Reimbursement policies of Swiss health insurances
for the surgical treatment of symptomatic breast hypertrophy: a retrospective
cohort study
DOI: https://doi.org/https://doi.org/10.57187/s.3923
Astrid Navarraab*,
Daniel Schmaussabc*,
Reto Wettsteind,
Yves Harderaef
a Department of Plastic, Reconstructive and Aesthetic Surgery,
Ospedale Regionale di Lugano, Ente Ospedaliero Cantonale (EOC), Lugano,
Switzerland
b Università della
Svizzera Italiana, Faculty of Biomedical Sciences, Lugano, Switzerland
c Department
of Plastic Surgery and Hand Surgery, Klinikum rechts der Isar, Technische
Universität München, Munich, Germany
d Department of Plastic, Reconstructive, Aesthetic and Hand Surgery, University Hospital
of Basel, Basel, Switzerland
e Department
of Plastic, Reconstructive, Aesthetic and Hand Surgery, Centre Hospitalier
Universitaire Vaudois (CHUV), Lausanne, Switzerland
f Faculty of
Biology and Medicine, University of Lausanne (UNIL), Lausanne, Switzerland
* contributed equally
Summary
BACKGROUND: Patients
with symptomatic breast hypertrophy typically suffer from chronic back pain,
recurrent skin irritation at the inframammary fold and/or low self-esteem
resulting in impaired quality of life. Reduction mammaplasty has been shown to effectively
treat symptomatic
breast hypertrophy with high patient satisfaction. Despite the obvious benefits, reimbursement
requests for reduction
mammaplasty are initially often refused by the patient’s health insurance
company, thereby frequently resulting in additional examinations and eventually
extra expenses. The study aim was to evaluate the reimbursement policy by health
insurance companies for treatment costs of reduction mammaplasty in a
patient cohort, to quantify the
generation of additional costs due to initial refusal of reimbursement, as well
as to assess back pain after surgical treatment.
METHODS: A
retrospective cohort study was conducted in two Swiss centres. Inclusion
criteria were a diagnosis of symptomatic breast hypertrophy, cost approval for reduction
mammaplasty by the health insurance between October 2014 and March 2021 and
informed consent for the study. The exclusion criteria were private payers for reduction
mammaplasty and patients aged below 18. Primary outcome measures included
median duration between the first request for reimbursement sent to the health
insurance and the receipt of its approval, the number of requests needed per
patient, as well as the number and type of additional outpatient visits
conducted by specialists other than plastic surgeons, including the need for
further diagnostic investigations and therapeutic measures. Secondary outcome
measures included the additional costs generated in patients with more than one
request. Finally, back pain after surgical treatment was assessed using a visual
analogue scale (VAS).
RESULTS: A
total of 46 patients with symptomatic breast hypertrophy and approval for
reimbursement were included in the study. The median duration to obtain cost
approval for reduction mammaplasty was 9.4 weeks (ranging from 1 to 154 weeks).
Reimbursement was approved after 1, 2, 3 or 4 requests in 26, 6, 11 and 3
patients, respectively. If the first request was refused, further clinical
evaluation by specialists, additional imaging of the cervical spine and
physiotherapy was necessary in 70%, 35% and 80% of the patients, respectively. A
patient requiring more than one request to obtain cost approval for reduction
mammaplasty generated additional mean costs of approximately 2400 CHF, i.e. 2181
CHF, 164 CHF and 46 CHF for ongoing physiotherapy, additional outpatient visit
by a specialist doctor and complementary imaging compared to patients needing
only one request for cost approval. The level of back pain could be reduced
from 7.0 before surgery to 1.6 after surgery.
CONCLUSION:
Patients with symptomatic breast hypertrophy who
needed more than one request for cost approval (43%) had to undergo further outpatient
visits and/or radiological examinations, as well as physiotherapy, despite a
clear indication for surgery, resulting in a prolonged symptomatology and
increasing healthcare costs.
Introduction
Symptoms caused by breast hypertrophy are
manifold, yet although not very specific they are at least quite typical and
consistent, including chronic back pain, recurrent tension headache and
stiffness of the neck, shoulder grooving, numbness of the upper extremities,
exercise intolerance, poor posture, including hyperkyphosis of the cervical
spine and anteversion of the shoulders, as well as recurrent skin irritation
ranging from cutaneous rash to superficial skin infections and possibly
ulcerations, typically located in the inframammary fold (figure 1).
Furthermore, symptomatic breast hypertrophy often affects the patient’s psyche,
causing low self-esteem and resulting in limitations of daily life (e.g.
difficulty in dressing appropriately, social isolation, tension in relationship)
[1, 2].
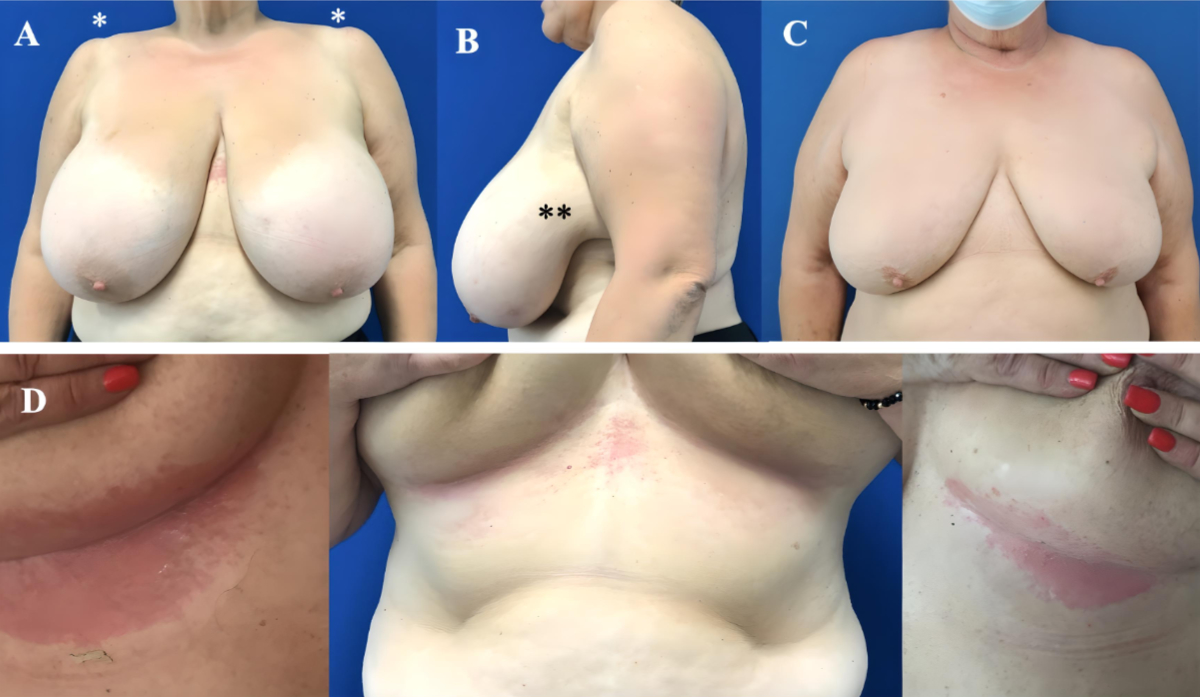
Figure 1(A) Patient with
hypertrophic, ptotic and heavy breasts with more than 1 kg excess breast tissue
per side. Note the bilateral shoulder groove resulting from the bra straps (*).
