Quantifying aminoglycoside resistance in extended-spectrum beta-lactamase (ESBL)-producing
Enterobacterales clinical isolates: a retrospective cohort study
DOI: https://doi.org/https://doi.org/10.57187/s.3904
Isabelle Vocka,
Lisandra Aguilar-Bulteta,
Nina Khannaa,
Adrian Eglib,
Elisabeth Wehrle-Wielanda,
Pranita D. Tammac,
Sarah Tschudin Sutterad
a Division
of Infectious Diseases and Hospital Epidemiology, University Hospital Basel,
University Basel, Basel, Switzerland
b Division
of Bacteriology and Mycology, University Hospital Basel, University Basel,
Basel, Switzerland
c Department
of Pediatrics, Johns Hopkins University School of Medicine, Baltimore,
Maryland, United States
d Department
of Clinical Research, University Hospital Basel, University Basel, Basel,
Switzerland
Summary
AIMS: Aminoglycoside resistance is
frequently detected in extended-spectrum-beta-lactamase (ESBL)-producing
Enterobacterales (ESBL-PE), questioning the appropriateness of aminoglycosides
as empiric therapy in patients with suspected ESBL-PE infections. Therefore, we
aimed to evaluate the frequency of aminoglycoside resistance in patients harbouring
ESBL-PE and identify patient-related risk factors associated with
aminoglycoside resistance to facilitate early detection of at-risk patients.
METHODS: This retrospective single-centre
cohort study included hospitalised patients aged ≥18 years with an ESBL-PE-positive
sample between January 2016 and
December 2018. Aminoglycoside resistance was defined according to the European
Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints
for Enterobacterales for the current year of testing.
RESULTS: Five hundred forty-four patients
met the eligibility criteria, of which 240 (44.1%) harboured aminoglycoside-resistant
ESBL strains. Identification of ESBL-Klebsiella pneumoniae was significantly
associated with aminoglycoside resistance (odds ratio [OR] = 2.64, 95%
confidence interval [CI] = 1.65–4.21, p <0.001) and an international travel
history within the past 12 months was marginally associated with aminoglycoside
resistance (OR = 1.51, 95% CI = 0.95–2.42, p = 0.084).
CONCLUSIONS: In a low ESBL endemicity setting,
aminoglycoside resistance in patients harbouring ESBL-PE is common, especially
ESBL-K. pneumoniae, and needs to be considered in clinicians’ decision-making
regarding empiric therapy regimens.
Introduction
Aminoglycosides are frequently administered
empirically in combination with beta-lactam agents to treat severe sepsis,
aiming to provide broad coverage for multidrug-resistant Gram-negative bacteria
[1, 2], including extended-spectrum beta-lactamase producing Enterobacterales
(ESBL-PE). While a number of host-related risk factors, such as immunosuppressive
therapy or haematologic malignancies, have been identified as risk factors for
carrying ESBL-PE [3], knowledge of their associations with aminoglycoside
resistance is incomplete. In Europe, the prevalence of aminoglycoside
resistance varies between countries, ranging from 0% to 67% for Klebsiella
pneumoniae and 5% to 34% for Escherichia coli (https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/data-ecdc,
accessed on 9th July 2022). These findings question the value of
empiric aminoglycoside therapy in patients with suspected ESBL-PE infections,
possibly enhancing their risk of adverse outcomes such as acute kidney injury
without necessarily improving clinical outcomes. Our study aimed to identify
patient-related risk factors associated with aminoglycoside resistance in ESBL-PE
carriers to optimise empiric antibiotic decision-making.
Methods
Setting and participants
This retrospective cohort study was
conducted at the University Hospital Basel, a tertiary care centre in Basel,
Switzerland. It included hospitalised patients aged ≥18 years with ESBL-PE detected
in any screening or clinical sample
between January 2016 and December 2018. Its sample size and study period were
determined based on a preexisting cohort in the ESBL-Infect study [4]. The
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)
guidelines were followed.
