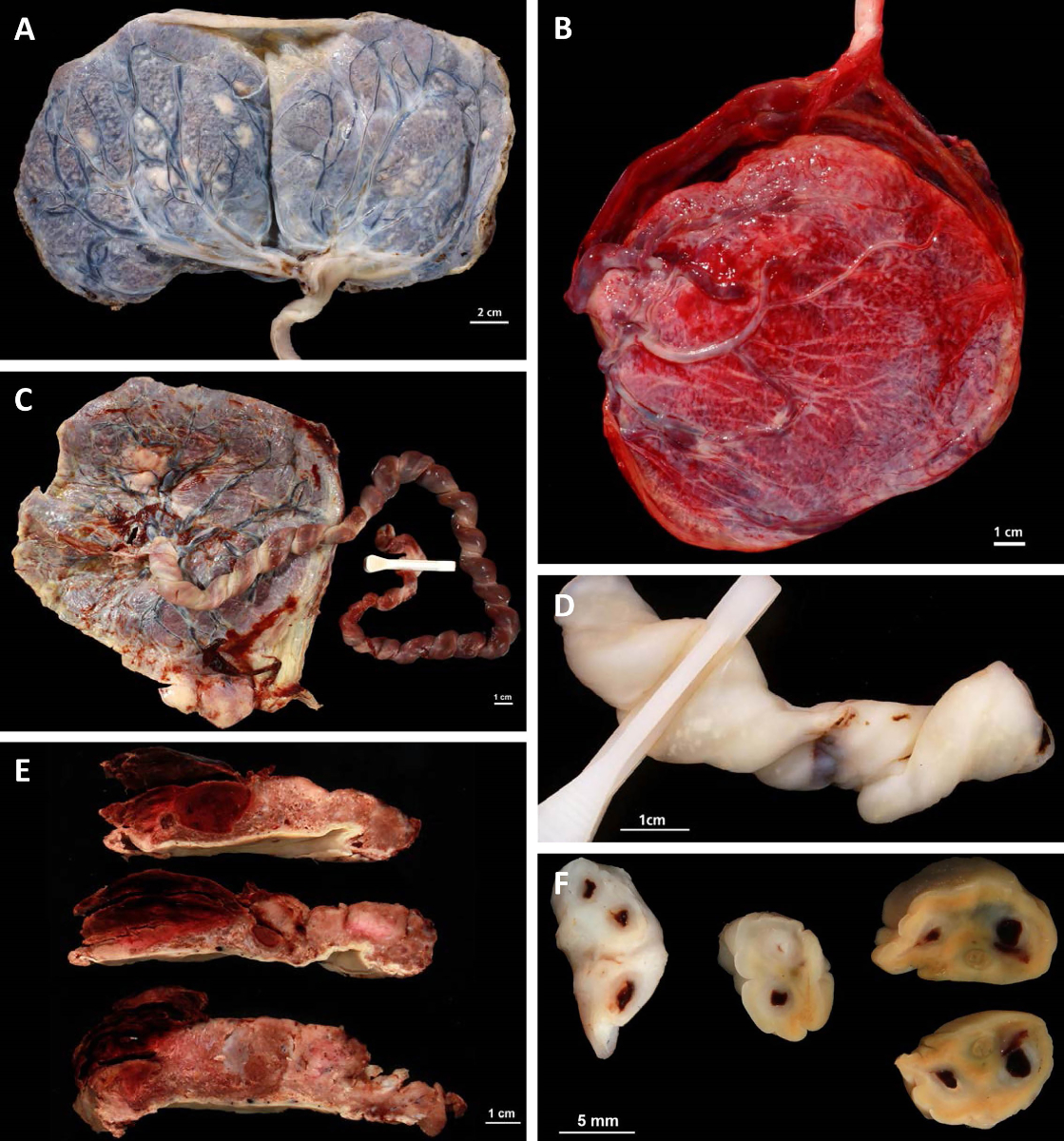
Figure 1Macroscopic placenta findings.
A: Placenta bipartita with marginal cord insertion.
B: Velamentous cord insertion.
C: Hypercoiling of the umbilical cord.
D: Candida funisitis.
E: Retroplacental haematoma.
F: Umbilical vessel thrombosis.
DOI: https://doi.org/https://doi.org/10.57187/s.3929
The placenta is a unique and complex organ that combines the circulatory systems of two or more individuals within a single dynamic organ with a set, short lifespan. A diverse spectrum of disorders, including infections as well as metabolic, genetic, circulatory, and maturation defects, may affect its function. The pathologic investigation of the placenta yields a wealth of information crucial for optimal patient treatment. Since the early descriptive days of placental pathology, enormous progress has been made towards an improved understanding of the pathogenesis and impact of morphologic phenotypes in relation to clinical conditions.
After the pioneering work of the College of American Pathologists based on the results of an interdisciplinary task force [1], several national and regional professional societies (e.g. the German Society for Pathology, The Royal Society of Pathologists, the American Academy of Pediatrics, the Section on Neonatal Perinatal Medicine, and the Society for Pediatric Pathology) published guidelines with indications for placental examination [2–4]. However, these guidelines come from countries with unique healthcare systems, or they are published in German. Therefore, a tailored set of guidelines is needed for our country as well.
Many clinicians in the field of perinatal medicine have reservations towards this examination, which may be based on historical doubts about the value of placental examination and the lack of a general application of the standards of the examination [5].
The Amsterdam Classification has developed more informative techniques and defined criteria to yield more useful results [6, 7], the significance of which has been documented (for example, in [8]). The comprehensive Human Placenta Projects of the NIH have also expressed the increased perception of the placenta as a source of information [9, 10]. Deficits and consequences of inadequate communication between clinicians and pathologists are apparent; to overcome these issues, mutually defined clinical data are needed in the requests for examinations, along with consistent nomenclature in examination reports [6, 11]. Patients expect a full and competent work-up of their health problems. Therefore, obstetricians and neonatologists may demand high-quality placental pathology examinations that provide relevant clinical information (see table 1) that includes information on the responsible pathologist at the receiving institution. Pathology reports should be standardised according to the new guidelines. The spectrum of thorough work-up has become so large that pathology departments should consider involving team members with special interests and expertise in placental examination [12, 13]. Without fulfilling of these two essential requirements, widespread acceptance of recommendations for placental examination cannot be expected.
Placental investigation aims to identify and classify previously unsuspected maternal or foetal disease requiring immediate attention, conditions with a high recurrence risk in subsequent pregnancies, implications for future pregnancy or patient management, and specific explanations for adverse pregnancy outcomes. This review focuses on the clinical indications for placental pathological examination and newly proposed classification schemes for placental pathology, endeavouring to unify nomenclature for facilitated communication between pathologists, obstetricians, and neonatologists. Thus, this review elucidates the contribution of placental investigation, ultimately benefiting the long-term interest of our patients, namely mothers and children.
Table 1Information required for the pathologist for placenta investigation.
| 1. Mother: age, parity, gestational age, and maternal pathology (diabetes mellitus, gestational diabetes, hypertensive disease, or other systemic maternal disease) |
| 2. Newborn: sex, birth weight, length, head circumference, pH of umbilical artery, and APGAR values |
| 3. Mode of delivery |
| 3. Course of pregnancy (uneventful, uterine bleeding, infection, peripartum fever, or abnormal amount of amniotic fluid) |
| 5. Results of foetal examinations (no abnormalities; abnormal laboratory or sonographic findings, including Doppler flowmetry) |
| 6. Multiple pregnancy: twin-specific identification of umbilical cord (for instance white clamp for twin A, blue clamp for twin B); chorionicity from ultrasound examination. |
| 7. Questions for the pathologist |
| 8. Addresses of recipients for copies of the pathology report, such as the neonatology department or referring external physicians (with the approval of the mother). |
This publication aims to provide standardised recommendations for placental investigation according to several indications, adapted to the literature [1–4, 13–15]. It offers a comprehensive list with specific clinical parameters for submitting a placenta for pathological examination (table S1 in the appendix). This list could be displayed in various areas in labour and delivery rooms to remind clinicians to submit placentas for examination. Some guidelines grade the strength of recommendation for different indications; however, this may be controversial in some cases, such as preterm births and other indications [2–4]. For practical purposes, it is more relevant to note that “placental pathology is most likely to provide explanatory data in these situations”:
When the placenta is still in the labour and delivery room, it is weighed with the membranes but manually cleaned of gross blood clots. This is recommended for practical reasons. The umbilical cord is lifted without tension from the placental disc so that the cord is not weighed. The result is recorded as the fresh weight of the placenta (table S2 in the appendix). In every obstetrical setting equipped with a balance, this value is easy to measure, and its documentation in the patient’s chart is mandatory. This practice corresponds to the traditional documentation of deliveries by physicians and midwives. For multiple births, the total weight of all placentas is recorded, and in cases of separate placentas, their respective weights may also be documented.
The umbilical cord is measured, including the placental and newborn components. Notes on abnormalities in placental shape or colour, the umbilical cord, and the membranes are made, including appropriate photographs.
When a pathological examination is indicated, oral informed consent must be obtained from the mother and documented in the file. According to Swiss legislation, in exceptional cases, competent and comprehensive information must be obtained before genetic analyses are performed, and written informed consent is mandatory. Information about this kind of additional analysis should be given at a suitable time before delivery or during postpartum, but not on the labour and delivery floor.
