
Figure 1(A) Third instar larvae just after collection; (B) one of the larvae after pupation; (C) detail of the anal plate of the pupa for species identification.
DOI: https://doi.org/https://doi.org/10.57187/s.3827
Myiasis is a parasitic infestation of living vertebrates by dipteran larvae. Human myiasis is usually caused by flies belonging to the families Calliphoridae, Sarcophagidae, Oestridae and Muscidae [1, 2]. Some species require a host for complete larval development (obligatory or specific myiasis) and can cause severe damage to healthy tissues [2]. Facultative or semi-specific myiasis may infest living tissue but can also thrive on decaying organic matter or carrion [2, 3]. Most human myiases are related to the infestation of necrotic lesions or body cavities with accumulated secretions.
A 1997 review of worldwide nosocomial myiasis reported 23 cases of nosocomial myiasis from 1945–1996, involving Lucilia sericata, Sarcophaga spp, Cochliomyia hominivorax and Musca domestica [4]. Since 1997, according to Bernhardt et al., 54 cases of autochthonous European myiasis were published [1]. Nosocomial myiasis is seldom reported in Europe, and intensive care unit (ICU)-acquired myiasis is even rarer.
We describe the first report of hospital-acquired oropharyngeal myiasis caused by Lucilia sericata occurring in a Swiss ICU. We provide a review of worldwide cases of ICU-acquired myiasis and discuss entomological aspects as well as treatment and prevention of this infestation.
A 78-year-old patient was admitted to hospital for heart failure. He was hospitalized and investigated for 10 days on the internal medicine ward and diagnosed with severe valvular disease. He underwent cardiac surgery on day 11 and was intubated in the operating theatre and admitted to the ICU after surgery. Sixty hours after the intubation, 13 live centimetric larvae were found in his oral cavity during usual mouth care by an assistant nurse.
A careful otorhinolaryngological examination revealed no significant oral lesion but only a small erosion in the retromolar trigone. Nasogastric tube aspiration and a routine bronchoscopy excluded further larvae infestation. The treatment consisted of mechanical larvae removal and further enhancement of oral hygiene with antiseptics. No further larvae emission was observed over the following days. The clinical course was then uneven, and the patient was discharged from the ICU two weeks later.
After their removal from the patient’s mouth, five larvae were placed in a small container by ICU staff and sent to the parasitology laboratory. Based on their morphological features, the maggots were identified as dipteran larvae in their third instar. The larvae were kept at room temperature but, lacking direct entomological supervision, only one pupated. The pupa was preserved in 90% ethanol and sent for further identification to the second author. The morphological characters of the pupa supported that the specimen belonged to Lucilia sericata (Calliphoridae; figure 1). This identification was confirmed by DNA barcoding of the mitochondrial cytochrome c oxidase subunit I (COI) gene using a novel primer, with the resulting sequence being identical to GenBank entry OQ611258 [5]. According to the data published by Grassberger et al.[6], it takes an average minimum of 30 hours after oviposition for L. sericata to reach the third instar at 34 °C. It stays in this stage for up to 57 hours, then reaches the post-feeding phase and starts to pupate 139 hours after oviposition at 34 °C. This is the approximate temperature assumed to be inside the patient’s oral cavity, where the larvae were found. Maggots were identified 60 hours after intubation, suggesting that oviposition occurred either in the operating room or shortly after ICU admission.

Figure 1(A) Third instar larvae just after collection; (B) one of the larvae after pupation; (C) detail of the anal plate of the pupa for species identification.
Usually, in our ICU, mouth care is performed three to six times a day by swabbing the patient’s oral cavity, but not the oropharynx, with an antiseptic solution. Selective oropharyngeal decontamination three times a day is also part of our ventilator-associated pneumonia (VAP) preventive bundle [7]. A staff and chart survey confirmed adherence to the mouth care protocol, whereas selective oropharyngeal decontamination, which may typically occur during the early ICU course, was not administered.
