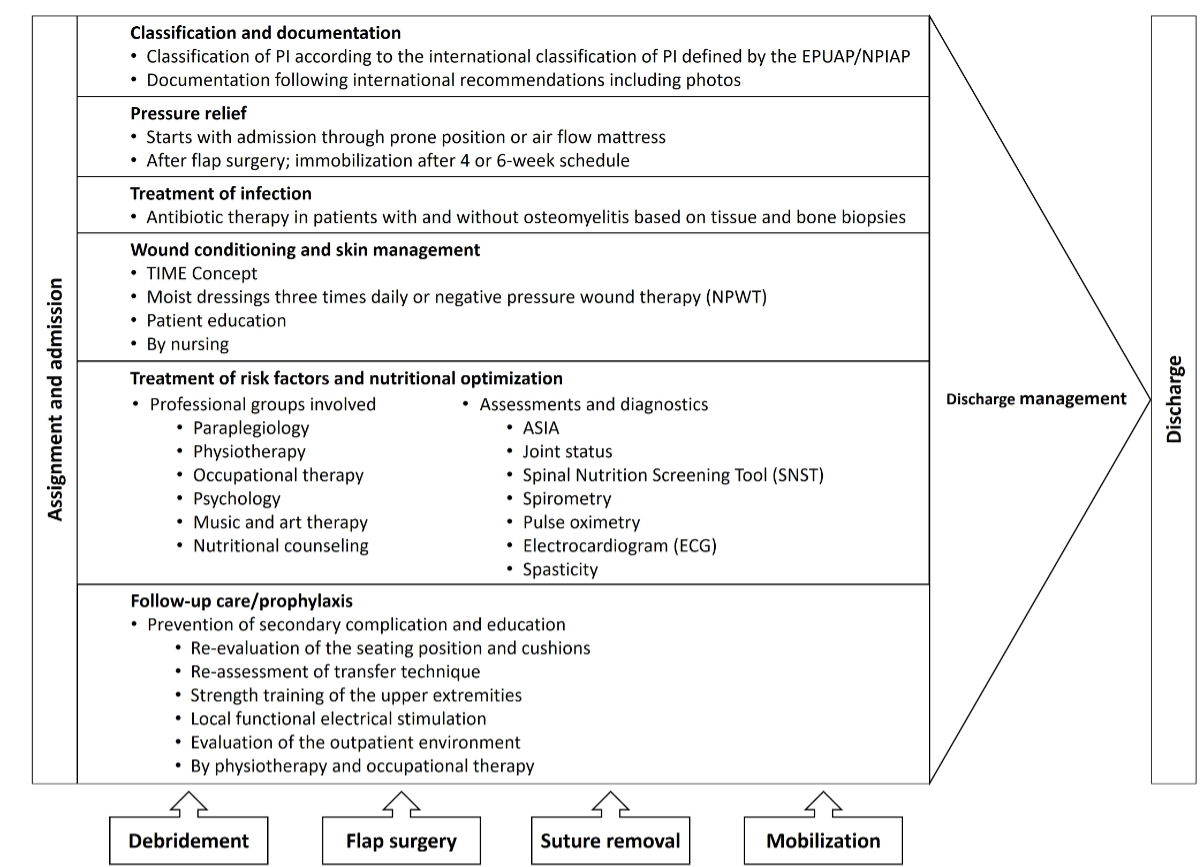
Figure 1Overview of the Basel Decubitus Approach. PI: pressure injury.
DOI: https://doi.org/https://doi.org/10.57187/s.3977
Among people with spinal cord injury or spinal cord disorder living in the community, the prevalence rates of pressure injury range from 26% to 54% within a one-year reporting period [1]. The lifetime incidence of at least one pressure injury in individuals with spinal cord injury/disorder is as high as 85%, and it is expected that 30% of these people will experience recurrent pressure injuries [1–4]. Pressure injuries often negatively affect an individual’s health, functioning and social participation [5, 6]. Stage III and IV pressure injuries, as categorised by the European Pressure Ulcer Advisory Panel, usually require surgical management [7]. The risk of postoperative complications such as hematoma formation, infection, wound dehiscence, partial flap necrosis and recurrence is high [8–10]. In the Clavien-Dindo classification, these postoperative complications are categorised as minor if they can be treated conservatively and major if they require reoperation [11]. Approximately 20% of flap surgeries in people with spinal cord injury/disorder and stage III and IV pressure injury result in a major complication [8, 12, 13]. Major complications not only prolong immobilisation and hospitalisation, thereby reducing quality of life, but also increase healthcare costs [8, 13–15]. Preoperative and postoperative care are crucial to the success of flap surgery [15]. Therefore, treatment approaches for stage III and IV pressure injuries, such as the Basel Decubitus Approach, have been developed, which consist of a multi-layered, coordinated involvement of different disciplines and professions [14–19]. Treatment approaches aim to address factors associated with postoperative complications [13]. Typical treatment elements include pressure relief, immobilisation, risk screening and optimisation of comorbidities, infection control, wound conditioning, debridement and flap surgery [13].
To reduce the incidence of major complications, decrease healthcare costs, and shorten hospital stays, it is necessary to identify risk predictors of major complications and modify clinical practice accordingly [20]. Based on the literature, there are several factors associated with postoperative complications in the treatment of stage III and IV pressure injuries in people with spinal cord injury/disorder, including older age, overweight and underweight, tensor fasciae latae flap, presence or absence of osteomyelitis, low serum albumin and pathological blood concentrations of cystatin C, calcium and vitamin B12 [9, 10, 13, 21–23]. However, it is unknown how the combination of these factors influences major complications [9, 10, 13, 21, 22]. In surgery, risk prediction models are used to predict the risk of an adverse outcome, such as major complications [24]. A risk prediction model is a statistical model that combines information from several different factors to estimate the probability of an individual experiencing a health outcome [24]. They are used to facilitate clinical decision making, such as preventive interventions for people at high risk [24, 25]. However, no risk prediction model exists for major postoperative complications in the treatment of stage III and IV pressure injuries in people with spinal cord injury/disorder. Thus, major complications sometimes occur unforeseeably. Therefore, this study aims to develop a risk prediction model for major complications in individuals with spinal cord injury/disorder and stage III or IV pressure injury at hospital admission using mixed effects logistic Bayesian Least Absolute Shrinkage and Selection Operator (LASSO) regression.
