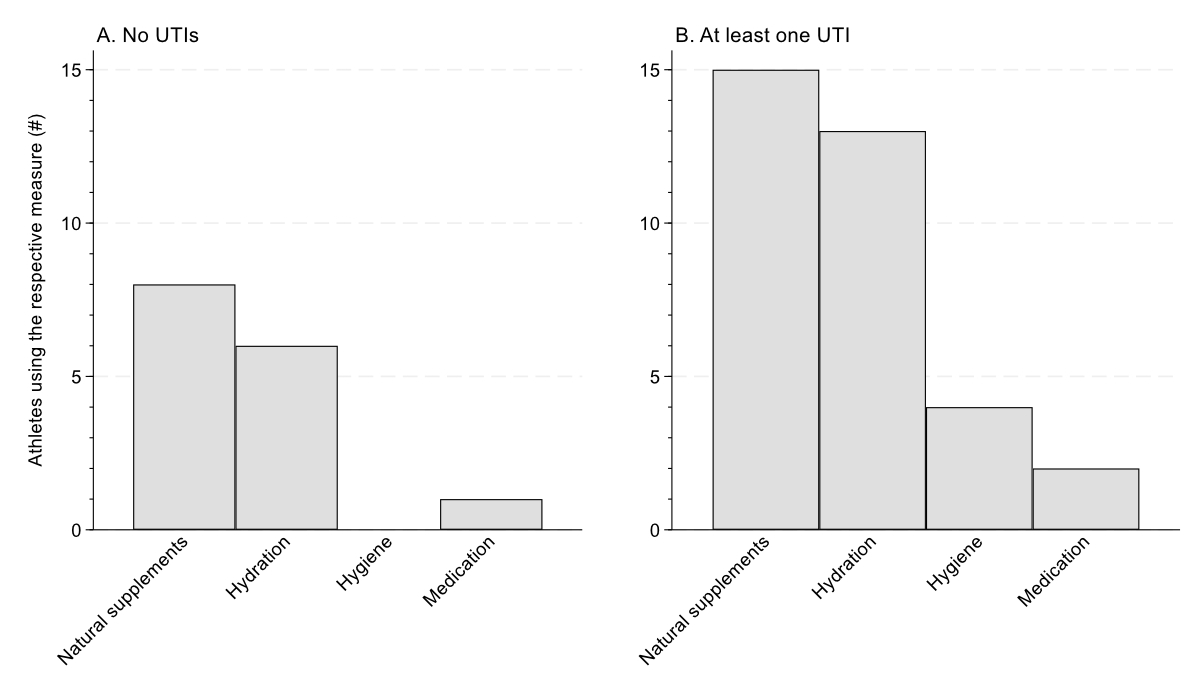
Figure 1Prophylaxis for urinary tract infections (UTIs) used by athletes. This was a multiple-answer question.
DOI: https://doi.org/https://doi.org/10.57187/s.4113
For athletes, illness can have a substantial impact on training and performance [1]. Para athletes have a higher risk of illness than Olympic athletes [2]. During several winter and summer Paralympic Games between 2012 and 2022, genitourinary illness was the fourth most commonly reported illness after respiratory, skin and gastrointestinal illnesses [2–4]. Genitourinary illness, which includes urinary tract infections, accounted for 7–11% of all events [2–4]. Symptoms of urinary tract infections in athletes with spinal cord injury include fever, malaise, increased spasticity and autonomic dysreflexia [5]. This makes urinary tract infections particularly burdensome for these athletes, potentially leading to decreased performance and missed training days, and ultimately affecting competition results.
Individuals with a neurogenic bladder, including those with spinal cord injury, spina bifida, cerebral palsy and multiple sclerosis, are at increased risk of developing a urinary tract infection [6, 7]. Depending on the bladder evacuation method, 10% to 50% of individuals with spinal cord injury experience recurrent urinary tract infections [8]. Impaired bladder storage and voiding function, intermittent catheterisation and catheter reuse are associated with an increased risk of urinary tract infections [5, 9–11]. Dehydration may also play a role, especially in athletes [2]. Several prophylactic measures can be taken to prevent urinary tract infections, including the intake of antibiotics or natural supplements, such as cranberry products [5, 12]. Nevertheless, evidence for the effectiveness of urinary tract infection prophylaxis is inconsistent [5]. Frequent bladder voiding by catheterisation has also been identified as an important preventive factor for urinary tract infections [5]. On the other hand, frequent catheterisation has been associated with an increased risk of cross-infection [5]. This highlights the complexity and multifactorial nature of this issue.
Although several studies have demonstrated an increased prevalence of urinary tract infections in individuals with spinal cord injury, findings in elite wheelchair athletes are rare. One study in international elite wheelchair athletes found that athletes who reused their catheters experienced more urinary tract infections [11]. Another study in international elite athletes with spinal cord injury using intermittent catheterisation found that 63% had experienced at least one urinary tract infection during the previous 12 months [10]. Besides knowledge regarding the occurrence of urinary tract infections, there is a lack of knowledge regarding the use of prophylaxis, potential causes and the impact of urinary tract infections on the performance of wheelchair athletes. The primary objective of this cross-sectional, retrospective, self-report study was to evaluate the occurrence of urinary tract infections in elite wheelchair athletes in the previous 12 months. We also evaluated the self-reported impact of urinary tract infections on performance and training. Additionally, we evaluated measures of prophylaxis implemented and potential reasons for the occurrence of urinary tract infections as reported by the athletes.
