Chatbots in medicine: certification process and applied use case
DOI: https://doi.org/https://doi.org/10.57187/s.3954
Mayssam Nehmea,
Franck Schneiderb,
Esther Amruthalingamc,
Elio
Schnarrenbergerd,
Raphaël Tremeaude,
Idris Guessousac
a Division
of Primary Care Medicine, Geneva University Hospitals, Geneva, Switzerland
b Direction
of Communication, Geneva University Hospitals, Geneva, Switzerland
c Faculty
of Medicine, University of Geneva, Geneva, Switzerland
d Säfeli
Sàrl, Granges-Paccot, Switzerland
e Promotion
Santé Suisse, Lausanne, Switzerland
Summary
Chatbots are computer
programs designed to engage in natural language conversations in an easy and
understandable way. Their use has been accelerated recently with the advent of large
language models. However, their application in medicine and healthcare has been
limited due to concerns over data privacy, the risk of providing medical diagnoses,
and
ensuring regulatory and legal compliance. Medicine and healthcare could benefit
from chatbots if their scope is carefully defined and if they are used appropriately
and monitored long-term.
The confIAnce chatbot, developed at the Geneva
University Hospitals and the University of Geneva, is an informational tool
aimed at providing simplified information to the general public about primary
care and chronic diseases. In this paper, we describe the certification and
regulatory aspects applicable to chatbots in healthcare, particularly in primary
care medicine. We use the confIAnce chatbot as a case study to explore the
definition and classification of a medical device and its application to
chatbots, considering the applicable Swiss regulations and the European Union
AI Act.
Chatbots can be classified anywhere from
non-medical devices (informational tools that do not handle patient data or
provide recommendations for treatment or diagnosis) to Class III medical
devices (high-risk tools capable of predicting potentially fatal events and enabling
a pre-emptive medical intervention). Key considerations in the definition and
certification process include defining the chatbot’s scope, ensuring compliance
with regulations, maintaining security and safety, and continuously evaluating
performance, risks, and utility. A lexicon of relevant terms related to artificial
intelligence in healthcare, medical devices, and regulatory frameworks is also presented
in this paper.
Chatbots hold potential for both patients and
healthcare professionals, provided that their scope of practice is clearly
defined, and that they comply with regulatory requirements. This review aims to
provide transparency by outlining the steps required for certification and
regulatory compliance, making it valuable for healthcare professionals,
scientists, developers, and patients.
Introduction
The use of chatbots has been accelerated in recent years, particularly
since the public deployment of ChatGPT [1]. Chatbots hold significant potential across
multiple fields, including in medicine and healthcare, as these domains depend
heavily on service delivery and information exchange. In recent years,
electronic patient messages have increased by 1.6 times, resulting in additional
time spent using electronic health records and increased after-hours work for
healthcare professionals [2, 3]. This surge
raises the risk of physician burnout [3, 4]. Moreover, unanswered
messages may lead to a decline in the physician-patient relationship. Chatbots
could serve as a complementary resource, reinforcing this relationship by addressing
some patient inquiries without attempting to diagnose or replace the
physician’s role. If chatbots can reduce even part of the messaging burden,
they could potentially improve time management, liberating some of physicians’
time, and allowing more meaningful interactions between physicians and patients.
In turn, this could facilitate patient acquisition of verified knowledge and
foster greater empowerment in healthcare. It is essential that physicians,
caregivers, and patients understand the core principles underpinning these
systems to make their operations more transparent, accessible, and easier to
grasp.
Large language
models (LLMs) encode vast amounts of text in a way that captures how words and
phrases relate to each other, allowing them to predict which words are likely
to follow. Driven by advanced algorithms, they have become more powerful than earlier
tools and are scalable for use in specific fields. The rapid advancements in
artificial intelligence (AI) have attracted significant attention. New
technologies using LLMs hold considerable promise for the efficient use of
chatbots in healthcare, including summarising clinical documentation or
research papers, answering patient-specific questions, and assisting with
appointments and workflow management in medical practices or hospitals.
Some studies have
evaluated the role of chatbots in managing and supporting patients in primary
care medicine. Chatbots can play a crucial role in chronic disease management,
providing support [5] and assisting patients with specific tasks such as
self-monitoring and self-management [6, 7]. The overall acceptance of chatbots
in primary care and chronic conditions appears promising, particularly in areas
such as cancer [8], hypertension [9], heart conditions [10, 11], pulmonary
conditions [10], mental health [12], and adherence to therapy [13, 14]. User
experience is one of the most frequently cited metrics in studies, which reflects
user satisfaction with perceived usefulness, ease of use, and improved quality
of life in managing their condition [15]. Studies have reported that patients
with chronic diseases may feel more comfortable using chatbots compared to
continuous in-hospital follow-ups [7]. For example, chatbots providing assistance
and follow-up to adults receiving cancer treatment have been shown to reduce
anxiety levels, limiting the need to contact healthcare professionals [16].
However, concerns about confidentiality and content quality arise with the use
of these technologies [17].
