Blood pressure control and antihypertensive treatment in Swiss general practice: a
cross-sectional study using routine data
DOI: https://doi.org/https://doi.org/10.57187/s.3898
Stefania Di Gangia*,
Roman Brennerb*,
Thomas Grischotta,
Jakob Martin Burgstallera,
Oliver Senna,
Thomas Rosemanna,
Stefan Markuna
a Institute of Primary Care,
University Hospital Zurich, University of Zurich, Zurich, Switzerland
b Department of Cardiology, Kantonsspital St. Gallen, St. Gallen, Switzerland
* Shared first authorship
Summary
AIMS OF THE
STUDY: Arterial hypertension is a major global health risk.
Global surveys indicate that only half of patients with arterial hypertension receive
pharmacotherapy, and only a quarter achieve the primary blood pressure target recommended
by guidelines. This study aimed to evaluate the achievement of the primary blood
pressure target in Swiss general practice, provide insights into arterial
hypertension treatment, and identify factors associated with achieving this goal.
METHODS: This cross-sectional study utilised data from a large Swiss primary
care database. Patients with arterial hypertension, aged ≥18 years, who underwent
blood pressure monitoring in 2021 were included. The primary observation was blood
pressure control, defined as the achievement of the primary blood pressure target
of systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm
Hg. Demographic data from physicians and patients, blood pressure measurements,
comorbidities, cardiovascular risk factors, and pharmacotherapy were collected,
and arterial hypertension stages were calculated. Unadjusted and multivariable-adjusted
mixed logistic regression models were used to identify factors associated with blood
pressure control.
RESULTS: A total of 49,290 patients were included, of whom 23,933 (48.6%) were
female. The median patient age was 71 years (interquartile range 61–80). Blood
pressure control was observed in 23,022 patients (46.7%), and 36,692 patients (74.4%)
had an antihypertensive pharmacotherapy prescription. In multivariable analysis,
blood pressure control was positively associated with arterial hypertension stage,
antihypertensive pharmacotherapy, the intensity of blood pressure monitoring, and
the number of blood pressure-increasing drugs, but negatively associated with a
long-standing arterial hypertension, female sex, and old age.
CONCLUSIONS: While general practitioners appear to consider
arterial hypertension stages in their treatment strategies, there is still room
for improvement in arterial hypertension care by prescribing pharmacotherapy, especially
in patients with long-standing arterial hypertension, female sex and old age.
Abbreviations
- ACEI:
-
angiotensin-converting enzyme inhibitor
- ARB:
-
angiotensin receptor blocker
- CCB:
-
calcium channel blocker
- ESC:
-
European Society of Cardiology
- ESH:
-
European Society of Hypertension
Introduction
Arterial hypertension affects approximately
10% of the global population and is the leading cause of cardiovascular diseases
and premature mortality worldwide [1, 2]. Along
with lifestyle modifications, lowering blood pressure with pharmacotherapy is the
mainstay of arterial hypertension treatment and is supported by strong evidence
demonstrating its beneficial effects on key outcomes [3, 4]. Numerous trials have
evaluated the optimal treatment goals for pharmacotherapy
in arterial hypertension, based on blood pressure levels and patient characteristics
[4–6].
However, the evidence is less clear for older patients [7–9]. Given the potential
side
effects of arterial hypertension pharmacotherapy and the risk of treatment discontinuation,
the 2018 European Society of Cardiology (ESC) / European Society of Hypertension
(ESH) guidelines for the management of arterial hypertension recommend a stepwise
approach to achieve blood pressure targets: a primary blood pressure goal, and for
patients who tolerate treatment well, a secondary goal [10].
Achieving the primary blood pressure target
of systolic blood pressure <140 mm Hg and diastolic blood pressure
<90 mm Hg is commonly referred to as blood pressure control [11]. The
proportion of arterial hypertension patients with blood pressure control is frequently
used as a performance indicator for the quality of hypertension care [11]. Despite
this, global surveys continue to show substantial evidence-to-practice gaps, with
only half of the patients with arterial hypertension receiving pharmacotherapy and
only a quarter with blood pressure control [12, 13]. In
Switzerland, a 2009 study reported blood pressure control in approximately 50% of
patients receiving antihypertensive pharmacotherapy [14]. However, blood pressure
control is influenced by both patient- and physician-related factors, which need
further exploration as potential enhancers or detractors. While limited data exist
on physician characteristics as predictors of blood pressure control, all relevant
variables should be assessed to identify potential targets for interventions aimed
at improving the quality of arterial hypertension care.
Therefore, to consolidate the epidemiological
basis for better arterial hypertension management, this study primarily seeks to
provide updated insights into blood pressure control in Swiss general practice and,
secondarily, to identify factors associated with blood pressure control, including
arterial hypertension stage, pharmacotherapy, and patient and physician characteristics.
Materials and methods
Study design, setting, participants and ethics statement
We conducted a cross-sectional
study based on data from Family Medicine Research using Electronic Medical Records
(FIRE), a large Swiss general practice database established in 2009 [15]. The database
currently includes data from 750 individual general practitioners and over 12 million
consultation records, including administrative information, laboratory test results,
vital sign measurements, and pharmacotherapy prescriptions. For this study, we included
general practitioners who contributed data in 2021. Patient inclusion criteria were
as follows: arterial hypertension diagnosis before 2021, age ≥18 years, and blood
pressure monitoring during 2021 (i.e., at least one office blood pressure measurement
recorded in 2021). Arterial hypertension was defined as at least one of the following
at any time during the patient's medical history: (1) antihypertensive drug therapy
as defined by the Swiss Pharmaceutical Cost Group [16]; (2) a general
practitioner-assigned reason for encounter codes K85, K86, or K87 of the International
Classification of Primary Care, 2nd edition (ICPC-2) [17]; or (3) at least two office-based
blood pressure measurements with systolic blood pressure ≥140 mm Hg or diastolic blood
pressure ≥90 mm Hg each, with the
second confirmatory measurement collected within 7 days to 6 months of the first,
or a single blood pressure measurement of systolic blood pressure ≥180 mm Hg or diastolic
blood pressure ≥110 mm Hg without
a second confirmatory measurement [10, 18].
The local ethics committee of the Canton of
Zurich waived approval for this study as it was based on anonymised data and thus
fell outside the scope of the Swiss Federal Act on Research Involving Human Beings
(BASEC-Nr. Req2017–00797).
Database query and definitions
For each patient, the last available blood
pressure measurement in 2021 was defined as the index measurement. The most recent
information prior to the index measurement was used to determine sex, age (continuous
and categorical variables: <30, 30–64, 65–80, >80 years, or <65, ≥65 years
as appropriate), and comorbidities (obesity, chronic kidney disease, dyslipidaemia,
diabetes mellitus, and cardiovascular disease, as defined in appendix table S1,
according to established methods [19, 20]). To capture
active antihypertensive pharmacotherapy at the time of the index measurement, we
queried all antihypertensive medications documented in the 5 years preceding the
index measurement that had not been subsequently discontinued, based on their Anatomical
Therapeutic Chemical (ATC) codes [21]. Data on potentially blood pressure-increasing
pharmacotherapy were considered from 3 months prior to the index measurement.