If the placenta is sent for histopathological examination, it should be submitted fresh and untrimmed to the pathology department. The placenta is then weighed without the membranes or umbilical cord, as most percentile curves for placentas are based on the trimmed weight.
The placenta may be stored unfixed at a temperature of 4–8 °C for 3–4 days, according to local possibilities and agreements either in the obstetrical or the pathology department, depending on available storage capacities and agreements between the departments regarding the storage of tissue that will not be submitted for histological exams. Some newborns present with problems several days after an apparently normal delivery, at which point a submission can still be made [17]. Histopathologic and molecular genetic examinations may be performed on placentas fixed after up to 7 days without a loss of information. However, for optimal RNA extraction, placental tissue should be snap-frozen or placed in an RNA protection medium as soon as possible, ideally within 30 minutes of delivery. The effect of longer intervals on other aspects, such as the proteomic profile, remains to be established, although initial data suggest potential significant effects [12]. For genetic counselling, “trio” sequencing (i.e. the analysis of the genomes of both parents and the foetus), a state-of-the-art technique, can be performed if sufficient resources are available [16].
Upon submission for pathology investigation, the routine macroscopic examination by the pathology laboratory, including the trimmed weight of the placenta, is performed according to the standards of the Swiss Pathology Society (table S3 in the appendix). The documentation includes the degree of coiling of the umbilical cord (normally 2–3 coils per 10 cm, i.e., a spiral or coiling index of 0.2) [3, 18–20]. Placental sampling follows the guidelines defined by the Swiss Pathology Society [21] in agreement with the Amsterdam Placental Workshop Group Consensus [6].
Mothers may opt for the personal use of their placenta. If the mother wishes to keep the placenta (whether a pathological examination is unnecessary or whether an explained examination is refused), it must be left to the mother
In recent years, progress in placental investigation has been made, and a standardised, reproducible, and biologically based classification system has been gradually accepted. To establish an updated and agreed-upon protocol for diagnostic criteria for placenta lesions, a group of placental and perinatal pathologists as well as foetal-maternal medicine specialists from across the world gathered in Amsterdam in 2014. The consensus criteria were published in 2016 [6] and constitute the current optimal international standard for diagnostic placental evaluation. Beyond the immediate diagnostic application, the proposed classification system is also expected to improve the comparability of studies. The classification categories are listed in detail in table 2.
Table 2Classification of placental pathologies.
| Placental vascular processes | |||
| Maternal stromal-vascular lesions | Developmental | Superficial implantation/decidual arteriopathy | |
| Increased immature extravillous trophoblast | |||
| Malperfusion | Global/partial | Early: distal villous hypoplasia | |
| Late: accelerated villous maturation | |||
| Segmental/ complete | Villous infarct(s) | ||
| Loss of integrity | Abruptio placentae (arterial) | ||
| Marginal abruption (venous) | Acute | ||
| Chronic | |||
| Foetal stromal-vascular lesions | Developmental | Villous capillary lesions | |
| Delayed villous maturation (maturation defect) | |||
| Dysmorphic villi | |||
| Malperfusion | Global/partial | Obstructive lesions of umbilical cord | |
| Recent intramural fibrin in large foetoplacental vessels | |||
| Small foci of avascular or karyorrhectic villi | |||
| Segmental/complete | Chorionic plate or stem villous thrombi | ||
| Large foci of avascular or karyorrhectic villi | |||
| Loss of integrity | Large vessel rupture (foetal haemorrhage) | ||
| Small vessel rupture (foeto-maternal haemorrhage) | |||
| Villous oedema | |||
| Placental inflammatory-immune processes | |||
| Infectious inflammatory lesions | Acute | Maternal inflammatory response: chorioamnionitis, subchorionitis | |
| Foetal inflammatory response: chorionic/ umbilical vasculitis | |||
| Chronic | Villitis (CMV, others) | ||
| Intervillositis (Malaria, others) | |||
| Immune/ idiopathic inflammatory lesions | Villitis of unknown aetiology and related/ associated lesions | Chronic villitis | |
| Chronic chorioamnionitis | |||
| Lymphoplasmacytic deciduitis | |||
| Eosinophil T-cell foetal vasculitis | |||
| Chronic histiocytic intervillositis | |||
| Other placental processes | |||
| Massive perivillous fibrin (oid) deposition (maternal floor infarction) | |||
| Abnormal placental shape or umbilical insertion site | |||
| Morbidly adherent placenta (PAS, placenta accreta spectrum) | |||
| Meconium-associated changes | |||
| Increased circulating nucleated red blood cells | |||
The normal placenta is characterised by low-velocity, high-volume blood flow. Maternal vascular malperfusion is understood as a consequence of abnormal spiral artery blood flow. This event often starts early in pregnancy because of developmental abnormalities leading to decidual arteriopathy (necrosis of decidual arteries). Global partial maternal vascular malperfusion results in accelerated villous maturation, as reflected by increased syncytial knots, increased intervillous fibrin, and decreased villous branching, leading to villous paucity. Over 30% of all distal villi affected are termed distal villous hypoplasia. Segmental complete maternal vascular malperfusion causes villous infarcts overlying occluded spiral arteries. Any infarction in a preterm placenta and any infarction affecting >5% of the placenta volume at term should be described.
Abruptio placentae is typically associated with pre-eclampsia due to atherosis/decidual arteriopathy or ischaemia-reperfusion in a central location with indentation of the basal plate and extension into the intervillous space. Marginal abruption is caused by the rupture of maternal veins at the periphery of the placenta. It may follow an acute or chronic course.
Delayed villous maturation is characterised by a monotonous villous population (at least 10 villi in at least 30% of one full-thickness parenchymal slide) with reduced numbers of vasculosyncytial membranes, a persistent continuous cytotrophoblast layer, and centrally placed capillaries. Focal delayed villous maturation is found in one parenchymal slide, whereas diffuse villous maturation is present in two or more parenchymal slides.
Global partial foetal vascular malperfusion is understood as being associated with potentially obstructive umbilical cord lesions, such as hypercoiling, stricture, abnormal umbilical cord insertion site, and long-standing entanglements, and is histologically characterised by scattered small foci of avascular villi and mural fibrin deposition in large foetoplacental veins. Segmental complete occlusion of large foetoplacental vessels by thrombi leads to larger foci of villi with stromal-vascular karyorrhexis. Furthermore, delayed villous maturation might also contribute to foetal hypoxemia due to reduced oxygen and nutrition supply.
Loss of foetal vascular integrity may result in haemorrhage or oedema of placental villi. Patchy oedema of distal villi is correlated with severe acidaemia in babies born at term [22] (figures 1 and 2).

Figure 1Macroscopic placenta findings.
A: Placenta bipartita with marginal cord insertion.
B: Velamentous cord insertion.
C: Hypercoiling of the umbilical cord.
D: Candida funisitis.
E: Retroplacental haematoma.
F: Umbilical vessel thrombosis.
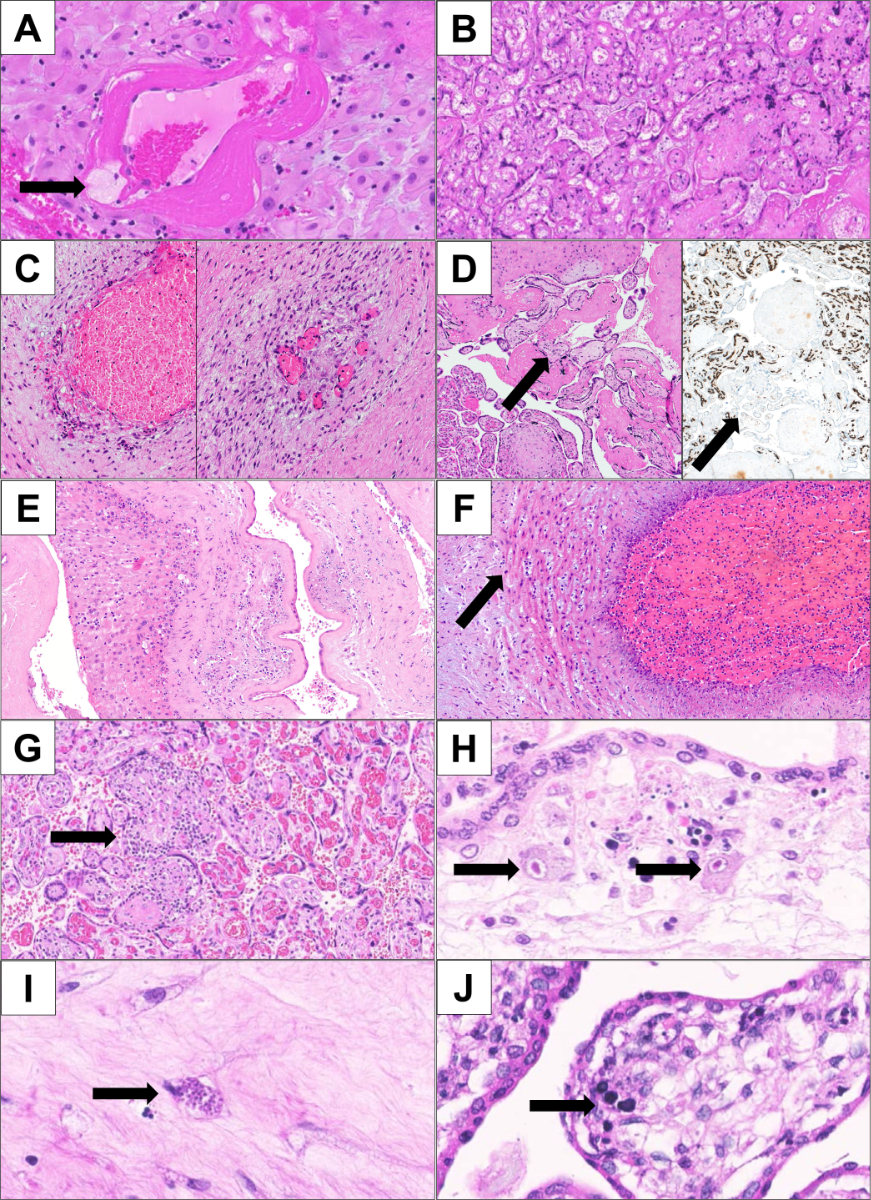
Figure 2Histological placenta findings.