We used a cross-sectional study design and adhered to the STROBE guidelines [13]. Data were collected from September 2022 to August 2023 at the Institute of Sports Medicine at the Swiss Paraplegic Centre, specialised in examination of wheelchair athletes. Wheelchair athletes include all athletes who compete in a variety of wheelchair sports. Wheelchair athletes who are members of the national team in their respective sport visit our institute at least once a year for a standardised medical examination and performance test. All the data analysed in this study were collected during these routine checkups. Compared to studies in able-bodied individuals, studies in wheelchair athletes are often limited by a small sample size – this is further complicated by the large variation in disease and classification categories. To maximise the sample size and diversity of the study population, all wheelchair athletes active in international and/or national competitions who attended one of these regular checkups at our institute during the study period were included. The time frame for data collection was chosen to resemble a full cycle of tests at our institute, i.e. at least one test performed in all our athletes.
Only one checkup for each athlete was included in the analyses. The following individual characteristics were collected from the athletes’ in-house medical files: sex, age, height, body mass, body mass index (BMI), regular use of medication, diagnosis, spinal cord injury lesion level, degree of impairment following the American Spinal Injury Association Impairment Scale (AIS), year of spinal cord injury onset and method of bladder emptying. All further parameters were collected using an in-house standardised questionnaire as part of the regular performance checkup of the athletes presenting to our institute. The questionnaire was completed together with the athlete, which provided the opportunity to resolve any issues or questions directly and resulted in no missing data. The following sports and urinary tract infection parameters were collected using questionnaires: type of sports, time spent at elite level, duration and number of weekly training sessions, total number of urinary tract infections in the previous 12 months, the month of manifestation, prophylaxis, potential reasons, symptoms, duration of symptoms, treatment and number of training days lost due to urinary tract infections. The impact of urinary tract infections on training volume and performance was assessed on a 5-point Likert scale ranging from “No impact at all” to “No participation possible”. Urine specific gravity was assessed from a 10 ml urine sample. The urine samples were handled and analysed according to routine clinical procedures in our in-house laboratory.
The diagnosis was categorised by presence of spinal cord injury, either “Yes” or “No”. The neurological level of spinal cord injury, the highest sensorimotor lesion level, was categorised as “tetraplegia” (C1–C8) or “paraplegia” (T1 or lower) [14]. The degree of sensory impairment was categorised as “complete” (A) or “incomplete” (B–D) [14]. The voiding method was categorised by use of catheterisation, either “Yes” (intermittent or indwelling catheter) or “No” (no catheter use at all), as any form of catheterisation increases the risk of urinary tract infections [15]. The type of sports was categorised as “Endurance” (cycling and wheelchair racing), “Team” (basketball and rugby) or “Skill” (alpine skiing, badminton, curling, fencing, shooting, table tennis, tennis and Wheelchair MotoCross [WCMX]). The occurrence of urinary tract infections was categorised as “None” (no urinary tract infections) or “At least one” (≥1 urinary tract infection), given that the occurrence of one urinary tract infection in athletes is already clinically relevant in terms of potential training disruptions and decreased performance. Prophylactic measures were categorised as “Natural supplements” (e.g. cranberry, vitamin C), “Hydration” (plenty or additional fluids), “Hygiene” (e.g. washing hands, appropriate catheter handling) or “Medication” (e.g. Uro-Vaxom®, methionine). Urine specific gravity was categorised as “Hydrated” (≤1.020 g/ml) or “Dehydrated” (>1.020 g/ml) [16].
Following the central limit theorem, parametric methods were applied. Data were reported as mean and standard deviation (SD) as well as count and percentage. Differences in the occurrence of urinary tract infections and the use of urinary tract infection prophylaxis between different groups (spinal cord injury yes/no and catheter yes/no) were evaluated using Pearson’s chi-squared test. Differences in missed training days, and the number and duration of urinary tract infections between different groups (spinal cord injury yes/no and catheter yes/no) were evaluated using the independent t-test. A Bonferroni correction was applied to the Pearson’s chi-squared and the independent t-tests, resulting in a significance level of p ≤0.013. A binary logistic regression was run to evaluate the effects of relevant predictors (sex, age, spinal cord injury, spinal cord injury duration, catheter use, training frequency, urinary tract infection prevention) on the occurrence of at least one urinary tract infection, entering all predictors into the model at once. One model was calculated with data from all athletes and a second model was calculated with data from athletes with spinal cord injury only. The Hosmer-Lemeshow test was used to assess the goodness of fit of the models. For the models, a p-value of ≤0.05 was considered statistically significant. Analyses were performed with Stata statistical software release 17.0 (StataCorp LLC, College Station, TX, USA).
