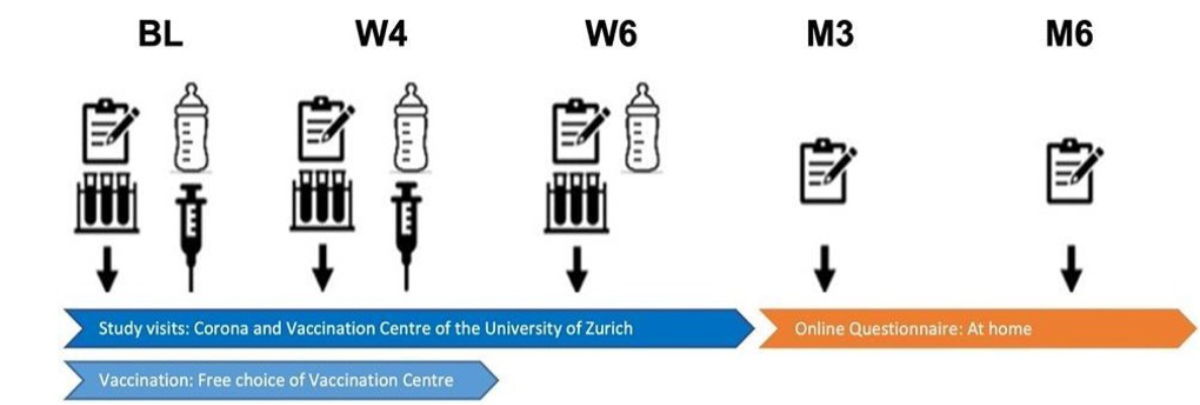
Figure 1Study flow and assessment time points. BL: Baseline visit; W4: Follow-up visit at approximately 4 weeks; W6: Follow-up visit at approximately 6 weeks; M3: Follow-up questionnaire at 3 months; M6: Follow-up questionnaire at 6 months.
DOI: https://doi.org/https://doi.org/10.57187/s.4207
The COVID-19 pandemic has posed significant health risks, especially to pregnant women and infants under 6 months, who faced high hospitalisation rates [2–5]. Antibodies play an important role in neonatal immunity, making detailed knowledge about immune responses in this population and protective immunity transferred through breast milk vital.
To date, the immune response of lactating women is subject to substantial research and not yet fully understood. Studies have shown that antibodies stimulated through vaccination transfer into breast milk and may protect the child. They could be detected in breast milk up to 8 months after receiving the first vaccine dose, although their concentrations decreased when compared with concentrations reached immediately after vaccination [6, 7]. Neutralising activity of vaccine-induced antibodies in breast milk is highly variable and generally low [8–12]. Breast milk IgG antibodies are synchronised with maternal serum IgG antibodies, and maternal serum antibody titres are equivalent to those of non-lactating women [11, 13–16]. There is no evidence of serious side effects of SARS-CoV-2 vaccinations in mothers or infants or significant impact on milk supply across numerous large studies and registries of COVID-19 vaccination in lactation [10, 15–20]. Until now, no clinical correlation has been established between clinical protection from COVID-19 infection in breastfed infants and breast milk antibody concentration of vaccinated lactating mothers [21, 22]. Only a few studies have compared immune responses in lactating vs non-lactating women and included longitudinal symptom evaluations. Despite increasing numbers of studies, further research needs to be conducted to better understand the complex topic of immune response after COVID-19 mRNA vaccination in lactating women.
This study aimed to characterise the antibody responses and neutralising antibody responses against different variants of SARS-CoV-2 in blood and breast milk after mRNA vaccination (Comirnaty® [BNT-162b2] or Spikevax® [mRNA-1273]) in lactating women. Further objectives were to compare the antibody responses to those in a matched cohort of non-lactating women, and to identify factors associated with antibody responses. Additionally, we assessed the occurrence of post-vaccination symptoms, health status trajectories, and SARS-CoV-2 infections among study participants and their children after vaccination.
A single-centre prospective cohort study of lactating women receiving an mRNA vaccine against SARS-CoV-2 was conducted at the Corona Centre of the University of Zurich, Switzerland, from 15 October 2021 to 21 February 2022 (recruitment period). Recruitment was stopped early due to a substantial decline in participant attendance, mainly because of changes in regulations (discontinuation of mandatory vaccination certificates, growing number of individuals with prior infection and therefore not needing vaccination). The study site operated as the reference vaccination centre in the Canton of Zurich, providing an optimal location for recruiting study participants. Participants were recruited on-site (flyers, direct approach by trained personnel) before vaccination or referred by external healthcare providers (whom we provided with information material beforehand). Approximately 65 healthcare providers (paediatricians and gynaecologists) in the city of Zurich were approached. During the recruitment period, two monovalent mRNA vaccines were available in Switzerland: BNT-162b2 and mRNA-1273 (both approved for individuals 12 years and older). Most of the data was collected during the first wave of infections with the omicron variant in Switzerland, which started in November 2021 [23].
Lactating women aged over 18 years, who had not previously received a vaccination against SARS-CoV-2 and who could follow the study procedures were included. Eligible women not completing at least two immunological assessments were excluded from the study.
The study was approved by the ethics committee of the Canton of Zurich (BASEC 2021-01835). All participants provided written informed consent to participate in the study. The study was registered in the International Standard Randomised Controlled Trial Number registry (ISRCTN12344753, accessible at https://doi.org/10.1186/ISRCTN12344753). A comprehensive project plan outlining the study design and methodology was developed and served as the equivalent of the study protocol. This project plan has, however, not been published online but is available upon request from the corresponding author.
The primary outcome was the antibody response (anti-S IgA, anti-S IgG and anti-N IgG), measured in mean fluorescence intensity (MFI) ratios (see below), in blood plasma and breast milk at baseline, 4 weeks and 6 weeks. Secondary outcomes included neutralising antibody responses against wildtype, delta and omicron variants of SARS-CoV-2 in blood plasma at 6 weeks, as well as self-reported post-vaccination symptoms over 6 weeks, health status trajectories (assessed using the EuroQol visual analogue scale [EQ VAS]) over 6 months, and SARS-CoV-2 infections in mothers and infants over 6 months of follow-up.
Study assessments were conducted at a total of five time points and included data collection using questionnaires, a symptom diary and the collection of biological samples (figure 1). The first visit, the baseline (BL), took place directly after study enrolment. The second visit (W4) was scheduled approximately 4 weeks after BL and the third visit (W6) was scheduled approximately 2 weeks after W4. At the first three visits, both questionnaire data and samples were collected. Further online follow-up assessments (exclusively questionnaire data) were conducted at approximately 3 months (M3) and 6 months (M6) after the BL visit. Symptom booklets were collected at 6 weeks during the last study visit.

Figure 1Study flow and assessment time points. BL: Baseline visit; W4: Follow-up visit at approximately 4 weeks; W6: Follow-up visit at approximately 6 weeks; M3: Follow-up questionnaire at 3 months; M6: Follow-up questionnaire at 6 months.
Participants were vaccinated with one of the two monovalent mRNA vaccines against SARS-CoV-2 available in Switzerland, BNT-162b2 or mRNA-1273, at BL and at W4. At both time points, vaccines were administered after blood withdrawal. Study participants could freely choose between the two vaccines.
The Research Electronic Data Capture (REDCap) survey system was used as a software solution to securely manage the study data [24].
The baseline questionnaire included questions about sociodemographic information of the mothers and their infants. Furthermore, SARS-CoV-2-related information was obtained, i.e. about previous episodes with possible COVID-19 symptoms or previous documented SARS-CoV-2 infection. Participants’ health status was assessed using the EuroQol visual analogue scale (EQ VAS), which was included as part of the EuroQoL 5-dimension 5-level (EQ-5D-5L) instrument (German version) [25]. The full baseline questionnaire can be found in the supplementary material at https://doi.org/10.57187/s.4207.
In addition, a symptom diary was handed out at the BL visit which aimed to collect information regarding post-vaccination symptoms. This included their nature, severity (based on participants’ self-assessment; 5-point Likert scale ranging from very mild to very severe) and medical consequence. There were separate sections for the mother and her infant. The symptom diary can be accessed in the supplementary material at https://doi.org/10.57187/s.4207.
At all follow-up time points (W4, W6, M3 and M6), follow-up questionnaires were distributed to participants to assess health status as well as new SARS-CoV-2 tests and potential infections over time.
At the study centre, peripheral venous blood samples were collected at BL, W4 and W6. For the collection of breast milk samples, study participants could either use a Medela® Symphony breast pump available at the study centre or bring cooled breast milk that they had collected on the day of the study visit. Hence, the maximum time difference between milk and blood sample collection was approximately 10 hours.
