Alveolar echinococcosis in the canton of Geneva between 2010 and 2021: a descriptive
analysis
DOI: https://doi.org/https://doi.org/10.57187/s.3863
Manon
Ollagnona,
Solange Bresson-Hadnibc,
Laurent Spahrac,
Laura Rubbia-Brandtad,
Christian Tosoae,
François Chappuisab
a Faculty of Medicine, University of Geneva, Geneva, Switzerland
b Division of Tropical and Humanitarian Medicine, University Hospitals
of Geneva, Geneva, Switzerland
c Division of Gastroenterology and Hepatology, University Hospitals of
Geneva, Geneva, Switzerland
d Division of Clinical Pathology, University Hospitals of Geneva, Geneva, Switzerland
e Division of Visceral Surgery, University Hospitals of Geneva, Geneva, Switzerland
Summary
BACKGROUND: Alveolar echinococcosis is a rare but
potentially severe parasitic disease caused by the larval stage of Echinococcus multilocularis, endemic in
many countries in the northern hemisphere, including Switzerland. While the
liver is most commonly affected, other organs can also be involved either by
contiguity or haematogenous spread. To date, there is no epidemiological or
clinical data on alveolar echinococcosis in the canton of Geneva.
OBJECTIVES: To describe the demographic,
epidemiological, clinical and therapeutic characteristics of alveolar
echinococcosis in the canton of Geneva between 2010 and 2021.
METHODS: An investigation was conducted
among physicians from Geneva University Hospitals (HUG) and the private sector
likely to encounter patients diagnosed with alveolar echinococcosis between
2010 and 2021. All patients being treated in the canton of Geneva were
included. After obtaining their consent, an epidemiological questionnaire was
completed by patients, and a clinical questionnaire by their referring
physicians. Demographic, epidemiological and clinical data were entered into
REDCap, then extracted and analysed.
RESULTS: Of a total of 27 patients diagnosed
with alveolar echinococcosis, 25 were included in the study; one patient did
not provide his consent and one patient could not be contacted. The annual
incidence of alveolar echinococcosis in the canton of Geneva was calculated at
0.24 cases per 100,000 inhabitants based on the subset (n = 14) domiciled in
Geneva. The vast majority of patients (n = 24; 96%) were followed at HUG. The
median age of patients was 55 years (range: 17–79) with a slight predominance
of women (56%). Reported risk factors for alveolar echinococcosis included
owning a vegetable garden (70.8%), often unfenced, practicing composting
(69.6%), and owning a dog (58.3%) or a cat (58.3%). Four patients (16%) had an
immunosuppressive condition. Only 52% of patients were symptomatic at the time
of diagnosis. The liver was affected in most cases (n = 24; 96%), but one
patient had a primary splenic location. Surgical resection for curative
purposes was performed in 13 patients (52%). All patients received
parasitostatic treatment with albendazole, discontinued in 5 patients (20%) due
to drug-induced hepatitis. Three patients died (12%), of which two directly
related to alveolar echinococcosis.
CONCLUSION: Alveolar echinococcosis, a rare but
severe disease, is endemic in the canton of Geneva. The establishment of
mandatory reporting of this disease in Switzerland would allow monitoring of its
epidemiological evolution. Primary and secondary prevention measures, currently
non-existent, could potentially lower the incidence and severity of the
disease.
Introduction
Alveolar
echinococcosis is a rare but potentially severe parasitic zoonosis caused by
the development in the liver of the larval stage (metacestode) of the cestode Echinococcus multilocularis. Human
contamination (accidental intermediate host) occurs through direct contact with
foxes, dogs or, to a lesser extent, cats infested with the tapeworm (definitive
host). More frequently, the contamination occurs through ingestion of raw
vegetables contaminated by the faeces of these carnivores containing the parasite’s
eggs. Various wild rodents (mainly voles) serve as the natural intermediate
hosts for this parasite. After ingestion, Echinococcus
multilocularis eggs release oncospheres that travel via the portal vein to the
liver, where they continue their larval development. Foxes feed on these
rodents, and the metacestodes evolve into adult worms in their intestines. The
last segment of these small tapeworms contains eggs released into the external
environment with fox faeces (figure 1).
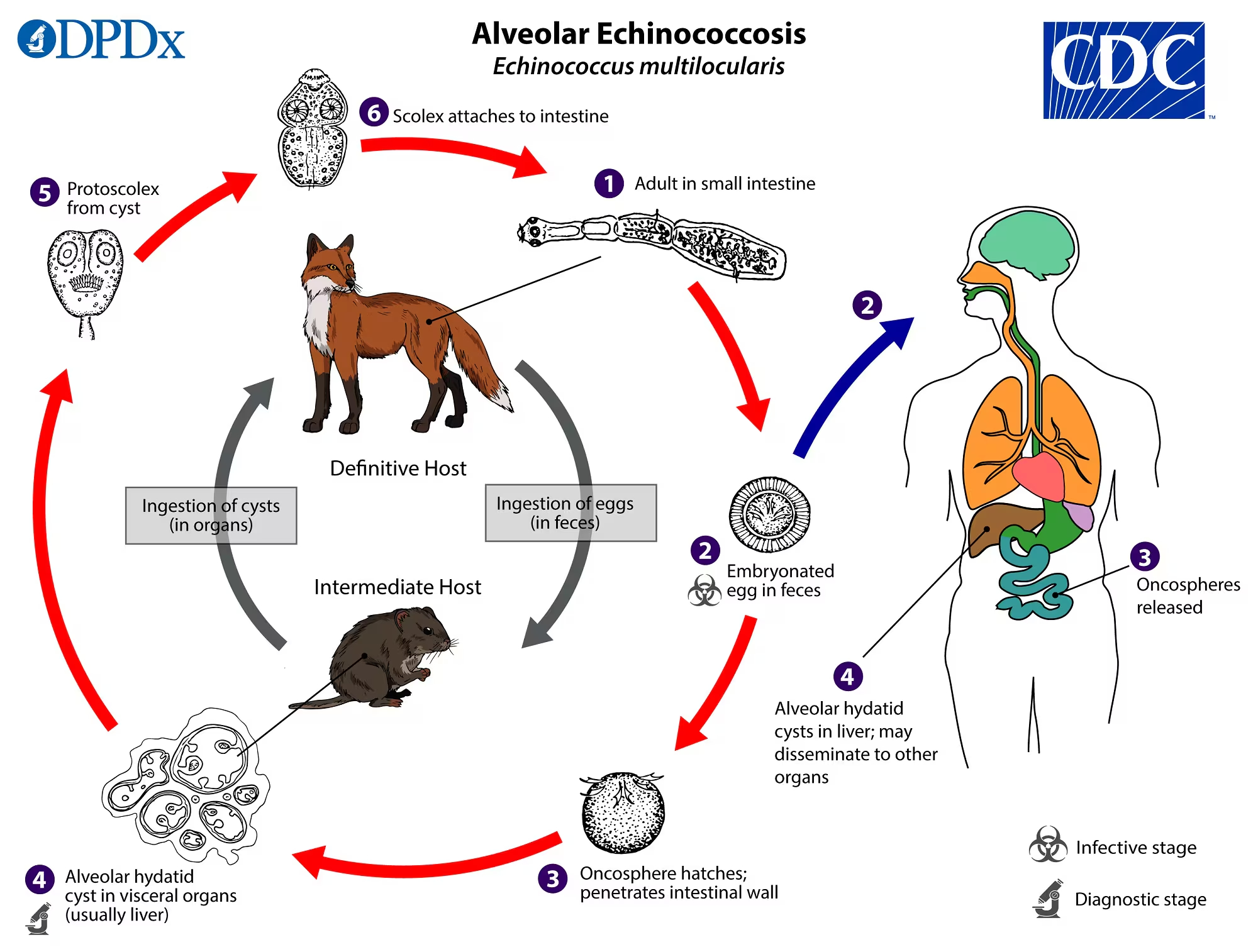
Figure 1Alveolar echinococcosis. Parasitic life cycle of Echinococcus multilocularis. (Source: Centers for Disease Control and Prevention (CDC):
https://www.cdc.gov/dpdx/echinococcosis/index.html. Reference to specific commercial products, manufacturers, companies, or trademarks
does not constitute its endorsement or recommendation by the U.S. Government, Department
of Health and Human Services, or Centers for Disease Control and Prevention.)
