigeminus in the picture, N. oculomotorius,
N. facialis) primarily compatible with the diagnosis of chronic inflammatory demyelinating
polyneuropathy (CIDP).
In a brain MRI performed
before any study interventions, enhancement of some cranial nerves was already
present. Neurographic examination with electroneurography and
electromyography revealed a severe sensorimotor mixed polyneuropathy in
all four extremities. CT of the chest and cervical region
did not evidence progression of melanoma. In an interdisciplinary evaluation,
the progressing sensorimotor polyneuropathy was interpreted as probable
exacerbation of the preexisting chronic inflammatory demyelinating
polyneuropathy. Accordingly, treatment with intravenous immunoglobulin was
started (0.5 g/kg, absolute dose: 40 g, for 4 consecutive days), with another
cycle (0.4 g/kg, absolute dose: 30 g, for 5 consecutive days) after 6 days.
Despite intravenous immunoglobulin treatment, there was a rapid deterioration
of the tetraparesis to a full plegia of the legs, dysphagia and eventually
respiratory muscle involvement with subsequent aspiration and respiratory
failure. The patient was transferred to the intensive care unit and required
respiratory support including mechanical ventilation. Lumbar puncture revealed
a normal cell count with cyto-albumin dissociation (a combination of elevated
protein level and normal cell counts in the cerebrospinal fluid), no evidence
of an infectious cause and interestingly negative results for autoantibodies
(including anti-gangliosides IgM/IgG) in the cerebrospinal fluid or serum (table
1). The clinical presentation was at this point resembling an acute
inflammatory demyelinating polyneuropathy of Guillain-Barré syndrome. Due to
the initial lack of response to intravenous immunoglobulin, we decided to add
high-dose methylprednisolone intravenously (500 mg, for 7 consecutive days). Given
the positive response to treatment, it was subsequently administered orally and
gradually tapered. In addition, antibiotic therapy was administered in the
context of nosocomial pneumonia. Despite the patient undergoing multiple
bronchoscopies due to massive secretions, no microbial pathogen
(viral, bacterial, fungal or parasitic) was identified. Antibiotic
therapy was terminated six days later in the context of clinical improvement
and a reduction in inflammatory indices.
Table 1Cerebrospinal fluid and serum results.
|
Value |
Normal range |
| Liquor |
| Leucocytes |
4 ×106/l |
<5 ×106/l |
| Mononuclear cells |
4 ×106/l |
<5 ×106/l |
| Polymorphonuclear cells |
0 |
|
| Erythrocytes |
0 |
|
| Lactate |
2.24 mmol/l |
1.1–1.9 mmol/l |
| Glucose |
4.3 mmol/l |
2.2–4.2 mmol/l |
| Lactate
quotient liquor/plasma |
1.9 |
|
| Glucose
quotient liquor/plasma |
0.6 |
>0.5 |
| Ferritin |
13 µg/l |
15 µg/l |
| Protein |
2850 mg/l |
150–500 mg/l |
| Albumin
quotient liquor/serum |
46.3 ×10-3 |
<6.5 ×10-3 |
| IgG index liquor-serum |
0.53 |
<0.70 |
| IgG liquor (Reiber diagram) |
<5% |
<10% |
| IgA liquor (Reiber diagram) |
<5% |
<10 |
| IgM liquor (Reiber diagram) |
<5% |
<10% |
| Oligoclonal IgG bands |
0 |
<1 |
| Syphilis |
Negative |
Negative |
| Mycoplasma pneumoniae |
Negative |
Negative |
| Meningoencephalitis panel PCR |
Negative |
Negative |
| Liquor culture |
Negative |
Negative |
| Liquor TBC culture |
Negative |
Negative |
| Autoantibodies panel* |
Negative |
Negative |
| Malignancy |
Negative |
Negative |
| Immunophenotyping |
No evidence of CNS lymphoma |
| Serum |
| Anti-ganglioside GM1 IgG/IgM |
<50% |
<50 |
| Anti-ganglioside GM2 IgG/IgM |
<50% |
<50% |
| Anti-ganglioside GD1a IgG/IgM |
<50% |
<50% |
| Anti-ganglioside GD1b IgG/IgM |
<50% |
<50% |
| Anti-ganglioside GQ1b IgG/IgM |
<50% |
<50% |
With the high-dose steroid treatment
ongoing, we observed a gradual improvement in respiratory and neurological
symptoms. Respiratory weaning was possible, and the patient extubated. On
transfer to the internal medicine unit, the patient benefited from
physiotherapy and ergotherapy until discharge. At that time, the patient was
able to walk with the help of a walker and outpatient physiotherapy was
continued.
