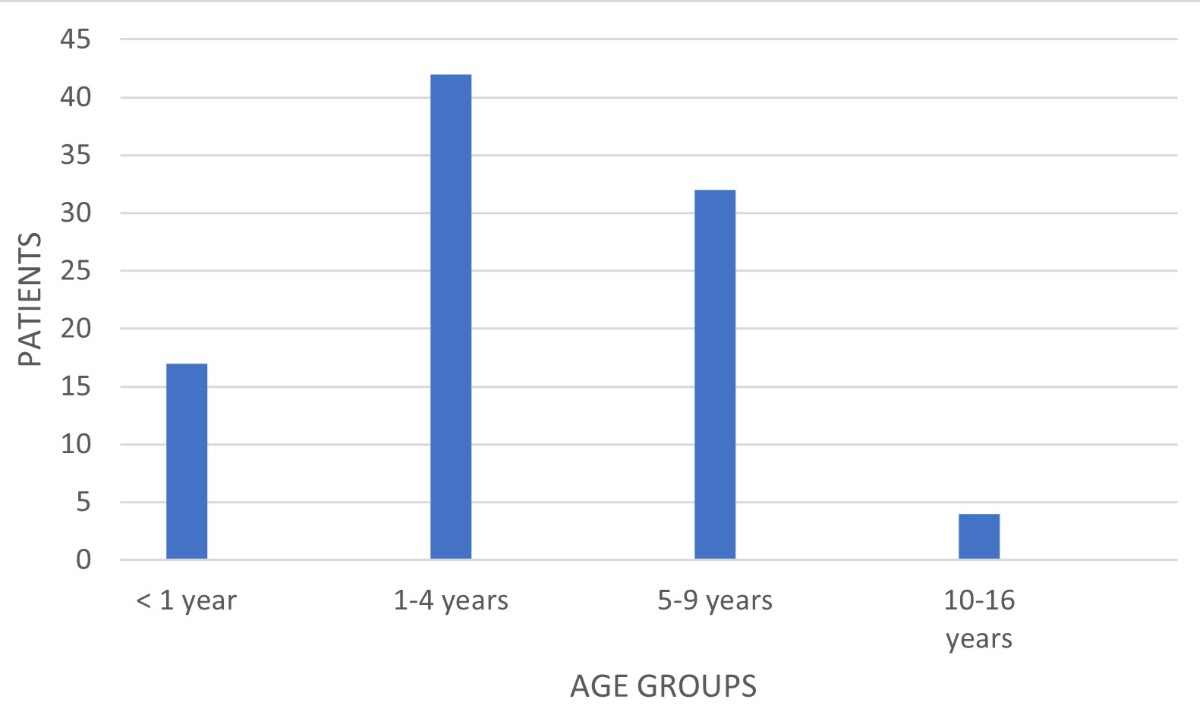Clinical outcomes and severe complications of hospitalised children and adolescents
with varicella in central Switzerland: a retrospective observational study
DOI: https://doi.org/https://doi.org/10.57187/s.3962
Jan Schwidetzkya,
Ulrich Heiningerbc,
Medea Salzmanna,
Thomas J. Neuhausa,
Michael Buettcherdef
a Department of Paediatrics, Children’s Hospital of Central Switzerland,
Lucerne, Switzerland
b Paediatric Infectious Diseases and Vaccinology, University Children’s
Hospital Basel (UKBB), Basel, Switzerland
c Medical Faculty, University of Basel, Basel, Switzerland
d Paediatric Infectious Diseases Unit, Department of Paediatrics,
Children’s Hospital of Central Switzerland, Lucerne, Switzerland
e Faculty of Health Sciences and Medicine, University Lucerne, Lucerne,
Switzerland
f Paediatric Pharmacology and Pharmacometrics Research Centre at the
University Children’s Hospital Basel (UKBB), Basel, Switzerland
Summary
AIM: Recent
data on clinical complications and mortality among hospitalised children and
adolescents due to varicella are unavailable in Switzerland. The aim of the study
was to explore data on severe
varicella complications in hospitalised children before the introduction of a universal
varicella vaccination recommendation, which the Swiss Federal Office of Public
Health implemented in January 2023.
METHODS: This
was a retrospective observational study of children hospitalised with varicella
between 01.01.2010 and 31.03.2020 at a tertiary children’s hospital in central
Switzerland serving approximately 10% of the Swiss population. The inclusion
criteria were acute varicella and/or related complications.
RESULTS: A
total of 95 patients were identified. The median age at onset was 4 years
(range: 2 months to 13 years) and the peak age of patients was between 1 and 4
years. 53 had mild and 42 patients had severe varicella-associated
complications (8 had >1 severe complication). The most common severe
complications were bacterial skin and soft tissue infections (n = 28), invasive
secondary bacterial infections (n = 18), and central nervous system-related
complications (n = 12). Admission to the paediatric intensive care unit and
surgical intervention were required in 11 (12%) and 16 (17%) patients, respectively.
Two previously healthy school-age children died because of secondary bacterial
infections.
CONCLUSION:
Our results demonstrate that varicella can cause severe and even fatal
complications in children living in a highly developed country. This study
provides valuable clinical data on severe
varicella complications in hospitalised children from a large catchment area of Switzerland,
facilitating future data comparison of the disease burden before and after the introduction
of universal varicella vaccination in Switzerland.
Introduction
Varicella
(chickenpox) is a common and highly contagious infectious disease caused by varicella
zoster virus (VZV). It manifests as a pruritic rash accompanied by fever and
other systemic signs and symptoms that usually are mild to moderate. The rash
is more intense on the trunk and head than on the extremities, and it typically
evolves as a series of “crops” over 1 to 3 days in non-immunocompromised hosts.
In the absence of universal varicella zoster vaccination, varicella occurs
primarily in young children, specifically, 52–78% of cases occur in children
younger than six years, and 89–96% of cases occur before adolescence [1]. Occasionally,
severe complications occur –
leading to hospitalisation, long-term sequelae, or death – not only in
immunocompromised but also in healthy, immunocompetent children [1]. Potential complications
include secondary
bacterial infections of the skin and soft tissue (e.g. impetigo, ecthyma,
abscess, cellulitis, and necrotising fasciitis), toxic shock syndrome, thrombocytopenia,
pneumonia (viral and bacterial), hepatitis, arthritis, cerebellitis with
ataxia, encephalitis with seizures and coma, and congenital varicella syndrome.
In Europe, annual incidence rates for varicella vary between 300 and 1291 per
100,000 population [2]. Data from
international surveillance studies show hospitalisation rates of 1.3 to 5.5 per
1000 VZV cases [3–6]. Approximately 70,000–85,000
individuals contract varicella in Switzerland each year [7, 8]. In Switzerland, the
most recent national data on VZV hospitalisations
of children were obtained in 2000–2003 through the Swiss Paediatric
Surveillance Unit (SPSU). This national surveillance was restarted by members
of our group in July 2021 and is currently ongoing. The
calculated hospitalisation rate during that 3-year period was 1.3 per 1000
cases [9]. In the USA and Germany, universal varicella vaccination was
introduced in 1996 and 2004, respectively, for children aged 11 months and
older. Thereafter, in the USA, the number of cases (all age groups) decreased
by 84% in 2000 compared with 1995/96 [10].
