Overview and evaluation of a nationwide hospital-based surveillance system for influenza
and COVID-19 in Switzerland (CH-SUR): 2018–2023
DOI: https://doi.org/https://doi.org/10.57187/s.4213
Jonathan A. Sobelab,
Marie-Céline Zanellabc,
Rebecca Grantb,
Camille B. Valeraa,
Mária Suvegesa,
Laura Urbinia,
Khaled Mostaguird,
Sara Boteroa,
Ursina Rodere,
Davide Bosettib,
Rami Sommersteinfg,
Ulrich Heiningerh,
Petra Zimmermanni,
Peter W. Schreiberj,
Domenica Fluryk,
Anita Niederer-Loherl,
Philipp Jentg,
Alexia Cusinim,
Didier Pittetb,
Stephan Harbarthb,
Anne Itenb*,
Olivia Keisera*
a Institute of
Global Health, University of Geneva, Geneva, Switzerland
b Infection Control
Programme and WHO Collaborating Centre on Infection Prevention and Control and
Antimicrobial Resistance, Geneva University Hospital and Faculty of Medicine,
Geneva, Switzerland
c Division of
Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland
d Clinical Research
Centre, Geneva University Hospitals and Faculty of Medicine, University of
Geneva, Geneva, Switzerland
e Swiss Federal
Office of Public Health, Bern, Switzerland
f Faculty of
Healthcare Sciences and Medicine, University of Lucerne, Lucerne, Switzerland
g Department of
Infectious Diseases, Bern University Hospital, University of Bern, Bern,
Switzerland
h Paediatric
Infectious Diseases and Vaccinology, University Children's Hospital Basel
(UKBB), Basel, Switzerland
i Department of
Community, Health Faculty of Science and Medicine, Department of Paediatrics,
Fribourg Hospital, University of Fribourg, Fribourg, Switzerland
j Department of
Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University
of Zurich, Zurich, Switzerland
k Division of
Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St.
Gallen, Switzerland
l Children's
Hospital of Eastern Switzerland, St Gallen, Switzerland
m Department of
Infectious Diseases, Cantonal Hospital Graubuenden, Chur, Switzerland
* Equal contribution as last authors
Summary
BACKGROUND: In 2018, a hospital-based
surveillance system for influenza (CH-SUR) was established in six tertiary care
hospitals in Switzerland. From March 2020 onwards, this surveillance system was
expanded to include more institutions, as well as COVID-19.
AIM: To quantitatively evaluate the
timeliness and completeness of CH-SUR data and to qualitatively assess
stakeholder perceptions of the importance, reliability and adaptability of the
surveillance system.
METHODS: All patients admitted to one of
the participating centres for more than 24 hours and who had a
laboratory-confirmed influenza virus or SARS-CoV-2 infection were included in
CH-SUR. For all cases, we evaluated the timeliness and completeness of
reporting to CH-SUR. A qualitative survey among CH-SUR stakeholders assessed
perceived importance, understanding, reliability and adaptability of CH-SUR.
RESULTS: Up to 20 centres participated in
CH-SUR. Between December 2018 and October 2023, 7375 cases of influenza were
reported and between March 2020 and October 2023, 49,235 cases of COVID-19 were
reported to CH-SUR. During the COVID-19 pandemic, time to data entry and
completeness improved over time; the median delay of data entry in CH-SUR was 5
days (interquartile range [IQR]: 2–23) for COVID-19 and 4 days (IQR: 2–15) for
influenza during the period 2018–2023. The completeness of variables was high
(99.4%), with the exception of COVID-19 or annual influenza vaccination status (respectively,
15% and 72% “Unknown” responses). Stakeholders perceived the system as
important, relevant, understandable and adaptable.
CONCLUSION: CH-SUR provided critical
epidemiological and clinical information on hospitalised influenza and COVID-19
cases across Switzerland during the pandemic. Our evaluation highlighted the
importance and relevance of this system among CH-SUR stakeholders, as well as
its importance for preparedness and response to future infectious disease
outbreaks.
Introduction
Epidemiological surveillance systems are
essential tools for monitoring and controlling the spread of diseases [1–4]. In
2018, a hospital-based surveillance system for influenza (CH-SUR), jointly
coordinated by Geneva University Hospitals (HUG), the Institute of Global
Health at the University of Geneva (IGH UNIGE) and the Federal Office of Public
Health (FOPH), was established in six tertiary care hospitals in Switzerland.
The CH‑SUR system was designed with the core objective of monitoring in real
time the clinical burden and course of hospitalised influenza and COVID‑19
cases, in order to support efficient healthcare resource planning and help evaluate
the impact of public health interventions.
A standardised, electronic case report form
was established across all centres, using REDCap[5]. This enabled the continuous
collection of demographic, clinical and epidemiological data from all patients
admitted to one of the participating centres for more than 24 hours and who had
a laboratory-confirmed influenza virus infection.
The first confirmed case of COVID-19 in
Switzerland was reported to the Federal Office of Public Health on 25 February
2020 [6]. As early as 2 March 2020, the CH-SUR system was expanded to include
all patients admitted to one of the participating centres for more than 24
hours and who had a laboratory-confirmed SARS-CoV-2 infection. From 15 November
2020 onwards, the influenza and COVID-19 databases in CH-SUR were merged into a
unified registry. The number of centres actively participating in CH-SUR
increased during 2020, up to 20 hospitals and clinics, covering approximately
97% of all hospitalisations related to SARS-CoV-2 in Switzerland; 17 centres continued
data collection until 2023 [1–4, 7, 8] (a map of participating centres is provided
in figure S1 in the appendix, available for download as a separate file at https://doi.org/10.57187/s.4213).
Together with the primary care surveillance
system (Sentinella) and community wastewater monitoring, CH-SUR served as one
of the key pillars contributing to comprehensive surveillance of influenza and
SARS-CoV-2 infections across Switzerland. The CH-SUR surveillance system was
intended to enable Swiss health authorities to monitor epidemiological trends
in hospitalised COVID-19 and influenza patients across Switzerland, to quantify
the disease burden among hospitalised patients and to assess healthcare
demands, thereby guiding the broader public health response to influenza and
COVID-19. During the COVID-19 pandemic, key findings from the CH-SUR system
were compiled into a monthly report published by Geneva University Hospitals, the
Institute of Global Health and the Federal Office of Public Health [9] until
September 2023.
To ensure that CH-SUR was meeting its stated
objectives, an evaluation of CH-SUR was initiated by Geneva University
Hospitals, the Institute of Global Health and the Federal Office of Public
Health. This study aimed (1) to quantify the timeliness and completeness of
data entry in CH-SUR for influenza and COVID‑19 cases, (2) to evaluate
stakeholder perceptions of the system’s importance, reliability and
adaptability and (3) to identify key strengths and opportunities for
improvement to guide future hospital‑based surveillance.
Methods
Study design
We conducted a retrospective quantitative
and qualitative evaluation of the nationwide hospital-based surveillance system
for influenza and COVID-19 in Switzerland from 1 December 2018 to 17 October
2023, informed by the CDC’s guidelines for evaluating public health
surveillance systems, which were used as a reference framework although not all
criteria were applied [10].