A: Decidual arteriopathy demonstrated by fibrinoid necrosis of the vessel wall and the presence of foam cells (arrow) (HE, 200×).
B: Infarct (HE, 40×).
C: Endangiopathia obliterans showing early changes (left; loss of endothelial integrity and erythrocyte extravasation) and late changes (right; fibroblast ingrowth and intraluminal septation) (HE, 100×).
D: Focus of avascular villi (arrow); the loss of capillaries is also visualised by immunohistochemical staining of the endothelial marker CD34 (right) (HE: 100×; immunohistochemistry for CD34: 40×).
E: Acute chorioamnionitis of the free membranes in ascending intrauterine infection (HE; 100×).
F: Foetal response to ascending infection: omphalovasculitis and debuting funisitis (HE, 200×).
G: Villitis of unknown aetiology (VUE) (the arrow shows lymphoid destructive aggregates in a villus) (HE, 100x).
H: CMV (the arrow shows two characteristic “owl eye” endothelial cells infected by CMV (HE, 400×).
I: Toxoplasma gondii (the arrow points to a toxoplasma cyst in the chorionic plate) (HE, 400×).
J: Parvovirus B19 (the arrow shows two characteristic “lampion” cells) (HE, 400×).
The placenta mediates between two organisms and the environment. This leads to increased susceptibility to infection and occasional immune-mediated allograft-type responses. Inflammation is the main abnormal non-vascular finding.
Acute inflammatory response in ascending infection and infection with haematogenous spread is described in detail below. Most importantly, villitis of unknown aetiology (VUE) consists of the chronic cellular inflammation of the villous stroma and sometimes the intervillous space and stem villus vessels. It is currently regarded as a maternal graft-versus-host-type reaction to foetal antigens. Approximately 5–10% of term placentas contain foci of villitis of unknown aetiology [15]. High-grade villitis of unknown aetiology, defined as at least one focus involving >10 contiguous villi, carries a significant recurrence risk (see below).
Massive perivillous fibrin(oid) deposition is considered an autoimmune-mediated process that contributes to a severe decrease in the exchange surface of the villi, leading to severe growth restriction or intrauterine demise [23]. The placenta might show a large variety of form anomalies because of a disrupted placentation process (myomas, scars, allo-foetal immune reaction). In addition, the placenta accreta spectrum (discussed at the end of this review) is associated with these conditions. Meconium is toxic to the amnion as well as fibroblasts and the smooth muscle cells of vessels [24]. The increase in nucleated red blood cells might raise the suspicion of foetal anaemia, prompting further investigations (e.g. ParvoB19 virus infection or foeto-maternal transfusion).
Table 3What to look for in the placenta as the cause of specific adverse outcomes (modified from [15]).
| Preterm foetal death | Maternal vascular malperfusion |
| Global/ partial foetal vascular malperfusion | |
| Abruption | |
| Placental insufficiency | |
| Umbilical cord complications | |
| Spontaneous preterm birth before 37 weeks of gestation | Acute chorioamnionitis |
| Marginal abruption | |
| Mild maternal malperfusion | |
| Foetal growth restriction/ indicated preterm birth before 37 weeks of gestation | Global/ partial maternal malperfusion (accelerated maturation) |
| Chronic villitis and intervillositis [79] | |
| Foetal vascular malperfusion | |
| Foetal stromal-vascular developmental lesions | |
| Placental insufficiency | |
| Umbilical cord complications | |
| Term foetal death | Abruption placentae |
| Global/partial foetal vascular malperfusion (umbilical cord accident) | |
| Foeto-maternal haemorrhage | |
| Delayed villous maturation | |
| Placental insufficiency | |
| Umbilical cord complications | |
| CNS injury at term | Complete/segmental foetal vascular malperfusion |
| Global/partial foetal vascular malperfusion (umbilical cord accident) | |
| Chronic villitis with obliterative foetal vasculopathy | |
| Acute chorioamnionitis with severe foetal cellular inflammatory response | |
| Multiple placental lesions |
Severe maternal vascular malperfusion is observed more frequently with maternal and obstetric disorders and can be the first indicator of maternal autoimmune disease. Investigations should include the evaluation of maternal cardiovascular status, glucose tolerance, thrombophilia, and renal function. These factors are associated with significant perinatal morbidity and mortality, including intrauterine growth restriction, foetal and neonatal demise, and foetal/neonatal neurocompromise (seizures and cerebral palsy). In addition, they have a recurrence risk ranging from 34% to 100%, and preventive measures can improve foetal and maternal outcomes in subsequent pregnancies [23]. The prophylactic use of acetylic salicylic acid, uterine artery Doppler, early third-trimester placental ultrasound, and indicated late-preterm and early-term deliveries in subsequent pregnancies may be recommended [15, 25].
In histologic chorioamnionitis with resulting spontaneous preterm delivery, neonatal antibiosis may be initiated, and the treatment of maternal conditions with an eventual causal relationship (e.g. periodontal or endometrial disease) may be considered [15].
Villitis of unknown aetiology, maternal floor infarction, and chronic histiocytic intervillositis should trigger maternal testing for autoimmune diseases, and low-molecular-weight heparin or intravenous immunoglobulin and heparin may be considered [15, 26]. Furthermore, a link between chronic histiocytic intervillositis and foetal and neonatal alloimmune thrombocytopenia, including the role of human platelet antigens, has been established. Therefore, the diagnosis of chronic histiocytic intervillositis should prompt a respective haematologic workup of the child [27, 28].
Findings for maternal antifoetal rejection in subsets of massive perivillous fibrin deposition/maternal floor infarction could lead to further diagnostic and therapeutic options in future [29].
Foetal vascular malperfusion, especially foetal thrombotic vasculopathy, is a red flag to exclude disorders of thrombophilia, including inherited disorders (e.g. factor V Leiden), maternal connective tissue disorders (anti-cardiolipin antibodies), and other causes of systemic thromboses, such as DIC. It also alerts clinicians that a thorough neonatal exam should be performed to exclude systemic thrombi in the brain, lungs, heart, or kidneys [2].
Term infants with high-grade foetal vascular pathology are at an increased risk of developing seizure disorders, developmental disability, and static neuromuscular conditions, such as cerebral palsy [30].
Delayed villous maturation is correlated with a decreased foetoplacental weight ratio; excessive villous stroma and centrally positioned capillaries lacking vasculosyncytial membranes, as seen in diabetes; foetal growth restriction; and chronic umbilical cord obstruction [14, 15]. Therefore, in subsequent pregnancies, delayed villous maturation should prompt testing for pregestational diabetes in early pregnancy, screening for gestational diabetes in the second trimester, serial ultrasound for foetal growth and amniotic fluid, and consideration of delivery before 40 weeks [15, 31].
If maternal placental perfusion or foetal circulation is affected, the placenta strives to modulate the effects of underlying disease. In a large cohort of term infants developing cerebral palsy, severe foetoplacental large-vessel lesions have profound effects on foetoplacental physiology and can be associated with the release of inflammatory mediators into the foetal circulation in 34% of patients [32]. Chronic processes that decrease the placental reserve are maternal vascular malperfusion, high-grade chronic villitis, increased perivillous fibrin deposition, chronic abruption, and distal villous immaturity; these are found in 23% of patients [32]. The placental indicators of protracted foetal hypoxia are increased circulating nucleated red blood cells and villous chorangiosis, which are present in 15% of patients [32]. In birth trauma or failed assisted vaginal delivery, placental findings may be lacking [32]. Multiple placental lesions are particularly important; in one study, 63% of patients had clinical or pathologic evidence of umbilical cord compromise [32]. Foetal vascular malperfusion, which is clinically correlated with chronic partial/intermittent umbilical cord obstruction due to hypercoiling, stricture, abnormal placental insertion sites, and long-standing foetal entanglements, has been associated with CNS injury [33, 34]. The histological features are venous dilatation, mural fibrin deposition of larger foetoplacental veins, and scattered foci of avascular villi, indicative of poor circulation of the distal villi. Extensive avascular villi have been termed foetal thrombotic vasculopathy and have been found to be associated with CNS injury and other adverse outcomes [14, 15] (figures 1 and 2).