All participants provided written informed consent. The study was performed in accordance with the standards of ethics outlined in the Declaration of Helsinki and national laws and was approved by the local ethics committee on 23 June 2023 (EKNZ, Basel, Switzerland, project-ID: 2023-01180).
Data were analysed from 81 athletes, ranging in age from 15 to 64 years (table 1). Some athletes had just joined the elite squad for the first year, while others had been competing at the elite level for up to 38 years. Most athletes were active in either basketball (n = 14, 17%) or cycling (n = 14, 17%). Forty-two (52%) athletes had a traumatic spinal cord injury, whereas other diagnoses included neural tube defect (n = 17, 21%), multiple sclerosis (n = 3, 4%), amputation (n = 3, 4%) or further diagnoses (n = 16, 20%) such as cerebral palsy or Guillain-Barré syndrome. Fifty-six (69%) athletes were using a catheter for bladder emptying of whom 52 (93%) were using intermittent catheterisation. Most of the athletes had spinal cord injury (n = 67, 83%), most of whom were using a catheter (n = 55, 82%). One athlete was dehydrated at the time of data collection and had a urine specific gravity of 1.025 g/ml.
Table 1Athlete characteristics. Data are presented as mean (standard deviation) or count (percentage). Percentages may not total 100 due to rounding.
| Overall (n = 81) | Spinal cord injury? | Using a catheter? | ||||
| Yes (n = 67) | No (n = 14) | Yes (n = 56) | No (n = 25) | |||
| Sex | Female | 24 (30%) | 15 (22%) | 9 (64%) | 15 (27%) | 9 (36%) |
| Male | 57 (70%) | 52 (78%) | 5 (36%) | 41 (73%) | 16 (64%) | |
| Age (years) | 35 ± 11 | 35 ± 11 | 33 ± 12 | 35 ± 12 | 36 ± 11 | |
| Height (cm) | 173 ± 13 | 174 ± 12 | 166 ± 13 | 173 ± 13 | 172 ± 13 | |
| Body mass (kg) | 68 ± 15 | 68 ± 16 | 70 ± 14 | 67 ± 16 | 70 ± 14 | |
| BMI (kg/m²) | 22.9 ± 4.4 | 22.3 ± 4.0 | 25.6 ± 5.7 | 22.4 ± 4.0 | 23.9 ± 5.2 | |
| Diagnosis | Traumatic spinal cord injury | 42 (52%) | 42 (63%) | – | 34 (61%) | 8 (32%) |
| Neural tube defect | 17 (21%) | 17 (25%) | – | 15 (27%) | 2 (8%) | |
| Multiple sclerosis | 3 (4%) | – | 3 (21%) | 1 (2%) | 2 (8%) | |
| Amputation | 3 (4%) | – | 3 (21%) | – | 3 (12%) | |
| Other* | 16 (20%) | 8 (12%) | 8 (57%) | 6 (11%) | 10 (40%) | |
| Time since spinal cord injury (years) | 18 ± 10 | 18 ± 10 | – | 17 ± 10 | 18 ± 13 | |
| Lesion level | Tetraplegia | 18 (27%) | 18 (27%) | – | 11 (20%) | 7 (58%) |
| Paraplegia | 49 (73%) | 49 (73%) | – | 44 (80%) | 5 (42%) | |
| Sensory impairment | Complete | 36 (54%) | 36 (54%) | – | 31 (56%) | 5 (42%) |
| Incomplete | 31 (46%) | 31 (46%) | – | 24 (44%) | 7 (58%) | |
| Sport | Endurance** | 27 (33%) | 26 (39%) | 1 (7%) | 22 (39%) | 5 (20%) |
| Team*** | 22 (27%) | 14 (21%) | 8 (57%) | 10 (18%) | 12 (48%) | |
| Skill**** | 32 (40%) | 27 (40%) | 5 (36%) | 24 (43%) | 8 (32%) | |
| Weekly training | Duration (h) | 9 ± 5 | 10 ± 5 | 8 ± 5 | 10 ± 5 | 8 ± 4 |
| Sessions (#) | 5 ± 3 | 6 ± 3 | 5 ± 3 | 6 ± 3 | 5 ± 2 | |
| Elite level athlete (years) | 5 ± 7 | 6 ± 7 | 3 ± 4 | 6 ± 7 | 4 ± 5 | |
BMI: body mass index.
* “Other” diagnosis = cerebral palsy, functional neurological disorder, Guillain-Barré syndrome and lower limb deficiency.
** Endurance sports = cycling and wheelchair racing.
*** Team sports = basketball and rugby.
**** Skill sports = alpine skiing, badminton, curling, fencing, shooting, table tennis, tennis and Wheelchair MotoCross (WCMX).