(B) Lateral adipoglandular tissue excess causing
discomfort (**). (C) Hypertrophic breasts are often associated with ptosis and
overweight of the patient. (D) Typical aspect of recurrent skin irritations at
the inframammary fold.
Nevertheless, reduction
mammaplasty is a commonly performed surgical procedure that may very effectively
achieve relief of symptoms and therefore significantly improve the quality of
life of affected patients [3–8].
Every citizen with residence in Switzerland
is obliged to insure himself by adhering to one of the health insurance
companies (HIC). Optionally, complementary insurance (Zusatzversicherung / assurance
complémentaire / assicurazione complementare) may be taken out to
cover medical treatments not covered by compulsory basic insurance. According
to the Federal Law of Health Insurance (Bundesgesetz über die
Krankenversicherung [KVG] / Loi Fédérale sur l'Assurance-Maladie / Legge
Federale sull'Assicurazione Malattie [LAMal]), health insurance companies
are obliged to reimburse all costs of any procedure required to diagnose or
treat a disease or its sequelae. To be reimbursed, the diagnostic procedure or
treatment has to cure or at least improve the medical state of the patient that
has to be associated with a burden (“value of disease” = Krankheitswert / valeur de maladie / valore di malattia). The examinations
and/or treatment options that are therefore needed can be defined by the
physician who examines the patient and establishes the diagnosis [9].
Social security law in Switzerland
states that any kind of diagnostic procedure or medical treatment offered to a patient
must be
effective, appropriate and economical (so-called WZW criteria: wirksam,
zweckmässig, wirtschaftlich / efficace, adéquat, économique / efficace,
adeguato, economico). If these criteria are fulfilled, the compulsory basic insurance (Grundversicherung / assurance de base / assicurazione di base) is obliged to
reimburse all costs related to the diagnostic procedure and/or medical
treatment.
Typically, patients with symptomatic breast
hypertrophy consult a board-certified plastic surgeon, who indicates reduction
mammaplasty to causally treat symptomatic breast hypertrophy. These patients have
often already undergone physiotherapy to strengthen the paravertebral
musculature and improve posture, recurrent treatment of skin rashes or
infections of the inframammary fold and/or some kind of imaging of the spine.
Yet, reimbursement for reduction mammaplasty is initially often refused by the medical
officer of the health insurance company. This results quite often in
reconsideration of the case, including re-evaluation by the board-certified
plastic surgeon, further investigation by other specialists (e.g. orthopaedic
surgeon, neurosurgeon, dermatologist, rheumatologist, psychiatrist etc),
continued physiotherapy, incapacity to work followed by absenteeism and – last
but not least – loss of time for the patient. This process is not only
frustrating for the patients, but could also be a source of increased costs for
the patient in particular and the healthcare system in general.
In case of symptomatic breast hypertrophy,
some more-or-less specific criteria shown in table 1 must be met for the health
insurance company to recognise reduction mammaplasty as a compulsory treatment
option. However, it does not seem to be mandatory that all defined criteria
must be fulfilled [10]. In some cases, the patients’ complementary insurances may
cover
the medical treatment that is not covered by the basic insurance, provided that this
particular diagnostical or therapeutical procedure (includes also surgery) will be
performed in a public hospital, as stipulated in the contracts between the caretaker
and the basic health insurance companies.
Table 1Criteria to be met for health
insurance companies to reimburse the costs of reduction mammaplasty in patients
with symptomatic breast hypertrophy.
| Large breasts cause regular physical or
psychological discomfort resulting in a medical condition with a “burden” |
| Causal relationship between the discomfort
and the large breasts |
| Procedure aims to eliminate the discomfort
|
| Removal of at least 500 grams of skin and
adipoglandular tissue per side |
| BMI must not exceed 25 kg/m² |
| Conservative measures (e.g. drugs,
physiotherapy or muscle training) have remained ineffective |
Unfortunately, these rather well-defined
criteria by the law may currently offer some “freedom for interpretation” when
individual cases are evaluated by the medical officer of the health insurance
company. This might be one of the reasons for “mutual misunderstanding” when a
board-certified plastic surgeon who has not only collected a detailed patient
history but also examined the patient submits an individual request for cost
approval for reduction mammaplasty to the medical officer for review, who
usually neither is a specialist in the field nor assesses the patient.
This
study aims to analyse the current reimbursement policy by health insurance companies
in Switzerland for the surgical treatment of reduction mammaplasty in a patient cohort
treated in
two Swiss centres, a Department of Plastic, Reconstructive and Aesthetic Surgery
in a public hospital and the practice of a board-certified plastic surgeon
operating in public and private hospitals.
Materials
and methods
Study design and setting
A retrospective and descriptive cohort study
was performed, including patients treated in the Department of Plastic,
Reconstructive and Aesthetic Surgery at the Ente Ospedaliero Cantonale (EOC) in
Lugano (Switzerland), as well as in a private practice in Lucerne (Wettstein
Plastic Surgery). Both centres, one public and one private, are representative of
the treatment of breast hypertrophy in Switzerland, in terms of both surgical
experience of the board-certified surgeons working in these institutions and patient
volume.
An application for approval to conduct the study
was submitted to the regional ethics committee (Comitato etico cantonale
ticinese) on 9 March 2021 (Req-2021-00309). The committee replied that ethical
approval was not necessary because, due to its economic nature, the study does
not fall within the scope of Article 3 of the Human Research Act. However, written
informed consent for participation in the study was obtained from all patients
included in the study, as required by the ethics committee. Separate informed
consent for the surgery was obtained.
Participants
Inclusion criteria were a diagnosis of symptomatic
breast hypertrophy, cost approval for reduction mammaplasty by the health
insurance company and informed consent. The recruitment period ran from October
2014 to March 2021. The exclusion criteria were patients paying for reduction
mammaplasty and patients aged under 18 years. The patients were referred by
general practitioners or other specialists, or presented themselves on the
advice of a third party to be seen in the outpatient clinics. If the clinical
diagnosis of symptomatic breast hypertrophy was confirmed by the board-certified
plastic surgeon, a letter was sent to the health insurance company requesting cost
approval for reduction mammaplasty according to the current SwissDRG (Diagnosis-Related
Group). After receiving confirmation for cost approval from the health
insurance company, reduction mammaplasty surgery was performed by the board-certified
plastic surgeons who had indicated surgery. Surgery was performed using the
standardised technique according to Elisabeth Hall-Findlay [11, 12]. The follow-up
visits were performed two weeks, six weeks, three
months and one year after surgery and consisted of assessment of patient
history, clinical examination, as well as quantification of current pain level.
A specific questionnaire was delivered to the patients at
the to assess surgery-induced changes in back pain
using a visual analogue scale (VAS). Furthermore, global patient satisfaction
concerning reduction mammaplasty was assessed. We were
aware of the potential symptom recall bias before surgery.