This retrospective cohort examined routinely collected clinical data in a Swiss acute and rehabilitation hospital for people with spinal cord injury/disorder using the Basel Decubitus Approach to manage stage III and IV pressure injuries [17, 19, 26]. This approach consists of pressure injury classification, pressure relief and immobilisation, debridement, flap surgery, wound conditioning before and after flap surgery, infection control, risk screening and optimisation of comorbidities (e.g. nutritional therapy) as well as prevention of secondary complications and education [26–28]. This approach also contains the four milestones of debridement, flap surgery, suture removal and mobilisation in a wheelchair (figure 1) [26, 27].

Figure 1Overview of the Basel Decubitus Approach. PI: pressure injury.
We collected data from all consecutive adults with spinal cord injury/disorder hospitalised between 1 January 2016 and 31 December 2022 for stage III or IV pressure injuries over the sacrum/coccyx, ischium or trochanter. We considered individuals who underwent their first flap surgery and data from subsequent treatment procedures in these individuals during this period. We predefined exclusion criteria: individuals who refused retrospective use of their data, those undergoing initial rehabilitation, those under 18 years of age, those with other neurological or malignant conditions, those treated conservatively, and those who died during treatment of another condition unrelated to a postoperative complication or pressure injury. Patients transferred to another hospital for severe additional comorbidities (e.g. vascular surgery) for which treatment is unavailable at our hospital were also excluded. These individuals had to be treated in another highly specialised academic hospital to achieve adequate safety.
As it is unknown how the combination of different possible risk factors influences major complications during the treatment of stage III and IV pressure injuries in people with spinal cord injury/disorder, we collected data on patients’ characteristics, pressure injury characteristics, comorbidities, surgical characteristics, and laboratory results. Patient characteristics included sex, age, years post-injury, lesion level, ASIA classification (A, B, C or D) and aetiology cause (transport activity, sports and leisure activity, fall, violence, other accident cause, inflammation/infection, bleeding, congenital, vascular disorders, other disease, caused by surgical intervention or unknown cause). The ASIA/ISCoS International Standard for Neurological Classification of SCI (ISNCSCI) was used to document neurological impairment, lesion level and completeness [29]. Pressure injury characteristics included pressure injury stage (III or IV), pressure injury location (trochanter, ischium or coccyx/sacrum), number of pressure injuries in these locations (1, 2, 3 or more), additional deep pressure injury in another location (e.g. foot) and recurrence of pressure injury (none, same location or different location). Comorbidities included the presence (yes or no) of osteomyelitis (based on tissue and bone biopsy in stage IV pressure injury), diabetes (6.5% HbA1c), psychiatric diagnosis, vascular comorbidity (peripheral arterial occlusive disease or chronic venous insufficiency), hypertension (>140 mm Hg), osteoarthritis, scoliosis, amputation, smoking, obstructive sleep apnoea and spasticity (based on ASWORTH scale). Surgical characteristics included type of flap surgery (fasciocutaneous gluteal rotation flap, fasciocutaneous posterior thigh flap, lateral advancement flap, local Limberg flap, other or unknown), number of previous flap surgeries, length of immobilisation and length of hospital stay. In addition, laboratory results were collected, including sodium (135–245 mmol/l), potassium (3.5–5.1 mmol/l), calcium (2.2–2.6 mmol/l), ferritin (15–150 ug/l), creatinine (59–104 µmol/l), cystatin C (0.61–0.95 mg/l), estimated glomerular filtration rate (eGFR) according to the cystatin formula (≥90 mg/l), total protein (64–83 g/l), albumin (32–50 g/l), C-reactive protein (CRP; <5 mg/l), total cholesterol (<5.2 mmol/l), HDL cholesterol (0.9–2.0 mmol/l), triglycerides (0.1–2.3 mmol/l), glucose (3.6–5.8 mmol/l mmol/l), vitamin D (25-hydroxy vitamin D; ≥75 nmol/l), vitamin B12 (200–1000 ng/l), folic acid (5–20 μg/l), haemoglobin (140–170 g/l), thyroid stimulating hormone (TSH; 0.4–4.0 mU/l) and international normalised ratio (INR; 0.9–1.3). Laboratory results were collected once at hospital admission. Data were retrieved from electronic databases used in clinical routine: KIS (Nexus, Switzerland), WicareDoc (Wigasoft, Switzerland), d.3one (D.velop, Switzerland) and ixserve.4 (ixmid Software Technologie GmbH, Germany). Due to the retrospective nature of this study, some factors such as pressure injury size, BMI or HbA1c could not be analysed due to missing or incomplete documentation.
We developed a risk prediction model to predict the occurrence of major postoperative complications between flap surgery and hospital discharge in people with spinal cord injury/disorder and stage III or IV pressure injury. The model is intended for use at hospital admission to assist physicians in making informed therapeutic decisions. The model was developed without external validation, using dependent data.
We conducted a mixed-effects logistic Bayesian LASSO regression according to Park and Casella [30]. The Bayesian LASSO method is robust for small sample sizes and can be combined with multiple imputation, mixed effects and logistic regression. Moreover, the LASSO model uses a shrinkage penalty that shrinks the coefficients of some features towards zero, thus leading to a parsimonious model. Therefore, LASSO is the method to use in case of multicollinearity. Unlike ordinary logistic regressions, the shrinkage penalty of the LASSO model results in a sparser model with fewer features having non-zero coefficients, which improves the interpretability and simplicity of the model and reduces overfitting compared to ordinary logistic regression. Furthermore, the odds ratios (OR) determined with the LASSO model have the same interpretation as those derived from ordinary logistic regression. Both represent the change in odds resulting from a one-unit change in the explanatory variable, assuming that the other variables are held constant. An OR of <1 indicates a negative effect and >1 a positive effect.
