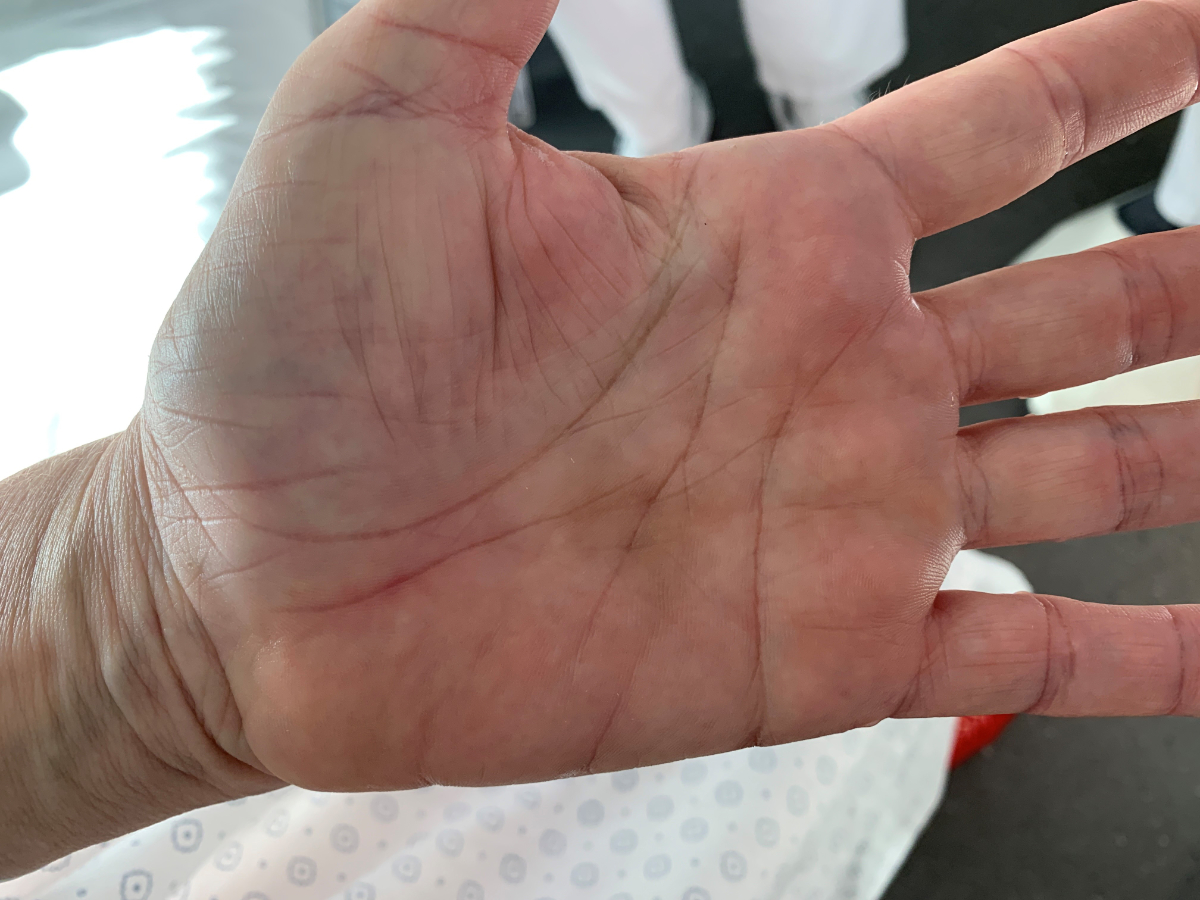
Figure 1Janeway lesions.
DOI: https://doi.org/https://doi.org/10.57187/s.4015
Bacillus cereus, a gram-positive rod-shaped bacterium, exhibits aerobic and facultative anaerobic characteristics. It generates dormant spores in response to adverse environmental conditions like heat and dryness, enabling its survival for extended periods. B. cereus belongs to the Bacillus cereus group, which also includes Bacillus anthracis, the causative agent of anthrax [1]. B. cereus was recognised as a pathogenic organism in 1963, and is no longer considered solely a contaminant [2]. It causes gastrointestinal issues, primarily through food-poisoning toxins [3], but is also an opportunistic pathogen that causes local infections and, less frequently, severe systemic infections [1]. Local infections can arise from post-surgical wounds, traumatic injuries, or burns [4]. Systemic infections include bacteraemia (more common in immunocompromised patients than intravenous drug users) [5], meningitis [6], encephalitis, respiratory infections, osteomyelitis, brain and liver abscess, pericarditis [7], and endocarditis [8–10].
Bacilli are ubiquitous and therefore frequently cause contamination in the laboratory and of paraphernalia used in intravenous drug consumption [11]. Bacillus sp. has historically been the most common bacterial contaminant, being found on 47% of injection paraphernalia [12].