Organisms present within the placental membranes may cause an inflammatory response called chorioamnionitis. This generally occurs during the second and third trimesters of gestation and constitutes the most common trigger of premature birth [35] [36]. The microbiological organisms involved are typically bacteria from the gastrointestinal and genitourinary tract, leading to an ascending infection [37–39]. The inflammatory response originates from both the mother and the foetus and is typically predominantly composed of neutrophil granulocytes.
The maternal inflammatory response leads to the migration of neutrophilic granulocytes from the intervillosum towards the chorionic plate and forms the earliest detectable inflammatory response in the form of subchorionitis. The ensuing migration towards the amniotic stroma forms the full picture of chorioamnionitis. The formation of subchorionic abscesses has been associated with adverse outcomes [40].
The foetal inflammatory response constitutes granulocyte infiltration of foetal vessels in the chorionic plate and umbilical cord [36]. Levels of circulating foetal interleukin 6 are found in inflammatory response with umbilical arteritis [41].
Microbiologic exams include a swab from the placenta in all premature children and cases of prolonged membrane rupture. The swabs are typically taken by obstetricians and should be obtained from both the maternal and the foetal sides of the membranes. For the neonatologist, atypical microbiological organisms (e.g. chlamydia) are especially relevant for further therapeutic decisions regarding the appropriate antibiotic therapy. In addition to bacterial ascending infection, haematogenous viral infections such as CMV, Zika, or COVID placentitis may arise.
CMV placentitis is characterised by marked chronic lymphohistiocytic villitis with a prominence of plasma cells and the deposition of haemosiderin in the villous stroma. Several endothelial cells might show typical cytomorphologic changes (“cytomegaly”). CMV infection is a leading cause of congenital deafness; therefore, detecting the transmission of CMV to the child in cases of CMV placentitis might help preserve the hearing capability of the infected child if they are adequately treated for CMV.
Parvovirus B19 affects the foetal erythroid cells leading to severe anaemia and hydrops fetalis. In the placenta, Parvovirus B19-infected cells appear as enlarged cells with ground glass nuclear inclusions. CMV diagnosis can be confirmed by immunohistochemical stains. Chronic villitis might also be present.
In Toxoplasma gondii infection, cysts containing the tachyzoites might be found in the subamnionic or subchorionic tissue and beneath the surface of the umbilical cord. In case of ruptured cysts, a granulomatous reaction may occur.
In the first waves of COVID-19 in 2020, most reported findings were non-specific findings, such as signs of maternal and foetal malperfusion or growth retardation; however, in 2021, several authors reported on intrauterine foetal demise (IUFD) in the wave of the Delta variant, showing a triad of prominent histiocytic intervillositis accompanied by extensive necrosis of the syncytiotrophoblast and fibrin deposition. Further studies showed that SARS-CoV-2 could be detected in syncytotrophoblastic and cytotrophoblastic cells, the villous stroma, and possibly Hofbauer cells [42]. IUFD could be correlated with these findings on the basis of acute placental insufficiency. Transmission of SARS-CoV-2 to the child has not been reported in most cases. Interestingly, in the wave of the Omicron variant, to date, no more cases of SARS-CoV-2-related placentitis have been reported [43, 44].
Chronic histiocytic intervillositis is rare, but it may recur in 75–90% of subsequent pregnancies [45] [46]. Similarly, massive perivillous fibrin deposition or maternal floor infarction may recur in 40–60% of subsequent pregnancies [15]. Commonly, high-grade villitis of unknown aetiology (25–50%), placenta accreta (25–30%), severe maternal malperfusion (10–25%), and spontaneous preterm birth with chorioamnionitis (10-25%) carry a significant risk of recurrence in subsequent pregnancies [47–50].
Foetal growth restriction (also called intrauterine growth restriction) means that a foetus was unable to reach its genetic growth potential because of interfering factors during pregnancy.
Foetal growth restriction has various definitions that vary between countries. To reach a consensus, the results of a Delphi process were published in 2016. The complexity of the phenomenon of foetal growth restriction is expressed in the mention of several foetal growth parameters as well as functional parameters (perfusion of the umbilical arteries) [51]. Foetal growth restriction is characterised by an increasing drop in sonographic growth parameters, especially the abdominal circumference, along with a foetal estimated weight below the expected values in serial measurements from early pregnancy. This observation is diagnostically more important than falling below a certain percentile (e.g. the 10th or 3rd percentile), as defined in various guidelines for the small-for-gestational-age (SGA) foetus or SGA newborn. This means that foetal growth restriction can also be present in a “normal weight” newborn. Conversely, an SGA newborn may have exhausted its genetic growth potential. Early-onset foetal growth restriction (<32 w) is distinguished from late-onset foetal growth restriction (≥32 w), with some authors defining the two ranges as ≤34 weeks vs. >34 weeks or <34 weeks vs. ≥34 weeks [52, 53].
Foetal growth restriction can be caused by maternal, foetal, or “genuinely” placental factors. Different factors may occur simultaneously.Descriptions of placental causes of foetal growth restriction in the literature are characterised by varying categorisations of findings by pathologists, such as vascular, macroscopic, or microscopic and congenital, acquired, or secondary abnormalities [53]. In turn, the assignment of “typical” histologic findings to specific clinical images is compromised because pathologists are typically informed about the clinical situation (i.e. not blinded) and most study designs are retrospective (based on case-series rather than case-control data) [12, 53].
Foetal growth restriction of any severity is an indication for placental examination. Many newborns with (intrauterine) foetal growth restriction come from pregnancies with pathologies that per se constitute an indication for placental examination, such as prematurity, maternal or other foetal pathology, or macroscopic abnormality of the placenta.
If the newborn’s weight is ≥10th (≥3rd) percentile (see table S1 in the appendix), foetal growth restriction cases that were not conspicuous antepartum are likely to remain undetected and, in the absence of any other indication, do not lead to placental examination.
The pathophysiologic processes in the placenta in foetal growth restriction are the result of complex trophoblast dysfunction. Trophoblasts in foetal growth restriction placentas exhibit reduced proliferation, increased apoptotic death, altered metabolism, senescence, and impaired invasive capacity. These cell-level changes underlie the gross anatomical changes seen in the foetal growth restriction, such as the deficient remodelling of the uterine spiral arteries supplying the placenta during early pregnancy [53, 54].
In cases of foetal growth restriction, the placenta shows a reduction in volume, surface area, and vascularisation of the intermediate and terminal villi [53]. Typically, placental weight and birth weight are highly correlated. Sonographic imaging in the first and the second trimester demonstrating a small placenta has predictive value regarding the development of foetal growth restriction. However, imaging using MRI in the third trimester may present foetal growth restriction placentas with a thickened globular appearance as opposed to the typical flattened disc seen in normal pregnancy, and the severity of growth restriction is significantly correlated with the percentage of placental volume affected by this morphology (literature cited by [54]).
Abnormal placental shapes (such as extrachorial or bilobate placentas) are associated with foetal growth restriction. Placental location on the lateral wall also carries a risk of foetal growth restriction up to four times higher compared with placental location on the anterior or posterior wall, but the data are conflicting [53].
Isolated small thromboses and infarcts may be found in placentas of uncomplicated pregnancies. Larger infarcts, often associated with intervillous thromboses and extensive fibrin deposition, are found in most pregnancies complicated by pre-eclampsia and foetal growth restriction. The frequently observed macroscopic vascular anomalies (lesions) in foetal growth restriction placentas are listed in table 4.
Table 4Pathophysiology and prenatal diagnosis of placental macroscopic vascular anomalies found in cases of foetal growth restriction [53].
| Type of anomaly | Pathophysiology | Prenatal ultrasound imaging |
| Intervillous thrombosis | Focal coagulation of maternal blood inside the intervillous space | Echogenic cystic lesions or hypoechoic areas on ultrasound |
| Breus’ mole | Extensive subchorial thrombosis involving at least 50% of the chorionic plate | Large echogenic lesions under the foetal placental plate |
| Infarcts | Villous necrosis due to obstruction of the uteroplacental artery | Complex echogenic intraplacental masses close to the basal plate |
| Maternal floor infarction | Lesion combining parabasal villous necrosis, fibrin deposition, thrombosis, and haematoma | Diffuse hyperechogenic lesions increasing with advancing gestation |
Many different microscopic placental lesions have been described in pregnancies complicated by foetal growth restriction (table 5). Most are non-specific and have been found in villous tissue from uncomplicated pregnancies, and the terminology used to describe them is highly variable. The distribution of these lesions depends on whether the restricted foetal growth is isolated or associated with pre-eclampsia; additionally, the distribution depends on gestational age at onset, with late onset leading to a more heterogeneous group with less characteristic histological changes [53].
Table 5Pathophysiology of placental microscopic lesions found in cases of foetal growth restriction [53].
| Type of lesion | Pathophysiology |
| Villous developmental defects (hypoplasia, dysmaturity, or capillary dysplasia) | Malperfusion of the intervillous space by maternal blood |
| Atherosis of spiral arteries | Failure of spiral artery remodelling in the placental basal plate |
| Villitis of unknown aetiology | Oxidative stress secondary to ischemia-reperfusion of the intervillous space |
Foetal growth restriction has been associated with abnormalities of the umbilical cord insertion, which in turn are often associated with abnormalities in placental shape. The absence of one of two umbilical arteries (SUA) is associated with foetal growth restriction; however, the strength of the association is controversial [53].