In Germany, varicella case rates per reporting physician decreased by 84%, from
3.6 per month in 2005 to 0.6 per month in 2012 [11].
During the years of our described cohort, the Swiss Federal Commission for
Immunisation recommended VZV vaccination (two doses, at least 4 weeks apart) as
a basic vaccine for adolescents aged 11–15 years without prior varicella. Furthermore,
VZV vaccination is recommended for high-risk individuals and healthcare workers
[12]. Only recently (January 2023) did Switzerland
introduce universal VZV vaccination. As no peer-reviewed paediatric varicella hospitalisation
data have been collected in Switzerland since 2003, we aimed to explore data on severe
varicella complications and clinical outcomes
in hospitalised children
from a large catchment area of Switzerland, facilitating future data comparison
before and after the introduction of universal varicella vaccination in Switzerland.
Materials and
methods
Study design
This retrospective
observational study of varicella-associated hospitalisations included children aged
0 to <16 years with clinical signs and symptoms of varicella who were hospitalised
at Children’s Hospital Lucerne between 01.01.2010 and 31.03.2020. Microbiological
confirmation of clinical varicella diagnosis was not a prerequisite. Screening
for eligibility was conducted using ICD-10 code B01 for a primary varicella diagnosis
(primary diagnosis for hospitalisation) or a secondary varicella diagnosis (varicella
as a concomitant disease during hospitalisation or recent preceding disease). Outpatients
were excluded. The output of this case capture was systematically reviewed and
validated by the first author (JS) and last author (MB), and all double entries
were removed. For this observational study, outcome measures were not
differentiated into primary or secondary outcomes. The outcome measures
included the exploration and description of varicella complications, clinical
course, risk factors, microbiology, and treatment in hospitalised children and
adolescents.
Data
collection was performed using a standardised clinical report form. Demographic
data (sex, age, and nationality), medical history (vaccination status,
hospitalisation duration, place of infection, and family medical history), risk
factors (immune status, co-morbidities, and atopic disease), clinical symptoms
(rash, fever before hospitalisation and on admission, vomiting, diarrhoea,
tachycardia, hypotension, dyspnoea, and others), diagnostics (microbiology: blood
culture, polymerase chain reaction (PCR), cerebrospinal fluid, wound swab,
radiology, and EEG), treatment (antipyresis, surgery, and antiviral and
antibacterial therapy), and associated complications (see definitions below) were
recorded from the case files and entered into an electronic database stored at
the children’s hospital.
Study setting
The study
was conducted in the Children’s Hospital of Central Switzerland (Lucerne) at
the Cantonal Hospital of Lucerne, which is a tertiary paediatric hospital
serving a catchment area of around 700,000 inhabitants from the entire canton
of Lucerne and the other five central Swiss cantons, accounting for
approximately 10% of the total Swiss population.
Analysis
Data were extracted
using Microsoft Excel tables, and statistical analysis was performed using IBM©
SPSS© Statistics versions 26 and 29. Statistics were descriptive.
Analyses were conducted by calculating the frequencies and percentages. No additional
software libraries,
frameworks, or packages were used in this study.
Definitions
Case
definition used for varicella: Clinician diagnosis of an acute illness with a
generalised vesicular or maculopapulovesicular rash with or without laboratory
confirmation (e.g. for positive VZV PCR) or a positive VZV PCR detected in the
diagnostic work-up (e.g. for cerebrospinal fluid or an atypical skin lesion) of
a patient.
Complications
were categorised as follows.
Severe
- Death
- Central
nervous system related complications: cerebellitis, meningoencephalitis, seizure,
stroke (vasculitis)
- Invasive
secondary bacterial infections of different organs: arthritis, endocarditis,
meningitis, osteomyelitis, and pneumonia
- Sepsis
(according to the Goldstein criteria) [13]
- Toxic
shock syndrome (with clinical and laboratory changes caused by Gram-positive
pathogens) [14]
- Bacterial
skin and soft tissue infections (including all classes, not subdivided) [15]
Mild
- Clinical
complications: dehydration, nausea, pain, and keratoconjunctivitis
- Laboratory
abnormalities: coagulation disorder, elevated liver enzymes, and thrombocytopenia
Ethics approval
The study
protocol was prepared by the first (JS) and last (MB) authors and was approved
by the Ethics Committee of Northwestern and Central Switzerland (EKNZ) (project
number: 2020-01367). General consent was not implemented in this tertiary hospital
during the
study period. According to article 34 a) HFG (swissethics.ch), the need for individual
consent was waived by the ethics committee.
Results
Study population
Screening
for eligibility by ICD-10 code resulted in 230 cases, 131 of whom had to be
excluded due to not meeting the case definition; 88 patients had complications not
related to varicella, and in 43 patients, varicella diagnosis was not confirmed
(figure 1). Four children were re-hospitalised with new complications due to
varicella during the study period; they were included in the analysis but counted
as one case. The median age of the remaining 95 patients was 4 years (range: 2
months to 13 years). Figure 2 shows the distribution of hospitalised patients
with varicella by age group. Between 2010 and 2020, 0.5 to 3.8 patients with VZV
complications were identified per 1000 inpatient admissions (table 1) in
Lucerne. Figure 3 provides an overview of patients with severe complications,
including complication categories. No children had congenital VZV infection.