A 62-year-old female presented to our emergency department with fever and chills. She reported feeling weak for several days and experiencing night sweats. She had a history of weekly intravenous cocaine use.
In 2021, the patient had Staphylococcus aureus bacteraemia with an epidural abscess in the cervical spine due to spondylodiscitis. At that time, there was also suspicion of tricuspid valve endocarditis. In 2022, the patient experienced a recurrence of S. aureus bacteraemia with probable mitral valve endocarditis, gonarthritis, and a cervical spine abscess.
Clinical examination revealed Janeway lesions and Osler nodes (figures 1 and 2) and possibly splinter haemorrhages, which were difficult to assess due to dry and brittle nails. Transoesophageal echocardiography showed a vegetation on the aortic valve (on the right coronary cusp, sized 8 × 6 mm) and changes of the mitral and tricuspid valve suggestive of involvement of these valves (figure 3). Laboratory results showed a slightly elevated CRP (25 mg/l) with a normal white blood cell count. Due to initial suspicion of recurrent S. aureus endocarditis, antibiotic therapy with co-amoxicillin 2.2 g quad 4 h was initiated upon admission.

Figure 1Janeway lesions.
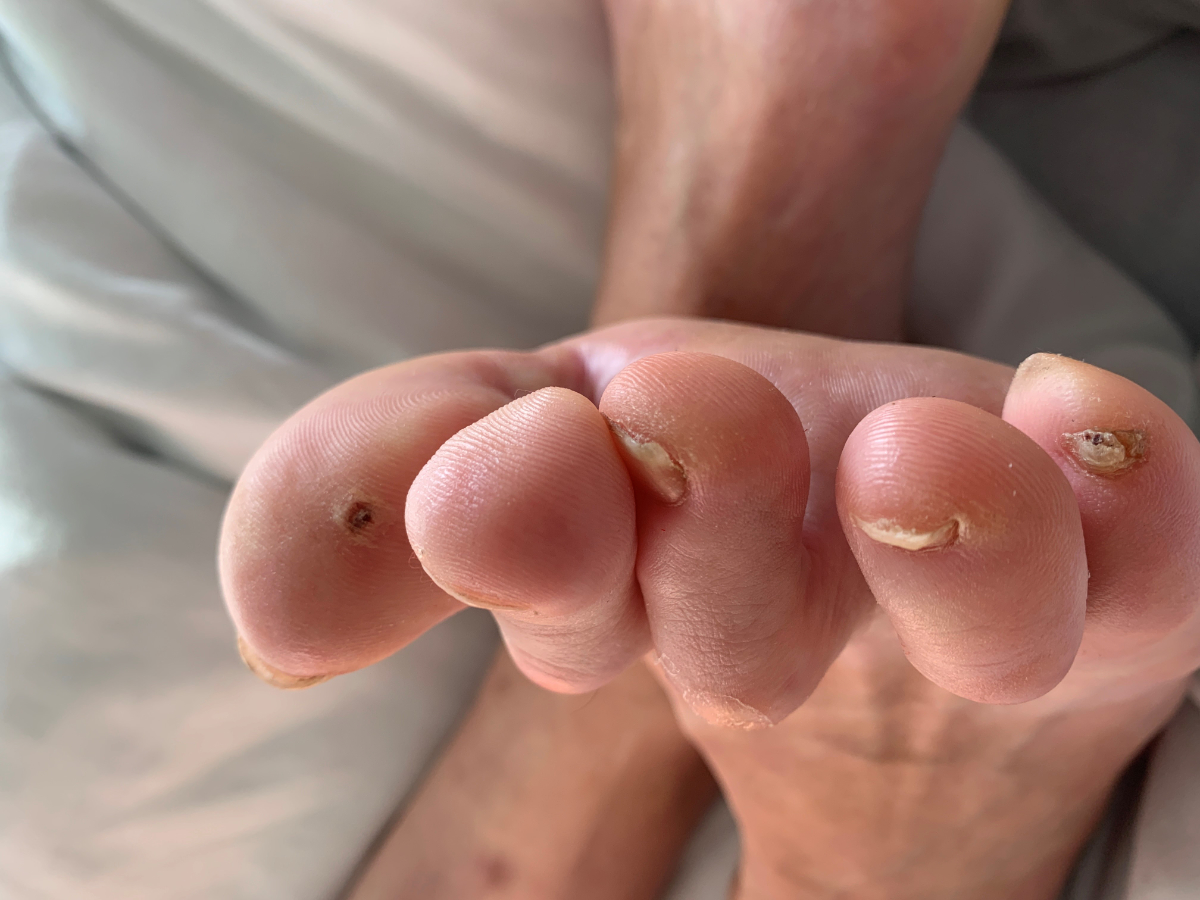
Figure 2Osler node.