Primary and secondary
outcome measures
Primary outcome measures were as follows:
(a) the total time in weeks, i.e. the median duration between the first request
for reimbursement sent by the board-certified plastic surgeon and the receipt
of cost approval for reduction mammaplasty by the health insurance company; (b)
the number of requests and reconsiderations needed until receipt of the written
cost approval by the health insurance company; (c) the number of additional
measures from the first request to cost approval and from the first refusal of
cost approval to final approval for the following three categories of measures:
outpatient visits by specialists other than plastic surgeons (i.e. orthopaedic
surgeons, neurosurgeons, dermatologists, psychiatrists), imaging studies including
X-ray, CT scan and MRI, and physiotherapy sessions.
Secondary outcome measures included the
costs generated by the additional visits, diagnostic procedures and therapeutic
measures performed during the evaluation period.
The costs were calculated using the following
methodology. Costs at the EOC for a specialist visit, for a cervical spine
X-ray, for a cervical spine CT scan and for a cervical spine MRI amounted, according
to medical fare structure TARMED for outpatient treatment, to 240.30 CHF,
125.00 CHF, 342.20 CHF and 415.00 CHF, respectively. These additional costs
were multiplied by the number of examinations performed for the
subgroup of 26 patients requiring one request for cost approval and in the
subgroup of 20 patients requiring multiple requests for cost approval. Costs
for physiotherapy sessions were calculated by multiplying the cost of a single
session of physiotherapy (69.30 CHF) by the mean number of sessions performed
per week and the median duration in weeks in the two subgroups. Finally, the cost
difference between these two subgroups was calculated, representing the
additional (“extra”) expenses in patients with more than one request for cost
approval for reduction mammaplasty, due to the initial refusal of cost coverage
by the health insurance company.
These additional costs per patient were
eventually added and compared to the Swiss DRG for reduction mammaplasty (DRG-J24A;
cost weight: 1.03; mean length of hospital stay: 2.7
days). Thereby, following mean base rates were taken into consideration: 9730 CHF
for cantonal hospitals (which corresponds to a mean base rate value of three
cantonal hospitals in Switzerland in 2023) and 11,005 CHF for university
hospitals (which corresponds to a mean base rate value of three university
hospitals in Switzerland in 2023) for reduction mammaplasty.
Furthermore, the following baseline factors
were evaluated: class of health insurance (3rd, 2nd and 1st
class), age, BMI at surgery, sternal notch-to-nipple distance, grade of ptosis
according to the classification of Regnault [13] and total weight of resected breast
tissue.
Finally, all
patients were asked whether their expectations had been met regarding their
breasts after reduction mammaplasty. Pre- and postoperative back pain using the
VAS were quantified. Moreover, BMI and weight of resected breast tissue are
decisive factors for the health insurance company during the decision process
regarding cost approval for reduction mammaplasty. The threshold values of the
health insurance company are a BMI of 25 kg/m and an expected
weight of breast tissue to be resected of 500 g or more. Thus, stratification
by BMI and weight of resected breast tissue were performed in order to describe
potential differences between patients in the two subgroups with a BMI of less
or more than 25 kg/m and a weight of resected breast tissue of less
or more than 500 g in terms of reduction of back pain.
Statistical
methods
Categorical
and numerical variables were expressed as counts or percentages. Statistical
analysis was performed using DATAtab (Online Statistics Calculator; Dr. Mathias
Jesussek, Seiersberg, Austria; URL https://datatab.net). For the evaluation of
pre-operative and post-operative pain, a t-test for dependent samples was used.
A p-value ≤0.05 was considered statistically significant.
Results
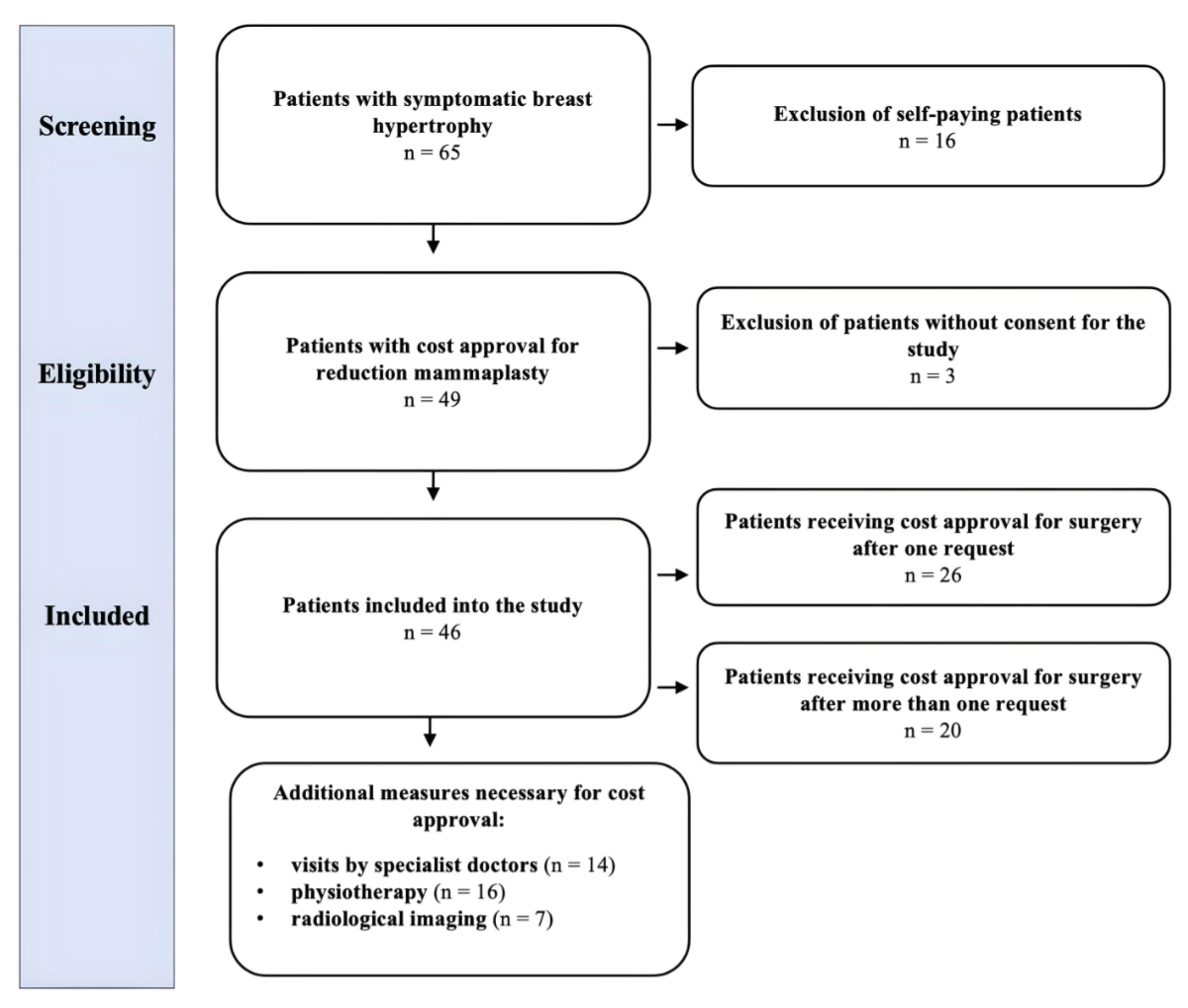
A total of 65 patients underwent reduction
mammaplasty for symptomatic breast hypertrophy in the abovementioned period of
time. Sixteen patients were excluded, since they did not receive cost approval for
reduction mammaplasty by the health insurance company despite multiple requests;
these patients eventually decided to pay the costs of surgery themselves.