In
immunocompetent humans, the metacestode develops very slowly but progresses
like a malignant tumour, invading the liver tissue, vessel and bile duct walls.
It can proliferate beyond the liver, invading adjacent structures (e.g.
diaphragm, peritoneum, pancreas) or spread through lymphatic or haematogenous
routes, forming distant metastases, mainly in the lungs. The initial symptoms
typically appear ten to fifteen years after contamination. Without treatment,
symptomatic alveolar echinococcosis is almost invariably fatal within a decade
[1]. Only complete surgical removal of the parasitic mass, when technically
feasible, can lead to cure. Albendazole, an antiparasitic from the
benzimidazole family, is only parasitostatic against Echinococcus multilocularis but remains the sole available drug and
represents the common denominator for all therapeutic options. It is
administered in conjunction with surgical resection to reduce the risk of
relapse. Complete surgical excision, combined with albendazole, usually allows
for the patient’s cure. Albendazole is also very useful in inoperable patients:
long-term – most often lifelong – administration stabilises the disease in the
majority of cases.
The
prognosis of this parasitic disease, once catastrophic 50 years ago, has
significantly improved thanks to earlier diagnoses, systematic administration
of albendazole, advances in hepatobiliary surgery and the development of
instrumental techniques to treat biliary complications. Multidisciplinary
management of alveolar echinococcosis, involving infectious disease
specialists, parasitologists, hepatogastroenterologists, radiologists and
surgeons, is also essential. Expert centres in Europe report excellent current
survival rates, around 90% at 5 years [2].
The disease
is present in the northern hemisphere only, and the global Disability-Adjusted
Life Year (DALY) burden has been estimated at 666,433 [3]. China constitutes
the largest global focus, with a prevalence of almost 3% on the Tibetan
Plateau. Alveolar echinococcosis is also present in Japan, Central Asia,
Europe, North America including Alaska. The detection of
the parasite in the Americas has only been recent [4, 5]. In Europe, alveolar echinococcosis
has
been on the rise since the beginning of the 21st century [6]. The annual
incidence varies by country, ranging between 0.03 and 1.2 per 100,000
inhabitants [3]. Several European centres have recently reported the emergence
of opportunistic forms in patients treated with chemotherapy for solid cancers
or haematological disorders, immunosuppressants or biotherapy for chronic
inflammatory diseases, contributing to the increased incidence [7, 8].
Switzerland
is endemic for alveolar echinococcosis, with a prevalence of Echinococcus multilocularis infestation
in foxes ranging between 30% and 70% in the Jura and 1% to 20% in the Alpine
regions. The Federal Office of Public Health (OFSP) reports between 10 and 28
new cases per year [1]. The incidence of human disease increased in Switzerland
in the early 2000s. The increase in fox populations, their rate of infestation
by Echinococcus multilocularis and
the appearance of opportunistic forms of alveolar echinococcosis could explain
this trend [9]. However, given that alveolar echinococcosis is not a notifiable
disease, there is currently no reliable and recent epidemiological data for the
Swiss population.
A better
understanding of the epidemiology of alveolar echinococcosis could identify
high-risk areas and behaviours, demographic trends (age at diagnosis, sex,
geographical location), clinical characteristics, therapeutic management and
propose potential preventive public health actions. The objective of the
present work was to describe the demographic, epidemiological, clinical and
therapeutic characteristics of alveolar echinococcosis between 2010 and 2021 in
the canton of Geneva.
Methods
Study design
This is a retrospective
cross-sectional survey conducted among practitioners
likely to be involved with patients with alveolar echinococcosis and the
patients themselves. To be included, patients had to be diagnosed between 2010
and 2021 and be medically followed in the canton of Geneva. We recorded the
evolution of the patients and of the disease during this period, analysing the
context in which the patients were living, their initial symptoms, the modes of
diagnosis of alveolar echinococcosis, the different therapeutic and palliative
treatment options and the prognosis.
Recruitment process
Data was collected
through two questionnaires: (1) an epidemiological questionnaire, completed by
patients, gathering data on their lifestyle and geographic and professional
contexts, investigating the possible circumstances of contamination; (2) a
medical questionnaire, sent to the referring physician, collecting data on the
circumstances of diagnosis, presence and degree of hepatic and/or extrahepatic
involvement, management modalities and clinical outcome.
Patients
were recruited from two sources (figure 2). The first source consisted of
patients followed at the University Hospitals of Geneva (HUG). We directly
asked patients for their oral consent and then sent them the consent form along
with an explanatory letter and an epidemiological questionnaire. The second
source included patients followed outside the HUG. To do this, we contacted and
sent a medical questionnaire to the physicians most likely to encounter this
parasitic disease – hepatogastroenterologists, infectious disease specialists
and abdominal surgeons – through the Geneva telephone directory,
hepatogastroenterology forums and the association of private
hepatogastroenterologists.
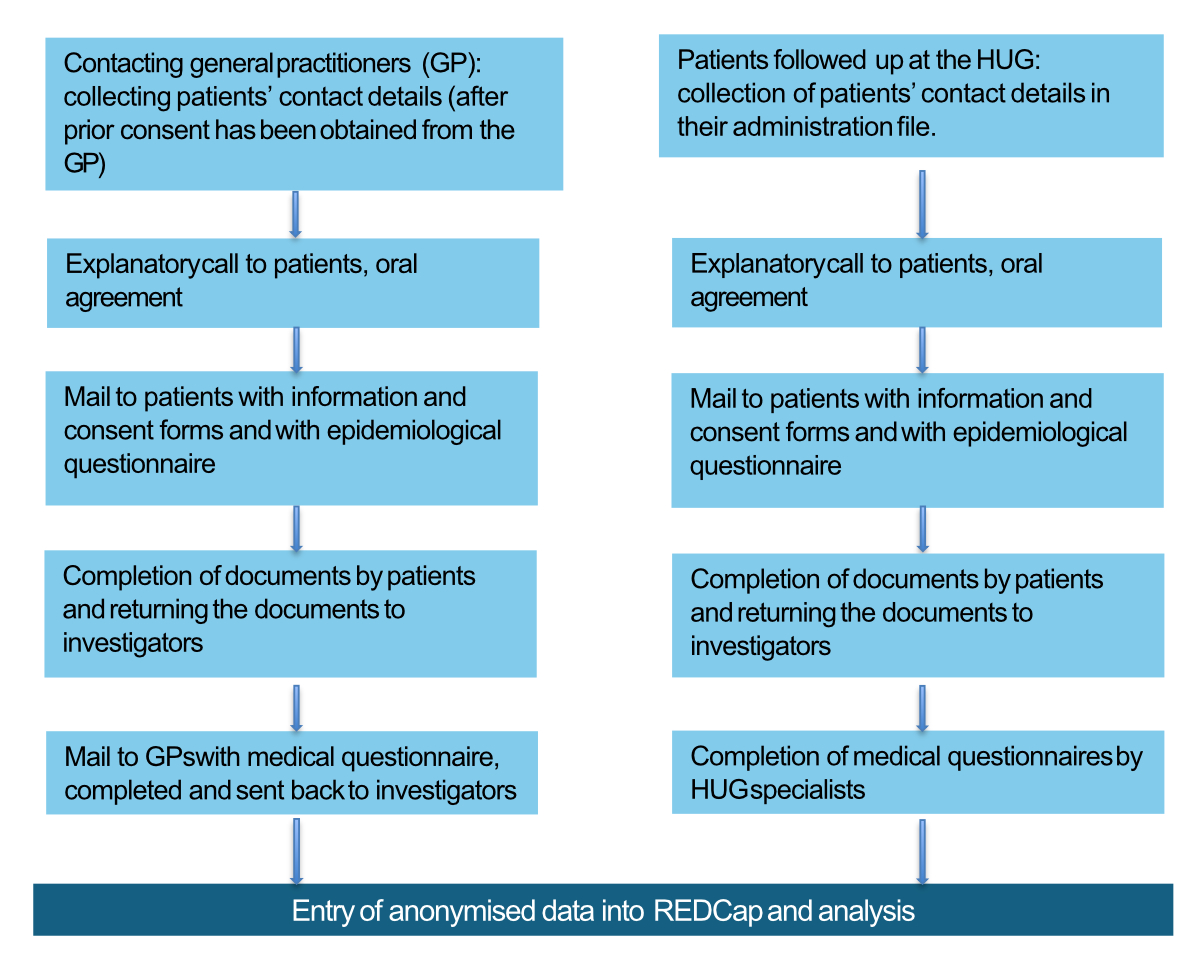
Figure 2Obtaining
patient consent and collecting data. Description of the step-by-step process for
obtaining consent from patients with alveolar echinococcosis and for collecting
data.