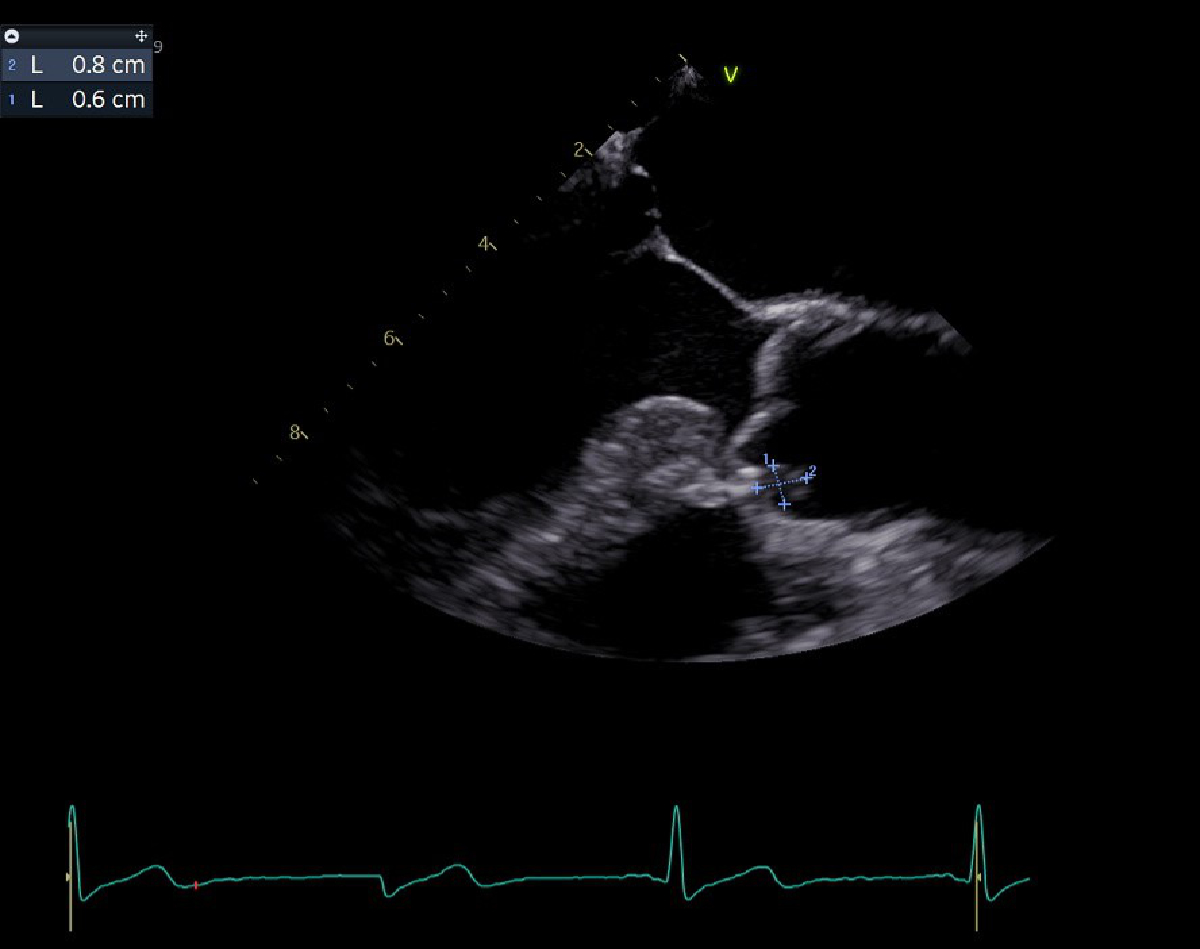
Figure 3Transoesophageal image of vegetation.
B. cereus was identified in 6/6 blood cultures collected upon the patient’s admission, and blood cultures obtained 48h later also yielded positive results. The antibiogram indicated that B. cereus was susceptible to vancomycin and clindamycin, and with increased dosage, also susceptible to ciprofloxacin and levofloxacin but resistant to imipenem and co-amoxicillin. With 2 positive major Duke criteria [13] (vegetations in echocardiography, positive blood cultures in 4 sets) and 4 fulfilled minor criteria (fever, Janeway lesions, previous suspicion of endocarditis, intravenous drug use), the diagnosis of endocarditis was confirmed.
The therapy was supplemented with vancomycin after the first positive blood culture result. Vancomycin monotherapy was continued throughout with twice daily administration with close monitoring of serum levels (2–3 g/d, target level 15–20 mg/l). Under this therapy, there were recurrent subfebrile temperatures without an increase in inflammatory markers. No new aspects such as septic emboli or other infection foci were identified.
