Treating Menière’s disease with rimegepant
DOI: https://doi.org/https://doi.org/10.57187/s.4147
Stefan C. A. Hegemannab,
Angela Schellcd
a Faculty of Medicine, University of Zurich, Zurich, Switzerland
b Balance Clinic Zurich, Zurich, Switzerland
c Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital Mannheim,
Mannheim, Germany
d Heidelberg University, Heidelberg, Germany
Summary
A recent
hypothesis states that Menière’s disease is caused by inappropriate expression,
i.e. enhanced release of the neurotransmitter calcitonin gene-related peptide. Here,
we tested this hypothesis by administering rimegepant, a new calcitonin
gene-related peptide antagonist approved for the acute treatment of migraine
and for the prevention of episodic migraine, to six patients with both Menière’s
disease and migraine. Two patients received the first dose of 75 mg rimegepant
to treat an acute attack of Menière’s disease. One of these two plus the
remaining four patients were treated with 75 mg rimegepant every other day for
secondary prevention. One patient developed an allergic reaction after the
first administration and was excluded from further treatment. In the two
patients treated during acute Menière’s disease, symptoms were relieved and
resolved about 30 min earlier than migraine symptoms. While all five patients
had reduced migraine, all completely resolved Menière's symptoms on preventive
therapy with rimegepant for up to eight months. These results support the idea
that calcitonin gene-related peptide is linked to the pathogenesis of Menière’s
disease and suggest that inhibition of calcitonin gene-related peptide signalling
may represent a promising therapeutic option for Menière’s disease patients.
Introduction
Menière's
disease is an inner-ear disorder characterised by attacks of vertigo lasting 20
minutes to 12 hours, accompanied by fluctuating low- and mid-frequency hearing
loss, increased tinnitus and/or ear pressure. It has an estimated prevalence
ranging from 3.5/100,000 adults in Japan [1] to 513/100,000 adults in southern Finland [2] or between 0.04% and 0.51%, respectively.
A
recent study in California [3] reported
190/100,000 or 0.19%. The prevalence of Menière’s disease appears to be lower
in Asian countries, although there are few epidemiological studies. Kim et al.
reported that the prevalence of Menière’s disease in Korea increased from 0.04%
in 2013 to 0.15% in 2017 [4].
Different
prevalences have also been reported for migraine. According to Burch et al. [5], the
global prevalence of migraine is 15%,
but with variations from 9% in the Western Pacific (China), 12% in the USA, 25–33%
in Southeast Asia to 35% in the European Union and Nepal. In a very recent
article on the prevalence of migraine in Asia, it was estimated to be 13.8% in
Asian countries.
Menière’s
disease and migraine are cross-correlated, with a 10% prevalence of migraine in
Korean patients with Menière’s disease compared with only 3.5% in a matched
control group [4]. The 3.5% migraine figure
is much lower than the prevalences mentioned above. Thus, the risk of migraine
would be 2.9 times higher in patients with Menière’s disease than in subjects
without Menière’s disease. Ghavami et al. [6]
reported that 51% of Menière’s disease patients also suffer from migraine.
Thus, it is generally accepted that there is a very high correlation between Menière’s
disease and migraine.
There also
exists a vestibular migraine, first listed in the 3rd edition of the
International Classification of Headache Disorders (ICHD-III) in 2018 [7]. As mentioned
in the previous publication
on calcitonin gene-related peptide as the cause of Menière’s disease, according
to Tabet and Saliba [8] “there are no
known definitive diagnostic tests that can reliably distinguish the two
conditions”. Nevertheless, we describe the typical combination of clinical Menière’s
disease symptoms in patients with migraine as two diseases.
Although its
first description dates back 164 years [9],
the aetiology and pathophysiology of Menière’s disease is still poorly
understood and no evidence-based effective oral or intravenous treatment is available.
Transtympanic or intratympanic injections of steroids were thought to be
promising according to a 2011 Cochrane review [10]
that included only one study, but the most recent Cochrane meta-analysis [11] of 10
included trials, all using
dexamethasone, concluded that intratympanic corticosteroids may make little or
no difference in the number of people reporting improvement in their vertigo at
6 to 12 months or more of follow-up.
Another
therapy, mostly third-line, is endolymphatic sac surgery. However, there is
considerable controversy about its efficacy and whether the sac should be
decompressed, opened or shunted [12].
Only
destructive procedures such as vestibular neurotomy, neurectomy,
labyrinthectomy or transtympanic injections of aminoglycosides, most commonly gentamicin,
have a significant effect on reducing the frequency of vertigo in Menière’s
disease [13]. However, these procedures carry
a significant risk of hearing loss.
Since all
or almost all patients with Menière’s disease show endolymphatic hydrops [14], it
is often assumed that Menière’s
disease is caused by endolymphatic hydrops. However, guinea pigs with endolymphatic
hydrops, induced by resection of the endolymphatic sac, do not develop Menière attacks
[15]. The only symptom which seemed to be
closely correlated with endolymphatic hydrops was low-frequency hearing loss [16],
but this correlation has also been questioned
[17].