_Hlk172275445_Hlk172275486However, there are also risks and challenges, along with concerns in the
medical and scientific communities about how to best utilise and regulate this
rapidly advancing technology [18]. LLM chatbots, for instance, can provide inaccurate
information and can
“hallucinate”, providing seemingly coherent but incorrect answers. This poses a significant
risk in healthcare, especially when
chatbots are used for diagnosis or treatment [19]. One way to mitigate these
risks is by restricting chatbots to a specific knowledge base through
retrieval-augmented generation (RAG). RAG is an AI technique that combines the capabilities
of LLMs with the specificity and accuracy of a verified knowledge base. The RAG
technique consists of limiting the chatbot and allowing it to answer only
within the predefined scope set by the developer. Other risk mitigation
strategies include ongoing supervision and surveillance of chatbots. In
September 2022, the U. S. Food and Drug Administration (FDA) issued guidance stating
that chatbots and AI-assisted devices should require approval as medical
devices unless their outputs are fully monitored by humans, who can “independently
review the basis for the recommendations presented by the software” [20]. Another
approach is to carefully define the chatbot’s scope. For instance, a chatbot
designed to provide general information is very different from one used for diagnosis
or for administrative purposes like scheduling appointments. The scope changes
not only the definition of the chatbot, but also its use, monitoring, and
regulation. Indeed, several important questions arise, such as whether the
chatbot includes identifiable information, making it as sensitive as electronic
health records, whether it functions as a clinical decision-making tool, requiring
consideration of medical responsibility, or whether it qualifies as a medical
device, thus subject to the same certification process as other medical devices
used in patient care. As a general approach, industry
standards and best practices that companies and industry groups can adopt are essential,
along with adaptive regulation and guidance from regulatory bodies.
The European Medicines Agency (EMA), through the Medical Device
Regulation (MDR) [21] and Medical Devices Ordinance (MedDO) [22] in Switzerland,
and the United States Food and Drug Administration (FDA) [23], are two of the
main regulatory bodies for medical devices worldwide. Both agencies share
similarities in their classification systems, although the MDR is considered
more stringent in its equivalency and surveillance processes [24].
Additionally, the EMA uses notified bodies (as of March 2024, there were 41
notified bodies designated under the MDR) [25], whereas the FDA centralises
this task under a single government authority. Both systems use the same risk classes
(I, II,
III) ranging from lowest to highest risk, with the EU MDR further subdividing
Class II into IIa and IIb. The certification process varies depending on the
risk class. Therefore, when developing chatbots, it is important to determine
whether they are classified as medical devices and, if so, which risk class
they fall under.
Based on our previous experience developing chatbots for post-COVID care
[26] (www.rafael-postcovid.ch) and for general contact
and administrative information (https://www.hug.ch/en/contact) at the Geneva University Hospitals, our team developed an
informational chatbot for primary care, addressing the challenges of
certification and validation. The chatbot, confIAnce, was developed by the
Division of Primary Care Medicine, the communication department, and several
stakeholders at the Geneva University Hospitals and the University of Geneva.
It aims to provide simplified information to the general public on primary care
and chronic diseases. The chatbot is designed for informational purposes only, using
a knowledge base owned and updated by the Division of Primary Care Medicine, which
reflects the most common pathologies and chronic conditions encountered in general
practice [27]. This knowledge base was initially created for healthcare
professionals and was adapted into layperson terms to serve the general
population. The chatbot uses retrieval-augmented generation
(RAG), limiting its scope to the verified database.
This paper aims to demystify the development
and certification process for healthcare chatbots, defining their boundaries
and capabilities within the framework of the MDR. Using the confIAnce chatbot
as a case study, this paper provides insights into the necessary steps and best
practices. Herein, we review the definition of a
medical device under the MDR, the classification of chatbots according to risk levels,
the certification process, including quality management systems for both
medical and non-medical chatbots, data protection considerations, and new
considerations under the EU AI act.
Definition of a medical
device
According to the MDR (Article 2 and Annex VIII) [21], a medical device
is “any instrument, apparatus, appliance,
software, implant, reagent, material, or other article intended by the
manufacturer to be used, alone or in combination, for human beings for one or
more specific medical purposes”. These purposes include
“diagnosis, prevention, monitoring, prediction, prognosis, treatment, or
alleviation of disease; diagnosis, monitoring, treatment, alleviation of, or
compensation for an injury or disability; investigation, replacement, or
modification of the anatomy or of a physiological or pathological process or
state; providing information by means of in vitro examination of specimens
derived from the human body”. Devices for controlling or supporting
conception, as well as those specifically intended for cleaning, disinfecting,
or sterilising other medical devices, are also included. Additionally, “software
intended to provide information which is used to take decisions with diagnosis
or therapeutic purposes, or to monitor physiological processes are also
considered a medical device”.
For a chatbot to qualify
as a medical device, its application must be explicitly medical, such as aiding
in the diagnosis, prevention, treatment, or management of diseases and medical
conditions. Scope is a determining factor: a chatbot designed solely to give
general health and wellness advice may not fall under the MDR classification,
whereas one designed to diagnose or monitor specific medical conditions, or
offer personalised medical advice, would. Moreover, understanding the
limitations of chatbots is essential. While they can process and analyse large
data sets and interact with patients, their ability to provide accurate medical
advice or diagnoses depends on the sophistication of their underlying
algorithms and the quality of the data they use. These limitations must be
clearly defined to ensure safe and effective use, avoiding over-reliance on
these digital tools for critical medical decisions.
