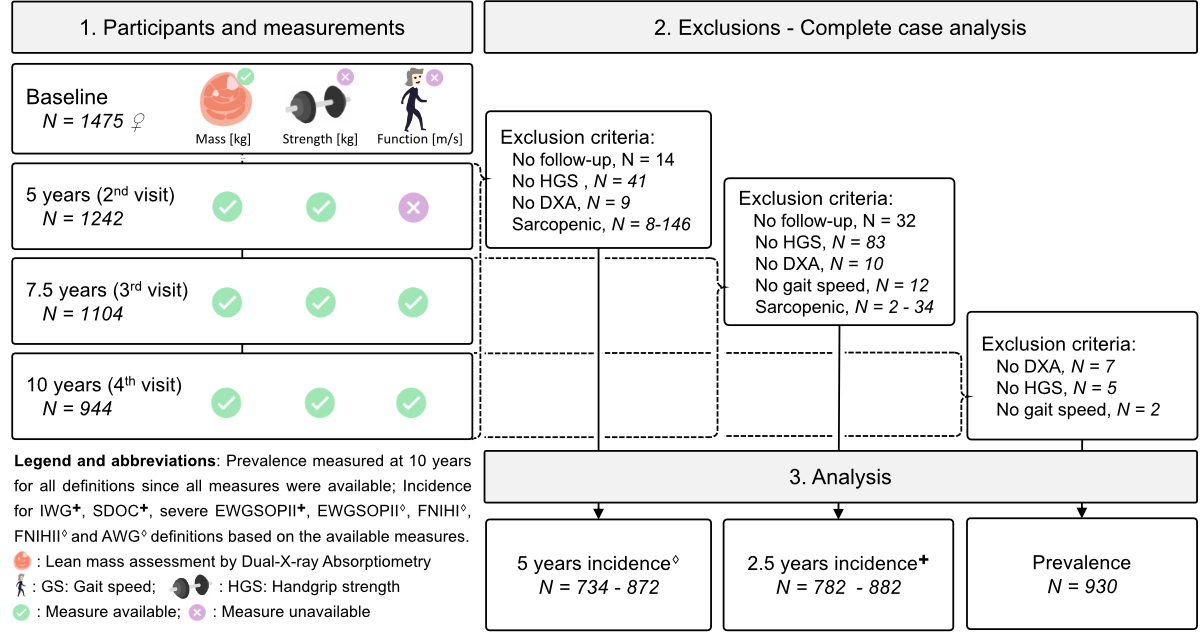
Figure 1Flowchart of study participants, data collection, exclusions and analysis.
DOI: https://doi.org/https://doi.org/10.57187/s.4034
Sarcopenia was first mentioned in 1989 by Rosenberg as the loss of muscle mass associated with ageing [1]. Since then, its operational definition has evolved to encompass the progressive and generalised decline in muscle mass, strength and function [2]. Beyond ageing, the multifactorial physiopathology of sarcopenia also includes a wide range of diseases and behaviours, including inflammatory, osteoarticular and neurologic conditions, physical inactivity, sedentary lifestyle and malnutrition [2]. Since 2016, sarcopenia has been recognised as a muscular disease with an ICD-10-MC diagnosis code, enabling care to be billed in some countries [3]. However, the conceptual and operational definitions of sarcopenia lack global consensus [4]. Sarcopenia is associated with an increased risk of falls, fragility fractures, hospitalisations and mortality [5–8]. It significantly impacts quality of life and limits daily activities for affected individuals. Although no widely accepted pharmacological treatment exists, outcomes related to sarcopenia are known to be reversible or preventable through appropriate nutrition and physical therapy [9]. In the United Kingdom, the additional costs associated with muscular weakness have been estimated at £2707 per person annually, leading to an overall cost of £2.5 billion per year [10]. With an ageing European population, this health, social and economic burden is expected to rise [11].
A 2019 meta-analysis of 58 cohorts from 26 countries estimated the prevalence of sarcopenia to range from 9.9% to 40.4% [12]. This variation largely stems from differences in age, sex and the operational definitions of sarcopenia employed. Existing definitions vary in the muscle health parameters considered, measurement techniques and threshold values [12]. Such variability introduces challenges in establishing reproducible and practical guidelines for sarcopenia management [4]. In the same year, the European Working Group on Sarcopenia in Older People (EWGSOPII) proposed a management algorithm comprising:
In Switzerland, the reported prevalence of sarcopenia ranges from 0.2% to 85%, depending on the population studied and the definition applied (table S1 in the appendix) [13–18]. None of these studies examined incidence, nor did any based on populational-wide cohorts; only two studies applied the most recent definitions from the Sarcopenia Definitions and Outcomes Consortium (SDOC) and the EWGSOPII [2, 6].
This study aims to describe sarcopenia prevalence, five-year incidence and agreement between different definitions, utilising the latest operational criteria in a Swiss population-based cohort of postmenopausal women.
The OsteoLaus study was approved by the Institutional Ethics Committee of the University of Lausanne (reference 215/09) and adheres to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines (see appendix).
