Exploring the real-world management of catheter-associated
urinary tract infections by Swiss general practitioners and urologists:
insights from an online survey
DOI: https://doi.org/https://doi.org/10.57187/s.3933
Iris Züntiab,
Emilio Arbelaezab,
Sarah Tschudin-Sutterbc,
Andreas Zellerbd,
Florian S. Halbeisenbe,
Hans-Helge
Seifertab,
Kathrin Bauschab
a Department of Urology, University Hospital Basel,
Basel, Switzerland
b University
of Basel, Basel, Switzerland
c Division
of Infectious Diseases and Hospital Epidemiology, University Hospital Basel,
Basel, Switzerland
d Centre
for Primary Health Care, University of Basel, Basel, Switzerland
e Surgical Outcome Research Center, Department of
Clinical Research, University Hospital Basel and University of Basel, Basel,
Switzerland
Summary
AIM: To assess and compare the real-world
management of catheters and catheter-associated urinary tract infections
(CAUTI) among Swiss general practitioners and urologists, encompassing diagnosis,
treatment and prophylaxis.
METHODS: An anonymised online
questionnaire was distributed among Swiss general practitioners and urologists between
January
and October 2023 via the networks of Sentinella and the Swiss Association of
Urology. The questionnaire consisted of questions on catheter management,
including diagnosis, treatment and prophylaxis of CAUTI. Analysis was performed
by discipline. Fisher’s exact test was applied for comparisons (statistical
significance with p <0.05).
RESULTS: Out of 175 participating physicians,
the majority were involved in catheter management. Urologists exhibited
significantly higher levels of competence as compared to general practitioners (67.1%
vs 20.9%).
Although no significant differences were observed regarding diagnostic
approaches between disciplines, unrecommended diagnostic methods were
frequently applied. general practitioners reported that they treated non-febrile CAUTI
for longer
durations, while urologists indicated that they treated febrile CAUTI longer.
Additionally, the use of fluoroquinolones was more prevalent among general practitioners
compared
to urologists, while prophylactic measures were more frequently applied by
urologists.
CONCLUSIONS: Catheter and CAUTI management
entail significant uncertainty for general practitioners. CAUTI management varied
notably between
general practitioners and urologists in terms of treatment and prophylaxis. The use
of non-recommended
diagnostic approaches and drugs was common. This trend, along with
inappropriate diagnostic methods and prophylaxis, may increase antimicrobial
resistance and CAUTI morbidity. The study emphasises the necessity for diagnostic
and antimicrobial stewardship interventions, and proper training in CAUTI
management for general practitioners and urologists.
Abbreviations
- CAUTI:
-
catheter-associated urinary tract infections
- EAU:
-
European Association of Urology
- EAUN:
-
European Association of
Urology Nurses
Introduction
Urinary tract infections (UTIs) are one of the
most common diseases, affecting more than 150 million people worldwide every
year [1]. Urinary tract infections rank among the top four most prevalent
nosocomial infections; 70–80% of urinary tract infections are catheter-associated
urinary tract infections
(CAUTIs) [2], which significantly increases morbidity and
mortality [3]. This imposes a substantial burden on the
healthcare system, leading to prolonged hospital stays and escalated costs [3].
Presence of a urinary catheter and duration of
catheterisation are the main risk factors for catheter-associated complications
[4]. Furthermore, studies indicate that between 21%
and 65% of all catheter insertions are not necessary [5, 6] and that prolonged catheterisation
without
indication is common [7].
Interventions such as confirming the need for catheterisation,
daily evaluation of this need and education on catheter management
significantly decrease catheter utilisation and non-infectious complications in
the hospital setting [2, 4]. These measures have also contributed to the
reduction of CAUTIs [2, 8]. However, there are patients in whom permanent
catheterisation is indicated but in whom such measures cannot be applied. This
cohort of patients is usually frail and has additional risk factors for infectious
complications such as immunosuppression, diabetes, advanced age or immobilisation
[9]. Therefore, comprehensive guidelines and
corresponding training for catheter and CAUTI management are urgently needed.
This patient cohort is primarily cared for by
general practitioners and urologists. Their decision-making is usually
guided by experience und guideline recommendations. Adherence to guideline
recommendations and antimicrobial stewardship programmes significantly reduce
the use of antimicrobials and the development of antimicrobial resistance [10]. Currently
there are various
guidelines for diagnosis, treatment and prophylaxis of uncomplicated urinary tract
infection [11–13] but only a few target urinary tract infections in catheterised
patients [11, 14–16]. Compared to urinary tract infections, the real-world management
of CAUTI is complicated by atypical symptoms, misleading diagnostic measures,
unclear guidance for treatment decisions and a lack of evidence on prophylaxis [17].
To establish a foundation for future antimicrobial stewardship
programmes and recommendations, our study aimed to
assess and compare real-world management of catheters and CAUTIs among Swiss
general practitioners and urologists encompassing diagnosis, treatment and prophylaxis.
Material and methods
Study design and setting
A web-based, anonymous survey was conducted
among general practitioners and urologists across Switzerland. In January 2023, the
survey,
hosted on the REDCap platform, was disseminated via the networks of Sentinella,
a surveillance system operated by the Swiss Federal Office of Public Health (Bundesamt
für Gesundheit) for gathering epidemiological data in family medicine [18], and the Swiss Association
of Urology (Schweizerische Gesellschaft für Urologie) through email. Participants were encouraged to
further distribute the survey within their respective institutions of general practitioners
and urologists,
with a follow-up reminder sent two weeks later.
The questionnaire comprised 6 demographic
questions, 3 about catheter management in general and 15 about CAUTIs.
Responses were structured with predefined options for most questions, while
some allowed for individual or multiple selections. The questionnaire was
provided in German, French and Italian, with the English version available in the
appendix.
Following the collection of baseline characteristics, participants
were queried about their involvement in catheter management. If respondents indicated
not being involved in catheter management, subsequent questions were omitted.
Incomplete questionnaires of participants who were involved in catheter
management were still included in the analysis. Participation was voluntary,
and to maintain anonymity no personal identifiers or written informed consent
were obtained from participants. Given the focus on general practitioners and urologists
and the absence
of patient data collection, ethical committee approval was deemed unnecessary
for this study. A study protocol has not been published or registered before.
The dataset can be obtained from the corresponding author upon request.