Application: The
confIAnce chatbot, designed for primary care medicine and chronic diseases,
does not diagnose or recommend treatment. Based on MDR regulations and the definition
of a medical device, confIAnce was classified as a non-medical device. Several
considerations were taken to ensure safety and scope of use: the chatbot’s scope
was restricted to a specific knowledge base, additional control layers were
added to fall back to no response when outside the scope of practice, and users
were explicitly informed that the chatbot is for informational purposes only. Safeguards
were implemented to maintain a non-personalised approach, reminding users that
they are interacting with a machine, and providing general information on chronic
diseases without interpreting patient data.
Classification of
chatbots as medical devices
If a chatbot is defined as a medical device
based on its application, it must then be classified under the MDR into one of
the risk classes (figure 1).
- Class I devices are low-risk,
and their certification process typically involves self-certification. Self-certification
is only applicable to Class I devices. This means the manufacturer applies the
best standards and practices to the product without requiring external
certification, though post-market surveillance and quality control are still necessary.
Examples include a chatbot that reminds patients to take medications or
schedules appointments without interpreting patient data or making clinical
decisions.
- Class IIa devices are medium
risk and require a conformity assessment, including a review of the quality
management system and product testing by third-party laboratories. Examples
include a chatbot integrated into a hypertension management program for patients
with high blood pressure, providing lifestyle recommendations based on patient data,
offering medication reminders, and sending alerts when readings fall outside
safe ranges. The chatbot’s role in guiding therapeutic decisions through the interpretation
of patient data and supporting patient self-management qualifies it as a Class
IIa medical device. Since hypertension is considered a low to medium-risk
condition, the device remains classified as Class IIa.
- Class IIb devices present
a higher medium risk and require rigorous third-party inspection and
examination due to the increased risk of harm if the device fails or
malfunctions. Examples include a chatbot integrated into a cardiac
insufficiency program, designed to provide therapeutic recommendations based on
patient data, with alerts to medical professionals if immediate intervention is
needed. The chatbot’s role in guiding therapeutic decisions through interpreting
patient data in a high-risk condition, and the high-risk nature of cardiac
management, qualifies this chatbot as a Class IIb medical device.
- Class III devices are high-risk
and must undergo pre-market approval, including detailed technical documentation
and clinical data. These devices support therapeutic or diagnostic decisions
that directly impact patient survival or could lead to death or irreversible health
deterioration. Examples include a chatbot that analyses high-risk health
indicators to predict acute events like sepsis or organ failure, potentially
hours before they occur, allowing for pre-emptive medical interventions.
Chatbots may also be classified as in-vitro diagnostic medical devices (IVDs)
if used for tests conducted on samples. Under the In-Vitro Diagnostic Medical
Device Regulation (IVDR) [28], devices are classified into four categories (A, B,
C, and D) based on risk, with Class A devices representing the lowest risk and
Class D the highest. Similarly to medical devices, this classification imposes
more stringent requirements as the risk increases.

Figure 1Definition and
classification of chatbots as medical devices. Further information on
the definition and classification of medical devices, including software, can
be found in MDR article 2, MDR Annex VIII [21], Guidance on Qualification and
Classification of Software in Regulation (EU) 2017/745 (MDR) and Regulation
(EU) 2017-746 (IVDR) [35], and the Medical Devices Legislation by the Federal
Office of Public Health [22].
Certification process
While the landscape of AI is rapidly evolving,
some certification standards should be considered by developers and product
owners in the development and dissemination of their tools. Once the initial development
phase is completed and relevant
standards are identified, the certification process begins with a clinical
evaluation and the definition of the intended medical use, functionality, and
market. These elements are integrated into the design, and a technical file or
dossier is compiled. This technical file provides detailed documentation of
the chatbot’s development, demonstrating its compliance with required standards
and regulations. The file is then submitted to a certified notified body
designated by the European Union to assess the conformity of the product. The
notified body grants certification once all requirements and standards are met,
followed by required post-market surveillance under the MDR to ensure continued
compliance. Finally, if the device is decommissioned, specific rules for ending
its active use must also be followed. Each step of the certification process after
the initial development phase is outlined below and in figure 2.
(1) Clinical evaluation should follow
the specifications outlined in Article 61 and Annex XIV of the MDR [21]. Article
61 defines clinical evaluation, criteria for exemption, and demonstration of
sufficient data access to justify claims of equivalence – an important factor
if the manufacturer can show that the clinical data used is based on a device
deemed equivalent. Clinical evaluation involves validating the clinical
association and scientific validity of Medical Device Software (MDSW). Valid
clinical association ensures that the device’s outcome aligns with the intended
clinical purpose, while scientific validity ensures that this association is
grounded in well-established scientific principles and evidence. Clinical
trials or investigations may be required to assess the device’s safety and
efficacy, subject to approval by ethics committees. Annex XIV provides
additional guidelines on evaluation methods, including reviewing scientific
literature, assessing safety and performance, defining the intended purpose,
target groups, and clinical benefits, and determining methods to assess safety
and benefit-risk ratios. It also includes an analysis of relevant clinical data
to draw conclusions about safety and clinical performance. Clinical data may
come from a device for which equivalence can be demonstrated (e.g., similar
design, conditions of use, specifications, principles of operation, and
critical performance requirements). The complete document can be found at (https://www.medical-device-regulation.eu/2019/08/14/annex-xiv/).
