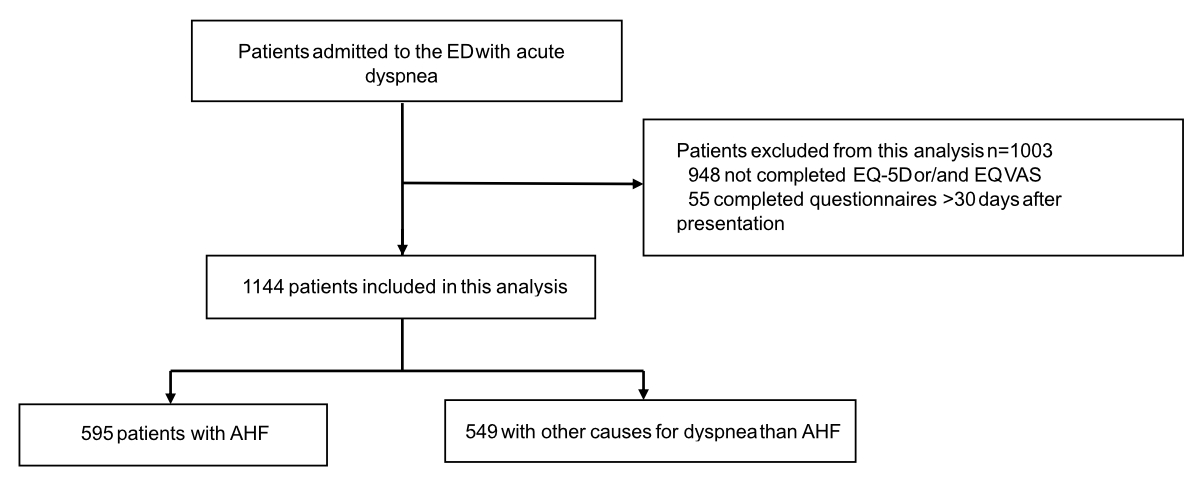
Figure 1Patients flow diagram.
DOI: https://doi.org/https://doi.org/10.57187/s.3785
area under the receiver-operating characteristics curve
Basics in Acute Shortness of Breath EvaLuation
B-type natriuretic peptide
chronic obstructive pulmonary disease
estimated glomerular filtration rate
EQ visual analogue scale
Geriatric Depression Scale – Short Form
N-terminal pro-B-type natriuretic peptide
visual analogue scale
Acute shortness of breath is one of the leading symptoms of patients presenting to the emergency department and is still associated with unacceptably high rates of mortality [1]. Risk stratification of patients presenting with acute dyspnoea is challenging, as underlying causes and urgency of intensified treatment vary. Uncertainty in risk stratification contributes to poor outcome [1]. Early identification of patients at highest risk for adverse events could help the treating physicians with initial triage and treatment decisions, and result in improved outcomes [2].
Scores and biomarkers have been suggested as simple, inexpensive, non-invasive tools to help physicians in the risk stratification of patients with acute dyspnoea [3–6]. B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP), quantitative markers of haemodynamic cardiac stress, provide moderate-to-good prognostic accuracy for the prediction of death, irrespective of whether acute dyspnoea was due to acute heart failure of other causes [3–7]. While no score has previously been established for all patients with acute dyspnoea, several scores, including the 13-item MEESSI-score, have been derived and validated for patients presenting with acute dyspnoea due to acute heart failure [8, 9].
We hypothesised that as an alternative to complex scores and biomarkers, self-perceived health-related quality of life may also provide prognostic value in patients with acute dyspnoea. This hypothesis is based on promising data in stable outpatients with chronic heart failure [10–15], and recent recommendations by the European Society for Cardiology and the American Heart Association to routinely evaluate self-perceived health status in patients with chronic heart failure [16–18]. In order to test this hypothesis, we aimed to evaluate the prognostic value of health-related quality of life as quantified by the established generic health-related quality of life instrument EQ-5D inclusive of the EQ visual analogue scale (EQ VAS) in patients presenting to the emergency department with acute dyspnoea and compare it to the established risk prediction marker NT-proBNP [19–21]. A secondary aim was to compare the health-related quality of life in patients adjudicated to have acute heart failure versus those with other causes of acute dyspnoea.
Basics in Acute Shortness of Breath EvaLuation (BASEL V) (ClinicalTrials.gov registry, number NCT01831115) was a prospective, multicentre, diagnostic and prognostic study enrolling adult patients presenting with acute dyspnoea as their primary complaint at the emergency departments of two University Hospitals in Switzerland (University Hospital Basel and University Hospital Zurich), both leading academic medical centres in Switzerland [7, 9, 22]. These hospitals are key referral centres for emergency care in the region, treating a wide range of acute conditions with state-of-the-art diagnostic tools. Patients with dyspnoea resulting from trauma or penetrating injuries were excluded, as these scenarios were beyond the study’s scope. Patients receiving renal replacement therapy were also excluded. Other than these groups, inclusion was open to all consecutive, unselected patients, ensuring minimal bias in the recruitment process. For this specific analysis, only patients with completed EQ-5D and EQ VAS were included. The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees (REC Zurich protocol code EK: 2011-0315, dated 25 August 2011, and REC Basel protocol code EK: 107/07, dated 26 July 2007). Written informed consent was obtained from all patients. The authors designed the study, gathered and reported the data in accordance with STROBE guidelines for cohort studies and the TRIPOD statement for studies reporting multivariable prediction models, vouched for the data and analysis, wrote the paper and made the decision to submit the article for publication.
The final diagnosis was centrally adjudicated by two independent cardiologists/internists who had access to all patients’ medical records including clinical history, physical examination, 12-lead electrocardiogram, laboratory findings, chest X-ray, echocardiography, lung function testing, computed tomography, response to therapy and also autopsy data for patients who died in hospital. All laboratory findings obtained through the clinician’s routine diagnostic workup were available for this study. These findings included one of the natriuretic peptides (BNP or NT-proBNP) that current guidelines recommended for diagnosing acute heart failure with a class I recommendation [16, 17, 23]. In situations of disagreement about the final diagnosis, cases were reviewed and adjudicated by a third cardiologist. Patients with an adjudicated diagnosis of acute heart failure were further classified as suggested by the European Society of Cardiology [24]. A detailed description of the clinical classification can be found in the supplementary material at https://doi.org/10.57187/s.3785.
Patients were contacted 3, 12 and 24 months after discharge by telephone or in writing. Information regarding death during follow-up was obtained from the hospital medical records, the general practitioner and the national mortality registry.
After patients had provided written informed consent, they received EQ-5D questionnaires and were asked to complete the self-administered forms without the help of external interviewers within the first days of hospitalisation. We used the EQ-5D-3L, which is a self-administered health-related quality of life instrument consisting of five questions regarding five dimensions (Mobility, Self-Care, Usual Activities, Pain/Discomfort and Anxiety/Depression) each with 3-levelled answers (figure S1 in the supplementary file) [19]. As recommended by the creators, responses can be converted to a single number, called the EQ-5D index, by applying a country-specific value set. Since there is no value set currently available for the German-speaking part of Switzerland, for this analysis the responses were weighted using the European VAS value set, which ranges from −0.074 to 1.0, with −0.074 indicating severe impairments over all five dimensions and a self-perceived health state worse than death. Additionally, patients were asked to mark their current health status on the 20 cm vertical EQ VAS ranging from 0 to 100, with 100 representing the best imaginable health state.
The validated 15-item Geriatric Depression Scale – Short Form (GDS-SF) (range 0–15) is a clinical screening tool that facilitates the assessment of depression [25]. GDS questionnaires were handed to the patients at the same time as the EQ-5D forms for self-administration. For the evaluation, patients were stratified according to their answers into four groups: probably no depression (GDS score 0 to <4.5), mild depression (GDS score 4.5 to <8.5), moderate depression (GDS score 8.5 to <11.5) and severe depression (GDS score 11.5 to 15). Questionnaires with five or fewer missing values were extrapolated as suggested by the developers. In patients with more than five missing answers, depression was not measured. The 15 questions of GDS and additional scoring information are shown in figure S2 in the supplementary file.