The ICU and infection and prevention control team conducted an environmental investigation. No specific environmental factors favouring fly entry in the ICU were identified, and no other patient cases were identified. This case was discussed during a multidisciplinary meeting to ensure adequate staff education and awareness. Mouth care and organic waste management education were reinforced.
We performed a literature search in PubMed using the following MeSH terms: “myiasis” combined with “intubation intratracheal”, “intensive care unit” or “nosocomial infection” (37 results). A second search was conducted using the following keywords: “myiasis” combined with “ICU”, “intensive care” or “intubat*” (39 results). The initial search yielded 69 papers. A selection was made using the following criteria: cases describing myiasis that occurred either during ICU stay (≥48 hours after admission) or within 48 hours of ICU discharge. We selected only papers with at least an abstract available in English.
Thirty-eight case reports of ICU-acquired myiasis were identified worldwide (figure 2). Among these, 14 were reported from high-income countries, and eight were from Europe. Ninety-two per cent of reports of ICU-acquired myiasis presented as oro- or nasopharyngeal. In rare instances, infections were also reported at tracheostomy sites, in the vagina or in wounds. The most frequently involved species was Lucilia sericata (45% of cases; figure 3). Mechanical ventilation was a common feature of all cases. Nasogastric tube insertion and the presence of periodontal disease also appeared as predisposing factors. The Appendix offers an overview of these selected cases.
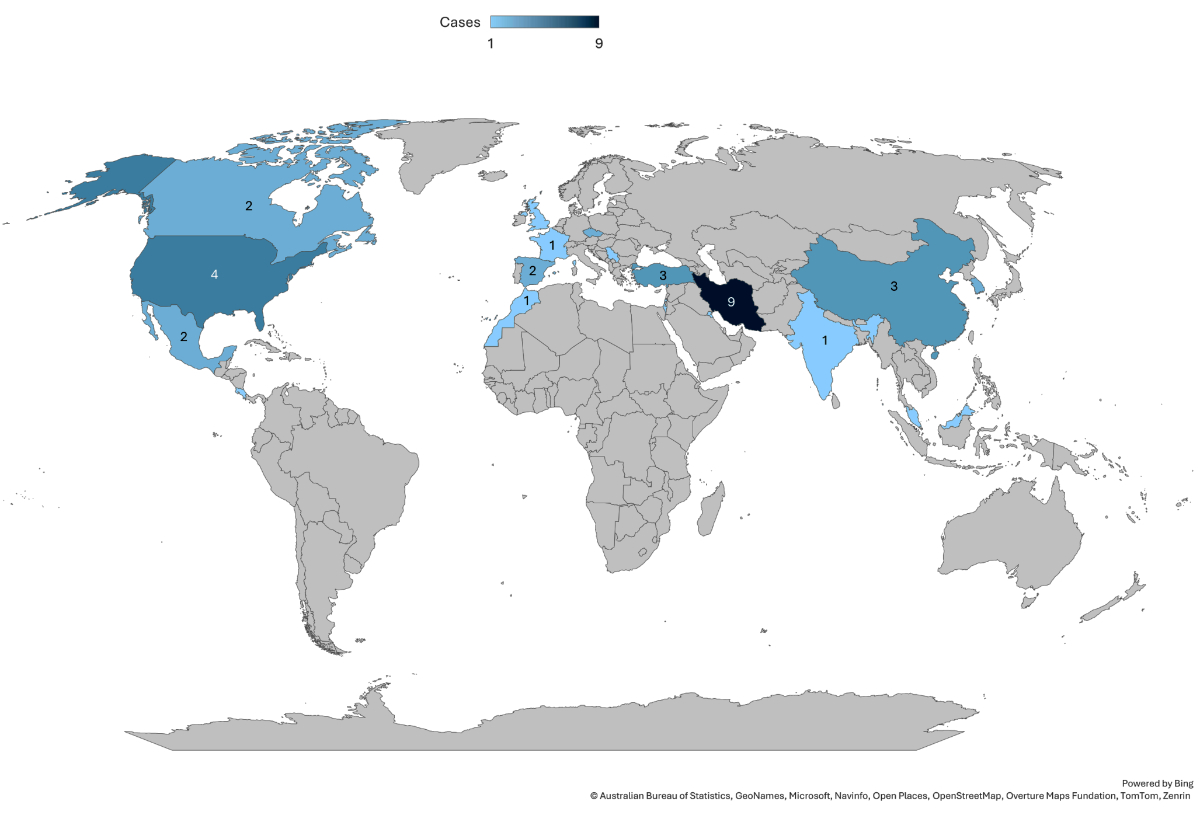
Figure 2World distribution of published cases of ICU myiases.