Case definitions
All patients admitted to one of the
participating centres for more than 24 hours and who had a laboratory-confirmed
influenza virus infection or laboratory-confirmed or clinically diagnosed
SARS-CoV-2 infection were included in CH-SUR. Patients who were seen in
outpatient settings, or who were hospitalised for less than 24 hours were not
included in CH-SUR. Within CH-SUR, cases were categorised as “nosocomial” if a
patient tested positive for influenza virus three days or more after hospital
admission, or for SARS-CoV-2 five days or more after hospital admission. Cases
transferred from other institutions were also considered as nosocomial cases.
Influenza cases were categorised by season,
while COVID-19 cases were categorised by nine distinct phases, determined by
the epidemiology and/or variant in circulation during the pandemic: (1) Spring
wave 2020 (24 February 2020 to 7 June 2020), (2) Autumn/winter wave 2020 (8
June 2020 to 14 February 2021), (3) Alpha wave (15 February 2021 to 20 June
2021), (4) Delta wave (21 June 2021 to 19 December 2021), (5) BA.1 (Omicron)
wave (20 December 2021 to 28 February 2022), (6) BA.2 (Omicron) wave (1 March
2022 to 5 June 2022), (7) BA.5 (Omicron) wave (6 June 2022 to 14 November
2022), (8) BQ.1 (Omicron) wave (15 November 2022 to 11 February 2023) and (9)
XBB wave (12 February 2023 to 17 December 2023). The classification of
SARS-CoV-2 variants was based on genomic sequencing data from Switzerland
submitted to the Global Initiative on Sharing All Influenza Data (GISAID) [11].
Data entry and validation
The CH-SUR surveillance system involved
multiple stakeholders (figure S2 in the appendix, available for download as a separate
file at https://doi.org/10.57187/s.4213), including the Federal Office of Public Health,
the Institute of Global Health, the clinical research centre of Geneva
University Hospitals and up to 20 hospitals across Switzerland. CH-SUR operated
under the aegis of the Federal Office of Public Health, which functioned as
sponsor and project manager. The principal investigators, one at each participating
centre, were responsible for implementation of the surveillance system at their
institution, as well as the collection and the quality of data; data collection
was mostly performed by research nurses at each institution. The Institute of
Global Health centralised data from case report forms for processing,
evaluation and reporting. The Institute of Global Health and the clinical
research centre of Geneva University Hospitals were responsible for database maintenance,
while the clinical research centre of Geneva University Hospitals was
responsible for the hosting and security of the collected data.
All participating centres were required to
complete an electronic case report form for all patients meeting the case
definitions above. The CH-SUR case report form had six components: inclusion
criteria; demographic information; case-related information; hospital admission
data and clinical data throughout the patient’s hospitalisation, including
treatments received; admission to intensive or intermediate care units (ICU/IMCU);
and outcome information. The CH-SUR codebook (version 2023) used during the
data extraction is provided in the supplementary material (see appendix, available
for download as a separate file at https://doi.org/10.57187/s.4213). The target for time elapsed from
patient admission to hospital (or positive test for nosocomial cases) to CH-SUR
data entry for admission-related information was 48 to 72 hours, while the
target for time to completion of data entry was 14 days. These targets were set
by the Federal Office of Public Health when CH-SUR was established in 2018. The
electronic case report form included internal consistency checks and pop-up
messages for incomplete data entry. There was also a distinction between “Unknown”
(information not available) and “NA” (missing data).
Data extraction
For all influenza and COVID-19 cases in the
CH-SUR database during the study period, we extracted the following variables:
hospital admission date, date of positive influenza/SARS-CoV-2 test; COVID-19
or influenza vaccination information; ICU or IMCU admission data; outcome
(in-hospital death, discharge alive). For COVID-19, the reason for
hospitalisation (because of COVID-19 or with COVID-19) was introduced into the
questionnaire in December 2021. “Because of COVID-19” referred to patients
hospitalised primarily due to symptoms caused by COVID-19 or chronic conditions
exacerbated by the virus, such as COPD or cardiac decompensation during
infection. “With COVID-19” denoted patients hospitalised for reasons deemed to
be unrelated to COVID-19 by the investigators but were SARS-CoV-2 test positive
during their stay, for example hospitalisation for surgical procedures or
infections other than SARS-CoV-2; this variable was also included in the data
extraction. We also extracted data on the completeness of each of the six
components of the case report form (see appendix, available at
https://doi.org/10.57187/s.4213). In the case report form, “NA” was used to
denote fields that were not applicable for a given patient, while “Unknown”
indicated that the information was unavailable at the time of data entry. For
our quantitative analyses, we treated both “NA” and “Unknown” as missing
values. Completeness for each variable was then calculated as the proportion of
entries that were neither “NA” nor “Unknown”. We did not perform any
imputation; all analyses of timeliness and completeness were conducted on
available‐case data only.
Qualitative assessment
We conducted a REDCap survey among CH-SUR stakeholders (CH-SUR principal
investigators, research nurses, data scientists/researchers; Federal Office of
Public Health/ Institute of Global Health coordinators). These four groups
supported the following CH-SUR activities: implementation of the surveillance
system in participating centres, data collection, data quality assessments,
clinical interpretation, reporting and coordination of the surveillance system.
The survey questionnaire was designed to assess multiple dimensions of the
CH-SUR project, including perceived importance, relevance, understanding and
adaptability. A total of 67 questions were structured to elicit responses
regarding the stakeholders’ perception of these aspects in relation to the
surveillance system. Twenty-four open-ended questions were also included to
allow participants to provide additional comments or suggestions. The full
survey is provided in the appendix, available
at https://doi.org/10.57187/s.4213.
Analyses
We described all influenza and COVID-19
cases in the CH-SUR database during the study period. We expressed continuous
variables as median and interquartile range, and categorical variables as
counts and percentages. Pearson’s chi-squared test or the Wilcoxon rank-sum
tests were used, using a significance threshold set at 0.05.
We assessed the delay to case entry in
CH-SUR, defined as the time from hospital admission (or from a positive
influenza/SARS-CoV-2 test for nosocomial infections) to date of data entry in
CH-SUR. We also assessed the completeness of each of the extracted variables,
calculated as the proportion of non-missing data for each of the extracted
variables among all cases in the database. For COVID-19 cases, we assessed the
completeness of data across the nine periods of the pandemic, described across different
COVID-19 waves; for influenza, we considered five seasons.