The outcome of this analysis was all-cause mortality within 90 and 720 days. Primarily, the EQ-5D index and the EQ VAS were analysed on a continuous scale. Adjusted Cox proportional hazards models were fitted to quantify the potential prognostic value of health-related quality of life on short-term (90-day) and long-term (720-day) mortality. Adjustments were made for predefined prognostic factors and confounders including age, sex, history of heart failure, systolic blood pressure at presentation, haemoglobin level, estimated glomerular filtration rate (eGFR) as per the Chronic Kidney Disease Epidemiology Collaboration and log-transformed NT-proBNP concentration. For the 720-day model, history of obstructive lung disease was added [26, 27]. Table S1 in the supplementary file summarises the covariates. The health-related quality of life scores were reversed, such that higher values indicated worse health-related quality of life. Additionally, z-transformation was applied to standardise the EQ-5D index and EQ VAS, enabling direct comparison of their hazard ratios (HRs) on a common scale. The linearity assumption was tested using Martingale residuals [28].
To obtain a measure for use in clinical practice, patients were stratified by the EQ-5D index and by the EQ VAS score into four groups: group 1 included patients with the lowest health-related quality of life (EQ-5D index: −0.074 to <0.25, EQ VAS: 0 to <25); group 2 included patients with moderately low health-related quality of life (EQ-5D index: 0.25 to <0.5, EQ VAS: 25 to <50); group 3 included patients with moderately high health-related quality of life (EQ-5D index: 0.5 to <0.75, EQ VAS: 50 to <75); and group 4 included patients with the highest health-related quality of life (EQ-5D index: 0.75 to 1.0, EQ VAS: 75 to 100). Adjusted Cox regression models were applied after stratification, and mortality was visualised with cumulative incidence curves. For internal validation, bootstrapping with 1000 resamples was performed to assess model robustness and to provide overoptimism-adjusted measures of discrimination and calibration. Subgroup analyses were conducted in patients with a final diagnosis of acute heart failure.
The accuracies of the EQ-5D index and the EQ VAS in predicting mortality were displayed using time-dependent area under the receiver-operating characteristics curve (AUC). A time-dependent receiver operating characteristic curve varies as a function of time and accommodates censored data [29]. Comparisons of AUC at 90- and 720-day follow-up between the EQ-5D index, the EQ VAS and NT-proBNP levels at presentation were performed by the DeLong test [30]. A combined impact was determined by integrating the EQ-5D index and EQ VAS into a single variable using a logistic regression model to reflect their overall effect.
Continuous variables are summarised as medians with interquartile ranges (IQR), and categorical variables by counts and percentages. Differences in baseline characteristics of the study population were assessed using the Mann-Whitney U or chi-squared test as appropriate. All hypothesis testing was two-tailed and p-values of less than 0.05 were considered to indicate statistical significance. If not explicitly mentioned, no adjustment for multiple testing was performed. Statistical analysis was performed with SPSS for Windows 28.0 (SPSS Inc., Chicago, IL, USA) and R statistical software version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Standard publicly available packages were used, including haven, dplyr, tidyr, ggplot2, eq5d, timeROC and boot.
This study was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the University of Basel, University Hospital Basel, Critical Diagnostics, Abbott, Alere, Beckman Coulter, BRAHMS, Roche and Singulex. None of these supporters had any role in designing the study, conducting the study, analysing the data or in the decision to submit this manuscript for publication.
Between April 2006 and February 2014, a total of 2153 patients with acute dyspnoea was enrolled at presentation to the emergency department. Of these, 1144 patients (median age 74 years, 42% female, median body mass index 25.8 kg/m2) were eligible for this analysis (figure 1). The baseline characteristics of the study patients are summarised in table 1. Acute heart failure was the most common adjudicated diagnosis (52%), followed by asthma/chronic obstructive pulmonary disease (20%) and pneumonia (14%). Table S2 in the supplementary file compares characteristics of patients with and without acute heart failure.

Figure 1Patients flow diagram.
Table 1Characteristics of patients at baseline. Values are medians (interquartile range) or counts (%). The p-values are for the comparison between patients alive vs deceased at day 720 of follow-up. The chi-squared test was performed for categorical variables and the Mann-Whitney U test for continuous variables.
| Characteristics and health-related quality of life at baseline | ||||||
| All patients, n = 1144 (100%) | Missing values, n (%) | Alive after 720 days, n = 829 (72%) | Died within 720 days, n = 315 (28%) | p-value* | ||
| Age in years | 74 (61–81) | 0 | 71 (59–79) | 79 (70–85) | <0.001 | |
| Female sex, n (%) | 481 (42%) | 0 | 364 (44%) | 117 (37%) | 0.045 | |
| Body mass index in kg/m2 | 25.8 (22.5–30.1) | 0 | 26.1 (23.0–30.8) | 24.7 (21.3–28.3) | <0.001 | |
| Medical history, n (%) | Diabetes | 264 (23%) | 0 | 179 (22%) | 85 (27%) | 0.064 |
| Hypertension | 783 (68%) | 0 | 539 (65%) | 244 (77%) | <0.001 | |
| Coronary artery disease | 401 (35%) | 0 | 251 (30%) | 150 (48%) | <0.001 | |
| Peripheral artery disease | 147 (13%) | 0 | 82 (10%) | 65 (21%) | <0.001 | |
| Previous stroke | 129 (11%) | 0 | 77 (9%) | 52 (17%) | 0.001 | |
| Atrial fibrillation | 332 (29%) | 0 | 207 (25%) | 125 (40%) | <0.001 | |
| History of heart failure | 394 (34%) | 0 | 236 (28%) | 158 (50%) | <0.001 | |
| COPD/Asthma | 387 (34%) | 0 | 270 (33%) | 117 (37%) | 0.164 | |
| Psychiatric disorder** | 253 (22%) | 0 | 171 (21%) | 82 (26%) | 0.059 | |
| Signs and symptoms | Systolic blood pressure in mm Hg | 138 (122–155) | 1 (0.5%) | 140 (125–156) | 131 (114–148) | <0.001 |
| SpO2 % | 96 (93–98) | 6 (0.4%) | 96 (93–98) | 95 (91–98) | 0.006 | |
| Oedema, n (%) | 475 (42%) | 0 | 307 (37%) | 168 (53%) | <0.001 | |
| Laboratory findings | Haemoglobin in g/l | 133 (117–146) | 7 (0.5%) | 136 (123–149) | 121 (108–137) | <0.001 |
| Sodium in mmol/l | 139 (136–141) | 7 (0.5%) | 139 (136–141) | 138 (136–141) | 0.271 | |
| eGFR in ml/min/m2 | 67 (45–88) | 1 (0.1%) | 72 (51–91) | 50 (34–78) | <0.001 | |
| NT-proBNP in ng/l | 1307 (235–5007) | 9 (0.7%) | 853 (1556–3645) | 3513 (950–9284) | <0.001 | |
| Chronic medication, n (%) | ACEIs/ARBs | 581 (51%) | 1 (0.1%) | 410 (50%) | 171 (54%) | 0.169 |
| Beta-blocking agents | 510 (45%) | 0 | 342 (41%) | 168 (53%) | <0.001 | |
| Diuretics | 582 (51%) | 0 | 367 (44%) | 215 (68%) | <0.001 | |
| Health-related quality of life | EQ-5D index | 0.637 (0.374–0.779) | 0 | 0.687 (0.480–0.779) | 0.480 (0.226–0.687) | <0.001 |
| EQ VAS | 50 (40–70) | 0 | 50 (40–70) | 50 (35–60) | <0.001 | |
ACEI: angiotensin-converting enzyme inhibitor; ARB: aldosterone receptor blocker; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate as per the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SpO2: oxygen saturation; VAS: visual analogue scale.
* p-values <0.05 were considered statistically significant. All hypothesis testing was two-tailed.
