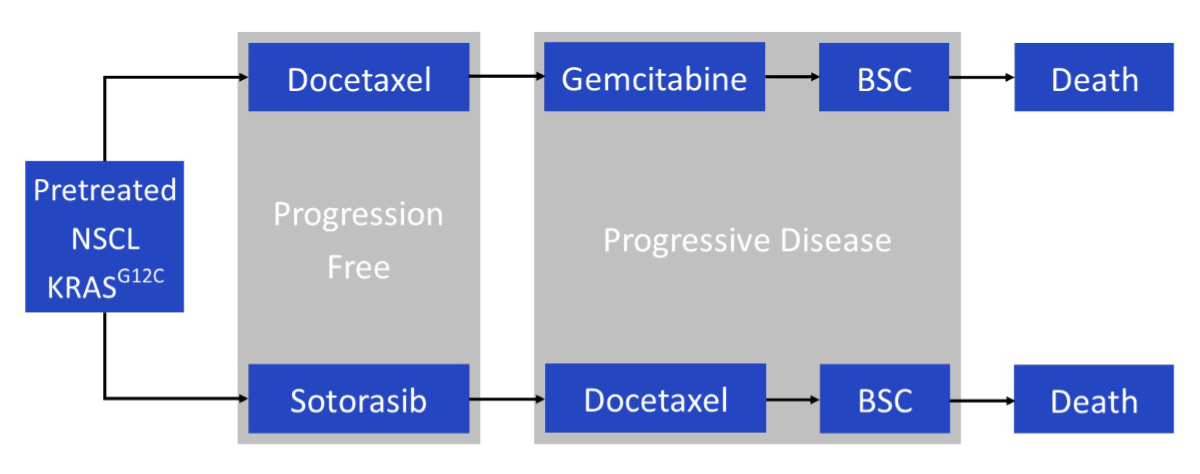
Figure 1Model structure. BSC: best supportive care; KRASG12C: G12C-mutated Kirsten rat sarcoma virus gene; NSCL: non-mall cell lung cancer.
DOI: https://doi.org/https://doi.org/10.57187/s.3777
Diagnosis-Related Group
European Society for Medical Oncology
incremental cost-effectiveness ratio
G12C-mutated Kirsten rat sarcoma virus gene
metastatic non-small cell lung cancer
programmed cell death protein 1
programmed death ligand 1
quality-adjusted life year
Lung cancer is one of the leading causes of cancer deaths, accounting for over 3500 deaths in Switzerland per year [1]. Most lung cancers are diagnosed when the disease has already metastasised [2]. The most frequent histological subtype is non-small cell lung cancer (NSCLC) (80–85%) [3]. Targeted therapies have become crucial in the treatment of patients receiving adjuvant therapy and those with metastatic non-small cell lung cancer (mNSCLC), and tumour genotyping has been incorporated into the clinical management of non-small cell lung cancer to personalise treatment.
The Kirsten rat sarcoma virus (KRAS) gene codes for an oncoprotein and is involved in cell growth and division. In non-small cell lung cancer, KRAS is the most frequently observed mutated oncogene, present in approximately 30% of patients. The KRASG12C mutation occurs in 11% of patients and is particularly common in current and former smokers [4]. It has been recognised for decades, but drug treatments have only become available recently [5].
For patients with mNSCLC patients with a KRASG12C mutation whose cancer has progressed after first-line platinum-based chemotherapy and programmed cell death protein 1 (PD-1) / programmed death ligand 1 (PD-L1)-based treatment, chemotherapy with docetaxel is recommended as second-line treatment in the current European Society for Medical Oncology (ESMO) guidelines [6]. Recently, the results of the CodeBreak 200 study were published. In this phase 3 randomised open-label trial, the efficacy of the new targeted treatment sotorasib was examined. Patients with mNSCLC harbouring the KRASG12C mutation who were pre-treated with platinum and PD-1 / PD-L1-based therapy received either sotorasib or docetaxel. The results showed a significant progression-free survival benefit of sotorasib versus docetaxel (5.6 months [95% confidence interval [CI] 4.3–7.8] versus 4.5 months [3.0–5.7], hazard ratio [HR] 0.66 [0.51–0.86], p = 0.0017). However, no difference in overall survival was observed. The advantages of the treatment with sotorasib are that it has fewer side effects and is convenient for patients, as it is an oral treatment instead of intravenous chemotherapy like docetaxel [7].
The Food and Drug Administration (FDA) approved sotorasib as a first-in-class treatment in the USA in May 2021, with the requirement that a post-marketing trial be conducted to investigate whether a lower dose than that used in CodeBreak 200 would have similar efficacy [8]. The results of this trial have been published at an ESMO Virtual Plenary session in November 2023. The trial was designed as a phase 2 randomised controlled open-label study to compare the efficacy and safety of the standard sotorasib dose (960 mg) and a lower dose (240 mg). The primary endpoint was the objective response rate. Compared with patients treated with 240 mg of sotorasib daily, patients treated with 960 mg daily had higher objective response rates (33% versus 25%). Regarding adverse events, the two patient groups were comparable, with slightly higher gastrointestinal toxicity observed in the higher-dose arm. No statistically significant differences in overall or progression-free survival were observed between the two treatment arms; however, these were only secondary endpoints, and the trial was not powered to show a difference or non-inferiority [9]. The two discussants of the trial stated that given the new results, the new standard dose of sotorasib should be 240 mg [10, 11].
In Switzerland, sotorasib received temporary approval from Swissmedic in December 2021 [12]. The temporary approval has not been extended; thus, a solution is now required to ensure that patients can continue receiving sotorasib. To date, the new drug has not been listed on the Swiss list of pharmaceutical specialities (“Spezialitätenliste”), and therefore, health insurance providers do not automatically reimburse it. However, exceptional remuneration is available in individual cases under Art. 71a-d “Verordnung über die Krankenversicherung” (KVV) after consultation with the independent medical officer of the health insurance provider [13].
To our knowledge, the cost-effectiveness of sotorasib has not been established in any country. Therefore, using the recently published phase 3 data from the CodeBreak 200 trial, this study analysed the cost-effectiveness of sotorasib treatment as a second-line mNSCLC therapy and tested different pricing models for patients in Switzerland.
A partitioned survival model was developed to project the costs and outcomes of sotorasib and docetaxel over 10 years according to published data from the CodeBreak 200 trial. Incremental cost-effectiveness ratios (ICERs), expressed as the cost per quality-adjusted life year (QALY) gained, were determined using published United Kingdom (UK) utility values. If QALYs were equal for both strategies, cost differences were reported. Prices and costs were assessed from the perspective of the Swiss healthcare system and compared with a hypothetical willingness-to-pay of CHF 100,000. Contributions from Swiss patients, insurers, the cantons, and the government are reflected from this perspective. Future costs and utilities were discounted by 3% per year, as this is the commonly used standard for health economic evaluations in Switzerland. All values and estimation steps of the models were checked and validated. The model was developed and implemented in R 4.3.1 [14] and Treeage Pro [15]. The reporting of the analysis followed the CHEERS principles [16] (appendix table S1).