Furthermore, three patients did not consent to the study. Consequently, a total
of 46 patients were included in the study
(figure 2).
The mean age at surgery was 45 years
(range: 16–76). The mean BMI was 26 kg/m2 (range: 18–40), indicating
only slight overweight. The mean sternal notch-to-nipple distance was 31 cm
(range: 23–45) bilaterally, indicating an intermediate-to-high grade of breast
ptosis of 3.3 (range: 1–4) according to the classification of Regnault.
Finally, the mean weight of resected breast tissue amounted to 558 g (range:
105–1750) per breast. In four patients, the information regarding the weight of
resected breast tissue was missing. The mean weight of resected breast tissue
between right and left breast was calculated for each patient. In 22 patients
(48%), the weight of resected breast tissue was less than 500 g with a mean
weight of 308 g (range: 105–499) per breast (table 2).
Table 2Baseline patient characteristics.
|
Mean |
Range |
| Patient age at
surgery (years) |
45 |
16–76 |
| Body mass index
at surgery (kg/m2) |
26 |
18–40 |
| Sternal
notch-to-nipple distance (cm) |
|
|
| Right breast |
31 |
23–43 |
| Left breast |
31 |
22–45 |
| Grade of ptosis
according to Regnault |
3.3 |
1–4 |
| Weight of
resected breast tissue (g) |
558 |
105–1750 |
| Weight of
resected breast tissue in patients undergoing reduction mammaplasty <500 g |
308 |
105–499 |
| Weight of
resected breast tissue in patients undergoing reduction mammaplasty >500 g |
833 |
501–1750 |
The insurance status of the patients was as
follows: the majority (35 patients or 76%) had only the compulsory 3rd
class basic insurance. The remaining patients further had a private 1st
or 2nd class health insurance. One patient with 1st class
health insurance coverage (i.e. the most expensive health insurance) received
cost approval with the following condition: surgical care could take place as
long as the patient would be treated as a 3rd class patient despite
paying an insurance fee for 1st class health care. Partial cost
coverage by the complementary insurance instead of the basic insurance occurred
in four cases (table 3).
Table 3Health insurance company
characteristics of patients.
| Class of health insurance |
n |
% |
| Class
I |
6 |
13% |
| Class
II |
5 |
11% |
| Class
III |
35 |
76% |
| Class
I–III |
46 |
100% |
| Cost coverage |
|
|
| 100% |
42 |
91% |
| 80%
(partial) |
3 |
7% |
| 50%
(partial) |
1 |
2% |
| 50–100% |
46 |
100% |
The number of requests needed to obtain cost
approval for reduction mammaplasty ranged from 1 to 4. In 26 patients (57%)
only 1 request was necessary, whereas multiple requests were needed for the
remaining 20 patients (43%), i.e. 2, 3 and 4 requests for 6, 11 and 3 patients,
respectively (figure 3). The median duration from the first request to final
approval was 9.4 weeks, with a range from 1 to 154 weeks, showing a clear
correlation between the number of requests and time to obtain final cost
approval (figure 4).
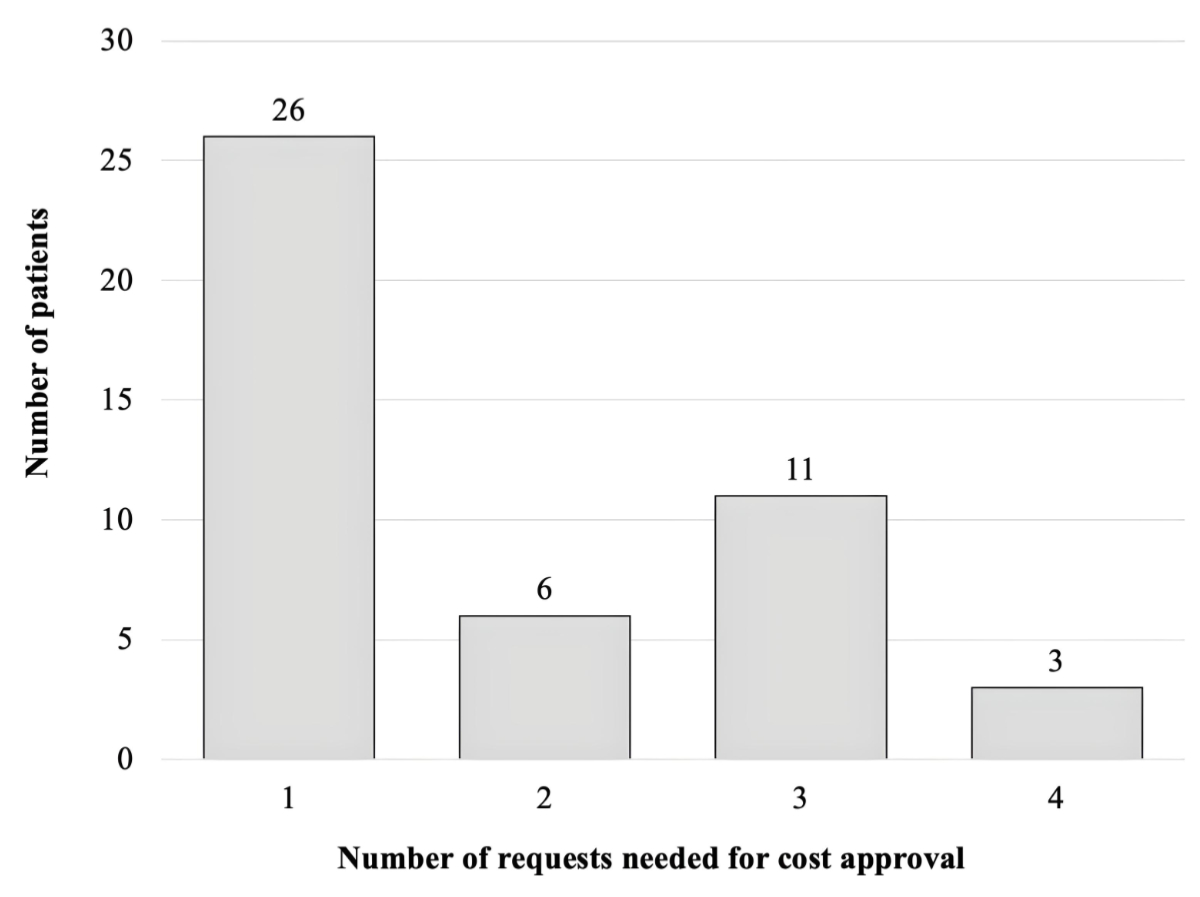
Figure 3Number of patients per subgroup needing 1 to 4 requests for cost
approval.