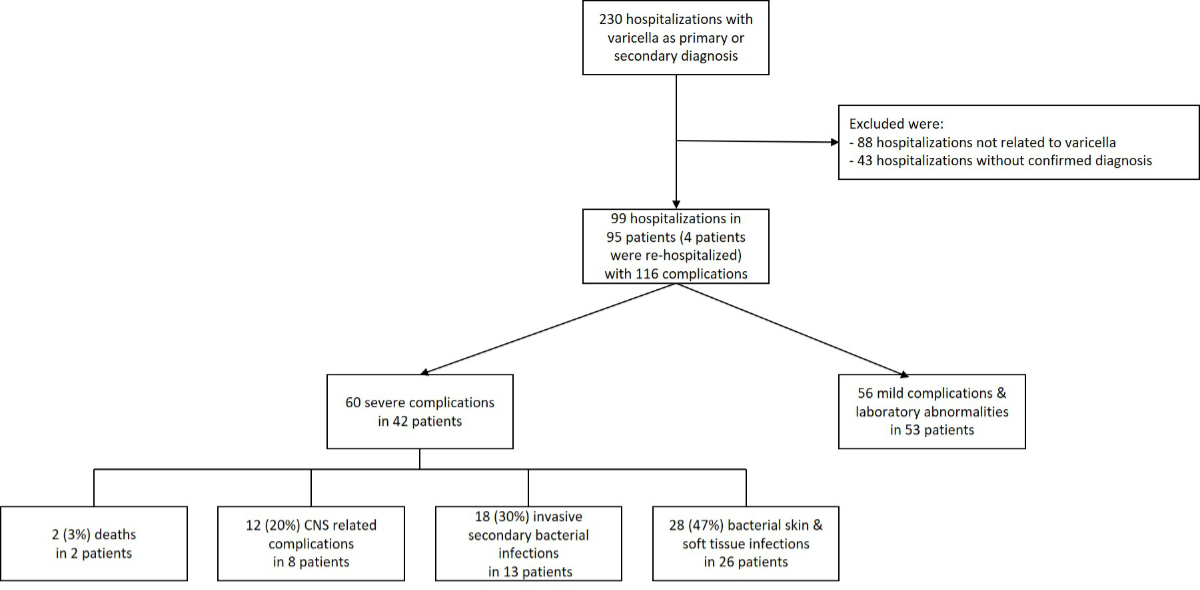
Figure 1Study population meeting the inclusion criteria. The four patients with
re-hospitalisation were counted as one case in the analysis. * Eight patients experienced
more than one severe
complication and are listed in more than one complication category. CNS:
central nervous system.
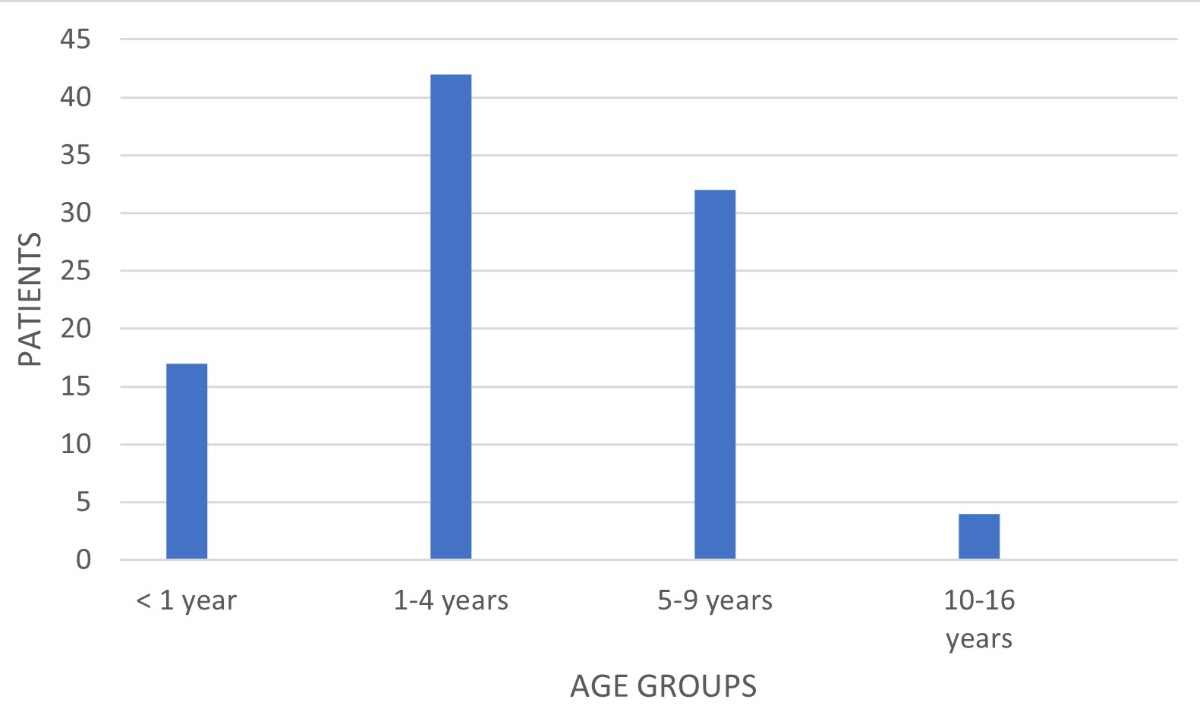
Figure 2Distribution of all hospitalised patients with varicella by age
group (n = 95).
Table 1Epidemiology
of varicella zoster virus hospitalisations at the Children’s Hospital of
Central Switzerland, Lucerne (2010–2020).
| Study year |
Number of hospitalisations |
Varicella-related hospitalisations |
Varicella-related hospitalisations per 1000
hospitalisations |
| 2010 |
3692 |
14 |
3.8 |
| 2011 |
3705 |
6 |
1.6 |
| 2012 |
3815 |
5 |
1.3 |
| 2013 |
3846 |
12 |
3.1 |
| 2014 |
4138 |
12 |
2.9 |
| 2015 |
3940 |
8 |
2 |
| 2016 |
4088 |
8 |
2 |
| 2017 |
4069 |
16 |
4 |
| 2018 |
4199 |
4 |
1 |
| 2019 |
4557 |
8 |
1.8 |
| 2020* |
1112 |
2 |
1.8 |
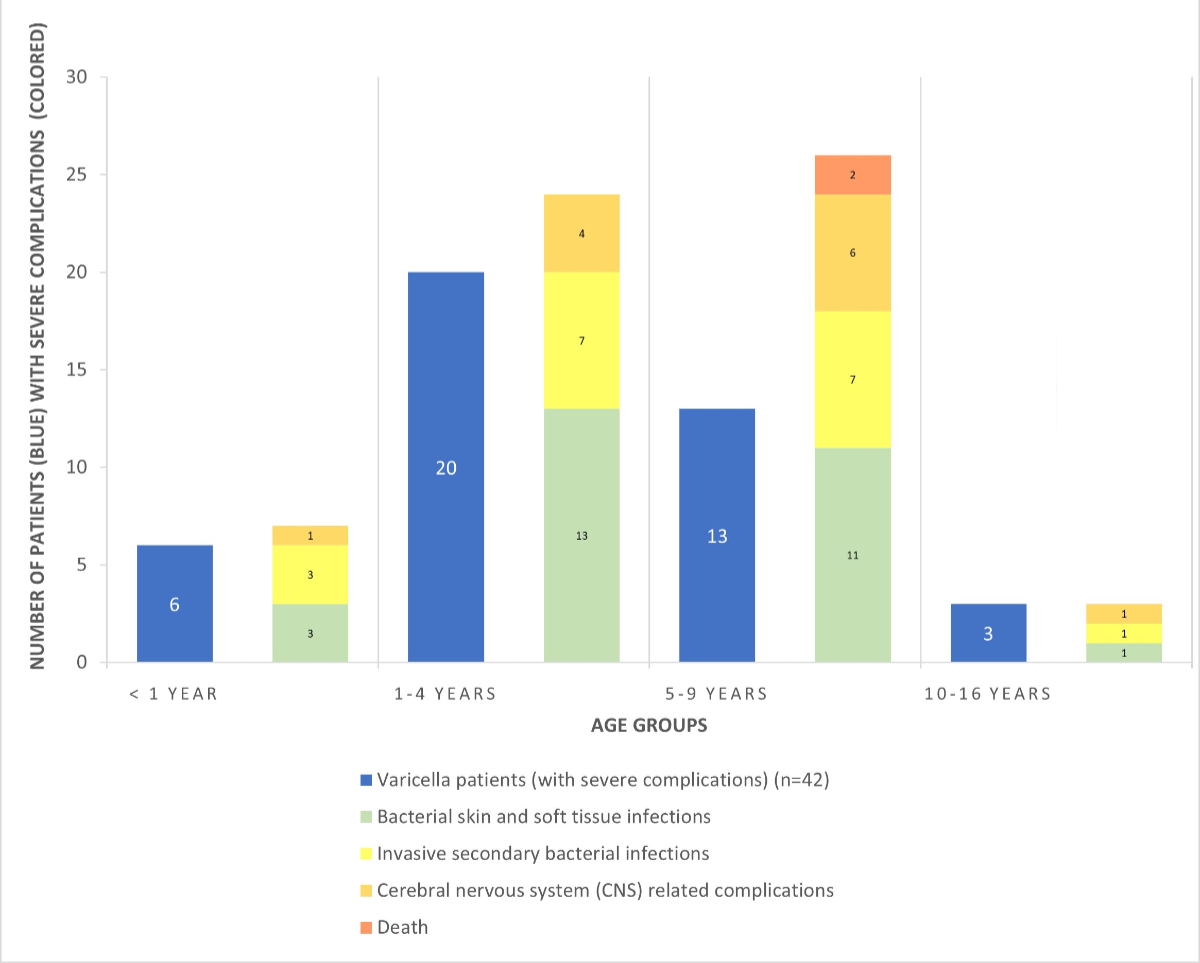
Figure 3Severe
varicella complications by age group. The blue
bars show the number of patients with severe varicella complications (n = 42); the
coloured bars show the numbers and categories of severe complications (n = 60)
in the respective age groups. Eight patients experienced more than one severe
complication.
Of the 95 children,
80 (84%) had no comorbidities, 12 (13%) had atopic eczema, and 3 (3%) had an
underlying oncologic disease. Vaccination status could be assessed in 84 patients;
none had received VZV vaccination, as documented by a certificate or reported
by the parents. A total of 79 had received other universal general vaccinations
according to age and the Swiss vaccination recommendations; two patients were incompletely
vaccinated; and three children had no vaccinations, including two infants who
had not yet received vaccination due to their young age. Three oncology patients
had been previously exposed to varicella and had received post-exposure
prophylaxis (VZV Immunoglobulin) at least 2 months before the current hospitalisation.
In 44 patients, exposure information was available: 32 children were exposed
within their families, and 12 were exposed in a public institution (e.g. nurseries,
kindergartens, or schools).
Hospitalisation duration
Eighty-six children
presented to our emergency care unit and were admitted. The median time from onset
of first symptoms to hospitalisation was 10 days (range: 0–150 days). Nine
patients had already been hospitalised initially for a “non-varicella”
diagnosis; however, during the course, they had varicella as a further
diagnosis. The median hospitalisation duration was 6 days in previously healthy
children and in children with atopic eczema (range: 1–31 and 2–18 days, respectively).
Oncology patients had a shorter median hospitalisation period of 4 days (range:
3–5 days).
Complications
A total of 60
severe complications occurred in 42 (44%) of the 95 patients (table 2),
including 8 (53%) of the 15 patients with comorbidities and 34 (42%) of the 80
patients without comorbidities. Their median age was 4 years (range 7 months to