The dataset contained missing values, which were addressed through multiple imputation according to Zhou and Reiter [31]. Repeated surgeries on the same patient were captured using random intercepts with normal priors with zero mean and inverse gamma (0.01, 0.01) prior for the variance. For the regression coefficients, we used the Laplace prior with zero mean and the scale parameter depending on the variance and the squared penalisation hyperparameter. Park and Casella suggested using the gamma prior with scale 1/1.78 as the prior for the squared penalisation hyperparameter. Moreover, for the intercept and variance, we used non-informative priors: normal (0, 1,000,000) and Jeffreys prior. For parameter estimation, we used Markov chain Monte Carlo (MCMC) with a burn-in period of 1000, thinning of 10, to obtain a sample of size 3000. All traces were visually checked for convergence and autocorrelation (Appendix). Afterwards, we selected variables with non-zero coefficients based on the posterior probabilities [32]. We calculated the odds ratios and 95% credible intervals (95% CI) of these variables.
Descriptive analysis was conducted to present the study population. Count (n) was used to present categorical variables, while median and interquartile range (IQR), according to the Shapiro-Wilk test, were used for continuous data. The data were analysed using the statistical program Stata/MP (version 17, 64-bit) for Windows.
This study was approved by the Swiss Ethics Committee for Northwest/Central Switzerland (trial registration number: 2014-107).
During the observational period, 397 flap surgeries were performed in 174 individuals for stage III or IV pressure injuries over the sacrum/coccyx, ischium or trochanter (figure 1). Of these, 29 individuals refused the use of their data and were excluded. Additionally, 14 individuals with minor complications were excluded. Four individuals died during the immobilisation phase, four to six weeks postoperatively, due to pneumonia or cardiac failure, and one individual died nine months after flap surgery due to pre-existing renal failure and pneumonia. Therefore, these five individuals were excluded from this study. Finally, we included 252 treatment procedures of 167 individuals (figure 2).
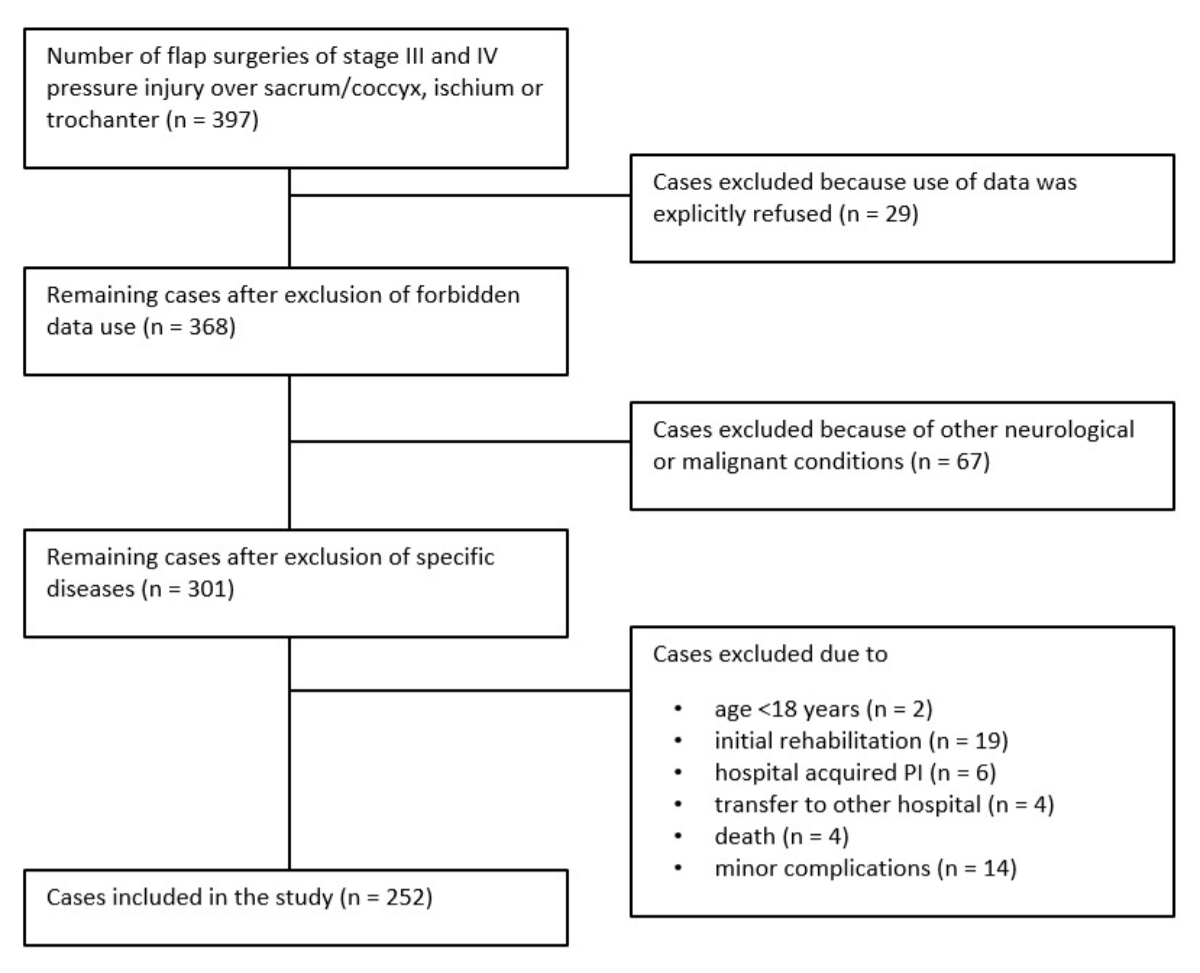
Figure 2Flow chart of case selection.
Of the 252 treatment procedures analysed, 48 (19%) resulted in major complications. The study population consisted of 189 (75%) men and 153 (61%) individuals with a traumatic spinal cord injury. Of the total, 168 (67%) had paraplegia, 192 (76%) had ISNCSCI A and 157 (62%) had a stage IV pressure injury. Additionally, 170 (67%) had only one pressure injury, while 44 (17%) had a further pressure injury at another location. Furthermore, 155 (62%) had no previous pressure injury, 145 (58%) had no osteomyelitis and 214 (85%) were non-smokers. Out of all individuals, 138 (55%) had a pressure injury over the ischium, 69 (27%) over the coccyx/sacrum and 45 (18%) over the trochanter. On average, the individuals were 60 years old, immobilised for 40 days, and hospitalised for 104 days. The mean time since injury was 23 years (table 1).