Data collection and outcomes
Pertinent clinical and microbiological data
were extracted from electronic medical records and entered into a secure REDCap® database [5]. The data were collected retrospectively, and missing data were categorised
as absent. The assessed variables were
- demographics;
- previous hospitalisations, defined as >1 night stay in an
acute-care facility within the past 12 months;
- comorbidities based on the
Charlson Comorbidity Index (CCI);
- travel history, defined as stay outside
of Switzerland within the past 12 months, and whether patients were hospitalised
abroad;
- surgical interventions within the prior three months and chronic
wounds (defined as ulcers or decubitus);
- indwelling hardware (transurethral
or suprapubic urinary catheterisation) within 30 days prior to the index sample
detecting ESBL-PE and vascular hardware (defined as a central venous catheter)
in place for at least seven days prior to the index sample;
- microbiologic
data;
- treatment data (antibiotic therapies, defined as any antibiotic
medication within three months prior to the index ESBL-PE-positive sample,
immunosuppressive therapy within the prior 12 months [e.g. long-term steroids,
cytostatics, biologicals/antibody therapies, mechanistic target of rapamycin
kinase (mTOR) inhibitors and calcineurin inhibitors] and concomitant medication).
Infections within the relevant hospitalisation were defined according to the Centres
for Disease Control and Prevention (CDC) guidelines [6]. The primary study outcome
was the frequency of aminoglycoside resistance, defined as resistance to at
least one of the tested aminoglycosides in patients with ESBL-PE isolates.
Microbiological testing
European Committee on Antimicrobial
Susceptibility Testing (EUCAST) clinical breakpoints for Enterobacterales were
used for minimal inhibitory concentration (MIC) breakpoints for the current year
of testing (www.eucast.org). Patients were categorised as aminoglycoside
resistant when ESBL-PE tested as “R – resistant” or “I – intermediate” for
tobramycin or amikacin, respectively. If multiple species of ESBL-PE were
isolated from the same patient, patients were categorised according to the more
resistant strain.
ESBL-PE testing was conducted as previously
described in the ESBL-Infect study at the University Hospital Basel, which was
part of the same cohort [4]: Samples were plated onto selective chromogenic
agar plates (chromID® ESBL; bioMérieux, Marcy-l’Étoile, France)
while species were identified using either matrix-assisted laser
desorption-ionisation time of flight (MALDI-TOF) mass spectrometry (Bruker
Daltonics, Bremen, Germany) or the Vitek 2™ System
(bioMérieux, Durham, NC, USA), which was also used for susceptibility testing.
ESBL production was suspected based on the detection of resistance to
cefpodoxime, ceftriaxone or ceftazidime and the ESBL-PE was phenotypically
confirmed using Etest® strips (bioMérieux, Marcy-l’Etoile, France) for
cefotaxime, ceftazidime or cefepime, each tested with and without clavulanic
acid or with disks using the Extended Spectrum β Lactamase Set (Mast
Diagnostika, Reinfeld, Germany). Indeterminate results were further evaluated
using the eazyplex Superbug CRE panel (AmplexDiagnostics, Gars-Bahnhof,
Germany), including the blaCTX-M-1
and blaCTX-M-9 genes,
based on our local epidemiology. If these genes were not present, isolates were
considered ESBL negative.
Statistical analyses
The overall distribution of numeric data
was compared using Fisher’s exact test and the Mann-Whitney-U test. Patients
with and without detected aminoglycoside-resistant ESBL-PE were compared using
univariable and multivariable logistic regression analyses. Variables differing
significantly in the multivariable analyses (except the outcome measures) were included
in
the multivariable model, which was checked using the Hosmer-Lemeshow
goodness-of-fit test. Statistical analyses were performed using STATA (version
16.1; StataCorp, College Station, TX, USA). A p-value of <0.05 was considered statistically
significant.
Ethics approval
This study was approved by the local ethics
committee (EKNZ-2017-00100).
Results
Five hundred forty-four patients met the eligibility
criteria, of which 240 (44.1%) harboured aminoglycoside-resistant ESBL-PE strains.
Their baseline characteristics are provided in table 1. Their
median age was 70 (interquartile range [IQR] = 56–81) years, and 49.3% were
male. In addition, 369 (67.8%) had a history of at least one prior hospitalisation
within the past 12 months. Moreover, 88 (16.2%) had a documented stay outside
of Switzerland, of which 35 (6.4%) were also hospitalised abroad. Furthermore, 258
(47.4%) had been administered antibiotic therapy within the previous three
months, of which 18 had received aminoglycosides (3.3%).