Statistical analysis
We conducted descriptive statistical analyses
to summarise the collected data. Categorical variables were presented as counts
and corresponding proportions, while appropriate descriptive statistics were
chosen for continuous variables based on data distribution; given the
non-normal distribution, we reported median and range.
To compare
characteristics between disciplines, two-sided Wilcoxon rank-sum tests were
applied for continuous variables, while two-sided Fisher’s exact tests were used
for categorical variables to detect significant differences in proportions
between urologists and general practitioners. Furthermore, Fisher’s exact tests were
separately
applied to each survey question to uncover discipline-specific variations in
responses, with separate tests conducted for each answer category in questions
with multiple options.
We considered a p-value less than 0.05 as
statistically significant. All statistical analyses were performed using R
statistical software (version 4.2.2, The R Foundation for Statistical
Computing, Vienna, Austria).
Results
In total, 175 questionnaires (93 general practitioners
and 82 urologists) were completed and 80% of them had no missing data. Seventeen
general practitioners indicated not being involved in catheter management and were
excluded from
further analysis. Urologists were younger than participating general practitioners
(table 1). The
majority of general practitioners and urologists reported that they replaced catheters
every 1–3
months, with urologists treating a higher number of patients with
catheter-related issues and more instances of CAUTIs (table 1). While
urologists generally expressed confidence in catheter management, over a
quarter of general practitioners did not feel competent in this area (table 1).
| Characteristic |
General practitioner (n = 93) |
Urology (n = 82) |
p-value |
Missing data |
| Age, median and range |
55 |
18–77 |
48 |
28–70 |
0.001 |
0 |
| Years of experience as a medical professional, median and range |
25 |
1–50 |
20 |
2–45 |
0.006 |
0 |
| Sex, n and % |
|
|
|
|
0.411 |
0 |
|
Female |
31 |
33.3% |
22 |
26.8% |
|
|
|
Male |
62 |
66.7% |
60 |
73.2% |
|
|
| Medical facility where you work, n and % |
|
|
|
|
<0.001* |
0 |
| General practitioner
practice |
93 |
100% |
|
|
|
|
|
Urological practice |
|
|
37 |
45.1% |
|
|
|
District hospital |
|
|
9 |
11.0% |
|
|
|
Cantonal hospital |
|
|
21 |
25.6% |
|
|
|
University hospital |
|
|
8 |
9.8% |
|
|
|
Rehabilitation hospital |
|
|
4 |
4.9% |
|
|
|
Other |
|
|
3 |
3.7% |
|
|
| Do you look after patients who are permanently supplied with a
urinary catheter? n and % |
|
|
|
|
<0.001* |
0 |
|
No |
17 |
18.3% |
0 |
|
|
|
|
Rarely |
40 |
43.0% |
1 |
1.2% |
|
|
|
Yes |
10 |
10.8% |
0 |
|
|
|
|
Yes and I change
transurethral |
5 |
5.4% |
0 |
|
|
|
|
Yes and I change
suprapubic |
1 |
1.1% |
0 |
|
|
|
|
Yes and I change both |
20 |
21.5% |
81 |
98.8% |
|
|
| At what interval do you usually perform catheter changes in
asymptomatic patients? n and % |
|
|
|
|
<0.001* |
17 |
|
<2 weeks |
|
|
1 |
1.2% |
|
|
|
2–4 weeks |
6 |
7.9% |
2 |
2.4% |
|
|
|
1–2 months |
33 |
43.4% |
41 |
50.0% |
|
|
|
2–3 months |
22 |
29.0% |
38 |
46.3% |
|
|
|
>3 months |
4 |
5.3% |
0 |
|
|
|
|
Only if needed |
11 |
14.5% |
0 |
|
<0.001* |
|
| During the past 12 months, how many patients with transurethral
and/or suprapubic catheters did you see on average per week for
catheter-related concerns? n and % |
|
|
|
|
|
|
|
<1 |
61 |
80.2% |
10 |
12.2% |
|
17 |
|
1–5 |
14 |
18.4% |
34 |
41.5% |
|
|
|
5–10 |
1 |
1.3% |
28 |
34.2% |
|
|
|
11–25 |
0 |
|
9 |
11.0% |
|
|
|
>50 |
0 |
|
1 |
1.2% |
|
|
| How often do you diagnose an urinary tract infection in a catheterised patient per
year? n and % |
|
|
|
|
0.089* |
24 |
|
<1/year |
19 |
25.7% |
14 |
18.2% |
|
|
|
1/year |
22 |
29.7% |
22 |
28.6% |
|
|
|
2–3/year |
22 |
29.7% |
19 |
24.7% |
|
|
|
4–5/year |
4 |
5.4% |
2 |
2.6% |
|
|
|
>5/year |
7 |
9.5% |
20 |
26.0% |
|
|
| Do you feel competent in managing catheters and recurrent
urinary tract infections in catheterised patients? n and % |
|
|
|
|
<0.001* |
25 |
|
No |
3 |
4.5% |
0 |
|
|
|
|
Rather no |
16 |
23.9% |
0 |
|
|
|
|
Rather yes |
34 |
50.8% |
24 |
32.9% |
|
|
|
Yes |
14 |
20.9% |
49 |
67.1% |
|
|
Figure 1 shows responses regarding CAUTI
diagnosis and compares respective disciplines. In terms of CAUTI symptoms,
there were only a few differences between disciplines except for confusion,
which was more frequently considered symptomatic by general practitioners, and testicular
pain,
which was seen as a typical symptom by urologists (figure 1A). The most
frequent methods used to diagnose CAUTI were symptoms and urine culture. In
both disciplines, dipstick test and urine status/sediment were often used to
diagnose CAUTI (figure 1B).