The model was populated with effectiveness estimates and proportions of grade ≥3 adverse events obtained from the CodeBreak 200 trial publication. This trial was used for the model because it is the only global phase 3 randomised controlled trial that has assessed sotorasib for previously treated advanced non-small cell lung cancer with the KRASG12C mutation [7]. Costs were estimated from publicly available Swiss sources. Considering patient life expectancy, a time horizon of 10 years was used for the base-case analysis to capture most future costs and outcomes associated with the treatment strategies.
In the CodeBreak 200 trial, patients received sotorasib 960 mg orally daily or docetaxel 75 mg/m2 intravenously every 3 weeks. In our model, patients received either sotorasib or docetaxel until disease progression. We considered two alternatives: (1) sotorasib 960 mg orally daily, in accordance with the CodeBreak 200 trial, and (2) sotorasib 240 mg orally daily, in accordance with the most recent FDA-requested trial [9]. Subsequently, patients in the intervention strategy received docetaxel 75 mg/m2 or best supportive care (with an assumed time to progression of 10.6 versus 6.7 weeks [17]). Under the docetaxel strategy, patients received gemcitabine 1000 mg/m2 as next-line treatment or best supportive care (no data on third-line chemotherapy are available; data were obtained from a randomised controlled trial of gemcitabine versus best supportive care (in previously untreated patients), which showed a median overall survival of 5.7 months (95% CI 4.6–7.6) for gemcitabine versus 5.9 months (95% CI 5.0–7.9) for best supportive care) [18]. The proportion of patients who were modelled to receive one further line of treatment was taken from the trial and was set at 39% for both treatment strategies. All patients were assumed to be treated with palliative care. These choices reflect the most likely standard clinical practice in Switzerland (figure 1 and appendix table S2).

Figure 1Model structure. BSC: best supportive care; KRASG12C: G12C-mutated Kirsten rat sarcoma virus gene; NSCL: non-mall cell lung cancer.
Model effectiveness parameters were based on published trial data on progression-free and overall survival (table 1). Parametric models were fitted to the Kaplan-Meier trial data for progression-free and overall survival as a basis for extrapolating effectiveness estimates from the short-term trial period (median follow-up of 17.7 months) to a 10-year period. Because the overall survival curves of sotorasib and docetaxel crossed two times and the hazard ratios were non-significant, the overall survival curves of the comparator (docetaxel) were used to model survival in both strategies. The method of Guyot [19] was used to construct potential underlying patient data, and survival curves were estimated using the “flexsurvreg” package in R [20]. In the fitting and selection of the models, the minimisation of the Akaike information criterion, combined with visual inspection of the closeness of parametric curves to Kaplan-Meier plots, was used to select the base-case parametric model from the following options: exponential, Weibull, Gompertz, log-logistic, log normal and generalised gamma. Projected survival was also compared with published data from the SEER cohort [21].
Table 1Key model parameters.
| Modelled strategy | Overall survival function | Progression-free survival function | |||
| Sotorasib | µ = 2.403; ơ = 1.437 | µ = 1.770; ơ = 0.970 | |||
| Docetaxel | µ = 1.409; ơ = 0.997 | ||||
| Utilities | Source | Mean EQ-5D-5L score (95% CI) | |||
| Sotorasib progression-free survival | NICE report ID3780 (CodeBreak 100) | 0.739 (0.704 to 0.774) | |||
| Sotorasib overall survival | NICE report ID3780 (CodeBreak 100) | 0. 66 (Utility SOC progression-free survival minus 0.084 [0.044, 0.123]) | |||
| Docetaxel progression-free survival | NICE report ID840 | 0.736 (0.719–0.754) | |||
| Docetaxel overall survival | NICE report ID840 | 0.67 (0.63–0.71) | |||
| Best supportive care | Nafees et al., 2008, estimate for progressive disease | 0.473 | |||
| Disutility adverse event | Nafees et al., 2008 | –0.06*** | |||
| Costs* | Cost (CHF) | Source | |||
| Scenario 1: Sotorasib drug cost (30 days) | 7870 | Based on published UK price [26] | |||
| Scenario 1: ¼ dose Sotorasib drug cost (30 days) | 1968 | Based on one-quarter of the published UK price | |||
| Docetaxel drug cost (per 3-week cycle) | 544 | Spezialitätenliste (see appendix) | |||
| Docetaxel drug administration | 371 | TARMED / cantonal hospital Graubünden | |||
| Sotorasib drug administration | 0 | ||||
| Consultation | 153 | TARMED / cantonal hospital Graubünden | |||
| CT | 975 | ||||
| MRI | 503 | ||||
| Best supportive care (monthly) | 2903 | [39] | |||
| Gemcitabine drug cost (per 4-week cycle) | 606 | TARMED / cantonal hospital Graubünden | |||
| Terminal care | 17,340 | [40] | |||
| Adverse events ≥3 | Sotorasib | Docetaxel | Ratio of inpatients to outpatients** | Inpatient cost per event (CHF) (DRG E71A) | Outpatient cost per event (CHF) (TARMED) |
| Diarrhoea | 12% | 2% | 100:0 | 13,743.02 | – |
| Alanine aminotransferase increased | 8% | 0% | 25:75 | 13,743.02 | 684.81 |
| Aspartate aminotransferase increased | 5% | 0% | 25:75 | 13,743.02 | 684.81 |
| Alkaline phosphatase increased | 3% | 0% | 0:100 | – | 684.81 |
| Decreased appetite | 2% | 0% | 80:20 | 13,743.02 | 684.81 |
| Neutropenia | 0% | 12% | 0:100 | – | 1005.51 |
| Fatigue | 0% | 6% | 20:80 | 13,743.02 | 684.81 |
| Febrile neutropenia | 0% | 5% | 80:20 | 13,743.02 | 684.81 |
| Anaemia | 0% | 3% | 20:80 | 13,743.02 | 1147.8 |
| Asthenia | 0% | 3% | 20:80 | 13,743.02 | 684.81 |
| Pneumonia | 0% | 3% | 80:20 | 13,743.02 | 684.81 |
CI: confidence interval; CT: CT: computed tomography; MRI: magnetic resonance imaging; SE: standard error.
* Where relevant, adjusted by inflation to 2023 values; for further details, see appendix.
** Assumption based on clinical experience.
*** Mean value of disutilities for adverse events, Nafees et al. [30], table 2.
Table 2Base-case results, average total per-patient costs (CHF) and quality-adjusted life years, sotorasib versus docetaxel.
| Sotorasib, scenario 1: 7870 CHF / 30 days | Sotorasib (one-quarter dose), scenario 2: 1967.5 CHF / 30 days | Docetaxel | Incremental sotorasib versus docetaxel | |
| Quality-adjusted life years | 1.28 | 1.28 | 1.28 | 0.00 |
| Total costs (CHF) | 138,894 | 82,741 | 80,383 | Base case 1: 58,511 |
| Base case 2: 2359 | ||||
| Drug costs (CHF) | 83,704 | 29,314 | 16,252 | |
| Drug administration (CHF) | 4900 | 4900 | 7504 | |
| Cost of diagnostics (CHF) | 20,865 | 20,865 | 22,716 | |
| Cost of disease management (CHF) | 4922 | 4922 | 8645 | |
| Adverse events cost (CHF) | 3889 | 2126 | 5164 | |
| Cost of 2nd line drugs (CHF) | 72,519 | 18,633 | 5685 | |
| Cost of 2nd line total (CHF) | 88,758 | 32,606 | 17,272 |
Cost of 2nd line includes costs without further lines of treatment; cost of diagnostics includes CT, etc.; cost of disease management includes consultation fees; cost of drug administration includes chemo applications. 2nd line: sotorasib or docetaxel.