Table 1Patients’ characteristics by complication.
| Patient characteristic | Total | No complication | Major complication | |||||
| n = 252 | n = 204 | n = 48 | ||||||
| n | (%) | n | (%) | n | (%) | |||
| Sex | Male | 189 | (75) | 155 | (76) | 34 | (71) | |
| Female | 63 | (25) | 49 | (24) | 14 | (29) | ||
| Aetiology of spinal cord injury or spinal cord disease | Traumatic SCI | Transport activity | 71 | (28) | 56 | (27) | 15 | (31) |
| Sports or leisure activity | 37 | (15) | 28 | (14) | 9 | (19) | ||
| Fall | 29 | (12) | 25 | (12) | 4 | (8) | ||
| Violence | 4 | (2) | 4 | (2) | 0 | (0) | ||
| Surgical intervention | 10 | (4) | 7 | (3) | 3 | (6) | ||
| Other accident cause | 12 | (5) | 10 | (5) | 2 | (4) | ||
| Non-traumatic SCI | Inflammation/Infection | 6 | (2) | 4 | (2) | 2 | (4) | |
| Bleeding | 2 | (1) | 2 | (1) | 0 | (0) | ||
| Congenital | 11 | (4) | 11 | (5) | 0 | (0) | ||
| Other disease (undefined) | 37 | (15) | 29 | (14) | 8 | (17) | ||
| Unknown cause | 33 | (13) | 28 | (14) | 5 | (10) | ||
| Neurological level of SCI | C1–C4 | 23 | (9) | 18 | (9) | 5 | (10) | |
| C5–C8 | 61 | (24) | 48 | (24) | 13 | (27) | ||
| T1–S5 | 168 | (67) | 138 | (68) | 30 | (63) | ||
| ISNCSCI | ISNCSCI A | 192 | (76) | 153 | (75) | 39 | (81) | |
| ISNCSCI B | 35 | (14) | 31 | (15) | 4 | (8) | ||
| ISNCSCI C | 17 | (7) | 14 | (7) | 3 | (6) | ||
| ISNCSCI D at any level | 8 | (3) | 6 | (3) | 2 | (4) | ||
| Pressure injury stage | III | 95 | (38) | 86 | (42) | 9 | (19) | |
| IV | 157 | (62) | 118 | (58) | 39 | (81) | ||
| Localisation of the pressure injury | Coccyx/Sacrum | 69 | (27) | 49 | (24) | 20 | (42) | |
| Ischium | 138 | (55) | 118 | (58) | 20 | (42) | ||
| Trochanter | 45 | (18) | 37 | (18) | 8 | (17) | ||
| Number of pressure injuries in this location | 1 | 170 | (67) | 133 | (65) | 37 | (77) | |
| 2 | 63 | (25) | 54 | (26) | 9 | (19) | ||
| ≥3 | 19 | (8) | 17 | (8) | 2 | (4) | ||
| A further pressure injury in another location | No | 208 | (83) | 171 | (84) | 37 | (77) | |
| Yes | 44 | (17) | 33 | (16) | 11 | (23) | ||
| Recurrence of previous pressure injury | No | 155 | (62) | 129 | (63) | 26 | (54) | |
| Yes | 97 | (38) | 75 | (37) | 22 | (46) | ||
| Same location | 50 | (20) | 37 | (18) | 13 | (27) | ||
| Different location | 47 | (19) | 38 | (19) | 9 | (19) | ||
| Osteomyelitis | No | 145 | (58) | 111 | (54) | 34 | (71) | |
| Yes | 107 | (42) | 93 | (46) | 14 | (29) | ||
| Smoking | No | 214 | (85) | 175 | (86) | 39 | (81) | |
| Yes | 38 | (15) | 29 | (14) | 9 | (19) | ||
| Age at admission (year): median (IQR) | 60 | (18) | 60 | (18) | 63 | (18) | ||
| Years post-injury: median (IQR) | 23 | (24) | 24 | (25) | 22 | (23) | ||
| Immobilisation (days): median (IQR) | 49 | (17) | 44 | (15) | 68 | (34) | ||
| Hospital stay (days): median (IQR) | 104 | (53) | 98 | (50) | 132 | (63) | ||
ISNCSCI; International Standard for Neurological Classification of Spinal Cord Injury; IQR: Interquartile range.
The mixed-effects logistic Bayesian LASSO regression identified eGFR, vitamin D, vitamin B12, sodium and CRP as relevant predictors. While eGFR (OR 0.91, CI 0.62–1.02), vitamin B12 (OR 0.91, CI 0.74–1.05), sodium (OR 0.75, CI 0.16–1.05) and CRP (OR 0.98, CI 0.79–1.07) had a negative effect on major postoperative complication, vitamin D (OR 1.05, CI 0.98–1.23) had a positive effect. However, although these variables have been selected as relevant predictors by Bayesian LASSO regression, their effects were not statistically significant as the credible interval included one (table 2). Therefore, their effects require further validation.
Table 2Selected variables with odds ratios and credible intervals.
| Variable | Odds ratio | CI (95%) | |
| eGFR* (per 10 ml/min) | 0.91 | 0.62 | 1.02 |
| Vitamin D** (per 10 nmol/l) | 1.05 | 0.98 | 1.23 |
| Vitamin B12 (per 100 ng/l) | 0.91 | 0.74 | 1.05 |
| Sodium (per 10 mmol/l) | 0.75 | 0.16 | 1.05 |
| CRP (per 10 mg/l) | 0.98 | 0.79 | 1.07 |
CI: Credible interval; CRP: C-reactive protein; eGFR: Estimated glomerular filtration rate.