Various
changes occur in the inner ears of Menière’s disease patients, including signs
of inflammation [18] and decreased blood
supply [19], and various causes, such as
viral [20] or autoimmune inflammation [18] or allergies [21, 22], have been suspected
of causing Menière attacks. Hence, Menière’s
disease has recently been called Menière’s syndrome, because it is suspected
that its symptoms may be due to disparate causes and aetiologies. In 1992,
Cutrer and Baloh first suspected a common involvement of neuropeptides like
“substance P, neurokinin A and calcitonin gene-related peptide” in Menière’s disease and migraine [23].
They also speculated that “calcitonin
gene-related peptide and possibly other neuropeptides released from trigeminal
afferents and vestibular efferents may increase excitability of the inner ear
vestibular receptors”.
In 2021, calcitonin
gene-related peptide was suspected of being the main cause of Menière’s disease
[24], because it is one of the main
transmitters in cochlear and vestibular efferents, thus explaining simultaneous
cochlear and vestibular symptoms. Calcitonin gene-related peptide is a potent
vasodilator and endolymphatic hydrops may be induced by dilation of capillaries
in the stria vascularis, which may induce endolymphatic hydrops like oedema
induced in other parts of the body. Calcitonin gene-related peptide is also
known to induce neurogenic inflammation [25, 26],
explaining the inflammatory signs described in the inner ears of Menière’s
disease patients. Later, Menière’s disease as well as isolated cochlear or
vestibular symptoms were suspected of being caused by inner ear migraine [27]. But
Frank et al. did not focus on calcitonin
gene-related peptide. Since we are still unable to distinguish Menière’s
disease from vestibular migraine as argued in the hypothesis that calcitonin
gene-related peptide causes Menière’s disease [24],
which is supported by other authors [6, 27, 28], we also see Menière’s disease as
a special
form of inner ear migraine. In their recent review, Baron and Steenerson [29] argue
that “given the frequent overlap of these two conditions, and the difficulty in
treating Menière’s disease, a patient with Menière’s disease who also has a
history of migrainous headaches could reasonably be trialled with targeted calcitonin
gene-related peptide therapy”.
The above
suggests that calcitonin gene-related peptide may be causally involved in Menière’s
disease and provides a rationale for treating Menière’s disease with calcitonin
gene-related peptide antagonists, which have been available since 2018.
However, the first calcitonin gene-related peptide antagonists (erenumab,
fremanezumab, galcanezumab, eptinezumab) are large monoclonal antibodies that
cross the blood-brain barrier only by 0.1–0.3% owing to their molecular weight
of 180–200 kDa [30]. Nevertheless, they work for the prevention of migraine because
the ganglion of the trigeminal nerve is situated outside the blood-brain
barrier [31]. Unfortunately, it has not yet been investigated whether they may
cross the blood-labyrinth barrier. Interestingly, severe disturbance of the blood-labyrinth
barrier in the cochlea has been reported in older patients with endolymphatic
hydrops [32] and a breakdown of the blood-labyrinth
barrier has recently been described in Menière’s disease [33].
More
recently, small molecule calcitonin gene-related peptide antagonists, so-called
gepants, have become available. Despite their small size of only 0.5–0.6 kDa [29],
these substances cross the blood-brain
barrier to a limited extent of about 1–3%. The spinal fluid concentrations of telcegepant
and olcegepant were only about 1.3% of plasma concentration [34], but
Hostetler et al. determined the in vivo cerebrospinal fluid / plasma ratio to
be between 2% and 3% and showed the highest level of binding in the cerebellum,
brainstem and meninges [35]. The 1–3% of plasma level in the CSF measured
for gepants is still small, but at least an order of magnitude higher than the
0.1–0.3% reported for galcanezumab [30]. The distribution volume in the inner ear
is
unknown. But even if the gepant molecules do not cross the normal blood-labyrinth
barrier, they may still reach the inner ears in Menière’s disease patients
because of the breakdown of the blood-labyrinth barrier in Menière’s disease [33].
Alternatively,
they could also reach the inner ear through the cochlear and vestibular
efferents, which arise from the superior olive in the brainstem, since calcitonin
gene-related peptide releasing neurons have been described in peri-olivary
locations [36]. We recommend the very recent review about calcitonin
gene-related peptide distribution and its effects on cochlear and vestibular
systems by Baron and Steenerson [29]. Nevertheless,
we assumed that gepants could reach the inner ear either way and hypothesise
that they may prevent Menière attacks.
Our first
goal was to determine in a small pilot study whether treating patients with Menière’s
disease and migraine with gepants would support the hypotheses that
- Menière’s disease is caused by calcitonin
gene-related peptide, and that
- gepants can reach the inner ear and
prevent Menière attacks.