Data collection
The data
collected in the epidemiological and medical questionnaires was checked,
sometimes supplemented by direct oral or written exchanges with the referring physician
or the patient, and then entered into REDCap, a secure database. The
radiological description and initial imaging were studied, allowing the
establishment of the PNM stage of the disease [10]. The P designates the
parasitic mass in the liver, its location and the presence or absence of
biliary and/or vascular invasions. The N designates the invasion of neighbouring
organs, and the M is determined by the presence or absence of
metastases in distant organs.
Statistical analysis
For the
descriptive analysis of demographic, epidemiological and clinical
characteristics, discriminative variables (e.g. presence of immunosuppression,
other exposure) were expressed in frequency (%) and continuous variables (e.g.
age) as mean (± standard deviation) and median
(range). To analyse the incidence of alveolar echinococcosis in the canton of
Geneva, expressed as the number of new cases diagnosed annually per 100,000
inhabitants, only patients domiciled in the canton of Geneva at the time of
diagnosis were included.
Ethics
The study
protocol was approved by the CCER (Commission cantonale d’éthique à la recherche or Cantonal Commission for Ethics in Research) on 15
September 2021 (BASEC ID: 2021-01307). The study protocol can be found
at the CCER and on the website of Swiss Ethics: https://ongoingprojects.swissethics.ch/runningProjects_list.php?q=%28BASECID~contains~2021-01307%29&orderby=dBASECID
Results
We
contacted 29 hepatogastroenterologists, 7 infectious disease specialists and 43
surgeons. Among these private practitioners (n = 79), 6 (7.6%) reported not
following patients with alveolar echinococcosis and 1 (1.3%) connected us with
one of his patients, diagnosed and treated in the private sector. Seventy-two
physicians (91.1%) did not respond. Apart from one patient, all patients were
recruited at Geneva University Hospital, which is the only centre in the canton
where multidisciplinary management for alveolar echinococcosis is available.
Between 1
January 2010 and 31 December 2021, a total of 27 patients were diagnosed and
managed in the canton of Geneva. Two patients were not included in the
analysis: one patient did not provide
his consent and one patient could not be contacted despite several attempts. Of these
27 patients, 14 resided in the canton of Geneva at the time of diagnosis. Based
on an averaged population of 485,321 inhabitants between 2010 and 2021, this
results in a mean incidence of 0.24 cases per 100,000 inhabitants.
Demographic and epidemiological
data
Of the 25
included patients, 14 (56%) were women and 11 (44%) men. The median age of
patients was 55 years (range: 17–83). The professional activities and
geographical distribution of patients’ residences are summarised in table 1.
Two city-dwelling patients reported having a country house in alveolar
echinococcosis risk areas.
Table 1Demographic data and medical history of 25 patients with alveolar
echinococcosis in the canton of Geneva (2010–2021).
| Variables |
Values |
| Age at
diagnosis in years, median (range) |
55 (17–83) |
| Sex, n (%) |
Male |
11 (44%) |
| Female |
14 (56%) |
| Place of residence at diagnosis*, n (%) |
Switzerland |
22 (88%) |
| Canton |
Geneva |
14 (56%) |
| Vaud |
3 (12%) |
| Valais |
2 (8%) |
| Fribourg |
2 (8%) |
| Neuchâtel |
1 (4%) |
| France |
2 (8%) |
| Department |
Ain |
1 (4%) |
| Moselle |
1 (4%) |
| Profession, n (%) |
Agricultural activity |
2 (8%) |
| Employee |
6 (24%) |
| Senior executive / Intellectual profession |
5 (20%) |
| Worker |
3 (12%) |
| Craftsman, retailer |
2 (8%) |
| Student |
1 (4%) |
| Jobless |
1 (4%) |
| Retired (except farmers) |
8 (32%) |
| Immunosuppression context** |
4 (16%) |
Four (16%)
patients were immunosuppressed at the time of diagnosis. Two patients had myelodysplastic
syndrome, one patient had ankylosing spondylitis treated with anti-tumour
necrosis factor (TNF) antibodies for 7 years, and one patient was treated with
tacrolimus and mycophenolate mofetil following renal transplant for
amyloidosis, 9 years prior to the incidental discovery of hepatic alveolar
echinococcosis. Most patients reported one or more other risk factor(s) for alveolar
echinococcosis, as detailed in table 2.
Table 2Risk
exposure of 25 patients with alveolar echinococcosis in the canton of Geneva
(2010–2021).
| Potential risk factor |
n (%) |
| Observation
of foxes around the house |
17 (68%) |
| Owning a vegetable garden |
|
17 (68%) |
| Without fence |
13 (76.5%) |
| With fence |
4 (23.5%) |
| Composting |
16 (64%) |
| Ownership of dog(s) |
14 (56%) |
| Ownership of cat(s) |
14 (56%) |
| Consumption
of uncooked wild berries |
12 (48%) |
|
Presence
of chicken / rabbits or other animals |
10 (40%) |
| Family
members suffering from alveolar echinococcosis* |
4 (16%) |
Clinical data
The clinical
circumstances leading to diagnosis are detailed in table 3. One patient
presented to the emergency room with left hypochondrial pain, four years after
superior mesenteric vein thrombosis. It was later revealed that the patient had
a primary splenic form of alveolar echinococcosis. Asymptomatic patients (n = 12;
48%) were most often diagnosed incidentally following blood tests revealing
liver test abnormalities or imaging studies ordered for other reasons. In
symptomatic patients, abdominal pain was the most frequent revealing symptom (n
= 13; 52%).
Table 3Circumstances
of alveolar echinococcosis diagnosis in 25 patients in the canton of Geneva
(2010–2021).
| Circumstances |
n (%) |
| Asymptomatic |
|
12 (48%) |
| Incidental discovery* |
10 (40%) |
| Serological screening** |
2 (8%) |
| Symptomatic |
|
13 (52%) |
| Abdominal pain |
13 (52%) |
| Impaired general condition |
|
5 (20%) |
| Asthenia |
2 (8%) |
| Weight loss |
3 (12%) |
| Fever |
1 (4%) |
| Jaundice |
4 (16%) |
| Cholangitis |
1 (4%) |
| Liver abscess |
1 (4%) |
| Hepatomegaly |
1 (4%) |
| Ascites |
1 (4%) |
| Splenomegaly |
1 (4%) |
Lesion description
The
characteristics of hepatic lesions in 24 of the 25 patients are described in table
4 and a detailed description for 5 patients is provided in figures 3 and 4.
Hepatic lesions invaded one or more adjacent organs in 3 patients: diaphragm (n
= 2), adrenal gland (n = 1), pericardium (n = 1) and abdominal wall (n = 1).
Another patient had pulmonary metastases. Only one patient presented a purely
extrahepatic location, in the form of a primary splenic alveolar echinococcosis
(figure 5). The PNM stages for the 24 patients with hepatic lesions are
indicated in table 5. The patient with primary splenic involvement could not be
classified as this system was conceptualised for liver lesions.
