Outcomes of coronary artery aneurysms: insights from the Coronary Artery Ectasia and
Aneurysm Registry (CAESAR)
DOI: https://doi.org/https://doi.org/10.57187/s.3857
Alessandro Candrevaab,
Jessica Huwilerc,
Diego Gallob,
Victor Schweigerc,
Thomas Gilhofera,
Roberta Leonea,
Michael Würdingera,
Maurizio Lodi Rizzinib,
Claudio Chiastrab,
Julia Stehlia,
Jonathan
Michela,
Alexander Gotschya,
Barbara E. Stähliac,
Frank Ruschitzkaac,
Umberto Morbiduccib,
Christian Templinde
a Department of Cardiology,
Zurich University Hospital, Zurich, Switzerland
b PolitoBIO Med
Lab, Department of Mechanical and Aerospace Engineering, Politecnico di Torino,
Torino, Italy
c University of Zurich, Zurich, Switzerland
d Department of Cardiology and Internal Medicine B, University Medicine Greifswald,
Greifswald, Germany
e Swiss CardioVascularClinic, Private Hospital Bethanien, Zurich, Switzerland
Summary
BACKGROUND: Coronary artery ectasias and aneurysms
(CAE/CAAs) are among the less common forms of coronary artery disease, with
undefined long-term outcomes and treatment strategies.
AIMS: To assess the clinical characteristics, angiographic patterns, and
long-term outcomes in patients with CAE, CAA, or both.
METHODS: This 15-year (2006–2021) retrospective
single-centre registry included 281 patients diagnosed with CAE/CAA via
invasive coronary angiography. Major adverse cardiovascular events included
all-cause death, non-fatal myocardial infarction, unplanned ischaemia-driven
revascularisation, hospitalisation for heart failure, cerebrovascular events,
and clinically overt bleeding. Time-dependent event risks for the CAE and CAA
groups were assessed using Cox regression models and Kaplan-Meier curves.
RESULTS: CAEs (n = 161, 57.3%) often had a multi-district distribution
(45.8%), while CAAs (78, 27.8%) exhibited a single-vessel pattern (80%). The co-existence
of CAAs and CAE was observed in 42 cases (14.9%), and multi-vessel obstructive coronary
artery disease was prevalent (55.9% overall). Rates of major adverse
cardiovascular events were 14.3% in-hospital and 38.1% at a median follow-up of
18.9 (interquartile range [IQR] 6.0–39.9) months. The presence of CAAs was associated
with increased major adverse cardiovascular events risk in comparison to CAE (hazard
ratio [HR] = 2.26, 95% confidence interval [CI] 1.38–3.69, p
= 0.001), driven by a higher hazard ratio of non-fatal myocardial infarctions (HR
= 5.00, 95% CI 1.66–15.0, p = 0.004) and unplanned ischaemia-driven revascularisation
in both dilated (HR = 3.23, 95% CI 1.40–7.45, p = 0.006) and non-dilated
coronary artery segments (HR 3.83, 95% CI 2.08–7.07, p = 0.001).
CONCLUSIONS: Overlap between obstructive and dilated coronary artery disease is
frequent. Among the spectrum of dilated coronary artery disease, the presence
of a CAA was associated with worse long-term outcomes.
Abbreviations
- CAA:
-
coronary artery aneurysm
- CAE:
-
coronary artery ectasia
Introduction
Expansive (or positive) coronary artery
remodelling occurs in the initial phase of atherosclerotic plaque formation [1].
The migration of leukocytes, foam cell formation in the vessel wall, and
subsequent extracellular matrix degradation are considered the fundamental
mechanisms underpinning this process [1]. This remodelling can maintain the
diameter of the vessel lumen, potentially acting as an early compensatory
mechanism to prevent luminal narrowing. However, dysregulation of the inflammatory
response and proteolysis of extracellular matrix proteins [2] might lead to reverse
remodelling with plaque deposition and luminal narrowing or a further increase
in the vessel’s lumen, locally enlarging the vessel’s diameter to the point where
it reaches the criteria for coronary ectasia [3]. Degradation or injury to any
of the vessel layers, particularly the media, can lead to the formation of an aneurysm
[4].
Coronary artery ectasias and aneurysms
(CAE/CAAs) have typically been defined as a diffuse or focal coronary dilation that
exceeds the diameter of normal adjacent segments or the diameter of the
patient’s largest coronary vessel by 1.5 times [5]. CAE/CAAs are uncommon forms
of coronary artery disease and have been diagnosed with increasing frequency since
the introduction of coronary angiography. Their incidence has been reported to
vary from 1.5% to 5.0%, with a suggested male predominance [6]. Although
several causes have been proposed, atherosclerosis accounts for more than 50%
of CAAs in adults [7]. Reported complications include thromboses and distal
embolisations, vasospasms, and ruptures that produce ischaemia, heart failure,
or arrhythmias [2]. The natural progression of CAE/CAAs and their long-term
outcomes remain uncertain due to the scarcity of definitive data, which are
often skewed by varying anatomical definitions and inclusion criteria.
Furthermore, a direct comparison between the two forms of dilated coronary
artery disease is lacking. Controversies also persist over the use of medical
treatment (such as anti-thrombotic therapy) or interventional/surgical
procedures.
Therefore, in this study, we aimed to delineate
the clinical and angiographic characteristics of patients presenting with CAE,
CAA or both, confirmed using invasive coronary angiography. Moreover, clinical
outcomes were assessed over an extended period, allowing for a more
comprehensive understanding of these conditions.
Methods
Study design
The coronary artery ectasia and aneurysm
registry (CAESAR) is a monocentric, retrospective registry that includes
all-comer patients with angiographical evidence of CAE or CAA based on invasive
coronary catheterisation.
Retrospective patient recruitment consisted
of (a) a clinical database query for a CAE/CAA diagnosis dating from January 1,
2006 to December 31, 2021 (see appendix),
(b) manual screening of electronic medical records and a review of
angiographies, and (c) visual confirmation of the diagnosis and classification of
CAE, CAA and CAA-CAE (if combined), performed by catheterisation laboratory
personnel. Any ambiguities were resolved through group consensus.