Although these guidelines were written before the emergence of AI technologies,
they still apply to the certification process of any medical device.
(2) Integrating the clinical evaluation
and specifications into the design and development of the device is an essential
step to ensure conformity and its potential use after certification while
meeting all required standards. During development, it is important to adhere
to existing norms, such as IEC 62304 for medical device software, and other
software development requirements, especially given the current lack of
specific standards for AI devices. Verification and validation should be completed
prior to deployment, along with appropriate user training to ensure the correct
and safe use of the device.
(3) Technical files
must be submitted to a certified notified body. A list of notified bodies can
be found at: https://health.ec.europa.eu/medical-devices-topics-interest/notified-bodies_en.
Notified bodies are responsible for a comprehensive evaluation process, which
includes reviewing the technical documentation of medical devices, auditing
manufacturers' quality management systems, and ensuring ongoing compliance with
applicable standards. This assessment is especially critical for higher risk
medical devices, where independent verification of safety and effectiveness is
essential. In contrast, Class I devices, which pose the lowest risk, may be
self-certified. Notified bodies also conduct audits and oversee post-market
surveillance to ensure continued compliance after certification is issued.
(4) Certification: Once a medical device successfully
passes this evaluation, the notified body issues a certificate of conformity.
This certificate is crucial, as it allows the manufacturer to affix the CE
marking, indicating that the device meets EU standards and can be marketed. After
receiving the certificate, the device must be registered with the appropriate
authorities (i.e., Swissmedic) before being introduced to the market.
(5) PMS is a systematic and essential
process, ensuring the continued safety and effectiveness of medical devices after
release to market. PMS involves real-world performance monitoring and mandatory
incident reporting. This includes monitoring the software’s use in various
clinical settings, gathering performance data, and collecting user feedback.
This step is crucial for quickly identifying and addressing potential issues to
ensure the software remains compliant with regulatory standards.
- Real-world
data utilisation: Analysis of real-world data helps refine the software and provides
crucial insights into its performance in diverse real-life scenarios, which can
lead to improvements and adaptations in functionality. _Hlk172277221
- Mandatory reporting of incidents: Reporting timeframes are determined based on the
incident’s severity. Any serious incidents and corrective actions taken to ensure
safety must be promptly reported to the relevant authorities.
The MDR, particularly in Article 83,
mandates that manufacturers establish a comprehensive PMS system as part of
their quality management system. Manufacturers are required to actively and
systematically collect, record, and analyse data on their device's quality,
performance, and safety throughout its life cycle. For class I devices, manufacturers
must compile a PMS report summarising the results and conclusions from the data,
along with any preventive or corrective actions taken. This report must be
updated as needed and made available to competent authorities upon request. For class
IIa, IIb, and III devices,
manufacturers are required to prepare a Periodic Safety Update Report (PSUR). The
PSUR provides comprehensive summaries of PMS data, findings from post-market
clinical follow-ups, sales volume, and estimates of the user population. For
class IIb and III devices, the PSURs are reviewed annually by the notified body
and made available to the competent authorities.
(6) The final phase
in the device’s lifecycle involves decommissioning. This process must ensure
data integrity, compliance with regulatory standards, and proper communication with
stakeholders.
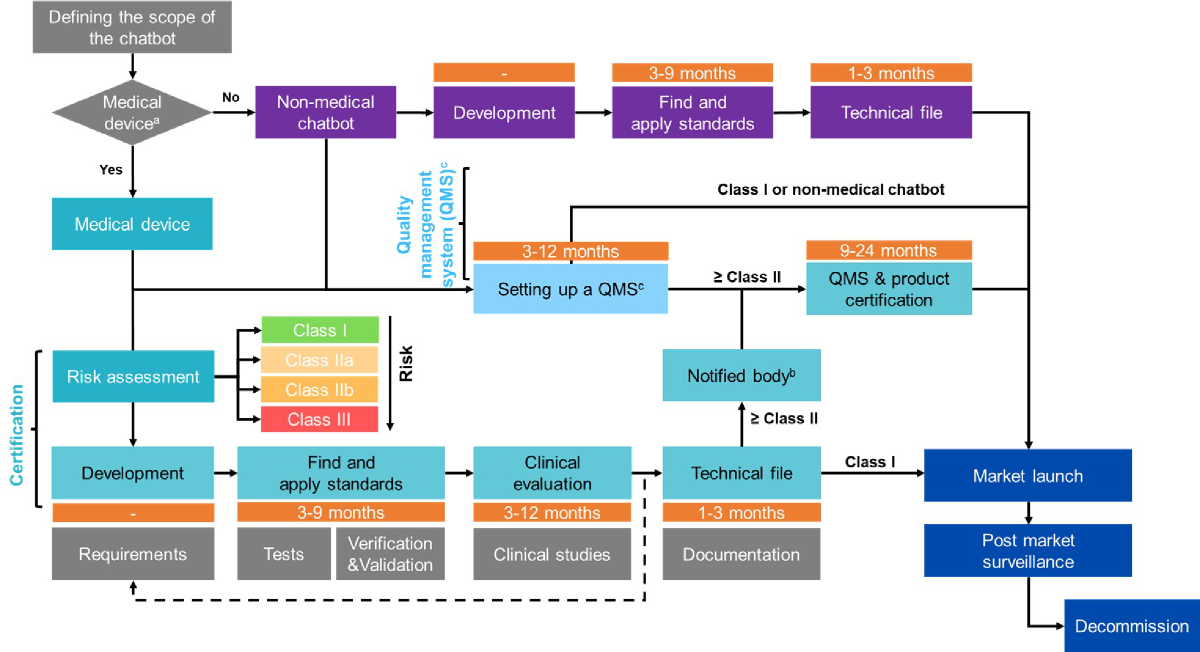
Figure 2Certification process,
quality management system, and post-market surveillance for medical and
non-medical chatbots.