Antihypertensive drugs were divided into the
following classes: angiotensin-converting enzyme inhibitors (ACEI), angiotensin
II receptor blockers (ARB), beta-blockers, calcium channel blockers (CCB),
diuretics, and other antihypertensives (list of ATC codes is provided in appendix
table S2). The following were classified as blood pressure-increasing drugs: antidepressants,
oestrogens or testosterone, stimulants, anti-obesity agents, decongestants, antipsychotics,
and systemic preparations of non-steroidal anti-inflammatory drugs (NSAIDs) and
steroids (table S2) [22]. For combination drugs, each antihypertensive/blood
pressure-increasing component was counted towards the total number of prescribed
medications. Antihypertensive treatment intensity was classified as monotherapy,
dual therapy, triple therapy, or more than three antihypertensive substances.
Arterial hypertension stages 1 to 3 were determined using the definitions outlined
in the 2018
ESC guidelines for the management of arterial hypertension [10] and adapted
to the variables available in FIRE (table S3 in the appendix). According to these
guidelines [10], arterial hypertension was classified as controlled when the primary blood pressure goal was achieved (systolic blood pressure [SBP] <140
mm Hg and diastolic blood pressure [DBP] <90 mm Hg), with further sub-classification
based on the achievement
of the secondary blood pressure goal (age <65 years: SBP <130 mm Hg and DBP
<80 mm Hg; age ≥65 years: SBP <140 mm Hg and DBP <80 mm Hg). Arterial
hypertension was classified as uncontrolled
when the primary blood pressure goal was not achieved (SBP ≥140 mm Hg or DBP
≥90 mm Hg), with further sub-classification into uncontrolled systolic (SBP ≥140 mm Hg and DBP <90 mm Hg), uncontrolled diastolic (SBP <140 mm
Hg and DBP ≥90 mm Hg) or uncontrolled combined
systolic-diastolic (SBP ≥140 mm Hg and DBP ≥90 mm Hg). Patients with uncontrolled
arterial hypertension were further classified as having resistant arterial hypertension if they were taking antihypertensive
medications from three or more different drug classes at the time of the index measurement,
including a diuretic.
The general practitioner characteristics collected
were: age (both continuous and categorical variables: ≤50, >50 years), sex, type
of practice organisation (single, double, or group practice), working position (employee,
self-employed), workload (expressed as percentage of full-time equivalent) in the
practice, and the practice’s postal code, which was used to identify the practice’s
location area (urban, suburban, rural) according to the Eurostat Degree of Urbanisation
index for Switzerland [23].
Observations
The primary observation was blood pressure
control, defined as the achievement of the primary blood pressure goal (yes, no).
Secondary observations included the achievement of the secondary blood pressure
goal (yes, no) and arterial hypertension pharmacotherapy (categorical), as defined
above in terms of drug classes and treatment intensity.
We considered the following variables as potential
factors associated with blood pressure control: (3a) patient-related:
age (categorical: <65, ≥65 years), sex, arterial hypertension stage, pharmacotherapy
for arterial hypertension (yes, no), number of blood pressure-increasing drugs used
(categorical: 0, 1, ≥2), long-lasting arterial hypertension diagnosis (≥5 years:
yes, no), intensity of blood pressure monitoring in 2021 (categorical: ≤5, >5
measurements); (3b) general practitioner-related: age (categorical),
sex, practice organisation, working position and workload, and practice location
area.
Statistical analysis
Summary statistics were presented as numbers
(n) and percentages (%) for categorical and binary variables, and as mean and standard
deviation (SD) or median and interquartile range (IQR), as appropriate, for continuous
variables. An available case analysis was performed. Blood pressure control and
the achievement of the secondary blood pressure goal were presented as proportions
of all included patients and within each arterial hypertension stage and age group,
with the latter stratification represented graphically. Descriptive statistics for
arterial hypertension pharmacotherapy were reported overall and stratified by patients
with and without blood pressure control. To identify factors associated with blood
pressure control, both unadjusted (univariable) and multivariable-adjusted mixed
logistic regression models were used. Random intercept effects were included at
the general practitioner level to account for correlations between patients cared
for by the same general practitioner. Predictors for the multivariable final model
were selected using a stepwise backward approach, starting from a full model that
included all variables with p <0.2 in univariable analyses. Multicollinearity
was assessed using the variance inflation factor (VIF), generalised for logistic
regression, for each predictor.
The results of the regression analyses were
reported as odds ratios (OR) with 95% confidence intervals (CI). The final model
results were represented in an odds ratio (OR) plot.
Test results were considered statistically significant
at p ≤0.05. All analyses were conducted
using the R statistical package, version 4.1.0 [24], with additional packages: dplyr version 1.1.2, tidyverse
version 2.0.0, tableone version 0.13.2, ggplot2
version 3.4.2, finalfit version 1.0.6,
ggVennDiagram version 1.2.2.
Results
Patient characteristics
We identified 458,240 eligible patients in the
FIRE database, with 49,290 meeting the inclusion criteria (figures S1–S2 in the
appendix, for the full inclusion process
and identification criteria). Patient characteristics, overall and stratified by
arterial hypertension stage, are shown in table 1. Of the total patient cohort, 23,933
(48.6%) were women. The
median patient age was 71 years (IQR 61–80). The most prevalent comorbidities were
dyslipidaemia, obesity, and cardiovascular disease, affecting 22,764 (46.2%), 14,582
(29.6%), and 13,631 (27.7%) patients, respectively.
Table 1Demographic characteristics, comorbidities, and
blood pressure characteristics at the index measurement of the 49,290 patients
included, overall and stratified by arterial hypertension stage.
|
Overall |
Stage 1 arterial hypertension |
Stage 2 arterial hypertension |
Stage 3 arterial hypertension |
| Total, n (%) |
49,290 |
24,302 (49.3) |
10,897 (22.1) |
14,091 (28.6) |
| Demographic characteristics |
Sex* (female) n (%) |
23,933 (48.6) |
12,454 (51.2) |
5533 (50.8) |
5946 (42.2) |
| Age (years) median (IQR) |
71 (61–80) |
66 (56–75) |
74 (63–82) |
77 (69–83) |
| Co-occurring conditions**, n (%) |
Obesity |
14,582 (29.6) |
6327 (26.0) |
4134 (37.9) |
4121 (29.2) |
| Chronic kidney disease |
7695 (15.6) |
511 (2.1) |
3402 (31.3) |
3782 (26.8) |
| Chronic kidney disease grade 3 or
higher |
6300 (12.8) |
0 (0.0) |
3171 (29.1) |
3129 (22.2) |
| Dyslipidaemia |
22,764 (46.2) |
7427 (30.6) |
5007 (45.9) |
10,330 (73.3) |
| History of smoking |
909 (1.9) |
347 (1.6) |
174 (1.6) |
388 (2.8) |
| Diabetes mellitus |
11,709 (23.8) |
0 (0.0) |
7063 (64.8) |
4646 (33.0) |
| Cardiovascular disease |
13,631 (27.7) |
0 (0.0) |
0 (0.0) |
13,631 (96.7) |
| – Heart failure or atrial fibrillation |
2027 (4.1) |
0 (0.0) |
0 (0.0) |
2027 (14.4) |
| – Obstructive atherosclerotic disease |
12,444 (25.2) |
0 (0.0) |
0 (0.0) |
12,444 (88.3) |
| – Pulmonary heart disease |
40 (0.0) |
0 (0.0) |
0 (0.0) |
40 (0.0) |
| Blood pressure index measurement,
mean (SD) |
Systolic blood pressure (mm Hg) |
139.9 (18.7) |
141.1 (18.2) |
139.1 (18.8) |
138.6 (19.3) |
| Diastolic blood pressure (mm Hg) |
81.9 (11.4) |
84.5 (10.9) |
80.6 (11.1) |
78.6 (11.3) |
| Blood pressure goal achievement, n
(%) |
Primary (i.e., blood pressure control) |
23,022 (46.7) |
10,379 (42.7) |
5439 (49.9) |
7204 (51.1) |
| Secondary |
12,411 (25.2) |
4619 (19.0) |
3127 (28.7) |
4665 (33.1) |
| Uncontrolled arterial hypertension, n
(%) |
Isolated systolic |
14,015 (28.4) |
6201 (25.5) |
3214 (29.5) |
4600 (32.6) |
| Isolated diastolic |
2610 (5.3) |
1698 (7.0) |
485 (4.5) |
427 (3.0) |
| Combined systolic-diastolic |
9643 (19.6) |
6024 (24.8) |
1759 (16.1) |
1860 (13.2) |
| Resistant arterial hypertension |
6326 (12.8) |
2282 (9.4) |
1464 (13.4) |
2580 (18.3) |
Blood pressure control
The average index measurement was 139.9 mm
Hg (SD 18.7) for systolic blood pressure and 81.9 mm Hg (SD 11.4) for diastolic blood
pressure (systolic and diastolic blood pressure distributions
are shown in figure S3–S4 in the appendix). Overall, blood pressure control was
observed in 23,022 patients (46.7%), while the secondary blood pressure goal was
met in 12,411 patients (25.2%). The percentages increased with the arterial
hypertension stage, with blood pressure control rates of 42.7%, 49.9%, and 51.1%,
and achievement of the secondary goal in 19.0%, 28.7%, and 33.1% of cases in arterial
hypertension stages 1 to 3, respectively. Blood pressure control and the achievement
of the secondary blood pressure goal, stratified by arterial hypertension stage
and age group, are shown in table 1 and figure 1, respectively.
