Updated recommendations for the treatment of light-chain amyloidosis from the Swiss
Amyloidosis Network
DOI: https://doi.org/https://doi.org/10.57187/s.4219
Max J. Rieger1,
Andreas J. Flammer2,
Sabine Gerull3,
Thomas Pabst4,
Holger W. Auner5,
Kaveh Samii6,
Felicitas Hitz7,
Ulrich Mey8,
Veronika Ballova9,
Raphael Battegay10,
Giorgia Melli11,
Dominik Benz12,
Yakup Yakupoglu13,
Christoph Gräni14,
Regina Schläger15,
Sarah Hugelshofer16,
Annina Studer17,
Luca Oechslin18,
Adam Bakula14,
Thomas M. Suter14, 19,
Julia Leo-Stickelberger1,
Manuela Averaimo20,
Thomas Fehr21, 22,
Hans H. Jung23,
Natallia Laptseva2,
Robert Manka2, 12,
Axel Rüfer24,
Adrian Schmidt25,
Harald Seeger26, 27,
Beat Müllhaupt28,
Simon F. Stämpfli29, 30,
Carmen de Ramon Ortiz6,
Marie Théaudin31,
Bernhard Gerber22, 32,
Rahel Schwotzer1
1 Department of Medical Oncology and Haematology,
University Hospital Zurich, Zurich, Switzerland
2 University Heart Centre, University
Hospital, Zurich, Switzerland
3 Department of Medical Oncology and Haematology,
Cantonal Hospital Aarau, Switzerland
4 Department of Oncology, University
Hospital Bern, Inselspital, Bern, Switzerland
5 Department of Haematology, Lausanne
University Hospital, Lausanne, Switzerland
6 Department of Haematology,
University Hospital of Geneva, Geneva, Switzerland
7 Department of Medical Oncology and Haematology,
Cantonal Hospital of St. Gallen, St. Gallen, Switzerland
8 Department of Oncology and Haematology,
Cantonal Hospital Graubünden, Chur, Switzerland
9 Department of Medical Oncology and Haematology,
Cantonal Hospital Baden, Baden, Switzerland
10 Department of Haematology,
University Hospital Basel, Basel, Switzerland
11 Department of Neurology and
Neurodegenerative Diseases Group, Laboratories for Translational Research,
Neurocentre of Southern Switzerland, Ente Ospedaliero Cantonale, Lugano,
Switzerland
12 Diagnostic and Interventional
Radiology, University Hospital Zurich, University of Zurich, Zurich,
Switzerland.
13 Division of Cardiology, Cantonal
Hospital Aarau, Switzerland
14 Department of Cardiology,
Inselspital, Bern University Hospital, University of Bern, Switzerland
15 Department of Neurology, University
Hospital Basel, Basel, Switzerland
16 Department of Cardiology, Lausanne
University Hospital, Lausanne, Switzerland
17 Department of Internal Medicine,
Clinic for Cardiology and Institute for Radiology and Nuclear medicine,
Stadtspital Zurich Triemli, Zurich, Switzerland
18 Hirlsanden Heart Centre, Zurich,
Switzerland
19 Lindenhofgruppe, Bern, Switzerland
20 Cardiocentro Ticino, Lugano,
Switzerland
21 Department of Internal Medicine,
Cantonal Hospital Graubünden, Chur, Switzerland
22 University of Zurich, Zurich, Switzerland
23 Department of Neurology, University
Hospital and University Zurich, Zurich, Switzerland
24 Department of Haematology, Cantonal
Hospital Lucerne, Lucerne, Switzerland
25 Department of Internal Medicine,
Clinic for Medical Oncology and Haematology, Stadtspital Zurich Triemli,
Zurich, Switzerland
26 Institute for Nephrology and
Dialysis, Cantonal Hospital Baden, Baden, Switzerland
27 Division of Nephrology, University
Hospital Zurich, Zurich, Switzerland
28 Department of Gastroenterology and
Hepatology, University Hospital Zurich, Zurich, Switzerland
29 Heart Centre Lucerne, Luzerner Kantonsspital,
Lucerne, Switzerland
30 Centre for Molecular Cardiology, University of Zurich, Zurich, Switzerland
31 Department of Clinical Neurosciences, Service of
Neurology, Lausanne University Hospital (CHUV) and University of Lausanne,
Lausanne, Switzerland
32 Clinic of Haematology, Ente Ospedaliero Cantonale, Bellinzona, Switzerland
Summary
Since the publication of the first Swiss recommendations
on systemic light-chain amyloidosis in 2020, treatment strategies have evolved.
As a result of the third joint meeting of the Swiss Amyloidosis Network, a multidisciplinary
and multicentre Swiss clinical consortium, in 2024, recommendations for the treatment
of light-chain amyloidosis were updated. They discuss the role of the new standard
first-line protocol Daratumumab, Cyclophosphamide, Bortezomib, Dexamethasone (Dara-CyBorD),
the timing and indication of high-dose treatment and potential second-line strategies
as well as emerging treatment options, with a special focus on multidisciplinary
supportive care measures. The update represents a synopsis of current evidence and
expert consensus and intends to provide general treatment guidance tailored to the
Swiss healthcare system. Nonetheless, treatment decisions should always be personalised
and involve a multidisciplinary approach. This update replaces the previous “therapeutic
recommendations” while the previous “diagnostic recommendations” remain valid.
Introduction
Treatment strategies for systemic light-chain
amyloidosis (AL) have evolved since the publication of the first consensus recommendations
by the experts of the Swiss Amyloidosis Network (SAN) in 2020 [1]. To address and
discuss the new aspects, the SAN held its third joint meeting
in April 2024 in Zurich, Switzerland. Participants were clinical specialists from
all fields of medicine involved in the care of amyloidosis patients. The discussion
resulted in an update of the SAN recommendations on light-chain
amyloidosis with a particular focus on treatment and supportive care. The following
update reflects the available published evidence, including the results of clinical
trials and recently published abstracts, as well as existing guidelines from international
societies and expert consensus.
The SAN recommendations
are tailored to address the structures of the Swiss healthcare system and aim to
provide guidance for clinical practitioners in Switzerland. To this end, the manuscript
avoids in-depth discussions of the literature and diagnostic considerations, instead
concentrating on practical “therapeutic recommendations”. This version is not intended
to be exclusive, particularly given the anticipation of continued rapid therapeutic
advancements.
Methodology
Prior to discussion within the SAN, we performed a PubMed search, focusing on studies
published since the release
of the first guidelines in 2020. This search included clinical trial publications,
treatment recommendations by other societies, review articles as well as published
meeting abstracts with high relevance to the field. Relevant publications on light-chain
amyloidosis in English were first reviewed and summarised by the first and last
author and later discussed, point by point, with experts of the SAN. To increase transparency,
the panel used the GRADE approach to assess the
underlying level of evidence [2] (table 1).
Table 1GRADE levels of evidence.
| Grade |
Level |
Origin of evidence |
| A |
I |
(Meta)-analysis
of ≥1 randomised controlled trial (RCT) |
| B |
IIA |
≥1 non-randomised
trial (incl. phase II and case-control) |
| IIB |
≥1 other prospective
non-experimental study (incl. observational studies) |
| III |
≥1 descriptive study
or abstract of meta-analysis and/or randomised controlled trial |
| C |
IV |
Expert consensus
or opinion statement and/or experience of respected authorities |
Where published
evidence was limited or insufficient, consensual expert opinions were included in
the recommendations. Evaluation of the resulting recommendations was performed by
all authors in a stepwise approach. The strength of a recommendation was graded
as either strong (Grade A or B; “The SAN recommends…”) or
conditional (Grade C; “The SAN suggests…”). To strengthen
methodological transparency and quality, the final update followed best practice
for guideline development according to the principles of AGREE II [3] (Appraisal
of Guidelines for Research and Evaluation). The manuscript was finally approved
by all active members of the SAN as listed in the author section.
In anticipation of an evolving treatment landscape
and more therapeutic advances, the recommendations will be considered current for
the following three years or until the publication of an earlier update by the SAN,
as future progress may require earlier revisions.
Considerations before and during treatment
Baseline risk stratification and response assessment
The revised
Mayo 2004 criteria and its European modification are the two best established staging
systems, both including cardiac biomarkers (NT-proBNP, Troponin-T), given that the
degree of cardiac involvement has been shown to be the most relevant prognostic
factor [4, 5]. For assessment of renal involvement, a separate renal staging system
has been developed predicting renal involvement and outcome [6]. Baseline assessment
generally also includes evaluation of “fitness” for high-dose (HD) melphalan treatment
and autologous stem cell transplantation (ASCT). This initial evaluation is crucial
as it affects intensity and sequence of treatment upfront and downstream. Criteria
for assessing fitness are subject to ongoing debate. In Switzerland, the criteria
proposed by the EHA-ISA working group are the best established [7, 8].