Table1Baseline characteristics of the study cohort (n = 544).
| |
n (%) or
median (IQR) |
| Age (years) |
70 (56–81) |
| Male sex |
268 (49.3) |
| Stay in an intensive
care unit |
141 (25.9) |
| History of
hospitalisation* |
369 (67.8) |
| Travel history* |
88 (16.2) |
| Hospitalisation
abroad* |
35 (6.4) |
| Charlson
Comorbidity Index |
2 (0–3) |
| Open wounds** |
60 (11.0) |
| Surgery*** |
154 (28.3) |
| Vascular
hardware# |
22 (4.0) |
| Dialysis |
10 (1.8) |
| Urinary
catheterisation## |
160 (29.4) |
| Solid organ
transplantation |
26 (4.8) |
| Allogenic stem cell
transplantation |
16 (2.9) |
| Antibiotic
therapy*** |
258 (47.4) |
| – Duration
(days) |
14 (6–34) |
| – Aminoglycosides |
18 (3.3) |
| –
Aminoglycosides: duration (days) |
3 (1–11) |
| Immunosuppressive
therapy* |
144 (26.5) |
| Proton pump
inhibitor* |
295 (54.2) |
| Death |
32 (5.9) |
| Length of
hospital stay (days) |
13 (8-23) |
| ESBL-PE species |
ESBL-Escherichia
coli |
475 (87.3) |
| ESBL-Klebsiella
pneumoniae |
93 (17.1) |
| ESBL-PE
Infection |
296 (54.4) |
| non-ESBL-PE-Infection |
293 (53.8) |
Proportions of
aminoglycoside resistance in patients harbouring ESBL-PE did not differ
significantly within the study period (p = 0.374; figure 1).
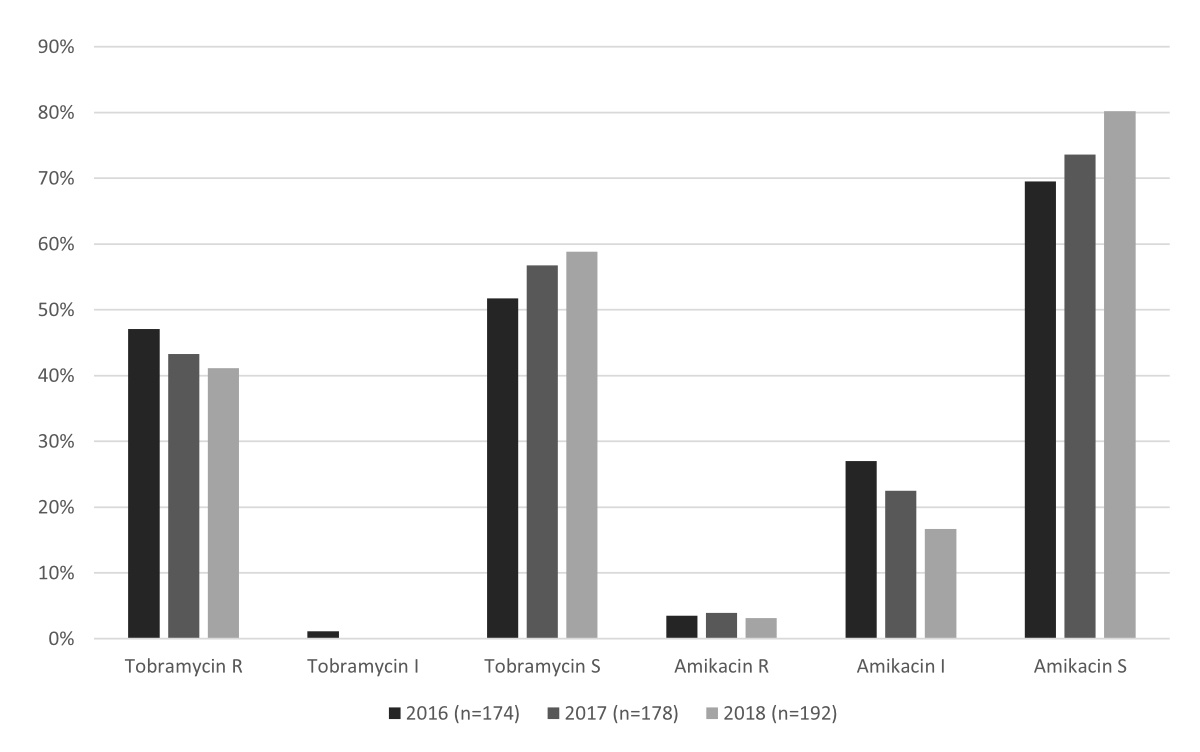
Figure 1The proportions of tobramycin and amikacin
resistance within the study period (01/2016-12/2018). The proportions of
susceptible isolates were compared between study years using Fisher’s exact
test: amikacin (p = 0.058), tobramycin (p = 0.374), and overall (p = 0.374).