CAA was visually defined as a focal
dilation of coronary segments of at least 1.5 times the adjacent normal
segment, whereas CAE was associated with a more diffuse dilatation (>20 mm
in length). Morphologically, CAAs were defined as “saccular” if the transverse diameter exceeded the longitudinal
diameter, “fusiform” in the opposite
case, and “giant” if the transverse
diameter exceeded 20 mm. CAEs were defined as “diffuse” if they involved more than one vessel segment and “focal” if confined within one vessel
segment. CAE “types” were defined
according to the combination of diffuse and focal components [2]:
- Type I: diffuse ectasia involving two or three vessels
- Type II: diffuse disease in one vessel accompanied by localized disease in another
vessel
- Type III: diffuse ectasia confined to one vessel
- Type IV: localized ectasia
We excluded patients with
lesions localised in a bypass graft, those upstream of a chronically occluded
vessel (CTO), or patients presenting a stent within the vessel dilation at the
time of the index coronary angiography. Patients without coronary angiograms
available for visual inspection were also excluded. Coronary artery disease was
identified and defined as a visual diameter stenosis above 50%.
Hospital records were screened for baseline
clinical characteristics and co-morbidities (including inflammatory and
oncologic diseases) as well as for cardiovascular and non-cardiovascular (i.e.
the de novo diagnosis of infectious, inflammatory and oncologic diseases) outcomes
for each enrolled patient, starting from the index invasive coronary
angiography. Major adverse cardiovascular events (MACE) were defined as a composite
of any incidental all-cause death, non-fatal myocardial infarctions or acute
coronary syndromes (ACSs), unplanned ischaemia-driven percutaneous coronary
interventions (PCIs), re-hospitalisation for heart failure (HF), acute
cerebrovascular events and clinically overt bleeding (Bleeding Academic
Research Consortium [Bleeding Academic Research Consortium] ≥2). Follow-up data
collection ended on December 31, 2022.
The study protocol conformed to the ethical
guidelines of the 1975 Declaration of Helsinki and was approved by the local
ethics committee (BASEC 2021-01119). Retrospective patient inclusion was
possible for patients who signed the general consent for research at the
University Hospital of Zurich and provided an informed written agreement for
clinical data usage for research purposes. Approval by the Data Governance
Board of the University Hospital of Zurich was obtained for database queries
and extractions.
Statistical analysis
All statistical analyses were performed at
a per-patient level to compare the three classification groups (CAE, CAA and
CAA-CAE). Continuous variables with normal distributions are presented as mean
± standard deviation (SD), and non-normally distributed variables as medians
(interquartile range [IQR]). Categorical variables are presented as
percentages. The Chi-squared test was used for comparing categorical variables,
while the Kruskal-Wallis test was used for continuous variables. Time-to-event
data are presented as Kaplan-Meier estimates. Follow-up calculations were based
on the time from the index invasive coronary angiography to the occurrence of a
major adverse cardiovascular event or the last follow-up date. Censoring events
included patients who were lost to follow-up or had not experienced a major
adverse cardiovascular event by the end of the study period. Cox
proportional-hazards regression models were used to compare the risk of incident
events by CAA or CAE. To avoid ambiguity, the CAA-CAE group was omitted from the
time-to-event and event prediction analyses. Anatomical and functional
variables presenting a univariate relationship with incident events were
included in the Cox proportional-hazards regression models. The proportional
hazards assumption was verified as part of the Cox regression analysis and the Schoenfeld
residual test. All analyses were performed using SPSS Statistics 29 (IBM Corp.
Armonk, NY, USA) and MATLAB (Version R2022, MathWorks, Natick, MA, USA).
Results
Over 15 years (figure 1), a total of 281
patients presented with either a CAA (27.8%), a CAE (57.3%) or both (14.9%).
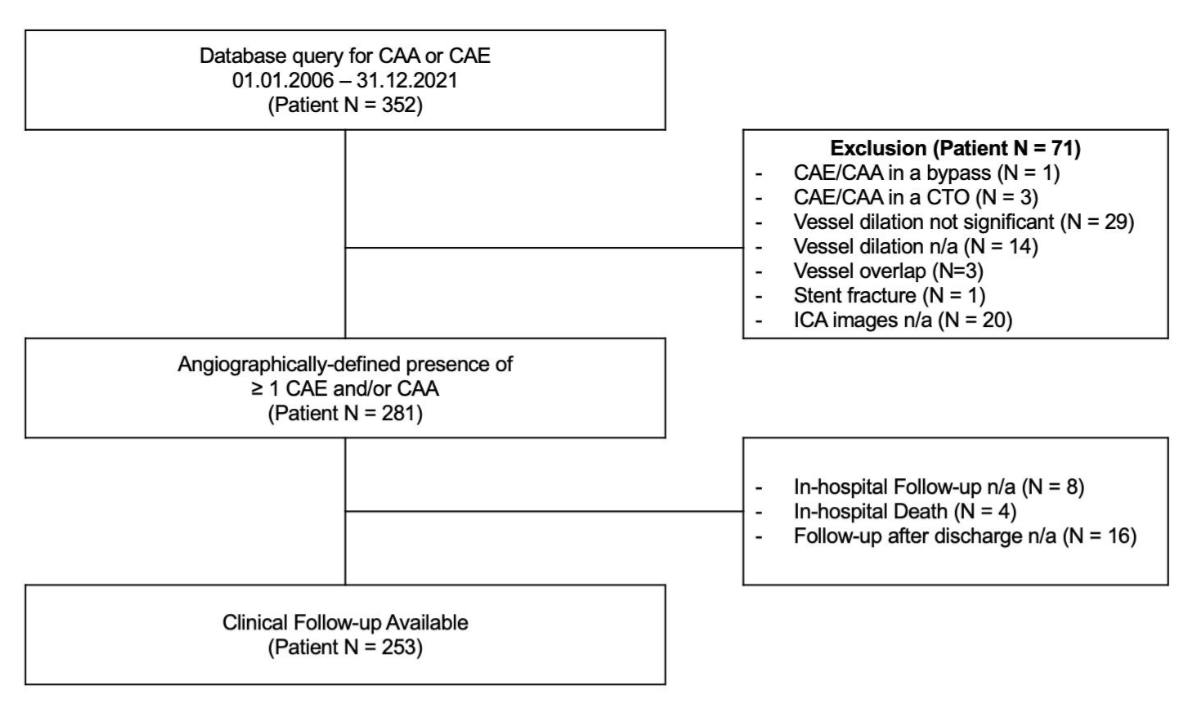
Figure 1Study flowchart. From the initial
352 patients presenting with a coronary aneurysm or ectasia according to the
database query, 71 met the study exclusion criteria, and 281 were included in
the study. Clinical follow-up was available in 253 cases. CAA: coronary artery
aneurysm; CAE: coronary artery ectasia; CTO: chronic total occlusion; ICA:
invasive coronary angiography; n/a: not available.