The SAN suggests the use of evaluation criteria for high-dose therapy
/ autologous stem cell transplantation as proposed by the EHA-ISA working group
[7, 8]. (Grade C, Level III)
Importantly, although clonal plasma cells are the amyloid-producing cell of origin
in most cases, the predominance of lymphoplasmacytic cells should always be excluded
in patients with IgM-related amyloidosis, as it modifies the proposed treatment
algorithm [9] (see section “IgM-directed treatment”). Organ response remains the ultimate
goal of treatment
and usually occurs within months after the start of treatment, depending on the
speed and depth of the haematological response [10, 11]. Criteria of haematological
and organ response have continuously been improved in recent years (table 2). Haematological
and organ response should be validated at least at 3 and 6 months after initiation
of therapy [12]. However, in routine care, assessments every 1–2 months are an established
practice. As the clinical outcome has been shown to be directly related to the depth
of response [10], obtaining at least a very good partial response (VGPR) is considered
standard. While achievement of a haematological complete
response is desirable, assessment of bone marrow minimal residual disease (MRD)
is the subject of clinical trials and not yet established as a routine response
parameter [13].
The SAN recommends assessment
of haematological and organ response at least at the beginning of every cycle and
at the end of treatment (every month under treatment, every 3–6 months under surveillance,
respectively). (Grade B, Level IIB)
Insufficient
response: The SAN suggests change of treatment if no haematological
very good partial response is reached within 3–4 months from initiation. (Grade
C, Level IV)
Table 2Response
criteria.
| Haematological* [12, 14] |
| Complete response (CR) |
1. Negative serum and urine immunofixation
AND |
| 2. Free light-chain ratio within reference
range OR uninvolved free light chain > involved free light chain |
| Very good partial response (VGPR) |
dFLC <40 mg/l |
| Partial response (PR) |
Decrease of dFLC ≥50% |
| No response (NR) |
All other |
| Organ response [6, 10, 15] |
| Complete response (CR) |
Heart |
NTproBNP: nadir
≤350 ng/l |
| Kidney |
Proteinuria: nadir
≤200 mg / 24 h |
| Liver |
Alkaline phosphatase:
nadir ≤2 × ULN |
| Very good partial response (VGPR) |
Heart |
NTproBNP: decrease
>60%, not meeting complete response |
| Kidney |
Proteinuria: decrease
>60% not meeting complete response |
| Liver |
Alkaline phosphatase:
decrease >60% not meeting complete response |
| Partial response (PR) |
Heart |
NTproBNP: decrease
of 30% to 60%, not meeting complete response |
| Kidney |
Proteinuria: decrease
of 30% to 60% of baseline OR <0.5 g/24 h if >0.5 g/24 h/d at baseline AND
no worsening of eGFR >25% of baseline |
| Liver |
Alkaline phosphatase:
decrease ≥50% OR decrease of liver size ≥2 cm |
| Peripheral nervous system |
Electromyoneurography:
any improvement |
| No response (NR) |
None of the response criteria is met |
First-line treatment of light-chain amyloidosis
For patients with rMayo Stage I–IIIa, as represented
in the ANDROMEDA trial [17], first-line treatment generally includes induction with
Dara-CyBorD (Daratumumab, Cyclophosphamide, Bortezomib and Dexamethasone). The potential
benefit of upfront high-dose therapy / autologous stem cell transplantation has
not been clearly established yet. The only RCT on this particular matter did not
show a survival benefit but was criticised for its unrepresentative inclusion criteria
[18]. The ongoing uncertainty has led to country-specific differences in the indication
for performing high-dose therapy / autologous stem cell transplantation. In general,
given the rarity of the disease and the many unknown variables, if available, treatment
in clinical trials should be considered. In Switzerland, treatment with Dara-CyBorD
requires prior health insurance approval, which is generally granted. The SAN has
agreed on the following recommendations regarding first-line
therapy (figure 1):
Patients with Stage I–III/IIIa
The SAN recommends induction
with Dara-CyBorD. (Grade A, Level I)
- The SAN suggests starting with reduced-dose dexamethasone
in patients with cardiac involvement: 10 to 20 mg/dose. (Grade C, Level IV)
For patients fit for high-dose therapy / autologous
stem cell transplantation, the SAN suggests early stem cell
collection after 2–4 cycles of induction. (Grade C, Level IV)
- The SAN recommends mobilisation with G-CSF (Grade
B, Level IIB) or plerixafor on demand. (Grade C, Level IV)
The SAN recommends upfront
treatment with high-dose therapy / autologous
stem cell transplantation after insufficient haematological response (no very good
partial response after 4 months) to induction or in patients with concomitant symptomatic
myeloma [8, 19–21]. (Grade B, Level IIB)
- The SAN recommends high-dose therapy / autologous
stem cell transplantation with standard dose melphalan 200 mg/m2 rather than dose reduction [8, 22]. (Grade B, Level
IIB)
- If high-dose therapy /
autologous stem cell transplantation is performed, the SAN
suggests it is followed by treatment-free surveillance. (Grade C, Level IV)
- The SAN
does not recommend tandem high-dose therapy / autologous stem cell transplantation
in light-chain amyloidosis [23]. (Grade B, Level III)
The SAN recommends deferral
of high-dose therapy / autologous stem cell transplantation in fit patients who
achieve at least very good partial response after 4 induction cycles [24]. (Grade
B, Level IIB)
If high-dose therapy / autologous stem cell
transplantation is not performed or is deferred, the SAN recommends
continuation of induction with Dara-CyBorD for a total of 6 cycles followed by 18
cycles of maintenance with daratumumab [17]. (Grade B, Level IIA)
Patients unfit for high-dose therapy / autologous stem
cell transplantation and/or Stage IV/IIIb
In Stage I–IIIa unfit for high-dose therapy;
the SAN recommends induction with 6 cycles of Dara SC-CyBorD
followed by 18 cycles of daratumumab maintenance [17]. (Grade A, Level I)
In Stage IIIb/IV with advanced cardiac involvement;
the SAN recommends a daratumumab-based induction treatment,
i.e. as monotherapy, in combination with dexamethasone [25], or as a modified, dose-reduced
Dara-CyBorD protocol [26]. (Grade B, Level III–IV)
- The SAN suggests
to start with bortezomib at an attenuated dose (0.7 mg/m2) followed by an increase every two weeks (+0.3 mg/m2) if tolerated. (Grade C, Level IV)
- The SAN suggests
to start with reduced-dose dexamethasone (4–10 mg). (Grade C, Level IV)
IgM-directed treatment
In IgM-type amyloidosis, non-Hodgkin’s lymphoma
(NHL) clonal B cells (less commonly plasma cells) are usually at the origin of the
disease. When clonal B cells are identified, rituximab-based treatment regimens
are established first-line therapies [9, 27, 28], combinations with Bruton’s tyrosine
kinase (BTK) inhibitors [29], bendamustin, cyclophosphamide, chlorambucil, bortezomib
and systemic corticosteroids may be appropriate depending on the biology of the
underlying clone [27, 29, 30].
Plasma cell-directed treatment may also be appropriate if plasma cells are (part
of) the identified clonal population.
The SAN recommends rituximab-based
induction therapy in the treatment of IgM-associated amyloidosis. (Grade B, Level
IIB)
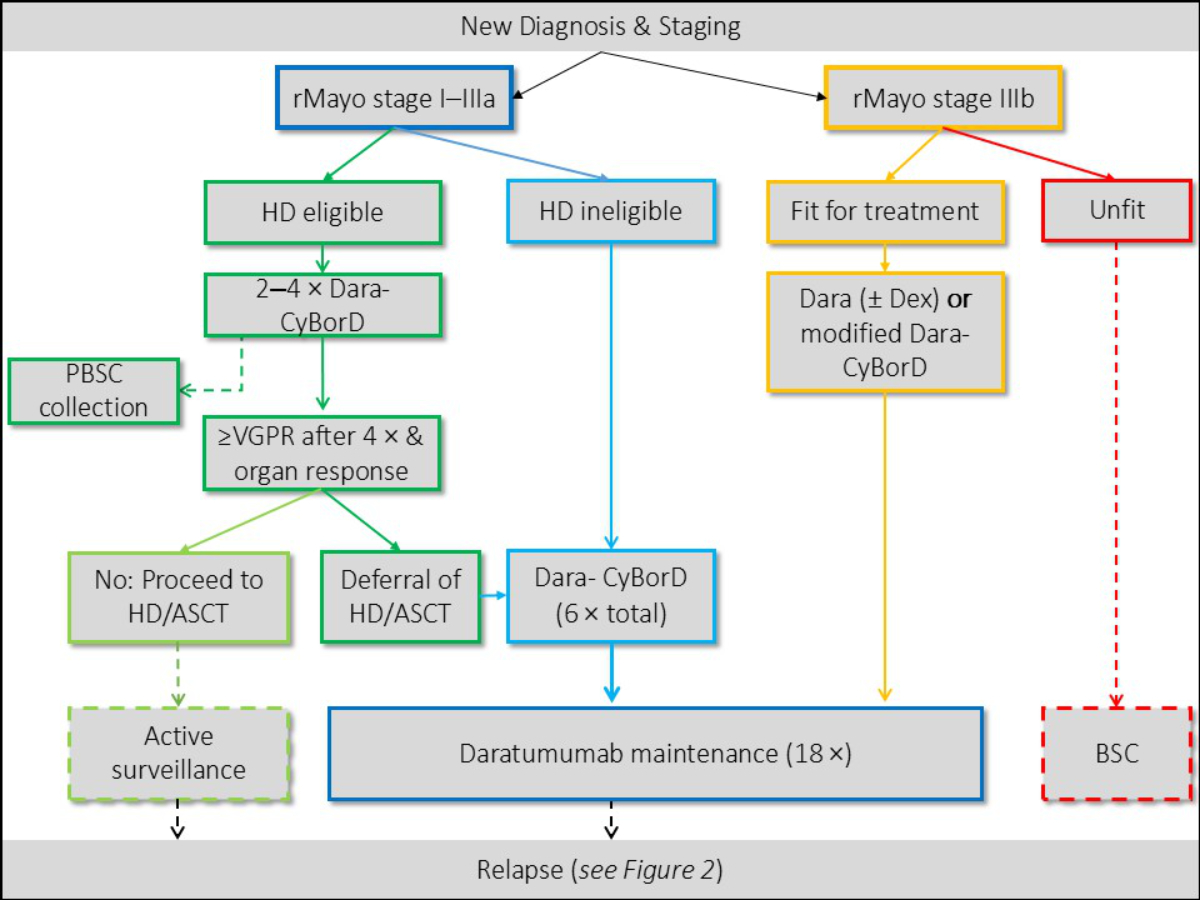
Figure 1First-line treatment algorithm as
proposed by the Swiss Amyloidosis Network (SAN). ASCT: autologous stem cell
transplantation; BSC: best supportive care; CyBorD:
cyclophosphamide-bortezomib-dexamethasone; Dara: daratumumab; Dex: dexmethasone; HD:
high-dose
therapy; PBSC: peripheral blood stem cells; rMayo: revised Mayo stage; VGPR: haematological
very good partial remission.