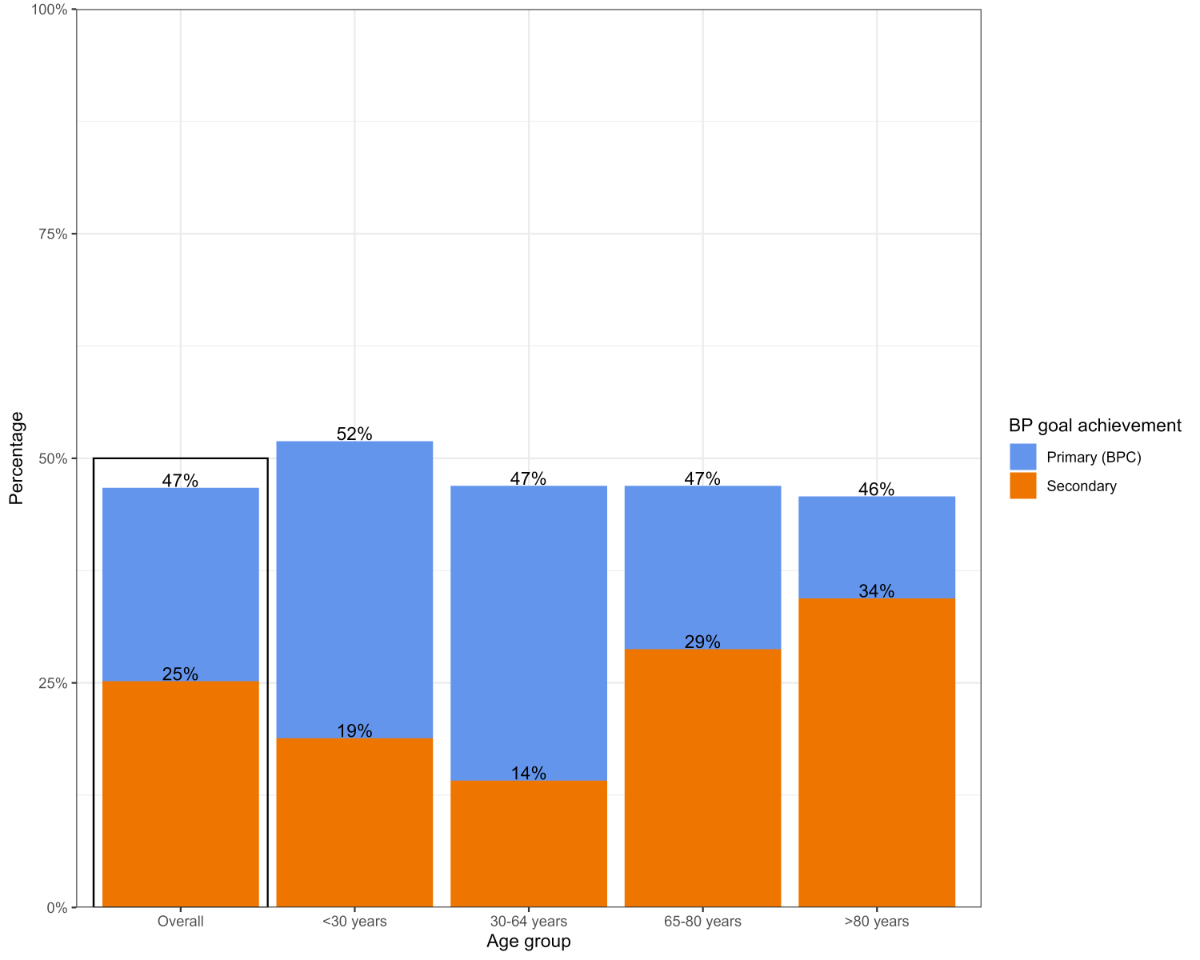
Figure 1Blood pressure control (BPC) (defined as
achieving systolic <140 mm Hg and diastolic <90 mm Hg) and achievement of
the secondary blood pressure goal (systolic <130 mm Hg, or <140 mm Hg if
aged ≥65 years, and diastolic <80 mm Hg). Percentages are reported overall
and stratified by patient age group. BP: blood pressure.
Antihypertensive pharmacotherapy
Overall, 36,692 patients (74.4%) had active
antihypertensive prescriptions at the time of the blood pressure index measurement.
Among the remaining 12,598 patients (25.6%), 6092 (12.4%) had never been treated,
and 6506 (13.2%) had previously been prescribed antihypertensive pharmacotherapy,
which was later discontinued. Regarding treatment intensity, 12,820 patients (26.0%)
were prescribed monotherapy, 11,937 (24.2%) were prescribed dual therapy, 7771 (15.8%)
received triple therapy, and 4164 (8.4%) received more than three antihypertensive
substances.
The most frequently prescribed drug class overall
was ARB, prescribed to 17,102 patients (34.7%), followed by beta-blockers to 15,756
patients
(32.0%), and ACEI to 14,956 patients (30.3%). The distribution of individual drug
classes across monotherapy, dual therapy, triple therapy, and four or more therapies
is shown in figure 2.