For all Kaplan-Meier curves from the trial, the log-normal function had the lowest Akaike information criterion and seemed to fit well visually (figure 2). Comparison with long-term survival data from SEER was acceptable (appendix table S3).
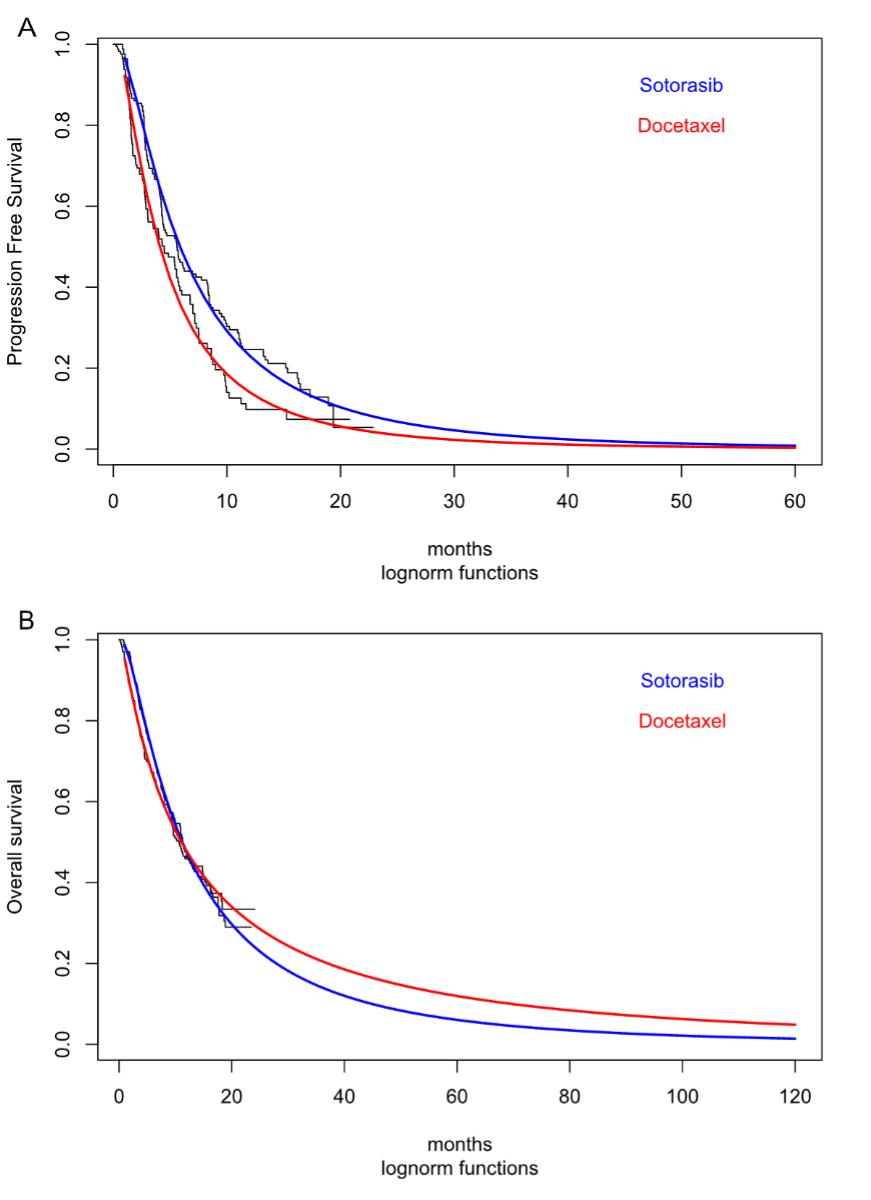
Figure 2Extrapolated survival modelling. (A) Progression free survival. (B) Overall survival (for model docetaxel overall survival curve used in both strategies since difference between Kaplan-Meier plots not significant in Codebreak 200).
Unit cost parameters were primarily obtained from the following Swiss data sources: “Spezialitätenliste” (list of specialities) [22] for drug costs, Swiss Diagnosis-Related Group (DRG) statistics for inpatient treatment costs, and TARMED [23] for outpatient treatment costs. Costs of consumables were calculated according to the current standard charges set by the cantonal hospital of Graubünden for insurers and patients. For some cost parameters, values from recently published studies were adopted for Switzerland. Costs assessed in previous years were adjusted for inflation [24]. Details are provided in table 1 and appendix table S4.
Unit costs related to drug acquisition and administration, post-discontinuation drugs, disease management, adverse event management, and terminal care were considered.
Swiss drug prices are determined through a combination of the Therapeutic Value Comparison (TQV) and the International Price Comparison (APV) [25], benchmarking prices against those in Germany, France, Austria, the Netherlands, Belgium, Denmark, Finland, Sweden, and the United Kingdom, with the possibility of an innovation surcharge. Switzerland has not established a price for sotorasib, and the only European country with an established price is the UK; therefore this price was used, as the price in the USA does not reflect European pricing conditions. Two base cases were tested: (1) the published expected monthly UK price for a 960 mg daily dose, equivalent to CHF 7870 [26] for a 30-day supply (table 1); and (2) one-quarter of that price (CHF 1968), reflecting the reduced dose used in the FDA-requested non-inferiority trial [9]. One-quarter of the sotorasib dose was assumed to be equally effective with one-quarter of the adverse events.
For the docetaxel strategy, the dosage was calculated using the estimated mean body surface area (BSA) of the Swiss general population aged 55–64 years of 1.88 m2 [27]. From this, the average cost of docetaxel was estimated at CHF 544 per 3-week cycle (appendix text S1 and appendix table S4).
For the sotorasib arm, this cost was estimated at CHF 799 per 3-week cycle, which included the costs of consultation, laboratory testing, a CT scan, and an MRI scan. Because sotorasib is a tablet taken orally, it does not have drug administration costs. In the docetaxel arm, this cost was estimated at CHF 1211 per 3-week cycle, including the costs of consultation, laboratory testing, a CT scan, an MRI scan, premedication (dexamethasone and ondansetron based on the public price of the list of specialities), and drug administration. Details on drug, drug administration, and post-discontinuation drug costs can be found in table 1 and appendix table S4.