Table 4Description of liver lesions in 24 patients with alveolar echinococcosis.
| Description |
n (%) |
| Presence of liver
lesions |
|
24 (96%) |
| Affected lobes |
Right lobe |
12 (50%) |
| Left
lobe |
1 (4.1%) |
| Left
and right lobe |
10 (41.7%) |
| Affected segments |
I |
8 (33.3%) |
| II |
10 (41.7%) |
| III |
10 (41.7%) |
| IV |
13 (54.2%) |
| V |
13 (54.2%) |
| VI |
10 (41.7%) |
| VII |
12 (50%) |
| VIII |
14 (58.3%) |
| Number of lesions (range) |
|
1–30 |
| Size of largest lesion |
<20 mm |
0 |
| 20–50 mm |
9 (37.5%) |
| 50–100 mm |
7 (29.2%) |
| >100 mm |
7 (39.2%) |
| Other features* |
Central
biliary or vascular infiltration of a lobe |
5 (20.8%) |
| Central
biliary or vascular infiltration of both lobes |
2 (8.3%) |
| Calcifications detected |
16 (66.7%) |
| Hepatic
lesion and vascular extension** |
8 (33.3%) |
| Intrahepatic bile duct dilation |
8 (33.3%) |
| Centroparasitic necrosis |
6 (25%) |
| Invasion
of the hepatic hilum |
7 (29.2%) |
| Pedicle flow |
3 (12.5%) |
| Infiltration of portal vein |
3 (12.5%) |
| Infiltration
of common hepatic artery |
2 (8.3%) |
| Infiltration of suprahepatic veins |
10 (41.8%) |
| Infiltration
of inferior vena cava |
6 (25%) |
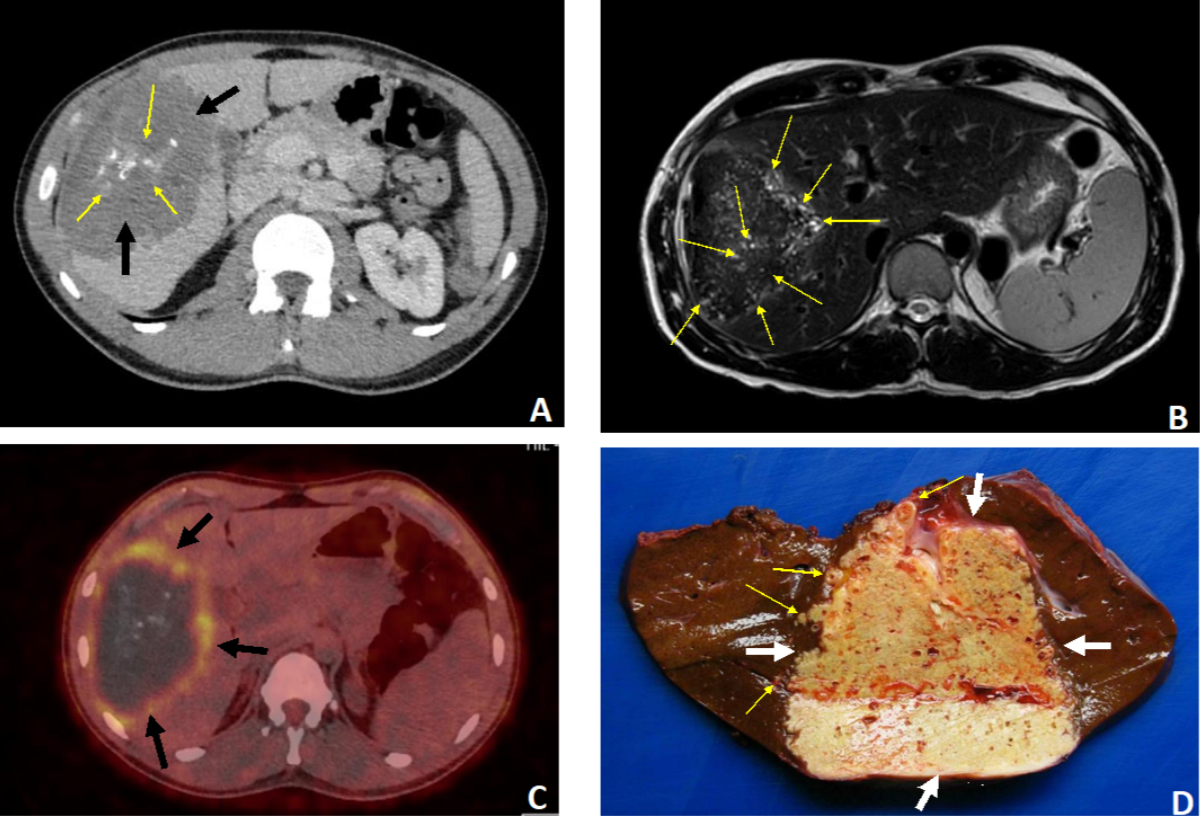
Figure 337-year-old
patient. Discovery of hepatic alveolar echinococcosis (AE) classified as P2N1M0
(stage IIIb) due to right hypochondrial pain. A–C: Radiological aspects
of the lesion invading the right lobe (segments IV, V, VI and VII). A:
Non-contrast CT scan, axial section: huge AE lesion (11 cm in greatest axis)
with heterogeneous content, central “crumb-like” calcifications (thin arrows)
and a hypodense peripheral component (thick arrows). Ill-defined margins. B:
MRI, T2-weighted sequence, axial section. Presence of numerous hyper-T2
microcysts within the lesion (arrows), indicative of the florid nature of AE. C:
PET-CT axial section: intense perilesional uptake of 18 fluorodeoxyglucose
(arrows), an indirect sign of an active AE. D: Right hepatectomy
specimen, macroscopic view: the AE lesion is located at the centre of the surgical
specimen (thick arrows). Chamois yellow in colour, it is filled with numerous
small cavities corresponding to parasitic microcysts. The lesion has irregular
limits and extends into the healthy hepatic parenchyma (thin arrows).