Clinical characteristics
Patients were predominantly male (87.9%),
with a median age of 66 (57.7–74.5) years. The CAA group had a higher percentage
of female patients (19.2% vs 10.6%, p = 0.045) and a higher median age
(70.9 vs 65.4 years, p = 0.077), see table 1. The CAA group also had a lower prevalence
of ST segment
elevation myocardial infarctions (STEMIs) at the index event than the CAA-CAE
and the CAE groups (7.7% vs 26.2% vs 14.3%, p = 0.022,
respectively). Among the 40 patients presenting with STEMI, the causal lesion
could be identified in 11 cases with an ectatic segment and in 3 cases with an
aneurysmatic segment. Approximately one-fourth of the patients were admitted
with symptoms or signs of acute heart failure (23.1% overall), while more than half
had a preserved left ventricular ejection fraction (median = 53% overall). Nearly
one-third of the patients presented ectatic or aneurysmatic vessels in other
body areas. CAA patients were more likely to have undergone previous cardiac
surgery (19.2% vs 0.0% vs 8.1%, p = 0.002). No differences were found regarding previous
occurrences of cancer (19.9% overall) or inflammatory diseases among the groups
(11.7% overall). High-sensitivity C-reactive protein (hs-CRP) concentration at baseline
was comparable between the groups (3.30 mg/l overall), see appendix table S1.
Table 1Baseline clinical characteristics.
|
Total |
“CAA” |
“CAA-CAE” |
“CAE” |
p-value |
| Patient n = 281 |
Patient n = 78 |
Patient n = 42 |
Patient n = 161 |
2-sided |
| Age, years |
66.1 (57.7–74.5) |
70.9 (58.4–76.3) |
63.01 (58.5–71.5) |
65.4 (57.2–73.2) |
0.077 |
| Female, n (%) |
34 (12.1) |
15 (19.2) |
2 (4.76) |
17 (10.6) |
0.045 |
| BMI, kg/m2 |
28.0 (24.9–31.3) |
28.31 (24.8–30.5) |
27.99 (25.2–32.7) |
27.85 (24.9–31.5) |
0.710 |
| Chest pain, n (%) |
183 (65.1) |
49 (62.8) |
37 (88.1) |
97 (60.3) |
0.003 |
| Dyspnoea, n (%) |
|
148 (52.7) |
47 (60.3) |
17 (40.5) |
84 (52.2) |
0.115 |
| NYHA class ≥III, n (%) |
62 (22.1) |
19 (24.4) |
8 (19.1) |
35 (21.7) |
0.666 |
| Stabile angina, n (%) |
65 (23.1) |
15 (19.2) |
11 (26.2) |
39 (24.2) |
0.608 |
| Acute coronary syndrome, n (%) |
|
115 (40.9) |
30 (38.5) |
23 (54.8) |
62 (38.5) |
0.142 |
| STEMI, n (%) |
40 (14.2) |
6 (7.7) |
11 (26.2) |
23 (14.3) |
0.022 |
| NSTEMI, n (%) |
59 (21.0) |
20 (25.6) |
10 (23.8) |
29 (18.0) |
0.354 |
| Unstable angina, n (%) |
16 (5.69) |
4 (5.13) |
2 (4.76) |
10 (6.21) |
0.907 |
| Acute heart failure, n (%) |
65 (23.1) |
21 (26.9) |
8 (19.1) |
36 (22.4) |
0.583 |
| LVEF, % |
53 (40–59) |
55 (42–60) |
55 (45–58) |
51 (39–58) |
0.497 |
| eGFR <30 ml/min, n (%) |
14 (4.98) |
5 (6.41) |
0 (0.00) |
9 (5.59) |
0.272 |
| Ectasia or aneurysm in other body
districts* |
|
84 (29.9) |
29 (37.2) |
11 (26.1) |
44 (27.3) |
0.252 |
| Thorax |
47 |
12 |
6 |
29 |
|
| Abdomen |
37 |
15 |
5 |
17 |
|
| Other |
14 |
7 |
1 |
6 |
|
| Hypertension, n (%) |
197 (70.1) |
55 (70.5) |
27 (64.3) |
115 (71.4) |
0.664 |
| Dyslipidaemia, n (%) |
178 (63.4) |
50 (64.1) |
26 (61.9) |
102 (63.4) |
0.972 |
| Type 2 diabetes mellitus, n (%) |
81 (28.8) |
22 (28.2) |
8 (19.1) |
51 (31.7) |
0.271 |
| Smoking, n (%) |
186 (66.2) |
52 (66.7) |
31 (73.8) |
103 (64.0) |
0.484 |
| Positive family history, n (%) |
98 (34.9) |
30 (38.5) |
13 (31.0) |
55 (34.2) |
0.697 |
| History of coronary artery disease, n (%) |
94 (33.5) |
30 (38.5) |
11 (26.2) |
53 (32.9) |
0.388 |
| History of cardiac surgery, n (%) |
28 (10.0) |
15 (19.2) |
0 (0.00) |
13 (8.07) |
0.002 |
| History of peripheral artery disease, n
(%) |
23 (8.19) |
10 (12.8) |
3 (7.14) |
10 (6.21) |
0.216 |
| History of cerebrovascular insult, n (%) |
21 (7.47) |
6 (7.69) |
2 (4.76) |
13 (8.07) |
0.759 |
| History of cancer, n (%) |
56 (19.9) |
14 (18.0) |
8 (19.0) |
34 (21.1) |
0.837 |
| History of inflammatory disease, n (%) |
33 (11.7) |
9 (11.5) |
8 (19.1) |
16 (9.9) |
0.263 |
Angiographic characteristics and treatment strategies
at the index event
At the angiographical evaluation, CAAs were
primarily found in a single vessel (80%), with a relatively low incidence in
all three coronary arteries (6.7%), as reported in table 2. Fusiform aneurysms were
the most common CAA type (65.8%).
The co-occurrence of fusiform and saccular CAAs within the same patient was
rare (5.0%). In contrast, CAE demonstrated a multi-district distribution in
nearly half of the cases (45.8%), with types 2 and 4 being the most frequently
observed (21.0% and 20.6%, respectively).