After 4 weeks of vancomycin treatment, a symmetrical generalised exanthema occurred, which we interpreted as vancomycin infusion reaction, which is a common adverse non-allergic reaction to vancomycin. It is characterised by flushing, erythema, and maculopapular rash as seen in our patient. There were no signs such as pustules or mucosa involvement, making a more severe differential diagnosis like acute generalised exanthematous pustulosis (AGEP) or drug reaction with eosinophilia and systemic symptoms (DRESS) highly unlikely. Due to the mild reaction, we followed Guidelines and continued vancomycin at a reduced infusion rate. We also initiated therapy with prednisone and an antihistamine, which resulted in a reduction of the rash.
From two days following the start of treatment, all subsequent blood cultures remained negative. After 5 weeks, the patient developed a fever, and SARS-CoV-2 was diagnosed. The infection progressed without complications in the triple-vaccinated patient. Vancomycin therapy was discontinued after 6 weeks. Follow-up echocardiography still showed possible small floating residual structures but no complications. Blood cultures 1 and 3 weeks after the end of antibiotic therapy were negative.
The hypothesis of pathogen introduction into the bloodstream through the utilisation of contaminated drug paraphernalia appears highly plausible. Additionally, a risk arises from the storage of cocaine in basements, where the conditions could support the growth of pathogenic spores, especially considering the widespread presence of B. cereus spores in soil and dust. A microbial assessment of the cocaine and paraphernalia used, though pivotal in elucidating causative agents, was unattainable in our case.
B. cereus endocarditis typically involves the mitral valve, succeeded by the aortic and tricuspid valves [14]. Vegetations on the right side of the heart have been associated with intravenous drug use, while those on the left side are predominantly linked to prosthetic valves or implanted devices [15]. In our case, the involvement of the aortic valve could be due to pre-existing damage associated with previous endocarditis, although involvement of the aortic valve was not detected echocardiographically in the earlier episodes.
B. cereus bacteraemia does not always result in severe disease, as evidenced by case reports in which patients refused antibiotic therapy and the bacteraemia was self-limiting and relatively benign [5, 16]. However, cases of fatal bacteraemia have also been described in immunocompetent patients without signs of endocarditis [17]. Therefore, treatment should be considered in patients even without signs of endocarditis in transoesophageal echocardiography, depending on the risk factors. Despite B. cereus’s occurrence as a common contaminant in hospital blood cultures, the presence of multiple positive bottles should be regarded as true bacteraemia [18]. In summary, endocarditis appears to be an unusual consequence of B. cereus bacteraemia [19].
A literature search found 35 reported cases, summarised in table 1. The first case with B. cereus bacteraemia and endocarditis was from 1974 in a female drug addict with atrial septal defect [20]. One year earlier, a case of endocarditis caused by B. subtilis had been reported with a patient with intravenous drug use [21]. Decades earlier three cases (1933–1951) with gram-positive Bacillus endocarditis were described [22]. Steen et al. [11] describes 10 cases of B. cereus endocarditis having been reported up to 1991, six of which were among people with intravenous drug use, and one each with rheumatic heart disease, mechanical mitral valve, porcine aortic valve, and permanent pacemaker. More cases of B. cereus endocarditis have been published since, describing intravenous devices and intravenous drug use as risk factors, as well as valvular and rheumatic heart disease [23] and immunosuppression [24]. In a summary of 38 cases of serious infectious caused by B. cereus, all but one patient had a risk factor [19], but multiple case reports have reported B. cereus endocarditis among patients without known risk factors [14, 25–30].
Table 1Overview of previously reported cases.