OsteoLaus is a sub-study of the CoLaus|PsyCoLaus study, a prospective populational-based cohort initiated in 2003 to investigate the determinants of cardiovascular and psychiatric diseases. This study enrolled 6733 men and women aged 35–75 years, residing in Lausanne, Switzerland, with follow-ups conducted every 5 years [19]. OsteoLaus is a prospective study focused on bone health, aiming to improve fracture risk modelling [20]. All women aged 50–80 years from the CoLaus|PsyColaus cohort were invited to participate in OsteoLaus. Of the 1704 women initially invited, 1500 (88%) accepted and 1475 were ultimately included in the study, with 98.4% of participants identifying as Caucasian. OsteoLaus follow-ups occurred every 2.5 years. A flowchart detailing the study population is shown in figure 1.

Figure 1Flowchart of study participants, data collection, exclusions and analysis.
This study includes the data and participants from the second, third and fourth OsteoLaus follow-ups, during which all sarcopenia parameters were available for a complete case analysis. Data from the baseline and first follow-up were excluded, as muscle status was not assessed and different DXA machines were used. The fourth visit served as the baseline for definitions involving gait speed (IWG, SDOC, severe sarcopenia in EWGSOPII), with a mean (standard deviation; SD) follow-up duration of 2.6 (0.4) years and final sample sizes ranging from 782 to 882 participants. The third visit was used for all other definitions, with a longer mean (SD) follow-up duration of 5.1 (0.4) years (April 2015 to October 2022) and final sample sizes from 734 to 872 participants (figure 1 and table 1).
Table 1Characteristics of the study population by analysis type.
| 5-year incidence | 2.5-year incidence | Prevalence | |
| Sample included [min – max] | 734–872 | 782–882 | 930 |
| Visit date range [min – max] | 04.2015–10.2022 | 01.2018–10.2022 | 06.2020–10.2022 |
| Age [years] | 67.7 (6.7) | 70.1 (6.6) | 72.9 (6.9) |
| Body Mass Index [kg/cm2] | 25.7 (4.5) | 25.8 (4.6) | 25.7 (4.8) |
| Appendicular Lean mass [kg] | 17.0 (2.5) | 17.0 (2.5) | 16.8 (2.5) |
| Handgrip strength [kg] | 25.0 (5.4) | 23.4 (5.9) | 21.2 (5.5) |
| Gait speed [m/s] | - | 1.1 (0.2) | 1.1 (0.2) |
| Follow-up duration [years] | 5.1 (0.4) | 2.6 (0.4) | - |
| Diabetes [Y/N] | 4.1% | 5.4% | 5.7% |
| Current tobacco use [Y/N] | 15.4% | 13.0% | 13.1% |
| Alcohol (over 3 units/day) [Y/N] | 4.1% | 3.7% | 3.9% |
| Malabsorption [Y/N] | 5.3% | 5.6% | 6.0% |
| Prolonged immobilisation [Y/N] | 2.8% | 3.0% | 2.9% |
| Glucocorticoids use [Y/N] | 5.2% | 5.7% | 6.7% |
Results expressed as mean (SD); Y/N: yes/no; 2.5-year incidence for IWG, SDOC and severe EWGSOPII definitions; 5-year incidence for EWGSOPII, FNIHI, FNIHII and AWG (figure 1).
Handgrip strength [kg] was measured once in CoLaus, corresponding to the third OsteoLaus follow-up, and twice directly in OsteoLaus during the fourth and fifth follow-ups (figure 1). A JAMAR Baseline® hydraulic hand dynamometer (Fabrication Enterprises, Inc., White Plains, NY, USA) was used. The examiner provided instructions and demonstrated the test before measurement. Each assessment was conducted in the morning, following the guidelines of the American Society of Hand Therapists [21]: participants were seated with shoulders adducted and neutrally rotated, elbows flexed at 90°, forearms in a neutral position, and wrists positioned between 0° and 30° dorsiflexion. During the OsteoLaus assessments, the examiner encouraged participants to exert maximum effort, with each test separated by a 30-second rest. The highest value from three consecutive measurements on the dominant hand was retained for analysis.
Appendicular lean mass (ALM, sum of lean mass in all four limbs) [g] and its indices (ALMI : ALM/ height2, ALM/BMI) were measured during total body composition assessments using DXA (GE Lunar iDXA™) at each OsteoLaus visit (figure 1). The procedure followed the guidelines of the International Society for Clinical Densitometry [22]. Participants wore medical gowns, removed all jewellery and lay supine at the centre of the scanning field with palms facing down and arms slightly separated from the trunk. Ankles were strapped to ensure proper positioning. If any condition was not met, the scan was restarted. Regions of interest (ROIs) were initially defined by the software and adjusted by the technician as necessary.
Gait speed (GS) [m/s] was assessed based on the average speed over a 6-metre walk at a normal pace, with participants wearing their own shoes and using any necessary assistive devices. Timing started with the participant's first movement and stopped once they crossed the 6-metre mark. Gait speed was measured at the third and fourth OsteoLaus visits (figure 1).