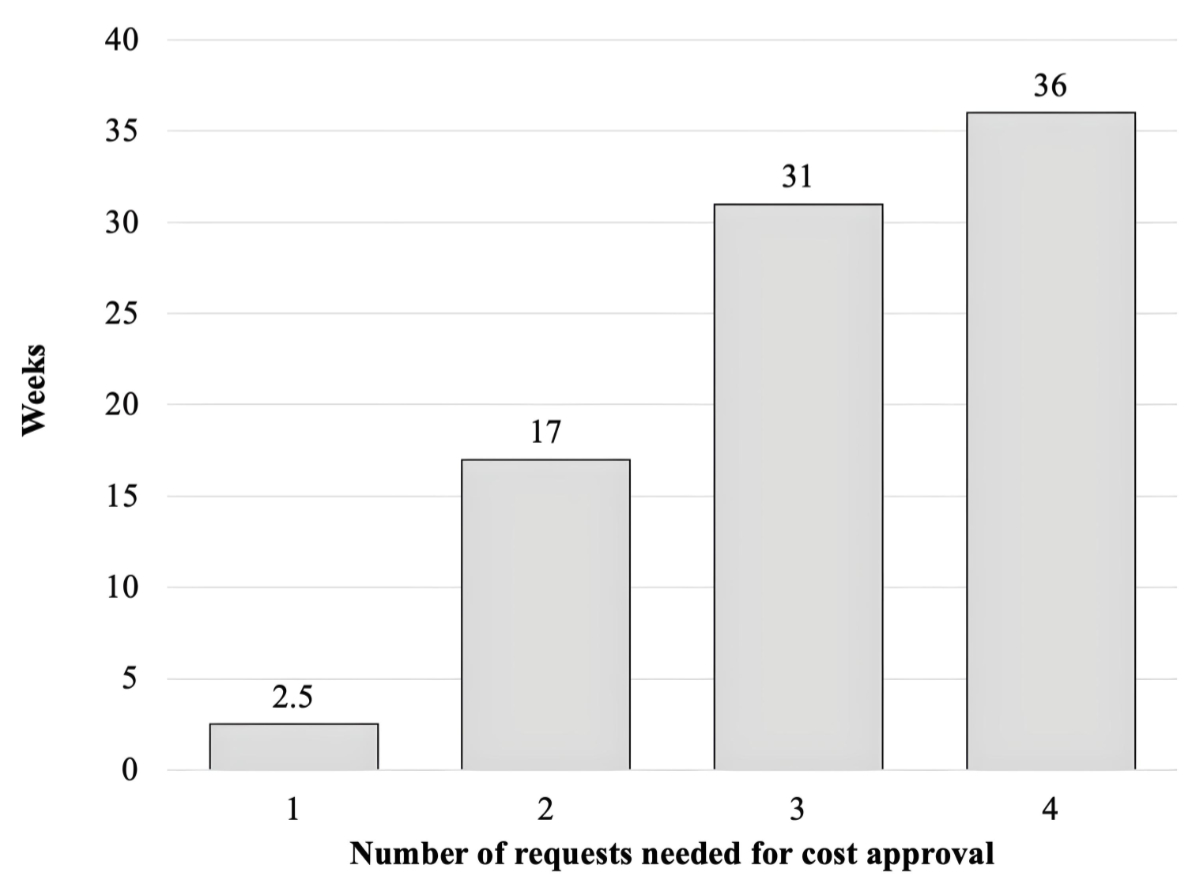
Figure 4Median duration in weeks until cost approval according to the number
of requests.
A more comprehensive analysis was conducted
in the subgroup of patients (n = 20 or 43%) with refusal of cost coverage by
the health insurance company following the first request despite unequivocal
diagnosis and indication for surgery. The number of additional visits by
specialists other than board-certified plastic surgeons concerned 14 patients (70%)
with a total of 26 additional outpatient visits with orthopaedic surgeons,
neurosurgeons, dermatologists and psychiatrists. Specifically, 5, 6 and 3
patients underwent 1, 2 and 3 additional visits, respectively. Of interest, in
7 of 20 patients (35%) the health insurance company requested specific imaging
of the spine, including 7 conventional X-rays, 1 CT scan and 3 MRI scans. In
addition, 16 of these 20 patients (80%) underwent physiotherapy before surgery
ranging from one (6 patients) to three sessions (2 patients) per week. In the
subgroup of 26 patients requiring only one request to obtain cost approval, fewer
visits by a board-certified specialist and fewer sessions of physiotherapy were
necessary, as shown in table 4.
Table 4Number of patients who needed
additional therapeutic and diagnostic measures.
|
(n = 26)* |
(n = 20)** |
| Physiotherapy |
16 (62%) |
16 (80%) |
| Visits by specialists (other than plastic surgeons) |
12 (46%) |
14 (70%) |
| Radiological imaging |
9 (35%) |
7 (35%) |
| Local skin therapy |
12 (46%) |
15 (75%) |
Accordingly, undergoing additional visits
by a specialist, pursuing physiotherapy, as well as performing complementary
imaging generated extra costs. Table 5 demonstrates the cost analysis in both subgroups
of 26 and 20 patients needing, respectively, one or more than one request for
cost approval.
Table 5Mean costs in CHF of additional
therapeutic and diagnostic measures.
|
Total costs (n = 26)* |
Costs per patient (n = 26)* |
Total costs (n = 20)** |
Costs per patient (n = 20)** |
Difference in costs |
Difference in costs per patient |
| Physiotherapy |
4418 |
170 |
47,020 |
2351 |
42,602 |
2181 |
| Visits by specialists |
3845 |
148 |
6248 |
312 |
2403 |
164 |
| Radiological imaging |
2000 |
77 |
2462 |
123 |
462 |
46 |
| Total costs |
10,263 |
395 |
55,730 |
2786 |
45,467 |
2391 |
Finally, 42 patients (91%) stated that
their expectations were met regarding the overall outcome of the reduction
mammaplasty and eventually would undergo this type of surgery again.
Objectively, surgery resulted in a significant reduction of high levels of chronic
back pain of 7.0 (range 0–10) before surgery to very low levels of infrequent
back pain of 1.6 (range 0–7) after surgery (p = 0.006; figure 5). Interestingly,
patients with total resection of breast tissue less than 500 g per breast (22
patients or 48%) showed a significant reduction in back pain after surgery
(mean [range] of VAS scores: 7.2 [0–10] preoperatively vs 2.0 [0–7]
postoperatively) that was similar in the 20 patients (52%) with resection of
more than 500 g breast tissue (7.0 [5–10] preoperatively vs 1.4 [0–7]
postoperatively).