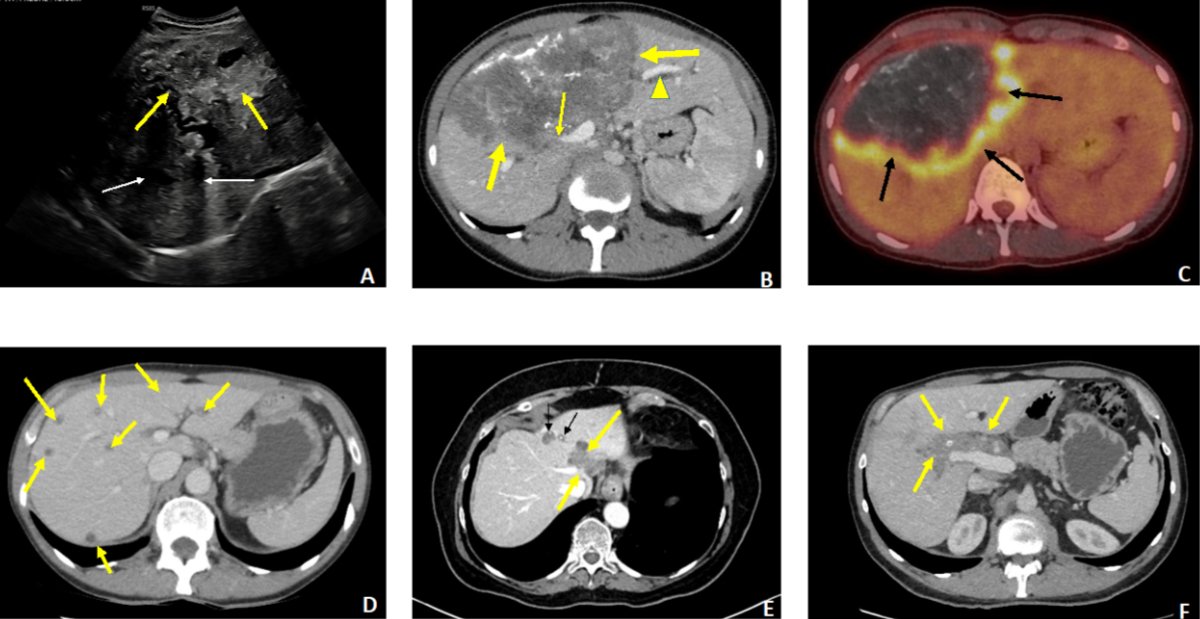
Figure 4A–C:
27-year-old female patient revealing advanced
hepatic alveolar echinococcosis (AE) with cholestatic jaundice. A: Ultrasound: extensive
heterogeneous
lesion of the right lobe with irregular contours (thick arrows), containing
numerous calcifications with posterior shadow cones (thin arrows). B: Contrast-enhanced
CT scan, portal
phase, axial section. The huge lesion (thick arrows) involves segments IV, V,
VI and VIII with invasion of the right portal branch (thin arrow) and the
hilum, causing dilation of the intrahepatic bile ducts in the non-infected left
liver (arrowhead). C: PET-CT, axial section. Intense
perilesional activity (arrows). D–F: Three cases of AE diagnosed at a pauci- or
asymptomatic stage. D: 56-year-old patient, abdominal
discomfort. Contrast-enhanced CT scan, portal phase, axial cut. Multiple small
scattered AE foci in both lobes, without calcified components. E: 66-year-old patient, renal transplant recipient. Incidental
discovery (imaging for sigmoiditis) of hepatic AE. Contrast-enhanced CT scan,
arterial phase, axial section. Two foci located in segment IV. Only the
anterior focus contains punctate calcifications (thin arrows). The posterior
focus invades the left and median suprahepatic veins (thick arrows). F: 45-year-old
patient. Discovery of AE during an annual routine
blood test showing a slight elevation of gamma-GT. Contrast-enhanced CT scan,
portal phase, axial section. Centrohepatic lesion with a low calcified
component, hilar and pedicular infiltration and invasion of the hepatic artery
(arrows).
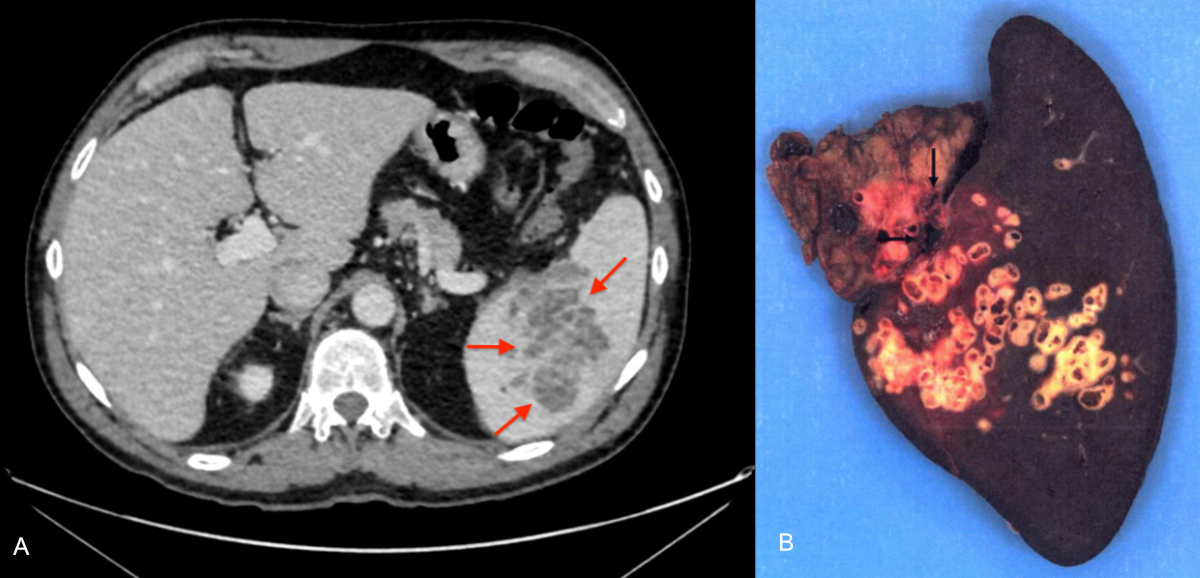
Figure 5A–B:
67-year-old patient admitted to the emergency department for a painful crisis
in the left hypochondrium occurring 4 years after superior mesenteric vein
thrombosis. A: Contrast-enhanced CT scan, portal phase, axial cut.
Multicystic hypodense splenic lesion (arrows). B: Total splenectomy
specimen, macroscopic view. The lesion is filled with multiple alveoli (arrows)
characteristic of alveolar echinococcosis, corresponding to parasitic
microcysts. No clear limits.
Table 5Classification
and staging of hepatic alveolar echinococcosis (24* patients).
| Classification |
n (%) |
Staging |
n (%) |
| P1N0M0 |
10 (41.7%) |
I |
11 (45.8%) |
| P1N1M0 |
1 (4.2%) |
|
|
| P2N0M0 |
1 (4.2%) |
II |
1 (4.2%) |
| P3N0M0 |
2 (8.3 %) |
IIIa |
2 (8.3%) |
| P2N1M0 |
4 (16.7%) |
IIIb |
7 (29.2%) |
| P3N1M0 |
1 (4.2%) |
|
|
| P4N0M0 |
3 (12.5%) |
|
|
| P3N0M1 |
2 (8.3%) |
IV |
3 (12.5%) |
| P4N1M1 |
1 (4.2%) |
|
|
Diagnosis
The methods
used to confirm the diagnosis of alveolar echinococcosis are summarised in table
6. The vast majority of patients were diagnosed through imaging and specific
serology, resulting in a probable alveolar echinococcosis diagnosis as defined
by the WHO consensus [11]. Histopathological confirmation through echo-guided
biopsy was necessary in only one patient. In another case, pathological
examination after surgical excision of a hilar lesion suspected of being a
cholangiocarcinoma led to the diagnosis of alveolar echinococcosis.
Table 6Methods used to confirm the diagnosis of alveolar echinococcosis (25 patients).
| Diagnostic methods |
n (%) |
| Imaging |
|
24* (96%) |
| CT |
22 (88%) |
| MRI |
22 (88%) |
| PET-CT |
21 (84%) |
| US |
18 (72%) |
| Serology |
|
25 (100%) |
| 1st line (ELISA) only |
4 (16%) |
| 2nd
line (Western blot) only** |
1 (4%) |
| Both 1st
and 2nd line |
19 (76%) |
| Anatomopathology |
Percutaneous biopsy |
1 (4%) |
| Surgical specimen |
14 (56%) |
| Molecular diagnosis
(PCR)*** |
|
2 (8.3%) |
Treatments
Treatment
modalities are summarised in table 7.
Table 7Treatment
modalities for the 25 patients with alveolar echinococcosis.
| Description |
n (%) |
| Liver surgery with curative intent* |
|
12 (48%) |
| Partial hepatectomy |
|
11 (91.6%) |
| Right
hepatectomy |
2 (16.7%) |
| Enlarged
right hepatectomy |
2 (16.7%) |
| Atypical
hepatectomy |
7 (53.8%) |
| Hepatic allotransplant |
1 (8.3%) |
| Additional
technical features |
Vascular reconstruction |
5 (41.7%) |
| Biliary reconstruction |
3 (25%) |
| Lymph node resection
|
1 (8.3%) |
| Total splenectomy with curative intent |
1 (4%) |
| Palliative
interventions |
|
5 (20%) |
| Laparotomy |
|
3 (60%) |
| Simple exploration |
1 (33.3%) |
| Surgical drainage** |
1 (33.3%) |
| Instrumental treatments |
Endoscopic
biliary procedure |
4 (80%) |
| Percutaneous procedure*** |
4 (80%) |
| Initiation of albendazole treatment**** |
25 (100%) |
Curative surgery: Curative surgical excision was possible in 13
patients (52%), including 11 patients by laparotomy (including 1 total
splenectomy) and 2 patients by laparoscopy. One patient with advanced alveolar
echinococcosis underwent liver transplantation.