Costs related to grade 3–5 adverse events were included in our analysis. The unit costs of adverse event inpatient events (i.e. hospitalisations) were based on codes from the Swiss DRG database statistics (table 1, appendix table S3) and weighed for the canton of Graubünden. Unit costs of adverse event outpatient events were based on TARMED. We made assumptions concerning the proportion of patients needing hospitalisation for each adverse event on the basis of our clinical experience (details can be found in appendix table S2). Costs were estimated for each scenario by multiplying adverse event unit costs for the inpatient or outpatient setting by the proportion of occurrences for each adverse event of the trial participants in each arm. For the docetaxel strategy, adverse event management costs due to gemcitabine, which was assumed to be administered as a post-discontinuation therapy, were also included (appendix table S5).
No EQ-5D (the EQ-5D is a standardised measure of health-related quality of life) values were available in the CodeBreak 200 trial. However, the EQ-5D-5L was administered to patients in the CodeBreak 100 trial, and the results were reported in a NICE report [28] (table 1). EQ-5D scores were assigned to patients for each of their disease states (progression-free survival, progressive disease, or death). A 2021 systematic review undertaken for the same NICE report found no other studies reporting health-related quality of life in patients with mNSCLC and the KRASG12C mutation. Because the phase 1 CodeBreak 100 trial was a single-arm trial, no EQ-5D values for the comparator arm were available. Therefore, utilities for patients in the progression-free survival and progressive disease health states from a different NICE report [29] were used for the comparator strategy with docetaxel (table 1), which was also considered a valid comparator in this analysis. Because the utilities from the trials used do not contain utility reductions for the duration of adverse events, disutilities for adverse events were considered equally. A disutility represents a decrement in the health state utility value. To compute the required decrements, the mean published disutility reported by Nafees et al. [30] was estimated at −0.06, and this utility decrement was subtracted for the respective proportion of patients with adverse events ≥ grade 3. The model assumed that the decrement lasted for one month and only in cases of adverse events requiring a hospitalisation (table 1). In a scenario analysis, the model was re-analysed without including disutilities, as they might already include the impact of adverse events to some extent.
In a scenario analysis, the alternative time horizons of 5 and 15 years were assessed to evaluate the sensitivity of the model results to the chosen time horizon. The impact of discount rates of 0% and 6% were also assessed for future costs and effects in secondary analyses. Further scenario analyses explored the effect of using the same utilities for both strategies (those for docetaxel), utilities from two alternative sources [30, 31], no dose reductions due to adverse events, no reduction in adverse events in the ¼-dose sotorasib base case, and a utility decrement under best supportive care. The effect of using the original sotorasib overall survival curve to model survival for sotorasib (and not the docetaxel overall survival curve for both strategies) was also assessed.
One-way sensitivity analyses were performed by implementing plausible variations of key input parameters to assess how this impacted the base-case incremental cost-effectiveness ratio (ICER). Costs were varied by ±20%; probabilities within their 95% confidence limits based on a ±20% standard deviation of the mean value, and utilities within their 95% confidence intervals (full details are provided in appendix tables S3–S5). The results of the one-way sensitivity analyses are presented in a Tornado diagram for the 10 most influential variables for both of our sotorasib price base cases (figure 3A and B).
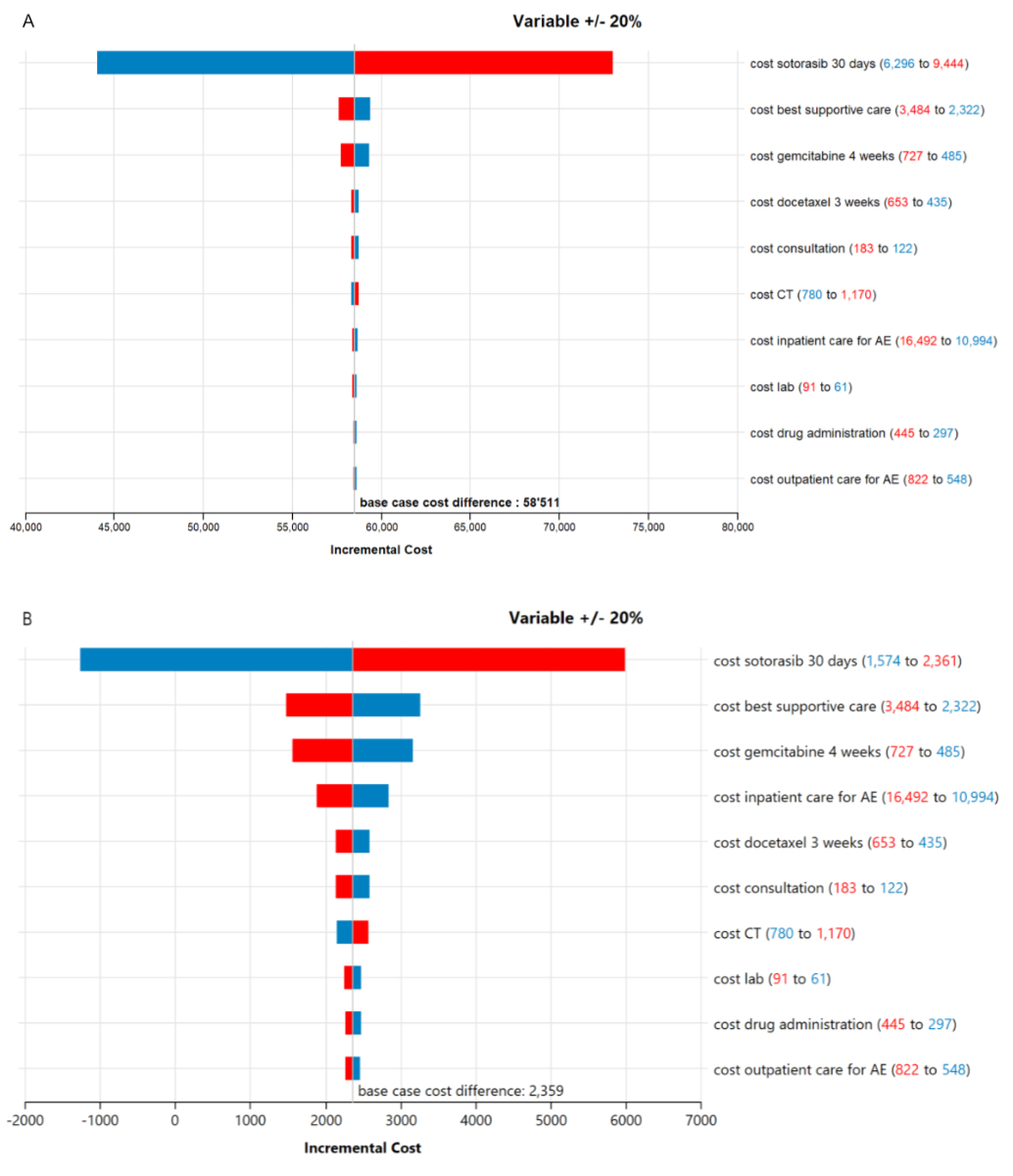
Figure 3Tornado diagrams both base cases: 10 most influential variables. (A) Base case 1: Sotorasib dose 90 mg at Swiss francs (CHF) 7870*. (B) Base case 2: Sotorasib 240 mg at CHF 1968 (zero line at CHF 1711)*. * blue: lower price leads to smaller cost difference; red: lower price leads to higher cost difference. CT: computed tomography; AE: adverse events.