Thirty-six (44%) athletes had experienced at least one urinary tract infection in the previous 12 months. Athletes with spinal cord injury (p = 0.013) and those using a catheter (p = 0.001) experienced urinary tract infections more often compared to athletes without spinal cord injury and those not using a catheter (table 2). In athletes with spinal cord injury and using a catheter, 31 (56%) had experienced at least one urinary tract infection. Of all athletes, 38 (47%) were using at least one type of urinary tract infection prophylaxis (table 2). Natural supplements, including cranberry juice and D-mannose, were used most often (figure 1). Of the athletes using prophylactic measures, twenty-five (69%) had experienced at least one urinary tract infection.
Table 2Characteristics of urinary tract infections during the previous 12 months, in all athletes. Data are presented as count (percentage).
| Overall | Spinal cord injury? | Using a catheter? | |||||
| Yes (n = 67) | No (n = 14) | p-value | Yes (n = 56) | No (n = 25) | p-value | ||
| Is using urinary tract infection prophylaxis | 38 (47%) | 36 (95%) | 2 (5%) | 0.007 | 33 (87%) | 5 (13%) | 0.001 |
| Has had at least one urinary tract infection | 36 (44%) | 34 (94%) | 2 (6%) | 0.013 | 32 (89%) | 4 (11%) | 0.001 |

Figure 1Prophylaxis for urinary tract infections (UTIs) used by athletes. This was a multiple-answer question.
Of the athletes who had experienced urinary tract infections, 13 (36%) reported one, while 2 reported having had 12 urinary tract infections. Urinary tract infections lasted a mean of 7 ± 5 days (table 2 and 3), with a range from 1 to 20 days. Thirty-four (94%) athletes with urinary tract infections had experienced at least one symptom. General malaise (28%), fever (25%) and pain (23%) were the most commonly reported symptoms, followed by spasm (13%) and incontinence (11%). Twenty-three (64%) athletes had received antibiotic treatment for their urinary tract infection. The urinary tract infections caused a mean loss of 4 ± 6 training days (table 3). Stress (34%), air travel (28%) and training intensity (17%) were the most commonly reported potential causes of the urinary tract infection, followed by competition (10%), nutrition (7%) and altitude (3%). Sixteen athletes did not mention a particular cause for their urinary tract infection. Most athletes mentioned that their urinary tract infection had at least some negative effect on their training volume (53%, figure 2A) or performance (61%, figure 2B).
Table 3Characteristics of urinary tract infections over the previous 12 months, in athletes with at least one episode. Data are presented as mean (standard deviation).
| Overall | Spinal cord injury? | Using a catheter? | |||||
| Yes (n = 34) | No (n = 2) | p-value | Yes (n = 32) | No (n = 4) | p-value | ||
| Number of urinary tract infections (mean ± SD) | 3 ± 3 | 3 ± 3 | 9 ± 4 | 0.002 | 3 ± 3 | 5 ± 5 | 0.31 |
| Duration of urinary tract infection (days, mean ± SD) | 7 ± 5 | 7 ± 5 | 4 ± 4 | 0.27 | 7 ± 5 | 6 ± 4 | 0.68 |
| Missed training due to urinary tract infection (days, mean ± SD) | 4 ± 6 | 4 ± 6 | 4 ± 5 | 0.94 | 4 ± 6 | 4 ± 7 | 0.91 |
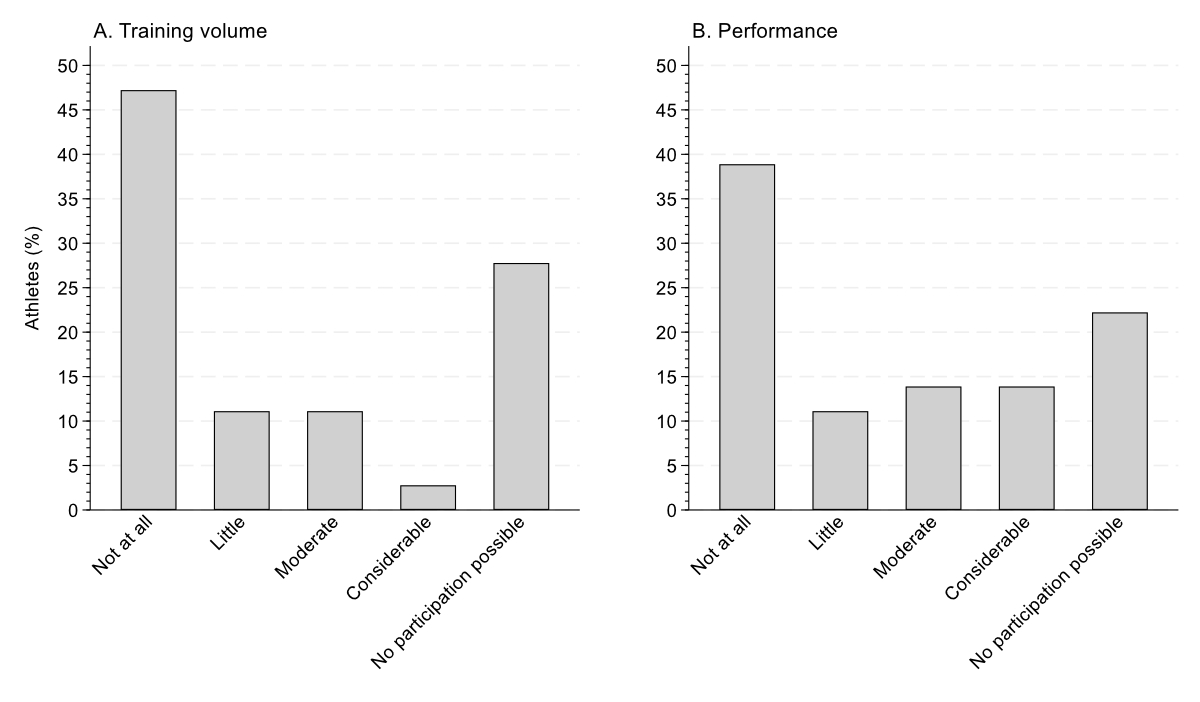
Figure 2Impact of urinary tract infections (UTIs) on (A) training volume and (B) performance among athletes with at least one urinary tract infection. These were multiple-choice questions.