The
analysis of the surgical specimen confirmed the diagnosis of alveolar
echinococcosis in 12 of the 13 patients for whom we had access to the
histopathological report. For 1 patient, who died, we were unable to retrieve
the report. One patient underwent surgery with an initial diagnosis of very
probable cholangiocarcinoma. Histopathological examination of the surgical
specimen, followed by specific serology, led to the diagnosis of alveolar
echinococcosis. The patient with primary splenic alveolar echinococcosis
underwent curative splenectomy (figure 5).
Instrumental treatments: Interventional radiology and/or
biliary endoscopy procedures were performed in 4 (16%) patients: percutaneous
drainage of dilated bile ducts or centro-parasitic abscesses, and endoscopic
placement of biliary stents.
Antiparasitic treatment: Treatment with albendazole was
initiated in all 25 patients. However, in 6 cases, surgical resection of the
lesion was performed without concomitant use of albendazole (due to prior albendazole
intolerance in 5 cases and an initial diagnosis of cholangiocarcinoma in 1 case).
One patient intolerant to benzimidazoles was operated on under liposomal
amphotericin B administration after obtaining informed consent because of the
off-label status of this indication. Pharmacological monitoring was established
for the 24 patients followed at HUG (plasma measurement of the active
metabolite, albendazole sulphoxide).
Patients
who underwent a curative surgical intervention received albendazole treatment
for an intended duration of 2 years. Eight patients completed the 2 years, and two
patients discontinued treatment after 3 and 12 months respectively due to the
occurrence of side effects. One patient continued albendazole beyond the
postoperative 2 years due to signs of persistent parasitic activity (celiac
lymph nodes). One patient did not receive postoperative albendazole due to
preoperative drug-induced hepatitis.
Inoperable
patients were directed towards lifelong treatment. One asymptomatic patient,
however, was able to stop treatment after 3 years. She had several small alveolar
echinococcosis foci. Serological (negativation) and morphological data
(especially PET-CT negativation) allowed treatment discontinuation while
continuing close surveillance.
Adverse
effects under albendazole occurred in 7 (28%) patients, the most common (n = 5)
being hepatic cytolysis with an increase in alanine aminotransferase (ALT) of more
than five times the normal value. Two of the 5 patients
switched to mebendazole, which could not be continued due to hepatic
intolerance as well. One patient could resume albendazole in a different
galenic form (syrup instead of tablets) without tolerance problems. One case of
haematological toxicity (agranulocytosis) and one case of alopecia were
reported.
Prognosis
Three
deaths occurred, two directly related to alveolar echinococcosis. These two
patients were diagnosed at an advanced stage (stage IV).
Discussion
This study
reports an annual incidence of 0.24 new cases of alveolar echinococcosis per
100,000 inhabitants in the canton of Geneva for the period 2010–2021, which is
similar to the nationwide incidence of 0.26 cases per 100,000 person-years
reported by Schweiger et al.
for the period 2001–2005 [9]. The latter thus showed a significantly increased
annual incidence compared with the period 1956–1992 (0.10 per 100,000). This
trend observed in our country may be linked to an increase in fox populations (due
to control of rabies and lack of natural
enemies), their
increasingly frequent presence in urban and peri-urban areas noted since the late
1990s, and
their infestation rates by Echinococcus multilocularis.
In the same time period, we note a rise in the number of immunosuppressed
patients, which may
also contribute to this progression [9, 12]. The most recent data on alveolar
echinococcosis incidence in Switzerland was published by the Swiss
echinococcosis network initiated in 2020 [13]. It was based on an exploratory survey
of 9 clinical centres (from 8 Swiss cantons) and the three main microbiology
laboratories involved in alveolar echinococcosis diagnosis in Switzerland. The network
collected 102 incident cases for the years 2020 and 2021, with 94 and 138 new
positive alveolar echinococcosis serologies respectively [13]. The cantons of
Zurich and Bern reported the majority of cases. While these are still very
preliminary results, the latest data suggests a continued increase in alveolar
echinococcosis incidence in Switzerland in recent years, with an estimated
annual incidence currently ranging from 0.58 to 1.33 per 100,000 inhabitants,
making Switzerland one of the most at-risk countries in Europe for this
parasitic disease.
In our
study, the majority of patients (n = 24) were followed at Geneva University
Hospitals (HUG). Only one patient was diagnosed and treated in the private
sector. Most patients were referred to HUG by their general practitioners. For
patients followed at HUG, therapeutic decisions were always made in
multidisciplinary meetings, following WHO recommendations [11]. The
female-to-male ratio is 1.03, with a slightly higher representation of women
(56%) than men. This is consistent with previous findings in other European
countries, such as France (54%) [13], as well as Canada (52%) [14]. In Germany,
a recent study suggested that the sex ratio was higher (1.4) in the period 1992–2000
than in the period 2000–2011 (1.25) [15]. In China, the region most heavily
endemic for alveolar echinococcosis, a meta-analysis published in 2020 [16],
focusing only on alveolar echinococcosis articles in that country, indicated
that alveolar echinococcosis prevalence was higher in women, suggesting that female
sex was a risk factor for alveolar echinococcosis (multiplied by 1.6). One
explanation could be related to specific lifestyle habits in that region of the
world, particularly regarding the distribution of domestic tasks. In at-risk
areas, especially Tibet, only women are responsible for taking care of dogs,
including feeding them [16].
We observed
that owning a vegetable garden was the most frequently reported known risk
factor. Additionally, 76.5% of patients who cultivated a vegetable garden had
not installed fences. Ownership of dogs and cats was found in over half of the
cases. This risk factor for alveolar echinococcosis has been emphasised in many
studies [17, 18, 19]. While the involvement of dogs in transmission is
well-established, it is more controversial for cats [20]. Indeed, cats are a
less favourable definitive host for Echinococcus
multilocularis, with the last segment of the tapeworm generally containing
few or no eggs [20]. However, an Austrian study identified cat ownership as a
potential risk factor [21], and a recent study examining carnivore faeces to
identify the presence of Echinococcus
multilocularis by polymerase chain reaction (PCR) highlighted its presence
in cat faeces, raising questions about its role in transmission to humans [22].
Even though a study assessing the level of alveolar echinococcosis knowledge in
the general population indicated that Switzerland performed better than in the
other studied countries (Czechia, France and Germany), it performed the worst
for the perception of severity of this condition [23]. Therefore, repeated
awareness campaigns about the disease and its potential severity, combined with
risk awareness by providing prevention advice (e.g. fencing vegetable gardens,
isolating composting points, regular deworming of pets), seem warranted.
Regarding
professions, most patients did not work in occupations known to be at risk of alveolar
echinococcosis (e.g. farmers). We report a high proportion of upper-level
executives and individuals with intellectual occupations and employees. These
individuals likely became infected during travels, stays in rural areas or in
their leisure activities (e.g. gardening, foraging, composting). In France,
based on data from the FrancEchino registry, an overrepresentation of
agriculture-related professions was observed in the past [13]. This trend has
been decreasing since 2010 [24]. This underlines the importance of not limiting
information to rural populations.
In our
study, the representation of familial forms was significant, accounting for 16%
of cases. Piarroux et al. reported 13% familial forms in a study analysing the
French registry cases [25]. Sharing risk factors, coupled with genetically
related patients with potential predisposing genetic factors can explain the occurrence
of alveolar
echinococcosis within a family [26, 27]. This emphasises the importance of
offering screening (by serology and abdominal echography) to relatives of an
index case who have shared or share the same risk factors, particularly to all
first-degree relatives.