All blood and breast milk samples were processed, aliquoted and stored at −20 °C in the biobank of the research laboratory attached to the study centre. Frozen blood plasma samples were subsequently transported to the Lausanne University Hospital (CHUV) for analysis. There, laboratory analyses for SARS-CoV-2-specific antibodies for both blood plasma and breast milk (anti-Spike [S] IgG, anti-S IgA, anti-Nucleocapsid [N] IgG) and neutralising antibodies (anti-wildtype, anti-delta, anti-omicron) were conducted as reported elsewhere [26–28]. In short, the analysis was performed using a Luminex binding assay, more specifically the Sensitive anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological (SenASTrIS), which has been shown to have a high specificity (99%) and sensitivity (97%) [28]. The mean fluorescence intensity ratio values obtained have been categorised into negative and positive according to the cut-off value of 6 (for anti-S IgG and anti-N IgG) or 6.5 (anti-S IgA). Values below the cut-off indicated no detection of SARS-CoV-2-specific antibodies and those above the cut-off were interpreted as presence of SARS-CoV-2-specific antibodies (i.e. seropositivity and hence functional immunity) in the analysed samples [26]. Anti-N IgG levels above the cut-off values were used as an indicator of a previous infection with SARS-CoV-2 (none of the applied mRNA vaccines contained nucleocapsid antigens). The same cut-off values for the mean fluorescence intensity values were used for blood plasma and breast milk analyses.
In order to determine the presence of SARS-CoV-2 neutralising antibodies against wildtype SARS-CoV-2 as well as two major variants of concern circulating in 2021 and early 2022 in Switzerland (delta, omicron BA-1), we used a cell- and virus-free surrogate assay based on the competitive inhibition of trimeric SARS-CoV-2 spike protein binding to the angiotensin-converting enzyme 2 (ACE2) receptor [29]. Neutralisation has been shown to occur at half-maximal inhibitory concentrations (IC50) above the cut-off value of 50 [26]. While a similar neutralisation assay in breast milk was initially planned, this method yielded unreliable results. After multiple unsuccessful attempts to implement the neutralisation assay in breast milk, we finally decided to omit it from the analysis.
Additionally, although planned a priori, an additional assessment of T cell responses in the study was not realised due to time and budget constraints.
To compare immune responses in blood of lactating mothers to those of non-lactating women of childbearing age, we used data from the Zurich SARS-CoV-2 Vaccination Cohort (ISRCTN15499304), an ongoing, population-based, longitudinal cohort of individuals vaccinated against SARS-CoV-2 [30]. From this cohort (total n = 575), a 1:1 propensity score-matched sample of women aged 20–45 years who were not pregnant and had received the mRNA-1273 or BNT-162b2 vaccines was drawn. Propensity score matching was performed based on age, smoking status, body mass index and presence of chronic comorbidities, using nearest-neighbour matching with a calliper of 0.2. The matching was implemented using the MatchIt package (v4.5.0) in R. Questionnaires, antibody and neutralising antibody testing protocols of this cohort were fully aligned with the cohort of lactating mothers to ensure comparability.
Participant characteristics, laboratory testing data and collected follow-up data were analysed using descriptive statistics. Minimum values were set to 1 (mean fluorescence intensity ratios) and 0.5 (IC50 values) for antibody and neutralising antibody responses, respectively, since lower measurement values were not considered clinically meaningful based on the laboratory tests used. Proportions with 95% Wilson confidence intervals (CIs) were calculated for individuals testing positive for anti-S IgA, anti-S IgG, anti-N IgG or neutralising antibodies, and reporting a SARS-CoV-2 infection at the different follow-up time points. Spearman correlation coefficients were calculated to investigate correlations of anti-S IgA, anti-S IgG and anti-N IgG between blood plasma and breast milk at different time points, as well as to investigate correlations of antibody and neutralising antibody responses in plasma at 6 weeks. The associations of different factors with post-vaccination (4 weeks and 6 weeks) anti-S IgG response in plasma and breast milk were evaluated using multivariable mixed (repeated-measures) linear regression models (mean fluorescence intensity ratios of anti-S IgG responses were log10-transformed). These models were mutually adjusted for the evaluated factors (age of mother, lactation duration, vaccine type, prior positive SARS-CoV-2 test, smoking status and body mass index). These variables were selected a priori based on clinical reasoning, since they were known to be associated with the strength of the antibody responses [31–34]. Models further included a random intercept for each individual in the study to account for correlation within individuals. Model assumptions were checked and considered to be reasonably met given the available sample size and exploratory nature of the analysis. We calculated 95% CIs and estimated two-sided p-values using Satterthwaite’s method [35]. We did not adjust p-values for multiple testing but instead interpreted them in terms of the strength of statistical evidence given the low statistical power of the study (no p-value threshold was applied; p >0.1: no evidence; p >0.05 and ≤0.1: weak evidence; p >0.01 and ≤0.05: moderate evidence; p >0.001 and ≤0.01: strong evidence; p <0.001: very strong evidence [36]). As missing data was sparse, no measures were taken to impute missing values and data was reported as recorded in the study.
All analyses were conducted using the free open-source software R (version 4.2.2) [37] and using the tidyverse (v2.0.0) and lmerTest (v3.1-2) packages.
Between 15 October 2021 and 21 February 2022, a total of 45 study participants were enrolled, of whom three dropped out at baseline and two after the first vaccination (did not complete at least two immunological assessments). One participant completed two immunological assessments but dropped out at 6 weeks of follow-up. Therefore, 40 study participants were included in the immune response analyses, and 39 were included in the other analyses (figure 2).
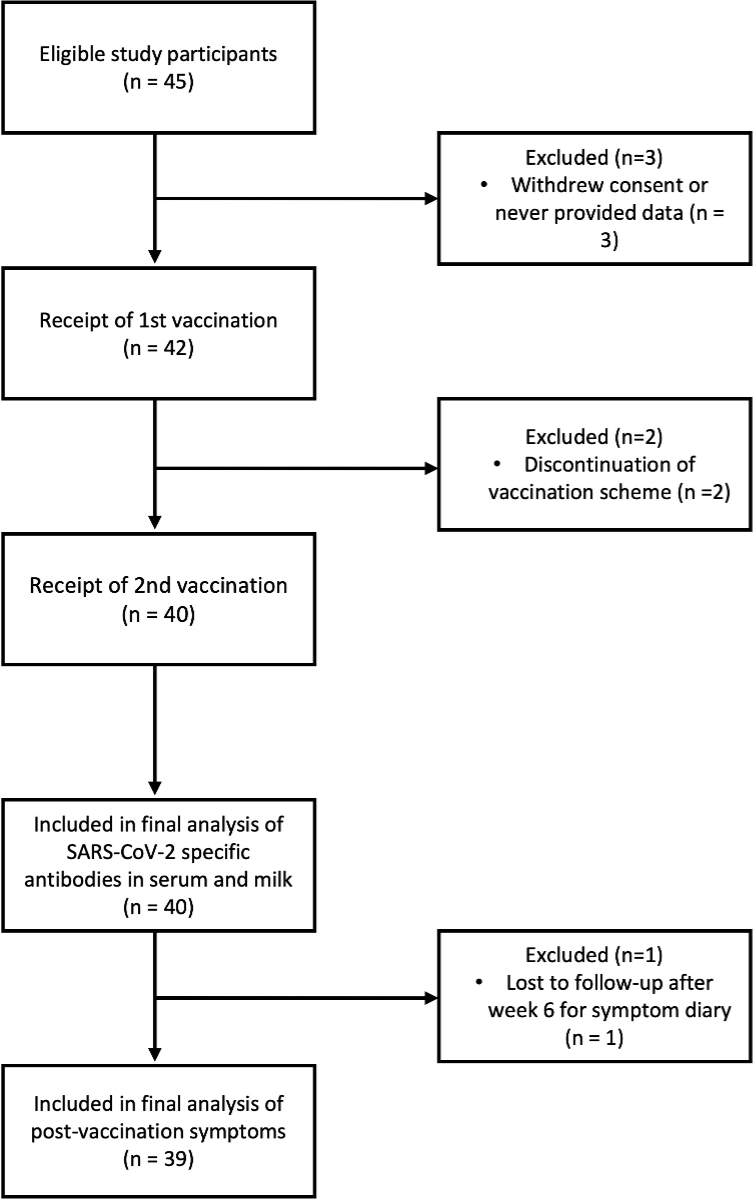
Figure 2Enrolment and follow-up.
Population characteristics are shown in table 1, with further details reported in appendix table S1. The median age of lactating mothers was 36 years (interquartile range [IQR]: 32–38). The median age of their infants at time of enrolment was 9 weeks (IQR: 4–17) with 61.5% (24/40) being female. The median duration of lactation at enrolment was 8.6 weeks (IQR: 4.3–17.7). Five mothers (5/39 or 12.8%, 1 missing) reported having ever tested positive for SARS-CoV-2 infection prior to enrolment.