For the analysis of the qualitative survey,
answers to the perceived importance, relevance, understanding and adaptability
of the CH-SUR system were expressed as counts and percentages, and by stakeholder
group (principal investigators, research nurses, data scientists/researchers,
coordinators/project managers). The responses to the open-ended questions were
analysed using natural language processing algorithms to identify keywords [12,
13]. These words were then visualised using a word cloud, with the size of each
word reflecting its frequency in the responses. This approach allowed for a
visual representation of the prominent themes and concerns expressed by the
participants. In addition, a classic qualitative content analysis (QCA) [14]
was conducted on the open-ended responses. The responses were carefully
reviewed and categorised into relevant themes and subthemes. These themes were
then analysed and interpreted to identify common patterns, recurring topics and
any noteworthy insights provided by the participants. All analyses were
performed using R version 4.2.1 [15] using the tidyverse suite (v1.3.1)
for data manipulation and the wordcloud package (v2.6) for text‑mining
visualisations. The complete analysis pipeline – including data importation,
cleaning, quantitative evaluation and qualitative survey processing – is
available under an MIT licence on GitHub
(https://github.com/jsobel1/CH-SUR_quality_analysis) and archived at Zenodo
(10.5281/zenodo.15325761).
Ethical considerations
The CH-SUR system was approved by the
Ethics Committee of the Canton of Geneva, Switzerland (CCER 2018–00577 and CCER
2020–00827). Data collection was also approved by all local ethics committees.
For the qualitative survey, all participants were informed about the purpose of
the survey, the voluntary nature of their participation and the confidentiality
of their responses.
Results
Quantitative evaluation of CH-SUR
Between November 2018 and October 2023, 7375
cases of influenza were reported to the nationwide CH-SUR surveillance system;
between March 2020 and October 2023, 49,235 cases of COVID-19 were reported.
Characteristics of influenza and COVID-19 patients are described in table 1.
The table shows that COVID-19 patients had a higher proportion of in-hospital
mortality (10%) than influenza patients (4%); however the COVID-19 case fatality
rate decreased over time. Figure 1A shows the cases of influenza reported to
CH-SUR for the past five seasons; figure 1B illustrates the number of COVID-19
cases reported to CH-SUR throughout the duration of the pandemic. For
influenza, the 2020/2021 season was notable for the near absence of cases
reported to CH-SUR, reflecting the impact of infection control and public
health measures for COVID-19 on influenza virus circulation.
Table 1Characteristics of COVID-19 (n = 49,235) and influenza (n = 7375) patients
reported through the nationwide hospital-based surveillance system in
Switzerland between 1 November 2018 and 17 October 2023. Counts and percentages
are shown for categorical variables, while medians and interquartile ranges are
shown for continuous variables.
| Characteristic |
COVID-19 (n = 49,235) |
Influenza (n = 7375) |
p-value |
| Sex |
|
|
|
<0.001 |
| Male |
26,754 (54%) |
3695 (50%) |
|
| Female |
22,481 (46%) |
3680 (50%) |
|
| Died |
|
|
|
<0.001 |
| Yes |
5039 (10%) |
295 (4.0%) |
|
| No |
43,705 (90%) |
7051 (96%) |
|
| Age in
years |
72 (55–82) |
67 (32–80) |
<0.001 |
| BMI in
kg/m2 |
25 (22–29) |
24 (20–28) |
<0.001 |
| Complications |
|
|
|
0.007 |
| No |
16,909 (35.6%) |
2674 (37%) |
|
| Yes |
29,986 (64%) |
4480 (62.4%) |
|
| Unknown |
182 (0.4%) |
41 (0.6%) |
|
| Comorbidities |
38,325 (81%) |
5204 (72%) |
<0.001 |
| Vaccination
received* |
|
|
|
0.8 |
| No |
26,838 (55%) |
1618 (22%) |
|
| Yes |
14,619 (30%) |
446 (6.1%) |
|
| Unknown |
7397 (15%) |
5200 (72%) |
|
| Intermediate
care stay |
|
|
|
<0.001 |
| No |
43,468 (92.1%) |
4292 (94%) |
|
| Yes |
3702 (7.8%) |
279 (6%) |
|
| Unknown |
12 (0.1%) |
0 (0%) |
|
| Intensive
care stay |
|
|
|
<0.001 |
| No |
41,194 (86.9%) |
6479 (90%) |
|
| Yes |
5889 (13%) |
703 (9.9%) |
|
| Unknown |
7 (0.1%) |
9 (0.1%) |
|
| Case
classification |
|
|
|
>0.9 |
| Community-acquired |
39,311 (80%) |
6091 (83%) |
|
| Nosocomial
from this hospital** |
7487 (15%) |
1095 (14.5%) |
|
| Nosocomial
from another institution |
1894 (3.8%) |
149 (2.0%) |
|
| Unknown |
529 (1.2%) |
40 (0.5%) |
|
| Delay
to data entry*** |
5 (2–23) |
4 (2–15) |
<0.001 |
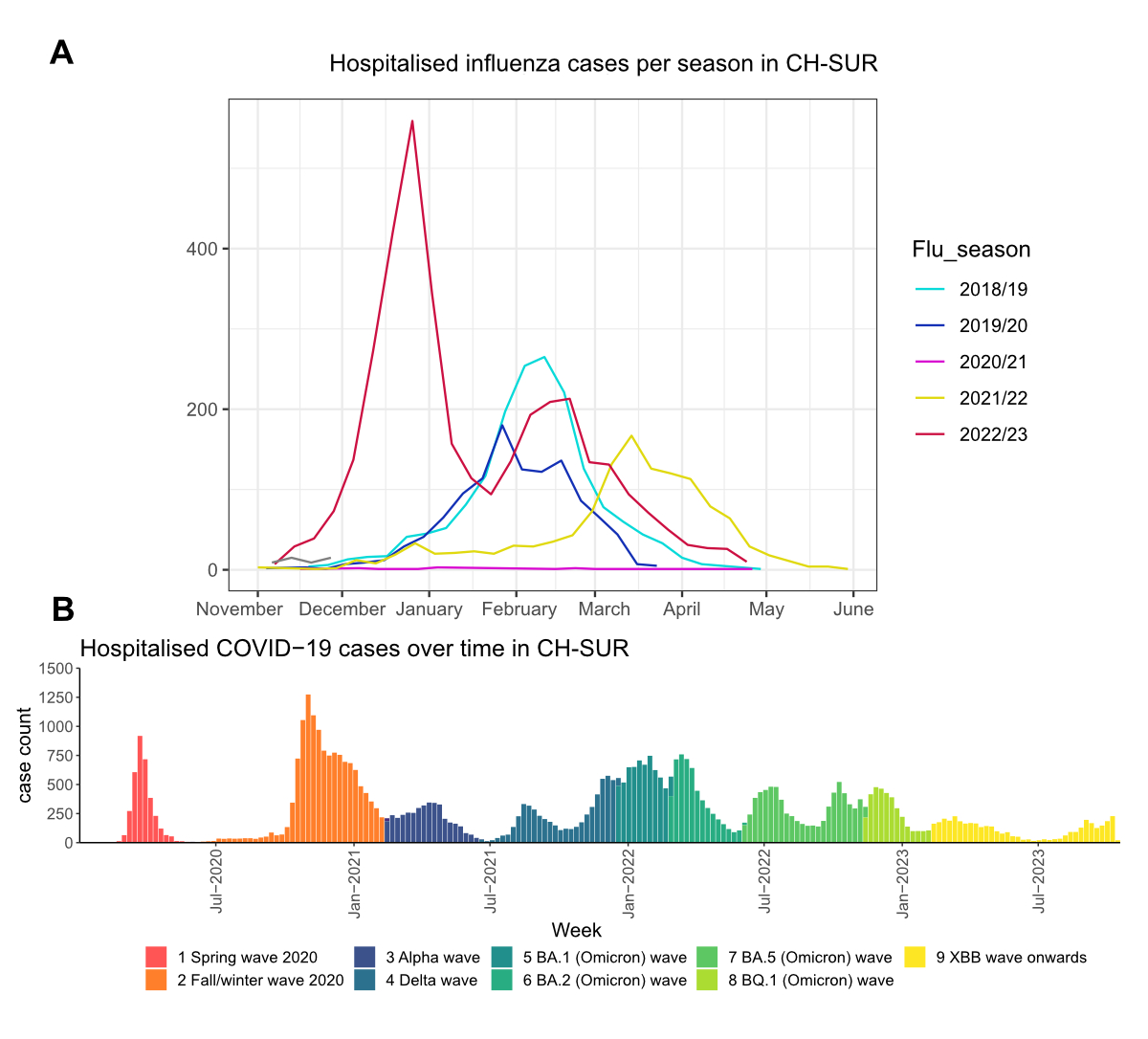
Figure 1Cases of influenza (flu) (A) and COVID-19 (B) reported
through the nationwide hospital-based surveillance system in Switzerland
between November 2018 and October 2023. The colours in (A) correspond to the
respective influenza season; the colours in (B) correspond to the nine
different time periods across the COVID-19 pandemic. CH-SUR: COVID-19 Hospital-Based
Surveillance.
