
Figure 1Heart failure (HF) categories according to European Society of Cardiology (ESC) Guidelines 2021 [4]. EF: ejection fraction; LVEF: left ventricular ejection fraction.
DOI: https://doi.org/https://doi.org/10.57187/s.4000
Diabetes is a significant risk factor for heart failure, along with hypertension, atherosclerotic cardiovascular disease, obesity, cardiotoxins (e.g. cardiotoxic anti-cancer therapies, alcohol) and familial predisposition [1–5]. This increased risk of heart failure in diabetes is also evident in the prevalence rates. In the general population, heart failure prevalence is estimated to be between 1.5% and 2%. However, this rate can increase to as high as 30% in individuals over the age of 60 who have diabetes [6, 7], with higher prevalence observed in those with type 1 diabetes compared to type 2 diabetes [8]. In cases of type 2 diabetes, heart failure often manifests as the first cardiovascular condition, preceding stroke, myocardial infarction or peripheral arterial disease [9]. The risk of developing heart failure correlates with the degree of deviation from normal blood glucose levels [8].
In Switzerland, the number of individuals with diabetes is increasing, currently estimated at around 500,000 people [10]. Out of these, approximately 40,000 individuals are affected by type 1 diabetes [10, 11]. The exact number of individuals with both diabetes and heart failure is not yet known. However, recent data from the SwissDiab study reveal that at least one in ten diabetes outpatients receiving tertiary care is affected by heart failure [12]. Within this group, three out of five cases of heart failure are newly diagnosed [12].
The pathophysiological processes in heart failure and diabetes are based on the interplay of various mechanisms [8]. This interaction may result in left ventricular systolic or diastolic dysfunction, occurring independently of other established heart failure aetiologies, such as coronary artery disease or hypertension [8]. Responsible factors include the activation of the renin-angiotensin-aldosterone system (RAAS), mitochondrial dysfunction, oxidative stress, inflammation and accumulation of advanced glycation end-products [8].
Women with diabetes often display more severe endothelial, coronary microvascular and diastolic abnormalities than men, which leads to an increased risk of heart failure in women with diabetes [8]. Moreover, individuals with type 1 diabetes often exhibit structural features of early heart failure with the preserved ejection fraction phenotype and increased left ventricular stiffness, which explains the higher prevalence of heart failure in type 1 diabetes compared to type 2 diabetes [8]. Furthermore, other major risk factors in addition to diabetes itself contribute to the development of heart failure in patients with diabetes, such as obesity, long-term and/or suboptimal diabetes management, insulin therapy, hypertension, hyperlipidaemia and present micro- and/or macrovascular complications (e.g. diabetic kidney disease, coronary artery disease) [8].
The European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic heart failure define heart failure as follows [4]:
Heart failure is not a single pathological diagnosis, but a clinical syndrome consisting of cardinal symptoms (e.g. breathlessness, ankle swelling and fatigue) that may be accompanied by other physical signs (e.g. elevated jugular venous pressure, pulmonary crackles and peripheral oedema). It is due to a structural and/or functional abnormality of the heart that results in elevated intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise.
The classification of heart failure into three phenotypes based on the measurement of left ventricular ejection fraction (LVEF) has become widely accepted in clinical practice (figure 1) [4]:

Figure 1Heart failure (HF) categories according to European Society of Cardiology (ESC) Guidelines 2021 [4]. EF: ejection fraction; LVEF: left ventricular ejection fraction.
Heart failure is a progressive disease and structural as well as functional changes start long before symptoms develop and hence before the definition of heart failure is fulfilled. The two asymptomatic stages before the development of overt heart failure have been named “at risk for heart failure” and “pre-heart failure” [1]. The presence of established diabetes alone indicates that an individual is at risk of heart failure. Additional risk factors (e.g. obesity) increase the likelihood for progression to symptomatic (overt) heart failure. If structural or functional cardiac changes occur in the absence of symptoms or signs, the “pre-heart failure” stage has been reached [1].
It is essential in general practice to identify these early, subclinical stages so that treatment can be started or intensified early, helping to prevent or delay heart failure [13, 14]. Various recommendations, including those from the German Society of Cardiology, the Austrian Diabetes Society, the Austrian Cardiology Society as well as the American Diabetes Association (ADA), emphasise the importance of early heart failure detection in individuals with diabetes [8, 15, 16].