Table 1Population characteristics at baseline.
| Mothers (n = 40) | Age in years, mean (SD), range | 34.9 (3.8), 27–42 | |
| BMI in kg/m2, mean (SD), range | 22.5 (2.9), 17–31 | ||
| Vaccine type, n (%) | BNT-162b2 (Comirnaty®, Pfizer/BioNTech) | 28 (70.0%) | |
| mRNA-1273 (Spikevax®, Moderna) | 12 (30.0%) | ||
| Lactation duration in weeks, mean (SD), range | 20.3 (41.9), 1–245 | ||
| Prior positive SARS-CoV-2 test, n (%) | 5 (12.8%) | ||
| Prior negative SARS-CoV-2 test, n (%) | 34 (87.2%) | ||
| Comorbidity, n (%) | 1 (2.6%) | ||
| Infants (n = 40) | Female sex, n (%) | 24 (61.5%) | |
| Age in weeks, mean (SD), range | 13.8 (14.0), 1–64 | ||
| Age in weeks by group, n (%) | <10 | 20 (54.1%) | |
| 10–19 | 9 (24.3%) | ||
| 20–29 | 1 (2.7%) | ||
| 30–39 | 5 (13.5%) | ||
| >39 | 2 (5.4%) | ||
BMI: body mass index; IQR: interquartile range; SD: standard deviation.
After vaccination, a relevant increase in anti-S IgA and anti-S IgG was observed in the blood plasma of the study participants (figure 3A). Anti-S IgA and anti-S IgG showed a continuous increase between baseline and W4 and between W4 and W6, respectively. At W4 and W6, all participants (100%, 39/39, 1 missing) tested seropositive for anti-S IgG antibodies. Three participants (7.5%, 3/40) tested anti-N IgG-positive at enrolment. The number of anti-N IgG-positive participants decreased by 4 weeks (2.5%, 1/40) and subsequently increased again by 6 weeks (7.8%, 3/39, 1 missing). Antibody positivity in blood plasma reached peak levels at W6 for anti-S IgA and at W4 for anti-S IgG (figure 3B).
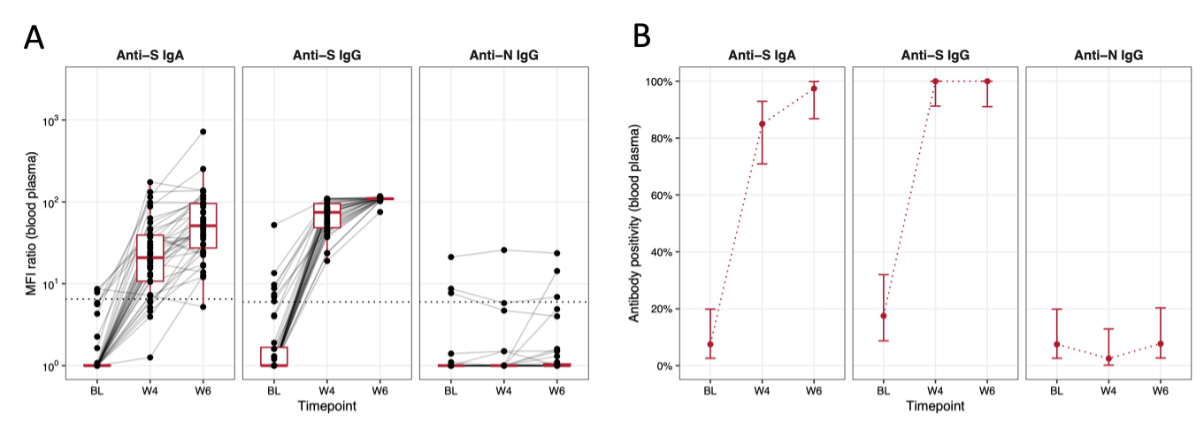
Figure 3Immune response in blood plasma over time. The x-axis represents the different time points (BL, W4, W6) at which the blood sampling was performed. (A) The y-axis shows the mean fluorescence intensity (MFI) ratio in the blood plasma on a log10 scale; the dotted line indicates the detection threshold for presence of SARS-CoV-2-specific antibodies (i.e. seropositivity and hence functional immunity). Individual measurements (MFI ratios) are depicted as black dots and measurements from the same individual are connected with grey lines. Boxplots depict median (red middle line), interquartile range (red box) and 1.5 × interquartile range (whiskers) of measurements across participants at each time point. (B) The y-axis displays the percentage of participants showing antibody positivity in blood plasma at time points BL, W4 and W6. The red dots represent the point estimate and red error bars represent 95% confidence intervals for proportions at each time point. Data is displayed for each antibody group individually (anti-S IgA, anti-S IgG and anti-N IgG).
In breast milk, a similar increase in anti-S IgG levels was observed. By 4 weeks, all participants tested positive for anti-S IgG antibodies. Meanwhile anti-S IgA showed only a minimal increase, with the majority remaining below the positivity threshold at 6 weeks (84.6%, 33/39, 1 missing) (figure 4A). Anti-N IgG antibody levels were consistently below the positivity threshold except for one participant. Antibody positivity reached high levels at W4 for anti-S IgG, while it stayed low for anti-S IgA (figure 4B).
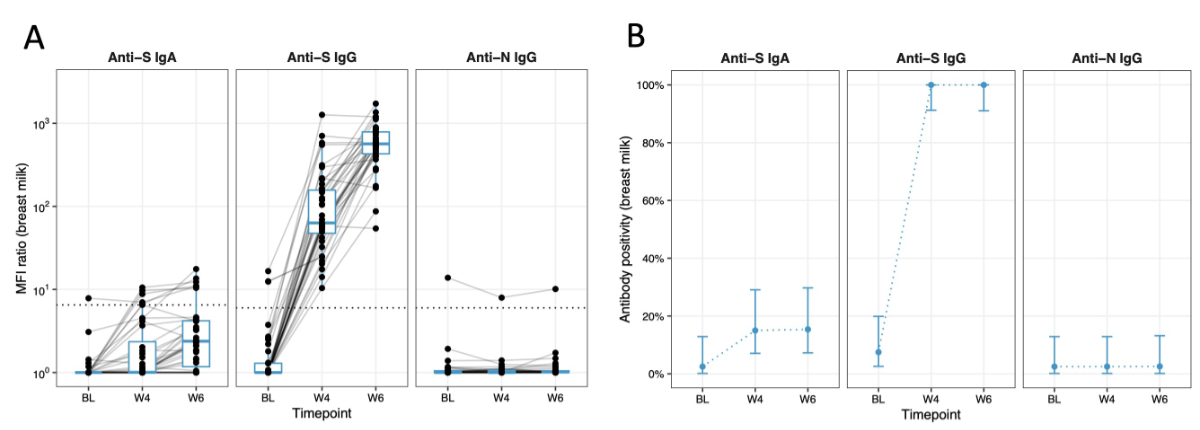
Figure 4Immune response in breast milk over time. The x-axis represents the different time points (BL, W4, W6) at which the breast milk sampling was performed. (A) The y-axis shows the mean fluorescence intensity (MFI) ratio in the breast milk on a log10 scale; the dotted line indicates the detection threshold for presence of SARS-CoV-2-specific antibodies. Individual measurements (MFI ratios) are depicted as black dots and measurements from the same individual are connected with grey lines. Boxplots depict median (red middle line), interquartile range (red box) and 1.5 × interquartile range (whiskers) of measurements across participants at each time point. (B) The y-axis displays the percentage of participants showing antibody positivity in breast milk. The blue dots represent the point estimate and blue error bars represent 95% confidence intervals for proportions at each time point. Data is displayed for each antibody group individually (anti-S IgA, anti-S IgG and anti-N IgG).
The correlation between antibody responses in blood plasma and in breast milk was moderate to very strong across time points, while it was greatest for anti-S IgG at all time points (appendix figure S1).
At 6 weeks, neutralising antibodies were detected in the blood against (in decreasing order) the wildtype, delta and omicron variant in 100% (39/39, 1 missing), 97.4% (38/39) and 64.1% (25/39) of participants, respectively (figures 5A and 5B). A strong correlation between anti-S IgG antibodies in the blood of the study participants and neutralising antibodies against the wildtype variant was observed (Spearman’s correlation coefficient 0.70, appendix figure S2). For the delta and omicron variants, moderate correlation was found (0.65 and 0.52, respectively). Correlation with neutralising antibodies was generally lower for anti-S IgA.