Second-line treatment of relapsed/refractory light-chain
amyloidosis
Although the pathological plasma cell clone
is generally responsive to multiple treatment options, many therapeutic agents are
poorly tolerated and disease- and treatment-related morbidity and mortality may
be significant, especially at the beginning of treatment. Multidisciplinary evaluation
and frequent adjustment of the therapeutic regimen are common, and individualised
symptom management is an integral part of therapy (figure 2).
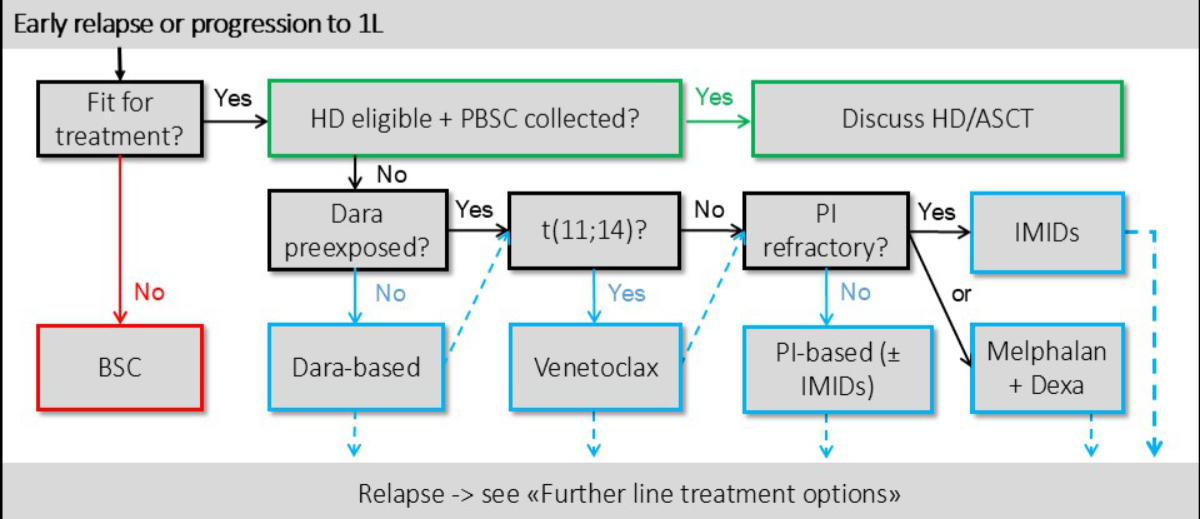
Figure 2Treatment algorithm for r/r light-chain amyloidosis (AL) as proposed
by the SAN (SAN). ASCT: autologous stem cell transplantation; BSC: best supportive
care; Dara: daratumumab;
Dexa: dexamethasone; HD: high-dose therapy;
IMIDs: immunomodulatory agents; PBSC: peripheral blood stem cells; PI: proteasome
inhibitor; r/r: relapsed/refractory; t(11;14): translocation 11;14; 1L: first-line
treatment.
Timing of relapse treatment
The optimal timing of second-line treatment
initiation is a matter of debate [31], weighing the apparent benefits (prevention
of organ deterioration ) against potential therapy-associated side effects. Palladini
et al. suggested treatment restart at high risk for progression, defined as a dFLC
of >20 mg/l, a level >20% of baseline and a >50% increase from the lowest
value reached [32].
The SAN recommends initiation
of rescue therapy in haematological relapse as proposed by Palladini et al. [32].
(Grade B, Level IIB)
- Criteria: dFLC of >20 mg/l AND
>20% of baseline AND >50% increase from the lowest value reached.
The SAN recommends earlier
initiation (at biochemical loss of response) of rescue therapy in patients with cardiac
involvement to prevent
cardiac deterioration [32]. (Grade B, Level III)
Treatment options
In the relapsed/refractory setting, a variety
of possible treatment options are available, most of which are derived from myeloma
treatment [31]. The optimal type and intensity of treatment depends on previous
exposure and response, time to relapse and current disease stage/comorbidities.
Access to treatment can be challenging however, as there is no guaranteed reimbursement
beyond first-line treatment in the absence of plasma cell myeloma in Switzerland.
Requests for cost coverage must be justified in accordance with Article 71 of the
Health Insurance Act [33].
The SAN recommends r/r
treatment with an anti-CD38-Ab and/or a proteasome inhibitor, if not already included
in previous therapy [34, 35]. (Grade B, Level IIB)
The SAN suggests retreatment
with the initial regimen in patients with good initial response and tolerance of
first-line and after at least 24 months of treatment-free remission. (Grade C, Level
IV)
If relapse occurs after the current standard-of-care,
Dara-CyBorD or similar protocols including an anti-CD38-Ab and proteasome inhibitor,
there is no universal standard for further-line therapy and the available evidence
is often limited to registry data and small case series. Therefore, the following
options are Grade B or C recommendations and are based on expert consensus within
the SAN.
Venetoclax: translocation t(11;14)
Venetoclax is a B-cell lymphoma-2 (BCL-2) inhibitor
active in plasma cell neoplasia such as myeloma (PCM), particularly those harbouring
t(11;14), which is associated with high BCL-2 expression [36–38]. Approximately
50% of patients with light-chain amyloidosis show t(11;14) [39, 40], making venetoclax
a valuable option. Growing evidence in a heavily pretreated patient population demonstrates
efficacy as a single agent or in combination with an acceptable safety profile,
with tumour lysis syndrome (TLS) being rare [36, 37]. If not already performed at
diagnosis, an iFiSH and/or BCL-2 expression analysis is a prerequisite for treatment
with venetoclax.
The SAN recommends second-line
treatment with venetoclax in patients with t(11;14) and previous exposure to an
anti-CD38-Ab. (Grade B, Level IIB)
- The SAN suggests
a target dose of 400 mg/d, consider ramp-up over 3 days with a starting dose of
100 mg/d under TLS monitoring. (Grade B, Level III)
The SAN suggests venetoclax
in patients with t(11;14), as monotherapy or in combination with an anti-CD38-Ab
and/or a proteasome inhibitor in case of no pre-exposure. (Grade C, Level IV)
Immunomodulatory agent-based treatment: no t(11;14) and adequate organ function
Various case series and phase II trials report
the efficacy of immunomodulatory agents (IMIDs) in pretreated patients. However,
unlike plasma cell myeloma, the tolerability of immunomodulatory agents in light-chain
amyloidosis is poor, the discontinuation rate is high (>40%) and reduced cardiac
and renal function as well as possible cytopenia are major limiting factors [41].
Published evidence favours pomalidomide over lenalidomide due to better tolerability
[41, 42]. Potential effects on volume homeostasis, cardiac function (biomarkers/rhythm)
and renal function should be closely monitored.
In patients with progression to standard treatment,
adequate organ function and without t(11;14), the SAN recommends
treatment with immunomodulatory agents ± dexamethasone as second-line treatment
[43, 44]. (Grade B, Level IIB)
- The SAN recommends
a preferential use of pomalidomide over lenalidomide [42, 45]. (Grade B, Level III)
- The SAN suggests
starting immunomodulatory agent treatment in reduced doses (pomalidomide; start
at 1 mg/d, lenalidomide; start at 5 mg/d) and subsequent dose escalation, if tolerated.
(Grade C, Level IV)
Oral melphalan + dexamethasone: no t(11;14) and immunomodulatory agents not
feasible
Melphalan alone or in combination with dexamethasone
(MelDex) has been studied quite extensively in the field of light-chain amyloidosis.
In selected patients, without significant comorbidities or advanced cardiac involvement,
it has been shown to be effective but is associated with relevant toxicity [46,
47].
The SAN recommends treatment
with melphalan/dexamethasone (MEL 0.22 mg/kg/d and Dex 20–40 mg/d , d1–4 every 28
days [46]) in patients without significant cardiac involvement (<revised Mayo
Stage III) and not qualifying for the other therapeutic options. (Grade A, Level
I)
High-dose therapy melphalan and autologous stem cell transplantation: only
selected patients in the r/r setting
If not already performed upfront, in patients
considered medically fit according to the proposed criteria (see above, “Considerations
before and during treatment”) and
when autologous stem cells are available, high-dose therapy and autologous stem
cell transplantation may be considered at relapse [24].