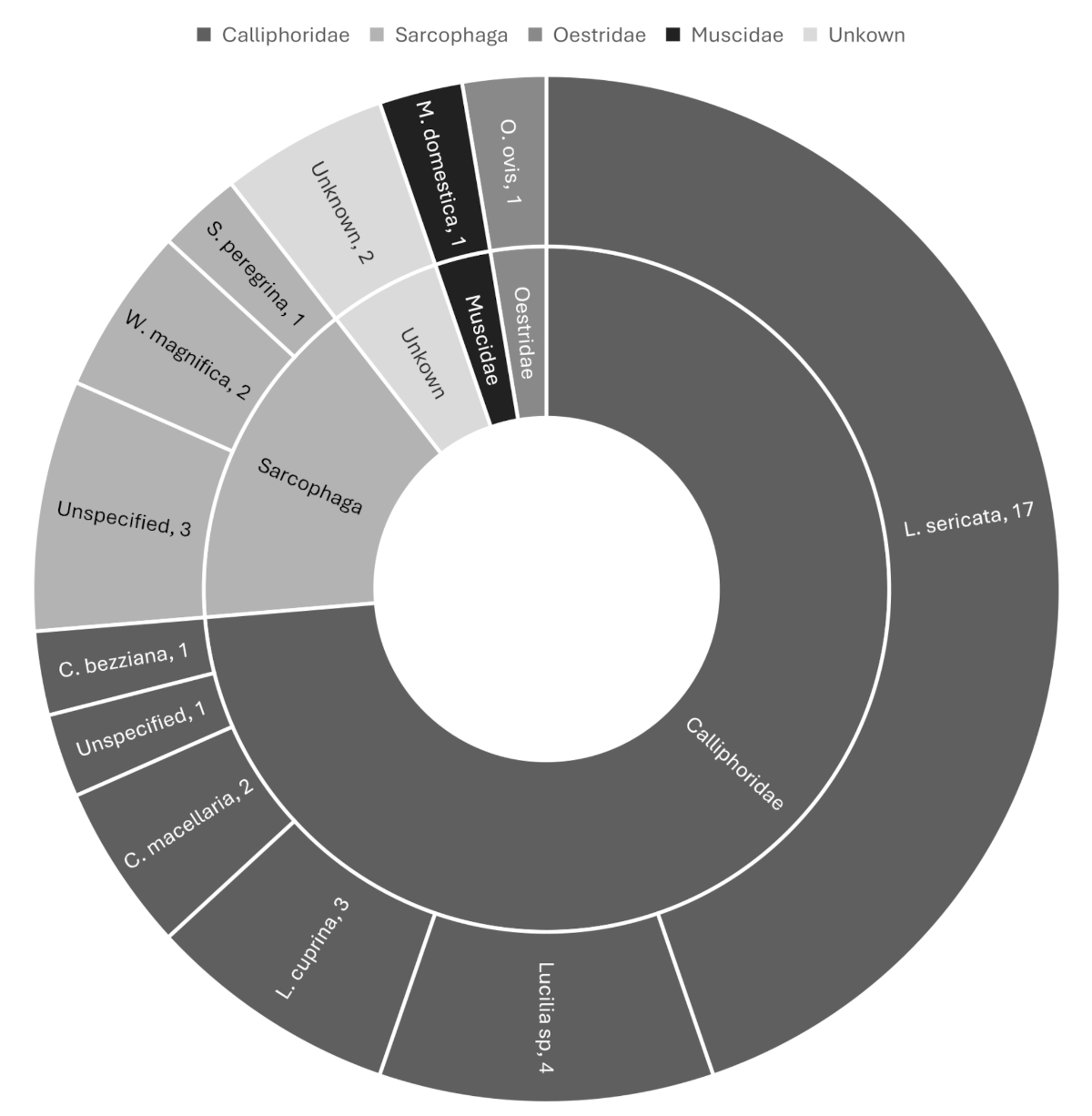
Figure 3Distribution of species in published cases of ICU-acquired myiasis.