However, these proposals lack a clearly defined algorithm for the early identification of heart failure in patients with diabetes that can be easily implemented in primary care settings. Thus, members of the Swiss Society of Endocrinology and Diabetology and the Swiss Society of Cardiology joined with general practitioners (GPs) to form a consensus group dedicated to developing a straightforward and effective recommendation for the early detection and prevention of heart failure in diabetes. A further goal was to raise awareness about the increased risk of heart failure in individuals with diabetes and to facilitate implementation of these recommendations.
A structured approach was chosen for the development of this consensus paper, based on established recommendations from organisations such as the European Society of Cardiology and the American Diabetes Association (ADA), and aimed to achieve an algorithm adapted to Switzerland. Where available, the class of recommendation and level of evidence are provided according to the guidelines. In addition, we clearly distinguish between evidence-based recommendations and expert opinions by appending “Swiss C” to statements that are based on the consensus of the expert group.
The natriuretic peptides (NPs) NT-proBNP and BNP are part of the diagnostic algorithm of the ESC in patients with suspected heart failure (Class I level of evidence B) [4]. If typical signs or symptoms are present, measuring natriuretic peptides as the initial diagnostic test is recommended [4]. In the non-acute setting, an NT-proBNP <125 ng/l and BNP <35 ng/l rules out heart failure hence other diagnoses must be considered. If the natriuretic peptides are higher than these cut-offs, heart failure is likely and needs to be confirmed by echocardiography [4].
As cardiomyocytes produce natriuretic peptides under stress due to volume or pressure overload of the heart, these markers serve as indicators of the molecular stress to which the heart is exposed [5]. Thus, natriuretic peptides are valuable for the detection of patients at increased risk of developing heart failure, which includes patients with asymptomatic cardiac dysfunction [1]. In patients at risk of developing heart failure, the American Heart Association (AHA) / American College of Cardiology (ACC) / Heart Failure Society of America (HFSA) Guidelines recommend the NT-proBNP- or BNP-based screening followed by team-based care, including a cardiovascular specialist (Class IIa, level of evidence B) [13].
The STOP-HF Randomized Trial demonstrated that natriuretic peptide screening in patients at risk of heart failure, followed by collaborative care, diagnostic evaluation and treatment for those with elevated levels, can reduce the combined rates of left ventricular systolic dysfunction, diastolic dysfunction and heart failure [17]. This approach has a high probability of being cost-effective [18]. Furthermore, the PONTIAC study showed that for individuals with diabetes without heart disease but with high NT-proBNP levels, quickly increasing the doses of renin-angiotensin-aldosterone system antagonists and beta-blockers to the highest tolerable amount is both safe and effective in preventing heart-related events [14].
Consequently, the two natriuretic peptides BNP and NT-proBNP are considered suitable candidates for the early identification of heart failure in patients with diabetes [19].
Patients with diabetes are more likely to develop heart failure, particularly if they have additional risk factors, and will progress to symptomatic disease earlier than the general population. Therefore, patients should be proactively questioned at every encounter regarding typical heart failure symptoms such as breathlessness, orthopnoea, paroxysmal nocturnal dyspnoea, reduced exercise tolerance, fatigue, tiredness, prolonged time to recover after exercise and swollen ankles.
If the patient does not report any of these typical heart failure symptoms, the Swiss consensus group recommends the following step-by-step algorithm (figure 2) based on age and natriuretic peptide levels.