Positive
results would support our second goal of organising a large prospective randomised
placebo-controlled clinical trial to test whether this drug will be the first
evidence-based effective oral treatment for Menière’s disease.
Methods
We describe
a series of six Menière’s disease patients who were treated with rimegepant, a
new medication for treating migraine. Treatment was in accordance with the Declaration
of
Helsinki as revised in 2023. An Institutional Review Board approval was
not required according to Kantonale Ethik Kommission Zurich
(BASEC_Req-2024.00515).
Patients with definite Menière’s
disease according to the 2015 Bárány criteria [37] were instructed to fill in a diary
listing
all migraine and Menière’s disease symptoms and their duration. In addition to
an intensive history of all symptoms of migraine and Menière’s disease, audiograms,
bilateral bithermal vestibular testing (calorics), video head impulse testing
(vHIT) as well as cervical and ocular vestibular evoked myogenic potentials (cVEMP,
oVEMP), subjective visual vertical (SVV) and fundus photography were performed.
All anonymised test results will be sent to interested readers on request.
In addition, patients were
specifically interviewed about migraine symptoms such as headache severity,
duration, and location, as well as associated symptoms such as increased
sensitivity to noise and light, and aura symptoms. If the patient met the ICHD-III
diagnostic criteria, a diagnosis of migraine was made [7]. If both diagnoses were
confirmed, he/she was asked to
participate in this pilot study. With simultaneous appearance of symptoms of Menière’s
disease and migraine
in 50% or more the patients also fulfilled the Bárány criteria for vestibular
migraine and discrimination between both diseases was not possible. All patients were
also asked about
previous treatments/medications to prevent migraine and/or Menière attacks and
their effects.
We first
wanted to treat patients with both Menière’s disease and migraine to avoid
off-label therapy. We enrolled six patients with the following history of Menière’s
disease: at least 3 Menière attacks per month in the last 6 months and rotatory
vertigo of at least 3 hours in most Menière attacks during the last 6 months.
As mentioned above, differentiation between Menière’s disease and vestibular
migraine was not possible due to the high overlap of symptoms in both diseases.
Even the very recent and thorough review by Baron and Steenerson does not
provide a specific test that clearly discriminates between the two diseases.
When we saw
the very impressive effect in the first four patients, we decided to also try
the same medication in one patient with Menière’s disease only. Two patients
were instructed to start their preventive medication at the beginning of their
next Menière’s attack to see if there was also an acute effect on the duration
of Menière’s symptoms.
Results
Six
patients agreed to take part in this pilot study. Five had migraine and Menière’s
disease and one (#6) had had migraine between the ages of 20 and 60 years,
approximately, and Menière’s disease since her 74th year of life. Table 1 shows
their characteristics.
Table 1Characteristics of patients
included in the study.
| Pat. # |
Sex |
Age at inclusion in study |
Age at onset / end of migraine |
Age at onset of Menière’s disease |
| 1 |
Female |
76 yr |
67 yr |
65 yr |
| 2 |
Female |
57 yr |
25 yr |
51 yr |
| 3 |
Female |
45 yr |
17 yr |
39 yr |
| 4 |
Male |
43 yr |
16 yr |
34 yr |
| 5 |
Male |
62 yr |
41 yr |
41 yr |
| 6 |
Female |
82 yr |
20/60 yr |
74 yr |
As
expected, in all five patients treated with rimegepant (Vydura®) every other day, the frequency of migraine attacks was significantly
reduced after the first dose of rimegepant. In addition, all five patients have
been free of Menière attacks since their first dose of rimegepant. After five
months, one of them (patient #2) was unable to continue the rimegepant regimen due
to a supply shortage. During the 3-week period without rimegepant, she had 8
migraine attacks and 6 Menière attacks; after the supply was restored, she had
no further migraine attacks or Menière attacks. One of the five patients was free
of Menière attacks for more than 8 months – however it is very likely that this
would also have been the case for patient #2 if her supply had not been interrupted.
Patient #3 has been free of Menière attacks for 7 months; patient #5 for more
than 6 months; and patient #6 for almost 5 months. Table 2 shows the duration
of freedom from Menière attacks and other descriptive data.
One patient
(#4 in table 1) developed a severe skin rash on the whole body as well as pain
in the right upper abdomen and diarrhoea. The medication was immediately
stopped.