In both logistic regression models (all athletes, only athletes with a spinal cord injury), the use of prophylaxis (p ≤0.02) was associated with higher odds of having at least one urinary tract infection (tables 4 and 5). None of the other investigated parameters were significant predictors (p ≥0.06, tables 4 and 5).
Table 4Binary logistic regression for having at least one urinary tract infection over the previous 12 months, including all athletes. χ2(6) = 21.31, p = 0.002, Hosmer-Lemeshow p = 0.77.
| Odds ratio | 95% confidence interval | p-value | |||
| Lower | Upper | ||||
| Age (years) | 1.02 | 0.98 | 1.07 | 0.33 | |
| Sex | Female | Reference | |||
| Male | 0.84 | 0.24 | 3.00 | 0.79 | |
| Spinal cord injury | No | Reference | |||
| Yes | 1.41 | 0.16 | 12.53 | 0.76 | |
| Using a catheter? | No | Reference | |||
| Yes | 4.43 | 0.93 | 20.98 | 0.06 | |
| Training (sessions/week) | 1.00 | 0.90 | 1.12 | 0.95 | |
| Urinary tract infection prophylaxis? | No | Reference | |||
| Yes | 3.49 | 1.22 | 10.02 | 0.02 | |
Table 5Binary logistic regression for having at least one urinary tract infection over the previous 12 months, including only athletes with spinal cord injury. χ2(7) = 15.28, p = 0.02, Hosmer-Lemeshow p = 0.37.
| Odds ratio | 95% confidence interval | p-value | |||
| Lower | Upper | ||||
| Age (years) | 1.03 | 0.98 | 1.09 | 0.25 | |
| Sex | Female | Reference | |||
| Male | 1.22 | 0.30 | 4.94 | 0.78 | |
| Spinal cord injury duration (years) | 1.01 | 0.95 | 1.07 | 0.85 | |
| Using a catheter? | No | Reference | |||
| Yes | 2.94 | 0.61 | 14.19 | 0.18 | |
| Training (sessions/week) | 1.01 | 0.89 | 1.34 | 0.42 | |
| Urinary tract infection prophylaxis? | No | Reference | |||
| Yes | 4.42 | 1.42 | 13.76 | 0.01 | |
This is the first study to investigate the occurrence of self-reported urinary tract infections in elite wheelchair athletes and their impact on performance and training. Thirty-six (44%) athletes had experienced at least one urinary tract infection during the previous 12 months. Urinary tract infections were more common in athletes with spinal cord injury and those using a catheter to empty their bladder. Most athletes experienced a reduction in training volume and performance due to urinary tract infections.
Urinary tract infections were common among our wheelchair athletes: 44% reported having had at least one urinary tract infection during the past 12 months. In this subgroup, 36% reported one infection episode (the most common frequency). In another cohort of international athletes with spinal cord injury using intermittent catheterisation, 63% reported having had at least one urinary tract infection during the past 12 months, with a median of 1 urinary tract infection [10]. In non-athletes with spinal cord injury, an incidence of 2 urinary tract infections per year has been reported [17]. In non-disabled individuals, both athletes and non-athletes, urinary tract infections are more common in females than in males [1, 18, 19]. Interestingly, sex was not a significant predictor for urinary tract infections in our study. However, this finding is consistent with other studies in individuals with spinal cord injury in which no difference was observed in the occurrence of urinary tract infections between sexes [8, 20, 21]. Corroborating previous findings [5, 11], we found that athletes with spinal cord injury or using a catheter were more likely to have had at least one urinary tract infection. Individuals with spinal cord injury are at increased risk of urinary tract infections due to a neurogenic bladder and the need to use catheterisation as a method of voiding the bladder [6, 9]. The cellular and humoral immune response may also be affected in spinal cord injury [22]. This results in a 2- to 3-fold higher level of circulating inflammatory markers compared to non-disabled individuals, which further increases the risk of urinary tract infections [22]. Further demographic or lesion characteristics did not predict the occurrence of a urinary tract infection, which was also found in a study in international athletes with spinal cord injury [11].