13 years) and 8 of them experienced more than one severe complication. No child
with an underlying oncological disease experienced a severe complication.
Table 2Summary of severe complications in healthy patients and those with atopic
eczema.
| Complication
categories |
Total patients |
No comorbidities |
Atopic eczema |
| n = 42 |
n = 34 |
n = 8 |
| Including specified
pathologies |
60 complications |
52 complications |
8 complications |
| Death |
|
2 |
2 |
– |
| Listeria
monocytogenes
meningitis |
1 |
1* |
|
| Group A Streptococcus
fulminant sepsis |
1 |
1* |
|
| Cerebral and nervous
system-related complications |
|
12 |
12 |
– |
| Meningoencephalitis |
4 |
4 |
|
| Cerebellitis |
4 |
4 |
|
| Febrile seizure |
2 |
2 |
|
| Stroke (vasculitis) |
2 |
2* |
|
| Invasive
secondary bacterial infections |
|
18 |
15 |
3 |
| Endocarditis |
1 |
1* |
– |
| Pneumonia |
5 |
4 |
1 |
| Sepsis or toxic shock syndrome |
9 |
8 |
1 |
| Arthritis |
2 |
1 |
1 |
| Osteomyelitis |
1 |
1 |
– |
| Bacterial
skin and soft tissue infections |
|
28 |
23 |
5 |
| Skin |
|
6 |
4 |
2 |
| Ecthyma |
1 |
1 |
– |
| Others |
5 |
3 |
2 |
| Soft tissue |
|
22 |
19 |
3 |
| Abscess |
4 |
3 |
1 |
| Necrotising fasciitis |
3 |
2* |
1 |
| Cellulitis/phlegmon |
11 |
11 |
– |
| Others |
4 |
3 |
1 |
Four
children with varicella were discharged and had to be readmitted within 4 weeks
because of newly occurring complications: two were re-hospitalised with new pathologies
(cerebral vasculitis (see case vignette 3) and osteomyelitis). Two had worsening
soft tissue infections (abscess; renewed inflammation and pectoral swelling).
Secondary
bacterial infections were the most common severe complications – both skin and
soft tissue infections and invasive infections. The median time from the onset
of the first symptoms to hospitalisation with a secondary bacterial infection was
6 days (range: 1–26 days). Of the 95 patients, 2 died from bacterial
complications (see case vignettes 1 and 2).
Case vignette 1: Fatal
Listeria monocytogenes meningitis
A previously
healthy 8-year-old boy died of Listeria monocytogenes meningitis. Fever
and typical varicella skin lesions had occurred two days before hospitalisation,
followed by vomiting, dehydration, neck pain, ataxia, somnolence, and
convulsions. In the emergency room, varicella encephalitis was suspected; immediate
intravenous therapy with acyclovir and ceftriaxone (standard local regimen for
suspected bacterial meningitis) was initiated, and the patient was admitted to
the paediatric intensive care unit. After a positive cerebrospinal fluid
culture for Listeria monocytogenes was received within 20 hours,
antibiotic therapy was immediately extended to intravenous amoxicillin and
amikacin. Rapid neurological deterioration (fixed pupils and apnoea) prompted magnetic
resonance imaging (MRI), which revealed cerebellar swelling with transforaminal
herniation. Despite an emergency bilateral craniectomy, the patient died within
24 hours of admission. No clinical or laboratory evidence indicated immunodeficiency,
and no varicella zoster virus was detected in the cerebrospinal fluid or the
brain.