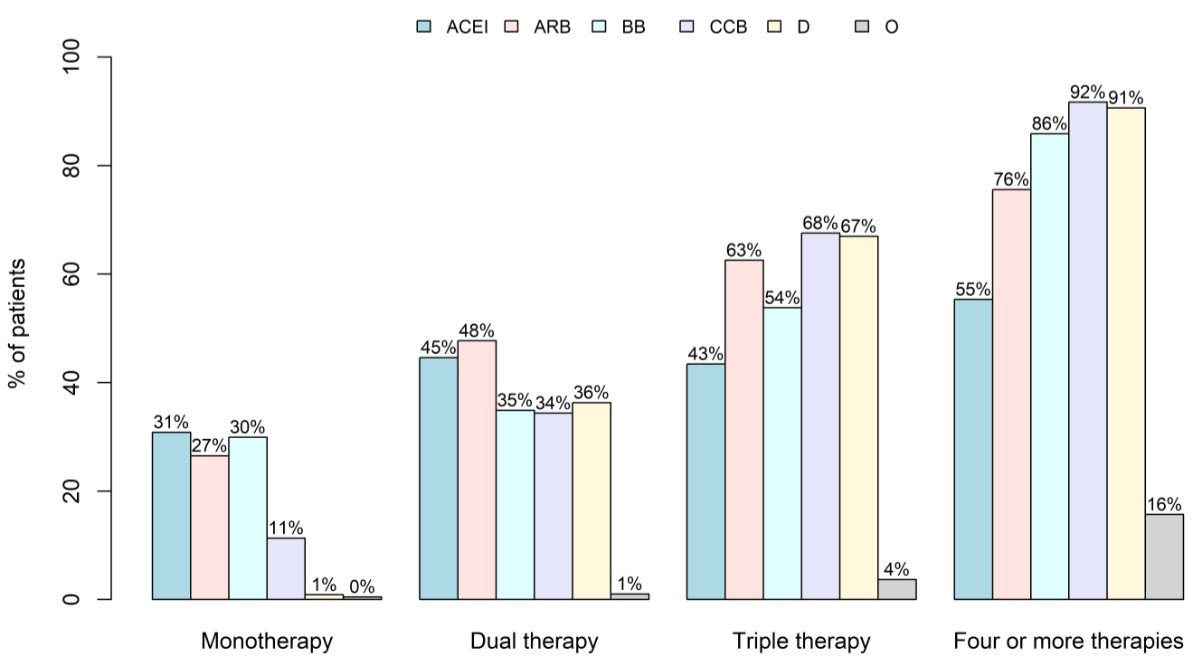
Figure 2Drug classes by intensity of prescribed
pharmacotherapy. Percentages are stratified across patients receiving
monotherapy, dual therapy, triple therapy, and four or more therapies. ACEI:
angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BB:
beta-blocking agent; CCB: calcium channel blocker; D: diuretic; O: other antihypertensives.
Table 2 describes the prescribed antihypertensive pharmacotherapies overall and stratified
by blood pressure control status (ATC codes listed in table S4 in the appendix).
Table 2Numbers (percentages) of patients with (or
without) specific antihypertensive pharmacotherapy at index measurement,
overall and stratified by blood pressure control. Only drug combinations
exceeding 1% within classes are shown.
| |
Overall |
Patients with BPC |
Patients without BPC |
| n = 49,290 |
n = 23,022 |
n = 26,268 |
| Pharmacotherapy |
| Active* antihypertensive pharmacotherapy n (%) |
36,692 (74.4) |
17,483 (75.9) |
19,209 (73.1) |
| No active antihypertensive pharmacotherapy n (%) |
12,598 (25.6) |
5539 (24.1) |
7059 (26.9) |
| – Never prescribed |
6092 (12.4) |
2243 (9.7) |
3849 (14.7) |
| – Prescribed but discontinued |
6506 (13.2) |
3296 (14.3) |
3210 (12.2) |
| Type of antihypertensive pharmacotherapy |
| Monotherapy n (%) |
Overall |
12,820 (26.0) |
6181 (26.8) |
6639 (25.3) |
| ACEI |
3953 (8.0) |
1793 (7.8) |
2160 (8.2) |
| ARB |
3399 (6.9) |
1479 (6.4) |
1920 (7.3) |
| Beta-blocker |
3836 (7.8) |
2174 (9.4) |
1662 (6.3) |
| CCB |
1453 (2.9) |
650 (2.8) |
803 (3.1) |
| Diuretic |
115 (0.2) |
53 (0.2) |
62 (0.2) |
| Dual therapy, n (%) |
Overall |
11,937 (24.2) |
5693 (24.7) |
6244 (23.8) |
| All fixed-dose combinations |
5779 (11.7) |
2615 (11.4) |
3164 (12.0) |
| ARB + diuretic, total |
2225 (4.5) |
970 (4.2) |
1255 (4.8) |
| … as fixed-dose combination |
2144 (4.3) |
944 (4.1) |
1200 (4.6) |
| ARB + CCB, total |
1791 (3.6) |
760 (3.3) |
1031 (3.9) |
| … as fixed-dose combination |
942 (1.9) |
412 (1.8) |
530 (2.0) |
| ARB + beta-blocker, total |
1298 (2.6) |
645 (2.8) |
653 (2.5) |
| … as fixed-dose combination |
– |
– |
– |
| ACEI + CCB, total |
1538 (3.1) |
674 (2.9) |
864 (3.3) |
| … as fixed-dose combination |
678 (1.4) |
309 (1.3) |
369 (1.4) |
| ACEI + beta-blocker, total |
1819 (3.7) |
1081 (4.7) |
738 (2.8) |
| … as fixed-dose combination |
– |
– |
– |
| ACEI + diuretic, total |
1607 (3.3) |
733 (3.2) |
874 (3.3) |
| … as fixed-dose combination |
1560 (3.2) |
709 (3.1) |
851 (3.2) |
| CCB + beta-blocker, total |
716 (1.5) |
343 (1.5) |
373 (1.4) |
| … as fixed-dose combination |
50 (0.1) |
27 (0.1) |
23 (0.1) |
| Triple therapy n (%) |
Overall |
7771 (15.8) |
3718 (16.1) |
4053 (15.4) |
| As single drugs |
2592 (5.3) |
1281 (5.6) |
1311 (5.0) |
| Including a dual fixed-dose combination |
3968 (8.1) |
1849 (8.0) |
2119 (8.1) |
| As a triple fixed-dose combination |
1211 (2.5) |
588 (2.6) |
623 (2.4) |
| ARB + CCB + diuretic, total |
1930 (3.9) |
846 (3.7) |
1084 (4.1) |
| … including a dual fixed-dose combination |
1010 (2.0) |
420 (1.8) |
590 (2.2) |
| … as triple fixed-dose combination |
890 (1.8) |
416 (1.8) |
474 (1.8) |
| ARB + CCB + beta-blocker, total |
992 (2.0) |
456 (2.0) |
536 (2.0) |
| … including a dual fixed-dose combination |
404 (0.8) |
180 (0.8) |
224 (0.9) |
| … as triple fixed-dose combination |
– |
– |
– |
| ARB + beta-blocker + diuretic, total |
1024 (2.1) |
500 (2.2) |
524 (2.0) |
| … including a dual fixed-dose combination |
977 (2.0) |
480 (2.1) |
497 (1.9) |
| … as triple fixed-dose combination |
– |
– |
– |
| ACEI + CCB + diuretic, total |
985 (2.0) |
457 (2.0) |
528 (2.0) |
| … including a dual fixed-dose combination |
631 (1.3) |
270 (1.2) |
361 (1.4) |
| … as triple fixed-dose combination |
321 (0.7) |
172 (0.7) |
149 (0.6) |
| ACEI + CCB + beta-blocker, total |
865 (1.8) |
453 (2.0) |
412 (1.6) |
| … including a dual fixed-dose combination |
275 (0.6) |
157 (0.7) |
118 (0.4) |
| … as triple fixed-dose combination |
– |
– |
– |
| ACEI + beta-blocker + diuretic, total |
731 (1.5) |
381 (1.7) |
350 (1.3) |
| … including a dual fixed-dose combination |
671 (1.4) |
342 (1.5) |
329 (1.3) |
| … as triple fixed-dose combination |
– |
– |
– |
| Four or more therapies n (%) |
4164 (8.4) |
1891 (8.2) |
2273 (8.7) |
| Number of drugs median (IQR) |
1 (0–2) |
1 (1–2) |
1 (0–2) |
The most frequently prescribed combinations
in patients who received dual therapy were ARB + diuretic and ACEI + beta-blocker
(prescribed to 4.5%
and 3.7% of patients, respectively). The most prescribed triple therapies were ARB
+ CCB + diuretic
(3.9%) and ARB + beta-blocker + diuretic (2.1%). Among patients on dual or triple
antihypertensive therapies,
10,958 (22.2% of all patients) were prescribed fixed-dose combinations.