Most athletes with spinal cord injury (95%) reported using prophylactic measures. In another study in athletes with spinal cord injury having neurogenic lower urinary tract dysfunction, 52% used antibiotics as prophylaxis [10]. None of our athletes used antibiotics as prophylaxis, but 64% received antibiotic treatment for their urinary tract infection. The presence of bacteria or leukocytes in the urine without symptoms, also known as asymptomatic bacteriuria and pyuria, is prevalent in 50–100% of individuals with spinal cord injury using a catheter [5]. Instead of medical treatment, this should be addressed by improving hydration status and hygiene during voiding routines [5, 23]. Though urine laboratory testing is recommended when athletes have urinary tract infection symptoms, dipstick testing or measuring urine specific gravity with a handheld refractometer provides an indication of hydration status that is practical and straightforward to implement in an athletic environment [5, 24].
Natural supplements and hygiene measures were the most commonly reported measures for preventing urinary tract infections. The prophylactic measures did not appear to be very successful in reducing the occurrence of urinary tract infections as 69% of athletes experienced at least one urinary tract infection despite implementing prophylactic measures. The use of urinary tract infection prophylaxis was also a significant predictor for the occurrence of urinary tract infections, which corresponds to findings in individuals with chronic neurogenic lower urinary tract dysfunction [8]. Nevertheless, the cross-sectional study design does not allow for causal conclusions to be drawn. We hypothesise that a previous history of recurrent urinary tract infections was the reason for taking prophylactic measures and not vice versa. Evidence regarding the effectiveness of urinary tract infection prophylaxis, including cranberries, D-mannose, vitamin C, methenamine or probiotics, in individuals with spinal cord injury remains inconclusive [5, 23, 25–30]. Optimising bladder management, hand hygiene and fluid intake appear to be the most important measures for preventing urinary tract infections in athletes with neurogenic bladder [5, 7]. Our findings provide valuable insights into potentially unsuccessful urinary tract infection prophylaxis practices used by elite wheelchair athletes, highlighting the need for further evaluation of the effectiveness of current prophylactic measures and the potential exploration of other strategies tailored to this specific population.
Causes of urinary tract infections may be different in athletes compared to non-athletes. Stress was mentioned most often as a potential cause of urinary tract infections by our athletes, followed by air travel, training intensity and competition. These self-reported potential causes provide valuable insights that, while warranting further research, can already inform practices that can be incorporated into training. In non-disabled athletes, changes in training load, increased exposure to non-exercise stressors, competing at major competitions and international travel are associated with a higher risk of illness [2, 31]. The impact of a spinal cord injury on daily life can also indirectly increase the risk of urinary tract infections, especially in athletes. Often more time is needed for activities of daily living, including nutrition and personal hygiene, compared with non-disabled athletes. This leaves less time for rest and recovery, which, combined with a high training load, can lead to immunosuppression and an increased risk of infection [22]. Interestingly, in elite non-disabled athletes, higher training loads are, up to a certain point, associated with a lower risk of infection [1, 31].
Even the occurrence of a single urinary tract infection is relevant for elite athletes. A seemingly minor decrease in training volume or performance can have huge consequences in elite sports, where the difference between winning and losing competitions can be very small [32]. In non-disabled endurance athletes, a short-term (0–4 weeks) reduction or complete cessation of training leads to decreased cardiorespiratory, muscular, metabolic and hormonal functioning [33]. In elite track and field athletes, every training week that had to be modified due to illness or injury led to a significant decrease in the achievement of performance goals during international competitions [34]. The majority of our athletes who had experienced a urinary tract infection reported a negative impact on training volume and performance. Furthermore, an average loss of four training days was reported. The high number of urinary tract infections, together with the impact on training and performance, highlights the relevance of this problem and the need for effective strategies to reduce the occurrence of urinary tract infections in this population.
This is the first study investigating the occurrence of self-reported urinary tract infections in a large cohort of elite wheelchair athletes. The cross-sectional design of our study prohibited any causal interpretation. Reuse of disposable catheters is a risk factor for developing urinary tract infections in elite wheelchair athletes [11], but this parameter was not collected in the current study and needs to be considered in future studies. The collected information was based on self-reported data which may be affected by recall and social desirability bias. The mediocre accuracy (66%) of self-prediction of a urinary tract infection further contributes to the uncertainty about the true number of urinary tract infections [35]. Nevertheless, as subjective measures may indeed surpass objective measures in athlete monitoring [36], we consider our collected data sufficiently reliable and clinically relevant. Future studies may investigate the incidence of urinary tract infections in wheelchair athletes longitudinally based on clinical symptoms and laboratory parameters, including leukocytes in the urine and bacterial culture.
Although many athletes used prophylaxis, urinary tract infections were common in elite wheelchair athletes and had a negative impact on training and performance. More education seems to be needed regarding prophylactic measures, both for athletes as well as their support teams. We recommend that basic prophylactic measures, such as proper catheter hygiene and adequate hydration, be implemented in the daily routine of every athlete. Urine specific gravity testing provides a straightforward indication of the current hydration status and can be easily implemented during training or while traveling. The effectiveness of existing prophylactic measures needs to be evaluated and new prophylactic measures may need to be developed.