Table 2List of patients, their estimated
frequencies of Menière and migraine attacks and their reduced frequencies after
starting rimegepant up to the time of the last data collection.
| Pat. # |
Estimated frequency of Menière
attacks (6 months ahead) |
Estimated frequency of migraine
attacks (6 months ahead) |
First rimegepant use (date) |
Duration of rimegepant use
(months) |
Number of Menière attacks
while using rimegepant |
Number of migraine
attacks while using rimegepant |
Previous treatments without success |
| 1 |
10–12 |
6–12 |
2023-11-08 |
>8 |
0 |
3 |
Betahistine,
sumatriptan, intratympanic steroids |
| 2 |
12–14 |
12–22 |
2023-11-12
(interrupted from 2024-04-12 to 2024-05-03)* |
>8 |
0 |
3 |
Betahistine, sumatriptan,
intratympanic steroids, topiramat, Mg++, erenumab, metoprolol |
| 3 |
7–9 |
8–9 |
2023-12-15 |
>7 |
0 |
2 |
Betahistine,
intratympanic steroids, metoprolol, erenumab |
| 4 |
12–16 |
12–16 |
2024-01-05 |
1 tablet
once |
12–16, no
rimegepant |
12–16, no
rimegepant |
Betahistine,
Mg++, riboflavin, intratympanic steroids, erenumab, fremanezumab |
| 5 |
8–10 |
10–20 |
2024-02-02 |
>6 |
0 |
0 |
Betahistine, Mg++,
riboflavin, intratympanic steroids, sumatriptan |
| 6 |
12–15 |
No
migraine since 22 years |
2024-03-12 |
>4 |
0 |
0 |
Betahistine,
intratympanic steroids, mirtazapine |
Patient #4
took the first dose of rimegepant at the start of a Menière attack with
simultaneous migraine. Although he showed the described allergic symptoms, his
Menière symptoms – usually lasting for 6–12 hours – started to improve after
about one hour and had completely resolved at 1.5 hours. Interestingly, his
migraine symptoms also improved, but about half an hour later than his Menière symptoms.
Table 3Report
of patient #4 (according to table 1) with effects of rimegepant on symptoms
during a Menière/migraine attack.
| Patient
#4 |
Date of Menière/migraine attack: 2024-01-05 |
| Symptom |
Strength
(0–10) |
Affected ear (right/ left/both) |
Time
when symptoms started |
Time
when rimegepant taken |
Time when symptoms started reducing |
Time
when symptoms resolved |
| Tinnitus |
9–10 |
Right |
15:45 |
15:50 |
16:40 |
18:30 |
| Pressure feeling |
7 |
Right |
15:50 |
16:40 |
17:30 |
| Hearing loss |
9–10 |
Right |
15:50 |
16:40 |
17:30 |
| Rotatory vertigo |
8 |
|
15:50 |
16:40 |
17:30 |
| Headache |
8 |
|
15:50 |
17:30 |
18:00 |
| Noise sensitivity |
8 |
Both |
15:50 |
17:30 |
18:00 |
| Light sensitivity |
8 |
|
15:50 |
17:30 |
18:00 |
Table 4Report
of patient #5 (according to table 1) with effects of rimegepant on symptoms
during a Menière/migraine attack.
| Patient #5 |
Date of Menière/migraine attack: 2024-02-02 |
| Symptom |
Strength
(0–10) |
Affected ear (right/left/both) |
Time when symptoms started |
Time when rimegepant taken |
Time when symptoms started reducing |
Time when symptoms resolved |
| Tinnitus |
7 |
Left |
9:39 |
9:53 |
10:30 |
11:00 |
| Pressure feeling |
5 |
Pulse for about 1s |
9:39 |
|
9:39 |
| Hearing loss |
? |
Left |
9:39 |
10:30 |
11:00 |
| Rotatory vertigo |
5 |
|
9:39 |
10:30 |
11:00 |
| Headache |
4 |
|
9:39 |
Sleep at 13:30 |
15:40 |
| Noise sensitivity |
|
Both |
9:39 |
|
15:30 |
| Light sensitivity |
4 |
|
9:39 |
|
15:30 |
Patient #5
also took the first dose 14 minutes after the start of Menière symptoms as well
as migrainous symptoms (see table 2). While his attacks of spinning vertigo usually
lasted 3–6 hours with increased tinnitus and hearing loss usually lasting much
longer than vertigo, he used sumatriptan spray before to reduce his symptoms. This
reduced the duration of symptoms, especially of vertigo, to 30–60 minutes. All
Menière symptoms started to improve 37 minutes after he had taken rimegepant and
had completely resolved at 1 hour.