** Psychiatric disorders included, among others, dementia as per the Mini-Mental State Examination, history of depression and behavioural disorders due to psychoactive substance use.
EQ-5D questionnaires were completed at a median of 2 days (IQR 1–5) after emergency department presentation, with 45% of patients completing it within the first two days, and 78% completing it within 5 days of hospitalisation, yielding a median EQ-5D index of 0.637 (IQR 0.374–0.779) and a median EQ VAS of 50 (IQR 40–70). Health-related quality of life was similar in patients with acute heart failure and those with other causes of acute dyspnoea (p-values 0.638 and 0.504). Responses to the five EQ-5D questions are displayed in figure 2. Within 90 and 720 days, 7% and 28% of patients had died, respectively. Survivors at both time points had significantly higher EQ-5D indices and EQ VAS scores at presentation compared to non-survivors (e.g. 90-day survivors: EQ-5D 0.664, VAS 50; 90-day non-survivors: EQ-5D 0.480, VAS 40; both p <0.001). Differences in health-related quality of life between survivors and non-survivors at 720 days are shown in figure 3. Nearly 50% of the study patients did not complete the EQ-5D. Mortality was notably higher among non-participants (e.g. 30-day: 10% vs 3%, appendix table S3).
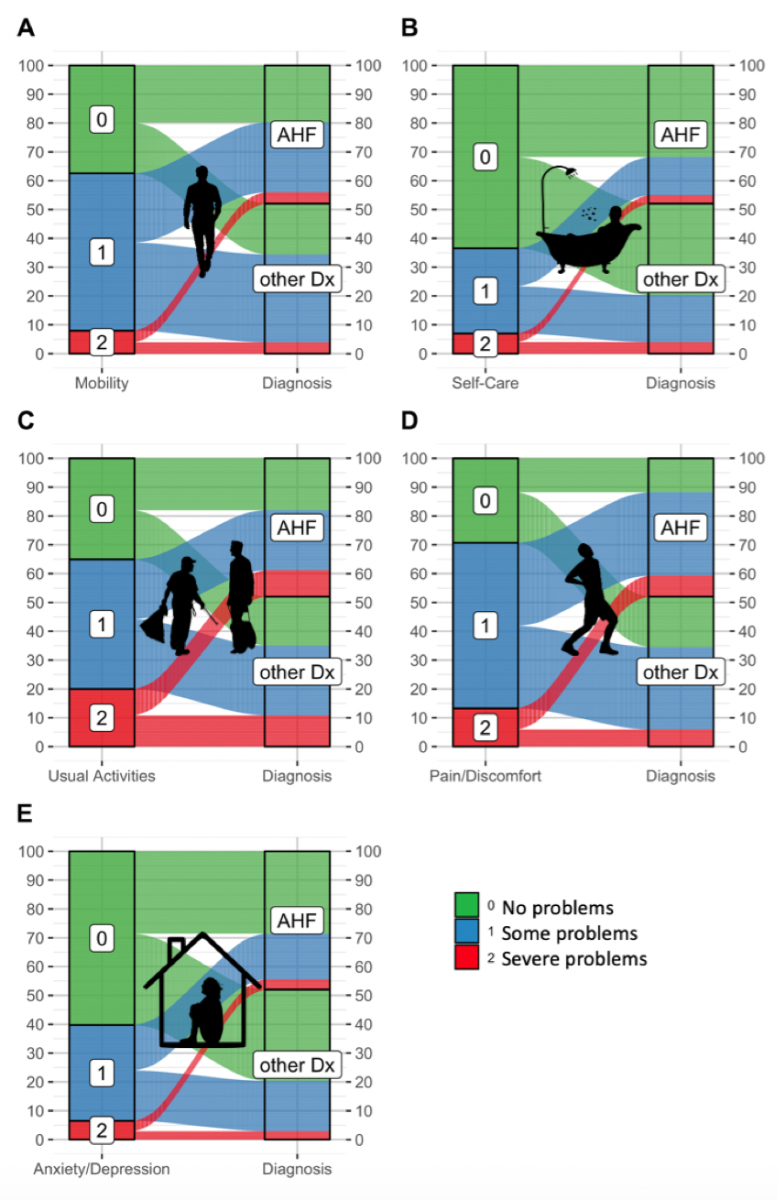
Figure 2Percentage of patients reporting no, some and severe impairments regarding (A) Mobility, (B) Self-Care, (C) Usual Activities, (D) Pain/Discomfort and (E) Anxiety/Depression according to the 3-levelled EQ-5D. Patients presenting with dyspnoea are stratified according to their diagnosis as acute heart failure (AHF) (n = 595) or other causes (Other diagnosis [Dx]) (n = 549).
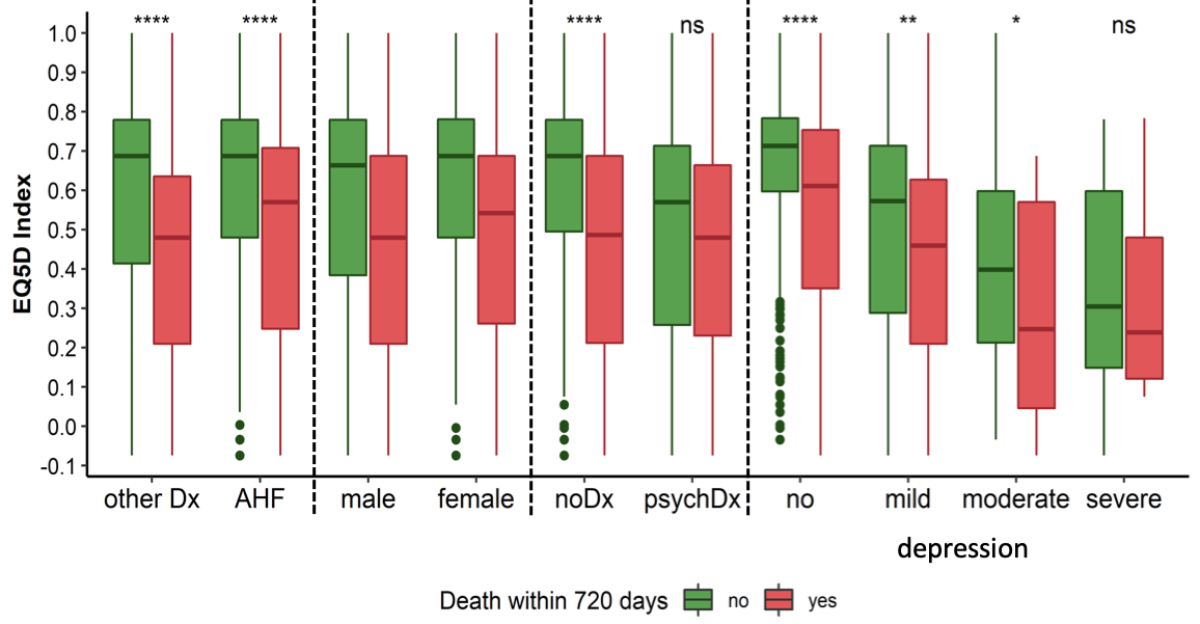
Figure 3EQ-5D index in different subgroups of patients presenting with acute dyspnoea. Patients (n = 1144) are stratified by mortality at day 720. Depression was measured with the Geriatric Depression Scale (n = 1123). Boxes represent medians and interquartile ranges, while whiskers display the smallest and the largest non-outliers and dots display outliers. P-values were calculated using the Mann-Whitney U test. Outliers in the figure are defined as data points that fall outside 1.5 times the interquartile range (IQR) from the first and third quartiles, represented as dots beyond the whiskers of the boxplots. Acute heart failure: acute heart failure (n = 549); noDx: no psychiatric diagnosis recorded (n = 891); psychDx: patients with psychiatric disorders (including dementia as per Mini-Mental State Examination, history of depression from medical records and behavioural disorders due to psychoactive substance use, n = 253); other Dx: other causes of acute dyspnoea (n = 595); male: n = 663; female: n = 481; no depression: n = 687; mild depression: n = 278; moderate depression: n = 106; severe depression: n = 52. **** p <0.0001; ** p <0.01; * p <0.05; ns p ≥0.05.