A probabilistic sensitivity analysis (PSA) with 1000 iterations was also conducted by assigning probability distributions to all key model input parameters reflecting the degree of variation used in the univariate sensitivity analysis (full details are provided in appendix tables S3–S5).
The model predicted equal QALYs gained for sotorasib treatment and docetaxel treatment (1.28 QALYs), and thus the ICER could not be calculated for the base case. Mean total per-patient costs were CHF 138,894 for the full sotorasib dose at UK prices, CHF 82,741 for the one-quarter dose, and CHF 80,383 for docetaxel. Therefore, with the full dose, docetaxel was projected to be CHF 58,511 cheaper per treated patient than docetaxel. When utilising one-quarter of the dose and cost for sotorasib, the difference between sotorasib and docetaxel was reduced to CHF 2359 (table 2).
The full results of the scenario analyses carried out for this study are provided in table 3. In the scenario analysis with a time horizon of 5 years, QALYs decreased to 1.08 for sotorasib and 1.07 for docetaxel. This led to ICERs of CHF 10,361,420 and CHF 453,721 per QALY gained for the full dose and one-quarter dose of sotorasib, respectively, compared with docetaxel. With a 15-year time horizon, our model predicted QALYs of around 1.36 for all strategies and mean cost differences of CHF 58,527 and CHF 2257 for the full and one-quarter dose of sotorasib, respectively. In the scenario with no discounting for costs or QALYs, more QALYs were gained (1.38) for sotorasib and docetaxel, and mean costs increased slightly, with cost differences of 60,026 for the full dose and 2325 for the one-quarter dose. Discounting costs and QALYs by 6% led to very similar mean cost differences (CHF 57,130 for the full dose and CHF 2381 for the one-quarter dose of sotorasib) and, again, no QALY differences. Because the modelled sotorasib overall survival curve crossed the modelled docetaxel curve, using the sotorasib overall survival curve (for the sotorasib arm) led to a negative QALY difference (−0.23) and mean cost differences of CHF 48,102 for the full dose and CHF −8127 for the one-quarter dose of sotorasib. Therefore, docetaxel dominated the comparison with the full sotorasib dose. Although the one-quarter dose of sotorasib was predicted to be less expensive than docetaxel, the negative QALY difference favoured docetaxel clinically (i.e. it was more expensive but generated more QALYs).
When we used the same utilities for both strategies (those observed with docetaxel), we calculated 1.29 QALYs gained for sotorasib and 1.28 QALYs gained for docetaxel. This resulted in ICERs of CHF 3,865,790 for the full dose and CHF 154,515 for the one-quarter dose of sotorasib. Using the utilities published by Nafees et al. 2008 [30], QALYs were reduced to 1.01 and 0.97 for the full dose and one-quarter dose, respectively, with ICERs of CHF 1,505,661 and CHF 60,499 for the full dose and one-quarter dose, respectively. Using the utilities published by Rothwell et al. [31], QALYs increased to 1.30 and 1.26 for the full dose and one-quarter dose, respectively, with ICERs of CHF 9,261,864 and CHF 72,516 for the full dose and one-quarter dose, respectively. Adding no utility reduction for adverse events led to only minor changes in utilities beyond the second decimal. In the last scenario, in which we reduced the dose of sotorasib to one-quarter while assuming that adverse events stayed the same, the mean cost differences increased slightly to CHF 4123.
The sensitivity analyses with the ten most influential parameters are presented in Tornado diagrams (figures 3A and 3B) for the two assumptions for sotorasib dosage. The variables that influenced the cost difference between sotorasib and docetaxel most were all ‘cost variables’, which were varied by ±20%. Varying the price of sotorasib led to the most notable changes in cost difference. For the alternative base case assuming the one-quarter sotorasib dose, an equal cost and utility of the sotorasib and docetaxel strategies could be achieved if the sotorasib price was set to CHF 1711 per 30 days. In the probabilistic sensitivity analysis for the full-dose scenario, sotorasib did not reach cost-effectiveness in 100% of the simulations when considering the hypothetical willingness-to-pay threshold of CHF 100,000 per QALY gained. For the one-quarter dose scenario, sotorasib did not reach cost-effectiveness in 62% of the simulations.
Targeted therapy approaches offer an ever-growing treatment portfolio for non-small cell lung cancer. With the approval of sotorasib, KRAS p.G12C mutations have become treatable with drugs. To the best of our knowledge, our analysis represents the world’s first cost-effectiveness study of sotorasib. To date, prices have yet to be set by the manufacturer and reimbursement authorities worldwide.
Our model predicted the same quality-adjusted life years (QALYs) in both strategies, even though toxicity parameters were different between the two arms. We considered the longer progression-free survival and better toxicity profile of sotorasib, but overall, the resulting differences were minor in this type of calculation. Over 10 years, these differences did not lead to an accumulation of more QALYs in the sotorasib strategy. However, patients’ preference for oral therapy over intravenous chemotherapy (although some patients might view the eight pills for the 960 mg dose as a disadvantage to their quality of life) is yet to be explored, and if such a preference exists, it may not be well reflected in EQ-5D-based QALYs. Oral treatment may require fewer clinical visits and diminish travel costs, making it less time-consuming for patients and any family members accompanying them. Our analysis did not consider such direct non-medical costs or any indirect costs.
There are two main reasons for the proximity of the minimal incremental QALYs and the therefore high incremental cost-effectiveness ratios (ICERs) in our analysis. The first is the lack of an overall survival benefit, given that no significant difference in overall survival was observed in the CodeBreak 200 trial, even though this was a secondary endpoint. An overall survival benefit would substantially impact the cost-effectiveness calculation. However, it must be stated that the primary endpoint of the CodeBreak 200 trial was progression-free survival, and the trial was not powered to detect a difference in overall survival. Second, the lower number of adverse events with sotorasib compared to docetaxel did not substantially impact our calculations or patients’ quality of life, as sotorasib’s adverse event profile is only slightly better than that of docetaxel.
For temporary approval, only phase 1 and 2 data were considered. According to these data, patients who were unfit to receive docetaxel could be included in these trials. In addition, sotorasib was administered not only as a second-line treatment but also in further therapy lines [32, 33]. Our analysis did not cover patients receiving sotorasib in further therapy lines, although in the real world, such patients will also be treated once the drug is commercially available and reimbursed.
If sotorasib was administered at the full dose and the assumed UK price was applied in Switzerland, the average per-patient cost difference compared with docetaxel, including subsequent treatment lines, would be approximately CHF 59,000. For the one-quarter doses, also assuming one-quarter of the adverse events and one-quarter of the UK price per dose, the cost difference would be CHF 2360 per patient. Because the newest data demonstrated only minor differences in adverse events when using the one-quarter dose [9], we also estimated the outcomes without reducing the toxicity. In this scenario, we estimated a price difference of CHF 4121. Furthermore, we estimated that the price of sotorasib (for 30 days) would need to be CHF 1711 for the cost to be equal to the current standard of care.
