Intravenous ferric carboxymaltose is associated
with lowering of plasma phosphate levels in patients with gastric bypass surgery:
a retrospective case series
DOI: https://doi.org/https://doi.org/10.57187/s.3771
Cindy Pereira
Portelaa,
Lucie Favreb,
Isabella
Locatellic,
Olivier Bonnyde
a University of Lausanne, Faculty of
Biology and Medicine, Lausanne, Switzerland
b Service of Endocrinology, Diabetes
and Metabolism, Department of Medicine, Lausanne University Hospital, Lausanne,
Switzerland
c Biostatistic Unit, Center for
Primary Care and Public Health (Unisanté), University of Lausanne, Lausanne,
Switzerland
d Service of Nephrology, Department of
Medicine, Lausanne University Hospital, Lausanne, Switzerland and Department of
Medical Biosciences, University of Lausanne, Lausanne, Switzerland
e Service of Nephrology, Department of
medicine, Fribourg State Hospital and University of Fribourg, Fribourg,
Switzerland
Summary
AIMS: Bariatric surgery induces several
micronutrient deficiencies that require supplementation. For iron, parenteral
infusions are usually preferred over oral supplementation. Ferric carboxymaltose
infusion has been associated with hypophosphataemia, mostly transient and
asymptomatic. However, in some cases, ferric carboxymaltose-induced hypophosphataemia
may persist for weeks to months and may induce muscle weakness, osteomalacia
and bone fractures. The aim of this study was to identify possible predictors
of a clinically relevant decrease in serum phosphate after ferric
carboxymaltose infusion in patients with previous Roux-en-Y
gastric bypass.
METHODS: Patients with previous Roux-en-Y
gastric bypass who received ferric carboxymaltose infusions between January 2018
and September 2019 and had recorded phosphataemia before and after ferric
carboxymaltose infusion at the Lausanne University Hospital, Lausanne,
Switzerland, were studied retrospectively. A multiple linear regression model
was built with delta phosphataemia as the outcome to investigate the factors related
to magnitude of serum phosphate lowering.
RESULTS: Seventy-seven patients (70 females
and 7 males) with previous Roux-en-Y gastric bypass were studied. Mean age (SD) was
43.2 (10.7) years and median BMI was 30.9 kg/m2 (IQR
27.9–36.4). Sixty-eight patients (88.3%) received an infusion of 500 mg ferric
carboxymaltose and 9 patients (11.7%) received 250 mg ferric carboxymaltose.
Forty-nine patients (63.6%) developed hypophosphataemia (<0.8 mmol/l) after ferric
carboxymaltose infusion. Median plasma phosphate significantly decreased by 0.33
mmol/l (IQR 0.14–0.49) (p<0.0001). Multiple linear regression identified the
ferric carboxymaltose dose as the only risk factor significantly associated
with the magnitude of serum phosphate lowering, with an additional mean loss of
0.26 mmol/l with a 500 mg infusion compared to a 250 mg infusion (p = 0.020).
CONCLUSION: Ferric carboxymaltose infusions
substantially decreased plasma phosphate levels in patients with previous Roux-en-Y
gastric bypass. Compared to a dose of 250 mg, infusion of a dose of 500 mg ferric
carboxymaltose decreased the plasma phosphate further in this population.
Introduction
Iron deficiency affects millions of people worldwide.
Causes are varied and include restricted food intake, increased blood loss, increased
needs or intestinal malabsorption. Patients with previous gastric bypass surgery
are at risk of micronutrient malabsorption, especially iron, for which the prevalence
is 16–42% [1, 2]. Given that oral
absorption is reduced after Roux-en-Y gastric bypass surgery, due to decreased
gastric acid secretion and bypass of the proximal small intestine, parenteral
iron formulations are often preferred to enteral ones. Ferric
carboxymaltose (FCM) is among the most prescribed intravenous iron
preparations due to its rapid infusion rate and overall safety profile [3–5]. Despite
its attractiveness, FCM was recently
associated with new-onset hypophosphataemia [5–10].
Mostly underdiagnosed, hypophosphataemia after FCM infusion is usually transient
and asymptomatic with a nadir about two weeks after infusion [7, 8, 11]. A prevalence
of FCM-induced hypophosphataemia
of up to 92% has been described when systematically sought [4, 6, 7, 11–15]. Increase
of the biologically
active phosphatonin fibroblast growth factor 23 (FGF23) appears to mediate hypophosphataemia
induced by FCM infusion [7, 9, 10, 14, 16].