Nearly half
of the patients were asymptomatic at the time of diagnosis, aligning with
observations from recent European series [15, 19]. In France, the proportion of
asymptomatic patients increased from 24% (1982–1992) to 50.2% (2003–2013), and
for the latest years (2014–2018), asymptomatic forms (60.4%) became clearly
more frequent than symptomatic forms [19]. In a German series published in
2017, 44% of patients were asymptomatic at diagnosis for the period 2000–2011,
compared to 21.3% for the earlier period, 1992–1999 [15]. In our study,
jaundice, a classic inaugural symptom of alveolar echinococcosis and a sign of
advanced disease, was present in only 4 cases. The most frequent revealing
symptom was abdominal pain (53% of cases), leading to imaging studies. Due to
the earlier diagnoses during the course of alveolar echinococcosis, low PNM
stage (I or II) was reported in nearly 50% of the patients, which is in
accordance with recent data reported in Germany [15]. Among these asymptomatic
forms, we noted a particular pattern in 3 patients that has been little
described to date: multiple small, minimally or non-calcified nodules scattered
in the hepatic parenchyma (figure 4D). This could represent an early stage of alveolar
echinococcosis, preceding the more typical appearance resulting from the
confluence of these lesions, associated with the progressive development of the
calcified component [19]. Interestingly, for these 3 patients, the therapeutic
orientation was long-term albendazole treatment due to the multifocal nature of
alveolar echinococcosis, with a fairly rapid observation of an objective
response. This allowed, in one case, an attempt to stop treatment after 3
years.
The
proportion of immunosuppressed patients (16%) in our cohort appears high but is
consistent with literature data [7, 8]. French data from the FrancEchino
registry reports a prevalence of 9.8% (1982–2012), but emphasise a clear
increase in the most recent period, with 84% of immunosuppressed patients
reported during the last decade [7]. Two Swiss teams recently confirmed this
observation. Lachenmayer et al.
[2] reported a proportion of 30% immunosuppressed patients among alveolar
echinococcosis cases diagnosed from 2008 to 2017 at Bern University Hospital;
Deibel et al. identified an immunosuppressed condition in 20% of alveolar
echinococcosis patients in the Zurich cohort [28]. In these reports,
solid cancers treated with chemotherapy and chronic inflammatory diseases
treated with various immunosuppressive drugs and/or biotherapies (e.g. anti-TNF
antibodies, anti-CD20 antibodies) were the most common situations [2, 7, 28].
In our study, two patients were immunosuppressed due to myelodysplastic
syndrome, and one patient had a renal transplant, situations also reported in
the literature [7]. In the latter patient, who underwent regular abdominal
morphological follow-ups, no suspicious liver lesions were reported 5 years
before the diagnosis, confirming the accelerated progression of the metacestode
in this context, a finding also observed in the French cohort [7]. The last
patient, suffering from spondyloarthritis for several years, had a long history
of immunosuppression due to treatment with anti-TNF-alpha antibodies. This patient
is one of the two most
severe cases in our study. He presented with inaugural jaundice, a sign of
severe alveolar echinococcosis. The lesions were diffuse, and death occurred a
few months after diagnosis. A recent literature review on this topic confirms
the emergence of alveolar echinococcosis in the context of immunosuppression
and highlights the diagnostic challenges in this situation, with a high
prevalence (48%) of atypical radiological images leading to confusion and
delayed diagnosis, and a low sensitivity of first-line serological tests (25%)
[8]. A recent small French-Swiss series of solid organ transplant patients who
developed alveolar echinococcosis under anti-rejection immunosuppressive
treatment confirms this data and emphasises the importance of performing a
second-line serological test by Western blot in these situations, when
first-line tests are inconclusive [29]. Increased mortality (20% vs 4% in
immunocompetent patients) was observed in Autier et al.’s review [8] and in the aforementioned
series [29]. Our
study aligns with this data since 2 of 4 patients (50%) with alveolar
echinococcosis in the context of immunosuppression died compared to 4.7% in
immunocompetent patients. All this information underscores the importance of
raising awareness among specialists caring for immunosuppressed patients about
the risk of opportunistic alveolar echinococcosis. Additionally, repeated
prevention advice is essential in this high-risk population.
We report
only one primary extrahepatic form (4%) as a splenic location. This result is
consistent with those of a large European series (n = 599) [30] and a French
series (n = 387) [25], reporting respectively 2% and 4% primary extrahepatic
locations, including the spleen. The patient described in our series had a
history of superior mesenteric vein thrombosis following laparoscopic resection
of the caecum performed 4 years before the diagnosis of splenic alveolar
echinococcosis. The mesenteric venous thrombosis likely facilitated redirection
of blood flow towards the splenic vein, thereby allowing the primary infection
of the spleen by the parasite. A case of primary vertebral alveolar
echinococcosis in a patient with liver cirrhosis complicated by portal
hypertension has recently been reported, probably involving the same mechanism
of portal flow diversion [31].
Albendazole
has become a pillar of alveolar echinococcosis therapy, as a complement or alternative
to curative surgery. This antiparasitic treatment has considerably improved the
prognosis of the disease. However, its long-term administration carries a
significant risk of toxicity and therefore requires regular monitoring of the
blood level of its active metabolite, albendazole sulphoxide, as well as liver
function tests and blood cell counts.
This study
has certain limitations that may have influenced the results, potentially
leading to an underestimation of the number of patients and the incidence of alveolar
echinococcosis. Indeed, only 6.3% of the contacted physicians actively
responded to our invitation letter. While it is likely that most non-responses
are related to the absence of alveolar echinococcosis patients followed during
the study period, we cannot rule out other causes of non-response, such as a
refusal to participate. However, since most patients were followed by specialised
university teams, the probability of alveolar echinococcosis patients in Geneva
who were never followed by HUG is probably low. Moreover, only specialists who
were most likely to follow alveolar echinococcosis patients
(hepatogastroenterologists, surgeons and infectious disease specialists) were
surveyed. Although this hypothesis seems unlikely, we cannot exclude that
physicians from other specialties (e.g. general internal medicine) may have
followed alveolar echinococcosis patients between 2010 and 2021. It is also
likely that some information provided by patients in the epidemiological
questionnaire (e.g. dates, locations of previous stays) may have been reported
inaccurately or forgotten given the considerable number of years (up to 11
years) covered by this retrospective study (recall bias) [32].
There is
currently no primary or secondary prevention strategy for alveolar
echinococcosis in the canton of Geneva. Various measures could be implemented,
such as (a) veterinary monitoring of infection rates in foxes, (b) public
awareness campaigns (e.g. fencing vegetable gardens, deworming dogs) and
awareness campaigns for at-risk patients (e.g. immunosuppressed individuals),
and (c) information for healthcare professionals (e.g. screening high-risk
individuals). The most recent data from the survey carried out by the Swiss
echinococcosis network suggests a very marked increase in the incidence of alveolar
echinococcosis in Switzerland in recent years, probably one of the highest in
Europe [12], as well as an increase in the number of patients with various
immunosuppressive conditions. Consequently, we believe that alveolar
echinococcosis should become a notifiable disease for the cantonal and federal
authorities (FOPH), in order to ensure adequate monitoring of the
epidemiological situation in Switzerland. Finally, similar to emerging viral
diseases that have been in the news in recent years, a One Health public health
approach involving human, animal and environmental dimensions intersectorally
appears to be the preferred path forward for this emerging anthropozoonosis.
Acknowledgments
Our
sincere thanks to Dr Philippe Zurbuchen, Dr Jean-Marc Schwob, Dr Andre Texeira
Antunes, Laurent Brodier, Prof. Gui Stoffels, Prof. Michel Boulvain and Dr
Sandrine Vijgen.
Prof. François Chappuis
Service de médecine tropicale et humanitaire
Hôpitaux Universitaires
de Genève
Rue Gabrielle-Perret-Gentil 4
CH-1205 Genève
Francois.chappuis[at]hug.ch
References
1. Fiche thématique sur l’Echinococcose. Office fédéral de la santé alimentaire et des
affaires vétérinaires (OSAV), online, 2011 consulted on 12.02.2021 https://www.blv.admin.ch/blv/fr/home/tiere/tierseuchen/uebersicht-seuchen/alle-tierseuchen/echinococcose.html
2. Lachenmayer A, Gebbers D, Gottstein B, Candinas D, Beldi G. Elevated incidence of
alveolar echinococcosis in immunocompromised patients. Food Waterborne Parasitol.