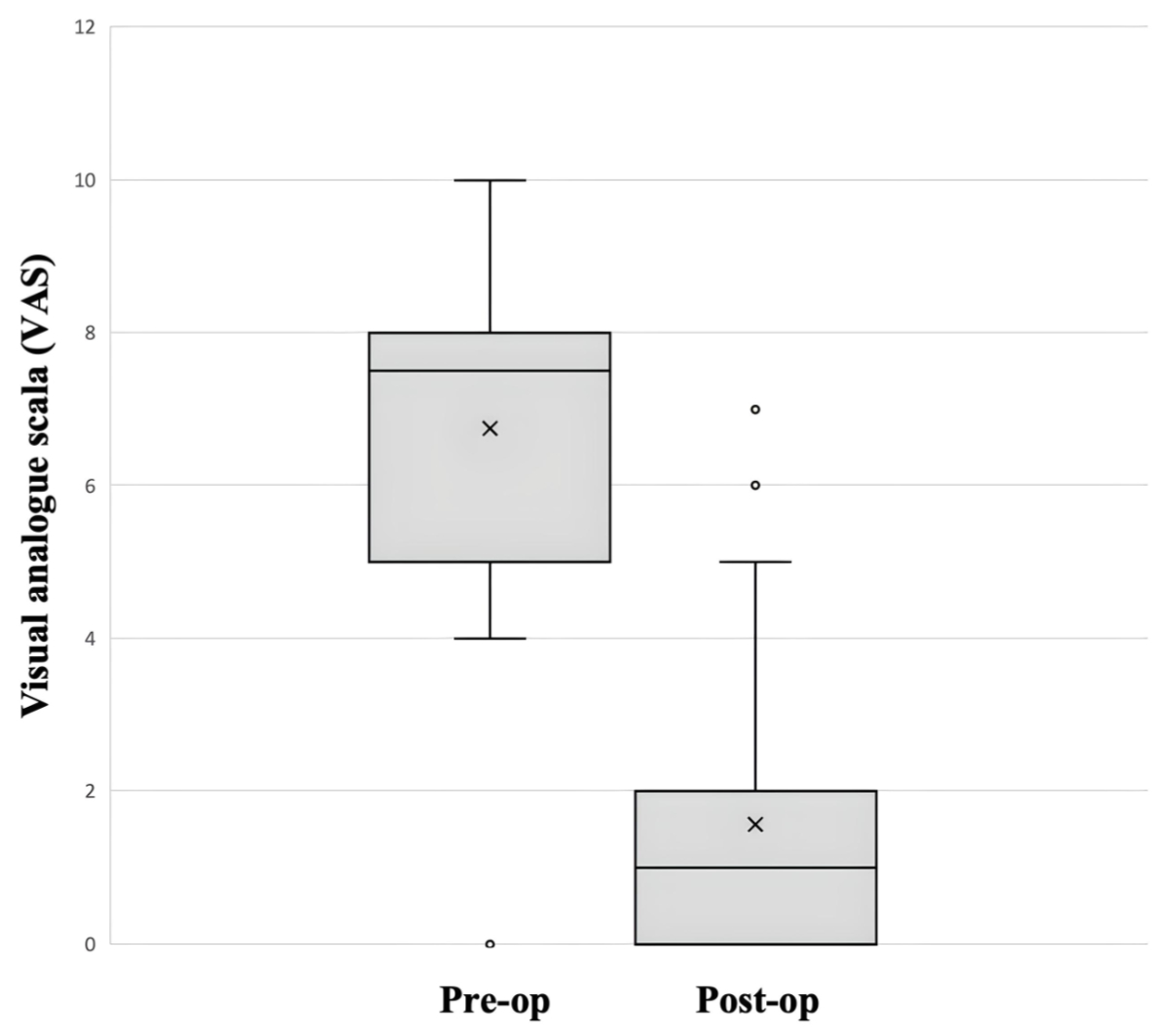
Figure 5Comparison of back pain before (pre-op) and after (post-op)
reduction mammaplasty using a visual analogue scale (VAS) from 0 (no pain at
all) to 10 (intolerable pain).
Moreover, the reduction in back pain on the
VAS after reduction mammaplasty did not differ between patients with a BMI ≤25 kg/m²
(22 patients or 48%) and those with a BMI >25 kg/m² (24
patients or 52%), as shown in table 6.
Table 6Back pain before and after
reduction mammaplasty, by body mass index at surgery.
| Body mass index at surgery (kg/m) |
≤25 (n = 22) |
>25 (n = 24) |
| Pain before reduction mammaplasty, mean (range)
VAS score |
6.77 (4–10) |
7.29 (0–10) |
| Pain after reduction mammaplasty, mean (range)
VAS score |
1.41 (0–5) |
1.83 (0–7) |
Discussion
Reduction mammaplasty is usually performed
in healthy and middle-aged patients with symptomatic breast hypertrophy and is considered
a safe procedure. Further, it is effective in decreasing symptoms and is associated
with a high grade of patient satisfaction [4–7],
also when performed in selected patient groups of younger age [14]
and/or obese women [15].
To be representative, we were particularly
interested in comparing our results with current evidence in the literature
from healthcare systems that, like Switzerland’s, use all-inclusive prices or
flat rates per case (Pauschale/forfait) according to DRGs, for
example those of Germany and Australia. Scholz et al. evaluated the total costs
that resulted from the assessment for symptomatic breast hypertrophy carried
out by specialists from conservative treatment, including physiotherapy,
massages, mud baths, as well as from anti-inflammatory and antalgic drug intake
over a six-month period. The authors demonstrated that conservative treatment
amounted to 4725 EUR compared to 3437 EUR for reduction mammaplasty. The
authors further evaluated administrative costs resulting from application for
reimbursement to the health insurance company as well as costs generated to
contest the decision of the health insurance company in case of refusal. These
costs included the applications for cost coverage, social medical reports by
the medical officer of the health insurance company, reports from additional
evaluations by specialists, lawyers’ fees, court costs and reached an
additional 3388 EUR per patient, almost amounting to the costs of reduction
mammaplasty. The authors therefore demonstrated that surgical treatment – notably
a causal treatment – was a safe and effective procedure, and is more
cost-effective than symptomatic treatment with conservative measures over a
longer period of time that would in most cases only act as a symptomatic
therapeutic approach [16]. This fact has recently been confirmed by Crittenden
et al., who conducted a cost-utility analysis in Australia. The authors could
show a gain in quality-adjusted life-years in operated patients compared to
non-operated patients [17]. Furthermore, it has been shown by Collins et al.
that conservative treatment measures, including weight loss, regular skin care
and physiotherapy, have significantly less impact on durable relief of symptoms
and have to therefore be considered only symptomatic and temporary [18]. This
is in line with the current study, showing that symptomatic patients who pursue
conservative treatment and undergo further examinations for reevaluation by the
medical examiner generate extra costs of almost 2392 CHF per patient without
persistent symptom relief. These extra costs have to be compared with the mean
total costs of reduction mammaplasty that usually amount to 9730 CHF in a cantonal
hospital in Switzerland. These extra costs of 2391 CHF are as high as 25% of
the total cost of the surgery and therefore particularly concerning in view of the
fact that ultimately the patients included in this study underwent surgery anyway.
These results confirm that reduction
mammaplasty is an effective treatment option for reduction mammaplasty with an
overall high grade of satisfaction following reduction mammaplasty in more than
90% of patients and a reduction of mean pain levels by 77% decreasing from
severe pain levels of 7.0 before surgery to levels of minor or almost no pain of
1.6 after surgery according to the VAS.
Treatment of symptomatic breast hypertrophy
may be considered from an aesthetic or from a functional point of view, as
health insurance companies try to clearly differentiate this aspect. Although
surgical treatment of symptomatic breast hypertrophy aims to reduce or even
eliminate symptoms, such as skin rashes, ulcerations, pain and/or tension, one
cannot underestimate the pure aesthetic aspect of reduction mammaplasty, as
many women’s self-esteem is strongly related to their breast appearance and
body image [19]. Despite the proven benefits of reduction mammaplasty in treating
symptomatic breast hypertrophy, many patients do not have access to reduction
mammaplasty, becauseit is still too often
considered an “aesthetic procedure” by the health insurance company and
therefore coverage of costs is refused. Basically, in Switzerland rather
well-defined criteria determine whether reduction mammaplasty to treat symptomatic
breast hypertrophy is reimbursed or not. If the patient meets the criteria – notably
not all the defined criteria have to be met – surgery is deemed an obligatory service
(Pflichtleistung / service obligatoire / servizio obbligatorio)
[10] and reimbursed by compulsory basic insurance. Accordingly, the
reimbursement should not be transferred to the complementary insurance, which is
specifically meant to reimburse services like medical treatment related to
alternative medicine, treatments at health resorts, dental treatments, preventive
health measures or rescue costs, such as emergency rescue and repatriation in case
of illness or accident abroad.