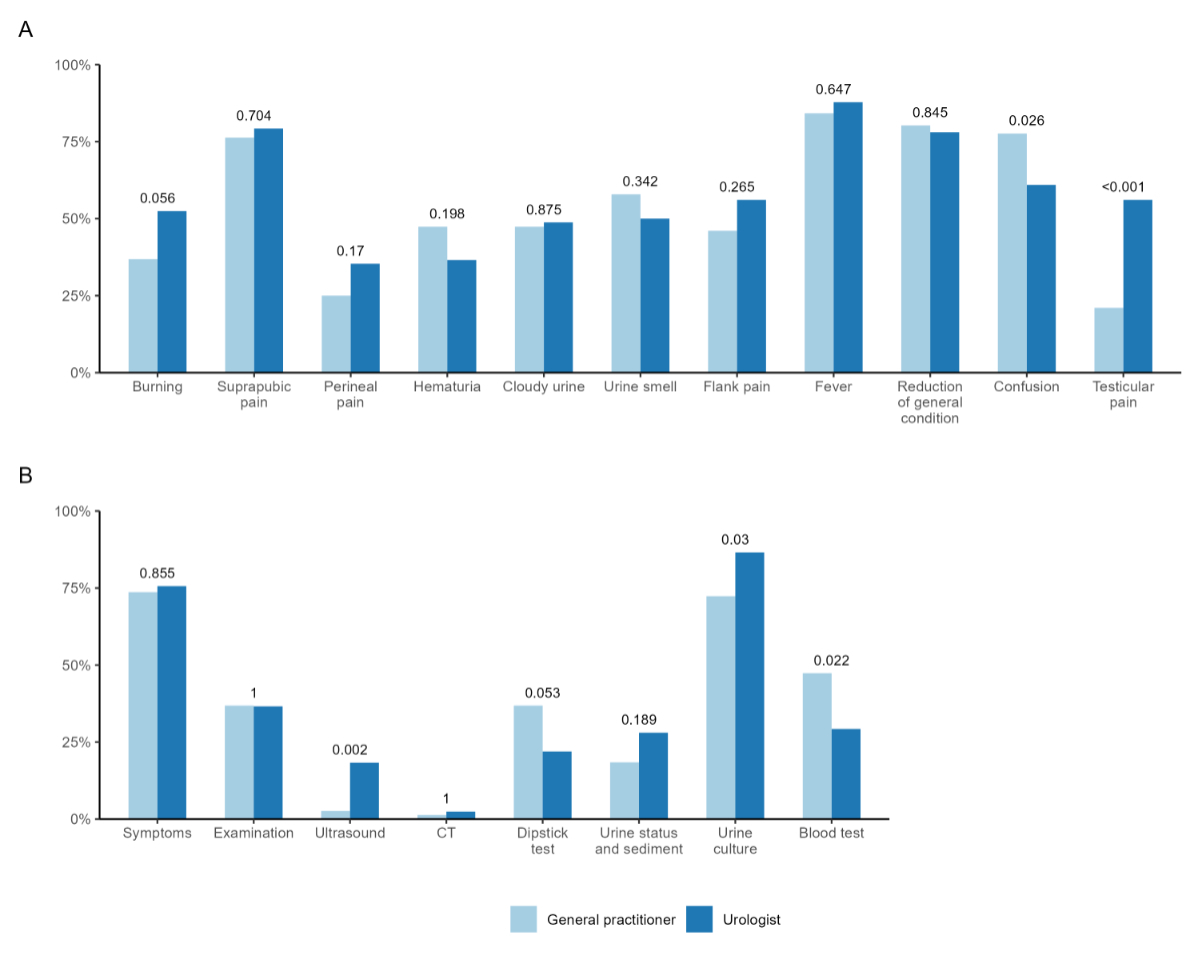
Figure 1Diagnosis of CAUTI. (A) Which signs/symptoms do you usually base your diagnose of an urinary tract infection
in a catheterised patient on? (B) How do you usually diagnose an urinary tract infection in a catheterised patient?
Answers depicted
by discipline (light blue, general practitioner; dark blue, urologists).
P-value based on available data, considered significant if ≤0.05. CT:
computed tomography.
Regarding CAUTI treatment, the majority of urologists
and general practitioners stated that they treated a catheterised patient less than
once per year
with antibiotics (figure 2A). Choice of antibiotic was primarily based on the
last urine culture by both disciplines (figure 2B). In non-febrile CAUTI,
urologists indicated that they favoured treating patients for 5 days, while general
practitioners
indicated 7 days (figure 2C). Co-trimoxazole was reported to be the mainly
prescribed antibiotic by both disciplines. While fluoroquinolones were still
frequently applied by general practitioners (17.1%), they were barely used by urologists
(2.4%) (figure
2D). In febrile CAUTI, general practitioners declared that they treat for around 7–10
days and
urologists preferred to treat for 10 days (figure 2E). In contrast to
urologists, fluoroquinolones were reported to be the most frequently prescribed
antibiotics for febrile CAUTI by general practitioners (36.8%). Urologists’ main choice
was intravenous
(i.v.) cephalosporins (figure 2F).