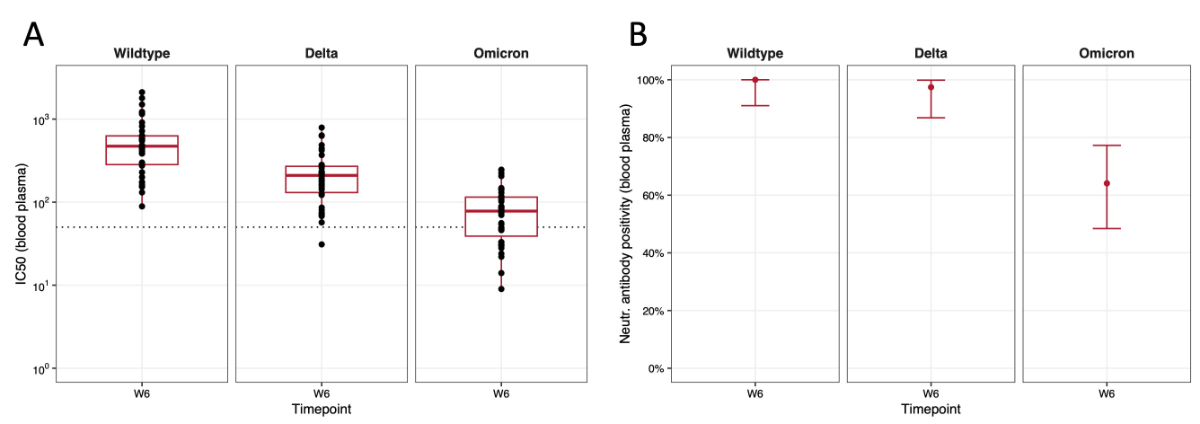
Figure 5Neutralising antibodies and antibody positivity against the different SARS-CoV-2 variants in blood plasma. The x-axis represents the time point (W6) at which the blood sampling was performed. (A) The y-axis shows the half-maximal inhibitory concentrations (IC50) in blood plasma on a log10 scale; the dotted line indicates the threshold for neutralisation capacity of the antibodies against the respective variants. Boxplots depict median (red middle line), interquartile range (red box) and 1.5 × interquartile range (whiskers) of measurements across participants. (B) The y-axis displays the percentage of participants showing neutralising antibody positivity in blood at W6, displayed for each variant individually. The red dots represent the point estimate and red error bars represent 95% confidence intervals for proportions.
The comparison of antibody and neutralising antibody responses in the blood of lactating mothers and non-lactating women from an otherwise comparable population-based cohort showed similar humoral immune response patterns between the two populations (appendix figures S3A and S3B). A detailed overview and the population characteristics of the matched sample can be found in the appendix tables S2 and S3.
Based on descriptive analyses, mRNA-1273 was found to induce slightly stronger antibody responses and neutralising antibody responses in blood plasma (appendix figure S4A) and breast milk (appendix figure S4B) when compared to BNT-162b2. However, there was no statistical evidence for a difference based on adjusted association analyses (figures 6A and 6B). With respect to neutralising antibodies, levels were higher with mRNA-1273 compared to BNT-162b2 for wildtype and delta SARS-CoV-2, but not for the omicron variant, based on descriptive analyses (appendix figure S6C).
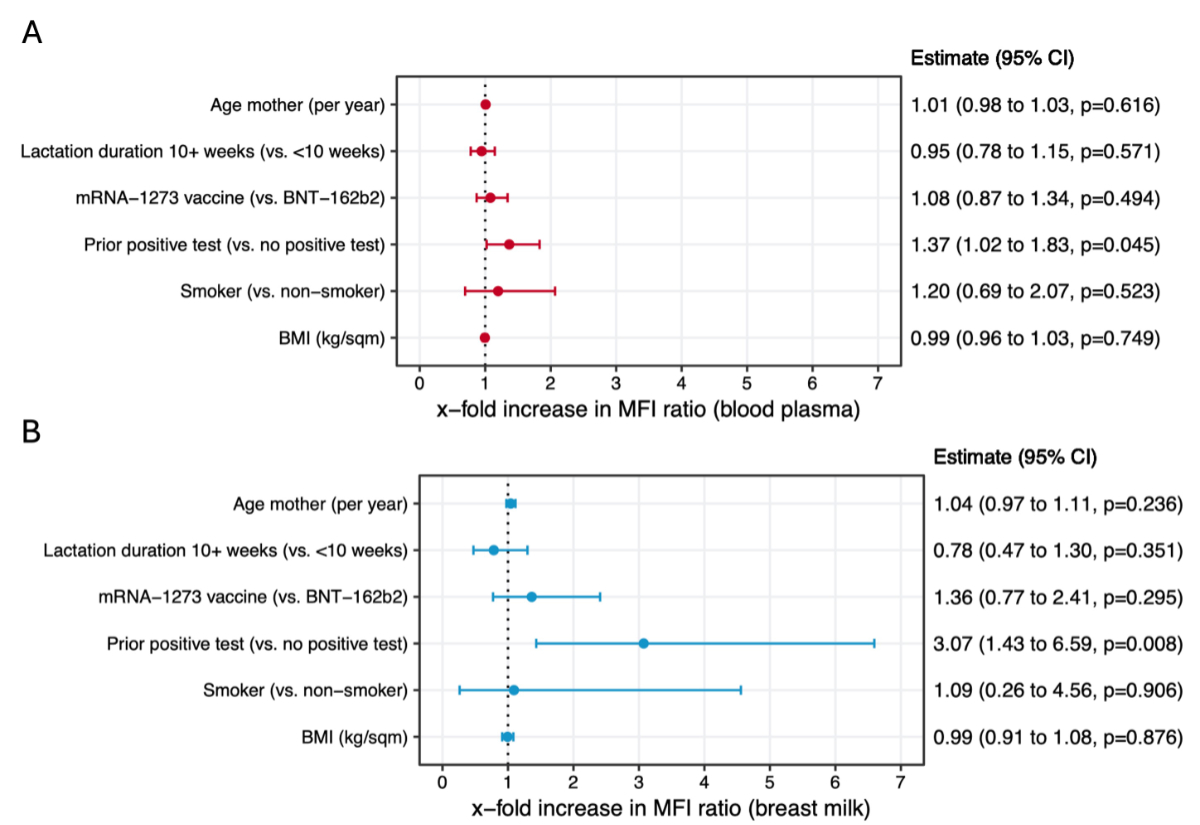
Figure 6Association analysis of factors potentially influencing immune responses in blood plasma (A) and breast milk (B). The x-axis in both panels represents the x-fold increase in mean fluorescence intensity (MFI) ratio for blood plasma (A) and breast milk (B), indicating the effect of different maternal characteristics or vaccine type on antibody levels. Results are based on adjusted (multivariable) mixed (repeated measures) linear regression models mutually adjusted for all reported variables. Dots depict point estimates and error bars represent 95% confidence intervals (CI) for the estimates derived from the model. Values >1 suggest a positive association (i.e. higher antibody levels in blood plasma or breast milk compared to the comparison group), while values <1 indicate a negative association. BMI: body mass index; BNT-162b2: Comirnaty®; mRNA-1273: Spikevax®
In adjusted association analyses, there was no statistical evidence that the age of the mother, lactation duration (subgroup analysis ≥10 weeks vs <10 weeks), vaccine type (mRNA-1273 vs BNT-162b2), smoking status or body mass index (BMI) was correlated with anti-S IgG antibody responses in blood and breast milk, based on mixed repeated-measures linear regression models mutually adjusted for all evaluated factors (figures 6A and 6B). Meanwhile, there was weak evidence for stronger anti-S IgG responses in blood (1.33-fold increase in mean fluorescence intensity ratio, 95% CI: 1.00–1.76, p = 0.059) and strong evidence for stronger anti-S IgG responses in breast milk (3.09-fold increase in mean fluorescence intensity ratio, 95% CI: 1.44–6.65, p = 0.007) among mothers with a prior positive test for SARS-CoV-2 compared to non-previously infected mothers.
In total, 173 post-vaccination symptoms were reported, of which 136 were in vaccinated mothers and 37 in their infants. The mean symptom duration was 2.3 days (SD: 2.8) in mothers and 4.2 days (SD: 4.3) in their infants, with a range of 0–15 days and 0–13 days, respectively (appendix table S4).
The three most common post-vaccination symptoms in mothers were pain in extremity (17.6%, 24/136), headache (16.2%, 22/136) and asthenia (11%, 15/136) (figure 7A). Most of the post-vaccination symptoms in mothers (70.7%, 70/99, 37 missing) resolved spontaneously or could be relieved by self-medication (28.3%, 28/99), while one single post-vaccination symptom required an emergency room or physician visit (1.0%, 1/99, symptom was diarrhoea) (figure 7B). Self-reported severity in mothers was very mild to medium in most cases (87.1%, 108/124, 12 missing), with fewer reported as severe to very severe (12.9%, 16/124) (figure 7C).