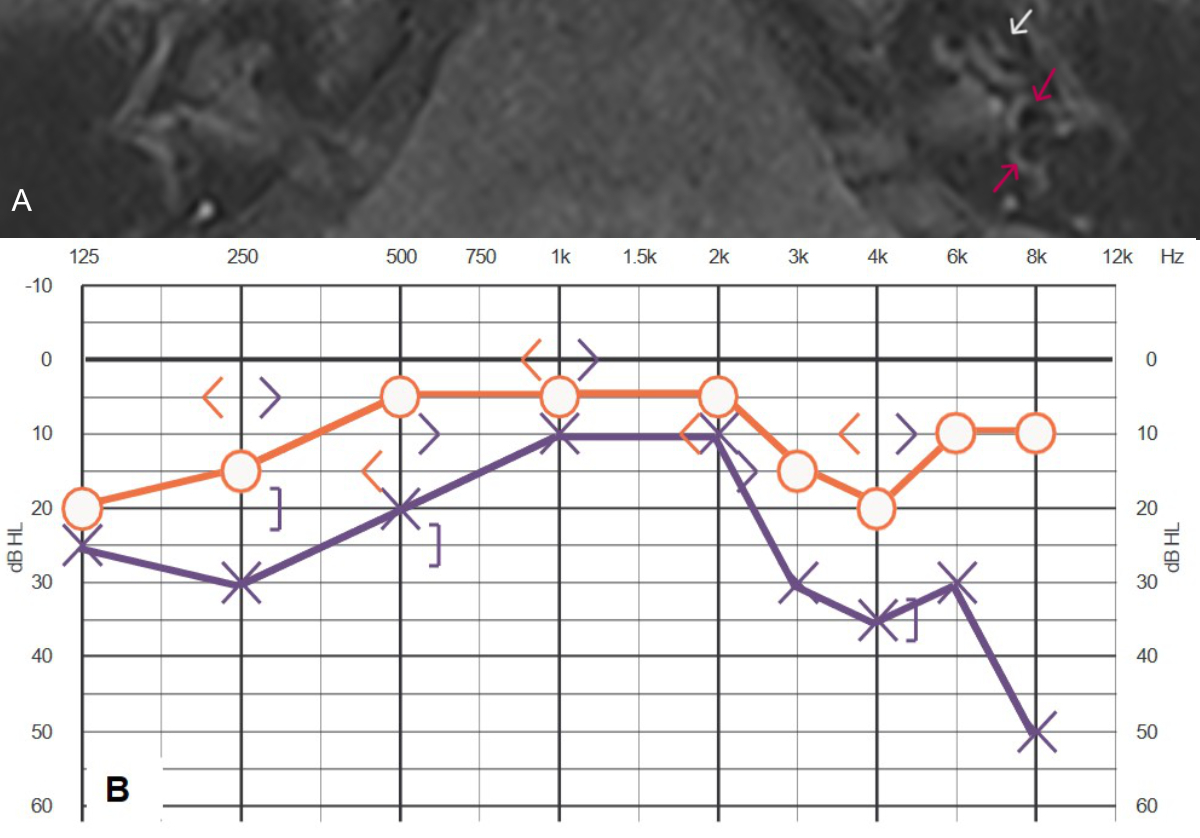
Figure 1MRI
and audiogram of patient #4 (table 1 and 2). (A) MRI 3D inversion recovery
sequence showing cochlear (white arrow) and vestibular (red arrows) endolymphatic
hydrops grade 1 in the left ear, according to Baráth et al. [38].
(B) Audiogram 5 years after the start of Menière’s disease showing low- and
high-frequency hearing loss called “peak.audigram”.
In both
patients, migrainous headache with increased light and noise sensitivity lasted
longer than the Menière’s disease symptoms. Patient #5 became tired and slept
for two hours. After this nap, his migrainous symptoms (headache as well as
increased sensitivity to noise and light) were also completely resolved.
Discussion
We describe
a small case series of six patients with Menière’s disease and migraine who
were treated with rimegepant. Only patient #6 had Menière’s disease only but had
had migraine until about 14 years before the first Menière symptoms appeared. Only
in this patient can a vestibular migraine be excluded.
We saw an
impressive positive effect of rimegepant on acute Menière attacks in two
patients, who took the first dose shortly after the combined onset of migraine
and Menière’s disease symptoms and whose Menière’s disease symptoms improved
even earlier than their migraine symptoms.
We also saw
a promising preventive effect in all six patients of our small first case
series. Five of these patients used it as a preventive treatment for both migraine
and Menière’s disease and patient #6 used it for prevention of Menière’s
disease only.
The effect
in migraine has already been described [39, 40] but we describe very promising effects
of rimegepant in both acute Menière attacks and the prevention of Menière’s
disease. It seems especially remarkable that migraine symptoms were
significantly reduced, but Menière’s disease symptoms were completely abolished
in all five patients during the preventive period, which is ongoing. And even
when treating an acute attack, symptoms improved better and faster than
migraine symptoms.
This study has several
limitations. First, the study sample is quite small. We know that such a small case
series constitutes
weak evidence and does not prove the effect statistically. Nevertheless, this
study was not designed to provide statistical evidence, but as a pilot study to
determine whether a larger randomised controlled trial is warranted for this
new medication. Therefore, one author (SH) tried to start prevention during an
acute attack in two patients with prolonged vertigo to see if an acute effect
could be observed and then included in a larger study. However, all
participants in the study were well characterised both clinically and with
audiovestibular function tests. Unfortunately, clear discrimination between
Menière’s disease and vestibular migraine is not possible, if symptoms of both
diseases occur simultaneously. Despite these
limitations, we believe that this small case series may be of interest to
clinicians who are managing this disabling condition and have no evidence-based
non-destructive medical therapy available.