Among the 1144 patients, 81% had a complete set of GDS, 18% had 5 or fewer missing values, and 2% had more than 5 missing values. After prorating, depression was measured in 98% patients of whom 60% indicated they were probably not depressed, 24% mildly depressed, 9% moderately depressed and 5% severely depressed according to the GDS scoring. Depression was distributed similarly between patients with acute heart failure and patients with acute dyspnoea due to other causes (appendix table S4). Patients with severe depression reported a significantly worse health-related quality of life, corresponding to a lower EQ-5D index (median 0.278, IQR 0.126–0.592) and EQ VAS (median 37, IQR 25–50) than patients without, with mild or moderate depression (median EQ-5D 0.664, IQR 0.395–0.779; median EQ VAS 50, IQR 40–70, both p-values for comparison <0.001, appendix table S5). Among the severely depressed patients, survivors and non-survivors had comparable EQ-5D indices and EQ VAS (figure 3 and table 2).
Table 2EQ-5D in patients stratified by survival status and severity of depression. The EQ-5D index and EQ visual analogue scale (VAS) in patients presenting with acute dyspnoea stratified by survivors and non-survivors and depression according to the Geriatric Depression Scale (n = 1123). Continuous variables are presented as medians with interquartile ranges (IQRs) and were compared by the Mann-Whitney U test.
| Survivors | Non-survivors | p-value* | |||
| 90-day mortality | No depression | n = 651 | n = 36 | ||
| EQ-5D index | 0.69 (0.57–0.78) | 0.570 (0.313–0.701) | 0.001 | ||
| EQ VAS | 60 (50–75) | 50 (40–66) | 0.023 | ||
| Mild depression | n = 254 | n = 24 | |||
| EQ-5D index | 0.558 (0.27–0.689) | 0.427 (0.187–0.570) | 0.019 | ||
| EQ VAS | 50 (35–60) | 40 (30–50) | 0.050 | ||
| Moderate depression | n = 93 | n = 13 | |||
| EQ-5D index | 0.421 (0.212–0.598) | 0.125 (−0.034–0.212) | <0.001 | ||
| EQ VAS | 45 (30–60) | 20 (15–40) | 0.004 | ||
| Severe depression | n = 48 | n = 4 | |||
| EQ-5D index | 0.258 (0.131–0.539) | 0.527 (0.281–0.643) | 0.415 | ||
| EQ VAS | 38 (23–51) | 37 (33–45) | 0.987 | ||
| 720-day mortality | No depression | n = 531 | n = 156 | ||
| EQ-5D index | 0.713 (0.596–0.783) | 0.611 (0.34–0.753) | <0.001 | ||
| EQ VAS | 60 (50–75) | 50 (45–70) | 0.004 | ||
| Mild depression | n = 182 | n = 96 | |||
| EQ-5D index | 0.573 (0.285–0.713) | 0.460 (0.21–0.63) | 0.003 | ||
| EQ VAS | 50 (33–60) | 49 (30–60) | 0.408 | ||
| Moderate depression | n = 72 | n = 34 | |||
| EQ-5D index | 0.399 (0.213–0.598) | 0.247 (0.043–0.57) | 0.021 | ||
| EQ VAS | 45 (30–60) | 40 (20–50) | 0.119 | ||
| Severe depression | n = 29 | n = 23 | |||
| EQ-5D index | 0.305 (0.149–0.598) | 0.239 (0.121–0.48) | 0.549 | ||
| EQ VAS | 40 (25–51) | 35 (20–50) | 0.739 | ||
VAS: visual analogue scale.
* p-value for comparison between the survivors and non-survivors.
When treated as a continuous variable, the reversed EQ-5D index (higher scores indicating worse health-related quality of life) showed strong associations with mortality, with adjusted hazard ratios (aHR) of 7.8 (95% confidence interval [CI] 3.4–17.9, p <0.001) for 90-day and 5.0 (95% CI 3.3–7.6, p <0.001) for 720-day mortality. Similarly, the EQ VAS had aHRs of 1.02 (95% CI 1.02–1.034, p = 0.004) for 90-day and 1.02 (95% CI 1.01–1.02, p <0.001) for 720-day mortality. After z-transformation, the EQ-5D index and the EQ VAS showed comparable predictive power (table 3 and appendix table S6).
Table 3Cox regression models for 720-day all-cause mortality with EQ-5D index and EQ VAS. EQ-5D index and EQ VAS reversed, with higher score indicating lower health-related quality of life and are treated as continuous variables. Hazard ratios and 95% confidence intervals derived from (A) univariable analyses, (B) multivariable analyses and (C) multivariable analyses with z-transformed EQ-5D index and EQ VAS. Adjustments made for age (years), sex, systolic blood pressure (mm Hg) at presentation, history of obstructive lung disease, history of heart failure, estimated glomerular filtration rate (GFR) as per CKD-EPI (ml/min/1.73m2), haemoglobin levels (g/l) and natural log of N-terminal pro-B-Type natriuretic peptide (NT-proBNP, ng/l) concentrations at presentation. Hazard ratios for continuous variables represent the risk per 1-unit increase, except for NT-proBNP, which was log-transformed and is expressed per unit increase in its natural logarithm. For categorical variables, the reference categories are: male for “Female sex”, absence of COPD/asthma for “COPD/asthma” and absence of heart failure for “Heart failure”.
| (A) Univariable analysis | (B) Multivariable analysis | (C) Multivariable analysis after z-transformation | ||||||||
| Outcome | Variables | HR | 95% CI | p-value* | aHR | 95% CI | p-value* | aHR | 95% CI | p-value* |
| 720-day mortality | EQ-5D index | 5.520 | (3.716–8.200) | <0.001 | 5.027 | (3.308–7.640) | <0.001 | 1.649 | (1.449–1.877) | <0.001 |
| Age | 1.049 | (1.038–1.060) | <0.001 | 1.041 | (1.029–1.054) | <0.001 | 1.041 | (1.029–1.054) | <0.001 | |
| Female sex | 0.776 | (0.617–0.975) | 0.029 | 0.637 | (0.503–0.808) | <0.001 | 0.637 | (0.503–0.808) | <0.001 | |
| Systolic blood pressure | 0.987 | (0.982–0.991) | <0.001 | 0.990 | (0.986–0.995) | <0.001 | 0.990 | (0.986–0.995) | <0.001 | |
| COPD/asthma | 1.164 | (0.926–1.463) | 0.193 | 1.258 | (0.991–1.597) | 0.059 | 1.258 | (0.991–1.597) | 0.059 | |
| Heart failure | 2.113 | (1.694–2.636) | <0.001 | 1.121 | (0.876–1.435) | 0.365 | 1.121 | (0.876–1.435) | 0.365 | |
| GFR | 0.983 | (0.978–0.987) | <0.001 | 1.001 | (0.995–1.006) | 0.785 | 1.001 | (0.995–1.006) | 0.785 | |
| Haemoglobin | 0.977 | (0.972–0.982) | <0.001 | 0.988 | (0.982–0.993) | <0.001 | 0.988 | (0.982–0.993) | <0.001 | |
| NT-proBNP | 1.406 | (1.314–1.503) | <0.001 | 1.186 | (1.082–1.300) | <0.001 | 1.186 | (1.082–1.300) | <0.001 | |
| 720-day mortality | EQ VAS | 1.013 | (1.008–1.018) | <0.001 | 1.015 | (1.009–1.020) | <0.001 | 1.358 | (1.209–1.525) | <0.001 |
| Age | 1.049 | (1.038–1.060) | <0.001 | 1.040 | (1.028–1.053) | <0.001 | 1.040 | (1.028–1.053) | <0.001 | |
| Female sex | 0.776 | (0.617–0.975) | 0.029 | 0.711 | (0.561–0.899) | 0.004 | 0.711 | (0.561–0.899) | 0.004 | |
| Systolic blood pressure | 0.987 | (0.982–0.991) | <0.001 | 0.990 | (0.985–0.995) | <0.001 | 0.990 | (0.985–0.995) | <0.001 | |
| COPD/asthma | 1.164 | (0.926–1.463) | 0.193 | 1.361 | (1.075–1.723) | 0.010 | 1.361 | (1.075–1.723) | 0.010 | |
| Heart failure | 2.113 | (1.694–2.636) | <0.001 | 1.094 | (0.853–1.403) | 0.479 | 1.094 | (0.853–1.403) | 0.479 | |
| GFR | 0.983 | (0.978–0.987) | <0.001 | 1.001 | (0.996–1.007) | 0.677 | 1.001 | (0.996–1.007) | 0.677 | |
| Haemoglobin | 0.977 | (0.972–0.982) | <0.001 | 0.985 | (0.980–0.991) | <0.001 | 0.985 | (0.980–0.991) | <0.001 | |
| NT-proBNP | 1.406 | (1.314–1.503) | <0.001 | 1.209 | (1.103–1.324) | <0.001 | 1.209 | (1.103–1.324) | <0.001 | |
aHR: adjusted hazard ratio; CI: confidence interval; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; VAS: visual analogue scale.