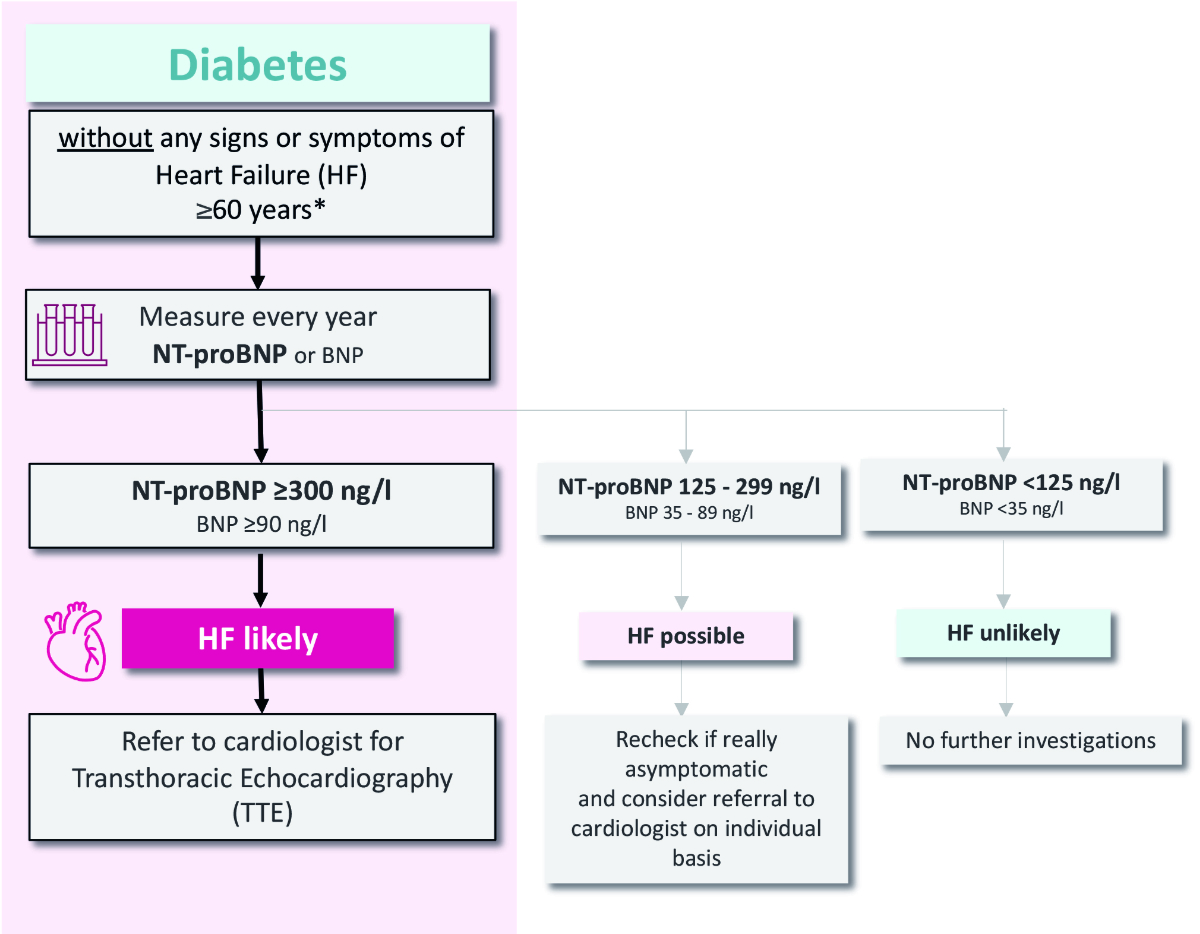
Figure 2Swiss recommendations 2024 with step-by-step algorithm for heart failure (HF) identification in diabetes patients. *If a patient has several additional heart failure risk factors such as obesity#, long-term and/or suboptimally managed diabetes therapy##, hypertension#, hyperlipidaemia or micro- and/or macrovascular complications (diabetic kidney disease [DKD], coronary artery disease [CAD])#, being female#, a determination of NT-proBNP or BNP may be considered individually for patients <60 years. #according to the Consensus Report of the American Diabetes Association; ##according to the consensus group Swiss Recommendations for Early Identification of Heart Failure in Patients with Diabetes.
Asymptomatic patients with diabetes younger than 60 years with no additional risk factors are unlikely to develop heart failure in the near future, as the incidence of heart failure rises with age. No specific measures need to be taken in this group apart from regular clinical checks for symptoms and signs of heart failure [20] (Swiss C).
Asymptomatic diabetes patients older than 60 years have a higher risk of undetected heart failure. In this second group, the Swiss Consensus Group recommends measuring natriuretic peptides once a year. According to the level of the natriuretic peptides, heart failure is deemed likely (NT-proBNP ≥300 ng/l, BNP ≥90 ng/l), possible (NT-proBNP 125–299 ng/l, BNP 35–89 ng/l) or unlikely (NT-proBNP <125 ng/l, BNP <35 ng/l) (Swiss C).