Cases of nosocomial myiasis are constantly reported worldwide but only occasionally reported in intensive care patients because safeguards prevent fly entry in ICUs compared to other locations in the hospital [8–12]. Our case involving Lucilia sericata is the first nosocomial myiasis reported in Switzerland.
Lucilia sericata, a common blowfly known as the green bottle fly, is a cosmopolitan species with immense veterinary, medical and forensic importance. It is known for causing serious myiasis in sheep (“blowfly strikes”), which causes huge economic impacts to affected regions (mainly Northern Europe). However, in human medicine, Lucilia sericata larvae have displayed therapeutic benefits and are used as larva therapy to help heal some chronic wounds because they can feed on necrotic tissues, display antimicrobial activity and produce enzymes that promote healing [13]. Lucilia sericata also plays a role in forensic science to estimate the minimum postmortem interval because it is one of the most common necrophagous species recorded from dead human bodies [14]. The life cycle of the fly goes through four developmental stages: egg, larva, pupa and adult fly. Larvae will moult twice to reach the third stage (instar) of larval development. After the third stage of the larval period, larvae leave their host to find a place to pupate. The length of the life cycle can last from 10.79 days at 34 °C to 35.08 days at 17 °C [6]. The duration of the particular developmental stages depends on many external factors, of which the most important is temperature [6]. Blowflies usually lay their eggs in batches directly onto food sources (decaying animal or vegetable matter). However, they can also occasionally deposit them onto human necrotic tissues or open neglected wounds (traumatic myiasis) or in various human body cavities – but not onto intact mucous membranes, unlike obligatory parasitic fly species [1].
Flies are present all year round but can proliferate during summer months with higher temperatures due to a shortened life cycle. In our case, the infestation happened during a mid-summer heat wave. As noted by Bernhardt et al. [1], it is not unlikely that future global warming might change the incidence of this condition in Switzerland. Additionally, though the vast majority of fly species in Europe are facultative parasites, some obligatory species have also emerged [1]. According to a recent review, obligatory oral myiasis is most frequently caused by Cochliomyia hominivorax and Chrysomya bezziana, with higher reporting rates in India and Brazil [15]. Neurological diseases associated with clinical conditions such as lip incompetence or mouth breathing can increase an individual’s risk of developing oral myiasis [15]. Unlike the present case of facultative oral myiasis caused by Lucilia sericata, these infestations can cause significant tissue damage and require aggressive treatment such as surgical extraction, necrotic tissue debridement and antiparasitic therapy [15].
ICU-acquired myiasis is predominantly oro- or nasopharyngeal. Mechanically ventilated patients, while sedated and paralysed, are defenceless against flies entering the pharyngeal sphere and are therefore at risk of infestation. Most cases of myiasis described in the ICU involve facultative parasites, such as Lucilia species, and are thus self-limited (figure 3). In rare instances, the infection is caused by obligatory parasites such as Chrysomya bezziana (figure 3). Due to the concern of local invasion and tissue destruction reported in nasal myiasis caused by obligatory species, some facultative ICU-acquired nasal myiases have been treated with invasive approaches such as sinus surgery [16].