Case vignette 2: Fatal
group A Streptococcus sepsis
A previously
healthy 5-year-old girl presented to the emergency room with septic shock and multiorgan
failure, followed by immediate intubation and admission to the paediatric
intensive care unit. Typical skin lesions had evolved three days earlier.
Intravenous antibiotic therapy included ceftriaxone, clindamycin, and
amoxicillin. Blood culture revealed group A Streptococcus. The patient
deteriorated rapidly with cardiac, respiratory, and renal failure and was
transferred to a university hospital for renal support (hemofiltration) and
extracorporeal membrane oxygenation (ECMO). However, the child died 5 days
later secondary to brain swelling and herniation
Case vignette 3: Cerebral
vasculitis and stroke
A previously
healthy 6-year-old girl presented to the emergency room with left hemiparesis
and choreoathetoid movement disorder. Immediate cranial MRI showed vascular stenosis
secondary to vasculitis of the right cerebral artery around segment M1. The
patient had experienced uncomplicated varicella 4 months prior. A positive VZV
PCR in the cerebrospinal fluid and a positive VZV immunoglobulin G (IgG) cerebrospinal
fluid / serum index were consistent with postinfectious varicella-associated
vasculitis. The girl recovered rapidly under high-dose intravenous methylprednisolone
therapy and acyclovir. Doppler sonographic and MRI angiographic control 4 weeks
later showed a new stenosis of the right anterior cerebral artery without
clinical symptoms in the presence of focal cerebral arteriopathy. The girl was
re-hospitalised for repeated steroid therapy over 4 days. Currently (5 years
later), the neurological status is unremarkable; the vasculitis changes on MRI
have resolved completely on secondary antithrombotic prophylaxis with acetylsalicylic
acid.
Case vignette 4: Group
A Streptococcus –
necrotising fasciitis and endocarditis
A
previously healthy 5-year-old boy presented to the emergency room with severe
gluteal pain. Typical skin lesions had evolved 2 days earlier. Gluteal necrotising
fasciitis was suspected, followed by immediate surgical intervention and administration
of intravenous antibiotics with clindamycin and cefuroxime. Blood culture and
wound swabs grew group A Streptococcus. Two further surgical
interventions were required. On day 6 of hospitalisation, the patient developed
a systolic murmur and respiratory and cardiac deterioration. Echocardiography revealed
rupture of the chordae tendineae with severe mitral valve prolapse. Antibiotic therapy
was switched to intravenous gentamycin and ceftriaxone, and the patient was
transferred to a cardiac surgery centre for reconstruction with intraoperative
evidence of bacterial endocarditis. Re-operation 2 years later for a new
rupture of the chordae tendineae was necessary. Currently (6 years later) the
boy has normal exercise tolerance, requires no medication, and has a residual
defect of moderate mitral regurgitation.
Microbiology
In the 42 patients
with severe complications, 35 blood samples, 5 cerebrospinal fluid samples, and
21 wound swab or tissue biopsy samples were analysed. Bacterial growth or viral
PCR was detected in 8 blood, 3 cerebrospinal fluid, and 16 tissue samples. Table
3 shows the distribution of pathogens by specimen and severe varicella complication
category. The most frequently detected pathogen was group A Streptococcus.
Table 3Pathogens detected and complication categories.
| Patient ID |
Specimen |
Pathogen |
Complication category |
| Death |
Central nervous system-related complications |
Invasive secondary bacterial infections |
Bacterial skin and soft tissue infections |
| 10-01 |
Swab |
Group A Streptococcus |
|
|
X |
X |
| 10-02 |
Swab |
Group A Streptococcus
and Pseudomonas aeruginosa |
|
|
|
X |
| 11-02 |
Blood (PCR) |
Varicella
zoster virus |
|
|
|
X |
| Swab |
Group A Streptococcus and Staphylococcus
aureus |
| 11-06 |
Swab |
Staphylococcus aureus and Stenotrophomonas maltophilia |
|
|
|
X |
| 13-01 |
Swab |
Staphylococcus aureus and Enterococcus faecalis |
|
|
X |
|
| 13-03 |
Blood (PCR) |
Varicella zoster virus |
|
X |
|
|
| Cerebrospinal fluid |
Varicella zoster virus |
| 13-11 |
Swab |
Staphylococcus aureus |
|
|
|
x |
| 13-12 |
Blood |
Listeria monocytogenes |
X |
X |
X |
|
| Cerebrospinal fluid |
Listeria monocytogenes |
| Swab |
Listeria monocytogenes |
| 14-07 |
Blood |
Group A Streptococcus |
X |
|
X |
X |
| Swab |
Group A Streptococcus and Staphylococcus
aureus |
| 14-11 |
Blood (PCR) |
Varicella zoster virus |
|
X |
|
|
| 16-07 |
Blood (PCR) |
Varicella zoster virus |
|
X |
|
|
| 17-02 |
Blood |
Group A Streptococcus |
|
|
X |
X |
| Swab |
Group A Streptococcus |
| 17-05 |
Swab |
Staphylococcus aureus |
|
|
X |
X |
| 17-07 |
Swab |
Group A Streptococcus |
|
|
X |
|
| 17-09 |
Swab |
Staphylococcus aureus and Enterococcus faecalis and
Pseudomonas aeruginosa |
|
|
|
X |
| 17-10 |
Swab |
Group A Streptococcus |
|
|
|
X |
| 17-14 |
Swab |
Group A Streptococcus |
|
|
|
X |
| 17-15 |
Cerebrospinal fluid |
Varicella zoster virus |
|
X |
|
|
| 18-02 |
Swab |
Staphylococcus aureus |
|
|
|
X |
| 19-01 |
Blood |
Group A Streptococcus |
|
|
|
X |
| 20-01 |
Swab |
Group A Streptococcus |
|
|
|
X |
Treatment
In 35 (84%)
of 42 patients with severe complications, intravenous antibiotic treatment was
administered for a median duration of 6 days (range: 1–16 days). No antibiotic
treatment was administered to five patients with non-bacterial central nervous
system-related complications (one with encephalitis, two with cerebellitis, one
with febrile seizure, and one with stroke) or two patients with bacterial soft
tissue infections (of the abdomen and knee). The two soft tissue infections did
not require antibiotics, as one was a superficial abscess that was locally
disinfected and rinsed during the treatment course, and the other was a knee
joint effusion without evidence of a pathogen after rinsing.
Surgery was
performed in 16 patients, including wound debridement in three because of cervical
(n = 1) or gluteal (n = 2) necrotising fasciitis. Six children with soft tissue
infection developed abscesses in the retro-auricular, periorbital, cheek, neck,
lower abdomen or thigh area; all abscesses were surgically drained. The other seven
surgical interventions included two joint punctures (knee and shoulder) and one
craniectomy (case vignette 1), lymph node extirpation, thoracic drainage, nail
extraction, and wound debridement, respectively.