Discussion
We presented the case of a patient with
pre-existing chronic inflammatory demyelinating polyneuropathy who developed an
acute polyneuropathy resembling Guillain-Barré syndrome in an acute
inflammatory demyelinating polyneuropathy variant form after receiving adoptive
cell therapy with tumour-infiltrating lymphocytes and the IL-2Rβγ binding agonist
ANV419 within a phase
I clinical study. The patient received the entire treatment as scheduled in the
study protocol and developed a progressive polyneuropathy after the second dose
of ANV419 (i.e. two weeks after tumour-infiltrating lymphocyte transfer and the
first dose of ANV419). Due to the initial assumption of an exacerbated chronic
inflammatory demyelinating polyneuropathy, a course of intravenous
immunoglobulin was administered, which, however, showed no effect – contrary to
the benefit of this therapy at initial diagnosis of chronic inflammatory
demyelinating polyneuropathy in the patient and, importantly, contrary to the
expected benefit of intravenous immunoglobulin treatment in classic Guillain-Barré
syndrome. Only after the initiation of high-dose steroid treatment did the patient’s
symptoms gradually improve.
To the best of our knowledge, there are
only a few patient cases that describe acute inflammatory demyelinating
polyneuropathy / Guillain-Barré syndrome after adoptive cell therapy. Orcurto
et al. presented the case of a patient with melanoma who developed Guillain-Barré
syndrome after TIL-ACT [10]. The patient was treated with intravenous
immunoglobulin resulting in progressive and full recovery. A cytomegalovirus
reactivation was detected in this patient and deemed to be the cause of Guillain-Barré
syndrome. In 2019, two cases of Guillain-Barré syndrome were reported following
adoptive transfer of autologous T lymphocytes transduced with a high-affinity
NY-ESO-1-reactive T cell receptor [11]. Both patients received intravenous
immunoglobulin with prompt response to the treatment. The authors attributed
the onset of acute inflammatory demyelinating polyneuropathy to NY-ESO-1-targeting
T cell therapy. In the cases described, there was a rapid and sustained
response to intravenous immunoglobulin, whereas in our case there was an
unexpected response to steroid treatment, suggesting a different
pathophysiology. An antibody-mediated event as a possible cause of the described acute
polyneuropathy is
possible, but we were not able to identify any antibodies in our assessments. Importantly,
we did not test for all possible autoantibodies, e.g. paranodal autoantibodies.
A respiratory infection during the immunosuppressive phase of the tumour-infiltrating
lymphocyte therapy may have triggered the development of acute inflammatory
demyelinating polyneuropathy / Guillain-Barré syndrome as a respiratory
infection/pneumonia was diagnosed following the lymphodepleting chemotherapy,
although no pathogen was identified. An antecedent gastrointestinal or other
infection was not present or could not be determined as the cause. No prior
vaccination occurred. No other triggering events were identified; in particular,
there was no disease progression in the imaging assessments, although disease
progression eventually occurred in the weeks following TIL-ACT. Other causes of
acute polyneuropathy must be considered. The electroneurography examination
performed in 2022 before study enrolment showed slowed NCV in the peroneal, tibial,
median and ulnar nerves with delayed
F-wave latencies. Already at that point, the amplitudes of the peroneal and
tibial nerves were decreased, which was considered to be consistent with
primary demyelination and secondary axonal damage. The electroneurography in
the acute setting showed markedly reduced NCV in the right median and ulnar
nerves and delayed F-wave latency for the median nerve, whereas no CMAPs were
measurable for the right peroneal and left tibial nerve (figure 4). In
conjunction with the rapid clinical deterioration, the cerebrospinal fluid
findings and the positive response to corticosteroids, the most likely
interpretation in our opinion is an inflammatory demyelinating process with
secondary axonal damage, rather than axonal damage on top of the
pre-existing demyelinating polyneuropathy, although we cannot confirm this
based on the available findings.