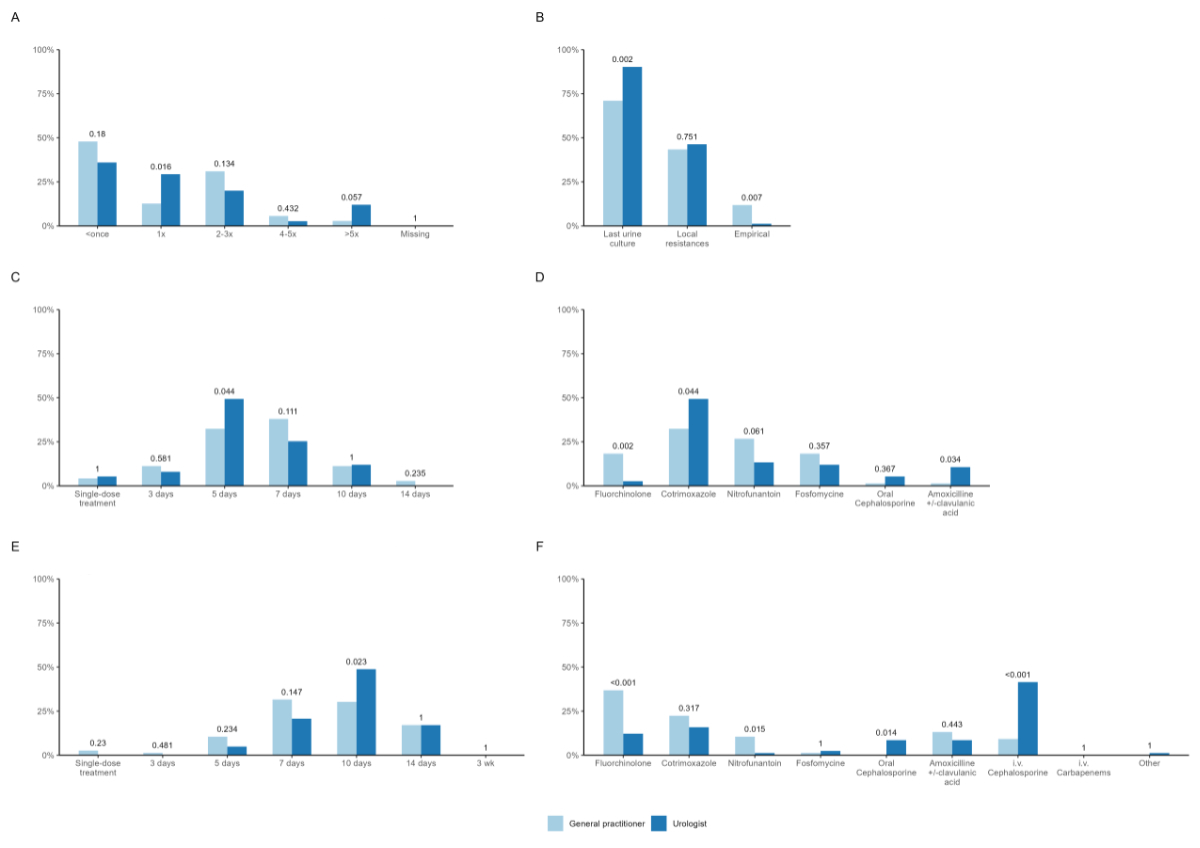
Figure 2Treatment of CAUTI. (A) How often do you prescribe an antibiotic for an urinary tract infection per catheterised
patient per year? (B) How do you choose the antibiotic for empiric therapy? (C) How long do you usually treat catheterised patients with an antibiotic for a non-febrile
urinary tract infection in average? (D) Which antibiotic do you usually choose for the empirical therapy of a non-febrile
urinary tract infection? (E) How long do you usually treat catheterised patients with an antibiotic for a febrile
urinary tract infection in average? (F) Which antibiotic do you usually choose for the empirical therapy of a febrile urinary
tract infection? Answers depicted
by discipline (light blue, general practitioner; dark blue, urologists).
P-value based on available data, considered significant if ≤0.05. i.v.: intravenous.
After empirical treatment, most questionees
would adjust the administered antibiotic according to urine culture (figure 3A,
table S1). Increasing fluid intake emerged as the primary prophylactic measure
(see figure 3B). With the exception of optimising catheter care and
vaccination, urologists statistically applied all prophylactic measures more
frequently (see figure 3B). If bladder irrigation was performed, general practitioners
reported preferring
saline solution and urologists tap water (figure 3C, table S2). General practitioners
indicated that
they refer patients to urologists for further management. Urologists performed
an ultrasound instead (figure 3D, table S3).
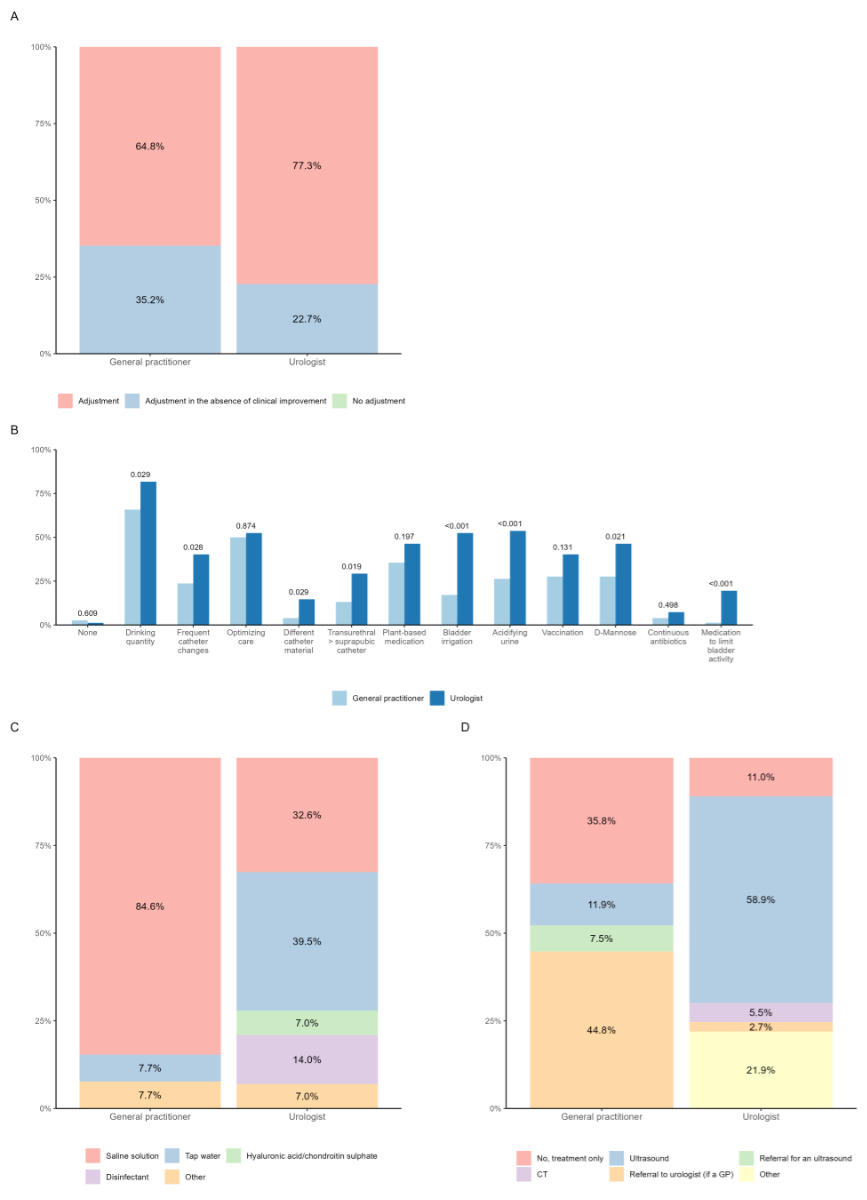
Figure 3Prophylaxis of CAUTI. (A) What do you usually do after receiving the results of the urine culture when the
detected germ proves to be resistant to the antibiotic administered? (B) Do you usually enact additional measures to reduce recurrent urinary tract infection
in catheterised patients? (C) If you perform regular bladder irrigation, which fluid do you use for this? (D) Do you initiate further diagnostic measures for recurrent urinary tract infection
in catheterised patients? Answers
depicted by discipline (light blue, general practitioner; dark blue, urologist).