Stratification into four health-related quality of life groups (figure 4 and 5, table 4) revealed steep gradients in mortality. For the EQ-5D index, the 90-day mortality ranged from 2% in the highest health-related quality of life group to 15.7% in the lowest (p <0.001), while the 720-day mortality ranged from 15% to 47% (p <0.001). Similar trends were observed with the EQ VAS. Multivariable Cox regression showed increasing aHRs for lower health-related quality of life groups, e.g. EQ-5D index aHRs for 90-day mortality rose from 2.7 (third group) to 6.7 (lowest group), with similar results for the EQ VAS and 720-day analyses. The timing of EQ-5D completion, whether within 5 days of presentation to the emergency department or later, did not significantly influence its prognostic accuracy for mortality prediction (appendix table S7).
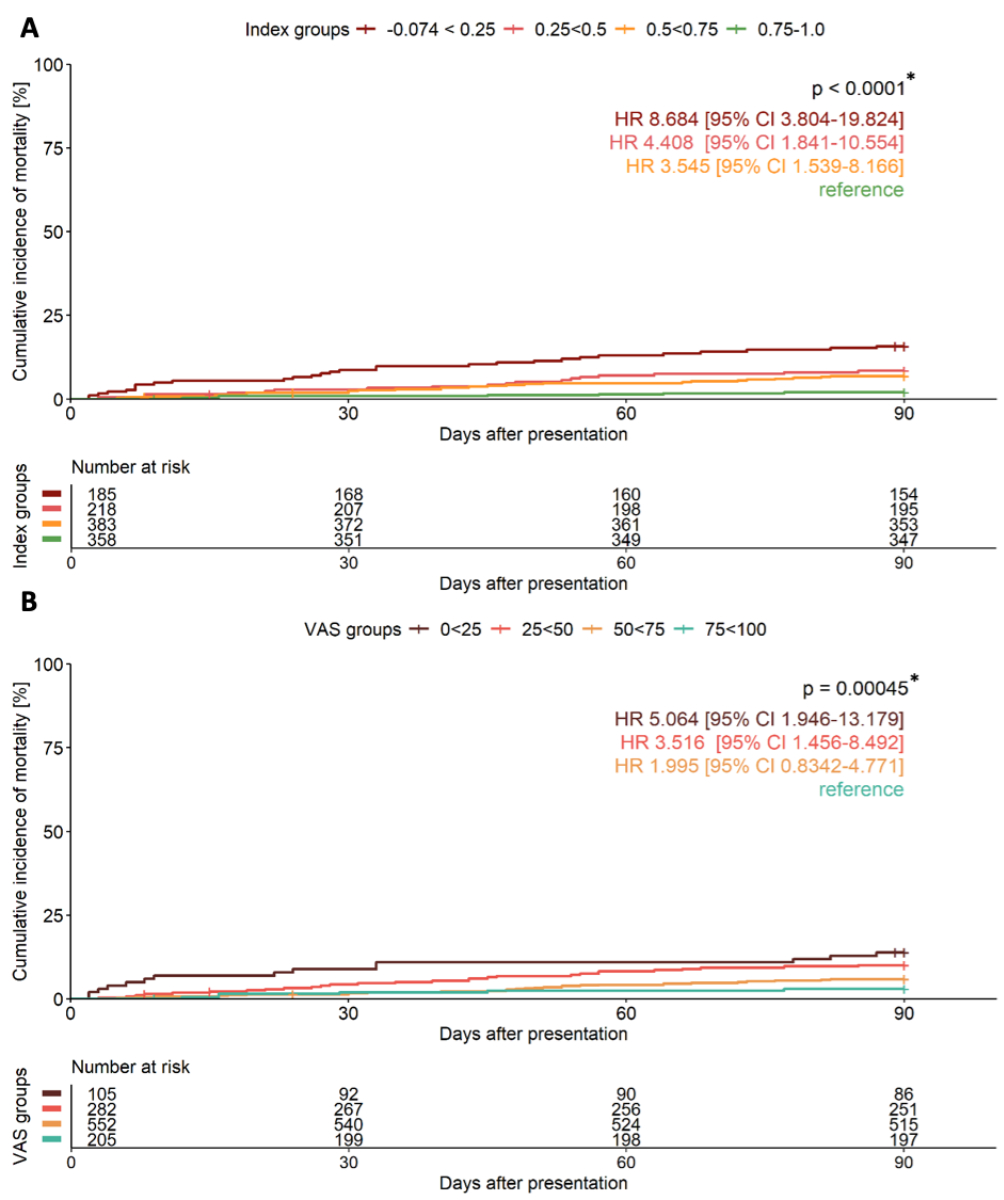
Figure 4Unadjusted cumulative incidence curves showing 90-day all-cause mortality in patients presenting with acute dyspnoea. Patients are stratified according to (A) EQ-5D index and (B) EQ VAS into 4 risk groups with corresponding numbers at risk and unadjusted hazard ratios (HR) and 95% confidence intervals (CI). Patients with highest health-related quality of life taken as reference group. Comparison made by log-rank test. VAS: visual analogue scale. * p-value for overall comparison.