* eGFR according to the cystatin formula
** 25-hydroxy vitamin D
Multidisciplinary treatment approaches have been developed to address factors associated with complications to reduce complications during the treatment of deep pressure injuries in people with spinal cord injury / disorder [26]. This study focused on a risk prediction that combines information from different factors to further develop the Basel Decubitus Approach [26]. The LASSO regression model identified eGFR, vitamin D, vitamin B12, sodium and CRP as predicting major postoperative complications at hospital admission. Although these factors were identified as risk predictors of major complications, their effects were not statistically significant.
Renal dysfunction, vitamin D and CRP are known to be associated with the presence of pressure injuries [5, 7, 33, 34], and might play a role in predicting major postoperative complications in people with spinal cord injury/disorder and deep pressure injuries. In our study, Bayesian LASSO regression identified the renal marker eGFR as a predictive variable of major postoperative complications. The eGFR is considered the best overall index of renal dysfunction [35, 36]. In this cohort, only 26% (65) of the individuals had a normal eGFR (≥90 ml/min), while the rest of the individuals showed mild to severe impairment. We found the eGFR to have a slight negative effect on major complications. For every 10 ml/min increase in the eGFR, the odds of having a major complication decreased by 9% (95% CI 0.62–1.02). According to the literature, renal dysfunction affects wound healing and increases the risk of developing postoperative wound infections [37, 38]. This notion is consistent with Fähndrich et al., who showed that cystatin C was associated with major complications after flap surgery in individuals with spinal cord injury/disorder and deep pressure injury [13]. The result of our study indicates that renal dysfunction and especially the eGFR should be assessed and monitored during the treatment of stage III and IV pressure injuries in people with spinal cord injury/disorder [13]. If possible, it should also be treated or integrated into the risk prediction before flap surgery is performed.
Based on our study, vitamin D is a risk predictor for major postoperative complications. Individuals with spinal cord injury/disorder are already at high risk for vitamin D deficiency (<75 nmol/l) [39]. If they also have a pressure injury, they are even more likely to have low vitamin D levels [40]. The odds of having a major complication increased by 5% (95% CI 0.98–1.23) for every 10 nmol/l increase in vitamin D. In contrast, the literature shows that low vitamin D levels are associated with pressure injuries and negatively impact wound healing [34, 41, 42]. Therefore, vitamin D supplementation during pressure injury treatment is recommended to achieve better wound healing [34, 43]. In our clinic, we have been supplementing vitamin D in those with a deficiency since 2016. Our results indicate that vitamin D plays a role in predicting major postoperative complications, which might be due to the treatment of vitamin D deficiency under the Basel Decubitus Approach. Nutritional status is routinely assessed at hospital admission and treated accordingly [17, 26]. In our cohort, 73% (146) of the individuals had a vitamin D deficiency. Individuals with high vitamin D levels at admission are not treated with vitamin D. Therefore, vitamin D supplementation might have been a protective factor for major complications because optimal levels were achieved during hospitalisation. It usually takes about 12 weeks to reach optimal vitamin D levels in deficient individuals [44]. However, we did not collect data on vitamin D levels during hospitalisation. Therefore, we do not know if normal vitamin D levels were achieved during treatment. We recommend observing vitamin D levels during hospitalisation regarding major complications.
Regarding vitamin B12, we found that 19% (37) of the individuals in our cohort had a deficiency (<200 ng/l). In our cohort, for every 100 ng/l increase in vitamin B12, the odds of having a major complication decreased by 9% (95% CI 07.74–1.07). As in the study by Fähndrich et al., our findings suggest that vitamin B12 deficiency is involved in predicting major complications [13]. No studies have been conducted on the association between vitamin B12 deficiency and surgical outcomes in individuals with spinal cord injury/disorder. Meyer et al. observed the impact of vitamin B12 deficiency in geriatric patients undergoing orthopaedic surgery [45]. They found that vitamin B12 deficiency did not impact complications [45]. The relationship between vitamin B12 deficiency and successful surgical outcomes in people with spinal cord injury/disorder needs to be elucidated in future studies.
According to our results, sodium is involved in predicting major complications. In our study, we found that the odds of having a major complication decreased by 25% (95% CI 0.16–1.05) for every 10 mmol/l increase in sodium. Furthermore, around 25% (63) of the individuals had low sodium levels. Teo et al. performed a systematic review and meta-analysis of 32 observational studies to estimate the association of hyponatremia (<135 mmol/l) with major postoperative complications [46]. They found that preoperative hyponatremia was associated with a 2.5-fold increased risk of having a major complication after surgical procedures [46]. Teo et al. also observed that hyponatremia was a specific risk predictor of major postoperative complications in individuals without spinal cord injury / disorder [46]. Moreover, it is known that individuals with spinal cord injury/disorder have an increased risk of hyponatremia compared to individuals without spinal cord injury / disorder and that the risk increases with higher lesion levels [47, 48]. Based on these findings, we recommend that hyponatremia should be considered as a risk predictor for major complications [46]. Thus, sodium levels should be assessed and treated before flap surgery [34, 46].
Finally, in our cohort, for every 10 mg/l increase in CRP, the odds of having a major complication decreased by 2% (95% CI 0.79–1.07). The effect of only 2% at an increase of 10 mg/l might not be large enough to play a role in clinical decision-making. Nevertheless, CRP was selected as a relevant risk predictor by Bayesian LASSO regression and is therefore discussed. Elevated CRP levels (>5 mg/l) are frequently observed in people with pressure injuries [33, 34, 49, 50]. In our cohort, 92% (231) of the individuals had elevated CRP levels. No studies have observed preoperative CRP levels to predict major complications after flap surgery in people with spinal cord injury/disorder. However, studies unrelated to spinal cord injury/disorder and pressure injury have shown that preoperative elevated CRP levels were predictors of increased major and minor postoperative complications [51, 52]. In contrast, we found a slight negative effect of high CRP levels on major complications. In the Basel Decubitus Approach, the CRP level is also routinely assessed at hospital admission [17, 26]. CRP is a non-specific inflammatory marker, indicating any acute inflammation, tissue damage or infection [33, 50]. Therefore, it is difficult to interpret our result as there are many reasons for elevated CRP levels at hospital admission. One possible explanation might be the association between high CRP levels and osteomyelitis. Rigazzi et al. found that CRP levels were statistically significantly higher in individuals with osteomyelitis than in individuals without osteomyelitis [22]. In addition, Fähndrich et al. found that people with osteomyelitis had fewer major complications than those without osteomyelitis with the Basel Decubitus Approach [13]. Individuals with osteomyelitis receive individualised antibiotic treatment based on tissue samples, as opposed to standardised treatment for those without osteomyelitis [13, 26]. Fähndrich et al. concluded that an individualised antibiotic treatment might have been the reason for fewer complications in individuals with osteomyelitis [13]. The same might apply to elevated CRP levels, as a high CRP level may indicate underlying osteomyelitis [22]. The individualised use of antibiotics might be more effective in treating the infection and, therefore, reduce major complications [13], which might explain the positive association between major complications and low CRP levels. However, based on our study, the effect of lower CRP levels on major postoperative complications cannot yet be explained because CRP levels are influenced by several factors. Therefore, further investigation is required to determine the role of CRP in major postoperative complications in individuals with spinal cord injury/disorder.