At each follow-up, height was measured with a portable stadiometer (Seca version 216, Seca, Chino, CA, USA) to a precision of 0.1 cm and body weight was measured with an electronic scale (Seca Clara 803, Seca, Chino, CA, USA) to a precision of 0.1 kg. Participants were barefoot and wore minimal clothing. Body mass index (BMI) was calculated as weight divided by height squared [kg/m2].
Sarcopenia was defined based on six sets of recommendations (11 definitions): The SDOC, 2020 [6]; the EWGSOPII, 2019 (five sub-definitions) [2]; the Asian Working Group on sarcopenia (AWG), 2019 [23]; the Foundation for the National Institutes of Health sarcopenia project (FNIHII), 2017 (2 sub-definitions) [24]; the FNIHI, 2014 [25]; and the International Working group on sarcopenia (IWG), 2011 [26].
The Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) definition was not included in the analyses, as it closely follows the EWGSOPII algorithm [27]. The criteria and components for each definition are summarised in table 2.
Table 2Prevalence and incidence of sarcopenia definitions in Swiss postmenopausal women from the OsteoLaus cohort.
| Definition, date [ref] | Criteria | Prevalence* (n = 930) | Incident cases* (n = 734 to 882) | Incidence rate*** | |
| 1 year | 5 years | ||||
| SDOC 2020 [6] | HGS <20 kg, Gait speed <0.8 m/s | 62 (6.7%), CI: 5.1–8.3% | 23 (2.9%)2.5yrs, CI: 1.9–4.0% | 1.18% | 5.9% |
| EWGSOP II 2019 [2], probable sarcopenia | HGS <16 kg | 114 (12.3%), CI: 10.2–14.4% | 79 (9.6%)5yrs, CI: 7.7–11.5% | 2.00% | 10.0% |
| Sarcopenia with ALMI | HGS <16 kg, ALM/ht2 <5.5 kg/m2 | 20 (2.2%), CI: 1.2–3.1% | 17 (2.0%)5yrs, CI: 1.1–2.8% | 0.39% | 1.9% |
| Sarcopenia with ALM | HGS <16 kg, ALM <15 kg | 53 (5.9%), CI: 4.2–7.2% | 40 (4.7%)5yrs, CI: 3.3–6.0% | 0.95% | 4.7% |
| Severe sarcopenia with ALMI | SarcopeniaALMI, Gait speed <0.8 m/s | 5 (0.5%), CI: 0.1–1.0% | 2 (0.3%)2.5yrs, CI: 0.0–0.6 | 0.10% | 0.5% |
| Severe sarcopenia with ALM | SarcopeniaALM, Gait speed <0.8 m/s | 14 (1.5%), CI: 0.7–2.3% | 8 (1.0%)2.5yrs, CI: 0.4–1.6% | 0.39% | 2.0% |
| AWG 2019[23] | HGS <18 kg, ALM/ht2 <5.4 kg/m2 | 26 (2.8%), CI: 1.7–3.9% | 20 (2.3%)5yrs, CI: 1.3–3.3% | 0.46% | 2.3% |
| FNIH II 2017 [24], sarcopenia with ALM/BMI | HGS <19.99 kg, ALM/BMI <0.591 | 125 (13.4%), CI: 11.2–15.6% | 80 (10.9%)5yrs, CI: 8.9–12.9% | 2.28% | 11.4% |
| Sarcopenia with ALM | HGS <19.99 kg, ALM <14.10 kg | 82 (8.8%), CI: 7.0–10.6% | 49 (5.8%)5yrs, CI: 4.3–7.3% | 1.18% | 5.9% |
| FNIH I 2014 [25] | HGS <16 kg, ALM/BMI <0.512 | 14 (1.5%) CI: 0.7–2.3% | 9 (1.1%)5yrs, CI: 0.4–1.8% | 0.22% | 1.1% |
| IWG 2011 [26] | ALM/ht2 ≤5.67 kg/m2, Gait speed <0.8m/s | 13 (1.4%) CI: 0.6–2.12% | 8 (0.9%)2.5yrs, CI: 0.3–1.5% | 0.36% | 1.8% |
Sarcopenia definitions, including their parameters and their epidemiology in the OsteoLaus cohort:
* prevalence (absolute cases and percentage with 95% confidence interval [CI]);
** incident cases (absolute cases and percentage with CI);
*** incident rates (new case over the estimated time of exposure);
5yrs: 5 to 10 years visits; 2.5yrs: 7.5 to 10 years visits; HGS: handgrip strength; ALM: appendicular lean mass; ALMI: ALM/height2; BMI: Body Mass Index; SDOC: Sarcopenia Definitions and Outcomes Consortium; EWGSOP: European Working Group on Sarcopenia in Older People II (2019); FNIH: Foundation for the National Institutes of Health Sarcopenia Project I (2014) and II (2017); IWG: International Working Group on Sarcopenia
The datasets and code used in this study are not publicly available but can be shared upon reasonable request (https://www.colaus-psycolaus.ch). Statistical analyses and data visualisations were conducted in Python (v3.10.13) using the pandas (v2.1.4), seaborn (v0.12.2), scipy.stats (v1.11.4) and sklearn (v1.3.0) libraries. As a preliminary qualitative assessment, the distribution and potential outliers of all included variables were visually examined using boxplots and quantile-quantile plots and assessed for normality using the Shapiro-Wilk Test (not shown).