Therefore, we
decided to publish these very promising first results in a very small group of
patients which support the previous hypothesis that Menière’s disease is mainly
caused by calcitonin gene-related peptide [24],
at least in patients with Menière’s disease and migraine and possibly also in
patients with Menière’s disease only. The efficacy in only one patient with Menière’s
disease only is even less evident than in the four with Menière’s disease and migraine,
but it shows at least a possible effect. Since other authors also argue for an
inner ear migraine [6, 27, 41, 42], we agree with this concept and we believe
that the effect of the therapy shown in this pilot study provides strong support for
further evaluating it in
a large randomised controlled trial.
With regard
to one patient from our small case series, we also suggest that rimegepant may
have a significant preventive effect not only in patients with Menière’s
disease and migraine, but also in patients with Menière’s disease only. This
will be an important question in a planned randomised controlled trial.
As
described in the Introduction, there are no evidence-based therapies described
for the treatment of an acute attack of Menière’s disease, and all preventive
treatments for Menière’s disease – except vestibular destructive procedures –
are controversial, so there are no generally accepted medications for the
treatment of Menière’s disease.
Conclusion
Despite the
abovementioned limitations of this very small case series and according to our
hypothesis, we would suggest treating patients with Menière’s disease and
migraine as well as those with isolated Menière’s disease (without migraine)
with rimegepant or other gepants, if they are available. We suggest that rimegepant
is a potentially strong medication for prevention of Menière’s disease as well
as for treating acute Menière attacks.
A larger,
double-blind, randomised, placebo-controlled trial is warranted and is about to
be initiated to provide scientific evidence of this impressive effect of
rimegepant. We will also evaluate the effect of rimegepant on hearing tests,
vestibular function tests and endolymphatic hydrops. We hope that we will soon
have scientific proof that the hypothesis that calcitonin gene-related peptide
causes Menière’s disease is correct and that this disabling disease can be
effectively treated, significantly improving the quality of life of Menière’s
disease patients. We are pleasantly surprised that rimegepant appears to be
even better at preventing Menière’s disease than migraine, for which it was
originally developed. And the faster and better effect in acute attacks of
Meniere’s disease is also very interesting and promising.
Acknowledgments
We thank
the pharmacy “City Apotheke zur Sihlporte” for providing the drug at cost (i.e.
without a profit margin). A philanthropic donor, who wishes to remain anonymous (but
was disclosed to the editorial board) paid the reduced price for the three Swiss patients
included.
Prof. Dr. med. Stefan Hegemann
Nüschelerstrasse 49
CH-8001 Zürich
s.hegemann[at]hin.ch
References
1. Shojaku H, Watanabe Y, Fujisaka M, Tsubota M, Kobayashi K, Yasumura S, et al. Epidemiologic
characteristics of definite Ménière’s disease in Japan. A long-term survey of Toyama
and Niigata prefectures. ORL J Otorhinolaryngol Relat Spec. 2005;67(5):305–9. 10.1159/000089413
2. Havia M, Kentala E, Pyykkö I. Prevalence of Menière’s disease in general population
of Southern Finland. Otolaryngol Head Neck Surg. 2005 Nov;133(5):762–8. doi: https://doi.org/10.1016/j.otohns.2005.06.015
3. Harris JP, Alexander TH. Current-day prevalence of Ménière’s syndrome. Audiol Neurootol.
2010;15(5):318–22. doi: https://doi.org/10.1159/000286213
4. Kim SY, Lee CH, Yoo DM, Kwon MJ, Kim JH, Kim JH, et al. Association Between Meniere
Disease and Migraine. JAMA Otolaryngol Head Neck Surg. 2022 May;148(5):457–64. doi: https://doi.org/10.1001/jamaoto.2022.0331
5. Wang Y, Liang J, Fang Y, Yao D, Zhang L, Zhou Y, et al. Burden of Common Neurologic
Diseases in Asian Countries, 1990-2019: An Analysis for the Global Burden of Disease
Study 2019. Neurology. 2023 May;100(21):e2141–54. doi: https://doi.org/10.1212/WNL.0000000000207218
6. Ghavami Y, Mahboubi H, Yau AY, Maducdoc M, Djalilian HR. Migraine features in patients
with Meniere’s disease. Laryngoscope. 2016 Jan;126(1):163–8. doi: https://doi.org/10.1002/lary.25344
7. Headache Classification Committee of the International Headache Society (IHS) The
International Classification of Headache Disorders. Headache Classification Committee
of the International Headache Society (IHS) The International Classification of Headache
Disorders, 3rd edition. Cephalalgia. 2018 Jan;38(1):1–211. doi: https://doi.org/10.1177/0333102417738202
8. Tabet P, Saliba I. Meniere’s Disease and Vestibular Migraine: Updates and Review of
the Literature. J Clin Med Res. 2017 Sep;9(9):733–44. doi: https://doi.org/10.14740/jocmr3126w
9. Ménière P. Mémoires sur des lesions de l'oreille interne donnant lieu á des symptômes
de congestion cérébrale apoplectiforme. lu à l'Académie impériale de médecine dans
la séance du 8 janvier 1861. Gazette medicale de Paris [Erstveröffentlichung]. 1861;16:597-601.