Paediatric
intensive care unit admission for a median stay of 3 days (range: 1–7 days) was
required for 11 (26%) of 42 patients with 23 severe complications (10 secondary
invasive bacterial infections, 7 central nervous system-related complications,
3 bacterial skin infections, 2 bacterial soft tissue infections, and 1
multiorgan failure). Seven patients in the paediatric intensive care unit experienced
more than one severe complication; two of them had five and four severe
complications at the same time, respectively. The median age of all paediatric
intensive care unit patients was 6 years (range: 2 to 12 years).
Discussion
In this comprehensive
retrospective observational study conducted over 10 years and 3 months (2010–2020),
we reviewed all varicella-associated paediatric hospitalisations at a large
tertiary hospital in central Switzerland serving approximately 10% of the Swiss
paediatric population. The study included 95 patients, nearly half of whom had severe
complications. Most of the hospitalised children were 0–9 years old, with a
peak in preschool age, which aligns with the overall age distribution of all
varicella cases in Switzerland during the pre-vaccination era [16]. This pattern was
also observed for severe
complications, paediatric intensive care unit stay, and surgery interventions. Our
observation is consistent with a former Swiss Paediatric Surveillance Unit
study [9], as well as studies from other
countries [11, 17–19]. At the time of
this study, no universal infant varicella immunisation recommendation was in
place in Switzerland. However, vaccination was recommended for children aged 11
years and older with no history of varicella. In our cohort, one patient (13
years old) should have been vaccinated but was not.
Since
January 2023, universal varicella immunisation has been recommended in
Switzerland, with two doses at 9 and 12 months of age and a catch-up program
for older children [20].
Analysis of
our cohort revealed that most patients with severe varicella zoster virus (VZV)
complications were previously healthy without pre-existing comorbidities, except
for a small subgroup with atopic eczema. Additionally, only 3% of patients hospitalised
for varicella were children with underlying oncological conditions. This is
consistent with previous studies conducted in Germany and New Zealand, which also
reported that most patients with complicated varicella were otherwise healthy.
However, patients with oncological diseases were older at the onset of
varicella complications, and the duration of hospitalisation was similar to
that of immunocompetent children [10, 21, 22].
Potential explanations for this observation in oncology patients include
earlier presentation to healthcare services, a lower threshold for hospitalisation,
and non-reluctant use of anti-viral medications.
Generally,
in secondary bacterial infections in previously healthy children and those with
underlying atopic eczema, the most prominent pathogen was group A Streptococcus,
which caused considerable morbidity and required surgical interventions and paediatric
intensive care unit admission. Group A Streptococcus was also the cause
of one fatal case in our study. The association of group A Streptococcus
as a secondary bacterial infection in varicella is well described [23–25]. In our
cohort, group A Streptococcus
was the most common pathogen (10 out of 24). Invasive bacterial infections affecting
the skin and soft tissue accounted for one-third of admissions in our cohort,
and the most common invasive complications were sepsis and pneumonia. Varicella
has been shown in various studies to be the most important risk factor for
developing invasive infection with group A Streptococcus (including
necrotising fasciitis), with the risk estimated to be 58–60-fold higher than in
the general population [23–25]. Children
may be colonised with virulent group A Streptococcus strains in their
oropharynx and transfer them to different body areas by scratching varicella
skin lesions, further breaking the skin barrier and facilitating invasive group
A Streptococcus infections and complications [26]. Notably, one case of mitral regurgitation and
papillary
muscle rupture due to a varicella complication with invasive group A Streptococcus
infection occurred during the study period and has been published elsewhere [27].
Furthermore,
children with atopic dermatitis are at increased risk for bacterial infections.
The risk factors are chronic inflammation, a dysfunctional skin barrier due to
altered skin pH values and lower epidermal antimicrobial peptides, and anti-inflammatory
medication (topical steroids) used for atopic dermatitis [28, 29]. VZV infection may
lead to transient
virus-induced alterations in the innate immune response [30].
The other
fatal case in our study had a combination of varicella with concurrent Listeria monocytogenes meningitis. However, risk factors such as immunosuppression
or associated underlying diseases (HIV, post-transplant status, and cancer)
were not present in this child. A previous literature review yielded only two
case reports of patients who developed Listeria
monocytogenes meningitis within 6 weeks of varicella.Despite a lack
of evidence of immunodeficiency, a transient T-cell abnormality due to VZV
infection could not be excluded as a risk factor for invasive listeriosis,
which can be a serious and life-threatening condition[31, 32]. Thus, it can be assumed
that
invasive infection with Listeria monocytogenes can occur as a
complication of VZV infection. Therefore, it may be prudent to broaden the
empirical treatment by including an antibiotic that also covers Listeria monocytogenes
(such as aminopenicillin) in children presenting with varicella and meningitis.
Central
nervous system complications were the second most common complication leading
to hospitalisation. Central nervous system complications have been described for
varicella, and their proportion among all complications varies between studies (9–61%)
[33–36]. Bonhoeffer et al. described a
large paediatric varicella cohort in Switzerland and reported that 25% of
complications involved the central nervous system (9). Encephalitis was the most frequent
manifestation [33].
Weeks to
months after varicella, patients are at an increased risk of vasculopathy and ischemic
stroke or transient cerebral arteriopathy. The pathogenesis of varicella zoster
virus vasculopathy involves viral invasion of blood vessels, in which the virus
spreads transaxonally from the ganglia to the vascular walls [37]. After reactivation
from the trigeminal or
upper cervical ganglia, the virus travels along neurites and infects the
adventitia of the cerebral arteries, causing vasculopathy [38].
Various studies
have reported an average duration of hospitalisation due to varicella of 4–8
days, and the results of our study are within this range [9, 22, 35, 36]. Most of
the children requiring
intensive care were previously healthy, (i.e. immunocompetent) (8 patients),
which is in accordance with other international studies [2, 9]. The average age of
the children who were admitted to the paediatric
intensive care unit was 6 years, slightly higher than the average age of the
overall study population (4 years). The results of Bonhoeffer et al. regarding the
mean age of admission to paediatric intensive care unit support this
observation [9]. The proportion of patients
that required admission to the paediatric intensive care unit was slightly higher
in our study (12%) than in the literature (3–9%) [9, 17, 22].