| Year | Author | Risk factors | Treatment | Outcome |
| 1974 | Craig et al. [20] | Intravenous drug use | Clindamycin | Recovered |
| 1978 | Block et al. [38] | Mechanical valve | Tobramycin, chloramphenicol | Died |
| 1978 | Tuazon et al. [8] | Intravenous drug use | Nafcillin | Recovered |
| Intravenous drug use | Clindamycin | Recovered | ||
| Intravenous drug use | Clindamycin | Recovered | ||
| Intravenous drug use | Chloramphenicol, gentamicin, erythromycin (patient suffered from endocarditis and endophthalmitis) | Recovered | ||
| 1979 | Wanvarie et al. [23] | Rheumatic heart disease | Penicillin, gentamicin, streptomycin | Died |
| 1979 | Weller et al. [16] | Intravenous drug use | Ampicillin, oxacillin, and gentamicin, then clindamycin and kanamycin, total 4 weeks, no vegetations in echo | Recovered |
| 1982 | Oster et al. [39] | Porcine aortic valve | Clindamycin, surgery | Recovered |
| 1987 | Sliman et al. [19] | ICD and breast implant | Clindamycin, surgery, 4 weeks | Recovered |
| 1992 | Steen et al. [11] | Mechanical valve | Vancomycin, surgery, 6 weeks | Recovered |
| 1993 | Tomomasa et al. [25] | No risk factors | Not reported | Recovered |
| 1994 | Yamamura et al. [40] | Mechanical valve | Amikacin, minocycline | Recovered |
| 1998 | Martin Cadenas et al. [41] | Mechanical valve | Vancomycin, gentamycin, rifampicin, surgery | Recovered |
| 1999 | Castedo et al. [42] | Mechanical valve | Vancomycin, gentamicin, rifampicin, surgery, 6 weeks | Recovered |
| 2005 | Cone et al. [24] | Leukaemia | Penicillin, vancomycin, ciprofloxacin | Died |
| 2007 | Shalev et al. [36] | ICD | Vancomycin, gentamicin, 6 weeks | Recovered |
| 2008 | Abusin et al. [35] | Pacemaker | Cefazolin, 6 weeks | Recovered |
| 2012 | Barraud et al. [43] | Pacemaker | Vancomycin (later replaced with amoxicillin, then piperacillin), gentamicin (later replaced with ofloxacin), surgery | Died |
| 2012 | Thomas et al. [37] | Not intravenous drug use | Daptomycin, ampicillin (later replaced with ceftriaxone), 6 weeks, surgery | Recovered |
| 2012 | Oh et al. [27] | No risk factors | Ceftriaxone, vancomycin, 6 weeks, surgery | Recovered |
| 2013 | Sharma et al. [44] | Leukaemia | Vancomycin, meropenem | Recovered |
| 2013 | Ngow et al. [15] | Former intravenous drug use | Cefuroxime, 6 weeks | Recovered |
| 2015 | Shah et al. [45] | Pregnant intravenous drug use | Daptomycin, then vancomycin, 5 weeks | Recovered |
| 2015 | Kitazawa et al. [26] | No risk factors | Vancomycin, 9 weeks, surgery | Recovered |
| 2016 | Wright et al. [32] | Central venous catheter | Vancomycin, piperacillin-tazobactam, 6 weeks, surgery | Recovered |
| 2017 | Soudet et al. [28] | No risk factors | Piperacillin-tazobactam + teicoplanin, changed to rifampicin + levofloxacine, 6 weeks | Recovered |
| 2018 | Gopinathan et al. [14] | Baby with VSD repair | i.v. vancomycin 10 days, then oral linezolid for 4 weeks | Recovered |
| No risk factors | i.v. vancomycin 6 weeks | Recovered | ||
| 2018 | Ren et al. [29] | No risk factors | Ampicillin, clindamycin, and vancomycin, then only vancomycin, 6 weeks | Recovered |
| 2020 | Nallarajah et al. [30] | No risk factors | Ciprofloxacin, 8 weeks | Recovered |
| 2021 | Meledathu et al. [46] | Mitral valve repair | Vancomycin and piperacillin-tazobactam, then meropenem, then ciprofloxacin, 6 weeks | Recovered |
| 2022 | Ribeiro et al. [47] | Central venous catheter | Vancomycin, 6 weeks | Recovered |
| 2023 | De Carvalho et a. [48] | Pacemaker | Daptomycin, 4 weeks | Recovered |
| 2023 | Current case | Intravenous drug use | Co-amoxicillin, then vancomycin, total 6 weeks | Recovered |
| 2024 | Fukushima [49] | Prosthetic aortic valve | Vancomycin, 6 weeks | Recovered |
ICD: implantable cardioverter-defibrillator; VSD: ventricular septal defect; i.v.: intravenous.
The first case of endocarditis with B. cereus was treated with intravenous clindamycin [20]. Most B. cereus strains seem to be in-vitro susceptible to clindamycin, vancomycin, imipenem, ciprofloxacin, erythromycin, tetracycline, and aminoglycosides (e.g. gentamicin, kanamycin) and chloramphenicol [11, 16, 19, 31]. According to Wright et al. [32], evidence from three studies [31, 33, 34] suggests B. cereus susceptibility to gentamicin, imipenem, and vancomycin, with all 240 strains tested responding to these antibiotics. Characteristically, the bacterium is resistant to beta-lactam antibiotics such as penicillin and cephalosporins due to the secretion of beta-lactamase enzymes [9, 11, 19, 31]. An exception to this appears to be susceptibility to mezlocillin. [31] Sensitivity to newer cephalosporins such as cefazolin has been described and successfully used in treatment [35]. Due to pronounced side effects (e.g. with aminoglycosides, and chloramphenicol), not all susceptible antibiotics are suitable for therapy.