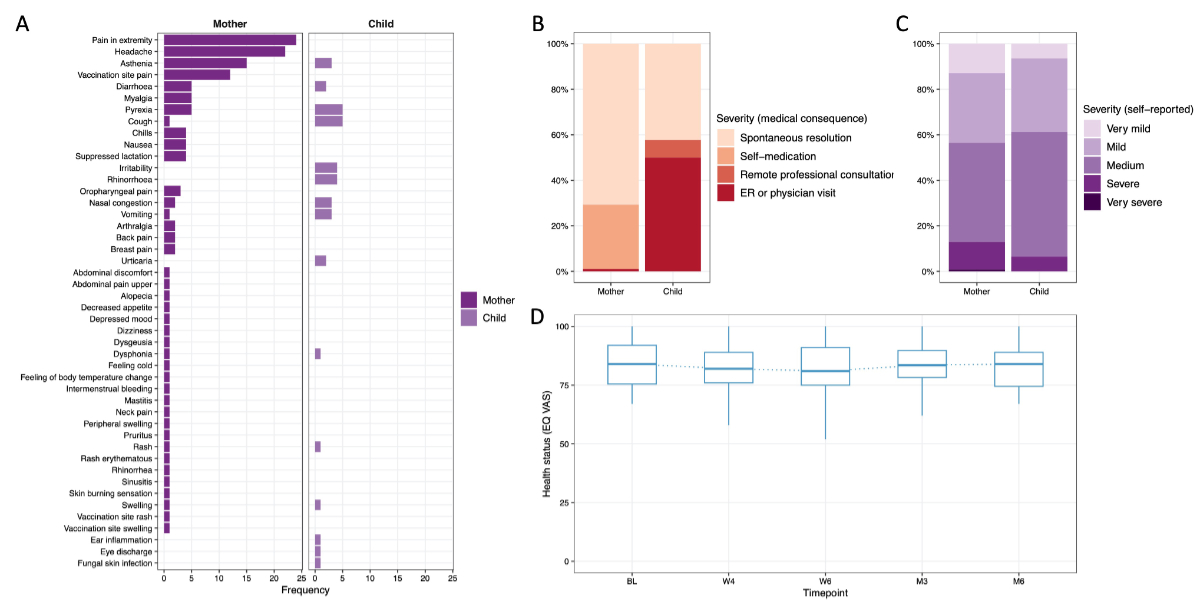
Figure 7Overview of self-reported post-vaccination symptoms (PVS) in mothers and their infants, their severity as well as health status (EuroQol visual analogue scale [EQ VAS]) over time. (A) Overview of the most common self-reported post-vaccination symptoms in the vaccinated mothers and their infants, with the frequency (in percentage) on the x-axis and symptoms on the y-axis. (B) Overview and breakdown (in percentage, y-axis) of the severity of post-vaccination symptoms based on their medical consequence reported by mothers (own post-vaccination symptoms as well as their infants’ post-vaccination symptoms). (C) Overview and breakdown (in percentage, y-axis) of the self-reported severity of the post-vaccination symptoms reported by mothers (own post-vaccination symptoms as well as their infants’ post-vaccination symptoms). (D) Health status measured by EQ VAS (0–100) over time with time points on the x-axis. Boxplots depict median (blue middle line), interquartile range (blue box) and 1.5 × interquartile range (whiskers) of measurements across participants.
Among the infants, the three most common symptoms after their mother’s vaccination were pyrexia and cough (both 13.5%, 5/37), irritability and rhinorrhoea (10.8%, 4/37) and asthenia, vomiting and nasal congestion (8.1% each, 3/37) (figure 7A). A large proportion of the post-vaccination symptoms (42.3%, 11/26, 11 missing) resolved spontaneously (figure 7B). In contrast to the mothers, 57.7% (15/26) of all post-vaccination symptoms in infants were reported to have led to the involvement of a professional healthcare worker (7.7%, 2/26 as a remote professional consultation; 50.0%, 13/26 as a visit in an emergency room or physician visit). Severity as reported by the mothers was very mild to medium (93.5%, 29/31, 6 missing) in most cases, and severe in two cases (6.5%, 2/31), with none being reported as very severe (figure 7C).
Analyses based on individual participants (mothers and infants) instead of individual symptoms are presented in appendix table S5. Full results including counts and percentages of symptoms are presented in appendix table S6.
EQ VAS scores were high at BL in all study participants (median: 85, IQR: 75.5–92). At time points W4 and W6, a minimal decrease in wellbeing was reported by the study participants (median: 82, IQR: 76–89 and median: 81, IQR: 75–91, respectively). In the longer-term follow-up, self-reported wellbeing returned to baseline levels by M6 (median: 84, IQR: 74.5–89) (figure 7D).
From W4 to M6, a continuous increase in positive SARS-CoV-2 tests (either polymerase chain reaction [PCR] or rapid antigen test) reported by participants was found, providing evidence for recent infection. At W4, one participant (2.6%, 1/39) reported a positive SARS-CoV-2 test, whereas at M6, 52% (13/25) of the remaining study participants reached for follow-up reported having had a positive SARS-CoV-2 test (appendix figure S5). The median duration from vaccination to infection among those reporting the date of diagnosis of infection (n = 5) was 33 days (IQR: 32–35).
This study of immune responses and post-vaccination symptoms in lactating mothers showed a relevant induction of an immune response (anti-S IgA and anti-S IgG) in the blood plasma of lactating women with anti-S IgG being the dominant subtype. This finding is coherent with previous studies and underlines the effectiveness of the mRNA vaccines in inducing a relevant immune response against SARS-CoV-2 in blood plasma, even in special populations like lactating women [12, 14–16, 22, 38–40).
In breast milk, a relevant and continuous increase in anti-S IgG was found, whereas anti-S IgA levels showed only a minimal increase over time. These findings correlate partly with previous findings, comprehensively summarised by Hunagund et al., who described an initial increase in anti-S IgG 14–21 days after the first vaccination followed by an increase and peak 7 days after the second vaccination, remaining at an elevated level for at least 6 weeks [41]. Additionally, anti-S IgA levels in breast milk generally peaked at 14–18 days after the first vaccination with a slight increase after the second vaccination for a duration of one week, followed by a decline. Our findings concerning anti-S IgG levels in breast milk hence correlate with the present literature on the topic, while the mostly non-relevant increase in anti-S IgA levels appears to stand in contrast. However, some studies showed similar results concerning anti-S IgA levels in breast milk. Scrimin et al. found no anti-S IgA in the breast milk samples of vaccinated mothers [38]. Similarly, Demers-Mathieu et al. described no change in titres of anti-S IgA levels in vaccinated mothers [42]. Golan et al. showed no detectable anti-S IgA in the breast milk in 25% of their vaccinated study participants [22]. In a recently published systematic review conducted by Nicolaidou et al., the intramuscular application of the vaccines and an antibody class switch to anti-S IgG was proposed to be responsible for the low levels of anti-S IgA in breast milk of vaccinated mothers [43]. This fact is supported by other authors stating that the lack of a robust anti-receptor binding domain (RBD) IgA response in breast milk and blood of vaccinated women could be attributed to the intramuscular route of administration of the mRNA vaccines [10, 44]. Another possible reason for the minimal increase of breast milk anti-S IgA levels in our study might be a low rate of previous SARS-CoV-2 infections in the mothers, as the post-infection antibody response in human milk is IgA-dominant [45–48]. Furthermore, the duration of lactation (in the present study, mean lactation duration was 20.3 weeks) might have had an impact, as antibody concentrations in the milk were shown to be significantly higher in breastfeeding periods >24 months [49]. However, we did not find evidence for an association of lactation duration and antibody responses in our study.
Besides antibody titre, antibody function is a key consideration in evaluating vaccine-induced antibody protection for both mothers and infants. New variants are evolving in SARS-CoV-2 and RBD mutations have been associated with a higher capacity to evade the immune system [50]. The data from the present study shows neutralising antibodies in the blood against the wildtype variant two weeks after the second vaccination in all mothers, and against the delta and omicron variants with an antibody positivity of 100%, 97.4% and 64.1%, respectively. A strong correlation between anti-S IgG in the blood and the wildtype variant could be shown. We did not measure neutralisation capacity in breast milk. However, it is worth mentioning that other studies measuring neutralising antibody responses in breast milk observed a high variability in these responses [8, 10, 11, 16]. It remains unclear whether COVID-19 mRNA vaccine-induced antibodies in breast milk can confer immune protection to the infant. Although breast milk antibodies might not be sufficient to directly neutralise SARS-CoV-2, cumulative transfer through repeated feeds might provide the infant with effective neutralisation capacity. This transfer of immunity to infants has been part of previous studies. Yeo et al. found no neutralising antibodies in the serum of five infants after maternal vaccination [12]. Schwartz et al. found anti-S IgG in the oral mucosa of 60% of breastfed infants, but no detectable amounts of anti-SARS-CoV-2 antibodies were found in their circulation [51]. In an analysis conducted by Narayanaswamy et al., anti-RBD IgG and anti-RBD IgA were detected in 33% and 30% of infant stool samples [10].
No relevant difference in immune response between lactating women and matched non-lactating women was found in the present study. This is in accordance with findings of previous studies showing similar immunogenicity after COVID-19 vaccination between lactating individuals and non-lactating controls [8, 16, 45]. The study group under Atyeo et al. has shown that after a booster dose, spike-specific total IgG, IgM and IgA levels and neutralising titres against omicron reached levels comparable to those in non-lactating women [52].