Table 2Angiographic characteristics at the
index coronary angiography.
|
Any CAA |
“CAA” |
“CAA-CAE” |
“CAE” |
p-value |
| Patient n = 120 |
Patient n = 78 |
Patient n = 42 |
Patient n = 161 |
2-sided |
| Number of coronary arteries presenting
CAA, n (%) |
|
|
|
|
|
0.621 |
| One |
96 (80.0) |
64 (82.1) |
32 (76.2) |
– |
| Two |
16 (13.3) |
10 (12.8) |
6 (14.3) |
– |
| Three |
8 (6.67) |
4 (5.13) |
4 (9.52) |
– |
| CAA type, n (%) |
|
|
|
|
|
<0.001 |
| Saccular |
35 (29.2) |
25 (32.1) |
10 (23.8) |
– |
| Fusiform |
79 (65.8) |
48 (61.5) |
31 (73.8) |
– |
| Combined |
6 (5.0) |
5 (6.41) |
1 (2.38) |
– |
|
Any CAE |
“CAA” |
“CAA-CAE” |
“CAE” |
p-value |
| Patient n = 203 |
Patient n = 78 |
Patient n = 42 |
Patient n = 161 |
2-sided |
| Number of coronary arteries presenting
CAE, n (%) |
|
|
|
|
|
0.291 |
| One |
110 (54.2) |
– |
27 (64.3) |
83 (51.6) |
| Two |
62 (30.5) |
– |
11 (26.2) |
51 (31.7) |
| Three |
31 (15.3) |
– |
4 (9.52) |
27 (16.8) |
| CAE type, n (%) |
|
|
|
|
|
<0.001 |
| Type 1 |
39 (13.9) |
– |
7 (16.7) |
32 (19.9) |
| Type 2 |
59 (21.0) |
– |
23 (54.7) |
36 (22.4) |
| Type 3 |
47 (16.7) |
– |
0 (0.00) |
47 (29.2) |
| Type 4 |
58 (20.6) |
– |
12 (28.6) |
46 (28.6) |
Over half of the patients (55.9%) exhibited
multi-vessel coronary artery disease, with a trend towards reduced prevalence
in those with CAE (absence of coronary artery disease: CAA 15.4% vs CAE
23.0%), although the differences between the three groups were not significant
(p = 0.061), as shown in table 3.
Table 3Coronary artery disease prevalence
and treatment strategy at the index coronary angiography.
|
|
Total |
“CAA” |
“CAA-CAE” |
“CAE” |
|
| Patient n = 281 |
Patient n = 78 |
Patient n = 42 |
Patient n = 161 |
2-sided |
| Number of coronary arteries presenting coronary
artery disease, n (%) |
|
|
|
|
|
0.061 |
| One |
73 (26.0) |
17 (21.8) |
10 (23.8) |
46 (28.6) |
| Two |
68 (24.2) |
22 (28.2) |
14 (33.3) |
32 (19.9) |
| Three |
89 (31.7) |
27 (34.6) |
16 (38.1) |
46 (28.6) |
| No coronary artery disease |
51 (18.2) |
12 (15.4) |
2 (4.8) |
37 (23.0) |
| Any percutaneous coronary intervention, n
(%) |
122 (43.4) |
32 (41.0) |
24 (57.2) |
66 (41.0) |
0.166 |
| Percutaneous coronary intervention of
CAE/CAA, n (%) |
|
57 (20.3) |
19 (24.4) |
15 (35.7) |
23 (14.3) |
0.853 |
| With BMS, n (%) |
3 (1.1) |
1 (1.3) |
1 (2.3) |
1 (0.6) |
0.954 |
| With drug-eluting stent, n (%) |
47 (16.7) |
15 (19.2) |
12 (28.6) |
20 (12.4) |
0.948 |
| With a covered stent, n (%) |
3 (1.1) |
2 (2.6) |
1 (2.3) |
0 (0.0) |
0.302 |
| With plain old balloon angioplasty, n (%) |
4 (1.4) |
1 (1.3) |
1 (2.3) |
2 (1.2) |
0.909 |
| Coronary artery bypass grafting, n (%) |
32 (11.4) |
13 (16.7) |
4 (9.52) |
15 (9.30) |
0.172 |
| Conservative treatment, n (%) |
127 (45.2) |
33 (42.3) |
14 (33.3) |
80 (49.7) |
0.138 |
A percutaneous coronary intervention was
conducted in 43.4% of the cases during the initial procedure, with no
significant distinctions between groups (p = 0.166), table 4. Interventions targeting
dilated coronary segments
occurred more frequently in CAA and CAA-CAE patients compared to those with CAE
alone (24.4% vs 35.7% vs 14.3%, p = 0.853, respectively) and
drug-eluting stents (DES) were generally used (47/57 of cases, p = 0.948). Patients
with CAE had the highest prevalence of conservative medical treatment (49.7%, p
= 0.138). Illustrated case examples of percutaneous coronary interventions on
dilated coronary arteries are presented in appendix figures S1–S6.
At the vessel level, both saccular and
fusiform CAAs were frequently identified in the left anterior descending (LAD)
artery (33.0% and 40.5%, p <0.001, respectively), whereas three out of the
four giant CAAs (75.0%, p <0.001) were localised in the right coronary
artery (RCA). CAEs were often detected in the right coronary artery (46.9%),
with type 2 being the most common CAE subtype (33.6%), as illustrated in appendix
table S2.
In-hospital and long-term outcomes
Clinical adverse events were documented in
80 cases (28.3%) prior to hospital discharge, with no significant differences between
the groups (33.8% vs 21.1% vs 29.1%, p = 0.369, respectively). Rates
were primarily driven by periprocedural bleeding (5.49%, p = 0.223) and acute
heart failure (6.23%, p = 0.841), as shown in appendix table S3.
Over a median follow-up period of 18.9
months (IQR 6.0–39.9), the incidence of major adverse cardiovascular events was
substantial (CAA 44.0%, CAA-CAE 40.5% and CAE 34.5%; p = 0.367). Patients
with the mixed disease form exhibited a higher rate of percutaneous coronary
interventions targeting the dilated coronary segment (10.7%, 27.0% and 9.7%; p
= 0.014), which was driven by a higher acute coronary syndrome rate (6.7%,
18.9% and 7.6%; p = 0.070), as detailed in table 4. Among the twelve reported patients
with
in-stent restenosis, four occurred in stents deployed in a dilated coronary
artery segment (two cases in CAA vessels, one case in a CAE vessel, and one in
a vessel presenting a mixed form). Only one stent thrombosis occurred in a
dilated coronary artery (an aneurysmatic vessel). The detection of de novo
cancers, inflammatory diseases and infectious diseases was reported in 8.2%,
3.1%, and 16.7% of cases for CAA, CAA-CAE, and CAE, respectively, with no
significant differences between the groups (p >0.05 in all groups), appendix table
S4.