https://www.ncbi.nlm.nih.gov/nlmcatalog/9805412
10. Phillips JS, Westerberg B. Intratympanic steroids for Ménière’s disease or syndrome.
Cochrane Database Syst Rev. 2011 Jul;(7):CD008514. doi: https://doi.org/10.1002/14651858.CD008514.pub2
11. Webster KE, Lee A, Galbraith K, Harrington-Benton NA, Judd O, Kaski D, et al. Intratympanic
corticosteroids for Ménière’s disease. Cochrane Database Syst Rev. 2023 Feb;2(2):CD015245.
12. Cooper MW, Kaylie DM. Is endolymphatic sac surgery beneficial for Meniere’s Disease? Laryngoscope.
2020 Dec;130(12):2738–9. doi: https://doi.org/10.1002/lary.28647
13. Schoo DP, Tan GX, Ehrenburg MR, Pross SE, Ward BK, Carey JP. Intratympanic (IT) Therapies
for Menière’s Disease: Some Consensus Among the Confusion. Curr Otorhinolaryngol Rep.
2017 Jun;5(2):132–41. doi: https://doi.org/10.1007/s40136-017-0153-5
14. Hallpike CS, Cairns H. Observations on the Pathology of Ménière’s Syndrome: (Section
of Otology) [Section of Otology]. Proc R Soc Med. 1938 Sep;31(11):1317–36. doi: https://doi.org/10.1177/003591573803101112
15. Kimura RS. Experimental blockage of the endolymphatic duct and sac and its effect
on the inner ear of the guinea pig. A study on endolymphatic hydrops. Ann Otol Rhinol
Laryngol. 1967 Aug;76(3):664–87. doi: https://doi.org/10.1177/000348946707600311
16. Gürkov R, Flatz W, Louza J, Strupp M, Krause E. In vivo visualization of endolyphatic
hydrops in patients with Meniere’s disease: correlation with audiovestibular function.
Eur Arch Otorhinolaryngol. 2011 Dec;268(12):1743–8. doi: https://doi.org/10.1007/s00405-011-1573-3
17. Huang Y, Zhao P, Han Z, Xie J, Liu Y, Gong S, et al. Evaluation of the relationship
between endolymphatic hydrops and hearing loss in Meniere’s disease based on three-dimensional
real inversion recovery sequence. Braz J Otorhinolaryngol. 2023;89(5):101314. doi: https://doi.org/10.1016/j.bjorl.2023.101314
18. Greco A, Gallo A, Fusconi M, Marinelli C, Macri GF, de Vincentiis M. Meniere’s disease
might be an autoimmune condition? Autoimmun Rev. 2012 Aug;11(10):731–8. doi: https://doi.org/10.1016/j.autrev.2012.01.004
19. Kariya S, Cureoglu S, Fukushima H, Nomiya S, Nomiya R, Schachern PA, et al. Vascular
findings in the stria vascularis of patients with unilateral or bilateral Ménière’s
disease: a histopathologic temporal bone study. Otol Neurotol. 2009 Oct;30(7):1006–12.
doi: https://doi.org/10.1097/MAO.0b013e3181b4ec89
20. Gacek RR. Ménière’s disease is a viral neuropathy. ORL J Otorhinolaryngol Relat Spec.
2009;71(2):78–86. doi: https://doi.org/10.1159/000189783
21. Derebery MJ. Prevalence of heat shock protein in patients with Meniere’s disease and
allergy. Otolaryngol Head Neck Surg. 2002 Jun;126(6):677–82. doi: https://doi.org/10.1067/mhn.2002.125297
22. Derebery MJ, Rao VS, Siglock TJ, Linthicum FH, Nelson RA. Menière’s disease: an immune
complex-mediated illness? Laryngoscope. 1991 Mar;101(3):225–9. doi: https://doi.org/10.1288/00005537-199103000-00001
23. Cutrer FM, Baloh RW. Migraine-associated dizziness. Headache. 1992 Jun;32(6):300–4.
doi: https://doi.org/10.1111/j.1526-4610.1992.hed3206300.x
24. Hegemann SC. Menière’s disease caused by CGRP - A new hypothesis explaining etiology
and pathophysiology. Redirecting Menière’s syndrome to Menière’s disease. J Vestib
Res. 2021;31(4):311–4. doi: https://doi.org/10.3233/VES-200716
25. Herbert MK, Holzer P. [Neurogenic inflammation. II. pathophysiology and clinical implications].
Anasthesiol Intensivmed Notfallmed Schmerzther. 2002 Jul;37(7):386–94. doi: https://doi.org/10.1055/s-2002-32701
26. Herbert MK, Holzer P. [Neurogenic inflammation. I. Basic mechanisms, physiology and
pharmacology]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2002 Jun;37(6):314–25.