The
distribution of aminoglycoside resistance patterns is summarised in table 2. Of the
240 patients harbouring aminoglycoside-resistant ESBL-PE strains,
amikacin-intermediate and tobramycin-resistant strains were the most common (n
= 117, 21.5%), followed by amikacin-susceptible and tobramycin-resistant strains
(n = 102, 18.8%), with 3.5% (n = 19) testing as resistant to both amikacin and
tobramycin.
Table 2Distribution of aminoglycoside resistance patterns in patients colonised with
ESBL-PE.
| Amikacin |
Tobramycin |
n = 544 |
% |
| S |
S |
304 |
55.9 |
| R |
R |
19 |
3.5 |
| I |
R |
117 |
21.5 |
| S |
R |
102 |
18.8 |
| I |
I |
2 |
0.4 |
The univariable analysis identified international
travel within the past 12 months as the only risk factor associated with
aminoglycoside resistance in patients with ESBL-PE colonisations (table 3).
Overall, 55.7% (n = 49) of patients travelled within Europe, 19.5% (n = 26)
within Asia, 6.8% (n = 6) within North and Central America, 2.3 % (n = 2)
within South America and 9.1% (n = 8) within Africa.
Table 3Comparison of patients colonised with aminoglycoside-resistant
and aminoglycoside-susceptible ESBL-PE.
|
Patients with aminoglycoside-resistant
ESBL-PE (n = 240) |
Patients with aminoglycoside-susceptible
ESBL-PE (n = 304) |
Univariable analysis |
| n (%) or median (IQR) |
n (%) or median (IQR) |
p-value |
OR (95% CI) |
| Age
(years)* |
60
(56–77) |
71 (57–81.5) |
0.075 |
0.99 (0.98–1.00) |
| Male sex |
112 (46.7) |
156 (51.3) |
0.282 |
1.20 (0.85–1.69) |
| Admission from another acute healthcare
facility |
47 (19.6) |
48 (15.8) |
0.248 |
1.30 (0.83–2.02) |
| Admission from a long-term care facility |
25 (10.4) |
36 (11.8) |
0.601 |
0.86 (0.50–1.49) |
| History of hospitalisation** |
163 (67.9) |
206 (67.8) |
0.970 |
1.01 (0.7–1.44) |
| Travel history** |
48 (20.0) |
40 (13.2) |
0.032 |
1.65
(1.04–2.61) |
| Hospitalisation abroad** |
20 (8.3) |
25 (8.2) |
0.112 |
1.75 (0.88–3.45) |
| Charlson Comorbidity Index |
2 (0–3) |
2 (0–3) |
0.971 |
1.00 (0.92–1.08) |
| Active open wounds*** |
29 (12.1) |
31 (10.2) |
0.486 |
1.21 (0.71–2.07) |
| Surgery |
68 (28.3) |
86 (28.3) |
0.991 |
1.00 (0.69–1.46) |
| Indwelling vascular hardware**** |
12 (5.0) |
10 (3.3) |
0.318 |
1.55 (0.66–3.65) |
| Dialysis |
6 (2.5) |
4 (1.3) |
0.315 |
1.92 (0.54–6.89) |
| Urinary catheterisation# |
75 (31.3) |
85 (28.0) |
0.403 |
1.17 (0.81–1.70) |
| Solid organ transplantation |
10 (4.2) |
16 (5.3) |
0.553 |
0.78 (0.35–1.76) |
| Allogenic stem cell transplantation |
6 (2.5) |
10 (3.3) |
0.590 |
0.75 (0.27–2.10) |
| Antibiotic therapy## |
|
118 (49.2) |
140 (46.1) |
0.470 |
1.13 (0.81–1.59) |
| Duration (days) |
14 (6–39) |
13 (6.5–29) |
0.370 |
1.00 (0.99–1.01) |
| Aminoglycosides |
10 (4.2) |
8 (2.6) |
0.324 |
1.61 (0.62–4.14) |
| Aminoglycosides duration (days) |
1.5 (1–14) |
3.5 (2.5–9) |
0.607 |
1.04 (0.90–1.19) |
| Immunosuppressive therapy** |
64 (26.7) |