FGF23 is produced by osteoblasts in response to high levels of plasma 1,25-(OH)2
vitamin D or of plasma phosphate. FGF23 inhibits renal reabsorption of
phosphate by downregulating the tubular co-transporters NaPiIIa (SLC34A1) and NaPiIIc (SLC34A3) and by decreasing plasma levels
of 1,25-(OH)2-vitamin D. Recent reports suggest that among known
iron preparations, FCM is more often linked to hypophosphataemia [5–7, 14] than ferric
saccharose [17], ferric isomaltoside [11, 18] or ferric dextran [19]. Most of the
patients did not display any
symptoms, even though in the presence of persistent and severe hypophosphataemia
some of them experienced bone pain, osteomalacia and even fractures [9, 10, 20]. Predictive
factors associated with
intravenous iron-induced hypophosphataemia have been identified, such as the
type of intravenous iron (FCM) [7, 10, 11],
low body weight [7, 8], high pre-perfusion
haemoglobin concentration [7], normal eGFR
[6, 7, 10] or abnormal uterine bleeding [6, 7]. Moreover, cumulative doses of iron
were
also associated with hypophosphataemia [8, 17].
Here, we performed a retrospective case series study in patients with previous Roux-en-Y
gastric bypass receiving FCM infusion, to identify predictive risk factors involved
in plasma phosphate lowering.
Method
Study design
The study recruited patients with previous Roux-en-Y
gastric bypass who received FCM infusion during their routine medical care
between January 2018 and September 2019 and had plasma phosphataemia
measured within three months before the infusion and up to one month after the
infusion. In patients who had received several
infusions of FCM with measurements of plasma phosphate levels during the study
period (n = 5), the infusion that resulted in the lowest plasma phosphate level
was arbitrarily selected.
Participants
The single-centre retrospective study
included patients who underwent Roux-en-Y gastric bypass at Lausanne University
Hospital, Lausanne, Switzerland. Laparoscopic Roux-en-Y gastric bypass was
performed by the same surgical team by creating a 15–20 ml gastric pouch, a
retrocolic 100–150 cm Roux alimentary limb, a 50 cm biliopancreatic limb, a 21
mm circular stapled gastrojejunostomy and a linear stapled jejunostomy. All
patients were followed up for obesity at the outpatient clinic of the hospital
and received supplements (daily multivitamins, calcium and vitamin D) according
to Swiss guidelines on obesity and post-bariatric treatment [21]. The indication for
iron infusion was
retained in patients with an established diagnosis of iron deficiency by: ferritin
<50 µg/l or serum ferritin ≤100 µg/l and and transferrin saturation ≤30% who have
failed to respond to oral iron supplementation, either because of insufficient
absorption or because of digestive adverse events leading to discontinuation of
oral iron supplementation. Phosphatemia was assessed systematically in this time
period for the following reasons: Roux-en-Y gastric bypass patients are at risk
of poor bone health due to malabsorption of several micronutrients; several iron
infusions per year are commonly required, particularly in premenopausal women
with Roux-en-Y gastric bypass; and FCM carries a marked risk of hypophosphataemia.
The study was approved by the institutional
ethics committee (CER-VD, project n° 2019-01064) and conducted in accordance
with the ethical standards of the Declaration of Helsinki.
Study variables
The anthropometric and biological
parameters obtained from patient charts and the accredited clinical chemistry
laboratory of Lausanne University Hospital were: age (years), sex (M/F), time
since bariatric surgery (years), weight (kg), height (m) and dose of FCM (mg). Baseline
plasma phosphate (mmol/l), ferritin (µg/l), haemoglobin (g/l) and calcium
corrected for albumin (mmol/l) were measured up to three months before the accounted
FCM infusion; eGFR (ml/min/1.73 m2) was
measured up to six months before FCM infusion; and 25-OH-vitamin D (µg/l) and parathyroid
hormone (ng/l) were measured up to one month before FCM infusion. Post-infusion
plasma phosphate was considered until one month after FCM infusion and the cumulative
dose of FCM was calculated up to one year prior the infusion considered in the
study.
Outcome
The primary outcome was phosphataemia
lowering following a single FCM infusion up to one month after that infusion.
Statistical analysis
Standard descriptive analyses were used to
summarise the study variables: means with standard deviations (SD) for
approximately normally distributed continuous variables; medians with interquartile
ranges (IQR) for continuous variables presenting skewness; and frequencies and
percentages for categorical variables. A p-value <0.05 was considered statistically
significant. To identify
predictors of magnitude of plasma phosphate lowering, we defined delta phosphataemia
as the outcome and examined baseline haemoglobin, baseline ferritin, baseline
eGFR, BMI, cumulative dose of FCM, dose of FCM and time between FCM infusion
and post-FCM phosphataemia measurement as predictor variables [7, 18] using multiple
linear regression. The
number of covariates was chosen to respect the general rule for linear
regression models, which is to include at most as many covariates as the number
of study subjects divided by ten. Absolute risk of hypophosphataemia according
to baseline phosphate levels was assessed by receiver operating characteristic
(ROC) analysis. Outliers were defined as patients with a delta phosphataemia
more than 3 standard deviations away from the mean. Outliers were kept for
descriptive statistics and removed for multiple linear regression model and ROC
analysis.