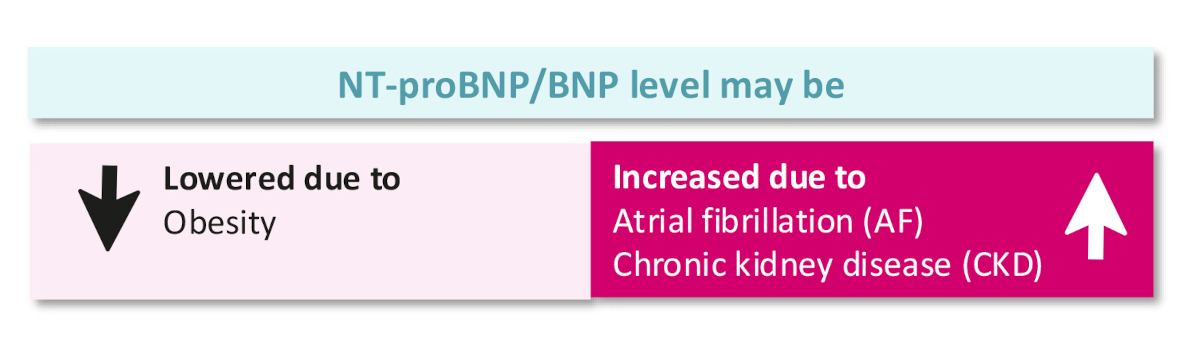
Figure 3Comorbidities and level of natriuretic peptides (NPs) (according to European Society of Cardiology heart failure guidelines 2021).
The ADA Consensus group recommends annual determination of natriuretic peptides in individuals with diabetes regardless of age [8]. The Swiss consensus group recommends regular natriuretic peptide measurements for asymptomatic patients with diabetes aged 60 years or older, as the prevalence of heart failure increases significantly from this age onward [6, 7] (Swiss C). The discussions around the age cut-off also focused on practical implementation in daily practice and minimisation of unnecessary investigations. Nevertheless, regular natriuretic peptide measurements should be considered at a younger age if several additional risk factors other than diabetes are present, since this increases the likelihood of developing heart failure earlier in life.
For asymptomatic patients with diabetes under 60 with multiple heart failure risk factors – including obesity, prolonged poor diabetes control (evidenced by continuous HbA1c elevation), insulin therapy, hypertension, hyperlipidaemia, micro- or macrovascular complications (chronic kidney disease [CKD], diabetic kidney disease [DKD] or coronary artery disease [CAD]) and being female – individual consideration for heart failure screening using natriuretic peptides is advised [8]. In patients with newly diagnosed diabetes, a single measurement of natriuretic peptides may be considered for the purpose of heart failure risk assessment and as a reference value for subsequent measurements depending on age and the presence of other heart failure risk factors (Swiss C).
If heart failure symptoms are evident, a NT-proBNP level of ≥125 ng/l or a BNP level of ≥35 ng/l suggests – but does not confirm – the presence of heart failure. Patients exhibiting elevated natriuretic peptides should be evaluated by a cardiologist using echocardiography, and classified and treated based on left ventricular ejection fraction as heart failure with reduced ejection fraction (HFrEF), heart failure with mildly reduced ejection fraction (HFmrEF) or heart failure with preserved ejection fraction (HFpEF) according to the ESC Guidelines 2021 [4] (Swiss C).
The primary aim of this consensus paper is to promote the early detection of heart failure in patients with diabetes. In addition, the following section provides a summary of key therapeutic principles for patients with diabetes at high risk of heart failure.
Risk factor management, including encouragement to engage in regular physical activity, should be the standard of care (figure 4). According to the Swiss Society for Endocrinology and Diabetes (SSED) Guidelines, a combination of metformin and a sodium-glucose co-transport 2 (SGLT2) inhibitor is generally advised for patients with type 2 diabetes to provide cardiovascular and renal protection [21]. For obese patients, a combination of metformin and a glucagon-like peptide-1 receptor agonist (GLP-1 RA) should be considered initially [21]. The use of SGLT2 inhibitors is discouraged in type 1 diabetes due to the increased risk of diabetic ketoacidosis [22].