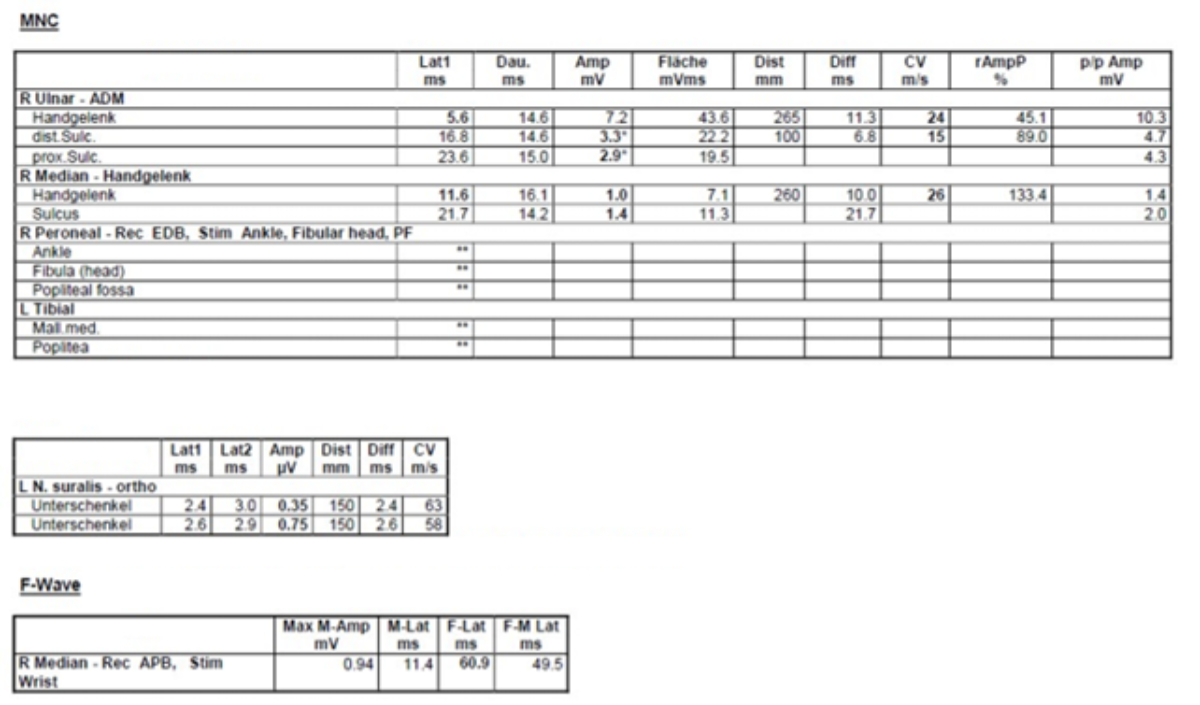
Figure 4Electroneurography.
Fludarabine-associated neurotoxicity seems implausible
given the reversible effect of high-dose steroids on the patient’s symptoms.
Fludarabine is known to cause dose-dependent neurological toxicity, usually
observed in the central nervous system (cerebellar syndrome, cognitive
disturbances, dizziness, depression), but also sensory neuropathy. The
predominant axonal damage caused by fludarabine chemotherapy rather than
demyelination (as opposed to the results in the patient) also point against a
chemotherapy-induced neurotoxicity [13–15]. The novel IL-2Rβγ binding agonist
ANV419, which was administered directly after and two weeks after tumour-infiltrating
lymphocyte-infusion, cannot be ruled out as the possible trigger of acute
inflammatory demyelinating polyneuropathy. However, the temporal correlation
with the onset of symptoms after the second administration of ANV419 (and not
immediately after the first administration) does not support this hypothesis.
Furthermore, no comparable events were documented in the phase I ANV419-001
study, which tested ANV419 in patients with various solid tumours [5]. Finally,
it must also be considered that an auto-reactive T cell clone may have been expanded
in the tumour-infiltrating lymphocyte product, cross-reacting with epitopes on
nerves and resulting in an autoimmune reaction. Again, the time interval to tumour-infiltrating
lymphocyte therapy does not seem quite appropriate. On the other hand, the
rapid response of the symptoms to steroid therapy may support this hypothesis.
In summary, the exact trigger of the
described acute inflammatory demyelinating polyneuropathy / Guillain-Barré
syndrome cannot be determined. Due to the expected increased implementation of
adoptive cellular therapies in clinical practice, especially tumour-infiltrating
lymphocyte-based therapies, it is important for clinicians to be aware of the
potential treatment-related adverse effects and complications associated with
this new treatment option, given the need for a rapid and effective treatment.