Table 4Events at follow-up.
|
Total |
“CAA” |
“CAA-CAE” |
“CAE” |
p-value |
| Patient n = 257 |
Patient n = 75 |
Patient n = 37 |
Patient n = 145 |
2-sided |
| Follow-up event, n (%) |
149 (58.0) |
44 (58.7) |
25 (67.6) |
80 (55.2) |
0.391 |
| Follow-up duration, days |
568 (180–1197) |
713 (225–1305) |
428 (182–1220) |
543 (161–1155) |
0.378 |
| Re-coronary angiography, n (%) |
96 (37.4) |
26 (34.7) |
17 (45.9) |
53 (36.6) |
0.487 |
| Major adverse cardiovascular events, n
(%) |
98 (38.1) |
33 (44.0) |
15 (40.5) |
50 (34.5) |
0.367 |
| All-cause death, n (%) |
49 (19.1) |
20 (26.7) |
5 (13.5) |
24 (16.6) |
0.126 |
| Cardiac death, n (%) |
10 (3.9) |
3 (4.0) |
1 (2.7) |
6 (4.1) |
0.920 |
| Acute coronary syndrome, n (%) |
23 (8.9) |
5 (6.7) |
7 (18.9) |
11 (7.6) |
0.070 |
| Percutaneous coronary intervention, n (%) |
59 (23.0) |
17 (22.7) |
12 (32.4) |
30 (20.7) |
0.316 |
| Percutaneous coronary intervention of
CAE/CAA, n (%) |
32 (12.5) |
8 (10.7) |
10 (27.0)* |
14 (9.7) |
0.014 |
| In-stent restenosis, n (%) |
12 (4.7) |
4 (5.3) |
4 (10.8) |
4 (2.8) |
0.111 |
| Stent thrombosis, n (%) |
3 (1.2) |
1 (1.3) |
0 (0.0) |
2 (1.4) |
0.774 |
| Cerebrovascular event, n (%) |
7 (2.7) |
2 (2.7) |
1 (2.7) |
4 (2.8) |
0.999 |
| Re-hospitalisation for heart failure, n
(%) |
9 (3.5) |
5 (6.7) |
1 (2.7) |
3 (2.1) |
0.205 |
| Bleeding, n (%) |
30 (11.7) |
7 (9.3) |
6 (16.2) |
17 (11.7) |
0.566 |
| Medication upon admission |
Single anti-platelet therapy, n (%) |
30
(11.7) |
9
(12.0) |
3
(8.1) |
18
(12.4) |
0.763 |
| Dual anti-platelet therapy, n (%) |
26
(10.1) |
7
(9.3) |
5
(13.5) |
14
(9.7) |
0.758 |
| Oral anticoagulant, n (%) |
17
(6.6) |
4
(5.3) |
4
(10.8) |
9
(6.2) |
0.524 |
| Dual anti-thrombotic therapy, n (%) |
13
(5.1) |
3
(4.0) |
1
(2.7) |
9
(6.2) |
0.606 |
| Triple anti-thrombotic therapy, n (%) |
2
(0.8) |
1
(1.3) |
0
(0.0) |
1
(0.7) |
0.739 |
| Medication at discharge |
Single anti-platelet therapy, n (%) |
14
(5.4) |
5
(6.7) |
1
(2.7) |
8
(5.5) |
0.684 |
| Dual anti-platelet therapy, n (%) |
43
(16.7) |
12
(16.0) |
8
(21.6) |
23
(15.9) |
0.690 |
| Oral anticoagulant, n (%) |
9
(3.5) |
1
(1.3) |
2
(5.4) |
6
(1.4) |
0.446 |
| Dual anti-thrombotic therapy, n (%) |
11
(4.3) |
3
(4.0) |
2
(5.4) |
6
(1.4) |
0.934 |
| Triple anti-thrombotic therapy, n (%) |
13
(5.1) |
4
(5.3) |
1
(2.7) |
8
(5.5) |
0.778 |
| Bleeding classification |
|
|
|
|
|
0.642 |
| BARC 1, n (%) |
2
(0.8) |
1
(1.3) |
0
(0.0) |
1
(0.7) |
|
| BARC 2, n (%) |
6
(2.3) |
2
(2.7) |
0
(0.0) |
4
(2.8) |
|
| BARC 3a, n (%) |
3
(1.2) |
1
(1.3) |
0
(0.0) |
2
(1.4) |
|
| BARC 3b, n (%) |
5
(1.9) |
1
(1.3) |
2
(5.4) |
2
(1.4) |
|
| BARC 3c, n (%) |
7
(2.7) |
2
(2.7) |
3
(8.1) |
2
(1.4) |
|
| BARC 4, n (%) |
1
(0.4) |
0
(0.0) |
0
(0.0) |
1
(0.7) |
|
| BARC 5a, n (%) |
1
(0.4) |
0
(0.0) |
0
(0.0) |
1
(0.7) |
|
| BARC 5b, n (%) |
5
(1.9) |
0
(0.0) |
1
(2.7) |
4
(2.8) |
|
Longitudinally, patients with
angiographically identified CAA exhibited worse clinical outcomes than those with
CAE, which reflected a higher incidence of acute coronary syndrome at follow-up
and higher rates of coronary re-intervention (either at the level of the
dilated coronary segment or in any normal coronary artery segment), figure 2 and appendix
figure S7.