a: A medical device, as defined by the Medical Device
Regulation (MDR), is an article, instrument, apparatus, or machine that is used
in the prevention, diagnosis, or treatment of illness or disease, or for
detecting, measuring, restoring, correcting, or modifying the structure or
function of the body for a health-related purpose.
b: A notified body is an organisation
designated to assess the conformity of certain products before they are placed
on the market.
c: The quality management system (QMS) process can
take longer for medical devices compared to non-medical devices. Setting up a QMS
can take between 3 to 12 months, while
QMS certification may take an additional 1 to 3 months.
Based on
the MDR, the confIAnce chatbot is considered a non-medical chatbot. However,
steps such as implementing a QMS and adhering to necessary standards for
product certification and data protection remain applicable.
Quality management system
A quality management system (QMS) is an
essential framework that ensures a product consistently meets required quality
and safety standards. Core elements of a QMS include the organisation's guiding
principles, processes to ensure consistency and quality – especially for
critical operations – and step-by-step procedures for carrying out specific
tasks, providing clear guidance for employees.
If a chatbot is not classified as a medical
device, quality management standards still apply. Relevant certifications may
include ISO 4213 (assessment of machine learning classification performance),
ISO 24027 (bias in AI systems and AI-aided decision making), ISO 24029 (robustness
assessment of neural networks), ISO 5469 (functional safety of AI systems), ISO
27563 (security and privacy in AI use cases), and ISO 8200 (controllability of
automated AI systems). Additionally, processes should comply with standards
such as ISO 5338 (AI system lifecycle processes), ISO 8183 (data lifecycle
framework), and ISO 23053 (framework for AI systems using machine learning).
The governing organisation should adhere to certifications like ISO 42001 (AI
management system), ISO 23894 (AI guidance on risk management), ISO 5259-5 (data
quality governance framework), and ISO DIS 5259-3 (data quality management
requirements and guidelines).
For chatbots classified as medical devices,
adherence to ISO 13485 is essential. This international standard outlines QMS
requirements, including defining roles and responsibilities, ensuring quality
control throughout design and development, planning validation and verification,
risk management, and regulatory compliance. It also covers supply chain management,
product realisation, and customer focus. ISO 13485 mandates regular monitoring
and measurement of processes and devices. Additional relevant standards for
medical software, which could apply to medical chatbots, include IEC 82304
(safety and security of health software), IEC 62304 (lifecycle requirements for
medical software), ISO 14971 (risk management for medical devices), IEC 62366-1
(usability of a medical device related to safety), ISO 20417 (identification
and labelling of medical devices), ISO 14155 (clinical investigation of medical
devices), and ISO TR 20416 (PMS).
Implementing a QMS requires organisation-wide
involvement, ensuring a unified approach to quality across all departments, employee
training, and engagement, as well as leveraging external QMS expertise.
Templates and tools can assist in implementation. QMS certification includes an
initial audit by a third-party organisation, an assessment of any
non-conformities, and the granting of certification. Ongoing surveillance
audits are also conducted to maintain certification.
Data protection
Chatbots considered non-medical devices remain
subject to regulations and standards including data protection, cybersecurity,
and quality management. Data privacy and security,
while not in the scope of this paper, must always comply with national or
regional rules, such as the General Data Protection Regulation (GDPR) in Europe
[29]. Key GDPR principles include consent, transparency, data minimisation,
the right to access and erase information, data portability, data protection
officers, security measures, and breach notification within 72 hours of
becoming aware of the breach [29]. Non-medical chatbots must also implement
strong cybersecurity measures to prevent data breaches, unauthorised access, or
other risks. Regular audits and stress tests should be conducted to ensure
compliance with security standards. ISO/IEC 27001 certification [30] is
applicable in this context, confirming that the chatbot has adequate
cybersecurity safeguards. Regular updates should be applied to comply with the
latest security standards to protect users, along with a clear incident
response plan in case of a breach.
_Hlk172294867Application: The confIAnce chatbot was
designed to be completely anonymous for security and privacy purposes, and it
does not collect any personally identifiable information. To enhance both
accessibility and anonymity, we chose to avoid sign-ins and downloadable
applications. The chatbot is hosted on the Geneva University Hospitals' website,
accessible to all without the need for sign-in or specific software. The confIAnce
chatbot has been reviewed by internal security officers
and complies with GDPR and the Swiss Federal Act on Data Protection, addressing
data transparency, security, quality, individual rights, and potential
penalties for non-compliance. Additionally, ISO/IEC 27001 certification is
applicable, ensuring that potential information security risks are identified
and managed.