The SAN recommends early
consideration of high-dose therapy / autologous stem cell transplantation as part
of relapse treatment for eligible patients. (Grade B, Level III)
Prior reinduction is optional. (Grade C, Level IV)
Ixazomib: if bortezomib is not feasible
Ixazomib-dexamethasone was evaluated in a phase
III study [48] (Tourmaline) where it showed no advantage in haematological response
over physician’s choice. As the proteasome-inhibitor bortezomib is often part of
first- or second-line treatment, an additional benefit of ixazomib at later time
points is expected only in very specific scenarios, i.e. if tolerability is of higher
priority [49] (see below, “Supportive care and treatment in advanced organ involvement”).
The SAN recommends treatment
with ixazomib only in selected scenarios and if bortezomib is not feasible. (Grade
A, Level 1)
Further-line treatment options
If patients progress after the proposed second-line
options and do not qualify for retreatment, the optimal treatment will again depend
on prior exposure, comorbidities and potential access to novel therapies, which
is often limited to patients with associated symptomatic myeloma.
Bispecific T-cell engagers (BiTe) and chimeric antigen receptor T-cells (CAR-T)
Early retrospective studies have been published
demonstrating efficacy and good tolerability of BCMA-directed bispecific antibodies
[50, 51]. Given the often low plasma cell burden in light-chain amyloidosis and
the therefore high “effector to target” ratio, they potentially reflect a very effective,
well-tolerated and promising treatment option.
The SAN suggests treatment
with BCMA-BiTe in eligible patients, relapsed/refractory or not qualifying to all
other established 2nd line treatment options. (Grade C, Level III)
Preliminary data on successful CAR-T cell treatment
has recently been published in a very selected patient population [52–54]. Given
its potential side effects in patients with severe organ involvement and its financial
burden, CAR-T cells are not yet established outside of clinical trials and rather
reflect a potential future option.
So far, the SAN does not
recommend treatment with CAR-T cells outside clinical trials. (Grade B, Level IIB)
Treatment options currently not recommended by the SAN
Carfilzomib has been shown to be associated
with a high rate of severe adverse events in patients with light-chain amyloidosis,
especially resulting from its potential cardiac toxicity [55].
If other options
are available, the SAN does not recommend carfilzomib-based
treatment. (Grade B, Level IIA)
Various light-chain amyloid-targeting agents
have been developed. To date, none of them has been translated into clinical practice.
A phase III study with birtamimab, a humanised IgG1 binding circulating and deposited
light-chain fibrils, is currently recruiting (AFFIRM-AL). Prior phase I/II studies
have shown somewhat inconsistent results [56–59].
Treatment of localised light-chain amyloidosis
Localised amyloidosis, if not involving a critical
site, generally does not impair survival of affected patients and almost never progresses
to a systemic disease [60]. The treatment of “amyloidoma” is therefore reserved
for symptomatic disease and usually involves surgical excision whenever possible
(although evidence is sparse) [61, 62]. In selected cases where surgery is not feasible,
radiotherapy or laser treatment can be an alternative local treatment options [63].
The SAN recommends local treatment (such as surgical resection) of localised light-chain
amyloidosis in symptomatic patients. (Grade B, Level III)
If local treatment is not feasible and need
for therapy is high, an individualised approach with systemic treatment may be considered.
(Grade C, Level IV)
The SAN recommends annual
follow-up to screen for local recurrence [64]. (Grade B, Level III)
During follow-up, the SAN
generally suggests not to perform repetitive extensive evaluation for systemic involvement,
but to perform a regular basic screening with NT-proBNP and albuminuria measurements.
(Grade B, Level III)
Supportive care and treatment in advanced organ involvement
Monitoring during follow-up
Supportive care requires a multidisciplinary
approach. Monitoring of organ function includes repetitive assessment of involved
organs and screening of new potential end-organ disease [65]. The SAN suggests the
following approach [66–68]:
The SAN suggests a comprehensive
cardiac assessment every 6–12 months (cardiac biomarkers, ECG, Holter ECG, echocardiography,
MRI if image quality is poor).
The SAN suggests routine
renal assessment at least every 3 months with standard measurements (eGFR, proteinuria
in spot urine) and a more comprehensive assessment every 6–12 months in case of
significant renal involvement or treatment toxicity.
The SAN suggests assessment
of liver enzymes at least every 3 months and a more comprehensive assessment every
12 months (sonography, liver stiffness) in case of involvement or associated toxicity.
The SAN suggests clinical
screening for polyneuropathy at least every 3 months and a comprehensive assessment
(clinical examination, autonomic testing and ENMG) every 12 months in case of organ
involvement or significant treatment-related neuropathy. Additional skin biopsy
for assessment of small-fibre neuropathy can be considered [69, 70].
General measures of supportive care and disease-modifying
therapy
Supportive care includes symptom management;
anti-infective strategies; management of cardiac and renal involvement, neuropathy
and GI dysfunction; and treatment of potential therapy-induced toxicities [65, 71].
Optimal supportive care is achieved through multidisciplinary care, preferably within
the SAN. Due to the rarity of the disease and the heterogeneity
of the clinical picture, studies focusing on supportive measures are rare. Therefore,
many of the following recommendations are extrapolated from similar scenarios in
other diseases. The few supportive care drugs that have been studied specifically
in light-chain amyloidosis include doxycycline and Epigallo-Catechin-Gallat (EGCG).
However, after promising early-phase study results, doxycycline failed to show significant
benefit in a phase III trial and should therefore no longer be part of supportive
care in light-chain amyloidosis [72]. Similarly, although often associated with
much patient hope, there is only insufficient evidence to support the use of Epigallo-Catechin-Gallat
or other green tea extracts [73, 74]. Patients should be advised not to co-administer
Epigallo-Catechin-Gallat with immunochemotherapy agents due to potential interactions.
The SAN recommends against
the use of doxycycline as a supportive agent in light-chain amyloidosis. (Grade
A, Level I)
The SAN suggests not to
use Epigallo-Catechin-Gallat in light-chain amyloidosis during chemo-immunotherapy.
(Grade C, Level IV)
Cardiovascular care
The most common early clinical manifestations
of cardiac involvement in light-chain amyloidosis are heart failure with preserved
ejection fraction (HFpEF), with predominant diastolic dysfunction and its consequences.
Fluid retention with elevated filling pressures together with structural alterations
in the atria increase the likelihood for atrial fibrillation and thromboembolic
stroke. Amyloid deposition can also lead to heart block and arrhythmia such as ventricular
tachycardia.
Heart failure and fluid management
As early mortality is high in patients with
advanced cardiac involvement (revised Mayo Stage IV / European modification Stage
IIIb), immediate initiation of plasma cell-directed therapy is crucial. Treatment
requires close collaboration with heart failure specialists and may even require
in-hospital monitoring during the first days of treatment. Daratumumab alone or
in combination with dexamethasone has been shown to be safe and effective as initial
treatment [25] (see section “First-line treatment of light-chain amyloidosis”).
In advanced cardiac involvement (rMayo IV),
the SAN suggests in-hospital monitoring during initiation
of treatment. (Grade C, Level IV)
Administration of diuretics is often required
(furosemide or torasemide ± spironolactone). However, careful dose titration
is advised to avoid symptomatic hypotension and significant reduction of preload,
which may further deteriorate cardiac function. Potential potassium supplementation
and restriction of salt and volume intake may be advisable [67].
Standard heart failure therapy including beta-blockers,
RAAS inhibitors and calcium-channel blockers should be used with great caution as
they are often not well tolerated and may worsen clinical symptoms (orthostatic
hypotension, volume overload) [75].
The SAN suggests initiation
of loop diuretics in patients with volume overload (low starting dose, e.g. torasemide
5–10 mg). (Grade C, Level IV)
The SAN suggests that
calcium-channel blockers should generally be avoided. RAAS inhibitors and beta-blockers
should only be used in selected cases and with great caution [76, 77]. (Grade C,
Level IV)
The SAN cannot recommend
for or against treatment with SGLT2 inhibitors due to insufficient evidence. (Grade
C, Level IV)
Heart transplant is an option for eligible patients
in the absence of severe other organ involvement [78, 79]. It should always be followed
by very close monitoring of haematological response and a low threshold for light-chain
amyloidosis directed treatment.
The SAN recommends evaluation
of heart transplantation in young patients. (Grade B, Level IIb)
Arrhythmia
Due to its frequency, arrhythmia should be actively
screened for (see section “Monitoring during follow-up” above). Antiarrhythmic therapy
should generally be limited to amiodarone. Rate control with a beta-blocker is also
an option, but heart rate should not be lowered too much as cardiac output depends
on heart rate with stroke volume fixed due to amyloid deposition [80].
If required, the SAN suggests
pharmacological antiarrhythmic therapy preferably with amiodarone [80, 81]. (Grade
C, Level IV)
With cardiac involvement, all patients with
atrial fibrillation (Afib) are at very high risk of thromboembolic events [82].
Oral anticoagulation, irrespective of the CHA2DS2-VA score,
is therefore generally advisable in the absence of overt bleeding or amyloidosis-associated
coagulopathies. Particular attention should also be given to ventricular arrhythmias
with the risk for sudden cardiac death [83]. Although an implanted cardiac defibrillator
(ICD) may potentially be life-saving, its long-term benefit on mortality has not
yet been proven in cardiac light-chain amyloidosis [84].