With increasing health expenditures in Switzerland and worldwide, drug costs are widely discussed. Switzerland’s healthcare system is based on the solidarity principle and is facing rapidly increasing costs. To maintain sustainability, the question is what prices of new medications can be justified if efficacy results from trials are very close to standard treatments. This is of special interest because some patients who receive sotorasib experience excellent benefits, and it is not currently possible to identify these patients with biomarkers. To avoid a scenario in which these patients cannot be treated for cost reasons, alternative pricing models such as Pay for Success should be further developed and discussed [34].
It will be interesting to observe the pricing of sotorasib across different countries in the next weeks and months and whether organisations such as NICE in the United Kingdom will reach similar conclusions.
One major strength of our study is that it is based on CodeBreak 200 data, which directly compared sotorasib to docetaxel. Another strength is that we conducted our analysis independently from the pharmaceutical industry using publicly available data. Costs, prices, probabilities, and therapy lines were researched in detail from reliable sources, and our modelling approach reflects the current standard for this type of analysis.
Our study also has several limitations. First, quality-of-life data were not available from the CodeBreak 200 trial, so we used data from the CodeBreak 100 trial. In this phase 2 trial, the therapeutic setting was different; patients were treated within various lines of therapy (second-, third-, and fourth-line therapy), whereas in CodeBreak 200, patients were treated only with second-line therapy. To take this into account, we tested the impact of different sets of utilities in three scenarios. In one scenario, we used published utilities for docetaxel for both strategies, and in the other two scenarios, we used different published utilities from an alternative source [31] and other estimation methods [30]. Although we observed a small QALY gain for sotorasib in the scenarios, this gain was minor. Thus, the main interpretation of our results does not change (table 3). However, using the one-quarter dose with the same price per unit of substance and assuming utilities from other publications, we estimated ICERs, which would probably be considered cost-effective given the hypothetical willingness-to-pay threshold in Switzerland (approximately CHF 60,000 per QALY gained using the utilities from Nafees et al. [30] and around CHF 72,000 per QALY gained using the published utilities from Checkmate 057 [31]). The underlying utilities from Nafees et al. [30] were assessed with the standard gamble approach in a general population sample and may thus be regarded as unsuitable for health technology assessment submissions [35]. The utilities from CheckMate 057 represent a similar patient population as that in CodeBreak 200. However, patients in CheckMate 057 were not pretreated with immunotherapy. For these reasons, we regarded these utilities as less suitable than the ones we used in our base case. However, this is an assumption, and results must be reevaluated in future when new utilities for patients with KRASG12C become available.
Table 3Scenario analyses: sotorasib versus docetaxel.
| Scenario | Sotorasib, base case 1: 7870 CHF / 30 days | Sotorasib (one-quarter dose), base case 1: 1967.5 CHF / 30 days | Docetaxel | Incremental sotorasib versus docetaxel | Incremental cost-effectiveness ratio | |
| Docetaxel utilities both strategies | QALYs | 1.29 | 1.29 | 1.28 | 0.02 | |
| Total costs (CHF) | 138,894 | 82,742 | 80,383 | Base case 1: 58,511 | Base case 1: 3,865,790 | |
| Base case 2: 2359 | Base case 2: 154,515 | |||||
| Nafees 2008 utilities both strategies* | QALYs | 1.01 | 1.01 | 0.97 | 0.04 | |
| Total costs (CHF) | 138,894 | 82,742 | 80,383 | Base case 1: 58,511 | Base case 1: 1,828,177 | |
| Base case 2: 2359 | Base case 2: 60,499 | |||||
| Utilities CheckMate 057** | QALYs | 1.30 | 1.30 | 1.26 | 0.03 | |
| Total costs (CHF) | 138,894 | 82,742 | 80,383 | Base case 1: 58,511 | Base case 1: CHF 9,261,864 | |
| Base case 2: 2359 | Base case 2: 72,516 | |||||
| Add utility best supportive care from Nafees 2008*** | QALYs | 1.16 | 1.16 | 1.12 | 0.04 | |
| Total costs (CHF) | 138,894 | 82,742 | 80,383 | Base case 1: 58,511 | Base case 1: 1,602,971 | |
| Base case 2: 2359 | Base case 2: 63,726 | |||||
| Sotorasib one-quarter dose, same adverse events | QALYs | 1.28 | 1.28 | 0.00 | # | |
| Total costs (CHF) | 84,504 | 80,383 | Base case 2: 4122 | |||
| No utility reduction for adverse events, both strategies | QALYs | 1.28 | 1.28 | 1.28 | 0.00 | # |
| Total costs (CHF) | 138,894 | 82,742 | 80,383 | Base case 1: 58,511 | ||
| Base case 2: 2359 | ||||||
| No discounting of costs or QALYs | QALYs | 1.38 | 1.38 | 1.38 | 0.00 | # |
| Total costs (CHF) | 146,195 | 88,494 | 86,169 | Base case 1: 60,026 | ||
| Base case 2: 2325 | ||||||
| 6% discounting of costs and QALYs | QALYs | 1.20 | 1.20 | 1.19 | 0.00 | # |
| Total costs (CHF) | 132,613 | 77,863 | 75,482 | Base case 1: 57,130 | ||
| Base case 2: 2381 | ||||||
| 5-year time horizon | QALYs | 1.08 | 1.08 | 1.07 | 0.01 | |
| Total costs (CHF) | 126,781 | 71,555 | 68,964 | Base case 1: 57,817 | Base case 1: 10,361,420 | |
| Base case 2: 2591 | Base case 2: 453,721 | |||||
| 15-year time horizon | QALYs | 1.36 | 1.36 | 1.36 | 0.00 | # |
| Total costs (CHF) | 143,345 | 87,074 | 84,817 | Base case 1: 58,527 | ||
| Base case 2: 2257 | ||||||
| Sotorasib overall survival curve | QALYs | 1.05 | 1.05 | 1.28 | −0.23 | |
| Total costs (CHF) | 128,485 | 72,256 | 80,383 | Base case 1: 48,102 | Base case 1: dominated | |
| Base case 2: −8127 | Base case 2: 36,190## |
CHF: Swiss francs; QALY: quality-adjusted life year.
* Progressive disease = 0.473; progression-free survival = 0.653 [30]
** Progressive disease = 0.688; progression-free survival = 0.713 [31]
*** Best supportive care = 0.473 [30]
# No incremental cost-effectiveness ratio can be reported because QALYs did not differ.
## In favour of docetaxel (docetaxel is a better value for money).
One of the standout benefits of sotorasib is its convenient, oral route of administration. However, our analysis could not account for this potential advantage of sotorasib, as we did not find appropriate studies that directly compared patients’ quality of life between IV and oral cancer treatment administration. Another possible benefit of oral therapy is that it saves personnel resources in hospitals. Given the current strain on healthcare professionals, this could represent an advantage that was not well reflected in our analysis. Furthermore, to make predictions, it was necessary to model future survival, which inherently involves high uncertainty. However, we used standard modelling approaches and compared our results with SEER data [21]. We chose the survival curves that best fit the Kaplan-Meier plot from the trial (Akaike information criterion). We made simplified assumptions about subsequent treatment lines; however, we tested the impact of cheaper and more expensive further treatments in sensitivity analyses and found their impact to be minor and not to change the main conclusions of our analyses.