Delay to case entry
Figure 2 shows the time from hospital
admission (or positive influenza / SARS-CoV-2 test for nosocomial infections)
to CH-SUR data entry for influenza patients and COVID-19 patients. The median
time to case entry for influenza throughout the monitoring period was low (3–8 days
from admission, figure 2A), although the absolute number of influenza cases was
also lower as compared to COVID-19. For COVID-19, the median time from
admission to case entry in CH-SUR was 5 days (interquartile range [IQR]: 2–23) and
was highest during the initial COVID-19 waves in 2020.
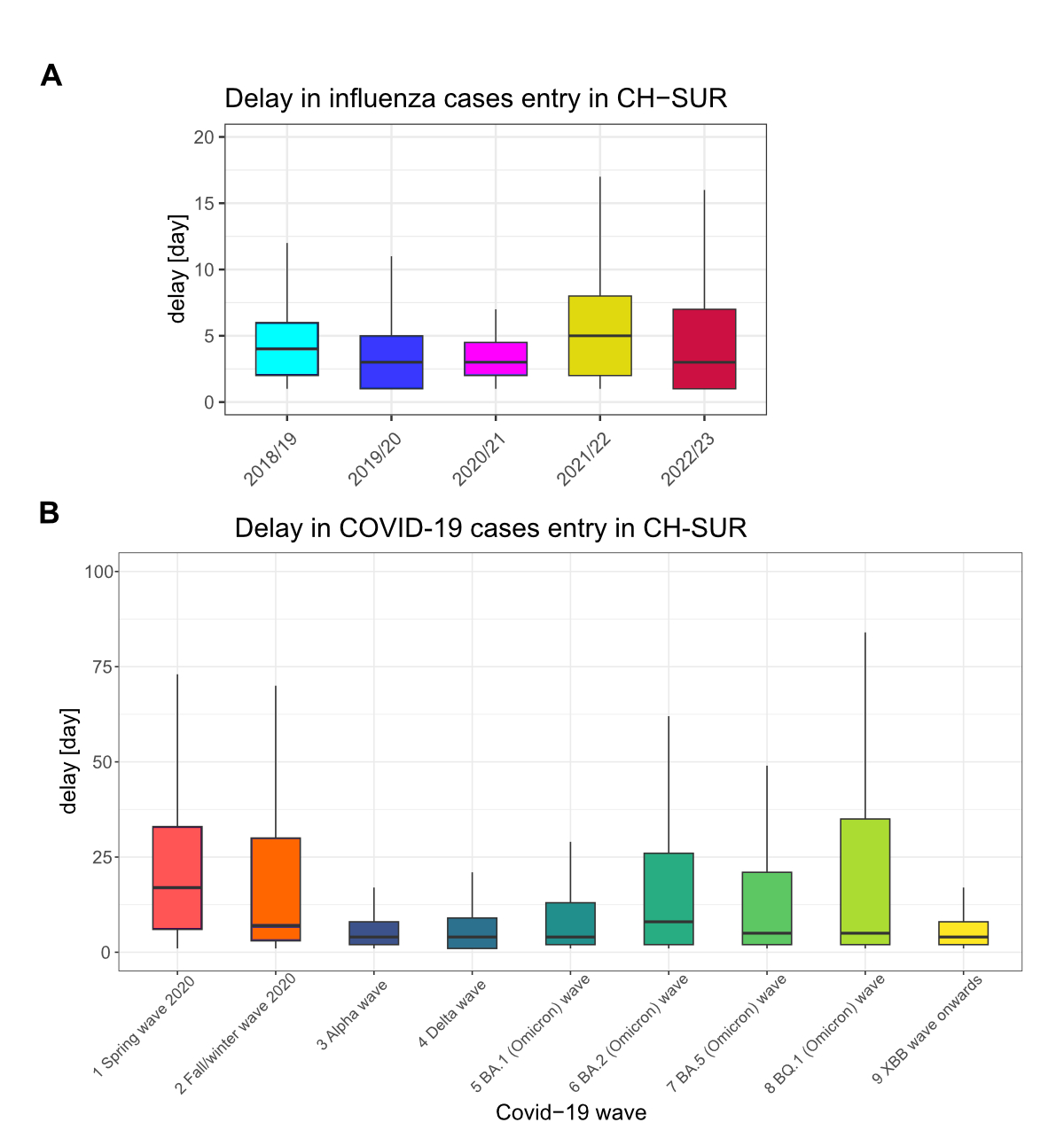
Figure 2Time from hospital admission (or a positive influenza
test/SARS-CoV-2 test for hospital-acquired infection) to CH-SUR data entry for (A)
influenza patients and (B) COVID-19 patients. Each box shows the median and interquartile
range (IQR) in days. For influenza, the data are shown by influenza season; for
COVID-19, the data are shown by nine different time periods across the COVID-19
pandemic. As a reminder: (1) Spring wave 2020 (24 February 2020 to 7 June
2020), (2) Autumn/winter wave 2020 (8 June 2020 to 14 February 2021), (3) Alpha
wave (15 February 2021 to 20 June 2021), (4) Delta wave (21 June 2021 to 19
December 2021), (5) BA.1 (Omicron) wave (20 December 2021 to 28 February 2022),
(6) BA.2 (Omicron) wave (1 March 2022 to 5 June 2022), (7) BA.5 (Omicron) wave
(6 June 2022 to 14 November 2022), (8) BQ.1 (Omicron) wave (15 November 2022 to
11 February 2023) and (9) XBB wave onwards (12 February 2023 to 17 December
2023). CH-SUR: COVID-19 Hospital-Based Surveillance.