The patient’s adverse reaction to vancomycin highlights the delicate equilibrium clinicians must maintain between drug efficacy and patient tolerance. Such side effects, while not life-threatening, can severely impact patient compliance and comfort. These concerns are particularly pronounced among intravenous drug users due to the challenges in ensuring adherence to treatment regimens. To mitigate these issues, we maintained the original antibiotic despite side effects and administered a six-week treatment within the hospital. This conservative approach was necessitated by limited literature on alternative antibiotics for this specific pathogen. Outpatient intravenous antibiotic treatment emerges as a possibility worthy of consideration. However, this is complicated by the need for twice-daily administration and for regular therapeutic drug monitoring to ensure appropriate dosing and monitoring for toxicity, thus reinforcing the need for inpatient oversight in complex cases such as this.
The successful non-surgical treatment of our patient highlights that conservative management with antibiotics is effective for native valve endocarditis and intravenous drug users. While B. cereus endocarditis typically carries a higher risk in prosthetic valve patients and often necessitates material replacement, conservative treatment can still succeed [35, 36], particularly in those who respond quickly to antibiotics or as an alternative among patients at high risk for peri-operative complications [36]. Nonetheless, valve replacement is sometimes imperative for native valve infections unresponsive to antibiotics alone [14, 27, 37].
One notable limitation is the lack of data, which makes it unclear whether all the reported cases met the Duke criteria for definitive endocarditis. Our case was diagnosed according to the updated Duke criteria 2023 [13].
In the clinical management of endocarditis, consideration of atypical aetiologies like B. cereus is critical, especially in intravenous drug users, who face an escalated risk of developing uncommon infections due to exposure to contaminated paraphernalia. Prompt initiation of antimicrobials attuned to the specific susceptibilities of the pathogen, toxicity profile, and local resistance patterns is essential. It is equally important to carefully assess potential adverse effects associated with the selected antibiotics. This case report, reinforced by a comprehensive literature review, underscores the imperative for ongoing research and the dissemination of knowledge to refine therapeutic strategies for rare infectious diseases and to provide alternatives when severe side effects arise.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
This article received no funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Ehling-Schulz M, Lereclus D, Koehler TM. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol Spectr. 2019 May;7(3):7.3.6. doi: https://doi.org/10.1128/microbiolspec.GPP3-0032-2018
2. Farrar WE Jr. Serious infections due to “non-pathogenic” organisms of the genus Bacillus. Review of their status as pathogens. Am J Med. 1963 Jan;34(1):134–41. doi: https://doi.org/10.1016/0002-9343(63)90047-0
3. Stenfors Arnesen LP, Fagerlund A, Granum PE. From soil to gut: bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev. 2008 Jul;32(4):579–606. doi: https://doi.org/10.1111/j.1574-6976.2008.00112.x
4. Drobniewski FA. Bacillus cereus and related species. Clin Microbiol Rev. 1993 Oct;6(4):324–38. doi: https://doi.org/10.1128/CMR.6.4.324
5. Curtis JR, Wing AJ, Coleman JC. Bacillus cereus bacteraemia. A complication of intermittent haemodialysis. Lancet. 1967 Jan;1(7482):136–8. doi: https://doi.org/10.1016/S0140-6736(67)91036-7
6. Siegman-Igra Y, Lavochkin J, Schwartz D, Konforti N. Meningitis and bacteremia due to Bacillus cereus. A case report and a review of Bacillus infections. Isr J Med Sci. 1983 Jun;19(6):546–51.
7. Fricchione LF, Sepkowitz DV, Gradon JD, Berkowitz LB. Pericarditis due to Bacillus cereus in an intravenous drug user. Rev Infect Dis. 1991;13(4):774. doi: https://doi.org/10.1093/clinids/13.4.774
8. Tuazon CU, Murray HW, Levy C, Solny MN, Curtin JA, Sheagren JN. Serious infections from Bacillus sp. JAMA. 1979 Mar;241(11):1137–40. doi: https://doi.org/10.1001/jama.1979.03290370041026
9. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010 Apr;23(2):382–98. doi: https://doi.org/10.1128/CMR.00073-09
10. Ihde DC, Armstrong D. Clinical spectrum of infection due to Bacillus species. Am J Med. 1973 Dec;55(6):839–45. doi: https://doi.org/10.1016/0002-9343(73)90266-0
11. Steen MK, Bruno-Murtha LA, Chaux G, Lazar H, Bernard S, Sulis C. Bacillus cereus endocarditis: report of a case and review. Clin Infect Dis. 1992 Apr;14(4):945–6. doi: https://doi.org/10.1093/clinids/14.4.945
12. Tuazon CU, Hill R, Sheagren JN. Microbiologic study of street heroin and injection paraphernalia. J Infect Dis. 1974 Mar;129(3):327–9. doi: https://doi.org/10.1093/infdis/129.3.327
13. Fowler VG Jr, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin Infect Dis. 2023 Aug;77(4):518–26. doi: https://doi.org/10.1093/cid/ciad271
14. Gopinathan A, Kumar A, Sen AC, Sudha S, Varma P, Gs S, et al. A Case Series and Review of Bacillus Cereus Endocarditis from India. Open Microbiol J. 2018 Mar;12(1):28–33. doi: https://doi.org/10.2174/1874285801812010028
15. Ngow HA, Wan Khairina WM. Bacillus cereus endocarditis in native aortic valve. J Infect Chemother. 2013 Feb;19(1):154–7. doi: https://doi.org/10.1007/s10156-012-0427-2
16. Weller PF, Nicholson A, Braslow N. The spectrum of Bacillus bacteremias in heroin addicts. Arch Intern Med. 1979 Mar;139(3):293–4. doi: https://doi.org/10.1001/archinte.1979.03630400025013
17. Lede I, Vlaar A, Roosendaal R, Geerlings S, Spanjaard L. Fatal outcome of Bacillus cereus septicaemia. Neth J Med. 2011;69(11):514–6.