P-value based on available data, considered significant if ≤0.05. CT:
computed tomography; GP: general practitioner.
Discussion
This study is the first real-world assessment of catheter and
CAUTI management among Swiss general practitioners and urologists. It reveals that
despite
uncertainties among general practitioners regarding CAUTI management, the diagnostic
approach for
CAUTI is similar between general practitioners and urologists. However, it also underlines
differences in CAUTI treatment and prophylaxis between the disciplines.
The inhomogeneity may stem from varying levels
of professional experience, training and decision-making between general practitioners
and
urologists. Guideline recommendations are a key basis for decision-making. A
handbook on urological diseases, including CAUTI, was recently published for
Swiss general practitioners but has not gained widespread recognition, partly because
it was not
endorsed by medical societies [19]. Furthermore, general practitioners, like urologists,
could
refer to international guidelines such as those from the European Association
of Urology (EAU), which summarise CAUTI management [11]. Additionally, the European
Association of
Urology Nurses (EAUN) recently issued recommendations on catheterisation,
defining CAUTI as a syndrome involving an indwelling catheter for over two days,
a positive urine culture for at least one pathogen and symptoms like fever,
flank pain or dysuria [16].
Other national European guidelines either lack
recommendations regarding catheter management and CAUTI [13] or contradict those of
the EAU and EAUN, as
seen in discrepancies regarding aspects such as the timing of urine sampling
and catheter changes, the duration of treatment and the choice of antimicrobial
agents [16, 20]. For example, the EAU and EAUN recommend taking
the urine sample after changing the catheter, treatment as recommended for
complicated urinary tract infections and choosing a corresponding antibiotic adjusted
to local
resistance patterns following the evidence-based medicine (EBM) recommendations.
[11, 12, 16, 21]. Whereas, for example, the French urological
guidelines recommend taking the sample from the indwelling catheter and
changing it 24 hours later [20]. While the EAU guidelines do not specify a
routine catheter replacement interval, the EAUN suggests that silicone catheters
can remain in place for up to 12 weeks according to manufacturer
recommendations. However, they emphasise that the decision should be
individualised based on factors like encrustation or CAUTIs [11, 16]. Ultimately,
in day-to-day practice,
especially for general practitioners, actively seeking out various guidelines proves
challenging.
They see, diagnose and manage a wide array of diseases and the workload
would simply be overwhelming.
Antimicrobial stewardship interventions, particularly
guideline adherence, reduce hospital stays, morbidity, antimicrobial use and
resistance rates [22, 23]. The absence of or discrepancies in these
recommendations can create uncertainty, as observed among general practitioners in
this study,
potentially leading to unnecessary treatment, increased morbidity and the
development of antimicrobial resistance in CAUTI management.
Good clinical practice relies not only on
guideline adherence but also on clinical routine. In this study, nearly half of
the general practitioners reported limited involvement in catheter management, with
urologists
handling more catheter-related cases and CAUTIs, which may contribute to the
higher level of uncertainty among general practitioners. Despite their relatively
limited
experience, general practitioners and urologists showed similarities in practices,
aligning with EAU
and EAUN guidelines, such as changing catheters every 3 months and basing
diagnoses on symptoms and urine culture [11, 16]. Surprisingly, both disciplines frequently
chose smelly urine and cloudy urine as diagnostic symptoms, even though it is
explicitly mentioned in guidelines that odorous or cloudy urine is neither a
sign for CAUTI nor urinary tract infection [11, 20, 23]. Interestingly, even rather
atypical symptoms
such as reduction of general condition and confusion were frequently considered
by both disciplines as being suggestive of CAUTI or urinary tract infection. Both
general practitioners and
urologists appear to recognise the importance of atypical CAUTI symptom
assessment in this frail patient cohort, understanding – [24, 25], as recommended
by EAUN guidelines [16] – that it extends beyond merely assessing
voiding symptoms.
Similarities between general practitioners and urologists were
also seen in the frequent use of dipstick and urine sediment, even though they
are highly unspecific in catheterised patients due to common asymptomatic
bacteriuria (ABU), pyuria and microhaematuria [11, 23]. Catheterisation leads to bacterial
colonisation
of the catheter and bladder within a few days, resulting in ABU. However, the
mere presence of ABU without symptoms indicative of an infection should not be
misinterpreted as a CAUTI. Basing decision-making for CAUTI treatment on these
diagnostic measures can lead to antibiotic treatment when not necessary, does
not provide benefits [23, 26] and can even harm due to side effects and the
development of antimicrobial resistance [27].
This study identified significant differences
in antimicrobial choices for CAUTI treatment. Both disciplines use co-trimoxazole
as the primary antibiotic for non-febrile CAUTIs, despite it not being a
first-line recommendation for complicated UTIs by the EAU. Urologists primarily
treat febrile CAUTIs with i.v. cephalosporins, aligning with EAU
recommendations and the logistical ease within a hospital setting [11]. General practitioners
frequently use fluoroquinolones for both
non-febrile and febrile CAUTIs. While the EAU still recommends fluoroquinolones
for complicated UTIs in non-hospitalised patients, the European Commission’s restriction
on fluoroquinolone use calls for highly restrictive application, especially in
frail, permanently catheterised patients due to rising antimicrobial resistance
and severe adverse events [28–30].
General practitioners and urologists reported different
durations of treatment, varying between 5–7 days for non-febrile and 7–10 days
for febrile CAUTIs. Guidelines recommend treatment for 7–14 days and indicate
that duration should be closely related to the underlying abnormality. If a
patient is haemodynamically stable and the prostate is not involved, a shorter
course of treatment can be considered [11].
In cases where empirical treatment proves to
be resistant, more than one-third of general practitioners and nearly a quarter of
urologists only
adjust treatment in the absence of clinical improvement. It is one of the
principles of antimicrobial stewardship to adjust to a less broader antibiotic [12]
and in case of resistant bacteria to minimise
early reinfection and selection of antimicrobial resistance [31–33].