The two vaccines BNT-162b2 and mRNA-1273 showed relatively similar immune response patterns in the present study. Antibody responses after mRNA-1273 vaccination appeared slightly elevated based on descriptive analyses. However, this difference could not be confirmed in adjusted regression analyses, which is likely due to the small sample size. At W6, mRNA-1273 seemed to induce higher levels of anti-S IgA in the blood compared to BNT-162b2. This correlates with the findings of a study conducted by Gray et al. which showed higher anti-S IgA responses in the blood of participants vaccinated with mRNA-1273 (2nd vaccination) than in those vaccinated with BNT-162b2 [45]. The observed elevated levels of antibody responses in breast milk in our study contrast with previous findings by Selma-Royo et al. showing no difference in antibody response (anti-S IgA, anti-S IgG) in breast milk when comparing BNT-162b2 and mRNA-1273 [47]. Yang et al. showed similar anti-S IgG titres in breast milk of participants receiving BNT-162b2 or mRNA-1273 [53]. Meanwhile, Juncker et al. found 1.5-fold higher anti-S IgG levels in breast milk of participants receiving mRNA-1273 in comparison to BNT-162b2, which corresponds well to our finding (1.52-fold higher). After 70 days, only participants vaccinated with mRNA-1273 showed detectable levels of anti-S IgA in breast milk [54]. Interestingly, neutralising antibody responses at W6 were also stronger for the wildtype and delta variant with mRNA-1273 compared to BNT-162b2, but not for omicron SARS-CoV-2.
Our analyses showed no association between the age of the mother, the lactation duration, the type of vaccine, the smoking status and the BMI and the antibody response in blood and breast milk – these findings were consistent with previous studies [8, 49, 55]. Exclusively, a prior positive SARS-CoV-2 test (PCR or rapid antigen test) showed a positive correlation in the antibody response in the present study. It has been shown in numerous studies that an infection with SARS-CoV-2 results in the production of antibodies against the virus [56]. A prior infection may have triggered an immune response that was later boosted by two mRNA vaccines, leading to higher antibody titres.
Post-vaccination symptoms turned out to be of mild to medium severity in most cases (in lactating mothers as well as in their breastfed infants). Except for one post-vaccination symptom, all post-vaccination symptoms experienced by the mothers could be handled by self-medication or resolved spontaneously. One of the study participants required medical care by a healthcare provider for the management of her post-vaccination symptoms and none required hospitalisation. This provides further evidence for the safety of the vaccines in lactating women, as described by previously published studies [18, 22, 46, 57].
In contrast, in 57.7% of cases of post-vaccination symptoms in infants, mothers sought medical care, though most were mild to moderate. Interpretation of these findings is complicated as the post-vaccination symptoms were greatly influenced by parental assessment and the fact that these post-vaccination symptoms may have also resulted from other early-life illnesses (not only maternal vaccination). Analysis of the health status during the vaccination period and during the follow-up period (6 months after first vaccination) showed no relevant difference between the first vaccination and 6 months after. At W6, a minimal decrease in median self-rated health status was detected – explainable by the occurrence of post-vaccination symptoms during the time of the vaccination period.
To our knowledge, this study is the first to assess the occurrence of SARS-CoV-2 infections in lactating women following an mRNA vaccination against SARS-CoV-2 in Switzerland. A continuous rise in self-reported positive SARS-CoV-2 tests (PCR or rapid antigen test) during the course of the present study was found. The study timeframe corresponded to a wave of high incidence of the omicron variant, spreading through the Swiss population rapidly because of its high transmissibility: according to the Swiss Science TaskForce, the omicron variant accounted for 67% of all sequenced probes in week 51 of 2021 [58]. This circumstance combined with the low neutralising capacity against omicron in the blood of participants at 6 weeks after baseline in comparison with wildtype and delta may explain the high rate of positive tests (52% of all study participants reported at least one positive test at M6).
Although SARS-CoV-2 infections, hospitalisations and deaths have decreased substantially to allow normal life to resume in most countries, this study’s findings remain important for healthcare providers and public health policies in managing future pandemic outbreaks.
First, post-vaccination symptoms seemed to appear in a similar pattern in the present study as in non-lactating individuals. As stated by the United States Food and Drug Administration (FDA) official briefing documents by Moderna and Pfizer on the website of the Centers for Disease Control and Prevention, the most common post-vaccination symptoms in persons ≥18 years of age were found to be injection site pain, fatigue and headache (in decreasing order, lactating individuals excluded) [59, 60]. Despite the small sample size, post-vaccination symptoms and immune responses in the blood in lactating women align with non-lactating women, supporting mRNA vaccine effectiveness in this specific subgroup.
Our study focused on immunoglobulins in human breast milk. However, it is important to consider that human breast milk is rich in other factors – immune cells such as monocytes/macrophages; neutrophils; cytotoxic, helper and regulatory T cells; natural killer (NK) cells; and B cells. These cells provide active immunity to neonates by their abilities to produce bioactive molecules such as lactoferrin, lysozyme, oligosaccharides, cytokines and others. Transfer of maternal lymphocytes via breast milk greatly assists the newborn’s immune system. It is postulated that lymphocytes survive the infant’s gastrointestinal tract and may be able to cross the infant’s gut mucosa and take up residence in infant tissues. As memory T cells are long-lived, this opens the possibility that milk-transferred protection might still be present in the infant even after weaning.
SARS-CoV-2 spike-specific T cells were detected in breast milk of mRNA-vaccinated mothers. Whether mRNA vaccines can elicit mammary MALT T and B cell responses that could be transferred to the infant via breast milk remains unknown [17].
To sum up, in the clinical setting, lactating women should be reassured and actively be informed about the safety and effectiveness of mRNA vaccination against SARS-CoV-2 during their lactation period. These findings could provide important data for establishing recommendations in future applications of mRNA vaccines in the vulnerable group of lactating women and their infants.
This study is based on a prospective evaluation of objective and self-reported measures over a total follow-up of 6 months. It provides a comprehensive evaluation of immune responses both in blood and breast milk, as well as data on post-vaccination symptoms in mothers and their infants, mothers’ longer-term health status and new SARS-CoV-2 infections after vaccination. It further includes a comparison with non-lactating women, providing further evidence for the interpretation of the study’s findings. Therefore, the current study not only contributes to the overall understanding of immune responses in lactating women and conferred protection in their infants as well as evidence-based clinical decision-making but also provides a foundation for future research in this field.
However, several limitations need to be considered when interpreting the findings of this study. First, the sample size is relatively small. However, despite difficulties with recruitment, we were able to enrol a comparable sample size to similar studies, even though there was more reluctance for study participation during the SARS-CoV-2 pandemic, especially in vulnerable individuals or their close relatives [61]. Second, selection bias may be present if those participating in our study are different to those not participating. Due to a lack of data on those not participating, we could not evaluate whether such differences exist. Meanwhile, the study population is rather homogeneous with respect to their educational level and health status (89.4%, 34/38, 2 missing, with an education level of higher technical school/college or university degree; 97.4%, 37/38, 2 missing, without comorbidities), as often observed in such studies [62]. This may limit the generalisability of our results. Third, five women did not complete at least two immunological assessments and thus were omitted from analyses. Additional drop-outs occurred after 6 weeks until 6 months of follow-up, which may have led to bias in the results related to the longer-term health status and post-vaccination infections. Fourth, the comparison group of non-lactating mothers may not have been fully comparable with the lactating mothers despite the use of a propensity score matching algorithm. While examined population characteristics were broadly comparable, there may still be other factors that may have biased the comparison. Similarly, there may be differences between participants who received BNT-162b2 and mRNA-1273. While it is unlikely that there are such differences that are also associated with differences in immune responses, the comparison may still suffer from confounding.
Fifth, interpretation of post-vaccination symptoms may be difficult as self-reported symptoms may also be attributable to causes other than the vaccination. Particularly for post-vaccination symptoms among children of study participants, it is likely that such symptoms occurred due to different circumstances (i.e. other diseases/infections) other than the vaccination of the mother.
Sixth, study participants could bring their milk collected on the same day, leading to a maximum time difference of 10 hours between milk and blood sample collection. The effect of the time difference on the outcome of the analysis however should be minimal, according to findings from Italianer et al.[63].
Lastly, the association analyses were adjusted for a limited set of potential confounders. Further residual confounding that influenced the results may have been present. However, it is unlikely that this would have altered the primary conclusions of this study.