Data completeness
While missing data for vaccination status
was low (ranging from 0 to 2.2% NAs), there was a high percentage of “Unknown” vaccination
status, up to 44% in the last period considered. Higher completeness was
observed for variables such as comorbidities, intermediate care or intensive
care stay, ranging from 0% to 8.6% NAs (figures 3A and 3B). The median
percentage of complete data was 99.4% across all time intervals and variables.
With respect to completeness of the components of the case report form,
clinical data, including outcome information, had the highest proportion of
incompleteness (15%) during the last period (Omicron XBB wave onwards),
although this likely reflects the fact that patients may have remained
hospitalised at the time of the data extraction. Taken together, the time
required for data entry and the completeness of the data both improved gradually
over time (figures 2 and 3).
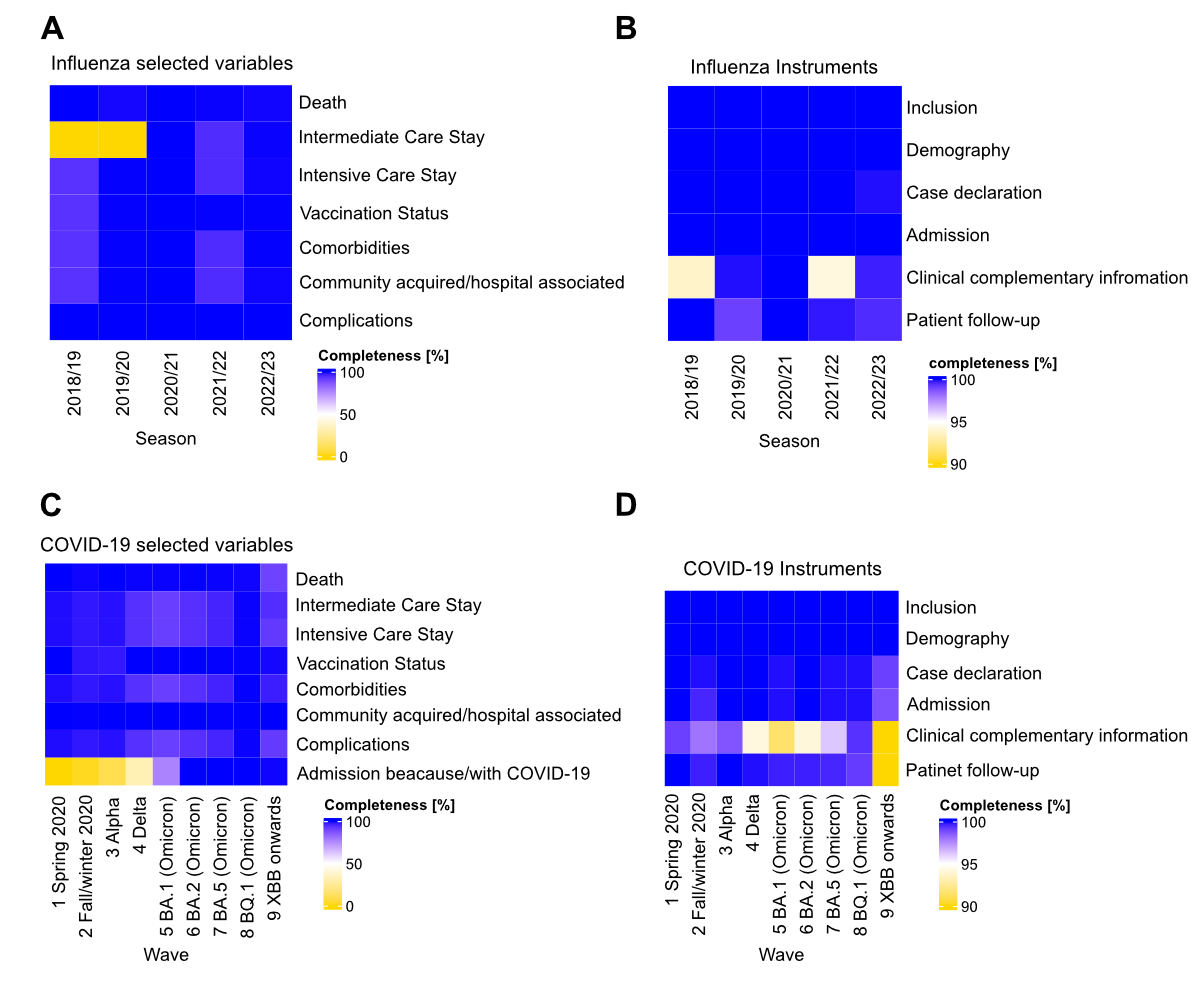
Figure 3Completeness of data in CH-SUR for influenza (A–B) and COVID-19
patients (C–D). The left panel shows the completeness of the following
variables: death, intensive care unit (ICU) / intermediate care unit (IMCU)
stay, comorbidity, complication, reason for admission “because” or “with”
COVID-19 and the classification between nosocomial or community-acquired, and
vaccination; the right panel shows the completeness of the REDCap case report form
components.
Qualitative evaluation of CH-SUR
The qualitative survey on CH-SUR was
conducted between 1 February 2023 and 1 April 2023. Forty-seven participants
responded (figure S3 in the appendix, available for download as a separate file at
https://doi.org/10.57187/s.4213):
13 principal investigators, 24 study nurses, 6 data scientists/researchers, 4
coordinators/project managers. Most principal investigators (9/13; 69%) and
researchers (5/6; 83%) had been working within the CH-SUR system for more than
2 years. About 40% of nurses had been involved in the CH-SUR system for 1–2
years (10/24; 41.6%) and the same proportion for more than 2 years (9/24;
37.5%).
Stakeholder perceptions
Most CH-SUR stakeholders who participated
in the survey judged CH-SUR to be important and relevant; most participants
expressed a good or excellent understanding of the system and found it reliable
and adaptable to the needs of their centre (figure 4). CH-SUR principal
investigators largely expressed support for expanding CH-SUR to respiratory
syncytial virus (RSV) (76.9%) and other diseases (53%). However, they were
largely unsupportive (85%) of an expansion of the CH-SUR case report form to
include other variables.