We however agree that it may often be
difficult to make a clear-cut decision, since the surgeon in charge of the
patient and the trusted physician of the health insurance company will again
and again be confronted with borderline cases, where it will not be easy to
differentiate between symptomatic breast hypertrophy and hypertrophic breast
associated with ptosis that has increased with age and weight.
Rawes et al. emphasised the increasing
difficulty of obtaining reimbursement for reduction mammaplasty in the United
States in response to rising costs in their healthcare system. Health insurance
companies therefore often include requirements for reimbursement of reduction
mammaplasty that go further than those published by the American Society of
Plastic Surgeons (ASPS) and are inconsistent with the current evidence. For
example, the ASPS guidelines neither include age-based limitations nor a
threshold for the amount of breast tissue to be resected. The authors however
demonstrated that one in five health insurance companies would cover the costs
only if patients were aged 18 years or over and a minimum of 500 g tissue per
breast would be excised. In order to achieve some clarity and homogeneity in
reimbursement policies for reduction mammaplasty, Rawes et al. summarised the
criteria that must be met to obtain cost approval for reduction mammaplasty,
such as the presence of symptomatic breast hypertrophy-related symptoms
(chronic neck and back pain and/or shoulder grooving and pain, tension
headache, skin irritations in the inframammary fold, etc.), documentation of
failed conservative treatments (topical dermal treatment, analgesic measures,
e.g. NSAIDs, massage, physiotherapy) and the performance of additional
diagnostics (radiographs showing acquired kyphosis). In fact, the
recommendations do not include either a minimum age or a minimal weight of
breast tissue to be resected [20].
At this point it is important to underline that
almost half (48%) of the patients included in the present study underwent
reduction of breast tissue of approximately 300 g per side, i.e. significantly
less than the 500 g per breast “required” for cost approval. This demonstrates
that the threshold of 500 g tissue to be removed per breast is only a relative
measure and the well-defined criteria by the law are only approximate. Though,
we are convinced that it is important to provide an approximate expected
resection weight per breast in the request for cost coverage, particularly if
breast size does not “allow” resection of 500 g or more per breast. This is
important, since a recent systematic review and meta-analysis by Wang et al.
including 28 publications demonstrated that patient-reported satisfaction with
their breasts after reduction mammaplasty did not correlate with the amount of
breast tissue resected [21].
Nevertheless, these criteria, which ultimately
determine whether symptomatic breast hypertrophy is considered a physical
condition with a “burden”, have to be used as a reference, rather than a
binding decision criterion. The observation in our subgroup of patients is of
particular importance, since there is clear evidence that patients with symptomatic
breast hypertrophy undergoing smaller resections of less than 250 g of breast
tissue still experienced significant improvement in several symptoms, including
back pain, rashes at the inframammary fold, headache, exercise intolerance and
lack of self-esteem as demonstrated by Strong et al. [22]. In addition, Spector
et al. analysed symptoms before and after surgery and compared them between
subgroups of patients undergoing resection of various weights of breast tissue
(per 2 breasts), including less than 1000 g, 1000–1500 g, 1500–2000 g and >2000
g. No significant difference was found between the subgroups with regard to
improvement of symptoms, including back pain, headache, skin changes, itching
and difficulty in running, indicating that it is more important to “shape” a
new and smaller breast that restores normal posture than to excise as much tissue
as possible [23].
Furthermore, Hernanz et al. could
demonstrate that obese women with a BMI higher than 30 kg/m also benefit from a persistently
improved quality of life following reduction
mammaplasty [15]. This indicates that surgery may also be the treatment of
choice with persistent symptom relief in overweight patients, i.e. a patient
group that does not meet the selection criteria used by health insurance
companies in Switzerland. Since overweight patients are particularly often affected
by refusal of cost approval, it has to be said that BMI does not always
correlate with breast volume, which is confirmed by the fact that patients with
low BMI and/or eating disorders may still have large and heavy breasts [24]. This
fact can be underlined by the current study, since back pain improved
significantly following reduction mammaplasty from very severe chronic pain
levels to almost no pain in patients with a BMI below and above 25 kg/m2.
Overweight patients are often penalised by the fact that health
insurance companies may require normalisation of weight as a prerequisite for paying
the costs of reduction mammaplasty. They thereby refer to the so-called
criteria to be met for health in order to reimburse the costs for reduction
mammaplasty. However, Geiker et al. could demonstrate that symptoms
related to breast hypertrophy persist even after weight loss, highlighting the
continued need of performing reduction mammaplasty, in selected cases also in
overweight patients [25].
At this point, it may be underlined that
the decision to reimburse or not is based on a letter including all relevant
patient information usually gathered by a plastic surgeon that is accompanied
by a set of standardised photographs of the patient’s current breast condition.
It is an absolute rarity that the patient be seen and examined by the medical
examiner of the health insurance company, also and particularly in cases with
borderline values and/or after initial refusal that needs reevaluation.
Finally, it is not only the decision of the health insurance company based on a
suggestion of the medical examiner that is subjective, but also the plastic
surgeon’s evaluation, which is inherent to the nature of the symptoms of BH.
Therefore, a fair evaluation for every single patient is probably an elusive
goal.
The limitations of this study might be the
small number of enrolled patients and the selection bias due to the 25% of
patients with symptomatic breast hypertrophy classified as eligible for reduction
mammaplasty by the board-certified plastic surgeon but who finally decided to
undergo surgery at their own expense, rather than reconsider the case and/or
wait. However, if we had included self-paying patients in this study,
we expect the median duration until cost approval for patients with multiple
requests would have been even higher. One potential bias affecting generalisability
of the findings is that patients were only enrolled in two cantons. In theory,
there should be no between-canton differences regarding cost approval for reduction
mammaplasty. However, we cannot prove this.
Conclusion
In this study, 57% of patients with symptomatic
breast hypertrophy received cost approval for reduction mammaplasty after a
median of 2.5 weeks following the first request. However, despite a clear diagnosis
and an indication for surgery, as
evaluated by board-certified plastic surgeons, the reimbursement was
initially refused in 43% of patients (median waiting
time: 23 weeks), resulting in further outpatient visits by specialists, as
well as additional complementary imaging and physiotherapy. This implied a 9.2-fold
prolonged median duration and
higher costs of 2391 CHF per patient compared to the patients who received cost
approval for reduction mammaplasty after the first request to the health
insurance company.
Prof. Dr Yves Harder, M.D.