Additional provisions under the EU AI act
The EU AI Act [31] builds on existing
regulations such as the MDR and IVDR, classifying AI systems into three
categories: (1) prohibited, (2) high-risk, and (3) low to minimal risk.
Prohibited systems are those that cannot be marketed due to the potential for physical,
psychological, or other forms of harm. High-risk systems, which include
products requiring third-party conformity assessment (Classes IIa to III under the
MDR), must undergo the full certification process. Low to minimal-risk systems
are not required to undergo the same level of scrutiny but are encouraged to
establish behavioural codes of conduct to promote adherence to legal
requirements applicable to high-risk systems. High-risk AI systems must follow the
certification process, including registration in the EU database. These systems
also require specific features, such as appropriate human-machine interface
tools, human oversight proportionate to the level of risk, and transparency in
providing information when necessary.
Table 1Lexicon in the
development of chatbots and certification process.
| Artificial intelligence
(AI) |
The theory and
development of computer systems capable of performing tasks that normally
require human intelligence. |
| Machine learning |
A branch of AI
and computer science focused on using data and algorithms to enable AI systems
to imitate humans learning, gradually improving accuracy [32]. |
| Deep learning |
A subset of
machine learning that uses large multilayered (artificial) deep neural networks
that compute with continuous (real number) representations, mimicking
hierarchically organised neurons in the human brain. It is particularly
effective at learning from unstructured data such as images, text, and audio
[33]. |
| Neural network |
A computational
model inspired by the structure and function of biological neurons [33]. |
| Large language models
(LLM) |
A neural
network trained on vast amounts of text to mimic human language. This type of
foundation model processes large volumes of unstructured text and learns
relationships between words tokens (portions of words) [33]. |
| Generative AI |
A form of
machine learning where AI platforms generate new outputs in response to
prompts, based on the data they were trained on [33]. |
| Retrieval-augmented generation
(RAG) |
A technique
that optimises the output of an LLM by referencing an external, authoritative
knowledge base before generating a response. |
| Fine tuning |
The process of
adapting a pre-trained model for specific tasks or use cases [34]. |
| Knowledge base |
A centralised
repository for information that can be integrated with AI technologies. |
| Prompt |
Instruction or question
provided to an AI system using natural language, rather than computer code. |
| Prompt engineering or prompt
design |
The process of
carefully constructing prompts or inputs for AI models to enhance their
performance on specific tasks [33]. |
| Prompt injection |
The process of
overriding original instructions in a prompt with a special user input. This
occurs when untrusted input is incorporated into the prompt. In a direct
prompt injection, hackers control the user input and feed the malicious
prompt directly to the LLM. |
| Bias |
A phenomenon where
AI systems produce results that are systematically unfair or inaccurate due
to erroneous assumptions or influences during machine learning. Bias in AI
can have negative impacts on individuals and society, such as discrimination,
misinformation, or loss of trust [33]. |
| Hallucination |
A phenomenon in
which an AI system produces outputs that are not based on reality or the
given context [33]. |
| Supervised learning |
A type of
machine learning that uses labelled datasets to train algorithms to classify
data or predict outcomes. The datasets are pre-labelled by humans [33]. |
| Unsupervised learning |
A type of
machine learning where algorithms learn patterns from unlabelled data,
without human guidance or feedback [33]. |
| AI-Assisted device |
A device that
leverages AI and machine learning algorithms to enhance or revolutionise its
functionality. |
| Medical device |
An article,
instrument, apparatus or machine used in the prevention, diagnosis, or
treatment of illness or disease, or for detecting, measuring, restoring,
correcting, or modifying the structure or function of the body for a health-related
purpose [28]. |
| Medical device regulation |
Regulation (EU)
2017/745 on the clinical investigation and sale of medical devices for human
use in the EU, repealing Directives 93/42/EEC (medical devices) and 90/385/EEC
(implantable medical devices), in effect since May 26th, 2021 [28]. |
| Food and Drug
Administration |
A U.S. federal agency
responsible for protecting public health by ensuring the safety, efficacy,
and truthful labelling of food, cosmetics, and nutritional supplements [23]. |
| European AI act |
A European
Union regulation establishing a common regulatory framework for artificial
intelligence, proposed on 21 April 2021 and passed on 13 March 2024 [31]. |
| General data protection
regulation (GDPR) |
A European
Union regulation governing data privacy and information security,
particularly Article 8 of the Charter of Fundamental Rights of the European
Union [29] . |
| Notified bodies |
Organisations
designated by EU countries to assess the conformity of certain products
before they are placed on the market [25]. |
| Class I; IIa; IIb; III |
Risk
classification for medical devices, ranging from low risk (Class I) to high
risk (Class III) [28]. |
| Certification |
The process
that certifies a device’s compliance with applicable regulations and
standards, guaranteeing the device’s safety and performance [28]. |
| Post-market surveillance
(PMS) |
A systematic
process that ensures the continued safety and effectiveness of medical
devices after they have been released onto the market [28] . |
| Quality management system
(QMS) |
A framework
that ensures a product consistently meets required quality and safety
standards (ISO/IEC). |
_Hlk172274439Application: Under the MDR, the confIAnce chatbot
was classified as an informational non-medical chatbot. This evaluation was
based on the fact that confIAnce provides users with information about chronic
diseases and is limited to a verified knowledge base. As the chatbot is not
designed to offer diagnosis or treatment options, it operates as an easily
accessible knowledge repository without delivering personalised information. Safeguards
include disclaimers, reminders of the chatbot's primary use, anonymisation of
all data, and fallback mechanisms for prompts outside its scope. These considerations
align with the current EU AI Act. Additionally, confIAnce undergoes continuous
monitoring with automated tests and a chatbot master who regularly evaluates
the prompts and answers for accuracy and harmlessness. Part of the ongoing quality
improvement process involves reviewing
recurring user prompts not yet included in the knowledge base.