18. McDowell RH, Sands EM, Friedman H. Bacillus Cereus. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Evan Sands declares no relevant financial relationships with ineligible companies. Disclosure: Harvey Friedman declares no relevant financial relationships with ineligible companies., 2023.
19. Sliman R, Rehm S, Shlaes DM. Serious infections caused by Bacillus species. Medicine (Baltimore). 1987 May;66(3):218–23. doi: https://doi.org/10.1097/00005792-198705000-00005
20. Craig CP, Lee WS, Ho M. Letter: bacillus cereus endocarditis in an addict. Ann Intern Med. 1974 Mar;80(3):418–9. doi: https://doi.org/10.7326/0003-4819-80-3-418
21. Reller LB. Endocarditis caused by Bacillus subtilis. Am J Clin Pathol. 1973 Nov;60(5):714–8. doi: https://doi.org/10.1093/ajcp/60.5.714
22. Finland M, Barnes MW. Changing etiology of bacterial endocarditis in the antibacterial era. Experiences at Boston City Hospital 1933-1965. Ann Intern Med. 1970 Mar;72(3):341–8. doi: https://doi.org/10.7326/0003-4819-72-3-341
23. Wanvarie S, Rochanawatanon M. Bacillus cereus endocarditis. J Med Assoc Thai. 1979 Jan;62(1):34–8.
24. Cone LA, Dreisbach L, Potts BE, Comess BE, Burleigh WA. Fatal Bacillus cereus endocarditis masquerading as an anthrax-like infection in a patient with acute lymphoblastic leukemia: case report. J Heart Valve Dis. 2005 Jan;14(1):37–9.
25. Tomomasa T, Itoh K, Matsui A, Kobayashi T, Suzuki N, Matsuyama S, et al. An infant with ulcerative colitis complicated by endocarditis and cerebral infarction. J Pediatr Gastroenterol Nutr. 1993 Oct;17(3):323–5.
26. Kitazawa T, Totsuka M, Yoshino Y, Koga I, Ota Y. Bacillus cereus native valve endocarditis with multiple brain infarctions: a case report and a review of the literature. Clin Res Infect Dis. 2015;2(2):1019.
27. Oh DH, Kim MH, Kim YC, Song JE, Ahn JY, Han SH, et al. A Case of Native Valve Infective Endocarditis Caused by Bacillus cereus. Infect Chemother. 2012;44(4):310–4. doi: https://doi.org/10.3947/ic.2012.44.4.310
28. Soudet S, Becquart C, Dezoteux F, Faure K, Staumont-Salle D, Delaporte E. [Bacillus cereus endocarditis and a probable cutaneous gateway]. Ann Dermatol Venereol. 2017 Jan;144(1):45–8. doi: https://doi.org/10.1016/j.annder.2016.09.045
29. Ren B, Lasam G. A Rare Case of Native Mitral Valve Bacillus Cereus Endocarditis Culminating Into a Cerebrovascular Infarction. Cardiol Res. 2018 Jun;9(3):173–5. doi: https://doi.org/10.14740/cr672w
30. Nallarajah J, Mujahieth MI. Bacillus cereus Subacute Native Valve Infective Endocarditis and Its Multiple Complications. Case Rep Cardiol. 2020 Jun;2020:8826956. doi: https://doi.org/10.1155/2020/8826956
31. Weber DJ, Saviteer SM, Rutala WA, Thomann CA. In vitro susceptibility of Bacillus spp. to selected antimicrobial agents. Antimicrob Agents Chemother. 1988 May;32(5):642–5. doi: https://doi.org/10.1128/AAC.32.5.642
32. Wright WF. Central Venous Access Device-Related Bacillus Cereus Endocarditis: A Case Report and Review of the Literature. Clin Med Res. 2016 Jun;14(2):109–15. doi: https://doi.org/10.3121/cmr.2016.1312
33. Luna VA, King DS, Gulledge J, Cannons AC, Amuso PT, Cattani J. Susceptibility of Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus pseudomycoides and Bacillus thuringiensis to 24 antimicrobials using Sensititre automated microbroth dilution and Etest agar gradient diffusion methods. J Antimicrob Chemother. 2007 Sep;60(3):555–67. doi: https://doi.org/10.1093/jac/dkm213