Factors associated with blood pressure control
The final adjusted regression model, shown in
figure 3, revealed that blood pressure control was positively associated with
arterial hypertension stage. Compared to stage 1 patients, stage 2 patients had
an OR of 1.38 (CI 1.31–1.45), and stage 3 patients had an OR of 1.46 (CI 1.39–1.54).
Other factors positively associated with blood pressure control included the intensity
of blood pressure monitoring in 2021, with an OR of 1.35 (CI 1.23–1.48) for patients
with more than five measurements compared to those with fewer. Treatment with blood
pressure-increasing drugs was also positively associated with blood pressure
control, with an OR of 1.06 (CI 1.01–1.12) for one blood pressure-increasing drug
prescribed, and 1.14 (CI 1.06–1.23) for more than one blood pressure-increasing
drug, compared to no blood pressure-increasing drugs. Conversely, blood
pressure control was negatively associated with female sex (OR 0.86, CI 0.83–0.90)
compared to male sex, patient age ≥65 years (OR 0.88, CI 0.84–0.91) compared to
younger age, lack of antihypertensive pharmacotherapy (OR 0.86, CI 0.82–0.91) compared
to being on antihypertensive pharmacotherapy, and a long-lasting diagnosis (OR 0.91,
CI 0.86–0.96) compared to a diagnosis within the last five years. Further results
are provided in table S5 in the appendix.
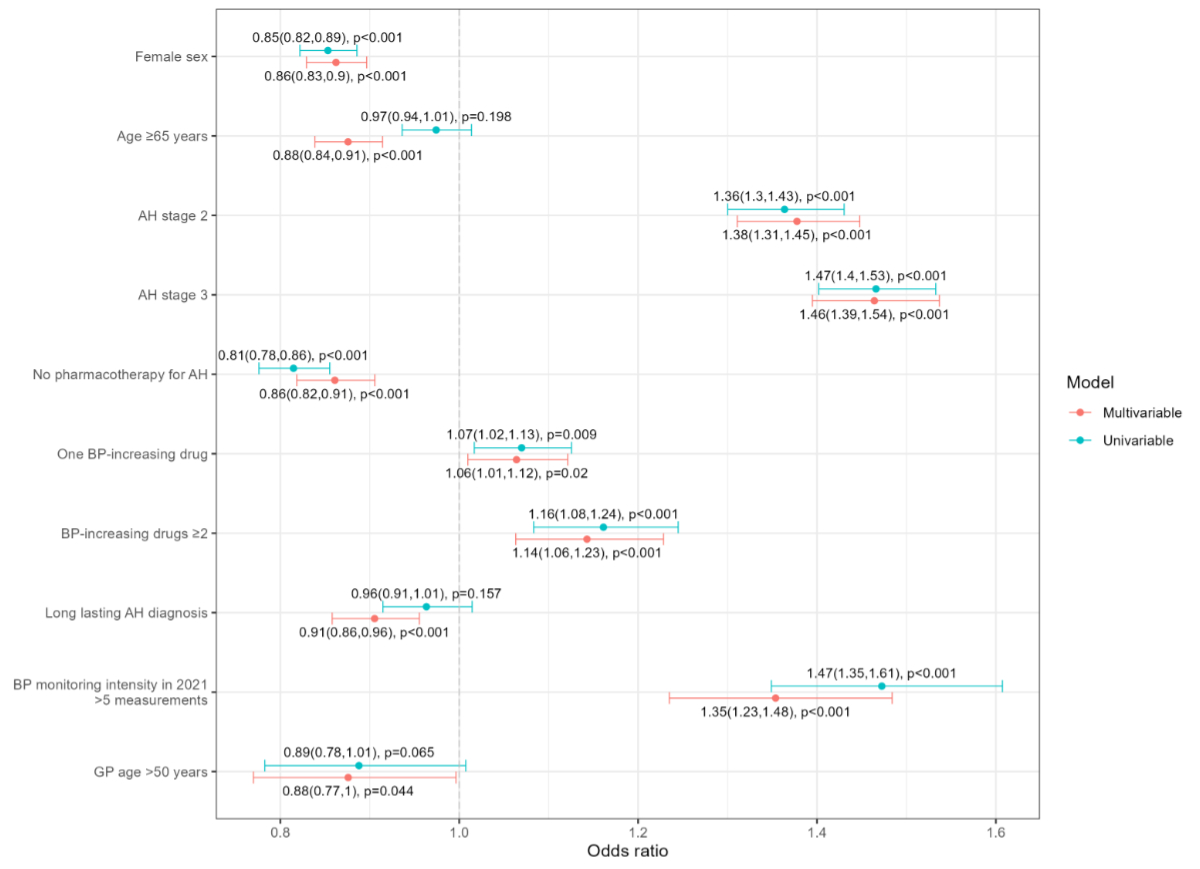
Figure 3Unadjusted (univariable) and multivariable
(adjusted) logistic mixed regression model (forest plot) of blood pressure
control. General practitioners (GPs) were considered as random effects. AH: arterial
hypertension; BP: blood pressure; OR:
odds ratio; CI: confidence interval.
Discussion
Main findings
In this study, we aimed to improve the understanding
of blood pressure control and pharmacotherapy in patients with arterial
hypertension treated in Swiss general practice. We found nearly half of the
patients with blood pressure control, and one in four met the secondary blood
pressure goal. Three-quarters of patients with arterial hypertension received antihypertensive
pharmacotherapy, predominantly in the form of monotherapy or dual therapy.
Blood pressure control was positively associated with arterial hypertension stage,
the intensity of blood pressure monitoring, and the number of blood pressure-increasing
drugs, but negatively associated with the absence of antihypertensive pharmacotherapy,
long-standing arterial hypertension diagnosis, female sex, and older age.
Patient selection and sample representativeness
We identified patients with arterial
hypertension from a large Swiss primary care database and found a prevalence of
approximately 22%. This figure aligns closely with the prevalence reported by Godwin
et al., who used highly comparable methods in a Canadian general practice-based
study [25].
Another study estimated the prevalence of arterial hypertension in Swiss general
practice to be 20% [26], supporting the validity of our data. The patients in our
study were, on average, more than five years older than those in similar studies
using routine general practice data [14, 25, 27]. This difference may be due to our
study design.
First, unlike Godwin et al., we did not impose an upper age limit. Second, our study
required patients to have undergone blood pressure monitoring in 2021, which may
have selected older patients requiring more intensive follow-up.