An increase in drinking quantity was the
primary prophylactic measure chosen. However, urologists implemented more
prophylactic measures than general practitioners, potentially because they are more
familiar with
managing prophylaxis in recurrent urinary tract infections. To date there are no recommended
prophylactic measures specific to CAUTI, which is reflected in the
inhomogeneity of our results. However, recently published EAUN guidelines
recommend an increase in drinking quantity to decrease catheter encrustation
whereas bladder irrigation is not recommended for preventing CAUTI [16]. Bladder irrigation
is commonly performed as
standard management of long-term urinary catheters, but it remains a
controversial method. A Cochrane review based on studies of poor methodological
quality found inconclusive evidence for the role of bladder irrigation in
preventing CAUTIs [34]. Even though not recommended by guideline
recommendations, bladder irrigation (with tap water) is frequently used in
Switzerland. A recent study showed that bladder irrigation with tap water
reduced CAUTI occurrence and antibiotic use [35].
In case of recurrent CAUTI, general practitioners usually referred
patients to urologists and urologists performed an ultrasound. According to "EbM Guidelines"
[12],
an ultrasound is the main diagnostic measure for recurrent CAUTI in order to
check for causative pathologies such as bladder stones. In 2005, a study showed that
approximately
one third of general practitioners had an ultrasound available [36]. This together
with the uncertainty in CAUTI
management could explain why general practitioners refer patients to the specialist.
One limitation of our study was
that it was not based on direct observations, which did not allow accounting
for recall and reporting biases. Further, our survey results may not adequately
represent CAUTI practices among general practitioners and urologists, as the overall
response
rate cannot be reproduced and was potentially low. However, it is conceivable
that compliance with CAUTI guidelines in non-respondents is not considerably
higher than in those respondents who are less interested in this topic. Lastly,
the CAUTI literature is highly heterogeneous, and even if the recommendations
are classified as strong, many critical clinical questions are not addressed,
which complicates the interpretation of
the study results regarding these recommendations.
In the real-world management of catheters and CAUTIs, similarities
and differences between general practitioners and urologists are evident. Some of
these align
with common recommendations and antimicrobial stewardship principles, while
others deviate from them. Catheter and CAUTI management entail significant
uncertainty for general practitioners. Even urologists, the presumed specialists,
do not always
act in accordance with recommendations and antimicrobial stewardship
principles.
CAUTI management varied notably between general practitioners and urologists in
terms of treatment and prophylaxis. Use of drugs not recommended by guidelines was
common. This trend, along with inappropriate diagnostic methods and prophylaxis, may
increase antimicrobial
resistance and CAUTI morbidity. general practitioners and urologists might benefit
from closer
collaboration and exchange of knowledge. Joint
training sessions could be organised in which specific clinical questions from
routine practice are discussed between the two specialties. Collaborative
conferences could be held, general practitioners could gain access to guidelines from
other
specialties and shared professional literature could be published. The study emphasises
the necessity for diagnostic
and antimicrobial stewardship interventions, and proper training in CAUTI
management for general practitioners and urologists.
PD. Dr. med.
Kathrin Bausch
Department of Urology
University Hospital Basel
Spitalstrasse
21
CH-4031 Basel
kathrin.bausch[at]usb.ch
References
1. Öztürk R, Murt A. Epidemiology of urological infections: a global burden. World J
Urol. 2020 Nov;38(11):2669–79. doi: https://doi.org/10.1007/s00345-019-03071-4
2. Schweiger A, Maag J, Marschall J. CAUTI Surveillance Teilnehmerhandbuch. Available
from: https://www.swissnoso.ch/fileadmin/module/cauti_surveillance/Dokumente_D/240101_Swissnoso_CAUTI_Surveillance_Handbuch_V2.1.pdf
3. Klevens RM, Edwards JR, Richards CL Jr, Horan TC, Gaynes RP, Pollock DA, et al. Estimating
health care-associated infections and deaths in U.S. hospitals, 2002. Public Health
Rep. 2007;122(2):160–6. doi: https://doi.org/10.1177/003335490712200205
4. Schweiger A, Kuster SP, Maag J, Züllig S, Bertschy S, Bortolin E, et al. Impact of
an evidence-based intervention on urinary catheter utilization, associated process
indicators, and infectious and non-infectious outcomes. J Hosp Infect. 2020 Oct;106(2):364–71.
doi: https://doi.org/10.1016/j.jhin.2020.07.002
5. Munasinghe RL, Yazdani H, Siddique M, Hafeez W. Appropriateness of use of indwelling
urinary catheters in patients admitted to the medical service. Infect Control Hosp
Epidemiol. 2001 Oct;22(10):647–9. doi: https://doi.org/10.1086/501837
6. Jain P, Parada JP, David A, Smith LG. Overuse of the indwelling urinary tract catheter
in hospitalized medical patients. Arch Intern Med. 1995 Jul;155(13):1425–9. doi: https://doi.org/10.1001/archinte.1995.00430130115012
7. Tiwari MM, Charlton ME, Anderson JR, Hermsen ED, Rupp ME. Inappropriate use of urinary
catheters: a prospective observational study. Am J Infect Control. 2012 Feb;40(1):51–4.
doi: https://doi.org/10.1016/j.ajic.2011.03.032
8. Saint S, Greene MT, Krein SL, Rogers MA, Ratz D, Fowler KE, et al. A Program to Prevent
Catheter-Associated Urinary Tract Infection in Acute Care. N Engl J Med. 2016 Jun;374(22):2111–9.