Ultimately, as eGFR, vitamin D, vitamin B12, sodium and CRP are routinely assessed at hospital admission for the Basel Decubitus Approach, the prediction model can be used by the physician’s at hospital admission to predict the patient’s risk of major postoperative complications during the treatment of stage III and IV pressure injuries in people with spinal cord injury/disorder.
This study was conducted in the context of the Basel Decubitus Approach. Therefore, a limitation of this study is that the results may not be applicable in settings without a similar treatment approach. Logistic Bayesian LASSO regression is a powerful technique, especially for variable selection and dealing with multicollinearity. However, we extracted data retrospectively from regular clinical documentation. Therefore, the quality of the data for some variables was limited due to the lack of standardised documentation, which required multiple imputations. Prospective cohort studies with a larger sample size and a pilot study using the risk prediction model at baseline are recommended for future research. Furthermore, we recommend prospective observation of other factors, such as the size of the pressure injury. Another limitation is that the results cannot be generalised to people with other severe additional comorbidities that require treatment in other hospitals, as we excluded them in this study.
For the Basel Decubitus Approach, the eGFR, vitamin D, vitamin B12, sodium and CRP at hospital admission were risk predictors for major postoperative complications. In this study, high eGFR, vitamin B12, sodium and CRP levels negatively affected major postoperative complications. Therefore, assessing the identified risk predictors during hospital stay should be considered during the Basel Decubitus Approach. In contrast, based on this study, high vitamin D levels positively affected major postoperative complications. Vitamin D supplementation in vitamin D-deficient individuals might be the reason for this finding. Furthermore, a lower CRP level was identified as a relevant risk predictor for major postoperative complications by Bayesian LASSO regression. One explanation for this finding might be underlying osteomyelitis, which is treated with individualised antibiotics, as opposed to standardised antibiotics for those without osteomyelitis. However, the effect of CRP on major postoperative complications cannot yet be explained because CRP levels at hospital admission are influenced by many factors. Therefore, the role of CRP in major complications needs to be further investigated.
All data are stored in Stata/MP (version 17, 64-bit) with the corresponding author. The deidentified study data are now available with the codebook and statistical analysis plan and can be requested directly (carina.faehndrich[at]paraplegie.ch).
All codes are stored with the corresponding author and can be requested directly (carina.faehndrich[at]paraplegie.ch).
CF developed the study, collected the data, performed the analyses, wrote the first draft and finalised the manuscript. AG conceptualised the study, supported the statistical analyses and provided valuable feedback on the manuscript. MB, MH, DJS and RW were involved in conceptualising the study and provided relevant feedback on the manuscript. ASS conceptualised the study and provided relevant feedback on the manuscript. All authors approved the final version of the manuscript.
This study was funded as part of the pressure injury rehabilitation management program through the Swiss Paraplegic Research.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. AG received consulting fees from AbbVie Switzerland that were unrelated to this article. No other potential conflicts of interest related to the content of this manuscript were disclosed.
1. Henzel MK, Bogie K. Medical management of pressure injuries in patients with spinal cord disorders. In: Kirshblum SC, editor. Spinal Cord Medicine. 3rd ed. New York: Demos Medical Publishing; 2019. pp. 516–43.
2. Ljung AC, Stenius MC, Bjelak S, Lagergren JF. Surgery for pressure ulcers in spinal cord-injured patients following a structured treatment programme: a 10-year follow-up. Int Wound J. 2017 Apr;14(2):355–9. doi: https://doi.org/10.1111/iwj.12609
3. Clark FA, Jackson JM, Scott MD, Carlson ME, Atkins MS, Uhles-Tanaka D, et al. Data-based models of how pressure ulcers develop in daily-living contexts of adults with spinal cord injury. Arch Phys Med Rehabil. 2006 Nov;87(11):1516–25. doi: https://doi.org/10.1016/j.apmr.2006.08.329
4. Ko HY. Preventing and managing pressure injuries in spinal cord injuries. A practical guide to care of spinal cord injuries. Singapore: Springer Nature; 2023. pp. 611–31.