Risk-stratified strategy for blood pressure control
In our study, 74% of patients received pharmacotherapy
for arterial hypertension, and nearly 50% achieved blood pressure control, a figure
consistent with studies from other high-income Western countries [13] and a previous
Swiss study [14]. We found a positive association between arterial hypertension
stage and blood pressure control, suggesting a risk-stratified treatment strategy.
This represents a shift from previous evidence from Swiss general practice in 2009,
where a negative association was observed [14]. However, a risk-stratified approach
was introduced in the 2013 ESC/ESH guidelines for arterial hypertension management
and appears to have been adopted by Swiss general practitioners [28].
This risk-stratified approach was even more
pronounced for the secondary blood pressure goal, with significant differences between
groups: 19% of arterial hypertension stage 1 patients and 33% of arterial
hypertension stage 3 patients achieved the secondary goal. However, it should be
noted that the secondary goal is age-dependent, and arterial hypertension stage
3 patients, who were on average six years older, had a more attainable target. Nevertheless,
the stage 3 group also had double the proportion of patients with resistant hypertension
compared to the stage 1 group (18% vs 9%), indicating further pharmaceutical escalation
in response to higher risk.
Paradoxically, patients receiving blood
pressure-increasing drugs were more likely to have blood pressure control. However,
for non-steroidal anti-inflammatory drugs, the most frequently prescribed blood
pressure-increasing drugs, the effects on blood pressure control are controversial
[29]. In our data, 86% of patients on blood pressure-increasing drugs also received
antihypertensive pharmacotherapy, compared with 70% of those not on blood
pressure-increasing drugs, potentially offsetting the blood pressure-increasing
effects. Moreover, it is possible that general practitioners were aware of the risks
and prescribed blood pressure-increasing drugs selectively to patients with relatively
low blood pressure levels.
Intensive blood pressure monitoring was also
positively associated with blood pressure control, consistent with findings from
previous studies that showed the benefits of monitoring interventions on blood
pressure control [30–33].
Factors negatively associated with blood pressure control
Other findings warrant attention and could inform
future quality improvement efforts. First, patients with long-standing arterial
hypertension were less likely to have blood pressure control. This may indicate
treatment inertia or status quo bias, which can result in a reluctance to adjust
treatment over time [34, 35]. Furthermore, long-standing arterial hypertension leads
to vascular remodelling, making it more challenging to treat [36]. Second, we found
that female patients were less likely than male patients to have blood
pressure control. This could be partly due to the higher proportion of older women
in our study, as hypertensive women tend to have stiffer large arteries and consequently
higher blood pressure than older men [37]. Although hypertension treatment and blood
pressure control are generally less common in men than in women in most countries,
this difference is small in high-income countries, and, in line with our findings,
a reverse pattern is observed in a few countries [13, 38]. This issue requires further
investigation and targeted quality initiatives, especially if unwarranted underuse
of guideline-recommended therapy in female patients is contributing to this disparity
[39]. Third, older patients were less likely to have blood pressure control.
This finding is consistent with previous studies [40] and may be attributed to vascular
ageing, degenerative processes [37], or reduced tolerability of antihypertensive
pharmacotherapy in older patients. Additionally, general practitioners may be more
reluctant to initiate or intensify treatment in older patients. However, the negative
association between age and blood pressure control was significant only after adjusting
for other potential confounders, suggesting that age alone is not a strong predictor
of blood pressure control. Importantly, for patients aged ≥80 years, the risk-benefit
ratio of antihypertensive pharmacotherapy remains unclear, but age alone should not
justify treatment
de-intensification [9, 10].
Finally, although the negative association between
general practitioner age and blood pressure control was statistically significant,
its relevance was limited. This is a novel finding that requires further research,
though it may be explained by the higher proportion of older patients cared for
by older general practitioners in our study.
Pharmacotherapy
Regarding pharmacotherapy, our findings were
consistent with those of the SWISSHYPE study [14], which examined general practice
patients receiving treatment for arterial hypertension in 2009: approximately one-third
of patients received monotherapy, one-quarter received dual therapy,
one-fifth were on triple therapy, and one-fifth received a fixed-dose combination.
Both low adherence to medication and a “sequential monotherapy” treatment strategy
may impede blood pressure control [10]. Most patients in randomised controlled arterial
hypertension trials ultimately required combination therapy to control their blood
pressure [41], and the PATHWAY study found that initial combination therapy resulted
in higher blood pressure control rates than sequential monotherapy [42], likely
due to the synergistic effects of different pharmacological mechanisms. Moreover,
a recent meta-analysis found that single-pill combination therapy was superior to
free-equivalent combination therapy in terms of drug adherence, persistence, and
blood pressure control [43]. Increasing the use of fixed-dose combinations is therefore
a promising strategy to improve blood pressure control, as recommended by the new
ESC guidelines [44]. Nonetheless, in our study, fixed-dose combinations were used
in only approximately 30% of treated patients. However, the availability of dual
and triple-fixed-dose antihypertensive combinations is steadily increasing, which
may increase the proportion of combination therapies.
The prevalence of beta-blocker prescriptions as monotherapy
was higher than in the SWISSHYPE study [14]. Since treatment with beta-blockers is
recommended
for arterial hypertension treatment only under specific conditions [44], our findings
suggest a gap between guidelines and practice in the management of arterial
hypertension that requires further investigation.
Strengths and limitations
The strengths of this study include its size
and representativeness, as it draws on the large FIRE database [45]. Furthermore,
we identified patients with arterial hypertension not only through diagnostic codes
and pharmacotherapy but also by including blood pressure measurements. This approach
provided valuable insights into the management of patients, including those who
did not receive pharmacotherapy, and allowed us to study different blood
pressure goals. Our approach to identifying patients based on electronic records
is highly reproducible and can be utilised in follow-up studies and interventional
studies aimed at improving the quality of care at the general practitioner level.
A further strength of this study is its novel consideration of both patient and
general practitioner characteristics as potential factors associated with blood
pressure control.
The main limitation of this study is the potential
for misclassification. Firstly, although there are established guidelines for measuring
blood pressure [46], we cannot confirm that a standardised measurement protocol
was always followed. However, it is highly likely that most blood pressure measurements
in the electronic medical records were office-based readings, which can detect “white
coat hypertension” in up to 24% of cases [47, 48]. This “white coat hypertension”
can be reproduced in about half of patients after a single measurement [49], and
since 11% of patients in our sample were identified solely by blood pressure measurement,
up to 5% may have been misclassified as having arterial hypertension, potentially
biasing the blood pressure control rate towards a lower proportion. Secondly, false-positive
identification based on pharmacotherapy cannot be ruled out, especially in cases
where antihypertensive drugs were prescribed primarily for other cardiovascular
indications, such as heart disease. However, since most cases of heart disease are
associated with arterial hypertension (even if not formally diagnosed), this may
be of limited concern. Thirdly, we used arterial hypertension staging criteria according
to the 2018 ESC/ESH guidelines [10], but without access to information on hypertension-mediated
organ damage, which was unavailable in our database. This may have led to an overestimation
of the number of stage 1 patients at the expense of stage 2 patients. However, given
that cardiovascular disease is likely to be accurately detected in our database,
the main results of our study are unlikely to be affected by this limitation.
Fourthly, we acknowledge that about half of
the patients with arterial hypertension were excluded because they did not have
any blood pressure measurements in 2021. This could affect the validity of our results
concerning blood pressure control, as blood pressure control is contingent
upon blood pressure measurements being recorded.