Conclusion
Chatbots are rapidly evolving
and, when used within their defined scope, hold significant potential in
healthcare. Specific safeguards, adapted regulations, and transparency are essential
to mitigate the risks and concerns regarding potential harm or misuse. The certification
process
applicable to medical devices provides a foundational understanding and
starting point; however, further provisions applying directly to AI systems are
still needed. In the meantime, clearly defining the scope of practice, implementing
risk-reduction tools and processes, and using chatbots for informational
purposes based on a verified knowledge base represents an effective way to complement
the relationship between healthcare professionals and patients, without making
medical decisions or replacing physicians. The next steps would involve
assessing the added value of integrating informational chatbots into general
practice and identifying any challenges or limitations when deployed on a
larger scale. In real-world scenarios, chatbots could
become valuable tools for physicians, helping to free up time and improve the
quality of care.
Mayssam Nehme
Division
of Primary Care Medicine
Geneva University Hospitals
Rue Gabrielle-Perret-Gentil 4
CH-1205 Genève
Mayssam.Nehme[at]hcuge.ch
References
1. Open AI. Introducing ChatGPT. 2022. https://openai.com/blog/chatgpt
2. Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact
of the COVID-19 pandemic on clinician ambulatory electronic health record use [Erratum
in: J Am Med Inform Assoc. 2022 Jan 10; PMID: 34888680; PMCID: PMC8689796]. J Am Med
Inform Assoc. 2022 Jan;29(3):453–60. 10.1093/jamia/ocab268
3. Tai-Seale M, Dillon EC, Yang Y, Nordgren R, Steinberg RL, Nauenberg T, et al. Physicians’
Well-Being Linked To In-Basket Messages Generated By Algorithms In Electronic Health
Records. Health Aff (Millwood). 2019 Jul;38(7):1073–8. 10.1377/hlthaff.2018.05509
4. Sinsky CA, Shanafelt TD, Ripp JA. The Electronic Health Record Inbox: recommendations
for Relief. J Gen Intern Med. 2022 Nov;37(15):4002–3. 10.1007/s11606-022-07766-0
5. Haque MD, Rubya S. An Overview of Chatbot-Based Mobile Mental Health Apps: Insights
From App Description and User Reviews. JMIR Mhealth Uhealth. 2023 May;11:e44838. 10.2196/44838
6. Hauser-Ulrich S, Künzli H, Meier-Peterhans D, Kowatsch T. A Smartphone-Based Health
Care Chatbot to Promote Self-Management of Chronic Pain (SELMA): Pilot Randomized
Controlled Trial. JMIR Mhealth Uhealth. 2020 Apr;8(4):e15806. 10.2196/15806
7. Bin Sawad A, Narayan B, Alnefaie A, Maqbool A, Mckie I, Smith J, et al. A Systematic
Review on Healthcare Artificial Intelligent Conversational Agents for Chronic Conditions.
Sensors (Basel). 2022 Mar;22(7):2625. doi: https://doi.org/10.3390/s22072625
8. Xu L, Sanders L, Li K, Chow JC. Chatbot for Health Care and Oncology Applications
Using Artificial Intelligence and Machine Learning: systematic Review. JMIR Cancer.
2021 Nov;7(4):e27850. 10.2196/27850
9. Lee N et al. Developing a Chatbot–Clinician Model for Hypertension Management. NEJM
Catal Innov Care Deliv 2022;3(11) DOI: 10.1056/CAT.22.0228 VOL. 3 NO. 11.
10. Ter Stal S, Sloots J, Ramlal A, Op den Akker H, Lenferink A, Tabak M. An Embodied
Conversational Agent in an eHealth Self-management Intervention for Chronic Obstructive
Pulmonary Disease and Chronic Heart Failure: Exploratory Study in a Real-life Setting.
JMIR Hum Factors. 2021 Nov;8(4):e24110. 10.2196/24110
11. Vaddadi G, et al. A Pilot Program Utilising a “Chatbot “ to Support Patients in the
First 30 Days Following an Admission to Hospital With Acute Decompensated Heart Failure.
Heart Lung and Circulation. July 2023. VOLUME 32, SUPPLEMENT 3, S139.