Clinicians should differentiate between facultative and obligatory oral myiasis because this can change their approach to treating the condition. In facultative cases, antiparasitic or antibiotic treatments are unlikely to add benefits after mechanical larvae removal. Liaising with an entomologist is invaluable for identifying species and establishing the timing of infection. Additionally, since myiasis can occur as an outbreak, any case of nosocomial myiasis should prompt consultation with infection prevention and control to evaluate environmental factors favouring fly entry and identify potential concurrent cases.
Unlike operating theatres, ICUs are usually devoid of airlock systems, and flies can therefore occasionally be observed within their closure despite sealed windows. Flies can enter the ICU via staff and visitor movements or imported resources such as food. One ICU nosocomial case has been described in a patient with a private windowless room [17]. ICU staff should be aware of the possibility of myiasis occurring in mechanically ventilated patients and should not overlook the presence of flies.
Fly prevention is part of a comprehensive strategy to minimise the risk of healthcare-associated infections, not only in operating theatres but also in ICUs and on the ward. In addition to causing incidental nosocomial myiasis, flies are also potential vectors of alert pathogens [18–20].
Though myiasis remains rare, reporting bias may be responsible for the lack of literature available, particularly in countries with high economic status, due to the poor image and risk of negative press it may lead to because this entity still carries the image of neglect and poor hygiene [21]. Our case is a reminder that myiasis is possible in high-income countries and is not invariably associated with neglect in care.
Author contributions: MM and VD conceived the idea and wrote the manuscript. JH and JMS identified the species and established the nosocomial nature of the infection. All the authors critically reviewed the manuscript and contributed substantially to its final form.
Written informed consent was obtained from the patient for the publication of this article.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Bernhardt V, Finkelmeier F, Verhoff MA, Amendt J. Myiasis in humans-a global case report evaluation and literature analysis. Parasitol Res. 2019 Feb;118(2):389–97.
2. Nigam Y, Dudley E, Bexfield A, Bond AE, Evans J, James J. Chapter 2 - The Physiology of Wound Healing by the Medicinal Maggot, Lucilia sericata. In: Simpson SJ(ed). Advances in Insect Physiology. Academic Press; 2010. p. 39–81. Available from: https://www.sciencedirect.com/science/article/pii/B9780123813879000026
3. David T. John M, Petri WA Jr. Markell and Voge’s Medical Parasitology. Elsevier Health Sciences; 2006. pp. 328–35.
4. Mielke U. Nosocomial myiasis. J Hosp Infect. 1997 Sep;37(1):1–5. doi: https://doi.org/10.1016/S0195-6701(97)90067-0
5. Hodecek J, Fumagalli L, Jakubec P. All insects matter: a review of 160 entomology cases from 1993 to 2007 in Switzerland-part I (Diptera). J Med Entomol. 2024 Mar;61(2):400–9. doi: https://doi.org/10.1093/jme/tjad164
6. Grassberger M, Reiter C. Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen- and isomorphen-diagram. Forensic Sci Int. 2001 Aug;120(1-2):32–6. doi: https://doi.org/10.1016/S0379-0738(01)00413-3
7. Landelle C, Nocquet Boyer V, Abbas M, Genevois E, Abidi N, Naimo S, et al. Impact of a multifaceted prevention program on ventilator-associated pneumonia including selective oropharyngeal decontamination. Intensive Care Med. 2018 Nov;44(11):1777–86. doi: https://doi.org/10.1007/s00134-018-5227-4
8. Leylabadlo HE, Kafil HS, Aghazadeh M, Hazratian T. Nosocomial oral myiasis in ICU patients: occurrence of three sequential cases. GMS Hyg Infect Control. 2015 Dec;10:Doc16.