Sarcopenia prevalence was measured cross-sectionally at the latest OsteoLaus follow-up visit, where all muscle assessments were available (figure 1). The final sample for prevalence calculations consisted of a complete case analysis, excluding participants with missing handgrip strength, appendicular lean mass or gait speed measurements. Sarcopenia prevalence was calculated as the number of participants with sarcopenia divided by the total number of participants, reported as a percentage with a confidence interval (CI).
For incidence calculations, sub-datasets were created by excluding participants with prevalent sarcopenia at baseline (from the second or third follow-up) for each sarcopenia definition (table 1). Incident cases represent new cases observed between the second and fourthfollow-ups for eight definitions and between the third and fourth follow-ups for the remaining three definitions (figure 1, table 2). Incidence rates were calculated as the number of new cases, adjusted for the observed duration per participant, measured in person-years. The observed duration for incident cases and participants lost to follow-up was set to half of their follow-up period, whereas non-cases were observed for the entire follow-up duration. Five-year incidence rates were recalculated for simplicity in representation and comparison. Prevalence and incidence for each definition were also analysed by age tertiles. The first and last age tertiles were compared using a two-sided Fisher’s exact test (p <0.05).
Agreement between sarcopenia definitions was visually examined using Venn diagrams to illustrate the overlap among definitions within EWGSOP I and II, FNIH I and II, American/Asian/International, and a combined group of SDOC, EWGSOP II and FNIH II definitions (figure 2). Visual and statistical agreement across all definition pairs was further assessed with pie charts and the Cohen Kappa Score, respectively. Agreement levels were categorised as follows: none (0.00–0.20), minimal (0.21–0.39), weak (0.40–0.59), moderate (0.60–0.79), strong (0.80–0.90) and almost perfect (0.90–1.00) (figure S1 in the appendix) [28].
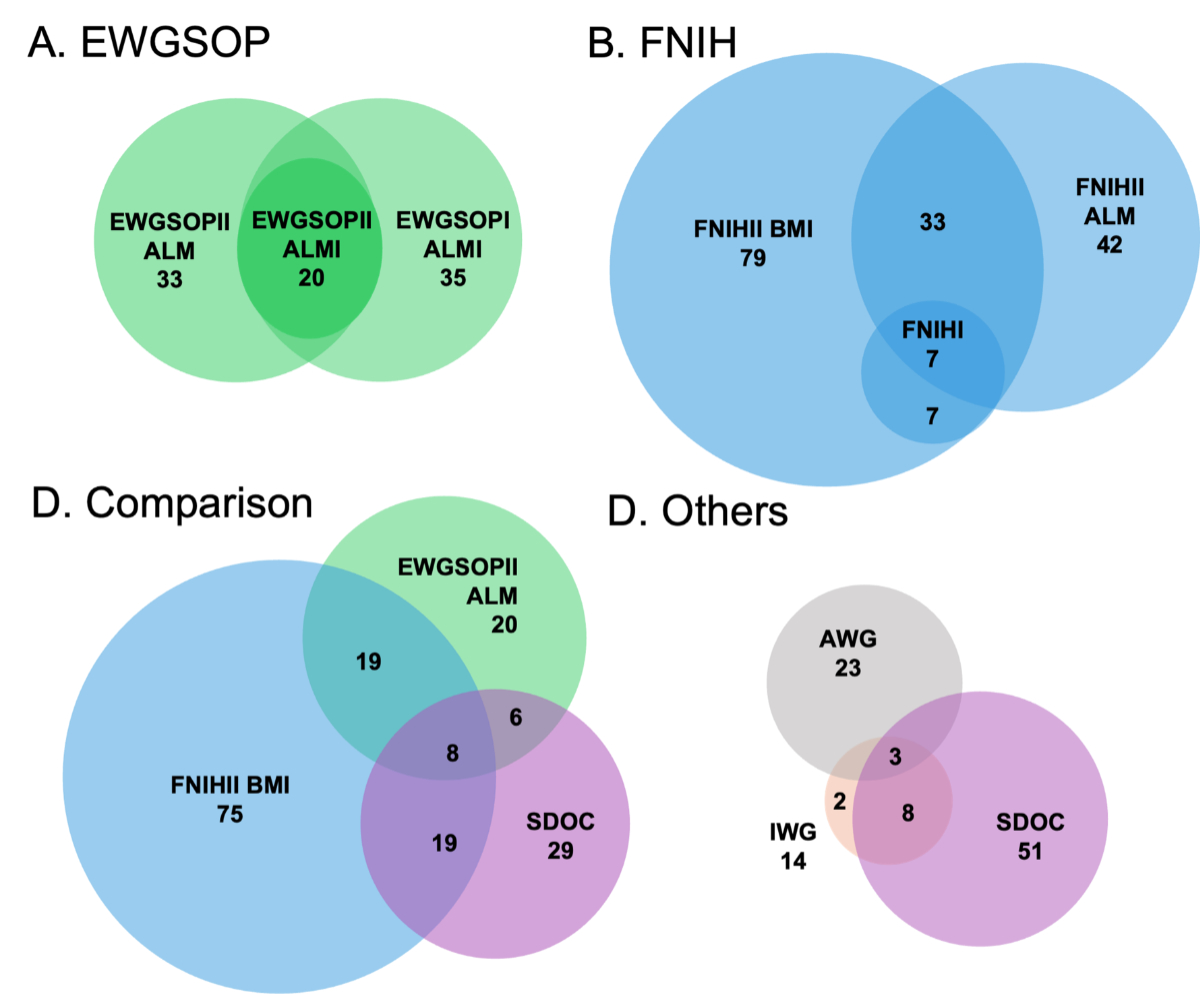
Figure 2Distribution and overlap of OsteoLaus participants based on classification across various sarcopenia definitions. Venn diagrams illustrate the distribution (circle size) and overlap between definitions among 930 Swiss postmenopausal women. See table 2 for complete sarcopenia definitions. EWGSOP: European Working Group on Sarcopenia in Older People; ALM: appendicular lean mass; ALMI: ALM/height2; FNIH: Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project; BMI: Body Mass Index; SDOC: Sarcopenia Definitions and Outcomes Consortium.
The study population for prevalence measurement included 930 postmenopausal women after excluding those with missing measurements of appendicular lean mass (n = 7), handgrip strength (n = 5) and gait speed (n = 2) (figure 1). The mean (SD) values were as follows: age 72.9 (6.9) years, BMI 25.7 (4.8) kg/m2, appendicular lean mass 16.8 (2.5) kg, handgrip strength 21.2 (5.5) kg and gait speed 1.1 (0.2) m/s (table 1). Additional participant characteristics are detailed in the OsteoLaus cohort profile [20]. Sarcopenia definitions, criteria, prevalence and incidence are summarised in table 2. The prevalence of sarcopenia varied depending on the definition used, ranging from 1.4% (IWG) to 13.4% (FNIHII). The SDOC definition, which incorporates gait speed and handgrip strength, classified 6.7% of women as sarcopenic.