doi: https://doi.org/10.1056/NEJMoa1504906
9. Jahromi MS, Mure A, Gomez CS. UTIs in patients with neurogenic bladder. Curr Urol
Rep. 2014 Sep;15(9):433. doi: https://doi.org/10.1007/s11934-014-0433-2
10. Cai T, Verze P, Brugnolli A, Tiscione D, Luciani LG, Eccher C, et al. Adherence to
European Association of Urology Guidelines on Prophylactic Antibiotics: An Important
Step in Antimicrobial Stewardship. Eur Urol. 2016 Feb;69(2):276–83. doi: https://doi.org/10.1016/j.eururo.2015.05.010
11. Bonkat G, Bartoletti R, Bruyère F, Cai T, Geerlings SE, Kranz J, et al. EAU Guidelines
on Urological Infections 2023. Uroweb - European Association of Urology. Available
from: https://uroweb.org/guidelines/urological-infections/chapter/the-guideline
12. Wuorela M. (HWI). In: Rabady S, Sönnichsen A, Kunnamo I (eds.) EbM-Guidelines. Evidenzbasierte
Medizin für Klinik und Praxis. 2023. Available from: https://www.ebm-guidelines.com/dtk/ebmde/avaa?p_artikkeli=ebd00206&p_haku=harnwegsinfekt
13. Leitlinienregister AW. Available from: https://register.awmf.org/de/leitlinien/detail/043-044
14. CAUTI Guidelines | Guidelines Library | Infection Control | CDC. 2019. Available from:
https://www.cdc.gov/infectioncontrol/guidelines/cauti/index.html
15. Bruyere F, Goux L, Bey E, Cariou G, Cattoir V, Saint F, et al. Infections urinaires
de l’adulte : comparaison des recommandations françaises et européennes. Par le Comité
d’infectiologie de l’Association française d’urologie (CIAFU). Prog Urol. 2020;30(8-9):472–81.
doi: https://doi.org/10.1016/j.purol.2020.02.012
16. Geng V. Indwelling catheterisation in adults – Urethral and suprapubic. European Association
of Urology Nurses - EAUN. Available from: https://nurses.uroweb.org/guideline/indwelling-catheterisation-in-adults-urethral-and-suprapubic/
17. Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al.; National
Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities.
NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated
infections: annual summary of data reported to the National Healthcare Safety Network
at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp
Epidemiol. 2008 Nov;29(11):996–1011. doi: https://doi.org/10.1086/591861
18. BAG B für G. Sentinella Meldesystem. Available from: https://www.sentinella.ch/de/info
19. Pro Medicus Gmb H. In a Nutshell - Urologie in der Hausarztpraxis. Available from:
https://www.inanutshell.ch/module/urologie-in-der-hausarzt-praxis/
20. Urofrance | Révision des recommandations de bonne pratique pour la prise en charge
et la prévention des Infections Urinaires Associées aux Soins (IUAS) de l’adulte.
- Urofrance. Available from: https://www.urofrance.org/recommandation/revision-des-recommandations-de-bonne-pratique-pour-la-prise-en-charge-et-la-prevention-des-infections-urinaires-associees-aux-soins-iuas-de-ladulte/
21. Kouri T. Harndiagnostik und Bakterienkultur. In: Rabady S, Sönnichsen A, Kunnamo I
(eds.) EbM-Guidelines. Evidenzbasierte Medizin für Klinik und Praxis. 2023. Available
from: https://www.ebm-guidelines.com/dtk/ebmde/avaa?p_artikkeli=ebd00205&p_haku=uti
22. Goebel MC, Trautner BW, Grigoryan L. The Five Ds of Outpatient Antibiotic Stewardship
for Urinary Tract Infections. Clin Microbiol Rev. 2021 Dec;34(4):e0000320. doi: https://doi.org/10.1128/CMR.00003-20
23. Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al.; Infectious
Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated
urinary tract infection in adults: 2009 International Clinical Practice Guidelines
from the Infectious Diseases Society of America. Clin Infect Dis. 2010 Mar;50(5):625–63.
doi: https://doi.org/10.1086/650482
24. Wojszel ZB, Toczyńska-Silkiewicz M. Urinary tract infections in a geriatric sub-acute
ward-health correlates and atypical presentations. Eur Geriatr Med. 2018;9(5):659–67.
doi: https://doi.org/10.1007/s41999-018-0099-2
25. Dutta C, Pasha K, Paul S, Abbas MS, Nassar ST, Tasha T, et al. Urinary Tract Infection
Induced Delirium in Elderly Patients: A Systematic Review. Cureus. 2022 Dec;14(12):e32321.
doi: https://doi.org/10.7759/cureus.32321
26. Köves B, Cai T, Veeratterapillay R, Pickard R, Seisen T, Lam TB, et al. Benefits and
Harms of Treatment of Asymptomatic Bacteriuria: A Systematic Review and Meta-analysis
by the European Association of Urology Urological Infection Guidelines Panel. Eur
Urol. 2017 Dec;72(6):865–8. doi: https://doi.org/10.1016/j.eururo.2017.07.014
27. Krzyzaniak N, Forbes C, Clark J, Scott AM, Mar CD, Bakhit M. Antibiotics versus no
treatment for asymptomatic bacteriuria in residents of aged care facilities: a systematic
review and meta-analysis. Br J Gen Pract. 2022 May;72(722):e649–58. doi: https://doi.org/10.3399/BJGP.2022.0059
28. Bausch K, Bonkat G. Fluoroquinolone antibiotics - what we shouldn’t forget two years
after the restriction by the European Commission. Swiss Med Wkly. 2022 Jan;152(0304):w30126–30126.
doi: https://doi.org/10.4414/SMW.2022.w30126
29. Classifying antibiotics in the WHO Essential Medicines List for optimal use—be AWaRe
- The Lancet Infectious Diseases. Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(17)30724-7/abstract
30. Humanmedizin R. ANRESIS. Available from: https://www.anresis.ch/de/antibiotikaresistenz/resistance-data-human-medicine/
31. Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding
the mechanisms and drivers of antimicrobial resistance. Lancet. 2016 Jan;387(10014):176–87.
doi: https://doi.org/10.1016/S0140-6736(15)00473-0
32.Core Elements of Antibiotic Stewardship for Health Departments | Antibiotic Use |
CDC. 2023. Available from: https://www.cdc.gov/antibiotic-use/core-elements/health-departments.html
33. Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing
an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of
America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016 May;62(10):e51–77.
doi: https://doi.org/10.1093/cid/ciw118
34. Shepherd AJ, Mackay WG, Hagen S. Washout policies in long-term indwelling urinary
catheterisation in adults. Cochrane Database Syst Rev. 2017 Mar;3(3):CD004012. doi: https://doi.org/10.1002/14651858.CD004012.pub5
35. van Veen FE, Den Hoedt S, Coolen RL, Boekhorst J, Scheepe JR, Blok BF. Bladder irrigation
with tap water to reduce antibiotic treatment for catheter-associated urinary tract
infections: an evaluation of clinical practice. Front Urol. 2023 Apr;3:1172271. Available
from: https://www.frontiersin.org/journals/urology/articles/10.3389/fruro.2023.1172271/full 10.3389/fruro.2023.1172271
36. Tschudi P, Rosemann T. Die Zukunft der Hausarztmedizin! Wie finden wir den Nachwuchs?
Womit können wir junge Ärztinnen und Ärzte für das Weiterbildungsziel «Hausärztin»
motivieren? 2010 Mar; Available from: https://www.zora.uzh.ch/id/eprint/33323
Appendix
Supplementary tables
Table S1What do
you usually do after receiving the results of the urine culture when the
detected germ proves to be resistant to the antibiotic administered?