Typically, the dysregulation of maternal glucose homeostasis first appears during pregnancy. However, diabetes type 1 and 2 (T1DM and T2DM) also occur in pregnant women, which means that the diabetes was already present before the onset of pregnancy (pregestational diabetes). Gestational diabetes mellitus (GDM) is considered transient insulin resistance most likely due to pregnancy hormones and resolves after delivery; however, the risk of developing diabetes type 2 after pregnancy is elevated in these women [55].
In contrast to pregestational diabetes, gestational diabetes mellitus is not associated with an increased risk of birth defects. Rather, a possible weak association is attributable to overweight and obesity or unrecognised pregestational diabetes [56].
Gestational diabetes mellitus is associated with foetal and neonatal complications, such as macrosomia and hypoglycaemia, but also maternal complications, such as hypertension, pre-eclampsia, and an increased risk of caesarean delivery. Existing placental histomorphology studies of maternal diabetes present varied and inconsistent findings regarding placental abnormalities [57]. However, the changes most often described in gestational diabetes mellitus are increases in placental weight, the frequency of immature villi, the mean number of redundant connections preterminal villi, the volume of parenchymal tissue, and the incidence of fibrinoid necrosis and chorangiosis [58–65]. When comparing pregestational diabetes (T1DM) with gestational diabetes mellitus, similar changes in the studied parameters of placental villi in the two conditions were observed, but the deviation of the morphometric parameters of placental villi was most pronounced in T1DM. The area and perimeter of the villi were reduced by 17% and 12%, respectively, in women with T1DM compared with 15% and 8%, respectively, in women with gestational diabetes mellitus [66]. In addition, placentas from women with T2DM had higher rates of decidual vasculopathy than those from women with gestational diabetes mellituswhen women with pre-eclampsia and diffuse chorangiosis were excluded, but they showed a lower rate of villous immaturity after full adjustment, indicating already-chronic damage of the maternal microvasculature in mothers with T2DM [67]. Interestingly, the correction of hyperglycaemia does not protect against placental abnormalities; several studies have shown that placental histopathologic changes exist even in pregnant women with well-controlled diabetes [58, 64, 68].
In summary, diabetes mellitus in pregnant women alters placental morphology, and morphological differences between different types of diabetes seem to exist, although the results of existing studies are not consistent.
A normally shaped placenta consists of a roundish disk-like organ with central or paracentral umbilical cord insertion. Abnormal placental shapes include placental lobation and elongation as well as peripheral, marginal, and membranous umbilical cord insertion. Placental shape anomalies are thought to result from disturbed development with a potential predisposition to preterm birth, foetal growth restriction, or adult cardiovascular disease [14, 69]. Accessory lobes are defined by entirely separate placental tissue foci within membranes. Placenta lobata denotes a placenta with over 50% septal incision. An elongated placenta is longer than broad, but the parameters are not yet clearly defined [14]. Peripheral umbilical cord insertion is defined as insertion less than three centimetres from the placental margin, whereas a marginal insertion is within two centimetres. Pathogenetically, currently, aberrant vasculogenesis of major chorionic vessels is implicated and correlated with adverse outcomes [70, 71].
The term placenta accreta has heterogeneous definitions [72]. Pathologists have differentiated three subtypes [73]. The most frequent form, placenta accreta, represents 75% of all cases and is defined as a lack of a decidua and direct contact between the chorion villi and the myometrium. Placenta increta is defined as an invasion or extension of the chorionic villi into the myometrium and represents approximately 18% of all reported cases. Placenta percreta is defined as a complete penetration of the myometrium and serosa. This form represents only 7% of cases. Abnormally invasive placentation is not due to the further invasion of extravillous trophoblast into the uterine wall; it likely arises due to scar dehiscence, allowing the development of chorionic villi deep within the uterine wall, including within its peripheral circulation [57]. The classification depends highly on the pathologist’s interpretation, the received specimens, and the sections taken [74]. In the instance of suspicion of placenta accreta, an average of five sections of basal plate are routinely taken from the placenta. Milder forms, in which only muscular fibres are involved, are termed basal plate myometrium (BPMYO) and represent clinical occult placenta accreta; alternatively, they might be a marker of previous abnormal placentation [75]. Placenta accreta has also been subdivided into total, partial, and focal, depending on the amount of placental tissue involved [76].
However, it is not known which threshold amount and/or depth of myometrial invasion must be reached to increase the risk of subsequent placenta accreta [74].
The recurrence rate of placenta accreta is as high as 18–28% [48, 77]. Results concerning the morbidity in subsequent pregnancies are inconsistent. Vinogard et al. described a history of previous placenta accreta as an independent risk factor for postpartum haemorrhage even without placenta accreta in the current pregnancy (adj. OR = 4.1, 95% CI 1.5–11.5). Additional risk factors were placenta accreta (adj. OR = 22.0, 95% CI 14.0–36.0) and placenta praevia (adj. OR = 7.6, 95% CI 4.4–13.2) in the current pregnancy. Interestingly, they described a reduced risk of pre-eclampsia with a history of placenta accreta (RR 0.51, 95% CI 0.26–0.98).
The presence of basal plate myometrium in a previous pregnancy is associated with an increased risk of a morbidly adherent placenta (MAP) in subsequent pregnancies; 76% of women with an MAP had basal plate myometrium in a previous pregnancy. However, basal plate myometrium was detected in 40% of women without an MAP. The histological finding of basal plate myometrium from previous placenta accreta in the context of clinical data increases the positive predictive value of MAP in the subsequent pregnancy by up to 85% [74].
In a retrospective study, Roeca et al. suggested that the risk for major morbidity after a prior pathologically diagnosed placenta accreta depends on the clinical context; 29% of women who had a placenta accreta and suffered from any morbidity during their index pregnancy had a major morbidity in the subsequent pregnancy [78].
No morbidities have been reported in patients in whom the index pregnancy had placenta accreta without any clinical signs, although careful assessment and management of these patients are warranted. Moreover, even a simple history of placenta accreta without recurrent disease is associated with an increased risk of obstetric complications in future pregnancies [76].
This article illustrates the consequences and possibilities of placental pathologic investigation. Table S1 in the appendix was created to serve as a short reminder for daily work in the labour and delivery room regarding when to send the placenta for histopathological evaluation. Many pathologic conditions of both the mother and the foetus can be addressed with modern therapies. They are associated with a specific histologic pattern of the placenta, which can be interpreted with higher accuracy. These morphologic changes can also be correlated with the clinical context, which further results in a more targeted therapy because the underlying cause is evident. This also has prognostic and predictive implications and the potential for more effective and faster clinical management, including risk stratification and further investigations.
There is a need for standardised guidelines and reproducible nomenclature are needed, as summarised in this review, serving as a powerful tool for clinicians to act in a reasonable and personalised way that prioritises the patients.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Langston C, Kaplan C, Macpherson T, Manci E, Peevy K, Clark B, et al. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med. 1997 May;121(5):449–76.
2. Roberts DJ, Baergen RN, Boyd TK, Carreon CK, Duncan VE, Ernst LM, et al. Criteria for placental examination for obstetrical and neonatal providers. Am J Obstet Gynecol. 2023 May;228(5):497–508.e4. doi: https://doi.org/10.1016/j.ajog.2022.12.017
3. S2k-Leitlinie Pathomorphologische Untersuchung der Plazenta. 2022. AWMF Registriernummer 035/005. available from: https://register.awmf.org/de/leitlinien/detail/035-005
4. Evans, C., et al., Tissue pathway for histological examination of the placenta. The Royal college of pathologists. G 108, 2022.
5. Polnaszek BE, Clark SL, Rouse DJ. Pathologic Assessment of the Placenta: Evidence Compared With Tradition. Obstet Gynecol. 2022 Apr;139(4):660–7. doi: https://doi.org/10.1097/AOG.0000000000004719
6. Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016 Jul;140(7):698–713. doi: https://doi.org/10.5858/arpa.2015-0225-CC
7. Slack JC, Parra-Herran C. Life After Amsterdam: Placental Pathology Consensus Recommendations and Beyond. Surg Pathol Clin. 2022 Jun;15(2):175–96. doi: https://doi.org/10.1016/j.path.2022.02.001
8. Zhou YY, Ravishankar S, Luo G, Redline RW. Predictors of High Grade and Other Clinically Significant Placental Findings by Indication for Submission in Singleton Placentas From Term Births. Pediatr Dev Pathol. 2020 Aug;23(4):274–84. doi: https://doi.org/10.1177/1093526620904801
9. Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014 May;35(5):303–4. doi: https://doi.org/10.1016/j.placenta.2014.02.012
10. Roberts JM, Hansson SR, Vaiman D, Redman CW; Global Pregnancy Collaboration. Global Pregnancy Collaboration symposium on placental health: summary and recommendations. Placenta. 2017 Apr;52:116–21. doi: https://doi.org/10.1016/j.placenta.2017.01.115
11. Odibo I, Gehlot A, Ounpraseuth ST, Magann EF. Pathologic examination of the placenta and its clinical utility: a survey of obstetrics and gynecology providers. J Matern Fetal Neonatal Med. 2016;29(2):197–201. doi: https://doi.org/10.3109/14767058.2014.998192
12. Sebire NJ. Implications of placental pathology for disease mechanisms; methods, issues and future approaches. Placenta. 2017 Apr;52:122–6. doi: https://doi.org/10.1016/j.placenta.2016.05.006
13. Redline RW, Roberts DJ, Parast MM, Ernst LM, Morgan TK, Greene MF, et al. Placental pathology is necessary to understand common pregnancy complications and achieve an improved taxonomy of obstetrical disease. Am J Obstet Gynecol. 2023 Feb;228(2):187–202. doi: https://doi.org/10.1016/j.ajog.2022.08.010
14. Redline RW. The clinical implications of placental diagnoses. Semin Perinatol. 2015 Feb;39(1):2–8. doi: https://doi.org/10.1053/j.semperi.2014.10.002
15. Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S21–8. doi: https://doi.org/10.1016/j.ajog.2015.05.056
16. Gabriel H, Korinth D, Ritthaler M, Schulte B, Battke F, von Kaisenberg C, et al. Trio exome sequencing is highly relevant in prenatal diagnostics. Prenat Diagn. 2022 Jun;42(7):845–51. doi: https://doi.org/10.1002/pd.6081