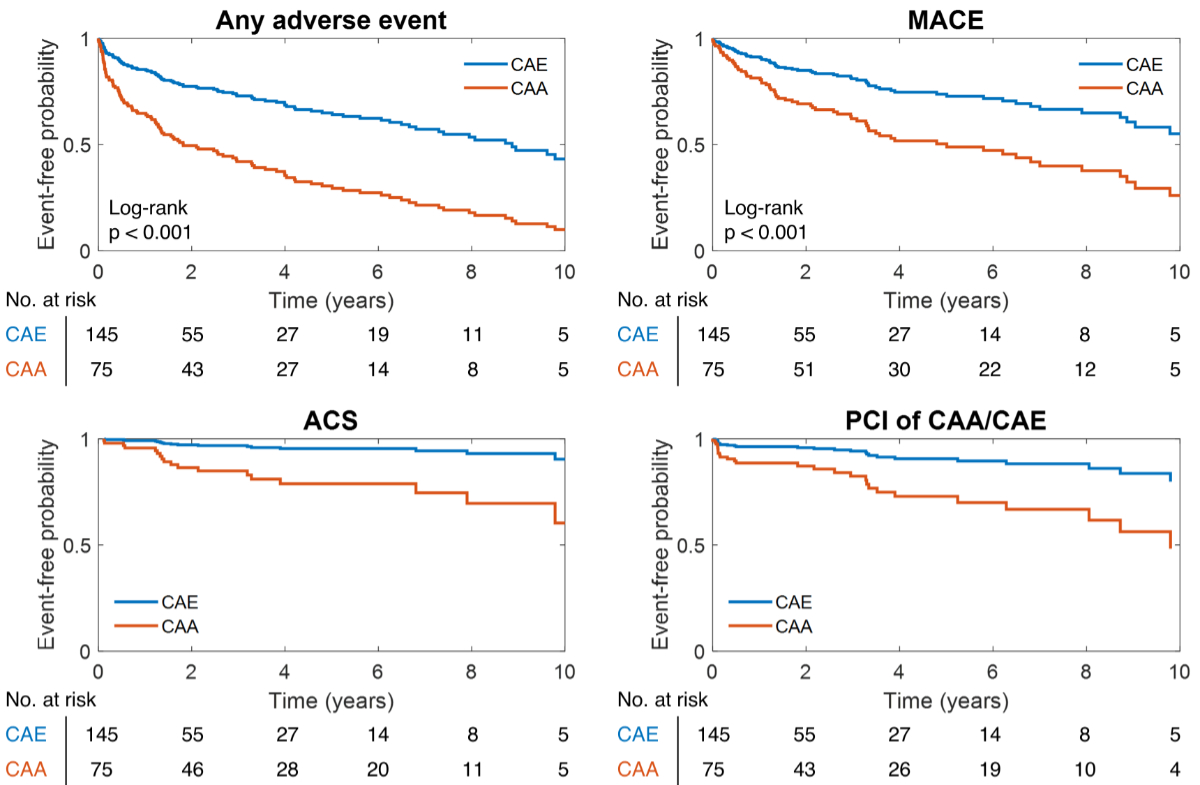
Figure 2Event incidence at the long-term
follow-up. Patients presenting either coronary artery ectasia (CAE) or coronary
artery aneurysm (CAA) showed an elevated incidence of adverse events at the
long-term follow-up. ACS: acute coronary syndrome; MACE: major adverse
cardiovascular events; PCI: percutaneous coronary intervention.
The Cox proportional hazards regression
analysis found that aneurysmatic coronary artery segments exhibited a higher
risk of adverse events compared to CAE (hazard ratio [HR] = 2.75, 95%
confidence interval [CI] 1.84–4.10), p <0.0001) and major adverse
cardiovascular events (HR = 2.26, 95% CI 1.38–3.69, p = 0.001), driven by a
higher acute coronary syndrome hazard (HR = 5.00, 95% CI 1.66–15.02, p = 0.004)
and percutaneous coronary intervention hazard (in a dilated coronary segment:
HR 3.23, 95% CI 1.40–7.45, p = 0.006; in any coronary segment: HR 3.83, 95% CI
2.08–7.07, p = 0.001), figure 3 and figure
S8 in the appendix. The presence of obstructive coronary artery disease
did not significantly influence the risk of major adverse cardiovascular events
at the follow-up in CAA or CAE patients, appendix figure S9.
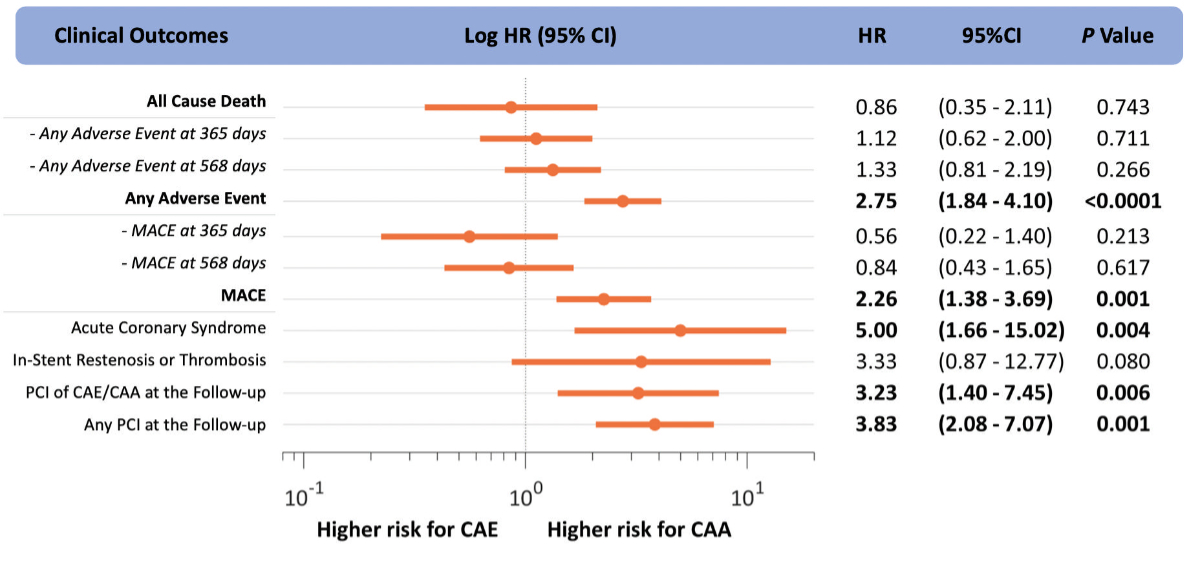
Figure 3Time-dependent event risk. Results
of the Cox models for coronary artery ectasia (CAE) or coronary artery aneurysm
(CAA) and the time-dependent hazard ratio (HR) of adverse events. In dash,
the shorter-term outcomes with respect to the one reported in bold (maximum
follow-up time). MACE: major adverse cardiovascular events; PCI:
percutaneous coronary intervention.
Discussion
In this study, a total of 281 patients with
dilated coronary artery disease were investigated, and clinical characteristics,
angiographic patterns and the long-term adverse outcomes of patients presenting
with CAE and CAA were described. The main results of the study were: (a) CAE
demonstrated a multi-district distribution, while CAAs were primarily isolated
to a single coronary segment; (b) multi-vessel obstructive coronary artery
disease was prevalent in more than half of the patients; (c) clinical adverse
events were common in-hospital and at the long-term follow-up; and (d) aneurysmatic
coronary artery segments were associated with a higher risk of adverse events
and major adverse cardiovascular events, which was driven by higher hazards of acute
coronary syndrome and percutaneous coronary intervention at the follow-up.