Currently, many different KRAS inhibitors are in development. Adagrasib is another inhibitor of KRASG12C, which received approval from the FDA in December 2022 based on the single-arm phase 2 trial KRYSTAL-1, for pretreated patients with KRASG12C-mutated metastatic non-small cell lung cancer (mNSCLC). The KRYSTAL-12 phase 3 trial, which has a similar design to the CodeBreak 200 study, is still awaiting results [36]. Many more substances are in preclinical and clinical development; these include drugs targeting not only the KRASG12C mutation but also other mutations, such as KRASG12D, as well as pan-RAS inhibitors. Furthermore, many different combinations are being tested, and although the initial results of sotorasib have shown a relatively modest clinical benefit, KRAS still appears to be a very promising target for new therapies [37]. Therefore, a final verdict on the efficacy and cost-effectiveness of this class of KRAS inhibitors is still pending.
Our analysis focused on Switzerland; prices may vary in other countries, but the main setup of our analysis will likely hold for other industrialised countries, and local prices can be adapted easily. Still, generalising our results to other countries requires caution.
In conclusion, sotorasib did not demonstrate cost-effectiveness regarding the hypothetical willingness-to-pay threshold at the full or one-quarter dose. Sotorasib’s overall response rate is superior to that of docetaxel (28.1% [95% CI 21.5–35.4] versus 13.2% [8.6–19.2]), as is its patient-reported improved quality of life (using an instrument other than the EQ-5D questionnaire) [38]; these are the primary reasons for clinicians to prescribe sotorasib. Taking this and the absolute price differences into account, we believe it would be reasonable to set sotorasib’s price at approximately one-quarter of the assumed UK cost.
This work was supported by the cantonal hospital of Graubünden, independent of any industry influence. We thank Arjun Bhadhuri and Leandra Rothweiler for their support with writing and the validation of the model.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Tämer El Saadany received travel support from Amgen and Sanofi. received consulting fees from Amgen, AstraZeneca, BMS, MSD, Pfizer, Takeda, and Roche and travel support from Astra Zeneca, Roche, and Takeda. Michaela Barbier has received personal fees from Vifor for participation in an advisory board meeting as well as funding from the Swiss Institute for Accident Insurance (Suva) via an employment institution, unrelated to the submitted work. Matthias Schwenkglenks received research funding from AbbVie, Biogen, Bristol Myers Squibb, Merck Sharpe and Dohme, Mundipharma, Novartis, Pfizer, and Roche, unrelated to the submitted work and paid to the institution, along with personal consulting fees from BMS and Sandoz. Roger von Moos serves on the advisory board for Amgen (Denosumab), BMS, Elli Lilly, Gilead, GSK, Innomedica, Merck, MSD, Novartis, Pharmamar, Roche, Seagen, and Vifor and received travel support from Takeda and reports lecturing activities for Pierre Fabre.
1. WorldHealthOrganisation. International Agency for Research on Cancer. Cancer Today. 2023; Available from: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=756&key=total&sex=0&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0#collapse-group-0-4
2. Casal-Mouriño A, Ruano-Ravina A, Lorenzo-González M, Rodríguez-Martínez Á, Giraldo-Osorio A, Varela-Lema L, et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 2021 Jan;10(1):506–18. doi: https://doi.org/10.21037/tlcr.2020.03.40
3. AmericanCancerSociety. What Is Lung Cancer? 2023; Available from: https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html
4. Reita D, Pabst L, Pencreach E, Guérin E, Dano L, Rimelen V, et al. Direct Targeting KRAS Mutation in Non-Small Cell Lung Cancer: focus on Resistance. Cancers (Basel). 2022 Mar;14(5):1321. doi: https://doi.org/10.3390/cancers14051321
5. Mullard A, Cracking KR. Cracking KRAS. Nat Rev Drug Discov. 2019 Nov;18(12):887–91. doi: https://doi.org/10.1038/d41573-019-00195-5
6. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al.; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023 Apr;34(4):358–76. doi: https://doi.org/10.1016/j.annonc.2022.12.013
7. de Langen AJ, Johnson ML, Mazieres J, Dingemans AC, Mountzios G, Pless M, et al.; CodeBreaK 200 Investigators. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial. Lancet. 2023 Mar;401(10378):733–46. doi: https://doi.org/10.1016/S0140-6736(23)00221-0
8. FDA. FDA Approves First Targeted Therapy for Lung Cancer Mutation Previously Considered Resistant to Drug Therapy 2021; Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-lung-cancer-mutation-previously-considered-resistant-drug
9. Hochmair, M.J., et al., VP4. Sotorasib 960 mg versus 240 mg in pretreated KRAS G12C advanced NSCLC. Ann Oncol. 2023.
10. Popat S. SOTORASIB 960MG VERSUS 240MG IN PRETREATED KRAS G12C ADVANCED NSCLCIs less, more? ESMO Virtual Plenary; 2023.
11. Arbour KC; Preliminary Clinical Activity of RMC-6236, a First-in-Class, RAS-Selective, Tri-Complex RASMULTI(ON) Inhibitor in Patients with KRAS-Mutant Pancreatic Ductal Adenocarcinoma (PDAC) and Non-Small Cell Lung Cancer (NSCLC). 2023, ESMO Congress 2023.
12. Swissmedic. Lumykras® (active substance: sotorasib). 2022; Available from: https://www.swissmedic.ch/swissmedic/en/home/about-us/publications/public-summary-swiss-par/public-summary-swiss-par-lumykras.html
13. Versicherungsärzte SG. Nutzenbewertung zur Vergütung von Arzneimitteln im Einzelfall (Art. 71a - 71d KVV). 2023; Available from: https://www.vertrauensaerzte.ch/expertcom/71kvv/updmay18/
14. RCoreTeam. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
15. TreeAgePro. 2021, R1. TreeAge Software, Williamstown, MA.
16. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al.; CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health. 2022 Jan;25(1):3–9. doi: https://doi.org/10.1016/j.jval.2021.11.1351
17. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000 May;18(10):2095–103. doi: https://doi.org/10.1200/JCO.2000.18.10.2095
18. Anderson H, Hopwood P, Stephens RJ, Thatcher N, Cottier B, Nicholson M, et al. Gemcitabine plus best supportive care (BSC) vs BSC in inoperable non-small cell lung cancer—a randomized trial with quality of life as the primary outcome. UK NSCLC Gemcitabine Group. Non-Small Cell Lung Cancer. Br J Cancer. 2000 Aug;83(4):447–53. doi: https://doi.org/10.1054/bjoc.2000.1307
19. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012 Feb;12(1):9. doi: https://doi.org/10.1186/1471-2288-12-9
20. Christopher Jackson, P.M., Jordan Amdahl, Matthew T. Warkentin, Michael Sweeting, Kevin Kunzmann, flexsurv: Flexible Parametric Survival and Multi-State Models. 2023.