17. Redline RW. Placental pathology: is it time to get serious? Contemp Ob Gyn. 2014;59(2):41–8.
18. Khong TY. Evidence-based pathology: umbilical cord coiling. Pathology. 2010 Dec;42(7):618–22. doi: https://doi.org/10.3109/00313025.2010.520309
19. Cromb D, et al. Clinical value of placental examination for paediatricians. Arch Dis Child Fetal Neonatal Ed. 2023.
20. de Laat MW, Franx A, van Alderen ED, Nikkels PG, Visser GH. The umbilical coiling index, a review of the literature. J Matern Fetal Neonatal Med. 2005 Feb;17(2):93–100. doi: https://doi.org/10.1080/jmf.17.2.93.100
21. Dirnhofer S, B.L., Lehr HA, Landau B, Zenklusen HR, Qualitätsrichtlinien SGPath. 2011.
22. Avagliano L, Locatelli A, Danti L, Felis S, Mecacci F, Bulfamante GP. Placental histology in clinically unexpected severe fetal acidemia at term. Early Hum Dev. 2015 May;91(5):339–43. doi: https://doi.org/10.1016/j.earlhumdev.2015.03.004
23. Chen A, Roberts DJ. Placental pathologic lesions with a significant recurrence risk - what not to miss! APMIS. 2018 Jul;126(7):589–601. doi: https://doi.org/10.1111/apm.12796
24. Kaspar HG, Abu-Musa A, Hannoun A, Seoud M, Shammas M, Usta I, et al. The placenta in meconium staining: lesions and early neonatal outcome. Clin Exp Obstet Gynecol. 2000;27(1):63–6.
25. Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011 Aug;118(2 Pt 1):323–33. doi: https://doi.org/10.1097/AOG.0b013e3182255999
26. Abdulghani S, Moretti F, Gruslin A, Grynspan D. Recurrent Massive Perivillous Fibrin Deposition and Chronic Intervillositis Treated With Heparin and Intravenous Immunoglobulin: A Case Report. J Obstet Gynaecol Can. 2017 Aug;39(8):676–81. doi: https://doi.org/10.1016/j.jogc.2017.03.089
27. Dubruc E, Lebreton F, Giannoli C, Rabilloud M, Huissoud C, Devouassoux-Shisheboran M, et al. Placental histological lesions in fetal and neonatal alloimmune thrombocytopenia: A retrospective cohort study of 21 cases. Placenta. 2016 Dec;48:104–9. doi: https://doi.org/10.1016/j.placenta.2016.10.009
28. Nedberg NH, Turowski G, Guz K, Przytuła E, Uhrynowska M, Roald B, et al. Platelet alloimmunization is associated with low grade chronic histiocytic intervillositis - A new link to a rare placental lesion? Placenta. 2021 Sep;112:89–96. doi: https://doi.org/10.1016/j.placenta.2021.07.291
29. Romero R, Whitten A, Korzeniewski SJ, Than NG, Chaemsaithong P, Miranda J, et al. Maternal floor infarction/massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol. 2013 Oct;70(4):285–98. doi: https://doi.org/10.1111/aji.12143
30. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005 Feb;192(2):452–7. doi: https://doi.org/10.1016/j.ajog.2004.07.030
31. Hösli I, et al. Gestationsdiabetes. Schweiz Arzteztg. 2023;103(38):88–90.
32. Redline RW. Disorders of placental circulation and the fetal brain. Clin Perinatol. 2009 Sep;36(3):549–59. doi: https://doi.org/10.1016/j.clp.2009.06.003
33. Clapp JF 3rd, Lopez B, Simonean S. Nuchal cord and neurodevelopmental performance at 1 year. J Soc Gynecol Investig. 1999;6(5):268–72.
34. Myers RE. Fetal asphyxia due to umbilical cord compression. Metabolic and brain pathologic consequences. Biol Neonate. 1975;26(1-2):21–43. doi: https://doi.org/10.1159/000240714
35. Guzick DS, Winn K. The association of chorioamnionitis with preterm delivery. Obstet Gynecol. 1985 Jan;65(1):11–6.
36. Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012 Feb;17(1):20–5. doi: https://doi.org/10.1016/j.siny.2011.08.003
37. Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O’shea TM, et al. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res. 2010 Jan;67(1):95–101. doi: https://doi.org/10.1203/PDR.0b013e3181bf5fab
38. Blanc WA. Pathology of the placenta and cord in ascending and in haematogenous infection. Ciba Found Symp. 1979;(77):17–38.
39. Kraus FT. R.R., Gersell DJ, Nelson DM, Dicke JM., Placental Pathology. Washington (DC): American Registry of Pathology; 2004.
40. Keenan WJ, Steichen JJ, Mahmood K, Altshuler G. Placental pathology compared with clinical outcome: a retrospective blind review. Am J Dis Child. 1977 Nov;131(11):1224–7. doi: https://doi.org/10.1001/archpedi.1977.02120240042009
41. Rogers BB, Alexander JM, Head J, McIntire D, Leveno KJ. Umbilical vein interleukin-6 levels correlate with the severity of placental inflammation and gestational age. Hum Pathol. 2002 Mar;33(3):335–40. doi: https://doi.org/10.1053/hupa.2002.32214
42. Fahmi A, Brügger M, Démoulins T, Zumkehr B, Oliveira Esteves BI, Bracher L, et al. SARS-CoV-2 can infect and propagate in human placenta explants. Cell Rep Med. 2021 Dec;2(12):100456. doi: https://doi.org/10.1016/j.xcrm.2021.100456
43. Stenton S, McPartland J, Shukla R, Turner K, Marton T, Hargitai B, et al. SARS-COV2 placentitis and pregnancy outcome: A multicentre experience during the Alpha and early Delta waves of coronavirus pandemic in England. EClinicalMedicine. 2022 May;47:101389. doi: https://doi.org/10.1016/j.eclinm.2022.101389
44. Schwartz DA, Avvad-Portari E, Babál P, Baldewijns M, Blomberg M, Bouachba A, et al. Placental Tissue Destruction and Insufficiency From COVID-19 Causes Stillbirth and Neonatal Death From Hypoxic-Ischemic Injury. Arch Pathol Lab Med. 2022 Jun;146(6):660–76. doi: https://doi.org/10.5858/arpa.2022-0029-SA
45. Boyd TK, Redline RW. Chronic histiocytic intervillositis: a placental lesion associated with recurrent reproductive loss. Hum Pathol. 2000 Nov;31(11):1389–96. doi: https://doi.org/10.1016/S0046-8177(00)80009-X
46. Bos M, Harris-Mostert ET, van der Meeren LE, Baelde JJ, Williams DJ, Nikkels PG, et al. Clinical outcomes in chronic intervillositis of unknown etiology. Placenta. 2020 Feb;91:19–23. doi: https://doi.org/10.1016/j.placenta.2020.01.001
47. Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007 Oct;38(10):1439–46. doi: https://doi.org/10.1016/j.humpath.2007.05.025
48. Sentilhes L, Kayem G, Ambroselli C, Provansal M, Fernandez H, Perrotin F, et al. Fertility and pregnancy outcomes following conservative treatment for placenta accreta. Hum Reprod. 2010 Nov;25(11):2803–10. doi: https://doi.org/10.1093/humrep/deq239
49. Lausman A, McCarthy FP, Walker M, Kingdom J. Screening, diagnosis, and management of intrauterine growth restriction. J Obstet Gynaecol Can. 2012 Jan;34(1):17–28. doi: https://doi.org/10.1016/S1701-2163(16)35129-5
50. Himes KP, Simhan HN. Risk of recurrent preterm birth and placental pathology. Obstet Gynecol. 2008 Jul;112(1):121–6. doi: https://doi.org/10.1097/AOG.0b013e318179f024
51. Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016 Sep;48(3):333–9. doi: https://doi.org/10.1002/uog.15884