EWGSOPII includes five definitions:
Prevalence significantly increased with age for most definitions (p <0.05), except for severe sarcopenia in EWGSOPII with ALMI and FNIHI definitions (table S2 in the appendix). Comparing the oldest and youngest age tertiles, prevalence was 2.9 (FNIHII with BMI) to 9.0 (SDOC) times higher in the oldest tertile. The prevalence of EWGSOPII with appendicular lean mass was 5.2 times higher in the oldest tertile compared to the youngest.
The study populations for incidence measurement varied due to exclusions based on missing measurements and the removal of baseline sarcopenic participants (figure 1). A detailed summary of incident cases and rates is provided in table 2. The 5-year incidence rate was 5.9% for the SDOC definition. For EWGSOPII, the corresponding incidence rates were 10.0% for probable sarcopenia, 1.9% for sarcopenia with ALMI, 4.7% with appendicular lean mass, 0.5% for severe sarcopenia with ALMI, and 2.0% with appendicular lean mass. Incidence rates for other definitions ranged from 1.1% to 11.4%. Incidence also significantly increased with age for most definitions (p <0.05), except for severe sarcopenia in EWGSOPII with ALMI and the FNIHI definitions (table S3 in the appendix). Comparing the oldest and youngest age tertiles, incidence was 2.3 (IWG) to 14.0 (SDOC) times higher in the oldest tertile. The incidence of EWGSOPII with appendicular lean mass was 5.5 times higher in the oldest tertile. In a supplementary analysis (figure S2 in the appendix), handgrip strength, appendicular lean mass and gait speed were all negatively associated with age, as shown by univariate linear regression (β coefficient: –0.36 to –0.01, p <0.001).
The greatest visual and numerical overlap occurred within definitions from the same working groups. In the Venn diagram, all participants classified as sarcopenic according to EWGSOPII with ALMI were also included within the EWGSOPI with appendicular lean mass and EWGSOPII with appendicular lean mass definitions (figure 2A). Similarly, the FNIHI definitions were fully encompassed by the FNIHII BMI definition (figure 2B). Comparisons across different consensus groups showed less overlap (figure 2C). Among the 55 possible combinations of definitions (figure S1), agreement levels were as follows: “none” for 31 combinations, “minimal” for 15 combinations, “weak” for 7 combinations and “moderate” for 2 combinations. No combination achieved “strong” or “almost perfect” agreement. Agreement scores between pairs of definitions ranged from 0.02, when comparing the FNIHII definition based on appendicular lean mass/BMI with the EWGSOPII severe sarcopenia with ALMI definition, to 0.69, when comparing EWGSOPII based on ALMI to the AWG definition.