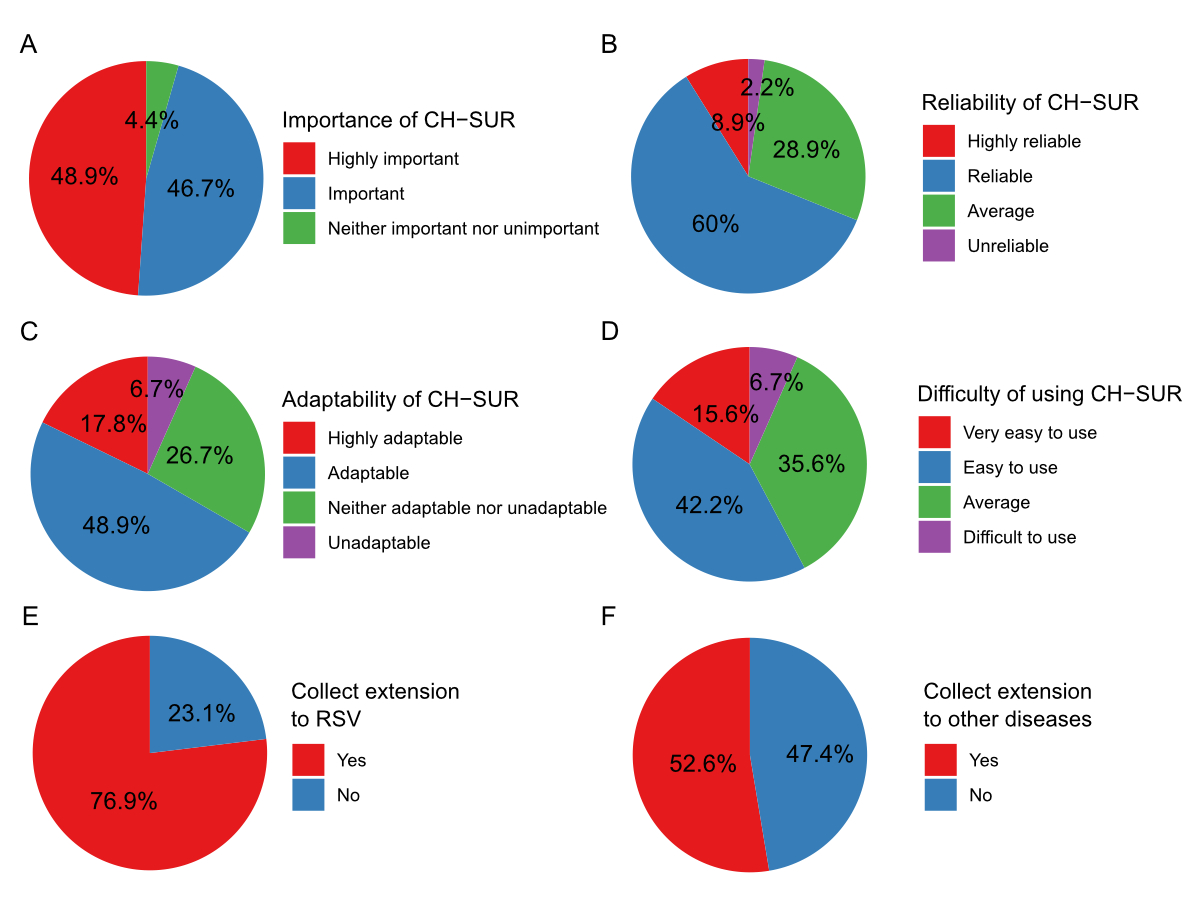
Figure 4Survey assessment of CH-SUR features
and extensions completed by CH-SUR stakeholders. This figure depicts
participant responses regarding various aspects of CH-SUR: (A) Perceived
importance of CH-SUR (n = 45). (B) Perceived reliability of CH-SUR (n = 45).
(C) Adaptability of CH-SUR in different contexts (n = 45). (D) Ease of use of
CH-SUR (n = 45). (E) Interest in adding respiratory syncytial virus (RSV)
monitoring to CH-SUR (n = 13). (F) Interest in including monitoring for other
diseases in CH-SUR (n = 19). Each pie chart represents the distribution of
participant opinions on the specified feature or potential extension of CH-SUR.
CH-SUR: COVID-19 Hospital-Based Surveillance.
The survey responses on nurse practice and
response timeliness of CH-SUR (figure S4 in the appendix, available for download as
a separate file at
https://doi.org/10.57187/s.4213) revealed both strengths in training and
follow-up practices, and areas for improvement in response timeliness and
efficiency.
The open-ended questions of the survey
identified several challenges faced by CH-SUR stakeholders. The first related
to personnel and the ability to hire nurses to enter data into the CH-SUR
system, as well as collaborators having to work overtime, particularly during
the early stages of the COVID-19 pandemic. This was closely linked to financial
challenges in resource allocation and budgeting for CH-SUR activities. CH-SUR
stakeholders reported challenges related to the electronic case report form,
including pop-up messages, violation rules and potential issues with missing or
incorrect values. The automation of data entry within the case report form also
presented a substantial challenge as only some of the larger institutions had
the capacity to partially automate the extraction of some of the variables from
patients’ electronic health records. The text mining and word cloud analysis of
responses to the open-ended questions in the survey showed that CH-SUR is
centred on “data” and “cases”. Among frequently cited themes, we observed the
terms “nurses”, “weekly”, “report” as well “automation”, “COVID-19”, “surveillance”,
“data quality”, “manpower” and “workload” (figure S5 in the appendix, available for
download as a separate file at
https://doi.org/10.57187/s.4213).
Discussion
We conducted an evaluation of the
nationwide hospital-based surveillance for influenza and COVID- 19 in
Switzerland from November 2018 to October 2023, inspired by the CDC’s
guidelines for evaluating public health surveillance systems [10]. In addition,
we conducted a qualitative stakeholder survey in February–April 2023. Initially
established for surveillance of influenza, the expanded surveillance system has
enabled Swiss health authorities to monitor epidemiological trends in
hospitalised influenza and COVID-19 patients across Switzerland, to quantify
the respective disease burden and to assess healthcare demands over time.
Within one month of the report of the first
confirmed case of COVID-19 in Switzerland, the surveillance system was expanded
to include what was then a novel respiratory disease. This demonstrates the
importance of an established network with an agile, adaptable surveillance
system and motivated participating hospitals to include other diseases beyond
the initial intention. Further expansion of CH-SUR to include RSV, for example,
may be considered in the future. In terms of preparedness for future pandemics,
it also demonstrates the time and resource advantages of adapting existing
surveillance systems, rather than having to establish a de novo surveillance system [16]. The flexibility of the
system was also demonstrated by its ability to capture COVID-19 vaccination
details as COVID-19 vaccines were introduced, and COVID-19 treatments as
different therapeutics were either repurposed to treat COVID-19 or developed
specifically for COVID-19 [17]. The 3-fold increase in the number of
participating centres after the onset of the pandemic is a testament to the
system’s ability to be implemented across healthcare settings: tertiary and
non-tertiary care settings; adult and paediatric settings. Completeness and
timeliness are two additional indicators of the quality of the surveillance
system. Our quantitative analyses demonstrated a high level of completeness for
variables within the case report form and timely reporting of cases throughout
most of the pandemic.
CH-SUR strengthened Switzerland’s
surveillance landscape by capturing data on hospitalised cases, complementing
systems focused on outpatient care and community circulation: while Sentinella
monitors influenza-like illness and circulating respiratory viruses in primary
care and wastewater surveillance tracks viral circulation at the population
level, CH-SUR provided critical data on the severity and healthcare burden of
COVID-19 and influenza, supporting public health decision-making. Beyond the
primary public health objectives of the surveillance system, the collection of
data on hospitalised patients also allowed important scientific questions to be
addressed. This included identifying risk factors for severe COVID-19 outcomes [18];
an evaluation of COVID-19 mortality over time [19]; a comparison of clinical
outcomes among community-acquired influenza and COVID-19; and a comparison of community-acquired
and healthcare-associated COVID-19 [20–21].