21. statistics, S.E.A.i.w.f.S.c. Surveillance Research Program, National Cancer Institute; 2023 Apr 19. 2023; Available from: https://seer.cancer.gov/statistics-network/explorer/
22. BAG. B.f.G. Spezialitätenliste. Available from: https://www.spezialitätenliste.ch/.
23. Tarmed FM. Available from: https://browser.tartools.ch/de/tarmed_kvg
24. FederalStatisticalOffice. HVPI Schweiz (2015=100), Totalindex und 12 Hauptgruppen, Veränderungsraten Totalindex. 2023; Available from: https://www.bfs.admin.ch/bfs/en/home/statistics/catalogues-databases/tables.assetdetail.24405716.html
25. Bundesamt für Gesundheit BAG. Preisfestsetzung von Arzneimitteln: Auslandpreisvergleich und therapeutischer Quervergleich. 2015; Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/kuv-leistungen/KUV-abgeschlossene%20Revisionen%20(Themen%20&%20Service)/Arzneimittel/29.%20April%202015/faktenblatt-auslandpreisvergleich-und-therapeutischer-quervergleich.pdf.download.pdf/faktenblatt-auslandpreisvergleich-und-therapeutischer-quervergleich.pdf
26. NICE. Sotorasib for previously treated KRAS G12C mutation-positive advanced non-small-cell lung cancer. 2022; Available from: https://www.nice.org.uk/guidance/ta781/documents/final-appraisal-determination-document-2
27. FederalStatisticalOffice. Durchschnittliche Körpergrösse (in cm). 2023; Available from: https://www.bfs.admin.ch/bfs/en/home/statistics/catalogues-databases.assetdetail.7586022.html
28. EXCELLENCE. N.I.F.H.A.C. Sotorasib for previously treated KRAS G12C mutated, locally advanced or metastatic non-small-cell lung cancer [ID3780]. [Single Technology Appraisal] 2021 30th June 2021; Available from: https://www.nice.org.uk/guidance/ta781/evidence/committee-papers-pdf-11014968349
29. NICE. Pembrolizumab for treating PD-L1-positive non-small-cell lung cancer after platinum-based chemotherapy [ID840]. 2016. Available from: https://www.nice.org.uk/guidance/ta428/documents/committee-papers
30. Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008 Oct;6(1):84. doi: https://doi.org/10.1186/1477-7525-6-84
31. Rothwell B, Kiff C, Ling C, Brodtkorb TH. Cost Effectiveness of Nivolumab in Patients with Advanced, Previously Treated Squamous and Non-squamous Non-small-cell Lung Cancer in England. PharmacoEconom Open. 2021 Jun;5(2):251–60. doi: https://doi.org/10.1007/s41669-020-00245-4
32. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020 Sep;383(13):1207–17. doi: https://doi.org/10.1056/NEJMoa1917239
33. Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med. 2021 Jun;384(25):2371–81. doi: https://doi.org/10.1056/NEJMoa2103695
34. vonMoos R. Was darf eine Krebstherapie kosten? Schweiz Arzteztg. 2021;102(3132):1007–9.
35. Paracha N, Abdulla A, MacGilchrist KS. Systematic review of health state utility values in metastatic non-small cell lung cancer with a focus on previously treated patients. Health Qual Life Outcomes. 2018 Sep;16(1):179. doi: https://doi.org/10.1186/s12955-018-0994-8
36. Mok TS, Lawler WE, Shum MK, Dakhil SR, Spira AI, Barlesi F, et al. KRYSTAL-12: A randomized phase 3 study of adagrasib (MRTX849) versus docetaxel in patients (pts) with previously treated non-small-cell lung cancer (NSCLC) with KRASG12C mutation. J Clin Oncol. 2021;39(15 suppl):TPS9129–9129. doi: https://doi.org/10.1200/JCO.2021.39.15_suppl.TPS9129
37. Mullard A. The KRAS crowd targets its next cancer mutations. Nat Rev Drug Discov. 2023 Mar;22(3):167–71. doi: https://doi.org/10.1038/d41573-023-00015-x
38. Waterhouse D, et al. Patient-reported outcomes from the CodeBreaK 200 phase 3 trial comparing sotorasib versus docetaxel in KRAS G12C-mutated NSCLC. European Lung Cancer Congress 2023, 2023.
39. Matter-Walstra K, Schwenkglenks M, Aebi S, Dedes K, Diebold J, Pietrini M, et al.; Swiss Group for Clinical Cancer Research. A Cost-Effectiveness Analysis of Nivolumab versus Docetaxel for Advanced Nonsquamous NSCLC Including PD-L1 Testing. J Thorac Oncol. 2016 Nov;11(11):1846–55. doi: https://doi.org/10.1016/j.jtho.2016.05.032
40. Barbier MC, Pardo E, Panje CM, Gautschi O, Lupatsch JE; Swiss Group for Clinical Cancer Research (SAKK). A cost-effectiveness analysis of pembrolizumab with or without chemotherapy for the treatment of patients with metastatic, non-squamous non-small cell lung cancer and high PD-L1 expression in Switzerland. Eur J Health Econ. 2021 Jul;22(5):669–77. doi: https://doi.org/10.1007/s10198-021-01282-4
41. Lippi G, Mattiuzzi C, Montagnana M. BRCA population screening for predicting breast cancer: for or against? Ann Transl Med. 2017 Jul;5(13):275. doi: https://doi.org/10.21037/atm.2017.06.71
42. Gamble C, Havrilesky LJ, Myers ER, Chino JP, Hollenbeck S, Plichta JK, et al. Cost Effectiveness of Risk-Reducing Mastectomy versus Surveillance in BRCA Mutation Carriers with a History of Ovarian Cancer. Ann Surg Oncol. 2017 Oct;24(11):3116–23. doi: https://doi.org/10.1245/s10434-017-5995-z
43. statistics, S.E.A.i.w.f.S.c. Adenocarcinoma of the Lung and Bronchus. SEER Relative Survival Rates by Time Since Diagnosis, 2000-2019. 2023; Available from: https://seer.cancer.gov/statistics-network/explorer/application.html?site=612&data_type=4&graph_type=6&compareBy=stage&chk_stage_106=106&sex=1&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&advopt_show_count=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0
44. Panje CM, Dedes KJ, Matter-Walstra K, Schwenkglenks M, Gautschi O, Siano M, et al.; Swiss Group for Clinical Cancer Research (SAKK). A cost-effectiveness analysis of consolidative local therapy in oligometastatic non-squamous non-small cell lung cancer (NSCLC). Radiother Oncol. 2018 Nov;129(2):257–63. doi: https://doi.org/10.1016/j.radonc.2018.07.017
45. Matter-Walstra KW, Achermann R, Rapold R, Klingbiel D, Bordoni A, Dehler S, et al. Delivery of health care at the end of life in cancer patients of four swiss cantons: a retrospective database study (SAKK 89/09). BMC Cancer. 2014 May;14(1):306. doi: https://doi.org/10.1186/1471-2407-14-306
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3777.