52. DGGG, O., SGGG, Intrauterine growth restriction. Guideline of the German Society of Gynecology and Obstetrics. 2016.
53. Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018 Feb;218(2S 2s):S745–61. doi: https://doi.org/10.1016/j.ajog.2017.11.577
54. Sun C, Groom KM, Oyston C, Chamley LW, Clark AR, James JL. The placenta in fetal growth restriction: what is going wrong? Placenta. 2020 Jul;96:10–8. doi: https://doi.org/10.1016/j.placenta.2020.05.003
55. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020 May;369:m1361. doi: https://doi.org/10.1136/bmj.m1361
56. Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008 Sep;199(3):237.e1–9. doi: https://doi.org/10.1016/j.ajog.2008.06.028
57. Parra-Herran C, Djordjevic B. Histopathology of Placenta Creta: Chorionic Villi Intrusion into Myometrial Vascular Spaces and Extravillous Trophoblast Proliferation are Frequent and Specific Findings With Implications for Diagnosis and Pathogenesis. Int J Gynecol Pathol. 2016 Nov;35(6):497–508. doi: https://doi.org/10.1097/PGP.0000000000000250
58. Evers IM, Nikkels PG, Sikkema JM, Visser GH. Placental pathology in women with type 1 diabetes and in a control group with normal and large-for-gestational-age infants. Placenta. 2003;24(8-9):819–25. doi: https://doi.org/10.1016/S0143-4004(03)00128-0
59. Asmussen I. Ultrastructure of the villi and fetal capillaries of the placentas delivered by non-smoking diabetic women (White group D). Acta Pathol Microbiol Immunol Scand [A]. 1982 Mar;90(2):95–101. doi: https://doi.org/10.1111/j.1699-0463.1982.tb00069_90A.x
60. Björk O, Persson B. Placental changes in relation to the degree of metabolic control in diabetes mellitus. Placenta. 1982;3(4):367–78. doi: https://doi.org/10.1016/S0143-4004(82)80030-1
61. Teasdale F. Histomorphometry of the human placenta in Class B diabetes mellitus. Placenta. 1983;4(1):1–12. doi: https://doi.org/10.1016/S0143-4004(83)80012-5
62. Jauniaux E, Burton GJ. Villous histomorphometry and placental bed biopsy investigation in Type I diabetic pregnancies. Placenta. 2006;27(4-5):468–74. doi: https://doi.org/10.1016/j.placenta.2005.04.010
63. Nelson SM, Coan PM, Burton GJ, Lindsay RS. Placental structure in type 1 diabetes: relation to fetal insulin, leptin, and IGF-I. Diabetes. 2009 Nov;58(11):2634–41. doi: https://doi.org/10.2337/db09-0739
64. Daskalakis G, Marinopoulos S, Krielesi V, Papapanagiotou A, Papantoniou N, Mesogitis S, et al. Placental pathology in women with gestational diabetes. Acta Obstet Gynecol Scand. 2008;87(4):403–7. doi: https://doi.org/10.1080/00016340801908783
65. Huynh J, Dawson D, Roberts D, Bentley-Lewis R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta. 2015 Feb;36(2):101–14. doi: https://doi.org/10.1016/j.placenta.2014.11.021
66. Dubova EA, Pavlov KA, Yesayan RM, Nagovitsyna MN, Tkacheva ON, Shestakova MV, et al. Morphometric characteristics of placental villi in pregnant women with diabetes. Bull Exp Biol Med. 2011 Sep;151(5):650–4. doi: https://doi.org/10.1007/s10517-011-1406-9
67. Huynh J, Yamada J, Beauharnais C, Wenger JB, Thadhani RI, Wexler D, et al. Type 1, type 2 and gestational diabetes mellitus differentially impact placental pathologic characteristics of uteroplacental malperfusion. Placenta. 2015 Oct;36(10):1161–6. doi: https://doi.org/10.1016/j.placenta.2015.08.004
68. Makhseed M, Musini VM, Ahmed MA, Al-Harmi J. Placental pathology in relation to the White’s classification of diabetes mellitus. Arch Gynecol Obstet. 2002 Jul;266(3):136–40. doi: https://doi.org/10.1007/s004040100232
69. Alwasel SH, Abotalib Z, Aljarallah JS, Osmond C, Al Omar SY, Harrath A, et al. The breadth of the placental surface but not the length is associated with body size at birth. Placenta. 2012 Aug;33(8):619–22. doi: https://doi.org/10.1016/j.placenta.2012.04.015
70. Yampolsky M, Salafia CM, Shlakhter O, Haas D, Eucker B, Thorp J. Centrality of the umbilical cord insertion in a human placenta influences the placental efficiency. Placenta. 2009 Dec;30(12):1058–64. doi: https://doi.org/10.1016/j.placenta.2009.10.001
71. Schwartz N, Mandel D, Shlakhter O, Coletta J, Pessel C, Timor-Tritsch IE, et al. Placental morphologic features and chorionic surface vasculature at term are highly correlated with 3-dimensional sonographic measurements at 11 to 14 weeks. J Ultrasound Med. 2011 Sep;30(9):1171–8. doi: https://doi.org/10.7863/jum.2011.30.9.1171
72. Hecht JL, Baergen R, Ernst LM, Katzman PJ, Jacques SM, Jauniaux E, et al. Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: recommendations from an expert panel. Mod Pathol. 2020 Dec;33(12):2382–96. doi: https://doi.org/10.1038/s41379-020-0569-1
73. Benirschke K. B.G., Baergen RN., Pathology of the human placenta. 6th edition. 2012.
74. Linn RL, Miller ES, Lim G, Ernst LM. Adherent basal plate myometrial fibers in the delivered placenta as a risk factor for development of subsequent placenta accreta. Placenta. 2015 Dec;36(12):1419–24. doi: https://doi.org/10.1016/j.placenta.2015.10.004
75. Miller ES, Linn RL, Ernst LM. Does the presence of placental basal plate myometrial fibres increase the risk of subsequent morbidly adherent placenta: a case-control study. BJOG. 2016 Dec;123(13):2140–5. doi: https://doi.org/10.1111/1471-0528.13579
76. Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012 Apr;33(4):244–51. doi: https://doi.org/10.1016/j.placenta.2011.11.010
77. Vinograd A, Wainstock T, Mazor M, Mastrolia SA, Beer-Weisel R, Klaitman V, et al. A prior placenta accreta is an independent risk factor for post-partum hemorrhage in subsequent gestations. Eur J Obstet Gynecol Reprod Biol. 2015 Apr;187:20–4. doi: https://doi.org/10.1016/j.ejogrb.2015.01.014
78. Roeca C, Little SE, Carusi DA. Pathologically Diagnosed Placenta Accreta and Hemorrhagic Morbidity in a Subsequent Pregnancy. Obstet Gynecol. 2017 Feb;129(2):321–6. doi: https://doi.org/10.1097/AOG.0000000000001843
79. Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2018 Feb;218(2S 2s):S803–17. doi: https://doi.org/10.1016/j.ajog.2017.11.575
80. Thompson JM, Irgens LM, Skjaerven R, Rasmussen S. Placenta weight percentile curves for singleton deliveries. BJOG. 2007 Jun;114(6):715–20.
81. Vogel M, Turowski G, eds. Clinical Pathology of the Placenta. Berlin: De Gruyter; 2019.
Table S1Recommendations for histopathological examination of the placenta (adapted from [2–4]).