The SAN suggests oral
anticoagulation irrespective of CHA2DS-VA in patients with
cardiac involvement and Afib (unless there is significant coagulopathy). (Grade
C, Level IV)
In selected patients with atrial mechanical
dysfunction and restrictive filling pattern (severe diastolic dysfunction), anticoagulation
can be evaluated in the absence of Afib. (Grade C, Level IV)
The SAN suggest the use
of ICD in cardiac amyloidosis only in selected cases and after interdisciplinary
discussion [85–87]. (Grade B, Level IIa)
The SAN cannot recommend
for or against catheter ablation or LAA-occlusion in patients with cardiac light-chain
amyloidosis. (Grade C, Level IV)
Renal support
Nephrotic syndrome
Fluid management and diuretic therapy (preferably
loop diuretics, may be combined with thiazides and/or spironolactone in diuretic
resistance) are among the mainstays of treatment. To date, evidence to support albumin
infusions is insufficient. In patients with progressive hypoalbuminaemia, management
of haemostasis can be particularly challenging (see section “Management of gastrointestinal
involvement and haemostasis”).
The SAN does not generally
suggest prophylactic anticoagulation in severe nephrotic syndrome with serum albumin
<25 g/l due to the potentially increased risk of bleeding. (Grade C, Level IV)
In patients with renal light-chain amyloidosis
and proteinuria, the SAN does not recommend anti-proteinuric
treatment with RAAS-blocking agents, as their potential harm in co-existing autonomic
dysfunction and cardiac amyloidosis may outweigh the potential benefits [88–90].
(Grade B, Level IIb)
The SAN suggests salt
and fluid restriction in resistant oedema. (Grade C, Level IV)
End-stage renal disease (ESRD)
RAAS-blocking agents may be introduced, but caution is advised
concerning hypotension and autonomic dysfunction (see above). Optimal control of
independent cardiovascular risk factors is recommended. Dialysis has been shown to
improve survival particularly in patients
without end-stage heart disease [91–94]. Peritoneal dialysis may be preferable over
haemodialysis in end-stage renal disease and heart failure, given its superior haemodynamic
tolerance [95]. Standard first-line Dara SC-CyBorD can be safely administered to
patients with end-stage renal disease with or without dialysis. However, many other
light-chain amyloidosis treatment options should be dose-reduced or omitted in patients
with end-stage renal disease.
The SAN recommends evaluation
of dialysis in end-stage renal disease. (Grade B, Level IIB)
Kidney transplantation has been shown to have
similar outcomes in renal light-chain amyloidosis compared to other causes of end-stage
renal disease [92, 96]. An adequate haematological response (very good partial response
or better) to light-chain amyloidosis treatment and/or promising further therapies
are prerequisites, as local recurrence is otherwise foreseeable.
The SAN recommends evaluation
of kidney transplantation in selected patients. (Grade B, Level IIB)
Management of gastrointestinal involvement and haemostasis
Management of gastrointestinal complaints is
challenging and often focuses on symptomatic treatment [97]. Malnutrition may require
nutritional counselling; evidence on parenteral nutrition is very sparse. Patients
with high bilirubin represent a particular challenge because liver-specific dose
modification for many established amyloidosis-directed treatment options remains
poorly studied.
In advanced amyloidosis, bleeding potentially
complicates severe hepatic failure or may be associated with amyloid vasculopathy,
factor X deficiency, hypofibrinogenaemia or other clotting factor deficiency [98].
However, risk of thrombosis may also be relevant due to the disease-associated immobility,
therapy (e.g. immunomodulatory agents) or possible nephrotic syndrome. The optimal
strategy for managing haemostasis therefore requires an individualised, multidisciplinary
consensus.
Polyneuropathy
Supportive care measures focus on symptom management
and prevention of further treatment-related damage. In patients with neuropathic
pain associated with peripheral neuropathy, symptomatic treatment is essential.
First-line treatments include serotonin-noradrenaline
reuptake inhibitors and gabapentinoids. Second-line treatments include
tramadol, tricyclic antidepressants (beware of hypotension), combinations and adjunctive
psychotherapy. Autonomic neuropathy presents a particular challenge (postural hypotension)
and may also require symptomatic treatment [99].
Amyloidosis-directed treatment in patients with
significant neuropathy should not include bortezomib. Daratumumab, alone or in combination
with cyclophosphamide and/or dexamethasone, as well as immunomodulatory agents are
feasible. In this specific patient population, the SAN also
considers ixazomib a reasonable alternative when bortezomib is not feasible [48].
The SAN recommends initial
treatment with single-agent daratumumab in patients with severe polyneuropathy (CTCAE
grade III). (Grade C, Level IV)
The SAN suggests symptomatic
treatment of peripheral nervous system involvement with gabapentin (start dose 100
mg 3 ×/d, ramp up to
max. 1200 mg 3 ×/d) and/or duloxetine (start dose 30 mg/d, ramp up to max. 120 mg/d)
under close blood pressure monitoring [81]. (Grade C, Level IV)
In symptomatic hypotension, pressure stockings,
abdominal binders and midodrine are valuable options [100] (starting dose 3 × 2.5
mg/d and escalation to a max. of 10 mg 3 ×/d). (Grade C, Level IV)
Infection prevention
Many forms of amyloid- and/or plasma cell-directed
treatment, particularly daratumumab, often cause profound hypogammaglobulinaemia,
increasing the risk of infections. Immunoglobulin substitution may be beneficial;
it is reimbursed in Switzerland in cases of total IgG <4 g/l and repetitive infections
or inadequate antibody response to vaccination [101].
The SAN suggests immunoglobulin
substitution in patients with total serum IgG <4 g/l and infectious complications.
(Grade C, Level IV)
The SAN recommends seasonal
vaccination against influenza and COVID infections during treatment and pre-therapeutic
invasive pneumococcal disease vaccination. (Grade B, Level III)
The SAN suggests Varicella zoster virus-reactivation
prophylaxis in light-chain amyloidosis patients undergoing plasma cell-directed
treatment; PJP prophylaxis may be considered. (Grade C, Level IV)
Conclusion
Fortunately, the evidence for upfront management
of patients with light-chain amyloidosis has improved significantly in recent years.
However, management in the relapsing/remitting setting, in patients with more significant
comorbidities or in those with very advanced disease, still requires expert consensus,
as the available evidence is limited to small prospective phase II or observational
studies. Supportive care should always involve a multidisciplinary team, as patterns
of organ involvement and clinical presentation are very heterogeneous. In particular,
management of the most advanced and morbid patients requires a comprehensive care
approach. Whenever possible, patients should be considered for clinical trials.
Author contributions
Conceptualisation: Rahel Schwotzer,
Max Rieger, Bernhard Gerber. Data curation, investigation and methodology: Rahel
Schwotzer, Max Rieger. Funding acquisition: Rahel Schwotzer. Drafting of the original
manuscript, project administration: Max Rieger, Rahel Schwotzer. Revision
and Editing: All authors. Visualisation: Max Rieger, Rahel Schwotzer. Administrative,
technical and material support: Max Rieger, Rahel Schwotzer. Supervision and validation:
Rahel Schwotzer, Bernhard Gerber.
Max J. Rieger
Department of
Medical Oncology and Haematology
University and
University Hospital of Zurich
Rämistrasse 100
CH-8091 Zurich
max.rieger[at]usz.ch
References
1. Schwotzer R, Flammer AJ, Gerull S, Pabst T, Arosio P, Averaimo M, et al. Expert recommendation
from the Swiss Amyloidosis Network (SAN) for systemic AL-amyloidosis. Swiss Med Wkly.
2020 Dec;150(4950):w20364. doi: https://doi.org/10.4414/smw.2020.20364
2. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al.; GRADE
Working Group. GRADE: an emerging consensus on rating quality of evidence and strength
of recommendations. BMJ. 2008 Apr;336(7650):924–6. doi: https://doi.org/10.1136/bmj.39489.470347.AD
3. Cluzeau F, et al.; AGREE Collaboration. Development and validation of an international
appraisal instrument for assessing the quality of clinical practice guidelines: the
AGREE project. Qual Saf Health Care. 2003 Feb;12(1):18–23. doi: https://doi.org/10.1136/qhc.12.1.18
4. Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac
troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary
systemic amyloidosis. J Clin Oncol. 2004 Sep;22(18):3751–7. doi: https://doi.org/10.1200/JCO.2004.03.029
5. Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A
European collaborative study of treatment outcomes in 346 patients with cardiac stage
III AL amyloidosis. Blood. 2013 Apr;121(17):3420–7. doi: https://doi.org/10.1182/blood-2012-12-473066
6. Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, et al. A staging system
for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis.
Blood. 2014 Oct;124(15):2325–32. doi: https://doi.org/10.1182/blood-2014-04-570010
7. Schönland SO, Dreger P, De Witte T, Hegenbart U. Current status of hematopoietic
cell transplantation in the treatment of systemic amyloid light-chain amyloidosis.
Bone Marrow Transplantat. 2012;47(7:895–905. doi: https://doi.org/10.1038/bmt.2011.152
8. Sanchorawala V. Summary of the EHA-ISA Working Group Guidelines for High-dose Chemotherapy
and Stem Cell Transplantation for Systemic AL Amyloidosis. HemaSphere. 2022 Jan;6(2):e681.