Department of Plastic, Reconstructive, Aesthetic and Hand Surgery
Centre Hospitalier Universitaire Vaudois (CHUV)
Rue du Bougnon 46
CH-1011 Lausanne
yves.harder[at]chuv.ch
References
1. Saariniemi KM, Keranen UH, Salminen-Peltola PK, Kuokkanen HO. Reduction mammaplasty
is effective treatment according to two quality of life instruments. A prospective
randomised clinical trial. J Plast Reconstr Aesthet Surg. 2008 Dec;61(12):1472–8.
doi: https://doi.org/10.1016/j.bjps.2007.09.024
2. Mundy LR, Homa K, Klassen AF, Pusic AL, Kerrigan CL. Understanding the Health Burden
of Macromastia: Normative Data for the BREAST-Q Reduction Module. Plast Reconstr Surg.
2017 Apr;139(4):846e–53e. doi: https://doi.org/10.1097/PRS.0000000000003171
3. Crittenden T, Watson DI, Ratcliffe J, Griffin PA, Dean NR; AFESA Research Group. Does
breast reduction surgery improve health-related quality of life? A prospective cohort
study in Australian women. BMJ Open. 2020 Feb;10(2):e031804. doi: https://doi.org/10.1136/bmjopen-2019-031804
4. Colohan SM, Massenburg BB, Gougoutas AJ. Breast Reduction: Surgical Techniques with
an Emphasis on Evidence-Based Practice and Outcomes. Plast Reconstr Surg. 2020 Sep;146(3):339e–50e.
doi: https://doi.org/10.1097/PRS.0000000000007263
5. Lin Y, Yang Y, Zhang X, Li W, Li H, Mu D. Postoperative Health-related Quality of
Life in Reduction Mammaplasty: A Systematic Review and Meta-Analysis. Ann Plast Surg.
2021 Jul;87(1):107–12. doi: https://doi.org/10.1097/SAP.0000000000002609
6. Hermans BJ, Boeckx WD, De Lorenzi F, van der Hulst RR. Quality of life after breast
reduction. Ann Plast Surg. 2005 Sep;55(3):227–31. doi: https://doi.org/10.1097/01.sap.0000171444.79737.70
7. Pérez-Panzano E, Güemes-Sánchez A, Gascón-Catalán A. Quality of Life Following Symptomatic
Macromastia Surgery: Short- and Long-term Evaluation. Breast J. 2016 Jul;22(4):397–406.
doi: https://doi.org/10.1111/tbj.12589
8. Crittenden T, Watson DI, Ratcliffe J, Griffin PA, Dean NR; AFESA Research Group. Does
breast reduction surgery improve health-related quality of life? A prospective cohort
study in Australian women. BMJ Open. 2020 Feb;10(2):e031804. doi: https://doi.org/10.1136/bmjopen-2019-031804
9. www.fedlex.admin.ch. [Internet]. Federal Law on Health Insurance (LAMal), chapter
8 of federal low in Switzerland [cited 2022 Dec 12]. Available from: https://www.fedlex.admin.ch/
10. www.vertrauensaerzte.ch/manual/chapter38.html [Internet]. 5th edition of the Manual of the Schweizerische Gesellschaft der Vertrauens-
und Versicherungsärzte (SGV), Chapter 38 [cited 2024 Oct 19]. Available from: www.vertrauensaerzte.ch/manual/chapter38.html
11. Hall-Findlay EJ. Pedicles in vertical breast reduction and mastopexy. Clin Plast Surg.
2002 Jul;29(3):379–91. doi: https://doi.org/10.1016/S0094-1298(02)00008-1
12. Hall-Findlay EJ. Vertical breast reduction with a medially-based pedicle. Aesthet
Surg J. 2002 Mar;22(2):185–94. doi: https://doi.org/10.1067/maj.2002.123052
13. Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg. 1976 Apr;3(2):193–203.
doi: https://doi.org/10.1016/S0094-1298(20)30220-0
14. Nuzzi LC, Firriolo JM, Pike CM, Cerrato FE, Webb ML, Faulkner HR, et al. The Effect
of Reduction Mammaplasty on Quality of Life in Adolescents With Macromastia. Pediatrics.
2017 Nov;140(5):e20171103. doi: https://doi.org/10.1542/peds.2017-1103
15. Hernanz F, Fidalgo M, Muñoz P, Noriega MG, Gómez-Fleitas M. Impact of reduction mammoplasty
on the quality of life of obese patients suffering from symptomatic macromastia: A
descriptive cohort study. J Plast Reconstr Aesthet Surg. 2016 Aug;69(8):e168–73. doi: https://doi.org/10.1016/j.bjps.2016.05.012
16. Scholz T, Diedrichson J, Olbrisch RR, Liebau J. [Cost analysis of conservative versus
operative therapy of macromastia] [German.]. Handchir Mikrochir Plast Chir. 2008 Apr;40(2):100–4.
doi: https://doi.org/10.1055/s-2008-1038475
17. Crittenden TA, Ratcliffe J, Watson DI, Mpundu-Kaambwa C, Dean NR. Cost-utility analysis
of breast reduction surgery for women with symptomatic breast hypertrophy. Med J Aust.
2022 Feb;216(3):147–52. doi: https://doi.org/10.5694/mja2.51343
18. Collins ED, Kerrigan CL, Kim M, Lowery JC, Striplin DT, Cunningham B, et al. The effectiveness
of surgical and nonsurgical interventions in relieving the symptoms of macromastia.
Plast Reconstr Surg. 2002 Apr;109(5):1556–66. doi: https://doi.org/10.1097/00006534-200204150-00011
19. Liao, Christopher D et al. “A Systematic Review of the Impact of Patient Factors on
BREAST-Q Outcomes After Reduction Mammoplasty.” Annals of plastic surgery vol. 90,6S
Suppl 5 (2023): S667-S673.
20. Rawes CM, Ngaage LM, Borrelli MR, Puthumana J, Slezak S, Rasko YM. Navigating the
Insurance Landscape for Coverage of Reduction Mammaplasty. Plast Reconstr Surg. 2020 Nov;146(5):539e–47e.
doi: https://doi.org/10.1097/PRS.0000000000007241
21. Wang AT, Panayi AC, Fischer S, Diehm YF, Tapking C, Hundeshagen G, et al. Patient-Reported
Outcomes After Reduction Mammoplasty Using BREAST-Q: A Systematic Review and Meta-Analysis.
Aesthet Surg J. 2023 Mar;43(4):NP231–41. doi: https://doi.org/10.1093/asj/sjac293
22. Strong B, Hall-Findlay EJ. How Does Volume of Resection Relate to Symptom Relief for
Reduction Mammaplasty Patients? Ann Plast Surg. 2015 Oct;75(4):376–82. doi: https://doi.org/10.1097/SAP.0000000000000190
23. Spector JA, Singh SP, Karp NS. Outcomes after breast reduction: does size really matter? Ann
Plast Surg. 2008 May;60(5):505–9. doi: https://doi.org/10.1097/SAP.0b013e31816f76b5
24. Losee JE, Jiang S, Long DE, Kreipe RE, Caldwell EH, Serletti JM. Macromastia as an
etiologic factor in bulimia nervosa: 10-year follow up after treatment with reduction
mammaplasty. Ann Plast Surg. 2004 May;52(5):452–7. doi: https://doi.org/10.1097/01.sap.0000123344.08286.3e
25. Geiker NR, Horn J, Astrup A. Preoperative weight loss program targeting women with
overweight and hypertrophy of the breast - a pilot study. Clin Obes. 2017 Apr;7(2):98–104.
doi: https://doi.org/10.1111/cob.12175