In the present study, immune responses in blood plasma and breast milk were found to be anti-S IgG-dominant. Neutralising capacity against the wildtype and delta variants was high, and no difference in antibody and neutralising antibody responses between lactating and non-lactating women was found. Post-vaccination symptoms were found to be largely of very mild to medium severity. Health status remained at a high level up to 6 months, with a short-term decrease around 4–6 weeks. Only mild infections after vaccination with a monovalent mRNA vaccine were reported. To conclude, the present data suggests that mRNA vaccinations against SARS-CoV-2 may be an effective and safe way to protect lactating women from a severe infection with SARS-CoV-2 and that an immune response in the breast milk is effectively triggered by vaccinating the mother. Further research needs to be conducted in order to analyse the exact mechanism of the immune transfer to infants by breastfeeding more precisely.
The datasets and code used in this study are available upon reasonable request from the corresponding author.
The authors thank Medela Schweiz for their contribution to the project by lending us a Symphony® breast milk pump and supplying the necessary milk bags and accessories. We also thank the study participants for their important contribution.
Author contributions: Conceptualisation: PW, JH and JSF; Methodology: JH, AF, JSF, DM, PW, DLC, CPy and CPe; Formal analysis: DM, PW and OB; Investigation: PW, JH and DM; Resources: PW, JH and DLC; Data curation: DM and PW; Writing – Original draft: PW, JH; Writing – Review and editing: PW, JH, OB, JK, DLC, OEJ, KDZ, CPy, CPe, DM, AF and JSF; Visualisation: PW and DM; Project administration: PW, JK, JH and JF; Funding acquisition: JSF; Supervision: DM and JSF.
This study is part of the Corona Immunitas research network, coordinated by the Swiss School of Public Health (SSPH+) and funded by fundraising of SSPH+ including funds from the Swiss Federal Office of Public Health and private funders (in compliance with the ethical funding policy of SSPH+), by funds of the Swiss Cantons of Vaud, Zurich and Basel and by institutional funds of universities. DM received funding by the University of Zurich Postdoc Grant, grant no. FK-22-053.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. – JSF received an allowance fee from the Federal commission for vaccination recommendation (EKIF) and research grants (paid to institution) from Gilead Sciences, MSD and ViiV Healthcare. – No other potential conflict of interest related to the content of this manuscript was disclosed.
1. Modi N, Ayres-de-Campos D, Bancalari E, Benders M, Briana D, Di Renzo GC, et al. Equity in coronavirus disease 2019 vaccine development and deployment. Am J Obstet Gynecol. 2021 May;224(5):423–7.
2. Atyeo C, Alter G. The multifaceted roles of breast milk antibodies [Cited]. Cell. 2021 Mar;184(6):1486–99.
3. Marchand G, Patil AS, Masoud AT, Ware K, King A, Ruther S, et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes up to June 3, 2021. AJOG Glob Rep. 2022 Feb;2(1):100049.
4. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al.; for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis [Cited]. BMJ. 2020 Sep;370:m3320.
5. Hamid S, Woodworth K, Pham H, Milucky J, Chai SJ, Kawasaki B, et al.; COVID-NET Surveillance Team. COVID-19-Associated Hospitalizations Among U.S. Infants Aged <6 Months - COVID-NET, 13 States, June 2021-August 2022. MMWR Morb Mortal Wkly Rep. 2022 Nov;71(45):1442–8.
6. Perez SE, Luna Centeno LD, Cheng WA, Marentes Ruiz CJ, Lee Y, Congrave-Wilson Z, et al. Human Milk SARS-CoV-2 Antibodies up to 6 Months After Vaccination. Pediatrics. 2022 Feb;149(2):e2021054260.
7. Golan Y, Ilala M, Li L, Gay C, Hunagund S, Lin CY, et al. Milk antibody response after 3rd COVID-19 vaccine and SARS-CoV-2 infection and implications for infant protection. iScience. 2023 Aug;26(10):107767.
8. Gonçalves J, Juliano AM, Charepe N, Alenquer M, Athayde D, Ferreira F, et al. Secretory IgA and T cells targeting SARS-CoV-2 spike protein are transferred to the breastmilk upon mRNA vaccination. Cell Rep Med. 2021 Dec;2(12):100468.
9. Young BE, Seppo AE, Diaz N, Rosen-Carole C, Nowak-Wegrzyn A, Cruz Vasquez JM, et al. Association of Human Milk Antibody Induction, Persistence, and Neutralizing Capacity With SARS-CoV-2 Infection vs mRNA Vaccination. JAMA Pediatr. 2022 Feb;176(2):159–68.
10. Narayanaswamy V, Pentecost BT, Schoen CN, Alfandari D, Schneider SS, Baker R, et al. Neutralizing Antibodies and Cytokines in Breast Milk After Coronavirus Disease 2019 (COVID-19) mRNA Vaccination. Obstet Gynecol. 2022 Feb;139(2):181–91.
11. Rosenberg-Friedman M, Kigel A, Bahar Y, Werbner M, Alter J, Yogev Y, et al. BNT162b2 mRNA vaccine elicited antibody response in blood and milk of breastfeeding women. Nat Commun. 2021 Oct;12(1):6222.
12. Yeo KT, Chia WN, Tan CW, Ong C, Yeo JG, Zhang J, et al. Neutralizing Activity and SARS-CoV-2 Vaccine mRNA Persistence in Serum and Breastmilk After BNT162b2 Vaccination in Lactating Women. Front Immunol. 2022 Jan;12:783975.
13. Lechosa-Muñiz C, Paz-Zulueta M, Mendez-Legaza JM, Irure-Ventura J, Cuesta González R, Calvo Montes J, et al. Induction of SARS-CoV-2-Specific IgG and IgA in Serum and Milk with Different SARS-CoV-2 Vaccines in Breastfeeding Women: A Cross-Sectional Study in Northern Spain. Int J Environ Res Public Health. 2021 Aug;18(16):8831.
14. Charepe N, Gonçalves J, Juliano AM, Lopes DG, Canhão H, Soares H, et al. COVID-19 mRNA vaccine and antibody response in lactating women: a prospective cohort study. BMC Pregnancy Childbirth. 2021 Sep;21(1):632.
15. Jakuszko K, Kościelska-Kasprzak K, Żabińska M, Bartoszek D, Poznański P, Rukasz D, et al. Immune Response to Vaccination against COVID-19 in Breastfeeding Health Workers. Vaccines (Basel). 2021 Jun;9(6):663.
16. Collier AY, McMahan K, Yu J, Tostanoski LH, Aguayo R, Ansel J, et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA. 2021 Jun;325(23):2370–80.
17. Shook LL, Fallah PN, Silberman JN, Edlow AG. COVID-19 Vaccination in Pregnancy and Lactation: Current Research and Gaps in Understanding. Front Cell Infect Microbiol. 2021 Sep;11:735394.
18. Bertrand K, Honerkamp-Smith G, Chambers CD. Maternal and Child Outcomes Reported by Breastfeeding Women Following Messenger RNA COVID-19 Vaccination. Breastfeed Med. 2021 Sep;16(9):697–701.
19. Low JM, Gu Y, Ng MS, Amin Z, Lee LY, Ng YP, et al. Codominant IgG and IgA expression with minimal vaccine mRNA in milk of BNT162b2 vaccinees. NPJ Vaccines. 2021 Aug;6(1):105.
20. McLaurin-Jiang S, Garner CD, Krutsch K, Hale TW. Maternal and Child Symptoms Following COVID-19 Vaccination Among Breastfeeding Mothers. Breastfeed Med. 2021 Sep;16(9):702–9.
21. Stafford LS, Valcarce V, Henry M, Neu J, Parker L, Mueller M, et al. Detection of SARS-CoV-2 IgA and IgG in human milk and breastfeeding infant stool 6 months after maternal COVID-19 vaccination. J Perinatol Off J Calif Perinat Assoc.; 2023.
22. Golan Y, Prahl M, Cassidy AG, Gay C, Wu AH, Jigmeddagva U, et al. COVID-19 mRNA Vaccination in Lactation: Assessment of Adverse Events and Vaccine Related Antibodies in Mother-Infant Dyads. Front Immunol. 2021 Nov;12:777103.
23. CoVariants [Internet] [cited 2023 Apr 17]. Available from https://covariants.org/
24. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001 Jul;33(5):337–43.
26. Frei A, Kaufmann M, Amati R, Dettwiler AB, von Wyl V, Annoni AM, et al. Development of hybrid immunity during a period of high incidence of infections with Omicron subvariants: A prospective population based multi-region cohort study. medRxiv. 2022;2022.10.14.22281076. doi: https://doi.org/10.1101/2022.10.14.22281076
27. Menges D, Zens KD, Ballouz T, Caduff N, Llanas-Cornejo D, Aschmann HE, et al. Heterogenous humoral and cellular immune responses with distinct trajectories post-SARS-CoV-2 infection in a population-based cohort. Nat Commun. 2022 Aug;13(1):4855.
28. Fenwick C, Croxatto A, Coste AT, Pojer F, André C, Pellaton C, et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol. 2021 Jan;95(3):e01828-20.