10.1097/HS9.0000000000000681
9. Sidana S, Larson DP, Greipp PT, He R, McPhail ED, Dispenzieri A, et al. IgM AL amyloidosis:
delineating disease biology and outcomes with clinical, genomic and bone marrow morphological
features. Leukemia. 2020 May;34(5):1373–82. doi: https://doi.org/10.1038/s41375-019-0667-6
10. Muchtar E, Dispenzieri A, Leung N, Lacy MQ, Buadi FK, Dingli D, et al. Depth of organ
response in AL amyloidosis is associated with improved survival: grading the organ
response criteria. Leukemia. 2018 Oct;32(10):2240–9. doi: https://doi.org/10.1038/s41375-018-0060-x
11. Palladini G, Merlini G. How I treat AL amyloidosis. Blood. 2022 May;139(19):2918–30.
doi: https://doi.org/10.1182/blood.2020008737
12. Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New
criteria for response to treatment in immunoglobulin light chain amyloidosis based
on free light chain measurement and cardiac biomarkers: impact on survival outcomes.
J Clin Oncol. 2012 Dec;30(36):4541–9. doi: https://doi.org/10.1200/JCO.2011.37.7614
13. Palladini G, Paiva B, Wechalekar A, Massa M, Milani P, Lasa M, et al. Minimal residual
disease negativity by next-generation flow cytometry is associated with improved organ
response in AL amyloidosis. Blood Cancer J. 2021 Feb;11(2):34. doi: https://doi.org/10.1038/s41408-021-00428-0
14. Palladini G, Schönland SO, Sanchorawala V, Kumar S, Wechalekar A, Hegenbart U, et
al. Clarification on the definition of complete haematologic response in light-chain
(AL) amyloidosis. Amyloid. 2021 Mar;28(1):1–2. doi: https://doi.org/10.1080/13506129.2020.1868810
15. Muchtar E, Dispenzieri A, Wisniowski B, Palladini G, Milani P, Merlini G, et al. Graded
Cardiac Response Criteria for Patients With Systemic Light Chain Amyloidosis. J Clin
Oncol. 2023 Mar;41(7):1393–403. doi: https://doi.org/10.1200/JCO.22.00643
16. Milani P, Basset M, Russo F, Foli A, Merlini G, Palladini G. Patients with light-chain
amyloidosis and low free light-chain burden have distinct clinical features and outcome.
Blood. 2017 Aug;130(5):625–31. doi: https://doi.org/10.1182/blood-2017-02-767467
17. Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, et al.; ANDROMEDA
Trial Investigators. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis.
N Engl J Med. 2021 Jul;385(1):46–58. doi: https://doi.org/10.1056/NEJMoa2028631
18. Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O, et al.; Myélome
Autogreffe (MAG) and Intergroupe Francophone du Myélome (IFM) Intergroup. High-dose
melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007 Sep;357(11):1083–93.
doi: https://doi.org/10.1056/NEJMoa070484
19. Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis,
and treatment. Am J Hematol. 2022 Jun;97(6):818–29. doi: https://doi.org/10.1002/ajh.26569
20. Sanchorawala V, Quillen K, Sloan JM, Andrea NT, Seldin DC. Bortezomib and high-dose
melphalan conditioning for stem cell transplantation for AL amyloidosis: a pilot study.
Haematologica. 2011 Dec;96(12):1890–2. doi: https://doi.org/10.3324/haematol.2011.049858
21. Basset M, Milani P, Nuvolone M, Benigna F, Rodigari L, Foli A, et al. Sequential response-driven
bortezomib-based therapy followed by autologous stem cell transplant in AL amyloidosis.
Blood Adv. 2020 Sep;4(17):4175–9. doi: https://doi.org/10.1182/bloodadvances.2020002219
22. Nguyen VP, Landau H, Quillen K, Brauneis D, Shelton AC, Mendelson L, et al. Modified
High-Dose Melphalan and Autologous Stem Cell Transplantation for Immunoglobulin Light
Chain Amyloidosis. Biol Blood Marrow Transplant. 2018 Sep;24(9):1823–7. doi: https://doi.org/10.1016/j.bbmt.2018.06.018
23. Sanchorawala V, Wright DG, Quillen K, Finn KT, Dember LM, Berk JL, et al. Tandem cycles
of high-dose melphalan and autologous stem cell transplantation increases the response
rate in AL amyloidosis. Bone Marrow Transplant. 2007 Sep;40(6):557–62. doi: https://doi.org/10.1038/sj.bmt.1705746
24. Manwani R, Hegenbart U, Mahmood S, Sachchithanantham S, Kyriakou C, Yong K, et al. Deferred
autologous stem cell transplantation in systemic AL amyloidosis. Blood Cancer J. 2018 Nov;8(11):101.
doi: https://doi.org/10.1038/s41408-018-0137-9
25. Oubari S, Hegenbart U, Schoder R, Steinhardt M, Papathanasiou M, Rassaf T, et al. Daratumumab
in first-line treatment of patients with light chain amyloidosis and Mayo stage IIIb
improves treatment response and overall survival. Haematologica. 2024 Jan;109(1):220–30.
26. Wechalekar AD, Sanchorawala V. Daratumumab in AL amyloidosis. Blood. 2022 Dec;140(22):2317–22.
doi: https://doi.org/10.1182/blood.2021014613
27. Palladini G, Foli A, Russo P, Milani P, Obici L, Lavatelli F, et al. Treatment of
IgM-associated AL amyloidosis with the combination of rituximab, bortezomib, and dexamethasone.
Clin Lymphoma Myeloma Leuk. 2011 Feb;11(1):143–5. doi: https://doi.org/10.3816/CLML.2011.n.033
28. Milani P, Schönland S, Merlini G, Kimmich C, Foli A, Dittrich T, et al. Treatment
of AL amyloidosis with bendamustine: a study of 122 patients. Blood. 2018 Nov;132(18):1988–91.
doi: https://doi.org/10.1182/blood-2018-04-845396
29. Buske C, Tedeschi A, Trotman J, García-Sanz R, MacDonald D, Leblond V, et al. Ibrutinib
Plus Rituximab Versus Placebo Plus Rituximab for Waldenström’s Macroglobulinemia:
Final Analysis From the Randomized Phase III iNNOVATE Study. J Clin Oncol. 2022 Jan;40(1):52–62.
doi: https://doi.org/10.1200/JCO.21.00838
30. Manwani R, Sachchithanantham S, Mahmood S, Foard D, Sharpley F, Rezk T, et al. Treatment
of IgM-associated immunoglobulin light-chain amyloidosis with rituximab-bendamustine.
Blood. 2018 Aug;132(7):761–4. doi: https://doi.org/10.1182/blood-2018-04-846493
31. Dima D, Mazzoni S, Anwer F, Khouri J, Samaras C, Valent J, et al. Diagnostic and Treatment
Strategies for AL Amyloidosis in an Era of Therapeutic Innovation. JCO Oncol Pract.
2023 May;19(5):265–75. doi: https://doi.org/10.1200/OP.22.00396
32. Palladini G, Milani P, Foli A, Basset M, Russo F, Perlini S, et al. Presentation and
outcome with second-line treatment in AL amyloidosis previously sensitive to nontransplant
therapies. Blood. 2018 Feb;131(5):525–32. doi: https://doi.org/10.1182/blood-2017-04-780544
33. Revision Art. 71 a/b KVV. Schweiz Arzteztg. 2017 Jan;98(4):122–5. doi: https://doi.org/10.4414/saez.2017.05084
34. Theodorakakou F, Fotiou D, Spiliopoulou V, Roussou M, Malandrakis P, Ntanasis-Stathopoulos I,
et al. Outcomes of patients with light chain (AL) amyloidosis after failure of daratumumab-based
therapy. Br J Haematol. 2023 Nov;203(3):411–5. doi: https://doi.org/10.1111/bjh.19042
35. Shragai T, Gatt M, Lavie N, Vaxman I, Tadmor T, Rouvio O, et al. Daratumumab for relapsed
AL amyloidosis-When cumulative real-world data precedes clinical trials: A multisite
study and systematic literature review. Eur J Haematol. 2021 Feb;106(2):184–95. doi: https://doi.org/10.1111/ejh.13535
36. Rieger MJ, Pabst T, Jeker B, Paul P, Bergamini F, Bühler MM, et al. Three years follow-up
of Venetoclax in advanced-stage, relapsed or refractory AL amyloidosis with cardiac
involvement and t(11;14) with BCL2 expression. Ann Hematol. 2024 Oct;103(10):4163–70.
doi: https://doi.org/10.1007/s00277-024-05901-x
37. Lebel E, Kastritis E, Palladini G, Milani P, Theodorakakou F, Aumann S, et al. Venetoclax
in Relapse/Refractory AL Amyloidosis-A Multicenter International Retrospective Real-World
Study. Cancers (Basel). 2023 Mar;15(6):1710. doi: https://doi.org/10.3390/cancers15061710
38. Le Bras F, et al. Venetoclax induces sustained complete responses in refractory/relapsed
patients with cardiac AL amyloidosis. J. Clin. Oncol. 2019;37_suppl.15.e19538. doi.org/10.1200/JCO.2019.37.15_suppl.e1953
39. Bochtler T, Hegenbart U, Heiss C, Benner A, Moos M, Seckinger A, et al. Hyperdiploidy
is less frequent in AL amyloidosis compared with monoclonal gammopathy of undetermined
significance and inversely associated with translocation t(11;14). Blood. 2011 Apr;117(14):3809–15.