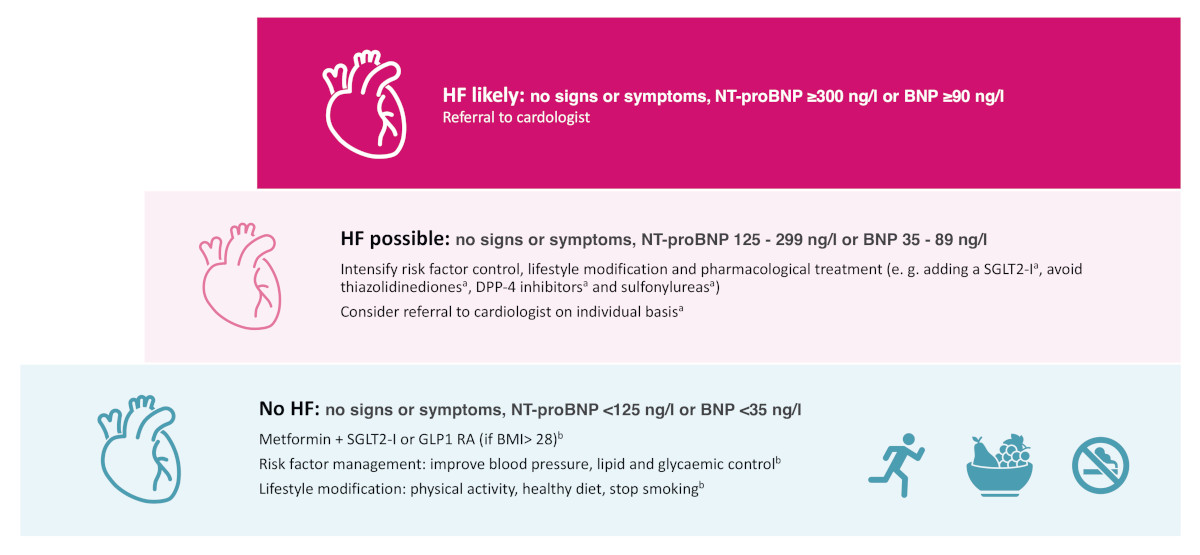
Figure 4Swiss recommendations 2024 on early heart failure (HF) management in patients with diabetes. a Recommendation of the consensus group Swiss Recommendations for Early Identification of Heart Failure in Patients with Diabetes; no clear scientific data available yet; b According to the Swiss recommendations of the Society for Endocrinology and Diabetes (SGED/SSED) for the treatment of type 2 diabetes mellitus (2023) [23]. DPP-4: Dipeptidyl peptidase 4; GLP-1 RA: Glucagon-like peptide-1 receptor agonist; SGLT2-I: dapagliflozin or empagliflozin as sodium-glucose transport protein 2 (SGLT2) inhibitor.
For asymptomatic patients with elevated natriuretic peptide levels, it is recommended to intensify risk factor management, encourage further lifestyle changes and review the pharmacological treatment of diabetes [21]. Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors (especially saxagliptin and alogliptin) and sulphonylureas should be avoided in these patients [8, 21]. While definitive scientific evidence is pending, the Swiss Consensus group suggests – in line with the ADA recommendation – adding an SGLT2 inhibitor for cardioprotection at this stage if not established yet [8]. Depending on the degree of natriuretic peptide elevation and other factors, a referral to a cardiologist is recommended as described above.
Figure 4 provides a summary of the main treatment standards, but for more comprehensive information, the ESC heart failure guidelines 2021 (including the 2023 update), the ADA consensus report and the recent ESC clinical consensus statement with practical algorithms for early diagnosis of heart failure should be consulted [4, 5, 8, 23].
Active monitoring for heart failure in diabetic care is strongly recommended to detect heart failure early. In addition to questioning about typical symptoms and examining for signs of heart failure at every encounter, the annual measurement of natriuretic peptides in patients older than 60 (or earlier if multiple risk factors for heart failure are present) should be a standard part of diabetes management. Early detection of patients at high risk of heart failure will delay or may even prevent progression to overt heart failure. Such a strategy can be expected to improve care before patients become symptomatic and to reduce the number of patients being diagnosed when admitted to hospital for acute heart failure, thereby reducing morbidity and mortality. The European Society of Cardiology promotes early diagnosis of heart failure not only in patients with diabetes but also in patients with other cardiovascular risk factors [5]. Further research is needed to better guide treatment of patients in stages preceding heart failure as well as to confirm the cost-effectiveness of such strategies.