The spectrum of coronary artery remodelling
The initial phase of atherosclerotic plaque
formation involves leukocyte migration, foam cell formation, and extracellular
matrix degradation, leading to expansive coronary remodelling that maintains
lumen diameter and prevents narrowing [1]. Dysregulation of these mechanisms by
injury or degradation of the vessel layers, especially the media, can cause
either reverse remodelling with luminal narrowing or further dilation,
resulting in coronary ectasia and aneurysm formation [2–4]. As such, both
obstructive and dilative coronary artery disease can co-exist within the same
coronary artery tree. In our analysis, only a small percentage of the vessels
included in the study failed to exhibit a (significant) obstructive coronary
artery disease form (18.2%). Instead, extensive three-vessel disease was the
most common coronary artery disease variant associated with either CAA (34.6%)
or CAE (28.6%). This, coupled with the high prevalence of traditional
cardiovascular risk factors in both dilative coronary artery disease subgroups,
supports the theory of a significant overlap in the pathobiology between
obstructive and dilative coronary artery disease [8]. Notably, this disease
form appears to predominantly affect males (ca. 88% of male patients in our
cohort), a trend consistent with the literature [9].
Coronary aneurysms and ectasia as two different biological
conditions
While sharing a similar pathophysiology, in
our analysis, CAAs and CAEs appeared to be distinct entities with marginally overlapping
clinical characteristics. In the study population, coronary aneurysms and
ectasia co-existed, however, only 14.9% of patients presented with both
conditions. The CAA group had a higher proportion of female patients, was older,
and was more likely to have undergone cardiac surgery than the CAE group. A similar
prevalence of inflammatory and oncological conditions was found in both groups.
A trend toward higher hs-CRP in the CAA group was also found, suggesting a higher
prevalence of residual inflammatory risk [10, 11].
Angiographic characteristics also varied
among groups. CAAs were primarily localised in a single vessel, whereas CAE
exhibited a multi-district distribution in nearly half of the cases. The
distribution of CAA was relatively balanced among the three major coronary
arteries, except for the giant form, which was almost invariably located in the
right coronary artery. Conversely, CAE was most frequently located in the right
coronary artery (46.9% of cases). Furthermore, CAAs had a more extensive
representation along the coronary tree, also appearing at the level of diagonal
and marginal side branches, a trait not observed in CAE cases. Nevertheless,
coronary ectasia had, in 79.0% of cases, a diffuse manifestation (i.e. type 1,
type 2 or type 3) with the involvement of more than one segment along the epicardial
vessel.
Coronary aneurysms and ectasia present different clinical
and risk profiles
The scientific literature regarding
clinical outcomes in patients with dilated coronary artery disease is typically
sparse, often biased by varying anatomical definitions and inclusion criteria,
and marked by controversial conclusions. Luo et al. demonstrated a lower
coronary flow (quantified in terms of TIMI frame counts) in patients with CAA
compared to those with CAE, suggesting that impaired intravascular haemodynamics
could lead to ischaemia [12]. Núñez-Gil et al. reported that morbidity and
mortality rates in large European and North American populations with CAA
exceeded 30% and 15%, respectively [9]. Several studies have indicated worse
post-procedural outcomes in the form of dilated coronaropathy than obstructive coronary
artery disease [2]. However, a comprehensive comparison of long-term clinical
outcomes in patients presenting with either CAA or CAE has not been adequately
addressed.
Our longitudinal analysis of patient
outcomes specifically underscored the distinct clinical behaviour of CAAs and
CAEs. In our cohort, patients with angiographically identified CAAs
demonstrated worse clinical outcomes than those with CAEs. This was not only
indicated by a higher incidence of acute coronary syndrome during follow-up but
also by an increased rate of coronary re-interventions involving dilated
coronary segments and other normal coronary artery segments. The Cox
proportional hazards regression analysis further corroborated these findings,
revealing that aneurysmal coronary artery segments were associated with a
significantly higher risk of adverse events and major adverse cardiovascular
events, predominantly driven by a higher incidence of acute coronary syndrome
and a subsequent requirement for a percutaneous coronary intervention during
follow-up. This was observed in a mixed, real-world study population where
patients were equally treated operatively and conservatively at baseline.
Elevated rate of stent failure in dilated coronary
disease
The high rates of in-stent restenosis and
stent thrombosis in our cohort were noteworthy. Considering only the sub-group
of patients who underwent a percutaneous coronary intervention at the baseline
coronary angiography, the rates of in-stent restenosis and stent thrombosis
were recorded at 9.8% and 2.5%, respectively, over a median follow-up period of
18.9 months. In particular, one-third of the recorded stent failures occurred
in stents positioned within a dilated coronary artery segment, where in-stent
restenosis and stent thrombosis rates reached 22.2% and 1.8%, respectively. The
causes of stent failure within an enlarged vessel segment may be secondary to
procedural factors, such as sub-optimal stent apposition, stent fracture or
edge dissection due to forceful post-dilation or a lack of post-procedural
imaging control [13]. Aneurysm ceilings with covered stents were rare, performed
in two out of nineteen treated CAAs. However, the reasons for the increased
risk in non-dilated coronary artery segments are less clear. Potential factors
could be alterations in intra-coronary haemodynamics, such as a decreased flow
speed downstream of a coronary aneurysm or secondary flow patterns with blood
re-circulation and platelet activation [14–16]. Alongside these factors, the
presence of a biologically favourable environment with heightened
pro-inflammatory activity – as the registered elevated hs-CRP levels might
suggest – could also play a role in affecting the long-term performance of
stents in these patients [17]. Larger studies are needed to substantiate the
evidence of an elevated stent failure rate in this population and to properly
address its aetiology.