doi: https://doi.org/10.1182/blood-2010-02-268987
40. Bochtler T, Hegenbart U, Kunz C, Benner A, Kimmich C, Seckinger A, et al. Prognostic
impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan:
a long-term follow-up study. Blood. 2016 Jul;128(4):594–602. doi: https://doi.org/10.1182/blood-2015-10-676361
41. Warsame R, LaPlant B, Kumar SK, Laumann K, Perez Burbano G, Buadi FK, et al. Long-term
outcomes of IMiD-based trials in patients with immunoglobulin light-chain amyloidosis:
a pooled analysis. Blood Cancer J. 2020 Jan;10(1):4. doi: https://doi.org/10.1038/s41408-019-0266-9
42. Wechalekar AD, Cibeira MT, Gibbs SD, Jaccard A, Kumar S, Merlini G, et al. Guidelines
for non-transplant chemotherapy for treatment of systemic AL amyloidosis: EHA-ISA
working group. Amyloid. 2023 Mar;30(1):3–17. doi: https://doi.org/10.1080/13506129.2022.2093635
43. Milani P, Sharpley F, Schönland SO, Basset M, Mahmood S, Nuvolone M, et al. Pomalidomide
and dexamethasone grant rapid haematologic responses in patients with relapsed and
refractory AL amyloidosis: a European retrospective series of 153 patients. Amyloid.
2020 Dec;27(4):231–6. doi: https://doi.org/10.1080/13506129.2020.1767566
44. Sanchorawala V, Shelton AC, Lo S, Varga C, Sloan JM, Seldin DC. Pomalidomide and dexamethasone
in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood. 2016 Aug;128(8):1059–62.
doi: https://doi.org/10.1182/blood-2016-04-710822
45. Wechalekar AD, Gillmore JD, Bird J, Cavenagh J, Hawkins S, Kazmi M, et al.; BCSH Committee.
Guidelines on the management of AL amyloidosis. Br J Haematol. 2015 Jan;168(2):186–206.
doi: https://doi.org/10.1111/bjh.13155
46. Palladini G, Milani P, Foli A, Obici L, Lavatelli F, Nuvolone M, et al. Oral melphalan
and dexamethasone grants extended survival with minimal toxicity in AL amyloidosis:
long-term results of a risk-adapted approach. Haematologica. 2014 Apr;99(4):743–50.
doi: https://doi.org/10.3324/haematol.2013.095463
47. Gibbs SD, Gillmore JD, Sattianayagam PT, Offer M, Lachmann HJ, Hawkins PN, et al. In
AL Amyloidosis, Both Oral Melphalan and Dexamethasone (Mel-Dex) and Risk-Adapted Cyclophosphamide,
Thalidomide and Dexamethasone (CTD) Have Similar Efficacy as Upfront Treatment. Blood.
2009 Nov;114(22):745. doi: https://doi.org/10.1182/blood.V114.22.745.745
48. Dispenzieri A, Kastritis E, Wechalekar AD, Schönland SO, Kim K, Sanchorawala V, et
al. A randomized phase 3 study of ixazomib-dexamethasone versus physician’s choice
in relapsed or refractory AL amyloidosis. Leukemia. 2022 Jan;36(1):225–35. doi: https://doi.org/10.1038/s41375-021-01317-y
49. Sanchorawala V, Wechalekar AD, Kim K, Schönland SO, Landau HJ, Kwok F, et al. Quality
of life and symptoms among patients with relapsed/refractory AL amyloidosis treated
with ixazomib-dexamethasone versus physician’s choice. Am J Hematol. 2023 May;98(5):720–9.
doi: https://doi.org/10.1002/ajh.26866
50. Chakraborty R, Bhutani D, Maurer MS, Mohan M, Lentzsch S, D’Souza A. Safety and efficacy
of teclistamab in systemic immunoglobulin light chain amyloidosis. Blood Cancer J.
2023 Nov;13(1):172. doi: https://doi.org/10.1038/s41408-023-00950-3
51. Forgeard N, Elessa D, Carpinteiro A, Belhadj K, Minnema M, Roussel M, et al. Teclistamab
in relapsed or refractory AL amyloidosis: a multinational retrospective case series.
Blood. 2024 Feb;143(8):734–7. doi: https://doi.org/10.1182/blood.2023022937
52. Das S, Ailawadhi S, Sher T, Roy V, Fernandez A, Parrondo RD. Anti-B Cell Maturation
Antigen Chimeric Antigen Receptor T Cell Therapy for the Treatment of AL Amyloidosis
and Concurrent Relapsed/Refractory Multiple Myeloma: Preliminary Efficacy and Safety.
Curr Oncol. 2023 Oct;30(11):9627–33. doi: https://doi.org/10.3390/curroncol30110697
53. Oliver-Caldes A, Jiménez R, Español-Rego M, Cibeira MT, Ortiz-Maldonado V, Quintana LF,
et al. First report of CART treatment in AL amyloidosis and relapsed/refractory multiple
myeloma. J Immunother Cancer. 2021 Dec;9(12):3783. doi: https://doi.org/10.1136/jitc-2021-003783
54. Lebel E, Asherie N, Kfir-Erenfeld S, Grisariu S, Avni B, Elias S, et al. Efficacy
and Safety of Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T-Cell for
the Treatment of Relapsed and Refractory AL Amyloidosis. J Clin Oncol. 2024 Dec;JCO2402252.
doi: https://doi.org/10.1200/JCO-24-02252
55. Cohen AD, Landau H, Scott EC, Liedtke M, Kaufman JL, Rosenzweig M, et al. Safety and
Efficacy of Carfilzomib (CFZ) in Previously-Treated Systemic Light-Chain (AL) Amyloidosis.
Blood. 2016 Dec;128(22):645. doi: https://doi.org/10.1182/blood.V128.22.645.645
56. Gertz MA, Landau H, Comenzo RL, Seldin D, Weiss B, Zonder J, et al. First-in-human
phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent
organ dysfunction. J Clin Oncol. 2016 Apr;34(10):1097–103. doi: https://doi.org/10.1200/JCO.2015.63.6530
57. Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, et al. Therapeutic
Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. 2015 Sep;373(12):1106–14.
doi: https://doi.org/10.1056/NEJMoa1504942
58. Merlini G, Liedtke M, Landau HJ, Comenzo RL, Sanchorawala V, Weiss BM, et al. The
PRONTO amyloidosis study: A randomized, double-blind, placebo-controlled, global,
phase 2b study of NEOD001 in previously treated subjects with light chain amyloidosis
and persistent cardiac dysfunction. J Clin Oncol. 2016 May;34(15) _suppl):TPS8073–8073.
doi: https://doi.org/10.1200/JCO.2016.34.15_suppl.TPS8073
59. Gertz MA, Cohen AD, Comenzo RL, Kastritis E, Landau HJ, Libby EN, et al. Birtamimab
plus standard of care in light-chain amyloidosis: the phase 3 randomized placebo-controlled
VITAL trial. Blood. 2023 Oct;142(14):1208–18. doi: https://doi.org/10.1182/blood.2022019406
60. Mahmood S, Sachchithanantham S, Bridoux F, Lane T, Rannigan L, Foard D, et al. Risk
Of Progression Of Localised Amyloidosis To Systemic Disease In 606 Patients Over 30
Years. Blood. 2013 Nov;122(21):3143. doi: https://doi.org/10.1182/blood.V122.21.3143.3143
61. Tahara S, Kohyama M, Nakamitsu A, Sugiyama Y, Tazaki T, Taogoshi H, et al. Surgical
strategies for localized colorectal amyloidosis. Surg Case Rep. 2023 Apr;9(1):66.
doi: https://doi.org/10.1186/s40792-023-01649-0
62. Kennedy TL, Patel NM. Surgical management of localized amyloidosis. Laryngoscope.
2000 Jun;110(6):918–23. doi: https://doi.org/10.1097/00005537-200006000-00005
63. Hall J, Rubinstein S, Lilly A, Blumberg JM, Chera B. Treatment of Localized Amyloid
Light Chain Amyloidosis With External Beam Radiation Therapy. Pract Radiat Oncol.
2022;12(6):504–10. doi: https://doi.org/10.1016/j.prro.2022.03.011
64. Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJ, Sekijima Y, et al. Amyloid
nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA)
nomenclature committee. Amyloid. 2018 Dec;25(4):215–9. doi: https://doi.org/10.1080/13506129.2018.1549825
65. Gertz MA, Lacy MQ, Dispenzieri A. Therapy for immunoglobulin light chain amyloidosis:
the new and the old. Blood Rev. 2004 Mar;18(1):17–37. doi: https://doi.org/10.1016/S0268-960X(03)00027-4
66. Maroun BZ, Allam S, Chaulagain CP. Multidisciplinary supportive care in systemic light
chain amyloidosis. Blood Res. 2022 Jun;57(2):106–16. doi: https://doi.org/10.5045/br.2022.2021227
67. Wong SW, Fogaren T. Supportive Care for Patients with Systemic Light Chain Amyloidosis.
Hematology/Oncology Clinics of North America. 2020;34(6:1177–1191. doi: https://doi.org/10.1016/j.hoc.2020.08.007
68. Jensen CE, Byku M, Hladik GA, Jain K, Traub RE, Tuchman SA. Supportive Care and Symptom
Management for Patients With Immunoglobulin Light Chain (AL) Amyloidosis. Front Oncol.