12. Griffin AC, Xing Z, Khairat S, Wang Y, Bailey S, Arguello J, et al. Conversational
Agents for Chronic Disease Self-Management: A Systematic Review. AMIA Annu Symp Proc.
2021 Jan;2020:504–13.
13. Blasco JM, Díaz-Díaz B, Igual-Camacho C, Pérez-Maletzki J, Hernández-Guilén D, Roig-Casasús S.
Effectiveness of using a chatbot to promote adherence to home physiotherapy after
total knee replacement, rationale and design of a randomized clinical trial. BMC Musculoskelet
Disord. 2023 Jun;24(1):491. 10.1186/s12891-023-06607-3
14. Schachner T, Keller R, V Wangenheim F. Artificial Intelligence-Based Conversational
Agents for Chronic Conditions: Systematic Literature Review. J Med Internet Res. 2020 Sep;22(9):e20701.
10.2196/20701
15. Kurniawan MH, Handiyani H, Nuraini T, Hariyati RT, Sutrisno S. A systematic review
of artificial intelligence-powered (AI-powered) chatbot intervention for managing
chronic illness. Ann Med. 2024 Dec;56(1):2302980. doi: https://doi.org/10.1080/07853890.2024.2302980
16. Greer S, Ramo D, Chang YJ, Fu M, Moskowitz J, Haritatos J. Use of the Chatbot “Vivibot”
to Deliver Positive Psychology Skills and Promote Well-Being Among Young People After
Cancer Treatment: Randomized Controlled Feasibility Trial. JMIR Mhealth Uhealth. 2019 Oct;7(10):e15018.
doi: https://doi.org/10.2196/15018
17. Thirunavukarasu AJ, Ting DS, Elangovan K, Gutierrez L, Tan TF, Ting DS. Large language
models in medicine. Nat Med. 2023 Aug;29(8):1930–40. 10.1038/s41591-023-02448-8
18. Meskó B, Topol EJ. The imperative for regulatory oversight of large language models
(or generative AI) in healthcare. NPJ Digit Med. 2023 Jul;6(1):120. 10.1038/s41746-023-00873-0
19. Webster P. Medical AI chatbots: are they safe to talk to patients? Nat Med. 2023 Nov;29(11):2677–9.
10.1038/s41591-023-02535-w
20. United States Food and Drug Administration. Clinical Decision Support Software. Guidance
for Industry and Food and Drug Administration Staff. September 2022. https://www.fda.gov/media/109618/download
21. Regulation (EU) 2017/745 of the European parliament and of the council https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745
22. Swiss Confederation - Federal Office of Public Health - Medical Devices Legislation
https://www.bag.admin.ch/bag/en/home/medizin-und-forschung/heilmittel/revision-med-prod-verord-mepv.html#:~:text=The%20EU%20Medical%20Devices%20Regulation,with%20developments%20in%20European%20law
23. Food and Drug Administration Regulations https://www.fda.gov/regulatory-information/fda-rules-and-regulations
24. Fink M, Akra B. Comparison of the international regulations for medical devices-USA
versus Europe. Injury. 2023 Oct;54 Suppl 5:110908. 10.1016/j.injury.2023.110908
25. European Commission. Notified Bodies https://health.ec.europa.eu/medical-devices-topics-interest/notified-bodies_en
26. Nehme M, Schneider F, Perrin A, Sum Yu W, Schmitt S, Violot G, et al. The Development
of a Chatbot Technology to Disseminate Post-COVID-19 Information: Descriptive Implementation
Study. J Med Internet Res. 2023 Jun;25:e43113. 10.2196/43113
27. Division of Primary Care Medicine, Geneva University Hospitals. Strategies for Primary
Care. https://www.hug.ch/medecine-premier-recours/strategies-medecine-premier-recours
28. European Union. Regulation (EU) 2017/746 of the European Parliament and of the Council
of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC
and Commission Decision 2010/227/EU.
29. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April
2016 on the protection of natural persons with regard to the processing of personal
data and on the free movement of such data, and repealing Directive 95/46/EC (General
Data Protection Regulation). https://eur-lex.europa.eu/eli/reg/2016/679/oj
30. ISO/IEC 27001:2022 Information security, cybersecurity and privacy protection. Information
security management systems Requirements. https://www.iso.org/standard/27001
31. European Parliament. Artificial Intelligence. https://www.europarl.europa.eu/topics/en/topic/artificial-intelligence
32. International Business Machines Corporation. IBM. What is Machine Learning (ML)? https://www.ibm.com/topics/machine-learning#:~:text=Machine%20learning%20(ML)%20is%20a,learn%2C%20gradually%20improving%20its%20accuracy. [Last accessed July 19, 2024].
33. International Monetary Fund. AI Lexicon. December 2023. Finance and Development Magazine.
https://www.imf.org/en/Publications/fandd/issues/2023/12/AI-Lexicon [last accessed July 19, 2024].
34. International Business Machines Corporation. IBM. What is fine tuning? https://www.ibm.com/topics/fine-tuning#:~:text=Fine%2Dtuning%20in%20machine%20learning,models%20used%20for%20generative%20AI. [Last accessed July 19, 2024].
35. Medical Device Coordination Group Document. MDCG 2019-11 Guidance on Qualification
and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU)
2017/746 – IVDR. October 2019.