9. Ahmadpour E, Youssefi MR, Nazari M, Hosseini SA, Rakhshanpour A, Rahimi MT. Nosocomial Myiasis in an Intensive Care Unit (ICU): A Case Report. Iran J Public Health. 2019 Jun;48(6):1165–8.
10. Katabi A, Aguirre M, Obeidat Y, Al-Ourani M, Assad S, Zeid F. Nasal myiasis in myasthenic crisis, a case report and literature review. Respir Med Case Rep. 2020 Sep;31:101212. doi: https://doi.org/10.1016/j.rmcr.2020.101212
11. Sun J, Fang K, Shi Z. Nosocomial infection with a crown. Intensive Care Med. 2019 Oct;45(10):1478–9. doi: https://doi.org/10.1007/s00134-019-05624-y
12. Martínez-Rojano H, Huerta H, Hernández-Triana LM, Ruiz Pérez EF, Sámano R. Nosocomial Myiasis Caused by Lucilia sericata (Diptera: Calliphoridae) in a Pediatric Patient in Mexico. Case Rep Infect Dis. 2020 Jan;2020:1285459. doi: https://doi.org/10.1155/2020/1285459
13. Sherman RA, Mumcuoglu KY, Grassberger M, Tantawi TI. Maggot Therapy. In: Grassberger M, Sherman RA, Gileva OS, Kim CM, Mumcuoglu KY, editors. Biotherapy - History, Principles and Practice: A Practical Guide to the Diagnosis and Treatment of Disease using Living Organisms. Dordrecht: Springer Netherlands; 2013. pp. 5–29.
14. Hodecek J, Jakubec P. Spatio-temporal distribution and habitat preference of necrophagous Calliphoridae based on 160 real cases from Switzerland. Int J Legal Med. 2022 May;136(3):923–34. doi: https://doi.org/10.1007/s00414-021-02769-8
15. Dos Passos JB, Coelho LV, de Arruda JA, Silva LV, do Valle IB, Santos MS, et al. Oral myiasis: analysis of cases reported in the English literature from 1990 to 2020. Spec Care Dentist. 2021 Jan;41(1):20–31. doi: https://doi.org/10.1111/scd.12533
16. Zobairy H, Modaresi P, Cabada MM, Sofi-Mahmudi A. Nasopharyngeal Myiasis in Intensive Care Unit (ICU) Patients: Report of Two Cases. Iran J Parasitol. 2023;18(1):113–8. doi: https://doi.org/10.18502/ijpa.v18i1.12388
17. Szakacs TA, MacPherson P, Sinclair BJ, Gill BD, McCarthy AE. Nosocomial myiasis in a Canadian intensive care unit. CMAJ. 2007 Sep;177(7):719–20. doi: https://doi.org/10.1503/cmaj.061598
18. Wiktorczyk-Kapischke N, Skowron K, Kwiecińska-Piróg J, Białucha A, Wałecka-Zacharska E, Grudlewska-Buda K, et al. Flies as a potential vector of selected alert pathogens in a hospital environment. Int J Environ Health Res. 2022 Aug;32(8):1868–87. doi: https://doi.org/10.1080/09603123.2021.1919605
19. Onwugamba FC, Fitzgerald JR, Rochon K, Guardabassi L, Alabi A, Kühne S, et al. The role of ‘filth flies’ in the spread of antimicrobial resistance. Travel Med Infect Dis. 2018;22:8–17. doi: https://doi.org/10.1016/j.tmaid.2018.02.007
20. Fürnkranz U, Walochnik J. Nosocomial Infections: Do Not Forget the Parasites! Pathogens. 2021 Feb;10(2):238. doi: https://doi.org/10.3390/pathogens10020238
21. Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012 Jan;25(1):79–105. doi: https://doi.org/10.1128/CMR.00010-11
The Appendix is available for download as a separate file at https://doi.org/10.57187/s.3827.