Elisa Canini
Department of Internal Medicine
University Hospital Basel
Petersgraben 4
CH-4031 Basel
elisacanini88[at]gmail.com
References
1. Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy
of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep;233(4770):1318–21.
doi: https://doi.org/10.1126/science.3489291
2. Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use
of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients
with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec;319(25):1676–80.
doi: https://doi.org/10.1056/NEJM198812223192527
3. Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et
al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating
lymphocytes and interleukin 2. J Natl Cancer Inst. 1994 Aug;86(15):1159–66. doi: https://doi.org/10.1093/jnci/86.15.1159
4. Rohaan MW, Borch TH, van den Berg JH, Met Ö, Kessels R, Geukes Foppen MH, et al. Tumor-Infiltrating
Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N Engl J Med. 2022 Dec;387(23):2113–25.
doi: https://doi.org/10.1056/NEJMoa2210233
5. Joerger M, Calvo E, Laubli H, Lopez J, Alonso G, Corral de la Fuente E, et al. Phase
1 first-in-human dose-escalation study of ANV419 in patients with relapsed/refractory
advanced solid tumors. J Immunother Cancer. 2023 Nov;11(11):e007784. doi: https://doi.org/10.1136/jitc-2023-007784
6. Rajabally YA, Attarian S. Chronic inflammatory demyelinating polyneuropathy and malignancy:
A systematic review. Muscle Nerve. 2018 Jun;57(6):875–83. doi: https://doi.org/10.1002/mus.26028
7. Dbouk MB, Nafissi S, Ghorbani A.Neurosciences (Riyadh). Chronic inflammatory demyelinating
polyneuropathy following malignant melanoma. 2012 Apr;17(2):167-70. Review.
8. Rousseau A, Salachas F, Baccard M, Delattre JY, Sanson M.J Neurooncol. Chronic inflammatory
polyneuropathy revealing malignant melanoma. 2005 Feb;71(3):335-6
9. van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré
syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014 Aug;10(8):469–82.
doi: https://doi.org/10.1038/nrneurol.2014.121
10. Orcurto A, Hottinger A, Wolf B, Navarro Rodrigo B, Ochoa de Olza M, Auger A, et al. Guillain-Barré
syndrome after adoptive cell therapy with tumor-infiltrating lymphocytes. J Immunother
Cancer. 2020 Aug;8(2):e001155. doi: https://doi.org/10.1136/jitc-2020-001155
11. Joseph J, Nathenson MJ, Trinh VA, Malik K, Nowell E, Carter K, et al. Guillain-Barre
syndrome observed with adoptive transfer of lymphocytes genetically engineered with
an NY-ESO-1 reactive T-cell receptor. J Immunother Cancer. 2019 Nov;7(1):296. doi: https://doi.org/10.1186/s40425-019-0759-x
12. Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete
responses in heavily pretreated patients with metastatic melanoma using T-cell transfer
immunotherapy. Clin Cancer Res. 2011 Jul;17(13):4550–7. doi: https://doi.org/10.1158/1078-0432.CCR-11-0116
13. Farook AM, Priyankara D, Aluwihare C, Mendis A. A Rare Case of Paraneoplastic Guillain-Barré
Syndrome in a Patient with Endometrial Cancer. Eur J Case Rep Intern Med. 2023 Nov;10(12):004077.
doi: https://doi.org/10.12890/2023_004077
14. Koike H, Sobue G. Paraneoplastic neuropathy. Handb Clin Neurol. 2013;115:713–26. doi: https://doi.org/10.1016/B978-0-444-52902-2.00041-2
15. Lehmann HC, Lopez PH, Zhang G, Ngyuen T, Zhang J, Kieseier BC, et al. Passive immunization
with anti-ganglioside antibodies directly inhibits axon regeneration in an animal
model. J Neurosci. 2007 Jan;27(1):27–34. doi: https://doi.org/10.1523/JNEUROSCI.4017-06.2007
16. Wanschitz J, Maier H, Lassmann H, Budka H, Berger T. Distinct time pattern of complement
activation and cytotoxic T cell response in Guillain-Barré syndrome. Brain. 2003 Sep;126(Pt
9):2034–42. doi: https://doi.org/10.1093/brain/awg207