5. Consortium for Spinal Cord Medicine Clinical Practice Guidelines. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2001;24(sup1 Suppl 1):S40–101. doi: https://doi.org/10.1080/10790268.2001.11753592
6. Stucki G, Cieza A, Melvin J. The International Classification of Functioning, Disability and Health (ICF): a unifying model for the conceptual description of the rehabilitation strategy. J Rehabil Med. 2007 May;39(4):279–85. doi: https://doi.org/10.2340/16501977-0041
7. European Pressure Ulcer Advisory Panel; NPIAP, Alliance PPPI. Prevention and treatment of pressure ulcers/injuries: Clinical practice guideline. The international guideline 2019: European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance 2019. Internet: https://internationalguideline.com/
8. Kreutzträger M, Voss H, Scheel-Sailer A, Liebscher T. Outcome analyses of a multimodal treatment approach for deep pressure ulcers in spinal cord injuries: a retrospective cohort study. Spinal Cord. 2018 Jun;56(6):582–90. doi: https://doi.org/10.1038/s41393-018-0065-3
9. Keys KA, Daniali LN, Warner KJ, Mathes DW. Multivariate predictors of failure after flap coverage of pressure ulcers. Plast Reconstr Surg. 2010 Jun;125(6):1725–34. doi: https://doi.org/10.1097/PRS.0b013e3181d51227
10. Biglari B, Büchler A, Reitzel T, Swing T, Gerner HJ, Ferbert T, et al. A retrospective study on flap complications after pressure ulcer surgery in spinal cord-injured patients. Spinal Cord. 2014 Jan;52(1):80–3. doi: https://doi.org/10.1038/sc.2013.130
11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004 Aug;240(2):205–13. doi: https://doi.org/10.1097/01.sla.0000133083.54934.ae
12. Schryvers OI, Stranc MF, Nance PW. Surgical treatment of pressure ulcers: 20-year experience. Arch Phys Med Rehabil. 2000 Dec;81(12):1556–62. doi: https://doi.org/10.1053/apmr.2000.17828
13. Fähndrich C, Gemperli A, Baumberger M, Harder M, Roth B, Schaefer DJ, et al. Risk factors of major complications after flap surgery in the treatment of stage III and IV pressure injury in people with spinal cord injury/disorder: a retrospective cohort study. Spinal Cord. 2024 Jan;62(1):34–41. doi: https://doi.org/10.1038/s41393-023-00944-9
14. Montroy R, Eltorai I, Garstang S. The surgical management of pressure ulcers. In: Lin V, editor. Spinal Cord Medicine: Principles and Practice. 2nd ed. New York: Demos Medical Publishing; 2010. pp. 673–91.
15. Maschke R, Scheel-Sailer A, Thumbikat P. Pressure ulcer and other dermatological complications. In: Chhabra H, editor. ISCoS textbook on comprehensive management of spinal cord injuries. Philadelphia: Lippincott Williams & Wilkins; 2015. pp. 733–60.
16. Kemp T, Wang C, Mathes D, Keys KA. The surgical management of pressure injuries. In: Kirshblum SC, Lin VW, editors. Spinal Cord Medicine. 3rd. New York: Demos Medical Publishing; 2019. pp. 544–58.
17. Meier C, Boes S, Gemperli A, Gmünder HP, Koligi K, Metzger S, et al. Treatment and cost of pressure injury stage III or IV in four patients with spinal cord injury: the Basel Decubitus Concept. Spinal Cord Ser Cases. 2019 Mar;5(1):30. doi: https://doi.org/10.1038/s41394-019-0173-0
18. Sgarzani R, Maietti E, Tedeschi S, Trapani FF, Battilana M, Landi S, et al. Multidisciplinary treatment protocol for ischiatic, sacral, trochanteric or other pressure injuries in people with spinal cord injury: a retrospective cohort study. Spinal Cord. 2023 Mar;61(3):204–10. doi: https://doi.org/10.1038/s41393-022-00869-9
19. Rieger U, Scheufler O, Schmid D, Zweifel-Schlatter M, Kalbermatten D, Pierer G. Die sechs Behandlungsprinzipien des Basler Dekubituskonzepts [Six treatment principles of the Basel pressure sore concept]. Handchir Mikrochir Plast Chir. 2007 Jun;39(3):206–14. doi: https://doi.org/10.1055/s-2007-965311
20. Neuhauser C, Sailer CO, Najmanova K, Baumberger M, Paez-Granados D, Schaefer DJ, et al. Risk constellation of hospital acquired pressure injuries in patients with a spinal cord injury/ disorder - focus on time since spinal cord injury/ disorder and patients’ age. Spinal Cord. 2023 Aug;61(8):453–9. doi: https://doi.org/10.1038/s41393-023-00910-5
21. Lindqvist EK, Sommar P, Stenius M, Lagergren JF. Complications after pressure ulcer surgery - a study of 118 operations in spinal cord injured patients. J Plast Surg Hand Surg. 2020 Jun;54(3):145–50. doi: https://doi.org/10.1080/2000656X.2020.1720700
22. Rigazzi J, Fähndrich C, Osinga R, Baumgartner S, Baumberger M, Krebs J, et al. Osteomyelitis and antibiotic treatment in patients with grade IV pressure injury and spinal cord lesion-a retrospective cohort study. Spinal Cord. 2022 Jun;60(6):540–7. doi: https://doi.org/10.1038/s41393-022-00758-1
23. Wettstein R, Tremp M, Baumberger M, Schaefer DJ, Kalbermatten DF. Local flap therapy for the treatment of pressure sore wounds. Int Wound J. 2015 Oct;12(5):572–6. doi: https://doi.org/10.1111/iwj.12166
24. Grant SW, Collins GS, Nashef SA. Statistical Primer: developing and validating a risk prediction model. Eur J Cardiothorac Surg. 2018 Aug;54(2):203–8. doi: https://doi.org/10.1093/ejcts/ezy180
25. Janes H, Pepe MS, Gu W. Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med. 2008 Nov;149(10):751–60. doi: https://doi.org/10.7326/0003-4819-149-10-200811180-00009
26. Fähndrich C, Gemperli A, Baumberger M, Bechtiger M, Roth B, Schaefer DJ, et al. Treatment approaches of stage III and IV pressure injury in people with spinal cord injury: A scoping review. J Spinal Cord Med. 2023;46(5):705–15. doi: https://doi.org/10.1080/10790268-2022.2108645
27. Lüscher NJ, de Roche R, Krupp S, Kuhn W, Zäch GA. The sensory tensor fasciae latae flap: a 9-year follow-up. Ann Plast Surg. 1991 Apr;26(4):306–10. doi: https://doi.org/10.1097/00000637-199104000-00004
28. Scheel-Sailer A, Koligi K, Lampart P, Fähndrich C, Gmünder HP, Metzger S, et al. Effect of a computerized decision support system on the treatment approach of stage III or IV pressure injury in patients with spinal cord injury: a feasibility study. BMC Health Serv Res. 2023 Jan;23(1):103. doi: https://doi.org/10.1186/s12913-023-09045-y
29. ASIA and ISCoS International Standards Committee. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)-What’s new? Spinal Cord. 2019 Oct;57(10):815–7. doi: https://doi.org/10.1038/s41393-019-0350-9
30. Park T, Casella G. The Bayesian lasso. J Am Stat Assoc. 2008;103(482):681–6. doi: https://doi.org/10.1198/016214508000000337
31. Zhou X, Reiter J. A note on Bayesian inference after multiple imputation. Am Stat. 2010;64(2):159–63. doi: https://doi.org/10.1198/tast.2010.09109
32. Efron B, Hastie T, Johnstone I, Tibshirani R. Least angle regression. Ann Stat. 2004;32(2):407–99. doi: https://doi.org/10.1214/009053604000000067
33. Wang N, Lv L, Yan F, Ma Y, Miao L, Foon Chung LY, et al. Biomarkers for the early detection of pressure injury: A systematic review and meta-analysis. J Tissue Viability. 2022 May;31(2):259–67. doi: https://doi.org/10.1016/j.jtv.2022.02.005
34. Lussi C, Frotzler A, Jenny A, Schaefer DJ, Kressig RW, Scheel-Sailer A. Nutritional blood parameters and nutritional risk screening in patients with spinal cord injury and deep pressure ulcer-a retrospective chart analysis. Spinal Cord. 2018 Feb;56(2):168–75. doi: https://doi.org/10.1038/s41393-017-0016-4
35. Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: part II. Glomerular filtration rate, proteinuria, and other markers. Am Fam Physician. 2004 Sep;70(6):1091–7.