Our qualitative evaluation of the CH-SUR
surveillance system provided further insights from CH-SUR stakeholders. It
highlighted that the expansion of the surveillance system to a novel disease
was not without notable challenges [22]. One difficulty stemmed from the extremely
high absolute number of COVID-19 cases requiring hospital-level care at each of
the participating centres – especially during the first pandemic phase – and
the increased scale and speed with which data needed to be reported. Although
not directly shown in our analysis, it has been shown that the protracted
nature of the COVID-19 pandemic has led to burnout among nurses and other
healthcare workers due to overtime work, lack of holidays and understaffing [23–25].
This underscores the need for strategic human resource planning and sufficient
financial resources to ensure the surveillance system can achieve its stated
objectives. Future cost-effectiveness analyses of CH-SUR may also help to correctly
size the surveillance system.
Data entry for CH-SUR currently relies on
study nurses retrieving data from patients’ (mostly computerised) medical files
and manually entering these data into the case report form. Unlike centralised
systems in other countries, such as France, Australia and New Zealand [26],
each centre participating in CH-SUR has its own system for managing patients’
medical data. In alignment with the digitisation strategy and automation of
surveillance led by Swissnoso and the Federal Office of Public Health,
automating data extraction and management may address some of the challenges
identified through our evaluation. This would not resolve the need for careful
interpretation and clinical judgement of some data, and there would be a
challenge in ensuring interoperability between different systems and databases.
However, pilot studies on automated surveillance are ongoing in Switzerland,
and CH-SUR may be able to benefit from the technological advances used in these
initiatives. The application of FAIR (Findable, Accessible, Interoperable,
Reusable) principles to biomedical databases is instrumental in enhancing the
value and utility of data [27] and it would be important to ensure that any
automated CH-SUR system adheres to these principles.
This study has some limitations. First, the
true number of hospitalised influenza and COVID-19 cases not reported to CH-SUR
remains unknown, potentially affecting the accuracy of the findings. Further,
the qualitative assessment was based on a convenience sample, which may not be
representative of all CH-SUR stakeholders, thus limiting the generalisability
of the results. These factors collectively highlight the need for caution in
interpreting the outcomes of this analysis.
Conclusions and perspectives
Overall, the nationwide hospital-based
surveillance for influenza was successfully adapted to COVID-19 [28] and has
provided important, actionable information on epidemiological trends in
hospitalised patients across Switzerland. This system has been instrumental in
understanding the disease burden among hospitalised patients and healthcare
demand, serving as a crucial resource for guiding evidence-based public health
decisions and preparing the Swiss health system for future respiratory virus
outbreaks. Our evaluation highlighted the significance and relevance of this
system among CH-SUR stakeholders, with identified challenges serving as
opportunities for improvement towards a sustainable and efficient respiratory
disease surveillance system in Switzerland.
Starting in January 2024, significant
changes were implemented in the CH-SUR system. Throughout the pandemic, the
system had been financed by temporary credits from the Federal Office of Public
Health, which were significantly reduced in 2024. The network was thus first
reduced to six hospitals from January 2024 and discontinued on 1 September 2024,
due to financial constraints. Looking ahead, further developments in collection
capabilities through increased automation for a future national hospital
surveillance system are recommended. This includes the implementation of CH-SUR
statistics on the Infectious Disease Dashboard of the Federal Office of Public
Health and the extension of surveillance to other diseases, such as RSV. These
advancements aim to enhance surveillance sustainability and resilience, and
data accuracy and accessibility, providing comprehensive insights into multiple
infectious diseases. The implementation of automated processes and
user-friendly dashboards will facilitate real-time monitoring and analysis,
ultimately strengthening public health surveillance and response strategies. By
embracing these innovations, Switzerland can ensure a robust and adaptable
surveillance system capable of addressing current and future public health
challenges [29].
Data sharing statement
The datasets presented in this article are not
publicly available. The data that support the findings of this study are
available from the Swiss Federal Office of Public Health on request for
specific research projects and at an aggregation level that ensures anonymisation.
Acknowledgments
We would like to thank the members of the
CH-SUR collaborative network who helped conduct the study at each of the study
sites. In particular, Mohamed Abbas, Jason Toko, Daniel Texiera, Michèle
Steiner, Marianne Rousseau-Schadegg, Anne-Flore Combaz, Audrey Aymon, Carlo
Balmelli, Manuel Battegay, Christoph Berger, Sara Bernhard-Stirnemann, Elfriede
Berwarth, Julia Bielicki, Michael Büttcher, Alexia Cusini, Lauro Damonti,
Philipp Kaiser, Stefan Keller, Henrik Köhler, Elia Lo Priore, Yvonne
Nussbaumer, Matthaios Papadimitriou, Felix Reichlin, Thomas Riedel, Susanne
Rüfenacht, Hanna Schmid, Markus Schneemann, Laurence Senn, Reto Stocker,
Nicolas Troillet, Sarah Tschudin-Sutter, Cathy Voide, Danielle Vuichard Gysin,
Konstanze Zoehrer and Franziska Zucol. We would also like to thank Céline
Gardiol and Jasmin Vonlanthen, Ornella Luminati, Carolina Agop Nersesian, Carla
Grolimund, Fabienne Krauer, Mirjam Mäusezahl and Katrin Schneider from the
Federal Office of Public Health of Switzerland and Maroussia Roelens and Amaury
Thiabaud from the University of Geneva. We acknowledge the contributions of the
Clinical Research Centre, Geneva University Hospitals and Faculty of Medicine,
Geneva.
Dr Jonathan Sobel,
PhD
Infection Control
Programme and WHO Collaborating Centre on Infection Prevention and Control and
Antimicrobial Resistance
Geneva University
Hospitals and Faculty of Medicine
CH-1205 Geneva
jonathanaryeh.sobel[at]hug.ch
References
1. Abbas M, Zhu NJ, Mookerjee S, Bolt F, Otter JA, Holmes AH, et al. Hospital-onset COVID-19
infection surveillance systems: a systematic review. J Hosp Infect. 2021 Sep;115:44–50.
doi: https://doi.org/10.1016/j.jhin.2021.05.016
2. Marcenac P, McCarron M, Davis W, Igboh LS, Mott JA, Lafond KE, et al. Leveraging international
influenza surveillance systems and programs during the COVID-19 pandemic. Emerg Infect
Dis. 2022 Dec;28(13 Suppl 1):S26–33. doi: https://doi.org/10.3201/eid2813.212248
3. Fougerolles de, Damm O, Ansaldi F, et al. National influenza surveillance systems
in five European countries: a qualitative comparative framework based on WHO guidance.
BMC Public Health. 2022;22(1):1–13.