|
n total |
General practitioner |
% |
Urologist |
% |
p-value |
| Adjustment |
104 |
46 |
64.8% |
58 |
77.3% |
0.136 |
| Adjustment in the
absence of clinical improvement |
42 |
25 |
35.2% |
17 |
22.7% |
|
| No adjustment |
0 |
0 |
0 |
0 |
0 |
|
Table S2If
you perform regular bladder irrigation, which fluid do you use for this?
|
n total |
General practitioner |
% |
Urologist |
% |
p-value |
| Saline solution |
25 |
11 |
84.6% |
14 |
32.6% |
0.018 |
| Tap water |
18 |
1 |
7.7% |
17 |
39.5% |
|
| Hyaluronic acid / chondroitin sulphate |
3 |
0 |
0 |
3 |
7% |
|
| Disinfectant |
6 |
0 |
0 |
6 |
14% |
|
| Other |
4 |
1 |
7.7% |
3 |
7% |
|
Table S3Do
you initiate further diagnostic measures for recurrent urinary tract infections in
catheterised
patients?
|
n total |
General practitioner |
% |
Urologist |
% |
p-value |
| No, treatment only |
32 |
24 |
35.8% |
8 |
11% |
<0.001 |
| Ultrasound |
51 |
8 |
11.9% |
43 |
58.9% |
|
| Referral for an
ultrasound |
5 |
5 |
7.5% |
0 |
0 |
|
| Computed tomography |
4 |
0 |
0 |
4 |
5.5% |
|
| Referral to urologist
(if a general practitioner) |
32 |
30 |
44.8% |
2 |
2.7% |
|
| Other |
16 |
0 |
0 |
16 |
21.9% |
|
Questionnaire
Questions and answer options in
brackets
- Which
country do you work in? [ Austria | France | Germany | Switzerland ]
- Age
[ Free-text answer ]
- Years of experience as a medical professional [ Free-text
answer ]
- Sex
[ Female | Male ]
- Which
type of medical facility do you work in? [ Urological practice | District
hospital | Cantonal hospital | University hospital | Rehabilitation hospital | Other
]
- Do
you care for patients using a permanent urinary catheter? [ No | Rarely | Yes |
Yes and I change transurethral | Yes and I change suprapubic | Yes and I change
both ]
- At
what interval do you usually perform catheter changes in asymptomatic patients?
[ <2 weeks | 2–4 weeks | 1–2 months | 2–3 months | >3 months | Only if
needed ]
- During
the past 12 months, how many patients with transurethral and/or suprapubic
catheters did you see on average per week for catheter-related concerns? [ <
1 | 1–5 | 5–10 | 11–25 | 26–50 | >50 ]
- How
often do you diagnose an urinary tract infection in a catheterised patient per year?
[ <1/year | 1/year
| 2–3/year | 4–5/year | >5/year ]
- Do
you feel competent in managing catheters and recurrent urinary tract infections in
catheterised
patients? [ Somewhat no | Somewhat yes |
Yes ]
- On
which signs/symptoms do you usually base your diagnosis of an urinary tract infection
in a catheterised
patient? [ Burning | Suprapubic pain | Perineal pain | Haematuria | Cloudy
urine | Urine smell | Flank pain | Fever | Reduction of general condition | Confusion
| Testicular pain ]
- How
do you usually diagnose an urinary tract infection in a catheterised patient? [ Symptoms | Examination
| Ultrasound | CT | Dipstick test | Urine status and sediment | Urine culture |
Blood test ]
- How
often do you prescribe an antibiotic for an urinary tract infection per catheterised
patient per
year? [ <1 | 1× | 2–3× | 4–5× | >5× ]
- How
do you choose the antibiotic for empirical therapy? [ Last urine culture | Local
resistances | Empirical ]
- On
average, how long do you usually treat catheterised patients with an antibiotic
for a non-febrile urinary tract infection? [ Single-dose treatment | 3 days | 5 days | 7 days | 10
days | 14 days ]
- Which
antibiotic do you usually choose for the empirical therapy of a non-febrile
urinary tract infection? [ fluoroquinolone | co-trimoxazole | nitrofurantoin | fosfomycin | oral cephalosporin
| amoxicillin ± clavulanic acid ]
- On
average, how long do you usually treat catheterised patients with an antibiotic
for a febrile urinary tract infection? [ Single-dose treatment | 3 days | 5 days | 7 days | 10 days
| 14 days | 3 weeks ]
- Which
antibiotic do you usually choose for the empirical therapy of a febrile urinary tract
infection? [ fluoroquinolone
| co-trimoxazole | nitrofurantoin | fosfomycin | oral cephalosporin | amoxicillin
± clavulanic acid | i.v. cephalosporin | i.v. carbapenems | other ]
- What
do you usually do after receiving the results of the urine culture when the
detected germ proves to be resistant to the antibiotic administered? [ Adjustment
| Adjustment in the absence of clinical improvement | No adjustment ]
- Do
you usually take additional measures to reduce recurrent urinary tract infections
in catheterised
patients? [ None | Drinking quantity | Frequent catheter changes | Optimising
care | Different catheter material | Transurethral -> suprapubic catheter | Plant-based
medication | Bladder irrigation | Acidifying urine | Vaccination | D-Mannose | Continuous
antibiotics | Medication to limit bladder activity ]
- If
you perform regular bladder irrigation, which fluid do you use for this? [ Saline
solution | Tap water | Hyaluronic acid/chondroitin sulphate | Disinfectant | Other
]
- Do
you initiate further diagnostic measures for recurrent urinary tract infections in
catheterised
patients? [ No | Treatment only | Ultrasound | Referral for an ultrasound | CT
| Referral to urologist (if a general practitioner) | Other ]