doi: https://doi.org/10.1055/s-2002-32233
27. Frank M, Abouzari M, Djalilian HR. Meniere’s disease is a manifestation of migraine.
Curr Opin Otolaryngol Head Neck Surg. 2023 Oct;31(5):313–9. doi: https://doi.org/10.1097/MOO.0000000000000908
28. Gürkov R. J. I [Vestibular function: migraine extends to the inner ear]. Laryngorhinootologie.
2014;93:814–5.
29. Baron R, Steenerson KK. A Review of Calcitonin Gene-Related Peptide and Its Implications
for Vestibular Disorders. Curr Treat Options Neurol. 2024;26(6):203–28. 10.1007/s11940-024-00792-9
30. Johnson KW, Morin SM, Wroblewski VJ, Johnson MP. Peripheral and central nervous system
distribution of the CGRP neutralizing antibody [125I] galcanezumab in male rats. Cephalalgia.
2019 Sep;39(10):1241–8. doi: https://doi.org/10.1177/0333102419844711
31. Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization
of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the
blood-brain barrier. Brain Res. 2015 Mar;1600:93–109. doi: https://doi.org/10.1016/j.brainres.2014.11.031
32. Yoshida T, Kobayashi M, Sugimoto S, Teranishi M, Naganawa S, Sone M. Evaluation of
the blood-perilymph barrier in ears with endolymphatic hydrops. Acta Otolaryngol.
2021 Aug;141(8):736–41. doi: https://doi.org/10.1080/00016489.2021.1957500
33. Zhang W, Xie J, Liu H, Wang M. Blood-labyrinth barrier breakdown in Meniere’s disease.
Eur Arch Otorhinolaryngol. 2024 May;281(5):2327–32. doi: https://doi.org/10.1007/s00405-023-08353-7
34. Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics. 2010 Apr;7(2):164–75.
doi: https://doi.org/10.1016/j.nurt.2010.02.004
35. Hostetler ED, Joshi AD, Sanabria-Bohórquez S, Fan H, Zeng Z, Purcell M, et al. In
vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant
in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232.
J Pharmacol Exp Ther. 2013 Nov;347(2):478–86. doi: https://doi.org/10.1124/jpet.113.206458
36. Wackym PA, Popper P, Micevych PE. Distribution of calcitonin gene-related peptide
mRNA and immunoreactivity in the rat central and peripheral vestibular system. Acta
Otolaryngol. 1993 Sep;113(5):601–8. doi: https://doi.org/10.3109/00016489309135871
37. Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al.; Classification
Committee of the Barany Society; Japan Society for Equilibrium Research; European
Academy of Otology and Neurotology (EAONO); Equilibrium Committee of the American
Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS); Korean Balance Society.
Diagnostic criteria for Menière’s disease. J Vestib Res. 2015;25(1):1–7. doi: https://doi.org/10.3233/VES-150549
38. Baráth K, Schuknecht B, Naldi AM, Schrepfer T, Bockisch CJ, Hegemann SC. Detection
and grading of endolymphatic hydrops in Menière disease using MR imaging. AJNR Am
J Neuroradiol. 2014 Jul;35(7):1387–92. doi: https://doi.org/10.3174/ajnr.A3856
39. Croop R, Goadsby PJ, Stock DA, Conway CM, Forshaw M, Stock EG, et al. Efficacy, safety,
and tolerability of rimegepant orally disintegrating tablet for the acute treatment
of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet.
2019 Aug;394(10200):737–45. doi: https://doi.org/10.1016/S0140-6736(19)31606-X
40. Yu S, Kim BK, Guo A, Kim MH, Zhang M, Wang Z, et al. Safety and efficacy of rimegepant
orally disintegrating tablet for the acute treatment of migraine in China and South
Korea: a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol.
2023 Jun;22(6):476–84. doi: https://doi.org/10.1016/S1474-4422(23)00126-6
41. Benjamin T, Gillard D, Abouzari M, Djalilian HR, Sharon JD. Vestibular and auditory
manifestations of migraine. Curr Opin Neurol. 2022 Feb;35(1):84–9. doi: https://doi.org/10.1097/WCO.0000000000001024
42. Moshtaghi O, Sahyouni R, Lin HW, Ghavami Y, Djalilian HR. A Historical Recount: Discovering
Menière’s Disease and Its Association With Migraine Headaches. Otol Neurotol. 2016 Sep;37(8):1199–203.
doi: https://doi.org/10.1097/MAO.0000000000001122