4. Quirós-González V, Rodríguez-Pérez P, Haro-Pérez AM, Jiménez-Rodríguez MM, Maderuelo-Fernández JÁ,
Eiros JM. Real-time surveillance systems: applicability for the control of influenza
in acute care. Influenza Other Respir Viruses. 2020 May;14(3):331–9. doi: https://doi.org/10.1111/irv.12720
5. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic
data capture (REDCap)—a metadata-driven methodology and workflow process for providing
translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81.
doi: https://doi.org/10.1016/j.jbi.2008.08.010
6. New Coronavirus 2019-nCoV: first confirmed case in Switzerland. Accessed June 5, 2024.
Available from: https://www.bag.admin.ch/bag/en/home/das-bag/aktuell/medienmitteilungen.msg-id-78233.html
7. Maximiano Sousa F, Roelens M, Fricker B, Thiabaud A, Iten A, Cusini A, et al.; Ch-Sur
Study Group. Risk factors for severe outcomes for COVID-19 patients hospitalised in
Switzerland during the first pandemic wave, February to August 2020: prospective observational
cohort study. Swiss Med Wkly. 2021 Jul;151(2930):w20547. doi: https://doi.org/10.4414/smw.2021.20547
8. Thiabaud A, Iten A, Balmelli C, Senn L, Troillet N, Widmer A, et al. Cohort profile:
SARS-CoV-2/COVID-19 hospitalised patients in Switzerland. Swiss Med Wkly. 2021 Feb;151(708):w20475.
doi: https://doi.org/10.4414/smw.2021.20475
9. Swiss Federal office of public health. Monthly COVID-19 situation in Switzerland. Published
online June 2023.
10. Centers for Disease Control and Prevention (CDC), Updated Guidelines for Evaluating
Public Health Surveillance Systems, Recommendations from the Guidelines Working Group,
2001
11. Shu Y, McCauley J. GISAID: global initiative on sharing all influenza data - from
vision to reality. Euro Surveill. 2017 Mar;22(13):30494. doi: https://doi.org/10.2807/1560-7917.ES.2017.22.13.30494
12. Fellows I. Wordcloud: Word Clouds.; 2018. Available from: https://CRAN.R-project.org/package=wordcloud
13. Feinerer I, Hornik K, Meyer D. Text Mining Infrastructure in R. J Stat Softw. 2008;25(5):1–54.
10.18637/jss.v025.i05
14. Schreier M. Qualitative Content Analysis. The SAGE Handbook of Qualitative Data Analysis.
SAGE Publications, Inc.; 2014. pp. 170–83. [ [cited 2024 Jun 5]]. 10.4135/9781446282243.n12
15. Team RC. A Language and Environment for Statistical Computing. R Foundation for Statistical
Computing; 2023. Available from: https://www.R-project.org/
16. Ricks PM, Njie GJ, Dawood FS, Blain AE, Winstead A, Popoola A, et al. Lessons learned
from CDC’s global COVID-19 Early Warning and Response Surveillance system. Emerg Infect
Dis. 2022 Dec;28(13 Suppl 1):S8–16. doi: https://doi.org/10.3201/eid2813.212544
17. Estill J, Venkova-Marchevska P, Günthard HF, Botero-Mesa S, Thiabaud A, Roelens M,
et al. Treatment effect of remdesivir on the mortality of hospitalised COVID-19 patients
in Switzerland across different patient groups: a tree-based model analysis. Swiss
Med Wkly. 2023 Aug;153(8):40095. 10.57187/smw.2023.40095
18. Fröhlich GM, De Kraker ME, Abbas M, Keiser O, Thiabaud A, Roelens M, et al. Hospital
outcomes of community-acquired COVID-19 versus influenza: insights from the Swiss
hospital-based surveillance of influenza and COVID-19. Euro Surveill. 2022 Jan;27(1):2001848.
doi: https://doi.org/10.2807/1560-7917.ES.2022.27.1.2001848
19. Roelens M, Martin A, Friker B, Sousa FM, Thiabaud A, Vidondo B, et al. Evolution of
COVID-19 mortality over time: results from the Swiss hospital surveillance system
(CH-SUR). Swiss Med Wkly. 2021 Nov;151(4748):w30105. doi: https://doi.org/10.4414/SMW.2021.w30105
20. Portmann L, de Kraker ME, Fröhlich G, Thiabaud A, Roelens M, Schreiber PW, et al.;
CH-SUR study group. Hospital Outcomes of Community-Acquired SARS-CoV-2 Omicron Variant
Infection Compared With Influenza Infection in Switzerland. JAMA Netw Open. 2023 Feb;6(2):e2255599–2255599.
doi: https://doi.org/10.1001/jamanetworkopen.2022.55599
21. Grant RL, Sauser J, Atkinson A, D’Incau S, Buetti N, Zanella MC, et al.; CH-SUR Collaborative
Network. Comparison of clinical outcomes over time of inpatients with healthcare-associated
or community-acquired coronavirus disease 2019 (COVID-19): A multicenter, prospective
cohort study. Infect Control Hosp Epidemiol. 2024 Jan;45(1):75–81. 10.1017/ice.2023.143
22. Bloom DE, Cadarette D. Strengthening the Global Response to Infectious Disease Threats
in the Twenty-First Century, with a COVID-19 Epilogue. Global Health. Cambridge University
Press; 2021. pp. 51–75. [ [cited 2024 Jun 13]]. 10.1017/9781108692137.004
23. Sullivan D, Sullivan V, Weatherspoon D, Frazer C. Comparison of nurse burnout, before
and during the COVID-19 pandemic. Nurs Clin North Am. 2022 Mar;57(1):79–99. doi: https://doi.org/10.1016/j.cnur.2021.11.006
24. Lasater KB, Aiken LH, Sloane DM, et al. Chronic hospital nurse understaffing meets
COVID-19: an observational study. BMJ Quality \& Safety. 2021;30(8):639-647.
25. Rivas N, López M, Castro MJ, Luis-Vian S, Fernández-Castro M, Cao MJ, et al. Analysis
of burnout syndrome and resilience in nurses throughout the COVID-19 pandemic: a cross-sectional
study. Int J Environ Res Public Health. 2021 Oct;18(19):10470. doi: https://doi.org/10.3390/ijerph181910470
26. Lee J, Park YT, Park YR, Lee JH. Review of National-Level Personal Health Records
in Advanced Countries. Healthc Inform Res. 2021 Apr;27(2):102–9. 10.4258/hir.2021.27.2.102
27. Queralt-Rosinach N, Kaliyaperumal R, Bernabé CH, Long Q, Joosten SA, van der Wijk HJ,
et al.; BEAT-COVID Group; COVID-19 LUMC Group. Applying the FAIR principles to data
in a hospital: challenges and opportunities in a pandemic. J Biomed Semantics. 2022 Apr;13(1):12.
doi: https://doi.org/10.1186/s13326-022-00263-7
28. World Health Organization, others. WHO Consultation to Adapt Influenza Sentinel Surveillance
Systems to Include COVID-19 Virological Surveillance: Virtual Meeting, 6–8 October
2020. World Health Organization; 2022.
29. World Health Organization, others. COVID-19 Strategic Preparedness and Response Plan:
Monitoring and Evaluation Framework, 11 May 2021. World Health Organization; 2021.
Appendix
The appendix is available for download as a separate file at https://doi.org/10.57187/s.4213.