The dataset generated and analysed during the current study is available from the corresponding author upon reasonable request.
We kindly thank all athletes who agreed to participate in this study. We thank Sophia Hürlimann, Joel Büttiker, Leonie Pfrunder and Anita Landolt for their support with data collection.
Author contributions: FA, AHG and CP designed the study. FA and AHG performed the data collection. JK provided clinical urology and statistical expertise. AHG performed the analyses and prepared the first draft of the manuscript. All authors were involved in the revision of the draft manuscript and approved the final content.
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Jaworski CA, Rygiel V. Acute Illness in the Athlete. Clin Sports Med. 2019 Oct;38(4):577–95. doi: https://doi.org/10.1016/j.csm.2019.05.001
2. Janse Van Rensburg DC, Schwellnus M, Derman W, Webborn N. Illness Among Paralympic Athletes: Epidemiology, Risk Markers, and Preventative Strategies. Phys Med Rehabil Clin N Am. 2018 May;29(2):185–203. doi: https://doi.org/10.1016/j.pmr.2018.01.003
3. Derman W, Runciman P, Eken M, Boer PH, Blauwet C, Bogdos M, et al. Incidence and burden of illness at the Tokyo 2020 Paralympic Games held during the COVID-19 pandemic: a prospective cohort study of 66 045 athlete days. Br J Sports Med. 2022 Dec;bjsports-2022-106312.
4. Derman W, Runciman P, Eken M, Boer PH, Blauwet C, Bogdos E, et al. Incidence of injury and illness at the Beijing 2022 Paralympic Winter Games held in a closed-loop environment: a prospective cohort study of 7332 athlete days. Br J Sports Med. 2024 Jul;58(15):836–43. doi: https://doi.org/10.1136/bjsports-2023-107525
5. Compton S, Trease L, Cunningham C, Hughes D. Australian Institute of Sport and the Australian Paralympic Committee position statement: urinary tract infection in spinal cord injured athletes. Br J Sports Med. 2015 Oct;49(19):1236–40. doi: https://doi.org/10.1136/bjsports-2014-094527
6. McKibben MJ, Seed P, Ross SS, Borawski KM. Urinary Tract Infection and Neurogenic Bladder. Urol Clin North Am. 2015 Nov;42(4):527–36. doi: https://doi.org/10.1016/j.ucl.2015.05.006
7. Dutton RA. Medical and Musculoskeletal Concerns for the Wheelchair Athlete: A Review of Preventative Strategies. Curr Sports Med Rep. 2019 Jan;18(1):9–16. doi: https://doi.org/10.1249/JSR.0000000000000560
8. Krebs J, Wöllner J, Pannek J. Risk factors for symptomatic urinary tract infections in individuals with chronic neurogenic lower urinary tract dysfunction. Spinal Cord. 2016 Sep;54(9):682–6. doi: https://doi.org/10.1038/sc.2015.214
9. Linsenmeyer TA. Catheter-associated urinary tract infections in persons with neurogenic bladders. J Spinal Cord Med. 2018 Mar;41(2):132–41. doi: https://doi.org/10.1080/10790268.2017.1415419
10. Walter M, Ruiz I, Squair JW, Rios LA, Averbeck MA, Krassioukov AV. Prevalence of self-reported complications associated with intermittent catheterization in wheelchair athletes with spinal cord injury. Spinal Cord. 2021 Sep;59(9):1018–25. doi: https://doi.org/10.1038/s41393-020-00565-6
11. Krassioukov A, Cragg JJ, West C, Voss C, Krassioukov-Enns D. The good, the bad and the ugly of catheterization practices among elite athletes with spinal cord injury: a global perspective. Spinal Cord. 2015 Jan;53(1):78–82. doi: https://doi.org/10.1038/sc.2014.208
12. Pannek J, Pannek-Rademacher S, Jus MS, Wöllner J, Krebs J. Usefulness of classical homeopathy for the prophylaxis of recurrent urinary tract infections in individuals with chronic neurogenic lower urinary tract dysfunction. J Spinal Cord Med. 2019 Jul;42(4):453–9. doi: https://doi.org/10.1080/10790268.2018.1440692
13. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007 Oct;335(7624):806–8. doi: https://doi.org/10.1136/bmj.39335.541782.AD
14. Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011 Nov;34(6):535–46. doi: https://doi.org/10.1179/204577211X13207446293695
15. Davis M, Jethani L, Robbins E, Kaner M. Is It Really the Foley? A Systematic Review of Bladder Management and Infection Risk. Top Spinal Cord Inj Rehabil. 2023;29(1):94–107. doi: https://doi.org/10.46292/sci22-00009
16. Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007 Feb;39(2):377–90.