Another limitation is the study design, which
did not allow for the investigation of causality, only associations with blood
pressure control. Moreover, we lacked information on several factors that could
have influenced blood pressure control, such as patient awareness of arterial
hypertension, lifestyle (diet, physical activity, stress), socio-economic and educational
status, non-pharmacological treatment, or compliance with pharmacotherapy [44, 50].
Conclusions
Our findings suggest that general practitioners
are adopting a risk-stratified management strategy for patients with arterial
hypertension, in line with the revised guidelines. This represents a paradigm shift
compared to the management strategies employed a decade ago. However, uncontrolled
arterial hypertension remains prevalent in Swiss general practice, and there is
significant potential to improve the quality of care, particularly for patients
not receiving arterial hypertension pharmacotherapy, those with long-standing arterial
hypertension, female sex or old age. The results of this study may inform policymakers
and health professionals in designing interventions to enhance blood pressure
control.
Open science
An unpublished research protocol was used to
guide the study. Data supporting the results are not publicly available due to institutional
data protection restrictions but can be obtained from the corresponding
author upon
reasonable request, along with the R-script used for the
statistical analysis.
Stefania Di Gangi
Institute for Primary Care
University Hospital Zurich
Pestalozzistrasse 24
CH-8091 Zurich
stefania.digangi[at]usz.ch
References
1. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk
factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income,
middle-income, and low-income countries (PURE): a prospective cohort study. Lancet.
2020 Mar;395(10226):795–808. doi: https://doi.org/10.1016/S0140-6736(19)32008-2
2. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global Burden
of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990-2015.
JAMA. 2017 Jan;317(2):165–82. doi: https://doi.org/10.1001/jama.2016.19043
3. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome
incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses
of randomized trials. J Hypertens. 2014 Dec;32(12):2285–95. doi: https://doi.org/10.1097/HJH.0000000000000378
4. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure
lowering for prevention of cardiovascular disease and death: a systematic review and
meta-analysis. Lancet. 2016 Mar;387(10022):957–67. doi: https://doi.org/10.1016/S0140-6736(15)01225-8
5. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering
in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015 Feb;313(6):603–15.
doi: https://doi.org/10.1001/jama.2014.18574
6. Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with
type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and
bayesian random-effects meta-analyses of randomized trials. Circulation. 2011 Jun;123(24):2799–810.
doi: https://doi.org/10.1161/CIRCULATIONAHA.110.016337
7. Thompson A, Barry AR. Should All Patients 75 Years of Age or Older Receive Intensive
Management for Hypertension? Can J Hosp Pharm. 2019;72(3):249–52. doi: https://doi.org/10.4212/cjhp.v72i3.2906
8. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et
al.; SPRINT Research Group. Intensive vs Standard Blood Pressure Control and Cardiovascular
Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016 Jun;315(24):2673–82.
doi: https://doi.org/10.1001/jama.2016.7050
9. Baffour-Awuah B, Dieberg G, Pearson MJ, Smart NA. Blood pressure control in older
adults with hypertension: A systematic review with meta-analysis and meta-regression.
Int J Cardiol Hypertens. 2020 Jul;6:100040. doi: https://doi.org/10.1016/j.ijchy.2020.100040
10. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al.; ESC
Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial
hypertension. Eur Heart J. 2018 Sep;39(33):3021–104. doi: https://doi.org/10.1093/eurheartj/ehy339
11. Gee ME, Campbell N, Sarrafzadegan N, Jafar T, Khalsa TK, Mangat B, et al. Standards
for the uniform reporting of hypertension in adults using population survey data:
recommendations from the World Hypertension League Expert Committee. J Clin Hypertens
(Greenwich). 2014 Nov;16(11):773–81. doi: https://doi.org/10.1111/jch.12387
12. Beaney T, Wang W, Schlaich MP, Schutte AE, Stergiou GS, Alcocer L, et al.; MMM Investigators.
Global blood pressure screening during the COVID-19 pandemic: results from the May
Measurement Month 2021 campaign. J Hypertens. 2023 Sep;41(9):1446–55. doi: https://doi.org/10.1097/HJH.0000000000003488
13. Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al.; NCD
Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence
and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201
population-representative studies with 104 million participants [published correction
appears in Lancet 2022 Feb 5; 399(10324):520]. Lancet. 2021 Sep;398(10304):957–80.
doi: https://doi.org/10.1016/S0140-6736(21)01330-1
14. Brenner R, Waeber B, Allemann Y. Medical treatment of hypertension in Switzerland.
The 2009 Swiss Hypertension Survey (SWISSHYPE). Swiss Med Wkly. 2011 Mar;141:w13169.
doi: https://doi.org/10.4414/smw.2011.13169
15. Chmiel C, Bhend H, Senn O, Zoller M, Rosemann T; FIRE study-group. The FIRE project:
a milestone for research in primary care in Switzerland. Swiss Med Wkly. 2011 Jan;140:w13142.
doi: https://doi.org/10.4414/smw.2011.13142
16. Huber CA, Szucs TD, Rapold R, Reich O. Identifying patients with chronic conditions
using pharmacy data in Switzerland: an updated mapping approach to the classification
of medications. BMC Public Health. 2013 Oct;13(1):1030. doi: https://doi.org/10.1186/1471-2458-13-1030
17.WHO. International Classification of Primary Care, Second edition (ICPC-2) 2020. Available
at: https://www.who.int/standards/classifications/other-classifications/international-classification-of-primary-care
18. Boffa RJ, Constanti M, Floyd CN, Wierzbicki AS; Guideline Committee. Hypertension
in adults: summary of updated NICE guidance. BMJ. 2019 Oct;367:l5310. doi: https://doi.org/10.1136/bmj.l5310
19. Meier R, Grischott T, Rachamin Y, Jäger L, Senn O, Rosemann T, et al. Importance of
different electronic medical record components for chronic disease identification
in a Swiss primary care database: a cross-sectional study. Swiss Med Wkly. 2023 Oct;153(10):40107.
doi: https://doi.org/10.57187/smw.2023.40107
20. Jäger L, Rosemann T, Burgstaller JM, Senn O, Markun S. Quality and variation of care
for chronic kidney disease in Swiss general practice: A retrospective database study.
PLoS One. 2022 Aug;17(8):e0272662. doi: https://doi.org/10.1371/journal.pone.0272662
21. WHO. Guidelines for ATC classification and DDD assignment. Available at https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/
22. Grossman A, Messerli FH, Grossman E. Drug induced hypertension--An unappreciated cause
of secondary hypertension. Eur J Pharmacol 2015; 763(Pt A):15-22.doi: https://doi.org/10.1016/j.ejphar.2015.06.027
23. Swiss Federal Statistics Office. Spatial divisions. Available at: https://www.bfs.admin.ch/bfs/en/home/statistics/cross-sectional-topics/regional-analyses/spatial-divisions.html
24. R Core Team. R: A Language and Environment for Statistical Computing. 2022. R Foundation
for Statistical Computing: Vienna, Austria. https://www.r-project.org/
25. Godwin M, Williamson T, Khan S, Kaczorowski J, Asghari S, Morkem R, et al. Prevalence
and management of hypertension in primary care practices with electronic medical records:
a report from the Canadian Primary Care Sentinel Surveillance Network. CMAJ Open.