80 (26.3) |
0.927 |
1.02 (0.69–1.49) |
| Proton pump inhibitor** |
129 (53.8) |
166 (54.6) |
0.842 |
0.97 (0.69–1.36) |
| Micriobiological data |
| ESBL-PE species###, #### |
Escherichia coli |
195 (81.3) |
280 (92.1) |
<0.001 |
0.37
(0.22–0.63) |
| Klebsiella pneumoniae |
60 (25.0) |
33 (10.9) |
<0.001 |
2.74
(1.71–4.36) |
| Other |
6 (2.5) |
6 (2.0) |
0.679 |
1.27 (0.41–4.00) |
| Outcomes |
| Death |
15 (6.3) |
17 (5.6) |
0.746 |
0.89 (0.43–1.81) |
| Death attributable to ESBL-PE infection |
9 (60.0) |
11 (64.7) |
0.784 |
0.82 (0.20–3.43) |
| Intensive care unit stay |
68 (28.3) |
73 (24.0) |
0.254 |
1.25 (0.85–1.83) |
| Length of hospital stay (days) |
13 (8–25) |
13 (7–22) |
0.232 |
1.00 (0.99–1.01) |
Colonisation with ESBL-K. pneumoniae
was associated with an increased probability of aminoglycoside resistance than colonisation
with ESBL-E. coli (odds ratio [OR] = 2.74, 95% confidence interval [CI]
= 1.71–4.36, p <0.001; table 1). In multivariable analyses including
travel history and ESBL-PE species, colonisation with ESBL-K. pneumoniae
remained associated with aminoglycoside resistance (OR = 2.64, 95% CI = 1.65–4.21,
p <0.001), while travel history only showed a trend towards an association
(OR = 1.51, 95% CI = 0.95–2.42, p = 0.084). The Hosmer-Lemeshow goodness-of-fit
test revealed adequate model fit (Hosmer-Lemeshow chi-square = 0.86, p = 0.649).
Discussion
Aminoglycosides are commonly administered
to patients with ESBL-PE infections. However, ESBL-PE infections are at risk of
aminoglycoside resistance. Understanding the patients at greatest risk of
aminoglycoside-resistant ESBL-PE is important to optimise patient outcomes. In
a cohort of 544 Swiss patients with ESBL-PE colonisation or carriage identified in
clinical
cultures, we found that 44% of isolates were aminoglycoside resistant.
Colonisation or infection with Klebsiella pneumoniae
was associated with 2.7 times greater odds of aminoglycoside resistance than
colonisation or infection with Escherichia coli. Moreover,
while not statistically significant, a travel history outside of Switzerland
trended towards being associated with aminoglycoside-resistant ESBL-PE
infections.
Our findings highlight the importance of
considering local antibiotic resistance patterns when adopting international
treatment guidelines. The European Conference on Infections in Leukemia (ECIL) and
Infectious Diseases Society of America (IDSA) guidelines recommend adding
aminoglycosides to empiric therapy for neutropenic fever in severely ill
patients, patients with a known history of colonisation with multidrug-resistant
Enterobacterales, or patients at increased risk of multidrug-resistant bacteria
infection [1, 2]. A 2021 propensity-matched cohort study found that combination
therapy with beta-lactam/aminoglycoside was associated with improved survival
in patients with hematologic malignancies and Gram-negative blood infections [7].