Although
often regarded as a benign illness, varicella imposes significant financial and
societal burdens. The direct healthcare costs range from €1000 to €25,000 per
hospital admission depending on the duration of stay and complications (with an
average cost of €1625), as demonstrated by a European study. Moreover, the
socioeconomic impact is considerable, as caregivers and parents often face
extended absences from work during their children’s illness [35]. When comparing the
costs of universal
varicella vaccination (universal varicella vaccination) (as recommended in Switzerland
since 2023) with the
previous vaccination strategy (vaccination at ages 11–40 in those with no history
of varicella), the direct costs – preventive vaccination versus healthcare
expenditures due to the disease – are comparable, at CHF 1.75 million and CHF
1.5 million, respectively. However, considering indirect costs, universal varicella
vaccination results in
an annual savings of approximately CHF 620,000. The reduced morbidity and
complication rates further support the implementation of universal varicella vaccination
in Switzerland [39].
With an
average of nine paediatric hospitalisations per year (95 patients over 11
years), our cohort in central Switzerland represents approximately 6% of the
146 annual varicella complication-related hospitalisations in Switzerland [7]. Most
of the complications in our catchment
area occurred in children younger than 11. These children were not vaccinated,
as per the national recommendations at that time. This observation calls for
the early introduction of universal varicella vaccination, ideally before a child’s
first birthday.
However, without knowing the total number of varicella cases in the whole
population and the respective age groups, we cannot use our study data to draw
firm conclusions and suggest a strategy for the optimal age to introduce
varicella vaccination. The recent recommendation in Switzerland for universal varicella
vaccination before a
child’s first birthday is based on the experience and evidence from other
countries, in which the introduction at an early age reduced the disease burden
and morbidity in subsequent age groups, including vulnerable patient groups
(young infants, non-immune pregnant women, and immunocompromised individuals) [39].
To the best
of our knowledge, none of the patients in our cohort had received the varicella
vaccine. The documentation concerning varicella vaccination status was
incomplete and likely not explicitly addressed in the medical history in many
cases, as it was not among the routinely recommended vaccinations at that time.
Consequently, our data provide limited utility for accurately comparing
vaccination rates before and after the implementation of the universal varicella vaccination.
Of note, our
analysis focused exclusively on inpatient VZV cases, excluding data from
outpatient consultations during the same period. As a result, we could not make
comparisons with complication-free outpatient cases. Furthermore, patients with
herpes zoster were not included in this study, which could have provided
valuable comparisons after universal varicella vaccination implementation. In addition,
the retrospective
nature of the data collection may have led to incomplete case identification
due to potential misdiagnoses or inadequate documentation. Nevertheless, the
long observation period and large cohort allowed us to gather comprehensive
clinical data on the complications and clinical outcomes associated with cases
of VZV requiring hospitalisation.
Conclusion
This
retrospective study provides a comprehensive description of the age, hospitalisation
duration, disease characteristics, severity of complications, and treatment of children
and adolescents hospitalised with varicella over 10 years and 3 months before
the introduction of universal varicella vaccination in Switzerland. These data will
be supplemented by current
ongoing, prospective nationwide surveillance and can be used in future for
evaluations of the impact of universal varicella vaccination introduction, particularly
the reduction in VZV-associated
hospitalisations and severe complications.
Open science
Anonymised study data can be shared
on request by contacting the corresponding author.
Acknowledgments
The
authors would like to thank Dr med. H.P. Kuen, Head of Centre for Congenital
Heart Defects, Children’s Hospital of Central Switzerland Lucerne, for review
of cases. The authors would also like to thank Ms. Janine Stritt, study
coordinator, Paediatric Research Centre, Children’s Hospital of Central Switzerland,
for contributing to data collection for this study.
Michael Buettcher
Paediatric
Infectious Diseases Unit
Department
of Paediatrics
Children’s
Hospital of Central Switzerland
CH-6000 Lucerne
Michael.Buettcher[at]luks.ch
References
1. Piazza MF, Amicizia D, Paganino C, Marchini F, Astengo M, Grammatico F, et al. Has
Clinical and Epidemiological Varicella Burden Changed over Time in Children? Overview
on Hospitalizations, Comorbidities and Costs from 2010 to 2017 in Italy. Vaccines
(Basel). 2021 Dec;9(12):1485. doi: https://doi.org/10.3390/vaccines9121485
2. Wutzler P, Bonanni P, Burgess M, Gershon A, Sáfadi MA, Casabona G. Varicella vaccination
- the global experience. Expert Rev Vaccines. 2017 Aug;16(8):833–43. doi: https://doi.org/10.1080/14760584.2017.1343669
3. Galil K, Brown C, Lin F, Seward J. Hospitalizations for varicella in the United States,
1988 to 1999. Pediatr Infect Dis J. 2002 Oct;21(10):931–5. doi: https://doi.org/10.1097/00006454-200210000-00009
4. Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, et al. Epidemiology of
varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect.
2001 Oct;127(2):305–14. doi: https://doi.org/10.1017/S0950268801005921
5. Boëlle PY, Hanslik T. Varicella in non-immune persons: incidence, hospitalization
and mortality rates. Epidemiol Infect. 2002 Dec;129(3):599–606. doi: https://doi.org/10.1017/S0950268802007720
6. Ratner AJ. Varicella-related hospitalizations in the vaccine era. Pediatr Infect Dis
J. 2002 Oct;21(10):927–31. doi: https://doi.org/10.1097/00006454-200210000-00008
7. Bundesamt für Gesundheit EKfIE. Windpocken (Varizellen)/ MMRV: Basisimpfung für Säuglinge.
Bern: Bundesamt für Gesundheit; 2023.
8. Lienert F, Weiss O, Schmitt K, Heininger U, Guggisberg P. Acceptance of universal
varicella vaccination among Swiss pediatricians and general practitioners who treat
pediatric patients. BMC Infect Dis. 2021 Jan;21(1):12. doi: https://doi.org/10.1186/s12879-020-05586-3
9. Bonhoeffer J, Baer G, Muehleisen B, Aebi C, Nadal D, Schaad UB, et al. Prospective
surveillance of hospitalisations associated with varicella-zoster virus infections
in children and adolescents. Eur J Pediatr. 2005 Jun;164(6):366–70. doi: https://doi.org/10.1007/s00431-005-1637-8
10. Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, et al. Varicella
disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA.
2002 Feb;287(5):606–11. doi: https://doi.org/10.1001/jama.287.5.606
11. Siedler A, Hecht J, Rieck T, Tolksdorf K, Hengel H. [Varicella vaccination in Germany.
A provisional appraisal in the context of MMR vaccination]. Bundesgesundheitsblatt
Gesundheitsforschung Gesundheitsschutz. 2013 Sep;56(9):1313–20. doi: https://doi.org/10.1007/s00103-013-1789-z
12. Bundesamt für Gesundheit EKfI. Kantonales Durchimpfungsmonitoring Schweiz. BAG-Bulletin.
2018. Bull BAG. 2018;(24):13.
13. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference:
definitions for sepsis and organ dysfunction in pediatrics. Pediatric critical care
medicine : a journal of the Society of Critical Care Medicine and the World Federation
of Pediatric Intensive and Critical Care Societies. 2005;6(1). doi: https://doi.org/10.1097/01.PCC.0000149131.72248.E6
14. Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009 May;9(5):281–90.
doi: https://doi.org/10.1016/S1473-3099(09)70066-0
15. Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA. Managing skin and soft
tissue infections: expert panel recommendations on key decision points. The Journal
of antimicrobial chemotherapy. 2003;52 Suppl 1. doi: https://doi.org/10.1093/jac/dkg466
16. A I, C A, K B, M B, AM S, U H. Prospective surveillance of varicella-zoster virus
infections in an out-patient setting in Switzerland. Hum Vaccin. 2009;5(12).