Limitations
This study acknowledges several
limitations. Firstly, patient selection was executed using a comprehensive
system query based on pre-defined keywords rather than a visual examination of
each coronary angiography performed at our institution. Nevertheless,
considering the extensive timeframe for patient inclusion (15 years) and the numerous
operators performing angiography procedures, the risk of selection bias is
minimal. Secondly, the classification of CAA or CAE was based on visual
assessments of the coronary angiograms rather than intravascular imaging (e.g.
intravascular ultrasound or optical coherence tomography). Consequently, the
precision of the assessment may be impaired, and the inadvertent inclusion of
coronary pseudoaneurysms cannot be ruled out. However, the implemented
methodology better represents current routine clinical practice. Third, the
study lacks a comparative analysis with a control group with obstructive coronary
artery disease without CAA/CAE. However, it should be noted that obstructive coronary
artery disease prevalence in the investigated cohort was large. This fact, in
conjunction with the high prevalence of traditional risk factors for coronary
artery disease, implies a significant overlap between the two types of coronary
diseases, making a direct comparison less informative. Finally, the observed
increased risk associated with CAA could be partially attributed to the longer
follow-up period for this group (a median follow-up duration of 713 days)
compared to the CAE group (a median follow-up duration of 543 days), highlighting
the importance of considering the follow-up duration when interpreting these
results.
Conclusions
Aneurysmatic coronary segments presented a
more aggressive clinical course compared to coronary ectasia, emphasising the
need for a nuanced approach to patient management that also accounts for the
different manifestations of dilative coronary artery disease. Further research
is necessary to elucidate the mechanisms behind these divergent outcomes and to
develop targeted therapeutic strategies for patients presenting with either
form of dilated coronary artery disease.
Prof. Christian Templin, MD, PhD, FESC
Department of Cardiology and Internal Medicine B
University Medicine Greifswald
Ellernholzstraße 1/2
DE-17489 Greifswald
ch.templin[at]gmx.de
References
1. Stary HC. Natural history and histological classification of atherosclerotic lesions:
an update. Arterioscler Thromb Vasc Biol. 2000 May;20(5):1177–8. doi: https://doi.org/10.1161/01.ATV.20.5.1177
2. Kawsara A, Núñez Gil IJ, Alqahtani F, Moreland J, Rihal CS, Alkhouli M. Management
of Coronary Artery Aneurysms. JACC Cardiovasc Interv. 2018 Jul;11(13):1211–23. doi: https://doi.org/10.1016/j.jcin.2018.02.041
3. Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006 Apr;47(8 Suppl):C7–12.
doi: https://doi.org/10.1016/j.jacc.2005.09.068
4. Antoniadis AP, Chatzizisis YS, Giannoglou GD. Pathogenetic mechanisms of coronary
ectasia. Int J Cardiol. 2008 Nov;130(3):335–43. doi: https://doi.org/10.1016/j.ijcard.2008.05.071
5. Scott DH. Aneurysm of the coronary arteries. Am Heart J. 1948 Sep;36(3):403–21. doi: https://doi.org/10.1016/0002-8703(48)90337-8
6. Núñez-Gil IJ, Nombela-Franco L, Bagur R, Bollati M, Cerrato E, Alfonso E, et al.;
CAAR Investigators. Rationale and design of a multicenter, international and collaborative
Coronary Artery Aneurysm Registry (CAAR). Clin Cardiol. 2017 Aug;40(8):580–5. doi: https://doi.org/10.1002/clc.22705
7. Syed M, Lesch M. Coronary artery aneurysm: a review. Prog Cardiovasc Dis. 1997;40(1):77–84.
doi: https://doi.org/10.1016/S0033-0620(97)80024-2
8. Vergallo R, Crea F. Atherosclerotic Plaque Healing. N Engl J Med. 2020 Aug;383(9):846–57.
doi: https://doi.org/10.1056/NEJMra2000317
9. Núñez-Gil IJ, Cerrato E, Bollati M, Nombela-Franco L, Terol B, Alfonso-Rodríguez E,
et al.; CAAR investigators. Coronary artery aneurysms, insights from the international
coronary artery aneurysm registry (CAAR). Int J Cardiol. 2020 Jan;299:49–55. doi: https://doi.org/10.1016/j.ijcard.2019.05.067
10. Carrero JJ, Andersson Franko M, Obergfell A, Gabrielsen A, Jernberg T. hsCRP Level
and the Risk of Death or Recurrent Cardiovascular Events in Patients With Myocardial
Infarction: a Healthcare-Based Study. J Am Heart Assoc. 2019 Jun;8(11):e012638. doi: https://doi.org/10.1161/JAHA.119.012638
11. Candreva A, Matter CM. Is the amount of glow predicting the fire? Residual inflammatory
risk after percutaneous coronary intervention. Eur Heart J. 2022 Feb;43(7):e10–3.
doi: https://doi.org/10.1093/eurheartj/ehy729
12. Luo Y, Tang J, Liu X, Qiu J, Ye Z, Lai Y, et al. Coronary Artery Aneurysm Differs
From Coronary Artery Ectasia: Angiographic Characteristics and Cardiovascular Risk
Factor Analysis in Patients Referred for Coronary Angiography. Angiology. 2017 Oct;68(9):823–30.
doi: https://doi.org/10.1177/0003319716665690
13. Esposito L, Di Maio M, Silverio A, Cancro FP, Bellino M, Attisano T, et al. Treatment
and Outcome of Patients With Coronary Artery Ectasia: Current Evidence and Novel Opportunities
for an Old Dilemma. Front Cardiovasc Med. 2022 Feb;8:805727. doi: https://doi.org/10.3389/fcvm.2021.805727
14. Fan T, Zhou Z, Fang W, Wang W, Xu L, Huo Y. Morphometry and hemodynamics of coronary
artery aneurysms caused by atherosclerosis. Atherosclerosis. 2019 May;284:187–93.
doi: https://doi.org/10.1016/j.atherosclerosis.2019.03.001
15. Kuramochi Y, Ohkubo T, Takechi N, Fukumi D, Uchikoba Y, Ogawa S. Hemodynamic factors
of thrombus formation in coronary aneurysms associated with Kawasaki disease. Pediatr
Int. 2000 Oct;42(5):470–5. doi: https://doi.org/10.1046/j.1442-200x.2000.01270.x
16. Burns JC, Glode MP, Clarke SH, Wiggins J Jr, Hathaway WE. Coagulopathy and platelet
activation in Kawasaki syndrome: identification of patients at high risk for development
of coronary artery aneurysms. J Pediatr. 1984 Aug;105(2):206–11. doi: https://doi.org/10.1016/S0022-3476(84)80114-6
17. Niccoli G, Montone RA, Ferrante G, Crea F. The evolving role of inflammatory biomarkers
in risk assessment after stent implantation. J Am Coll Cardiol. 2010 Nov;56(22):1783–93.
doi: https://doi.org/10.1016/j.jacc.2010.06.045
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3857.