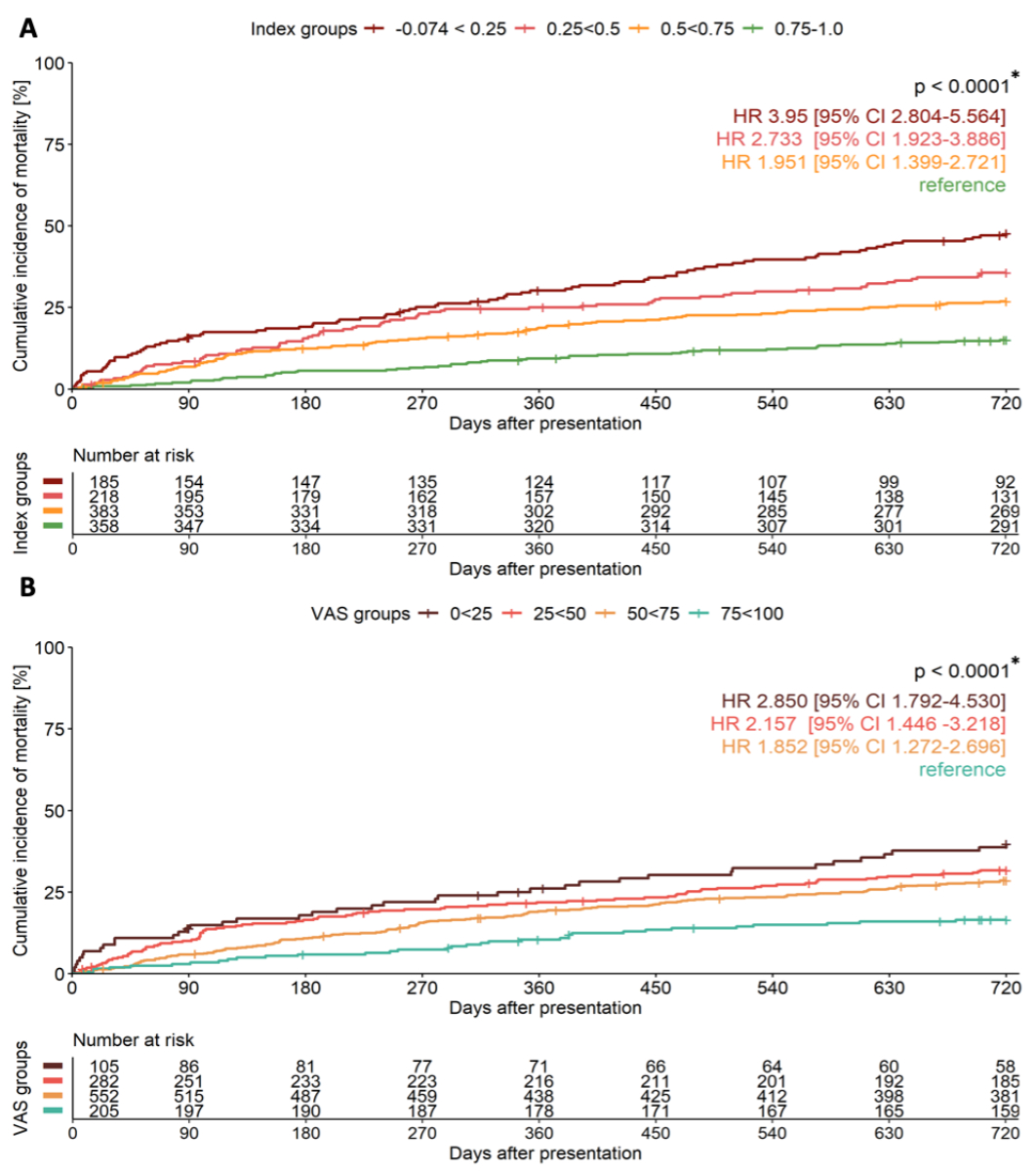
Figure 5Unadjusted cumulative incidence curves showing 720-day all-cause mortality in patients presenting with acute dyspnoea. Patients are stratified according to (A) EQ-5D index and (B) EQ VAS into 4 risk groups with corresponding numbers at risk and unadjusted hazard ratios (HR) and 95% confidence intervals (CI). Patients with highest health-related quality of life taken as reference group. Comparison made by log-rank test. VAS: visual analogue scale. * p-value for overall comparison.
Table 4Hazard ratios (HR) for 90- and 720-day all-cause mortality of patients stratified into low, moderately low, moderately high and high health-related quality of life according to (A) the EQ-5D index and(B) the EQ VAS. HRs and 95% confidence intervals (CI) derived from multivariable adjusted Cox regression models. Adjustments made for age (years), sex, systolic blood pressure (mm Hg) at presentation, history of heart failure, estimated glomerular filtration rate (GFR) as per CKD-EPI (ml/min/1.73m2), haemoglobin levels (g/l) and natural log of N-terminal pro-B-Type natriuretic peptide (NT-proBNP, ng/l) concentrations at presentation and history of obstructive lung disease for 720-day mortality.
| Outcome | Variable | HR | 95% CI | p-value | Outcome | HR | 95% CI | p-value |
| A | ||||||||
| 90-day mortality | Index group 1* | 6.707 | (2.907–15.474) | <0.001 | 720-day mortality | 3.379 | (2.373–4.811) | <0.001 |
| Index group 2** | 3.603 | (1.485–8.741) | 0.005 | 2.440 | (1.701–3.499) | <0.001 | ||
| Index group 3*** | 2.676 | (1.149–6.230) | 0.022 | 1.582 | (1.126–2.222) | 0.008 | ||
| Index group 4**** | reference | |||||||
| Age | 1.030 | (1.006–1.054) | 0.012 | 1.041 | (1.028–1.054) | <0.001 | ||
| Female sex | 0.567 | (0.349–0.921) | 0.022 | 0.648 | (0.512–0.820) | <0.001 | ||
| Systolic blood pressure | 0.982 | (0.972–0.993) | 0.001 | 0.990 | (0.986–0.995) | <0.001 | ||
| COPD/asthma | 1.284 | (1.013–1.628) | 0.039 | |||||
| Heart failure | 1.187 | (0.717–1.964) | 0.506 | 1.121 | (0.876–1.435) | 0.365 | ||
| GFR | 0.247 | (0.995–1.018) | 0.247 | 1.001 | (0.995–1.006) | 0.799 | ||
| Haemoglobin | 0.986 | (0.975–0.996) | 0.008 | 0.987 | (0.982–0.993) | <0.001 | ||
| NT-proBNP | 1.250 | (1.042–1.500) | 0.016 | 1.188 | (1.084–1.301) | <0.001 | ||
| B | ||||||||
| 90-day mortality | EQ VAS group 1* | 5.305 | (2.030–13.858) | 0.001 | 720-day mortality | 2.926 | (1.832–4.673) | <0.001 |
| EQ VAS group 2** | 2.988 | (1.227–7.275) | 0.016 | 2.029 | (1.351–3.047) | 0.001 | ||
| EQ VAS group 3*** | 1.735 | (0.722–4.171) | 0.218 | 1.614 | (1.102–2.365) | 0.014 | ||
| EQ VAS group 4**** | reference | |||||||
| Age | 1.031 | (1.007–1.055) | 0.010 | 1.040 | (1.027–1.053) | <0.001 | ||
| Female sex | 0.636 | (0.390–1.036) | 0.069 | 0.719 | (0.568–0.909) | 0.006 | ||
| Systolic blood pressure | 0.982 | (0.972–0.993) | 0.001 | 0.990 | (0.985–0.995) | <0.001 | ||
| COPD/asthma | 1.365 | (1.078–1.729) | 0.010 | |||||
| Heart failure | 1.130 | (0.679–1.880) | 0.639 | 1.089 | (0.849–1.397) | 0.502 | ||
| GFR | 1.007 | (0.996–1.019) | 0.187 | 1.001 | (0.9896–1.007) | 0.672 | ||
| Haemoglobin | 0.982 | (0.972–0.992) | 0.001 | 0.985 | (0.980–0.991) | <0.001 | ||
| NT-proBNP | 1.3106 | (1.089–1.574) | 0.004 | 1.121 | (1.106–1.328) | <0.001 | ||
* low health-related quality of life (group 1): EQ-5D index −0.074 to <0.25 (n = 185); EQ VAS 0 to <25 (n = 105)
** moderately low health-related quality of life (group 2): EQ-5D index 0.25 to <0.5 (n = 218); EQ VAS 25 to <50 (n = 282)
*** moderately high health-related quality of life (group 3): EQ-5D index 0.5 to <0.75 (n = 383); EQ VAS 50 to <75 (n = 552)
**** highest health-related quality of life (group 4): EQ-5D index 0.75 to 1.0 (n = 358); EQ VAS 75 to 100 (n = 205)
Internal validation confirmed the robustness of the results. aHRs from the bootstrapped datasets closely aligned with those from the original dataset. For example, the EQ-5D index aHR for 720-day mortality was 5.031 (95% CI 3.344–7.794) in the bootstrapped analysis versus 5.027 (95% CI 3.308–7.640) in the original. Cox regression coefficients and HRs remained consistent across analyses, strengthening confidence in the findings (table S8, appendix figures S3 and S4).