29. Fenwick C, Turelli P, Pellaton C, Farina A, Campos J, Raclot C, et al. A high-throughput cell- and virus-free assay shows reduced neutralization of SARS-CoV-2 variants by COVID-19 convalescent plasma. Sci Transl Med. 2021 Aug;13(605):eabi8452.
30. Bürzle O, Menges D, Maier JD, Schams D, Puhan MA, Fehr J, et al. Adverse effects, perceptions and attitudes related to BNT162b2, mRNA-1273 or JNJ-78436735 SARS-CoV-2 vaccines: population-based cohort. NPJ Vaccines. 2023 Apr;8(1):61.
31. Wei J, Stoesser N, Matthews PC, Ayoubkhani D, Studley R, Bell I, et al.; COVID-19 Infection Survey team. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021 Sep;6(9):1140–9.
32. Shrotri M, Fragaszy E, Nguyen V, Navaratnam AM, Geismar C, Beale S, et al. Spike-antibody responses to COVID-19 vaccination by demographic and clinical factors in a prospective community cohort study. Nat Commun. 2022 Oct;13(1):5780.
33. Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine [Internet]. EClinicalMedicine. 2021 Jun;36:100928. [cited 2025 May 18]
34. Notarte KI, Ver AT, Velasco JV, Pastrana A, Catahay JA, Salvagno GL, et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: a systematic review. Crit Rev Clin Lab Sci. 2022 Sep;59(6):373–90.
35. Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Softw. 2017;82(13):1–26. doi: https://doi.org/10.18637/jss.v082.i13
36. Bland M. An Introduction to Medical Statistics. 4th ed. UK: Oxford University Press; 2015.
37. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria 2017. [Internet] https://www.R-project.org/
38. Scrimin F, Campisciano G, Comar M, Ragazzon C, Davanzo R, Quadrifoglio M, et al. IgG and IgA Antibodies Post SARS-CoV-2 Vaccine in the Breast Milk and Sera of Breastfeeding Women. Vaccines (Basel). 2022 Jan;10(1):125.
39. Atyeo C, DeRiso EA, Davis C, Bordt EA, DeGuzman RM, Shook LL, et al. COVID-19 mRNA vaccines drive differential Fc-functional profiles in pregnant, lactating, and non-pregnant women. BioRxiv Prepr Serv Biol. 2021;2021.04.04.438404. doi:
40. Juncker HG, Mulleners SJ, van Gils MJ, Bijl TP, de Groot CJ, Pajkrt D, et al. Comparison of SARS-CoV-2-Specific Antibodies in Human Milk after mRNA-Based COVID-19 Vaccination and Infection. Vaccines (Basel). 2021 Dec;9(12):1475.
41. Hunagund S, Golan Y, Asiodu IV, Prahl M, Gaw SL. Effects of Vaccination Against Influenza, Pertussis, and COVID-19 on Human Milk Antibodies: Current Evidence and Implications for Health Equity. Front Immunol. 2022 Jul;13:910383.
42. Demers-Mathieu V, Hakansson AP, Hall S, Lavangnananda S, Fels S, Medo E. Functional Antibodies Against SARS-CoV-2 Receptor Binding Domain Variants with Mutations N501Y or E484K in Human Milk from COVID-19-Vaccinated, -Recovered, and -Unvaccinated Women. Breastfeed Med. 2022 Feb;17(2):163–72.
43. Nicolaidou V, Georgiou R, Christofidou M, Felekkis K, Pieri M, Papaneophytou C. Detection of SARS-CoV-2-Specific Antibodies in Human Breast Milk and Their Neutralizing Capacity after COVID-19 Vaccination: A Systematic Review. Int J Mol Sci. 2023 Feb;24(3):2957.
44. Joseph NT, Dude CM, Verkerke HP, Irby LS, Dunlop AL, Patel RM, et al. Maternal Antibody Response, Neutralizing Potency, and Placental Antibody Transfer After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Obstet Gynecol. 2021 Aug;138(2):189–97.
45. Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021 Sep;225(3):303.e1–17.
46. Perl SH, Uzan-Yulzari A, Klainer H, Asiskovich L, Youngster M, Rinott E, et al. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA. 2021 May;325(19):2013–4.
47. Selma-Royo M, Bäuerl C, Mena-Tudela D, Aguilar-Camprubí L, Pérez-Cano FJ, Parra-Llorca A, et al. Anti-SARS-CoV-2 IgA and IgG in human milk after vaccination is dependent on vaccine type and previous SARS-CoV-2 exposure: a longitudinal study. Genome Med. 2022 Apr;14(1):42.
48. Fox A, Marino J, Amanat F, Krammer F, Hahn-Holbrook J, Zolla-Pazner S, et al. Robust and Specific Secretory IgA Against SARS-CoV-2 Detected in Human Milk. iScience. 2020 Nov;23(11):101735.
49. Romero Ramírez DS, Lara Pérez MM, Carretero Pérez M, Suárez Hernández MI, Martín Pulido S, Pera Villacampa L, et al. SARS-CoV-2 Antibodies in Breast Milk After Vaccination. Pediatrics. 2021 Nov;148(5):e2021052286.
50. Kudriavtsev AV, Vakhrusheva AV, Novosеletsky VN, Bozdaganyan ME, Shaitan KV, Kirpichnikov MP, et al. Immune Escape Associated with RBD Omicron Mutations and SARS-CoV-2 Evolution Dynamics. Viruses. 2022 Jul;14(8):1603.
51. Schwartz A, Nir O, Toussia-Cohen S, Leibovich L, Strauss T, Asraf K, et al. Presence of SARS-CoV-2 antibodies in lactating women and their infants following BNT162b2 messenger RNA vaccine. Am J Obstet Gynecol. 2021 Nov;225(5):577–9.
52. Atyeo C, Shook LL, Nziza N, Deriso EA, Muir C, Baez AM, et al. COVID-19 booster dose induces robust antibody response in pregnant, lactating, and nonpregnant women. Am J Obstet Gynecol. 2023 Jan;228(1):68.e1–12.
53. Yang X, Fox A, DeCarlo C, Norris C, Griffin S, Wedekind S, et al. Comparative Profiles of SARS-CoV-2 Spike-Specific Human Milk Antibodies Elicited by mRNA- and Adenovirus-Based COVID-19 Vaccines. Breastfeed Med. 2022 Aug;17(8):638–46.
54. Juncker HG, Mulleners SJ, Ruhé EJ, Coenen ER, Bakker S, van Doesburg M, et al. Comparing the human milk antibody response after vaccination with four COVID-19 vaccines: A prospective, longitudinal cohort study in the Netherlands. EClinicalMedicine. 2022 May;47:101393.
55. Trofin F, Nastase EV, Iancu LS, Constantinescu D, Cianga CM, Lunca C, et al. Anti-RBD IgA and IgG Response and Transmission in Breast Milk of Anti-SARS-CoV-2 Vaccinated Mothers. Pathogens. 2022 Feb;11(3):286.
56. Scourfield DO, Reed SG, Quastel M, Alderson J, Bart VM, Teijeira Crespo A, et al.; Oxford-Cardiff COVID-19 Literature Consortium. The role and uses of antibodies in COVID-19 infections: a living review. Oxf Open Immunol. 2021 Jan;2(1):iqab003.
57. Romero Ramírez DS, Suárez Hernández MI, Fernández Vilar AM, Rivero Falero M, Reyes Millán B, González Carretero P, et al. Evaluation of Adverse Effects in Nursing Mothers and Their Infants After COVID-19 mRNA Vaccination. Breastfeed Med. 2022 May;17(5):412–21.
58. Lagebeurteilung E. 3. Januar 2022 – Swiss National COVID-19 Science Task Force [Internet]. [cited 2023 Apr 25]. Available from: https://sciencetaskforce.ch/epidemiologische-lagebeurteilung-3-januar-2022/
59. Moderna COVID-19 Vaccine’s Reactions and Adverse Events | CDC [Internet]. 2022 [cited 2023 May 22]. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
60. Pfizer-BioNTech COVID-19 Vaccine Reactions & Adverse Events | CDC [Internet]. 2023 [cited 2023 May 22]. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
61. Sathian B, Asim M, Banerjee I, Pizarro AB, Roy B, van Teijlingen ER, et al. Impact of COVID-19 on clinical trials and clinical research: A systematic review. Nepal J Epidemiol. 2020 Sep;10(3):878–87.
62. Spitzer S. Biases in health expectancies due to educational differences in survey participation of older Europeans: it’s worth weighting for. Eur J Health Econ. 2020 Jun;21(4):573–605.
63. Italianer MF, Naninck EF, Roelants JA, van der Horst GT, Reiss IK, Goudoever JB, et al. Circadian Variation in Human Milk Composition, a Systematic Review [Internet]. Nutrients. 2020 Aug;12(8):2328.
The appendix is available in the PDF version of the article and the supplemenary files can be downloaded as a separate file at https://doi.org/10.57187/s.4207.