However, with up to 44% aminoglycoside resistance among those infected with ESBL-PE
in our setting, empiric therapy including an aminoglycoside might risk
inadequate empirical antibacterial treatment, leading to poor clinical
outcomes, while also unnecessarily exposing patients to toxicities such as
acute kidney injury. This issue is particularly important because beta-lactam
agents commonly used in combination with aminoglycosides, such as
piperacillin-tazobactam or cefepime, are generally considered inadequate for
treating invasive ESBL-PE infections, potentially leaving vulnerable patients
with several days of inadequate coverage against ESBL-PE.
Colonisation with ESBL-K. pneumoniae
was independently associated with aminoglycoside resistance in our cohort compared
to colonisation with ESBL-E. coli. While high levels of aminoglycoside
resistance in ESBL-K. pneumoniae isolates have been previously reported in
Taiwan [8], to our knowledge, its frequency has not been compared to that of ESBL-E.
coli. However, our results are consistent with a previous report indicating
greater resistance to aminoglycosides for Klebsiella pneumoniae compared
to Escherichia coli among European isolates [9]. Travel history was the
only host factor identified in our cohort that may increase the odds of
aminoglycoside-resistant ESBL-PE infections. This finding appears to agree with
a previous study reporting an 8%–40% increase in gentamicin resistance in Escherichia
coli in returning travellers [10]. We did not find an association between
prior aminoglycoside exposure and aminoglycoside resistance. Interestingly, aminoglycoside
resistance has been described as infrequently identified after aminoglycoside
therapy, unlike with other antibiotic agents, where previous exposure is
generally a key resistance determinant [11].
This was a single-centre observational
study, potentially introducing biases and limiting its generalisability. Its data
collection was limited to the years 2016–2018;
however, since the resistance rates of Gram-negative bacteria, analysed annually
by our microbiology laboratory, remained consistent since 2016, we believe that
incorporating more recent data would not significantly alter the outcomes of
our study. Missing data might have led to underestimating associations for some
parameters with aminoglycoside resistance in ESBL-PE carriers. Since collecting
information on the included variables is standard practice, we consider the
respective impact on our findings minor. Furthermore, this limitation is
unlikely to have biased our results since it affects both patients with aminoglycoside-resistant
and
aminoglycoside-susceptible ESBL-PE colonisation. These limitations notwithstanding,
our
study indicates that aminoglycoside resistance is frequently detected in
ESBL-PE, especially ESBL-K. pneumoniae. Estimating the risk of carrying ESBL-PE
based on risk scores validated for local epidemiology might help guide
clinicians’ decision-making on empiric therapy regimens [12], along with infection
severity.
Availability of data and materials
The dataset used and analysed during this
study is available from the corresponding author upon reasonable request.
Author contributions: IV collected and analysed data, interpreted the results and wrote
the manuscript. LAB, NK, EWW and PDT critically revised the manuscript. AE
described the microbiological analyses and critically revised the manuscript.
STS conceived and supervised the study, analysed data, interpreted the results
and critically revised the manuscript.
Prof. Sarah Tschudin-Sutter, MD, MSc
Division of Infectious Diseases and
Hospital Epidemiology
University Hospital Basel
Petersgraben 4
CH-4031 Basel
sarah.tschudin[at]usb.ch
References
1. Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, et al.; ECIL4,
a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN. European guidelines for
empirical antibacterial therapy for febrile neutropenic patients in the era of growing
resistance: summary of the 2011 4th European Conference on Infections in Leukemia.
Haematologica. 2013 Dec;98(12):1826–35. doi: https://doi.org/10.3324/haematol.2013.091025
2. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al.; Infectious
Diseases Society of America. Clinical practice guideline for the use of antimicrobial
agents in neutropenic patients with cancer: 2010 update by the infectious diseases
society of america. Clin Infect Dis. 2011 Feb;52(4):e56–93. doi: https://doi.org/10.1093/cid/cir073
3. Isendahl J, Giske CG, Tegmark Wisell K, Ternhag A, Nauclér P. Risk factors for community-onset
bloodstream infection with extended-spectrum β-lactamase-producing Enterobacteriaceae:
national population-based case-control study. Clin Microbiol Infect. 2019 Nov;25(11):1408–14.