36. Kumaresan R, Giri P. A comparison of serum cystatin C and creatinine with glomerular filtration rate in Indian patients with chronic kidney disease. Oman Med J. 2011 Nov;26(6):421–5. doi: https://doi.org/10.5001/omj.2011.107
37. Maroz N, Simman R. Wound healing in patients with impaired kidney function. J Am Coll Clin Wound Spec. 2014 Jun;5(1):2–7. doi: https://doi.org/10.1016/j.jccw.2014.05.002
38. Maroz N. Impact of renal failure on wounds healing. J Am Coll Clin Wound Spec. 2018 Jan;8(1-3):12–3. doi: https://doi.org/10.1016/j.jccw.2018.01.004
39. Flueck JL, Perret C. Vitamin D deficiency in individuals with a spinal cord injury: a literature review. Spinal Cord. 2017 May;55(5):428–34. doi: https://doi.org/10.1038/sc.2016.155
40. Zhou XJ, Vaziri ND, Segal JL, Winer RL, Eltorai I, Brunnemann SR. Effects of chronic spinal cord injury and pressure ulcer on 25(OH)-vitamin D levels. J Am Paraplegia Soc. 1993 Jan;16(1):9–13. doi: https://doi.org/10.1080/01952307.1993.11735877
41. Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol. 2016 Nov;164:379–85. doi: https://doi.org/10.1016/j.jsbmb.2015.08.011
42. Smith K, Hewlings S. Correlation between vitamin D levels and hard-to-heal wounds: a systematic review. J Wound Care. 2020 Jul;29 Sup7:S24–30. doi: https://doi.org/10.12968/jowc.2020.29.Sup7.S24
43. Burkievcz CJ, Skare TL, Malafaia O, Nassif PA, Ribas CS, Santos LR. Vitamin D deficiency in patients with chronic venous ulcers. Rev Col Bras Cir. 2012;39(1):60–3. doi: https://doi.org/10.1590/S0100-69912012000100012
44. Bauman WA, Emmons RR, Cirnigliaro CM, Kirshblum SC, Spungen AM. An effective oral vitamin D replacement therapy in persons with spinal cord injury. J Spinal Cord Med. 2011;34(5):455–60. doi: https://doi.org/10.1179/2045772311Y.0000000032
45. Meyer M, Leiss F, Greimel F, Renkawitz T, Grifka J, Maderbacher G, et al. Impact of malnutrition and vitamin deficiency in geriatric patients undergoing orthopedic surgery. Acta Orthop. 2021 Jun;92(3):358–63. doi: https://doi.org/10.1080/17453674.2021.1882092
46. Teo CB, Gan MY, Tay RY, Loh WJ, Loh NW. Association of preoperative hyponatremia with surgical outcomes: A systematic review and meta-analysis of 32 observational studies. J Clin Endocrinol Metab. 2023 Apr;108(5):1254–71. doi: https://doi.org/10.1210/clinem/dgac685
47. Frisbie JH. Salt wasting, hypotension, polydipsia, and hyponatremia and the level of spinal cord injury. Spinal Cord. 2007 Aug;45(8):563–8. doi: https://doi.org/10.1038/sj.sc.3101984
48. Sica DA, Midha M, Zawada E, Stacy W, Hussey R. Hyponatremia in spinal cord injury. J Am Paraplegia Soc. 1990 Oct;13(4):78–83. doi: https://doi.org/10.1080/01952307.1990.11735824
49. Scivoletto G, Fuoco U, Morganti B, Cosentino E, Molinari M. Pressure sores and blood and serum dysmetabolism in spinal cord injury patients. Spinal Cord. 2004 Aug;42(8):473–6. doi: https://doi.org/10.1038/sj.sc.3101622
50. Houghton PE, Campbell KE, Panel C. Canadian best practice guidelines for the prevention and management of pressure ulcers in people with spinal cord injury. A resource handbook for clinicians2013 02.05.2024. Available from: https://estim4wounds.ca/wp-content/uploads/TheCanadianBPGforPreventionandManagementofPressureUlcersinPeoplewithSCI.pdf
51. Alsaif SH, Rogers AC, Pua P, Casey PT, Aherne GG, Brannigan AE, et al. Preoperative C-reactive protein and other inflammatory markers as predictors of postoperative complications in patients with colorectal neoplasia. World J Surg Oncol. 2021 Mar;19(1):74. doi: https://doi.org/10.1186/s12957-021-02142-4
52. Zhu Y, Chen J, Lin S, Xu D. Risk factor for the development of surgical site infection following ileostomy reversal: a single-center report. Updates Surg. 2022 Oct;74(5):1675–82. doi: https://doi.org/10.1007/s13304-022-01335-0
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3977.