In this study of 930 Swiss postmenopausal women, the prevalence of sarcopenia was 2.2% based on the latest EWGSOPII definition with ALMI, with an incidence of 1.9% over the previous 5 years using the same definition. In comparison, the DO-HEALTH study, which included 549 Swiss community-dwelling men and women with a mean age of 74.0 years, reported a sarcopenia prevalence of 0.9% using the EWGSOPII with the ALMI definition [13]. In another study examining a subset of Swiss women using the same definition, prevalence was 9.1% among 66 women in a geriatric rehabilitation hospital with a mean age of 84.4 years [18]. In the oldest age tertile mean age 80.7 (SD 3.5) of the current OsteoLaus study, the prevalence of sarcopenia by EWGSOPII with ALMI was 4.5%. The higher prevalence in the previous study may be explained by the older age and higher comorbidities in a hospitalised population, both of which increase sarcopenia risk [18].
Unlike most previous Swiss studies, our participants were not selected via convenience sampling or hospitalisation. Additionally, while cohort studies typically include healthier individuals than the general population, the proportions of participants with diabetes, alcohol consumption and tobacco use in this study were comparable to a national survey for similar age and sex demographics [29]. Therefore, it is likely that the reported prevalence and incidence rates are only slightly underestimated. Considering these sampling differences and the 5-year incidence of 1.9% for EWSOPII with ALMI, our findings are comparable to the previous similar study [18]. Other Swiss studies used older definitions, included men, and are thus not directly comparable [14–17] (table S1).
Both prevalence and incidence rates increased across age tertiles for most definitions (tables S2 and S3 in the appendix). More specifically, greater incidence rates and differences across age tertiles were observed with definitions incorporating muscle strength (SDOC, probable sarcopenia) or higher muscle mass thresholds (FNIHII, AWG), as opposed to definitions with lower muscle mass thresholds (FNIHI, EWGSOPII). As shown by the linear regression in figure S2 in the appendix and reported in previous population studies, muscle strength declines more rapidly with age than muscle mass [30, 31]. Moreover, previous studies have suggested that age is linearly or even exponentially associated with the rate of muscle mass loss [32]. In line with these hypotheses, the prevalences measured at the end of the OsteoLaus follow-up were similar to the incidences over the 5-years period, suggesting that most women developed sarcopenia during the follow-up. This decline in muscle health appears to accelerate from the seventh decade, as indicated by our findings and previous studies [30, 31]. Further studies are needed to continuously monitor and estimate the population trends in muscle health, particularly in high-risk subgroups.
Definitions with poorer muscle health cutoffs were generally encompassed within those with healthier cutoffs, as reflected by the greater overlap in Venn diagrams and higher Kappa Scores. However, the Venn diagrams typically showed limited overlap, and most agreements between definitions were classified as “none” or “minimal,” suggesting that the various definitions may not be capturing the same construct [12]. The debate on the definitions of sarcopenia extends beyond the inclusion of the different parameters (muscle mass, strength and/or function), also encompassing their possible correction for body morphology (height and weight), and the statistical basis of their thresholds based on population lower standard deviations [2] or the discrimination of adverse events [6]. Additionally, there is ongoing discussion regarding the independent, additive or synergistic roles of sarcopenia in relation to closely linked conditions such as physical inactivity, sedentary behaviour, cachexia, malnutrition and frailty [33, 34]. To address these points, the Global Initiative on Sarcopenia (GLIS) was established in 2021, comprising a large panel of international societies and experts involved in previous definitions. GLIS aims to establish a consensus on the conceptual and operational definitions of sarcopenia [4, 35]. The conceptual framework proposed by GLIS includes muscle mass, strength and muscle-specific strength (e.g. muscle strength relative to muscle size) as defining elements, while muscle function is considered as an outcome rather than a defining criterion. This approach contrasts with definitions from SDOC and EWGSOPII, where muscle function is a core component. The next phase for GLIS is to develop a new operational definition of sarcopenia that is broadly accepted worldwide [4].
A broader discussion on the high prevalence and incidence rates of sarcopenia is essential, given its substantial economic and societal burden [5, 10]. At the individual level, a systematic review of 130 studies on sarcopenia risk factors and consequences has highlighted its additional negative impact on multiple acute and chronic health conditions [8]. Consequently, the presence of sarcopenia can become a critical factor in medical decision-making and treatment allocation. Currently, the most effective response to sarcopenia lies in public health strategies, as prevention and management are largely behavioural, focusing on optimising physical activity and nutrition [36, 37]. No pharmacological treatments are available [38], and an operational consensus on its definition is yet to be reached [4]. For example, the WHO Global Action Plan on Physical Activity 2018–2030 advocates for the development and implementation of national guidelines, broad communication campaigns on physical activity, mass participation events and accessible and affordable physical activity opportunities [39]. By establishing the current prevalence and incidence of sarcopenia in Switzerland, this study provides essential data to support ongoing and future public health initiatives with potential benefits for sarcopenia and muscle health more broadly. Additionally, these solutions can target muscle health in older people as well as across the lifespan, including at younger ages during peak muscle mass formation [40].