Financial support was provided by AstraZeneca Schweiz and Roche Diagnostics Schweiz for the expert group meeting and medical writing (by medQuest GmbH). However, the views expressed in this article reflect the consensus of the authors and participants of the expert group meeting, based on available literature.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Matthias Paul: Advisory Boards and Lectures for AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Servier, Vifor. Research conflicts: none. Michael Brändle: Advisory Boards and Lectures for AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, E. Lilly, Novartis, Novo Nordisk. Research conflicts: none. Peter Wiesli: Advisory Boards and Lectures for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, E. Lilly, Mundipharma, Medtronic, Novo Nordisk, Roche, Sanofi. Research conflicts: none. Giacomo Gastaldi: Advisory Boards and Lectures for Abbott, Dexcom, Roche, Medtronic, Insulet, Ypsomed, AstraZeneca, Sanofi, E. Lilly, Novo Nordisk. Research conflicts: none. Mattia Arrigo: No conflict of interest. No personal fees from industry. Honoraria are fully devolved to the Stiftung Stadtspital Zürich. Philippe Meyer: No personal fees from industry. Honoraria are fully devolved to a private research foundation of the University Hospitals of Geneva Cardiology Service (GEcor foundation). Christian Müller: Research support from the Swiss National Science Foundation, the Swiss Heart Foundation, Innosuisse, the University of Basel, the University Hospital Basel, AstraZeneca, Beckman Coulter, Boehringer Ingelheim, Idorsia, LSI-Medience, Novartis, Ortho Cinical Diagnostics, Quidel, Roche, Siemens, Singulex, Sphingotec and SpinChip, as well as speaker honoraria/consulting honoraria from Acon, Amgen, AstraZeneca, Boehringer Ingelheim, Bayer, BMS, Idorsia, Novartis, Osler, Roche, Sanofi and SpinChip, outside of the submitted work, and all paid to the institution. Roger Lehmann: Advisory Boards and Lectures for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, E. Lilly, Mundipharma, Medtronic, Novo Nordisk, Roche, Sanofi. Research conflicts: none. No other potential conflicts of interest related to the content of this manuscript were disclosed.
1. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021 Mar;23(3):352–80. doi: https://doi.org/10.1002/ejhf.2115
2. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020 Aug;22(8):1342–56. doi: https://doi.org/10.1002/ejhf.1858
3. Ceriello A, Catrinoiu D, Chandramouli C, Cosentino F, Dombrowsky AC, Itzhak B, et al.; D&CVD EASD Study Group. Heart failure in type 2 diabetes: current perspectives on screening, diagnosis and management. Cardiovasc Diabetol. 2021 Nov;20(1):218. doi: https://doi.org/10.1186/s12933-021-01408-1
4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; Authors/Task Force Members; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022 Jan;24(1):4–131. doi: https://doi.org/10.1002/ejhf.2333
5. Bayes-Genis A, Docherty KF, Petrie MC, Januzzi JL, Mueller C, Anderson L, et al. Practical algorithms for early diagnosis of heart failure and heart stress using NT-proBNP: A clinical consensus statement from the Heart Failure Association of the ESC. Eur J Heart Fail. 2023 Nov;25(11):1891–8. doi: https://doi.org/10.1002/ejhf.3036
6. Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, et al.; National Heart Failure Societies of the ESC member countries (see Appendix). The Heart Failure Association Atlas: Heart Failure Epidemiology and Management Statistics 2019. Eur J Heart Fail. 2021 Jun;23(6):906–14. doi: https://doi.org/10.1002/ejhf.2143
7. Boonman-de Winter LJ, Rutten FH, Cramer MJ, Landman MJ, Liem AH, Rutten GE, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012 Aug;55(8):2154–62. doi: https://doi.org/10.1007/s00125-012-2579-0
8. Pop-Busui R, Januzzi JL, Bruemmer D, Butalia S, Green JB, Horton WB, et al. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care. 2022 Jul;45(7):1670–90. doi: https://doi.org/10.2337/dci22-0014
9. Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GC, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: A large multinational cohort study. Diabetes Obes Metab. 2020 Sep;22(9):1607–18. doi: https://doi.org/10.1111/dom.14074
10. Schweizerische Diabetesgesellschaft. Über Diabetes - Schweizerische Diabetesgesellschaft. Available from: https://www.diabetesschweiz.ch/ueber-diabetes.html
11. Gregory GA, Robinson TI, Linklater SE, Wang F, Colagiuri S, de Beaufort C, et al.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study [Erratum in: Lancet Diabetes Endocrinol. 2022 Nov; 10] [11] [:e11]. Lancet Diabetes Endocrinol. 2022 Oct;10(10):741–60. doi: https://doi.org/10.1016/S2213-8587(22)00218-2
12. Knaus L, Quarella M, Buser M, Maeder MT, Renström F, Brändle M. Screening for heart failure in patients with diabetes mellitus in tertiary care - A SwissDiab study. Diabetes Res Clin Pract. 2024 Mar;209:111565. doi: https://doi.org/10.1016/j.diabres.2024.111565
13. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [Erratum in: Circulation. 2022 May 3;145] [18] [:e1033. Erratum in: Circulation. 2022 Sep 27;146] [13] [:e185. Erratum in: Circulation. 2023 Apr 4;147] [14] [:e674]. Circulation. 2022 May;145(18):e895–1032. doi: https://doi.org/10.1161/CIR.0000000000001063
14. Huelsmann M, Neuhold S, Resl M, Strunk G, Brath H, Francesconi C, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013 Oct;62(15):1365–72. doi: https://doi.org/10.1016/j.jacc.2013.05.069
15. Schütt K, Aberle J, Bauersachs J, Birkenfeld A, Frantz S, Ganz M, et al. Positionspapier Herzinsuffizienz und Diabetes. Kardiologie. 2022;16(5):358–71. doi: https://doi.org/10.1007/s12181-022-00562-4
16. Kaser S, Hülsmann M, Siostrzonek P, Clodi M. Mörtl. D, Sourij H. Positionspapier der ÖDG und ÖKG Diabetes mellitus und Herzinsuffizienz. Chronic heart failure and diabetes. A position paper. J. Kardol. 2021;28(1-2):14–20.
17. Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013 Jul;310(1):66–74. doi: https://doi.org/10.1001/jama.2013.7588
18. Walter E, Arrigo M, Allerstorfer S, Marty P, Hülsmann M. Cost-effectiveness of NT-proBNP-supported screening of chronic heart failure in patients with or without type 2 diabetes in Austria and Switzerland. J Med Econ. 2023;26(1):1287–300. doi: https://doi.org/10.1080/13696998.2023.2264722
19. Gallagher J, Watson C, Campbell P, Ledwidge M, McDonald K. Natriuretic Peptide-based Screening and Prevention of Heart Failure. Card Fail Rev. 2017 Nov;3(2):83–5. doi: https://doi.org/10.15420/cfr.2017:20:1
20. Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al.; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct;44(39):4043–140. doi: https://doi.org/10.1093/eurheartj/ehad192
21. Gastaldi G, Lucchini B, Thalmann S, Alder S, Laimer M, Brändle M, et al.; Working group of the SGED/SSED. Swiss recommendations of the Society for Endocrinology and Diabetes (SGED/SSED) for the treatment of type 2 diabetes mellitus (2023). Swiss Med Wkly. 2023 Apr;153(4):40060. doi: https://doi.org/10.57187/smw.2023.40060
22. Deutsche Diabetes Gesellschaft. S3-Leitlinie Therapie des Typ-1-Diabetes. Available from: https://register.awmf.org/assets/guidelines/057-013l_S3-Therapie-Typ-1-Diabetes_2023-09_1.pdf
23. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific Document Group. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [Erratum in: Eur Heart J. 2024 Jan 1;45] [1]. Eur Heart J. 2023 Oct;44(37):3627–39. doi: https://doi.org/10.1093/eurheartj/ehad195
24. Klein L, Gheorghiade M. Coronary artery disease and prevention of heart failure. Med Clin North Am. 2004 Sep;88(5):1209–35. doi: https://doi.org/10.1016/j.mcna.2004.03.002
25. Christ E, Brändle M, Czock A, Diem P, Fischer-Taeschler D, Gastaldi G, et al. HagonTraub I, Margrit Hasler M, Zanella I; Arbeitsgruppe Disease Management Diabetes (DMD) der Schweizerischen Gesellschaft für Endokrinologie und Diabetologie (SGED/SSED). Kriterien für ein «gutes» Disease Management Diabetes in der Grundversorgung. Überarbeitete Version Juni 2017, verabschiedet 23. August 2017. Available from: https://www.sgedssed.ch/fileadmin/user_upload/6_Diabetologie/64_Ressourcen_Hausarzt/Diabetes_Kriterien_2017_SGED_def.pdf