In the overall cohort, the prognostic accuracies calculated by a time-dependent AUC for 90-day mortality were 0.68 and 0.66 for the EQ-5D index and the EQ VAS, respectively (p-value for comparison 0.350). Combining both variables into a logistic regression model did not improve prognostic accuracy significantly (AUC: 0.69). For 720-day mortality, the EQ-5D index showed a higher prognostic accuracy than the EQ VAS (AUC: 0.65 vs 0.59, p <0.001) and, again, combining both variables did not improve the AUC of the EQ-5D index. In comparison to the prognostic accuracy for mortality of NT-proBNP concentrations at presentation (AUC for 90-day mortality: 0.69, 95% CI 0.63–0.75 and for 720-day mortality: 0.69, 95% CI 0.66–0.73), the EQ-5D index performed similarly (p-values for comparison 0.915 and 0.121, respectively). Figure S5 displays the AUCs of the EQ-5D index, EQ VAS and NT-proBNP concentration over 720 days of follow-up taking into account censored patients.
In the 595 patients with acute heart failure, similar findings emerged as in the overall cohort: cumulative mortality increased gradually and significantly over all four groups of the EQ-5D index for 90 and 720 days of follow-up with, respectively, 4% and 23% deceased patients in the fourth group (n = 181), 9% and 32% in the third group (n = 212), 10% and 44% in the second group (n = 106) and 16% and 55% patients in the first group (n = 96, p-value by overall log-rank test 0.008 and <0.001 for 90 and 720 days, respectively). The prognostic accuracies of the EQ-5D index in patients with acute heart failure were moderate with an AUC of 0.63 (95% CI 0.55–0.71) and 0.63 (95% CI 0.59–0.68) for 90- and 720-day mortality (figure S6), while NT-proBNP showed slightly higher AUCs of 0.65 (95% CI 0.60–0.69) and 0.68 (95% CI 0.62–0.73) without significant differences between the two measures. Tables S9–S11 in the supplementary file explore the prognostic accuracy of EQ-5D in patients with acute heart failure stratified into different subgroups.
This secondary analysis from a large, prospective, multicentre, diagnostic and prognostic study using central adjudication was performed to assess the prognostic value of health-related quality of life with the use of the validated EQ-5D in patients presenting with acute dyspnoea to the emergency department. We report five major findings:
First, health-related quality of life measured by the EQ-5D index and the EQ VAS was comparable between patients with acute dyspnoea due to acute heart failure and patients with other causes of dyspnoea and profoundly lower than the EQ-5D index obtained in the general European population (e.g. the mean EQ-5D index is 0.938 in Germany and 0.892 in France) [31]. Over 60% of patients with acute dyspnoea reported issues in three key domains: Pain/Discomfort, Usual Activities and Mobility. Second, a pronounced difference was found in the EQ-5D index and in the EQ VAS between survivors and non-survivors. Patients who died within 90 or 720 days after presenting with acute onset of dyspnoea indicated significantly and substantially worse health-related quality of life at presentation than patients who survived. One exception to this observation were patients with previously diagnosed psychiatric disorders and severely depressed patients according to the validated Geriatric Depression Scale (GDS). Third, low health-related quality of life was strongly associated with short- and long-term mortality: even following adjustments for previously implemented predictors including age, sex, history of chronic heart failure, chronic obstructive pulmonary disease (COPD), systolic blood pressure, eGFR, haemoglobin and NT-proBNP concentration, the EQ-5D index and the EQ VAS remained significant predictors of death. After standardisation of both measurements, the EQ-5D index showed a more pronounced increase in the aHR per unit than the EQ VAS. The robustness of the results was confirmed by internal validation. Fourth, in patients assigned to four groups according to their EQ-5D index and EQ VAS, the aHR increased distinctly and significantly from the group with the highest health-related quality of life to the group with the lowest health-related quality of life. Patients who indicated lowest health-related quality of life (EQ-5D index −0.074 to <0.24 or EQ VAS 0 to <25) were at significantly higher risk of death within 90 as well as 720 days, with a cumulative mortality of almost 50% after 720 days. Fifth, the prognostic accuracy achieved by the EQ-5D index in the overall cohort was moderate-to-good and comparable to that of NT-proBNP concentration which has previously been suggested as a possible reference standard.
These findings corroborate and extend previous insights obtained on the utility of health-related quality of life and self-estimated exercise capacity as a prognostic marker in patients with acute dyspnoea [14, 32, 33]. In a large multicentre observational cohort including 9098 acute heart failure patients, the 10-item Barthel Index, another validated and in particular in elderly or chronically ill patients applied health-related quality of life instrument, showed a comparable moderate-to-good prognostic accuracy for 30-day mortality when obtained at baseline (AUC by 10-point interval: 0.698), and a good prognostic accuracy when obtained a few hours after presentation to the emergency department (AUC: 0.743) [32, 33]. On the basis of its strong prognostic value, the Barthel Index is part of the 13-item MEESSI-AHF risk model with excellent 30-day mortality prediction for patients presenting with acute heart failure (c-statistic without and with Barthel Index: 0.80 and 0.836, respectively) [8, 9]. Due to its generalisability, shorter form and very close resemblance to the initial nurse-led functional and social assessment routinely performed for hospitalised patients in many institutions, the EQ-5D questionnaire might be even easier to include in everyday clinical practice, further increasing its attractiveness. Although there is overlap in the EQ-5D scores between survivors and non-survivors, the tool remains useful for predicting outcomes in many cases. The overlap might benefit from further clinical refinement and the inclusion of other risk factors. Additional implementation studies seem warranted to determine easy-to-interpret thresholds of the EQ-5D index for reliable risk stratification and to evaluate the best strategy for implementing EQ-5D into clinical practice. Inclusion of health-related quality of life as an endpoint in future trials is strongly advocated since the burden of cardiorespiratory disease, chronic heart failure in particular, continues to grow. Moreover, and perhaps more importantly, it has previously been shown that patient-reported outcomes differ from assessed adverse events which should raise more awareness on patients’ self-perceived health state [34].
Despite the apprehension that a generic health-related quality of life instrument may be easily affected by depression as a comorbidity, only in severely depressed patients was no significant difference found in the EQ-5D index between survivors and non-survivors [30]. We assume that severe depressive symptoms or anxiety can easily lead to behavioural disengagement and treatment non-compliance, which can result in poorer health-related quality of life and also higher mortality in this group of patients. Additionally, a history of psychiatric disorders – including among others dementia as per the Mini-Mental State Examination, a history of depression from medical records and behavioural disorders due to psychoactive substance use – had a profound influence on the prognostic effect of health-related quality of life. Measurement of health status in patients with dementia has shown that cognitive impairment influences the reliability of self-reported health-related quality of life [31]. Further research is needed to establish cognition thresholds beyond which a patient is unable to reliably self-report their own health-related quality of life.
Some limitations might merit consideration when interpreting our findings:First, our findings are limited to patients presenting to the emergency department whose clinical condition is stable enough to provide informed consent. Accordingly, we cannot comment on the performance of the EQ-5D index in haemodynamically unstable patients or in patients with mild acute heart failure presenting to a general practitioner. Additionally, nearly 50% of patients did not complete the EQ-5D and/or the EQ VAS, likely because they were too unwell or in acute distress during their emergency department visit. Mortality was substantially higher among non-participants than participants, emphasising the need to account for these groups when generalising results. Second, we cannot comment on the performance of the EQ-5D index in patients on renal replacement therapy, since these patients were not included in our study. Third, the EQ-5D evaluates the self-perceived health state on the day of administration of the questionnaire depicting health-related quality of life at a specific time point rather than a period. However, EQ-5D’s temporal specificity can be advantageous in evaluating patients with acute conditions, making it an even more suitable instrument for the emergency department setting. Fourth, although we used one of the most stringent methods to adjudicate the presence or absence of acute heart failure, including central adjudication by two independent experienced cardiologists/internists including BNP/NT-proBNP concentrations in all patients, we may still have misclassified a small number of patients.
In conclusion, self-reported health-related quality of life assessed by the generic EQ-5D provides moderate-to-high prognostic accuracy, comparable to that of NT-proBNP, in patients presenting with acute dyspnoea to the emergency department and may aid physicians in risk stratification.