The primary strength of this study is the recency of the OsteoLaus cohort, which minimises historical bias. Additionally, it is the first study in Switzerland to assess sarcopenia prevalence and incidence in a general population recruited via random sampling. The OsteoLaus cohort design also offers several advantages, including a large sample size, high-quality data collection and close collaboration with its umbrella cohort, the CoLaus study.
The main limitations include the absence of male participants and limited ethnic diversity in the OsteoLaus cohort. Prevalence is known to vary by sex and population, but the homogeneity of this sample reduces the need for stratification. Another limitation is the lack of gait speed assessment at baseline, which required prevalence to be measured at the most recent visit to allow for comparison across all sarcopenia definitions, including IWG, SDOC and severe sarcopenia from EWGSOPII. Lastly, prevalence may be underestimated in the severe sarcopenia definition (EWGSOPII), as additional physical performance tests beyond gait speed could be used for the severity criterion.
This study of 930 Swiss postmenopausal women demonstrated a 2.2–5.7% prevalence of sarcopenia based on the EWGSOPII definition, with an incidence of 1.9–4.7% over 5 years. A tenfold variation was observed across different definitions and a tenfold increase in prevalence was noted when comparing the oldest to the youngest age subgroups. Given current demographic shifts and increasing life expectancy, the societal and individual burden of sarcopenia is expected to grow and should be carefully monitored. The lack of consensus and minimal agreement across definitions highlights the need for a standardised operational definition, which would improve clinical management and refine research priorities. The EWGSOPII definition is particularly suitable for further European studies, as the most recent and European-centred standard.
Considering the multiple adverse outcomes associated with sarcopenia, the absence of pharmacological therapies and the lack of a clear clinical implementation pathway, this study emphasises the importance of public health strategies in promoting and preserving muscle health.
We thank Marie Metzger, the study nurse who organised and performed all OsteoLaus visits throughout the study, as well as the CoLaus cohort team for their important contributions to the OsteoLaus cohort. We also acknowledge the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF) for recognising this work through a Young Investigator Award at the WCO-IOF-ESCEO 2023 Congress in Barcelona.
This study was funded by the Swiss National Science Foundation (SNSF 32473B_156978 and 320030_188886).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr;147(8):755–63.
2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al.; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan;48(1):16–31.
3. B. Vellas, R.A. Fielding, C. Bens, R. Bernabei, P.M. Cawthon, T. Cederholm, et al. Implications of ICD-10 for sarcopenia clinical practice and clinical trials: report by the international conference on frailty and sarcopenia research task force 2017. https://doi.org/. doi: https://doi.org/10.14283/jfa.2017.30
4. Kirk B, Cawthon PM, Arai H, Ávila-Funes JA, Barazzoni R, Bhasin S, et al.; Global Leadership Initiative in Sarcopenia (GLIS) group. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing. 2024 Mar;53(3):afae052.
5. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS One. 2017 Jan;12(1):e0169548.
6. Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: an SDOC Analysis. J Am Geriatr Soc. 2020 Jul;68(7):1429–37.
7. Vendrami C, Shevroja E, Gonzalez Rodriguez E, Gatineau G, Elmers J, Reginster J, et al. Muscle parameters in fragility fracture risk prediction in older adults: A scoping review. J Cachexia Sarcopenia Muscle 2024:jcsm.13418. https://doi.org/.
8. Yuan S, Larsson SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. 2023 Jul;144:155533.
9. Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al.; IOF-ESCEO Sarcopenia Working Group. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017 Jun;28(6):1817–33.
10. Pinedo-Villanueva R, Westbury LD, Syddall HE, Sanchez-Santos MT, Dennison EM, Robinson SM, et al. Health Care Costs Associated With Muscle Weakness: A UK Population-Based Estimate. Calcif Tissue Int. 2019 Feb;104(2):137–44.
11. OECD. OECD Economic Surveys: Switzerland 2019. OECD; 2019. https://doi.org/.
12. Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019 Jan;48(1):48–56.
13. Stuck AK, Tsai LT, Freystaetter G, Vellas B, Kanis JA, Rizzoli R, et al. Comparing Prevalence of Sarcopenia Using Twelve Sarcopenia Definitions in a Large Multinational European Population of Community-Dwelling Older Adults. J Nutr Health Aging. 2023;27(3):205–12.
14. Bertschi D, Kiss CM, Beerli N, Kressig RW. Sarcopenia in hospitalized geriatric patients: insights into prevalence and associated parameters using new EWGSOP2 guidelines. Eur J Clin Nutr. 2021 Apr;75(4):653–60.
15. Wearing J, Konings P, de Bie RA, Stokes M, de Bruin ED. Prevalence of probable sarcopenia in community-dwelling older Swiss people - a cross-sectional study. BMC Geriatr. 2020 Aug;20(1):307.