17. Blumental S, Sabbe M, Lepage P; Belgian Group for Varicella. Varicella paediatric
hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016 Jan;101(1):16–22.
doi: https://doi.org/10.1136/archdischild-2015-308283
18. Turel O, Bakir M, Gonen I, Hatipoglu N, Aydogmus C, Hosaf E, et al. Children Hospitalized
for Varicella: Complications and Cost Burden. Value Health Reg Issues. 2013;2(2):226–30.
doi: https://doi.org/10.1016/j.vhri.2013.05.003
19. Chang LY, Huang LM, Chang IS, Tsai FY. Epidemiological characteristics of varicella
from 2000 to 2008 and the impact of nationwide immunization in Taiwan. BMC Infect
Dis. 2011 Dec;11(1):352. doi: https://doi.org/10.1186/1471-2334-11-352
20. Bundesamt für Gesundheit EKfI. Schweizerischer Impfplan 2023. Richtlinien und Empfehlungen.
Bern: Bundesamt für Gesundheit; 2023.
21. Hagemann C, Krämer A, Grote V, Liese JG, Streng A. Specific Varicella-Related Complications
and Their Decrease in Hospitalized Children after the Introduction of General Varicella
Vaccination: Results from a Multicenter Pediatric Hospital Surveillance Study in Bavaria
(Germany). Infect Dis Ther. 2019 Dec;8(4):597–611. doi: https://doi.org/10.1007/s40121-019-00273-6
22. Wen SC, Best E, Walls T, Dickson N, McCay H, Wilson E. Prospective surveillance of
hospitalisations associated with varicella in New Zealand children. J Paediatr Child
Health. 2015 Nov;51(11):1078–83. doi: https://doi.org/10.1111/jpc.12937
23. Abuhammour W, Hasan RA, Unuvar E. Group A beta-hemolytic streptococcal bacteremia.
Indian J Pediatr. 2004 Oct;71(10):915–9. doi: https://doi.org/10.1007/BF02830836
24. Laupland KB, Davies HD, Low DE, Schwartz B, Green K, McGeer A; Ontario Group A Streptococcal
Study Group. Invasive group A streptococcal disease in children and association with
varicella-zoster virus infection. Pediatrics. 2000 May;105(5):E60. doi: https://doi.org/10.1542/peds.105.5.e60
25. Imöhl M, van der Linden M, Reinert RR, Ritter K. Invasive group A streptococcal disease
and association with varicella in Germany, 1996-2009. FEMS Immunol Med Microbiol.
2011 Jun;62(1):101–9. doi: https://doi.org/10.1111/j.1574-695X.2011.00788.x
26. Coleman S. The association between varicella (chickenpox) and group A streptococcus
infections in historical perspective. SAGE Open Med. 2016 Jul;4:2050312116658909.
doi: https://doi.org/10.1177/2050312116658909
27. Savoia P, Heininger U, Buettcher M. Streptococcus pyogenes Endocarditis Associated With Varicella-Case Report and Review of the Literature.
Front Pediatr. 2019 Dec;7:500. doi: https://doi.org/10.3389/fped.2019.00500
28. Kienast AK, Kreth HW, Höger PH. Varicella vaccination in children with atopic eczema.
J Dtsch Dermatol Ges. 2007 Oct;5(10):875–80. doi: https://doi.org/10.1111/j.1610-0387.2007.06488.x
29. Han JH, Yoon JW, Yook HJ, Bang CH, Chun JH, Lee JY, et al. Evaluation of Atopic Dermatitis
and Cutaneous Infectious Disorders Using Sequential Pattern Mining: A Nationwide Population-Based
Cohort Study. J Clin Med. 2022 Jun;11(12):3422. doi: https://doi.org/10.3390/jcm11123422
30. Gerada C, Campbell TM, Kennedy JJ, McSharry BP, Steain M, Slobedman B, et al. Manipulation
of the Innate Immune Response by Varicella Zoster Virus. Front Immunol. 2020 Jan;11:1.
doi: https://doi.org/10.3389/fimmu.2020.00001
31. Tim MW, Jackson MA, Shannon K, Cohen B, McCracken GH Jr. Non-neonatal infection due
to Listeria monocytogenes. Pediatr Infect Dis. 1984;3(3):213–7. doi: https://doi.org/10.1097/00006454-198405000-00006
32. Wright H, MacGregor A. A case of meningitis due to Bacterium monocytogenes. J Pathol
Bacteriol. 1939;48(2):48. doi: https://doi.org/10.1002/path.1700480220
33. Science M, MacGregor D, Richardson SE, Mahant S, Tran D, Bitnun A. Central nervous
system complications of varicella-zoster virus. J Pediatr. 2014 Oct;165(4):779–85.
doi: https://doi.org/10.1016/j.jpeds.2014.06.014
34. Ziebold C, von Kries R, Lang R, Weigl J, Schmitt HJ. Severe complications of varicella
in previously healthy children in Germany: a 1-year survey. Pediatrics. 2001 Nov;108(5):E79.
doi: https://doi.org/10.1542/peds.108.5.e79
35. Bernal JL, Hobbelen P, Amirthalingam G. Burden of varicella complications in secondary
care, England, 2004 to 2017. Euro Surveill. 2019 Oct;24(42):1900233. doi: https://doi.org/10.2807/1560-7917.ES.2019.24.42.1900233
36. Gowin E, Wysocki J, Michalak M. Don’t forget how severe varicella can be—complications
of varicella in children in a defined Polish population. Int J Infect Dis. 2013 Jul;17(7):e485–9.
doi: https://doi.org/10.1016/j.ijid.2012.11.024
37. Guedes M, Filipe R, Costa A, Soares C, Sarmento A, Tavares M. Central nervous system
varicella zoster vasculopathy in an immunocompromised patient. IDCases. 2018 Dec;15:e00483.
doi: https://doi.org/10.1016/j.idcr.2018.e00483
38. Nagel MA, Niemeyer CS, Bubak AN. Central nervous system infections produced by varicella
zoster virus. Curr Opin Infect Dis. 2020 Jun;33(3):273–8. doi: https://doi.org/10.1097/QCO.0000000000000647
39. Banz K, Iseli A, Aebi C, Brunner M, Schmutz AM, Heininger U. Economic evaluation of
varicella vaccination in Swiss children and adolescents. Hum Vaccin. 2009 Dec;5(12):847–57.
doi: https://doi.org/10.4161/hv.9898