Study protocol: As BASEL V is a registered trial, the study protocol can be consulted at ClinicalTrials.gov with the following registry number: NCT01831115.
Statistical code: Restricted access is available by contacting Maria Belkin (e-mail: maria.belkin[at]usb.ch).
Dataset: Not available.
We thank the patients who participated in the study, the members of the participating emergency departments, the research coordinators and the laboratory technicians for their most valuable efforts. MB, DW, EM, ZS and CM had full access to all the data in the study and assume responsibility for the integrity of the data and the accuracy of the data analysis.
This study was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the University of Basel, University Hospital Basel, Roche, Abbott, BRAHMS (primary funding source) and Critical Diagnostics, Alere, Beckman Coulter and Singulex.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. P. Lopez-Ayala reports research grants from the Swiss Heart Foundation (FF20079 and FF21103) and speaker honoraria from Quidel, paid to the institution and outside the submitted work. N. Kozhuharov acknowledges research grants from the Swiss National Science Foundation (grant no P400PM-194477 and grant no P5R5PM_210856), Gottfried und Julia Bangerter-Rhyner-Stiftung, Freiwillige Akademische Gesellschaft, L. & Th. La Roche Stiftung and the European Society of Cardiology. M. Diebold reports research grants from the Swiss National Science Foundation. T. Breidthardt reported research grants from the Swiss National Science Foundation, University Hospital Basel, the Department of Internal Medicine, University Hospital Basel, Abbott, and Roche. C. Mueller reported research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the KTI, University Hospital Basel, the University of Basel, Abbott, Beckman Coulter, bioMérieux, BRAHMS, Ortho Clinical, Quidel, Novartis, Roche, Siemens, Singulex and Sphingotec. All other authors declare that they have no conflict of interest related to the content of this study. The sponsors had no role in the design and conduct of the study; nor in the collection, management, analysis and interpretation of the data; nor in the preparation, review and approval of the manuscript.
1. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al.; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012 Feb;185(4):435–52.
2. Jaarsma T, van der Wal MH, Lesman-Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, et al.; Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Investigators. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med. 2008 Feb;168(3):316–24.
3. Wussler D, Michou E, Belkin M, Kozhuharov N, Diebold M, Gualandro DM, et al. Mortality prediction in acute heart failure: scores or biomarkers? Swiss Med Wkly. 2020 Aug;150(3334):w20320–20320.
4. Christ M, Laule-Kilian K, Hochholzer W, Klima T, Breidthardt T, Perruchoud AP, et al. Gender-specific risk stratification with B-type natriuretic peptide levels in patients with acute dyspnea: insights from B-type natriuretic peptide for acute shortness of breath evaluation study. J Am Coll Cardiol. 2006;48(9):1808–12.
5. Christ M, Thuerlimann A, Laule K, Klima T, Hochholzer W, Perruchoud AP, et al. Long-term prognostic value of B-type natriuretic peptide in cardiac and non-cardiac causes of acute dyspnoea. Eur J Clin Invest. 2007 Nov;37(11):834–41.
6. Kozhuharov N, Sabti Z, Wussler D, Nowak A, Badertscher P, Twerenbold R, et al.; BASEL V Investigators. Prospective validation of N-terminal pro B-type natriuretic peptide cut-off concentrations for the diagnosis of acute heart failure. Eur J Heart Fail. 2019 Jun;21(6):813–5.
7. Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004 Feb;350(7):647–54.
8. Miró Ò, Rossello X, Gil V, Martín-Sánchez FJ, Llorens P, Herrero-Puente P, et al.; ICA-SEMES Research Group. Predicting 30-Day Mortality for Patients With Acute Heart Failure in the Emergency Department: A Cohort Study. Ann Intern Med. 2017 Nov;167(10):698–705.
9. Wussler D, Kozhuharov N, Sabti Z, Walter J, Strebel I, Scholl L, et al. External Validation of the MEESSI Acute Heart Failure Risk Score: A Cohort Study. Ann Intern Med. 2019 Feb;170(4):248–56.
10. Alla F, Briançon S, Guillemin F, Juillière Y, Mertès PM, Villemot JP, et al.; EPICAL Investigators. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail. 2002 Jun;4(3):337–43. doi: https://doi.org/10.1016/S1388-9842(02)00006-5
11. Moradi M, Daneshi F, Behzadmehr R, Rafiemanesh H, Bouya S, Raeisi M. Quality of life of chronic heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. 2020 Nov;25(6):993–1006.
12. Belkin M, Michou E, Mueller C. Letter by Belkin et al Regarding Article, “Increased Myocardial Stiffness in Patients With High-Risk Left Ventricular Hypertrophy: The Hallmark of Stage-B Heart Failure With Preserved Ejection Fraction”. Circulation. 2020 May;141(20):e820–1.
13. Belkin M, Wussler D, Gualandro DM, Shrestha S, Strebel I, Goudev A, et al. Effect of a strategy of comprehensive vasodilation versus usual care on health-related quality of life among patients with acute heart failure. ESC Heart Fail. 2021 Oct;8(5):4218–27.
14. Belkin M, Wussler D, Michou E, Strebel I, Kozhuharov N, Sabti Z, et al. Prognostic Value of Self-Reported Subjective Exercise Capacity in Patients With Acute Dyspnea. JACC Adv. 2023 May;2(3):100342.
15. Wussler D, Belkin M, Mueller C. Letter by Wussler et al Regarding Article, “Challenging the Hemodynamic Hypothesis in Heart Failure With Preserved Ejection Fraction: Is Exercise Capacity Limited by Elevated Pulmonary Capillary Wedge Pressure?”. Circulation. 2023 Aug;148(7):619–619.
16. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep;42(36):3599–726.
17. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al.; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May;145(18):e895–1032.
18. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, et al.; American Heart Association Council on Quality of Care and Outcomes Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Stroke Council. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013 Jun;127(22):2233–49.
19. EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990 Dec;16(3):199–208.
20. Boczor S, Daubmann A, Eisele M, Blozik E, Scherer M. Quality of life assessment in patients with heart failure: validity of the German version of the generic EQ-5D-5L™. BMC Public Health. 2019 Nov;19(1):1464.
21. Nagy KV, Merkely B, Rosero S, Geller L, Kosztin A, McNitt S, et al. Quality of life predicting long-term outcomes in cardiac resynchronization therapy patients. Europace. 2019 Dec;21(12):1865–75.
22. Wussler D, Kozhuharov N, Tavares Oliveira M, Bossa A, Sabti Z, Nowak A, et al. Clinical Utility of Procalcitonin in the Diagnosis of Pneumonia. Clin Chem. 2019 Dec;65(12):1532–42.
23. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JG, Kozhuharov N, et al.; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019 Jun;21(6):715–31.
24. Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al.; ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008 Oct;29(19):2388–442.
25. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37–49.
26. Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail. 2014 Oct;2(5):429–36.
27. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail. 2017 May;19(5):627–34.
28. Therneau TM, Grambsch PM, Fleming TR. Martingale-Based Residuals for Survival Models. Biometrika. 1990;77(1):147–60. doi: https://doi.org/10.1093/biomet/77.1.147
29. Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017 Apr;17(1):53.
30. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep;44(3):837–45.
31. Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019 Mar;20(2):205–16.
32. Rossello X, Miró Ò, Llorens P, Jacob J, Herrero-Puente P, Gil V, et al.; ICA-SEMES Research Group. Effect of Barthel Index on the Risk of Thirty-Day Mortality in Patients With Acute Heart Failure Attending the Emergency Department: A Cohort Study of Nine Thousand Ninety-Eight Patients From the Epidemiology of Acute Heart Failure in Emergency Departments Registry. Ann Emerg Med. 2019 Jun;73(6):589–98.
33. Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. 1965 Feb;14:61–5.
34. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail. 2017 Sep;19(9):1095–104.
The supplementary material is available in a separate pdf file at https://doi.org/10.57187/s.3785.