16. Hars M, Biver E, Chevalley T, Herrmann F, Rizzoli R, Ferrari S, et al. Low Lean Mass Predicts Incident Fractures Independently From FRAX: a Prospective Cohort Study of Recent Retirees. J Bone Miner Res. 2016 Nov;31(11):2048–56.
17. Graf CE, Pichard C, Herrmann FR, Sieber CC, Zekry D, Genton L. Prevalence of low muscle mass according to body mass index in older adults. Nutrition. 2017 Feb;34:124–9.
18. Stuck AK, Mäder NC, Bertschi D, Limacher A, Kressig RW. Performance of the EWGSOP2 Cut-Points of Low Grip Strength for Identifying Sarcopenia and Frailty Phenotype: A Cross-Sectional Study in Older Inpatients. Int J Environ Res Public Health. 2021 Mar;18(7):3498.
19. Firmann M, Mayor V, Vidal PM, Bochud M, Pécoud A, Hayoz D, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008 Mar;8(1):6.
20. Shevroja E, Marques-Vidal P, Aubry-Rozier B, Hans G, Rivadeneira F, Lamy O, et al. Cohort profile: the OsteoLaus study. Int J Epidemiol. 2019;48(4):1046–1047g.
21. Therapists AS of H. MacDermid J, Solomon G, Valdes K. Clinical Assessment Recommendations. American Society of Hand Therapists; 2015.
22. Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, et al. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J Clin Densitom. 2019;22(4):453–71.
23. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300–307.e2.
24. Shaffer NC, Ferrucci L, Shardell M, Simonsick EM, Studenski S. Agreement and Predictive Validity Using Less Conservative FNIH Sarcopenia Project Weakness Cutpoints. J Am Geriatr Soc. 2017;65:574–9.
25. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014 May;69(5):547–58.
26. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al.; International Working Group on Sarcopenia. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011 May;12(4):249–56.
27. Daly RM, Iuliano S, Fyfe JJ, Scott D, Kirk B, Thompson MQ, et al. Screening, Diagnosis and Management of Sarcopenia and Frailty in Hospitalized Older Adults: Recommendations from the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) Expert Working Group. J Nutr Health Aging. 2022;26(6):637–51.
28. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–82. doi: https://doi.org/10.11613/BM.2012.031
29. Healthcare - Pocket Statistics. 2024. Neuchâtel: Bundesamt für Statistik (BFS); 2024.
30. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006 Oct;61(10):1059–64.
31. Kim KM, Lim S, Oh TJ, Moon JH, Choi SH, Lim JY, et al. Longitudinal Changes in Muscle Mass and Strength, and Bone Mass in Older Adults: Gender-Specific Associations Between Muscle and Bone Losses. J Gerontol A Biol Sci Med Sci. 2018 Jul;73(8):1062–9.
32. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012 Jul;3:260.
33. G. Faxén-Irving, Y. Luiking, H. Grönstedt, E. Franzén, Å. Seiger, S. Vikström, et al. Do malnutrition, sarcopenia and frailty overlap in nursing-home residents? 2020. https://doi.org/. doi: https://doi.org/10.14283/jfa.2020.45
34. Ida S, Imataka K, Morii S, Katsuki K, Murata K. Frequency and Overlap of Cachexia, Malnutrition, and Sarcopenia in Elderly Patients with Diabetes Mellitus: A Study Using AWGC, GLIM, and AWGS2019. Nutrients. 2024 Jan;16(2):236.
35. Cawthon PM, Visser M, Arai H, Ávila-Funes JA, Barazzoni R, Bhasin S, et al. Defining terms commonly used in sarcopenia research: a glossary proposed by the Global Leadership in Sarcopenia (GLIS) Steering Committee. Eur Geriatr Med. 2022 Dec;13(6):1239–44.
36. Shen Y, Shi Q, Nong K, Li S, Yue J, Huang J, et al. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J Cachexia Sarcopenia Muscle. 2023 Jun;14(3):1199–211.
37. Nasimi N, Sohrabi Z, Nunes EA, Sadeghi E, Jamshidi S, Gholami Z, et al. Whey Protein Supplementation with or without Vitamin D on Sarcopenia-Related Measures: A Systematic Review and Meta-Analysis. Adv Nutr. 2023 Jul;14(4):762–73.
38. Bahat G, Ozkok S. The Current Landscape of Pharmacotherapies for Sarcopenia. Drugs Aging. 2024 Feb;41(2):83–112.
39. World Health Organization. Global action plan on physical activity 2018–2030: more active people for a healthier world. Geneva: World Health Organization; 2018.
40. Office fédéral du sport OFSPO, Office fédéral de la santé publique OFSP, Promotion Santé Suisse, Bureau de prévention des accidents bpa, Réseau suisse Santé et activité physique hepa. Recommandations suisses en matière d’activité physique - Bases. Macolin: OFSPO; 2022.
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.4034.