2022;12:907584. doi: https://doi.org/10.3389/fonc.2022.907584
69. Cheshire WP et al. Electrodiagnostic assessment of the autonomic nervous system: A
consensus statement endorsed by the American Autonomic Society, American Academy of
Neurology, and the International Federation of Clinical Neurophysiology. Clinical
Neurophysiology. 2021;132(2):666–682. doi: https://doi.org/10.1016/j.clinph.2020.11.024
70. Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic
criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008 Jul;131(Pt
7):1912–25. doi: https://doi.org/10.1093/brain/awn093
71. Cibeira MT, Ortiz-Pérez JT, Quintana LF, Fernádez De Larrea C, Tovar N, Bladé J. Supportive
care in AL amyloidosis. Acta Haematologica. 2020;143(4):335–342. doi: https://doi.org/10.1159/000506760
72. Shen KN, Fu WJ, Wu Y, Dong YJ, Huang ZX, Wei YQ, et al. Doxycycline Combined With
Bortezomib-Cyclophosphamide-Dexamethasone Chemotherapy for Newly Diagnosed Cardiac
Light-Chain Amyloidosis: A Multicenter Randomized Controlled Trial. Circulation. 2022 Jan;145(1):8–17.
doi: https://doi.org/10.1161/CIRCULATIONAHA.121.055953
73. Hora M, Carballo-Pacheco M, Weber B, Morris VK, Wittkopf A, Buchner J, et al. Epigallocatechin-3-gallate
preferentially induces aggregation of amyloidogenic immunoglobulin light chains. Sci
Rep. 2017 Jan;7(1):41515. doi: https://doi.org/10.1038/srep41515
74. Fernandes L, Cardim-Pires TR, Foguel D, Palhano FL. Green Tea Polyphenol Epigallocatechin-Gallate
in Amyloid Aggregation and Neurodegenerative Diseases. Frontiers in Neuroscience.
2021;15. doi: https://doi.org/10.3389/fnins.2021.718188
75. Gertz MA, Dispenzieri A, Sher T. Pathophysiology and treatment of cardiac amyloidosis.
Nature Reviews Cardiology. 2015;12(2):91–102. doi: https://doi.org/10.1038/nrcardio.2014.165
76. Shams P, Ahmed I. Cardiac Amyloidosis. StatPearls Publishing; 2024.
77. Gertz MA, Falk RH, Skinner M, Cohen AS, Kyle RA. Worsening of congestive heart failure
in amyloid heart disease treated by calcium channel-blocking agents. Am J Cardiol.
1985 Jun;55(13 Pt 1):1645. doi: https://doi.org/10.1016/0002-9149(85)90995-6
78. Dubrey SW, Burke MM, Khaghani A, Hawkins PN, Yacoub MH, Banner NR. Long term results
of heart transplantation in patients with amyloid heart disease. Heart. 2001 Feb;85(2):202–7.
doi: https://doi.org/10.1136/heart.85.2.202
79. Kumar S, Li D, Joseph D, Trachtenberg B. State-of-the-art review on management of
end-stage heart failure in amyloidosis: transplant and beyond. Heart Fail Rev. 2022;27(5):1567–1578.
doi: https://doi.org/10.1007/s10741-021-10209-3
80. Ashraf I, Peck MM, Maram R, Mohamed A, Ochoa Crespo D, Kaur G, et al. Association
of Arrhythmias in Cardiac Amyloidosis and Cardiac Sarcoidosis. Cureus. 2020 Aug;12(8):e9842.
doi: https://doi.org/10.7759/cureus.9842
81. Weber N, et al. Management of systemic AL amyloidosis: Recommendations of the Myeloma
Foundation of Australia Medical and Scientific Advisory Group. Intern Med J. 2015;45(4):371–382.
doi: https://doi.org/10.1111/imj.12566
82. Feng D, Edwards WD, Oh JK, Chandrasekaran K, Grogan M, Martinez MW, et al. Intracardiac
thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007 Nov;116(21):2420–6.
doi: https://doi.org/10.1161/CIRCULATIONAHA.107.697763
83. Palladini G, Malamani G, Cò F, Pistorio A, Recusani F, Anesi E, et al. Holter monitoring
in AL amyloidosis: prognostic implications. Pacing Clin Electrophysiol. 2001 Aug;24(8
Pt 1):1228–33. doi: https://doi.org/10.1046/j.1460-9592.2001.01228.x
84. Dhoble A, Khasnis A, Olomu A, Thakur R. Cardiac amyloidosis treated with an implantable
cardioverter defibrillator and subcutaneous array lead system: report of a case and
literature review. Clin Cardiol. 2009 Aug;32(8):E63–5. doi: https://doi.org/10.1002/clc.20389
85. Kristen AV, Dengler TJ, Hegenbart U, Schonland SO, Goldschmidt H, Sack FU, et al. Prophylactic
implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis
and high risk for sudden cardiac death. Heart Rhythm. 2008 Feb;5(2):235–40. doi: https://doi.org/10.1016/j.hrthm.2007.10.016
86. Varr BC, Zarafshar S, Coakley T, Liedtke M, Lafayette RA, Arai S, et al. Implantable
cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm.
2014 Jan;11(1):158–62. doi: https://doi.org/10.1016/j.hrthm.2013.10.026
87. ZeppenfeldK, et al. 2022 ESC Guidelines for the management of patients with ventricular
arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43(40):3997–4126.
doi: https://doi.org/10.1093/eurheartj/ehac262
88. Mayr M, Dickenmann MJ. [Further evidence for the renoprotective effect of ACE inhibitors:
ramipril protects against the progression of chronic renal insufficiency in non-diabetic
nephropathy with nephrotic proteinuria]. Schweiz Med Wochenschr. 2000 Apr 1;130(13):491.
doi:https://pubmed.ncbi.nlm.nih.gov/10812646/
89. Paydas S. Report on 59 patients with renal amyloidosis. Int Urol Nephrol. 1999;31(5):619–31.
doi: https://doi.org/10.1023/A:1007152320216
90. Gentile G, Remuzzi G, Ruggenenti P. Dual renin-angiotensin system blockade for nephroprotection:
still under scrutiny. Nephron J. 2015;129(1):39–41. doi: https://doi.org/10.1159/000368331
91. Gertz MA, Kyle RA, O’Fallon WM. Dialysis support of patients with primary systemic
amyloidosis. A study of 211 patients. Arch Intern Med. 1992 Nov;152(11):2245–50. doi: https://doi.org/10.1001/archinte.1992.00400230061010
92. Pinney JH, Lachmann HJ, Bansi L, Wechalekar AD, Gilbertson JA, Rowczenio D, et al. Outcome
in renal Al amyloidosis after chemotherapy. J Clin Oncol. 2011 Feb;29(6):674–81. doi: https://doi.org/10.1200/JCO.2010.30.5235
93. Herrmann SM, Gertz MA, Stegall MD, Dispenzieri A, Cosio FC, Kumar S, et al. Long-term
outcomes of patients with light chain amyloidosis (AL) after renal transplantation
with or without stem cell transplantation. Nephrol Dial Transplant. 2011 Jun;26(6):2032–6.
doi: https://doi.org/10.1093/ndt/gfr067
94. Angel-Korman A, Stern L, Sarosiek S, Sloan JM, Doros G, Sanchorawala V, et al. Long-term
outcome of kidney transplantation in AL amyloidosis. Kidney Int. 2019 Feb;95(2):405–11.
doi: https://doi.org/10.1016/j.kint.2018.09.021
95. Kunin M, Klempfner R, Beckerman P, Rott D, Dinour D. Congestive heart failure treated
with peritoneal dialysis or hemodialysis: typical patient profile and outcomes in
real-world setting. Int J Clin Pract. 2021 Mar;75(3):e13727. doi: https://doi.org/10.1111/ijcp.13727
96. Sattianayagam PT, Gibbs SD, Pinney JH, Wechalekar AD, Lachmann HJ, Whelan CJ, et al. Solid
organ transplantation in AL amyloidosis. Am J Transplant. 2010 Sep;10(9):2124–31.
doi: https://doi.org/10.1111/j.1600-6143.2010.03227.x
97. Fritz CD, Blaney E. Evaluation and Management Strategies for GI Involvement with Amyloidosis.
Am J Med. 2022 Apr;135 Suppl 1:S20–3. doi: https://doi.org/10.1016/j.amjmed.2022.01.008
98. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms
and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol.
2000 Aug;110(2):454–60. doi: https://doi.org/10.1046/j.1365-2141.2000.02183.x
99. Merlini G, Dispenzieri A, Sanchorawala V, Schönland SO, Palladini G, Hawkins PN, et
al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018 Oct;4(1):38.
10.1038/s41572-018-0034-3
100. Cruz DN. Midodrine: a selective alpha-adrenergic agonist for orthostatic hypotension
and dialysis hypotension. Expert Opin Pharmacother. 2000 May;1(4):835–40. doi: https://doi.org/10.1517/14656566.1.4.835
101. Bundesamt für Gesundheit (BAG). Spezialitätenliste (SL) - Präparate. Available from:
https://spezialitaetenliste.ch/ShowPreparations.aspx?searchType=SUBSTANCE