2015 Jan;3(1):E76–82. doi: https://doi.org/10.9778/cmajo.20140038
26. Excoffier S, Herzig L, N’Goran AA, Déruaz-Luyet A, Haller DM. Prevalence of multimorbidity
in general practice: a cross-sectional study within the Swiss Sentinel Surveillance
System (Sentinella). BMJ Open. 2018 Mar;8(3):e019616. doi: https://doi.org/10.1136/bmjopen-2017-019616
27. Paulsen MS, Andersen M, Thomsen JL, Schroll H, Larsen PV, Lykkegaard J, et al. Multimorbidity
and blood pressure control in 37 651 hypertensive patients from Danish general practice.
J Am Heart Assoc. 2012 Dec;2(1):e004531. doi: https://doi.org/10.1161/JAHA.112.004531
28. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC
guidelines for the management of arterial hypertension: the Task Force for the Management
of Arterial Hypertension of the European Society of Hypertension (ESH) and of the
European Society of Cardiology (ESC). Eur Heart J. 2013 Jul;34(28):2159–219. doi: https://doi.org/10.1093/eurheartj/eht151
29. Rivasi G, Menale S, Turrin G, Coscarelli A, Giordano A, Ungar A. The Effects of Pain
and Analgesic Medications on Blood Pressure. Curr Hypertens Rep. 2022 Oct;24(10):385–94.
doi: https://doi.org/10.1007/s11906-022-01205-5
30. Steurer-Stey C, Zoller M, Chmiel Moshinsky C, Senn O, Rosemann T. Does a colour-coded
blood pressure diary improve blood pressure control for patients in general practice:
the CoCo trial. Trials. 2010 Apr;11(1):38. doi: https://doi.org/10.1186/1745-6215-11-38
31. Zuo HJ, Ma JX, Wang JW, Chen XR, Hou L. The impact of routine follow-up with health
care teams on blood pressure control among patients with hypertension. J Hum Hypertens.
2019 Jun;33(6):466–74. doi: https://doi.org/10.1038/s41371-018-0158-7
32. He J, Muntner P, Chen J, Roccella EJ, Streiffer RH, Whelton PK. Factors associated
with hypertension control in the general population of the United States. Arch Intern
Med. 2002 May;162(9):1051–8. doi: https://doi.org/10.1001/archinte.162.9.1051
33. Huguet N, Green BB, Voss RW, Larson AE, Angier H, Miguel M, et al. Factors Associated
With Blood Pressure Control Among Patients in Community Health Centers. Am J Prev
Med. 2023 May;64(5):631–41. doi: https://doi.org/10.1016/j.amepre.2022.11.002
34. De Backer T, Van Nieuwenhuyse B, De Bacquer D. Antihypertensive treatment in a general
uncontrolled hypertensive population in Belgium and Luxembourg in primary care: therapeutic
inertia and treatment simplification. The SIMPLIFY study. PLoS One. 2021 Apr;16(4):e0248471.
doi: https://doi.org/10.1371/journal.pone.0248471
35. Augustin A, Coutts L, Zanisi L, Wierzbicki AS, Shankar F, Chowienczyk PJ, et al. Impact
of Therapeutic Inertia on Long-Term Blood Pressure Control: A Monte Carlo Simulation
Study. Hypertension. 2021 Apr;77(4):1350–9. doi: https://doi.org/10.1161/HYPERTENSIONAHA.120.15866
36. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial Stiffness and Cardiovascular
Risk in Hypertension. Circ Res. 2021 Apr;128(7):864–86. doi: https://doi.org/10.1161/CIRCRESAHA.121.318061
37. Pinto E. Blood pressure and ageing. Postgrad Med J. 2007 Feb;83(976):109–14. doi: https://doi.org/10.1136/pgmj.2006.048371
38. Bager JE, Manhem K, Andersson T, Hjerpe P, Bengtsson-Boström K, Ljungman C, et al. Hypertension:
sex-related differences in drug treatment, prevalence and blood pressure control in
primary care. J Hum Hypertens. 2023 Aug;37(8):662–70. doi: https://doi.org/10.1038/s41371-023-00801-5
39. Santilli F, D’Ardes D, Guagnano MT, Davi G. Metabolic Syndrome: Sex-Related Cardiovascular
Risk and Therapeutic Approach. Curr Med Chem. 2017;24(24):2602–27. doi: https://doi.org/10.2174/0929867324666170710121145
40. Andrade SE, Gurwitz JH, Field TS, Kelleher M, Majumdar SR, Reed G, et al. Hypertension
management: the care gap between clinical guidelines and clinical practice. Am J Manag
Care. 2004 Jul;10(7 Pt 2):481–6.
41. Mensah GA, Bakris G. Treatment and control of high blood pressure in adults. Cardiol
Clin. 2010 Nov;28(4):609–22. doi: https://doi.org/10.1016/j.ccl.2010.08.002
42. MacDonald TM, Williams B, Webb DJ, Morant S, Caulfield M, Cruickshank JK, et al.;
British Hypertension Society Programme of Prevention And Treatment of Hypertension
With Algorithm‐based Therapy (PATHWAY). Combination Therapy Is Superior to Sequential
Monotherapy for the Initial Treatment of Hypertension: A Double-Blind Randomized Controlled
Trial. J Am Heart Assoc. 2017 Nov;6(11):e006986. doi: https://doi.org/10.1161/JAHA.117.006986
43. Parati G, Kjeldsen S, Coca A, Cushman WC, Wang J. Adherence to Single-Pill Versus
Free-Equivalent Combination Therapy in Hypertension: A Systematic Review and Meta-Analysis.
Hypertension. 2021 Feb;77(2):692–705. doi: https://doi.org/10.1161/HYPERTENSIONAHA.120.15781
44. Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH
Guidelines for the management of arterial hypertension The Task Force for the management
of arterial hypertension of the European Society of Hypertension: Endorsed by the
International Society of Hypertension (ISH) and the European Renal Association (ERA).
J Hypertens. 2023 Dec;41(12):1874–2071. doi: https://doi.org/10.1097/HJH.0000000000003480
45. FIRE. Family medicine Research using Electronic medical records project. Available
at https://www.fireproject.ch/en
46. Stergiou GS, Parati G, McManus RJ, Head GA, Myers MG, Whelton PK. Guidelines for blood
pressure measurement: development over 30 years. J Clin Hypertens (Greenwich). 2018 Jul;20(7):1089–91.
doi: https://doi.org/10.1111/jch.13295
47. Hsu C, Hansell L, Ehrlich K, Munson S, Anderson M, Margolis KL, et al. Primary care
physician beliefs and practices regarding blood pressure measurement: results from
BP-CHECK qualitative interviews. BMC Prim Care. 2023 Jan;24(1):30. doi: https://doi.org/10.1186/s12875-022-01950-1
48. Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure
measured in the office, at home and during ambulatory monitoring in older patients
in general practice. J Hum Hypertens. 2005 Oct;19(10):801–7. doi: https://doi.org/10.1038/sj.jhh.1001903
49. de la Sierra A, Vinyoles E, Banegas JR, Parati G, de la Cruz JJ, Gorostidi M, et al. Short-Term
and Long-Term Reproducibility of Hypertension Phenotypes Obtained by Office and Ambulatory
Blood Pressure Measurements. J Clin Hypertens (Greenwich). 2016 Sep;18(9):927–33.
doi: https://doi.org/10.1111/jch.12792
50. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et
al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention,
Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of
the American College of Cardiology/American Heart Association Task Force on Clinical
Practice Guidelines. J Am Coll Cardiol. 2018 May;71(19):e127–248. doi: https://doi.org/10.1016/j.jacc.2017.11.006
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3898.