doi: https://doi.org/10.1016/j.cmi.2019.04.002
4. Vock I, Aguilar-Bultet L, Egli A, Tamma PD, Tschudin-Sutter S. Infections in Patients
Colonized With Extended-spectrum Beta-Lactamase-Producing Enterobacterales: A Retrospective
Cohort Study. Clin Infect Dis. 2021 Apr;72(8):1440–3. doi: https://doi.org/10.1093/cid/ciaa895
5. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic
data capture (REDCap)—a metadata-driven methodology and workflow process for providing
translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81.
doi: https://doi.org/10.1016/j.jbi.2008.08.010
6. Centers for Disease Control and Prevention. CDC/NHSN Surveillance Definitions for
Specific Types of Infections. 2022. Available from https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf
7. Albasanz-Puig A, Gudiol C, Puerta-Alcalde P, Ayaz CM, Machado M, Herrera F, et al. Impact
of the Inclusion of an Aminoglycoside to the Initial Empirical Antibiotic Therapy
for Gram-Negative Bloodstream Infections in Hematological Neutropenic Patients: a
Propensity-Matched Cohort Study (AMINOLACTAM Study). Antimicrob Agents Chemother.
2021 Jul;65(8):e0004521. doi: https://doi.org/10.1128/AAC.00045-21
8. Ma L, Lin CJ, Chen JH, Fung CP, Chang FY, Lai YK, et al.; Taiwan Surveillance of Antimicrobial
Resistance Project. Widespread dissemination of aminoglycoside resistance genes armA
and rmtB in Klebsiella pneumoniae isolates in Taiwan producing CTX-M-type extended-spectrum
beta-lactamases. Antimicrob Agents Chemother. 2009 Jan;53(1):104–11. doi: https://doi.org/10.1128/AAC.00852-08
9. Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide
source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017 May;41(3):252–75.
doi: https://doi.org/10.1093/femsre/fux013
10. Kennedy K, Collignon P. Colonisation with Escherichia coli resistant to “critically
important” antibiotics: a high risk for international travellers. Eur J Clin Microbiol
Infect Dis. 2010 Dec;29(12):1501–6. doi: https://doi.org/10.1007/s10096-010-1031-y
11. Karlowsky JA, Zelenitsky SA, Zhanel GG. Aminoglycoside adaptive resistance. Pharmacotherapy.
1997;17(3):549–55. doi: https://doi.org/10.1002/j.1875-9114.1997.tb03063.x
12. Vock I, Aguilar-Bultet L, Egli A, Tamma PD, Tschudin-Sutter S. Independent, external
validation of clinical prediction rules for the identification of extended-spectrum
β-lactamase-producing Enterobacterales, University Hospital Basel, Switzerland, January
2010 to December 2016. Euro Surveill. 2020 Jul;25(26):1900317. doi: https://doi.org/10.2807/1560-7917.ES.2020.25.26.1900317
Appendix: Definitions
History of hospitalisation: Hospitalisation
lasting ≥2 days within the past 12 months prior to index hospitalisation.
Travel history: A stay outside of
Switzerland within 12 months prior to the index hospitalisation.
Hospitalisation abroad: Hospitalisation
outside of Switzerland within 12 months prior to the index hospitalisation.
Active open wounds: Decubitus or ulcers.
Surgery: Within three months prior to index
hospitalisation.
Vascular hardware: Central venous catheters
in place for at least seven days prior to index sample.
Urinary catheterisation: Suprapubic or
transurethral catheterisation within 30 days prior to the index sample
detecting ESBL-PE.
Antibiotic therapy: Any antibiotic
medication within three months prior to the index sample detecting ESBL-PE.
Duration of antibiotic therapy: Cumulative
duration (days) of any antibiotic therapy within the three months prior to the index
sample detecting ESBL-PE.
Immunosuppressive therapy: Within 12 months
prior to index sample (e.g. long-term steroids, cytostatics,
biologicals/antibody therapies, mTOR-Inhibitors and calcineurin-inhibitors).
Proton pump inhibitor: Within 12 months
prior to the index sample.
Infections: Acute infection within the relevant
hospitalisation, defined according to the CDC guidelines.