| A: Maternal indications | ||
| Indication | Restrictions/comments | Common underlying placental findings |
| Preterm delivery <37 0/7 weeks gestation | Acute chorioamnionitis, marginal abruption, mild global/partial maternal malperfusion (accelerated maturation) | |
| Systemic disorders, gestational or underlying, with concern for mother or infant (e.g., pregestational or poorly controlled gestational diabetes mellitus, severe hypertensive disorders, autoimmune disorders, collagen disorders, severe anaemia [<90 g/l], malignant diseases) | Malignant diseases: excl. cured malignancies according to clinical definitions/conventions | |
| Unexplained or recurrent pregnancy complications | E.g., late spontaneous abortion (14 0/7 to 21 6/7 weeks gestation1) or early spontaneous abortion | Global/partial maternal vascular malperfusion (accelerated maturation), global/partial fetal vascular malperfusion (umbilical cord accident), abruptio placentae, maternal floor infarction, chronic histiocytic intervillositis |
| Amnion infection syndrome, peripartum fever >38.5 °C | Incl. clinical suspicion of chorioamnionitis | Acute: maternal (chorioamnionitis, subchorionitis) or fetal inflammatory response (chorionic/umbilical vasculitis). – Chronic: villitis (e.g., CMV), intervillositis. – Potential identification of specific agent |
| Abruptio placentae | Incl. suspicion of abruption during delivery | Loss of vascular integrity: abruptio placentae (central, arterial); marginal abruption (venous); chronicity (circumvallate membrane insertion, organizing marginal blood clots, hemosiderin deposition) |
| Excessive uterine bleeding of unknown aetiology during 2nd and 3rd trimester | ||
| Thick or prolonged (viscid) meconium | Meconium-associated changes (meconium phagocytosis as sign of chronic or recurrent hypoxia) delayed villous maturation (maturation defect) | |
| History of maternal substance abuse | If suspected relevance for fetal development | |
| Suspicion of placental injury following invasive procedure | If suspected relevance for fetal development | Hematoma (timing) |
| Maternal abdominal trauma in pregnancy | If suspected relevance for fetal development | Hematoma (timing) |
| Maternal death | ||
| B: Fetal-neonatal indications | ||
| Indication | Restrictions/comments | Common underlying placental findings |
| Stillbirth or neonatal death | Stillbirth ≥22 0/7 weeks gestation | Preterm fetal death: global/partial maternal vascular malperfusion (accelerated maturation), global/partial fetal vascular malperfusion (UC accident), abruptio placentae; term fetal death: abruptio placentae, global/partial fetal vascular malperfusion (UC accident), fetomaternal haemorrhage, delayed villous maturation |
| Fetal growth restriction (FGR) or small for gestational age (SGA) (<10. p.)2 | Global/partial maternal malperfusion (accelerated maturation), chronic villitis of unknown aetiology (VUE), complete/segmental fetal vascular malperfusion (fetal thrombotic vasculopathy), fetal stromal-vascular developmental lesions | |
| Embryo-fetal infection, incl. suspicion of infection TORCH infections, Zika, COVID-19 | Acute: maternal (chorioamnionitis, subchorionitis) or fetal inflammatory response (chorionic/umbilical vasculitis). – Chronic: villitis (e.g., CMV), intervillositis. – Potential identification of specific agent | |
| Hydrops fetalis of unknown aetiology | ||
| Major congenital anomalies | May be omitted if known aneuploidy | |
| Dysmorphic phenotype of unknown aetiology | ||
| Suspicion of diabetic fetopathy | Independent of birthweight and maternal diagnosis of DM or GDM; incl. distribution of body fat, face, repeated hypoglycaemia, polyglobulia | |
| Haemolytic disease due to maternal alloimmunisation | May be omitted in mild disease manifestation during pregnancy and early postpartum | |
| Admission to NICU | ||
| Compromised clinical condition e.g., non-reassuring fetal heart rate requiring urgent or immediate delivery | pH umbilical artery <7.0, Apgar score ≤6 at 5 min or ventilatory assistance >10 min | In case of antepartum hypoxemic episodes: possible strongly clotting blood and/or impression of basal plate; maternal or fetal stromal-vascular lesions, esp. malperfusion; meconium-associated changes |
| Neonatal haematocrit <35% | Erythroblastosis in fetal vessels | |
| Infection or sepsis | Restricted to 72 h postpartum | |
| Neonatal seizures | ||
| Suspected meconium aspiration syndrome | Meconium-associated changes (e.g., meconium phagocytosis, meconium associated myonecrosis and ulceration of the umbilical cord) | |
| Anomalies not diagnosed antepartum | ||
| Complications associated with multiple gestation, e.g., weight difference >20% (base: larger fetus)3 | Distribution of placental area, feto-fetal vascular anastomoses | |
| Multiple pregnancy with same sex and macroscopically fused placentas | May be omitted if chorionicity was determined by ultrasound antenatally | Chorionicity (confirmation of monozygosity), feto-fetal vascular anastomoses |
| Neonate with known or suspected malignancy | Placental metastases affect prognosis and have the potential to metastasize to the mother | For diagnoses of pathologic causes of adverse outcome, critical values and findings associated with maternal and neonatal long-term morbidity |
| C: Placental indications | ||
| Indication | Restrictions/comments | Common underlying placental findings |
| Unusual findings in any aspect of the placenta gross examination by experienced examiner e.g., abnormal weight of placenta4 | If neonatal pathology present (birth weight <10. or >90. p. or disturbed adaptation) | Small placenta, e.g., maternal vascular malperfusion; large placenta, e.g., oedema of chorionic villi; delayed villous maturation (maturation defect); partial hydatidiform mole; placental mesenchymal dysplasia |
| Structural abnormalities or masses involving the placental disc, umbilical cord or membranes | Cord: incl. thrombosis, abnormal coloration, malodour, single artery, absence of Wharton’s jelly; excl. true and false knots of cord, amniotic band syndrome, accessory lobe, uncomplicated velamentous cord | Abnormal colour of cord, e.g., fungal infection; abnormal colour of placenta (pale): disturbed maturation of villi |
| Morbidly adherent placenta | ||
| History of a placenta with pathology known to recur | ||
| Termination of pregnancy for obstetrical or maternal indications | ||
1 Recommended definition of late abortion: 14 0/7 to 21 6/7 weeks gestation. Limits are not uniform in literature.
2 All charts used in Swiss University Hospitals are accepted.
3 Birth weight discordance: (larger twin weight − smaller twin weight)/larger twin weight × 100 [74]. The ≥ 20% level for defining birth weight discordance agrees with the ACOG-SMFM recommendation [75]. A Delphi procedure consensus recommends a ≥ 25% difference for the sonographically estimated fetal weight [76].
4 Weighing the placenta and measuring the length of the cord are not as standardized in the obstetric department as in a department of pathology after trimming, i.e., cutting the membranes and cord. Results from standardized techniques should be declared as such. If the placental weight is taken as criterion, use of the 10th and 90th percentile of the weight distribution curves is recommended for untrimmed placentas published by [77] and for trimmed placentas published by [78].
Table S2Normal weights of untrimmed placentas (from: Thompson JM, Irgens LM, Skjaerven R, Rasmussen S. Placenta weight percentile curves for singleton deliveries. BJOG. 2007 Jun;114(6):715-20. https://doi.org/10.1111/j.1471-0528.2007.01327.x [80], reprint with permission by the publisher).
| Gestational weeks | Male infants | Female infants | ||||||||
| 3rd p. | 10th p. | 50th p. | 90th p. | 97th p. | 3rd p. | 10th p. | 50th p. | 90th p. | 97th p. | |
| 24 | 150 | 180 | 260 | 380 | 460 | 130 | 160 | 240 | 350 | 400 |
| 25 | 150 | 180 | 270 | 400 | 470 | 130 | 170 | 260 | 370 | 430 |
| 26 | 150 | 190 | 290 | 420 | 490 | 140 | 180 | 270 | 400 | 460 |
| 27 | 160 | 200 | 310 | 450 | 520 | 150 | 190 | 300 | 430 | 490 |
| 28 | 170 | 220 | 340 | 480 | 550 | 170 | 210 | 320 | 460 | 530 |
| 29 | 190 | 240 | 360 | 510 | 590 | 190 | 230 | 350 | 500 | 570 |
| 30 | 210 | 270 | 400 | 550 | 630 | 210 | 260 | 390 | 540 | 610 |
| 31 | 240 | 290 | 430 | 590 | 670 | 240 | 290 | 420 | 570 | 660 |
| 32 | 260 | 320 | 460 | 620 | 710 | 260 | 320 | 450 | 610 | 700 |
| 33 | 290 | 350 | 500 | 660 | 750 | 290 | 350 | 490 | 650 | 740 |
| 34 | 320 | 380 | 530 | 700 | 790 | 320 | 380 | 520 | 690 | 780 |
| 35 | 350 | 410 | 560 | 740 | 830 | 350 | 410 | 560 | 730 | 820 |
| 36 | 370 | 440 | 590 | 770 | 870 | 370 | 440 | 590 | 760 | 860 |
| 37 | 400 | 460 | 620 | 810 | 900 | 400 | 460 | 610 | 800 | 890 |
| 38 | 420 | 490 | 650 | 840 | 930 | 420 | 480 | 640 | 820 | 920 |
| 39 | 440 | 510 | 670 | 860 | 960 | 440 | 500 | 660 | 840 | 950 |
| 40 | 460 | 530 | 690 | 880 | 980 | 460 | 520 | 670 | 860 | 960 |
| 41 | 470 | 540 | 700 | 890 | 990 | 470 | 530 | 680 | 870 | 970 |
| 42 | 480 | 540 | 700 | 900 | 1000 | 470 | 530 | 690 | 870 | 980 |
| 43 | 480 | 540 | 700 | 890 | 1000 | 470 | 530 | 680 | 870 | 980 |
| 44 | 470 | 540 | 690 | 880 | 980 | 460 | 520 | 670 | 860 | 960 |
Table S3Normal weights of trimmed placentas (from: Vogel M, Turowski G. Clinical Pathology of the Placenta. Berlin: De Gruyter; 2019, https://doi.org/10.1515/9783110452600, [81], reprint with permission of the publisher).
| Gestational week | Trimmed placental weight | ||
| 10th p. | 50th p. | 90th p. | |
| 15/16 | 45 | 70 | 115 |
| 17 | 50 | 100 | 125 |
| 18 | 65 | 105 | 155 |
| 19 | 90 | 125 | 160 |
| 20 | 105 | 140 | 165 |
| 21 | 110 | 145 | 215 |
| 22 | 115 | 165 | 230 |
| 23 | 120 | 180 | 240 |
| 24 | 120 | 205 | 250 |
| 25 | 145 | 210 | 300 |
| 26 | 155 | 230 | 300 |
| 27 | 165 | 220 | 305 |
| 28 | 170 | 255 | 345 |
| 29 | 185 | 295 | 350 |
| 30 | 225 | 285 | 375 |
| 31 | 230 | 335 | 420 |
| 32 | 265 | 320 | 400 |
| 33 | 295 | 370 | 465 |
| 34 | 285 | 365 | 490 |
| 35 | 300 | 390 | 495 |
| 36 | 340 | 435 | 555 |
| 37 | 345 | 470 | 550 |
| 38 | 375 | 460 | 605 |
| 39 | 395 | 490 | 620 |
| 40 | 405 | 500 | 625 |
| 41 | 415 | 515 | 650 |
| 42 | 410 | 495 | 625 |