34. Veysseyre F, Fourcade C, Lavigne JP, Sotto A. Bacillus cereus infection: 57 case patients and a literature review. Med Mal Infect. 2015;45(11-12):436–40. doi: https://doi.org/10.1016/j.medmal.2015.09.011
35. Abusin S, Bhimaraj A, Khadra S. Bacillus Cereus Endocarditis in a permanent pacemaker: a case report. Cases J. 2008 Aug;1(1):95. doi: https://doi.org/10.1186/1757-1626-1-95
36. Shalev A, Gilad J, Riesenberg K, Borer A, Kobal S, Schlaeffer F, et al. Conservative management of implantable cardioverter defibrillator-related endocarditis due to Bacillus spp. Infection. 2007 Apr;35(2):114–7. doi: https://doi.org/10.1007/s15010-007-5061-z
37. Thomas BS, Bankowski MJ, Lau WK. Native valve Bacillus cereus endocarditis in a non-intravenous-drug-abusing patient. J Clin Microbiol. 2012 Feb;50(2):519–21. doi: https://doi.org/10.1128/JCM.00657-11
38. Block CS, Levy ML, Fritz VU. Bacillus cereus endocarditis. A case report. S Afr Med J. 1978 Apr;53(14):556–7.
39. Oster HA, Kong TQ. Bacillus cereus endocarditis involving a prosthetic valve. South Med J. 1982 Apr;75(4):508–9. doi: https://doi.org/10.1097/00007611-198204000-00044
40. Yamamura M, Aoki K, Takanashi S, Tadokoro M, Furuta S, Mizokami T. [A case of Bacillus cereus prosthetic valve endocarditis]. Kyobu Geka. 1994 Mar;47(3):232–4.
41. Martín Cadenas P, Sánchez Alor G, Aguilar Ruiz JC, Castedo E, Daza R, Mendaza P. [Endocarditis by Bacillus cereus 1 in prosthetic mitral valve]. Enferm Infecc Microbiol Clin. 1998 Feb;16(2):102–4.
42. Castedo E, Castro A, Martin P, Roda J, Montero CG. Bacillus cereus prosthetic valve endocarditis. Ann Thorac Surg. 1999 Dec;68(6):2351–2. doi: https://doi.org/10.1016/S0003-4975(99)01163-7
43. Barraud O, Hidri N, Ly K, Pichon N, Manea P, Ploy MC, et al. Pacemaker-associated Bacillus cereus endocarditis. Diagn Microbiol Infect Dis. 2012 Nov;74(3):313–5. doi: https://doi.org/10.1016/j.diagmicrobio.2012.08.002
44. Meghana A, Aparna Y, Chandra SM, Sanjeev S. Emergency preparedness and response (EP&R) by pharmacy professionals in India: lessons from the COVID-19 pandemic and the way forward. Res Social Adm Pharm. 2020.
45. Shah M, Patnaik S, Wongrakpanich S, Alhamshari Y, Alnabelsi T. Infective endocarditis due to Bacillus cereus in a pregnant female: A case report and literature review. IDCases. 2015 Oct;2(4):120–3. doi: https://doi.org/10.1016/j.idcr.2015.10.003
46. Meledathu S, Denyer R, Roberts A, Simon G. Polymicrobial native valve endocarditis due to Bacillus cereus and Cardiobacterium hominis. BMJ Case Rep. 2021 Dec;14(12):e245417. doi: https://doi.org/10.1136/bcr-2021-245417
47. Ribeiro RL, Bastos MO, Blanz AM, Rocha JA, Velasco NA, Marre AT, et al. Subacute infective endocarditis caused by Bacillus cereus in a patient with Systemic Lupus Erythematosus. J Infect Dev Ctries. 2022 Apr;16(4):733–6. doi: https://doi.org/10.3855/jidc.15685
48. de Carvalho MT, Fernandes LV, Cavalcante MF, Távora RV, Farias LA, de Carvalho BM. Infectious endocarditis due to Bacillus Cereus in a patient with a pacemaker: case report and literature review. Journal of Health & Biological Sciences. 2023;11(1):1–4. doi: https://doi.org/10.12662/2317-3076jhbs.v11i1.4903.p1-4.2023
49. Fukushima A, Kobayashi T, Otsuka Y, Hosokawa N, Moody S, Takagi M, et al. A case of prosthetic valve endocarditis and aortic abscess due to Bacillus cereus. IDCases. 2024 Apr;36:e01940. doi: https://doi.org/10.1016/j.idcr.2024.e01940