17. Garcia-Arguello LY, O’Horo JC, Farrell A, Blakney R, Sohail MR, Evans CT, et al. Infections in the spinal cord-injured population: a systematic review. Spinal Cord. 2017 Jun;55(6):526–34. doi: https://doi.org/10.1038/sc.2016.173
18. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002 Jul;113(1 Suppl 1A):5S–13S. doi: https://doi.org/10.1016/S0002-9343(02)01054-9
19. Deltourbe L, Lacerda Mariano L, Hreha TN, Hunstad DA, Ingersoll MA. The impact of biological sex on diseases of the urinary tract. Mucosal Immunol. 2022 May;15(5):857–66. doi: https://doi.org/10.1038/s41385-022-00549-0
20. Togan T, Azap OK, Durukan E, Arslan H. The prevalence, etiologic agents and risk factors for urinary tract infection among spinal cord injury patients. Jundishapur J Microbiol. 2014 Jan;7(1):e8905. doi: https://doi.org/10.5812/jjm.8905
21. Sartori AM, Padilla-Fernández B, ’t Hoen L, Blok BF, Castro-Díaz DM, Del Popolo G, et al. Definitions of Urinary Tract Infection Used in Interventional Studies Involving Neurourological Patients-A Systematic Review. Eur Urol Focus. 2022 Sep;8(5):1386–98. doi: https://doi.org/10.1016/j.euf.2021.07.012
22. Sellami M, Puce L, Bragazzi NL. Immunological Response to Exercise in Athletes with Disabilities: A Narrative Review of the Literature. Healthcare (Basel). 2023 Jun;11(12):1692. doi: https://doi.org/10.3390/healthcare11121692
23. Pannek J, Wöllner J. Management of urinary tract infections in patients with neurogenic bladder: challenges and solutions. Res Rep Urol. 2017 Jul;9:121–7. doi: https://doi.org/10.2147/RRU.S113610
24. Hertig-Godeschalk A, Perret C. How Elite Athletes with a Spinal Cord Injury Sweat during Exercise-An Exploratory Study. Sports (Basel). 2024 Mar;12(3):81. doi: https://doi.org/10.3390/sports12030081
25. Williams G, Stothart CI, Hahn D, Stephens JH, Craig JC, Hodson EM. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2023 Nov;11(11):CD001321.
26. Hill TC, Baverstock R, Carlson KV, Estey EP, Gray GJ, Hill DC, et al. Best practices for the treatment and prevention of urinary tract infection in the spinal cord injured population: the Alberta context. Can Urol Assoc J. 2013;7(3-4):122–30. doi: https://doi.org/10.5489/cuaj.337
27. Everaert K, Lumen N, Kerckhaert W, Willaert P, van Driel M. Urinary tract infections in spinal cord injury: prevention and treatment guidelines. Acta Clin Belg. 2009;64(4):335–40. doi: https://doi.org/10.1179/acb.2009.052
28. Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2012 Oct;10(10):CD003265. doi: https://doi.org/10.1002/14651858.CD003265.pub3
29. Toh SL, Lee BB, Ryan S, Simpson JM, Clezy K, Bossa L, et al. Probiotics [LGG-BB12 or RC14-GR1] versus placebo as prophylaxis for urinary tract infection in persons with spinal cord injury [ProSCIUTTU]: a randomised controlled trial. Spinal Cord. 2019 Jul;57(7):550–61. doi: https://doi.org/10.1038/s41393-019-0251-y
30. Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018 Dec;15(12):750–76. doi: https://doi.org/10.1038/s41585-018-0106-x
31. Schwellnus M, Soligard T, Alonso JM, Bahr R, Clarsen B, Dijkstra HP, et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br J Sports Med. 2016 Sep;50(17):1043–52. doi: https://doi.org/10.1136/bjsports-2016-096572
32. Perret C. Elite-adapted wheelchair sports performance: a systematic review. Disabil Rehabil. 2017 Jan;39(2):164–72. doi: https://doi.org/10.3109/09638288.2015.1095951
33. Barbieri A, Fuk A, Gallo G, Gotti D, Meloni A, La Torre A, et al. Cardiorespiratory and metabolic consequences of detraining in endurance athletes. Front Physiol. 2024 Jan;14:1334766. doi: https://doi.org/10.3389/fphys.2023.1334766
34. Raysmith BP, Drew MK. Performance success or failure is influenced by weeks lost to injury and illness in elite Australian track and field athletes: A 5-year prospective study. J Sci Med Sport. 2016 Oct;19(10):778–83. doi: https://doi.org/10.1016/j.jsams.2015.12.515
35. Massa LM, Hoffman JM, Cardenas DD. Validity, accuracy, and predictive value of urinary tract infection signs and symptoms in individuals with spinal cord injury on intermittent catheterization. J Spinal Cord Med. 2009;32(5):568–73. doi: https://doi.org/10.1080/10790268.2009.11754562
36. Saw AE, Main LC, Gastin PB. Monitoring the athlete training response: subjective self-reported measures trump commonly used objective measures: a systematic review. Br J Sports Med. 2016 Mar;50(5):281–91. doi: https://doi.org/10.1136/bjsports-2015-094758