2019 May;16:e00060. 10.1016/j.fawpar.2019.e00060
3. Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis.
PLoS Negl Trop Dis. 2010 Jun;4(6):e722. 10.1371/journal.pntd.0000722
4. Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, et al. Echinococcosis: advances
in the 21st Century. Clin Microbiol Rev. 2019 Feb;32(2):e00075-18. 10.1128/CMR.00075-18
5. Polish LB, Pritt B, Barth TFE, Gottstein B, O’Connell EM, Gibson PC. European haplotype
of Echinococcus multilocularis in the United States. N Engl J Med. (November 17th);
387;20, 2022. DOI: 10.1056/NEJMc2210000.
6. Bresson-Hadni S, Spahr L, Chappuis F. Hepatic alveolar echinococcosis. Semin Liver
Dis. 2021 Aug;41(3):393–408. 10.1055/s-0041-1730923 doi: https://doi.org/10.1055/s-0041-1730925
7. Chauchet A, Grenouillet F, Knapp J, Richou C, Delabrousse E, Dentan C, et al.; FrancEchino
Network. Increased incidence and characteristics of alveolar echinococcosis in patients
with immunosuppression-associated conditions. Clin Infect Dis. 2014 Oct;59(8):1095–104.
10.1093/cid/ciu520
8. Autier B, Gottstein B, Millon L, Ramharter M, Gruener B, Bresson-Hadni S, et al. Alveolar
echinococcosis in immunocompromised hosts. Clin Microbiol Infect. 2023 May;29(5):593–9.
10.1016/j.cmi.2022.12.010
9. Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, et al. Human
alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis.
2007 Jun;13(6):878–82. 10.3201/eid1306.061074
10. Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, et al. WHO classification of
alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283–7.
10.1016/j.parint.2005.11.041
11. Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for
the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta
Trop. 2010 Apr;114(1):1–16. 10.1016/j.actatropica.2009.11.001
12. Bresson-Hadni S; Swiss Echinococcosis Network. Alveolar echinococcosis in Switzerland.
Leading Opinions Internal Medicine 2023, Universimed-https://www.universimed.com/ch/epaper
13. Piarroux M, Piarroux R, Knapp J, Bardonnet K, Dumortier J, Watelet J, et al.; FrancEchino
Surveillance Network. Populations at risk for alveolar echinococcosis, France. Emerg
Infect Dis. 2013 May;19(5):721–8. 10.3201/eid1905.120867
14. Houston S, Belga S, Buttenschoen K, Cooper R, Girgis S, Gottstein B, et al. Epidemiological
and Clinical Characteristics of Alveolar Echinococcosis: An Emerging Infectious Disease
in Alberta, Canada. Am J Trop Med Hyg. 2021 Mar;104(5):1863–9. 10.4269/ajtmh.20-1577
15. Grüner B, Kern P, Mayer B, Gräter T, Hillenbrad A, Barth TE, et al. Comprehensive
diagnosis and treatment of AE: a single center, long- term observational study of
312 patients in Germany. GMS Infect Dis., 2017, DOI: 10.3205/id000027
16. Wang X, Dai G, Li M, Jia W, Guo Z, Lu J. Prevalence of human alveolar echinococcosis
in China: a systematic review and meta-analysis. BMC Public Health. 2020 Jul;20(1):1105.
10.1186/s12889-020-08989-8
17. European Food Safety Authority (EFSA),Zancanaro G. Annual assessment of Echinococcus
multilocularis surveillance reports submitted in 2020 in the context of Commission
Delegated Regulation (EU), 2018/772. EFSA J. 2021. Jan.10.2903/j.efsa.2021.6382
18. Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, et al.; interdisciplinary
Echinococcosis Working Group Ulm. Worldwide literature on epidemiology of human alveolar
echinococcosis: a systematic review of research published in the twenty-first century.
Infection. 2019 Oct;47(5):703–27. 10.1007/s15010-019-01325-2
19. Bresson-Hadni S, Bellanger AP, Knapp J, Grenouillet F, Blagosklonov O, Millon L, et
al. Echinococcose alvéolaire. EMC Hépatol; 2020. 10.1016/S1155-1976(20)42252-1
20. Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, et al. Potential
risk factors associated with human alveolar echinococcosis: Systematic review and
meta-analysis, PLoS Negl Trop Dis., 2017, DOI: 10.1371/journal. pntd.0005801
21. Kreidl P, Allerberger F, Judmaier G, Auer H, Aspöck H, Hall AJ. Domestic pets as risk
factors for alveolar hydatid disease in Austria. Am J Epidemiol. 1998 May;147(10):978–81.
10.1093/oxfordjournals.aje.a009388
22. Knapp J, Combes B, Umhang G, Aknouche S, Millon L. Could the domestic cat play a significant
role in the transmission of Echinococcus multilocularis? A study based on qPCR analysis
of cat feces in a rural area in France. Parasite. 2016;23:42. 10.1051/parasite/2016052
23. Hegglin D, Bontadina F, Gloor S, Romig T, Deplazes P, Kern P. Survey of public knowledge
about Echinococcus multilocularis in four European countries: need for proactive information.
BMC Public Health. 2008 Jul;8(1):247. 10.1186/1471-2458-8-247
24. Knapp J, Demonmerot F, Lallemand S, Richou C, Heyd B, Montange D. Registre français
de l’échinococcose alvéolaire : 776 patients et 35 ans de recueil de données épidémiologiques
et cliniques. CO31. Congrès annuel de l’Association Française pour l’Etude du Foie.
2021.https://afef.asso.fr
25. Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, et al. Clinical features
and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a
survey in 387 patients. J Hepatol. 2011 Nov;55(5):1025–33. 10.1016/j.jhep.2011.02.018
26. Yang YR, Ellis M, Sun T, Li Z, Liu X, Vuitton DA, et al. Unique family clustering
of human echinococcosis cases in a chinese community. Am J Trop Med Hyg. 2006 Mar;74(3):487–94.
doi: https://doi.org/10.4269/ajtmh.2006.74.487
27. Vuitton DA, Zhang SL, Yang Y, Godot V, Beurton I, Mantion G, et al. Survival strategy
of Echinococcus multilocularis in the human host. Parasitol Int. 2006;55 Suppl:S51–5.
10.1016/j.parint.2005.11.007
28. Deibel A, Meyer Zu Schwabedissen C, Husmann L, Grimm F, Deplazes P, Reiner CS, et
al. Characteristics and Clinical Course of Alveolar Echinococcosis in Patients with
Immunosuppression-Associated Conditions: A Retrospective Cohort Study. Pathogens.
2022 Apr;11(4):441. 10.3390/ pathogens11040441 doi: https://doi.org/10.3390/pathogens11040441
29. Marquis B, Demonmerot F, Richou C, Thiéfin G, Millon L, Wallon M, et al.; Swiss Transplant
Cohort Study; FrancEchino Network. Alveolar echinococcosis in solid organ transplant
recipients: a case series from two national cohorts. Parasite. 2023;30:9. 10.1051/parasite/2023008
30. Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, et al. European registry:
Human AE, Europe, 1982. Emerg Infect Dis. 2000;2003: 10-3201/eid0903.020341.
31. Faucher JF, Descotes-Genon C, Hoen B, Godard J, Félix S, Aubry S, et al. Hints for
control of infection in unique extrahepatic vertebral alveolar echinococcosis. Infection.
2017 Jun;45(3):365–8. 10.1007/s15010-016-0974-z
32. Hassan E.S, Recall Bias can be a Threat to Retrospective and Prospective Research
Designs, Internet J. Epidemiology, 2005, DOI: 10.5580/2732