The analysis was performed by the Biostatistical
Platform from UniSanté, using the software R (version 3.5.2) and GraphPad Prism
(version 8.01, GraphPad Software, San Diego, California, United States).
Results
Seventy-seven patients (70 females and 7 males) with a mean age (SD) of 43.2 (10.7)
and a median BMI of 30.9 kg/m2 (IQR 27.9-36.4) were included in the study. A
description of baseline and post-FCM infusion characteristics is shown in table
1. Most patients (88.3%) were
prescribed 500 mg FCM at the discretion of the treating physician. A decrease
in plasma phosphate was measured in 90.9% of patients within a median of 13
days (IQR 10.0–15.0) after FCM infusion and 63.6% of them developed new-onset hypophosphataemia;
40.8% had mild hypophosphataemia (0.6–0.8 mmol/l), 53.1% severe hypophosphataemia
(0.3–0.59 mmol/l) and 6.1% critical hypophosphataemia (<0.3 mmol/l). Five
patients were already mildly hypophosphataemic and one patient presented severe
hypophosphataemia (0.55 mmol/l) before FCM infusion. The variation in plasma phosphate
concentrations pre- and post-FCM infusion are shown in figure 1. Delta phosphataemia
was found to be approximately normally distributed, except for a severe outlier
in the left part of the distribution (a patient having phosphataemia increased by
approximately 1.5 mmol/l). Median delta phosphataemia (IQR) was 0.33 mmol/l
(0.14–0.49), with the first quartile rising to 0.17 mmol/l if the outlier is
excluded. Nearly one-fifth of patients had received a cumulative dose of FCM greater
than 500 mg in the year preceding the iron infusion. Mean eGFR (SD) for the
whole cohort was normal at 101.5 ml/min/1.73 m2 (19.2). Median ferritin level was low at 27.5 µg/l (IQR 18.3–43.8; reference range:
30–300 µg/l).
Table 1Characteristics of the
participants and treatment. Reference range for phosphataemia: 0.8–1.4 mmol/l.
Reference range for ferritin: 30–300 µg/l. Reference range for haemoglobin in women:
117–157 g/l, and in men: 133–177 g/l. Reference range for calcium corrected for
albumin: 2.10–2.50 mmol/l. Reference range for 25-OH-vitamin D: 8.4–52.3 µg/l. Reference
range for parathyroid hormone: 10–70 ng/l.
| Age
(y), mean (SD) |
43.2
(10.7) |
| Sex,
n (%) |
70 F / 7 M (90.9%/9.1%) |
| Time
from surgery (years), median (IQR; range) |
4.6
(2.6–10.0; 0.4–18.5) |
| BMI (kg/m2),
median (IQR; range) |
30.9 (27.9–36.4;
22.0–46.9) |
| Ferritin (µg/l),
median (IQR; range) (n = 72) |
27.5 (18.3–43.8;
5.0–176.0) |
| Haemoglobin (g/l),
mean (SD) (n = 62) |
131.0 (12.5) |
| eGFR (ml/min/1.73 m2),
mean (SD) (n = 66) |
|
101.5 (19.2) |
| CKD G1* (eGFR >90 ml/min/1.73 m2),
n (%) |
48 (72.8%) |
| CKD
G2* (eGFR 60–89 ml/min/1.73 m2), n (%) |
16 (24.2%) |
| CKD G3* (eGFR 30–59 ml/min/1.73 m2),
n (%) |
2 (3%) |
| Calcium corrected for
albumin (mmol/l), mean (SD) (n = 58) |
2.2 (0.07) |
| 25-OH-vitamin D
(µg/l), mean (SD) (n = 39) |
38.3 (8.0) |
| Parathyroid hormone
(ng/l), mean (SD) (n = 35) |
58.7 (29.7) |
| Pre-FCM phosphataemia (mmol/l), mean (SD) |
1.0 (0.2) |
| Cumulative dose of FCM>500 mg, n (%) |
15 (19.5%) |
| Dose of FCM: 500 mg, n (%) |
68 (88.3%) |
| Dose of FCM: 250 mg, n (%) |
9 (11.7%) |
| Time between FCM infusion and post-FCM phosphataemia
(days), median (IQR; range) |
13 (10–15; 0.4–18.5) |
| Post-FCM phosphataemia
(mmol/l), mean (SD) |
0.7 (0.3) |
| Delta (pre/post
infusion) phosphataemia (mmol/l), median (IQR; range) |
0.33 (0.14–0.49; -0.29
– 1.14) |
| Post-FCM infusion hypophosphataemia
(<0.8 mmol/l), n (%) |
|
49 (63.6%) |
| Mild (0.6 – <0.8 mmol/l), n
(%) |
20 (40.8%) |
| Severe (0.3 – <0.6 mmol/l),
n (%) |
26 (53.1%) |
| Critical (<0.3 mmol/l), n (%) |
3 (6.1%) |
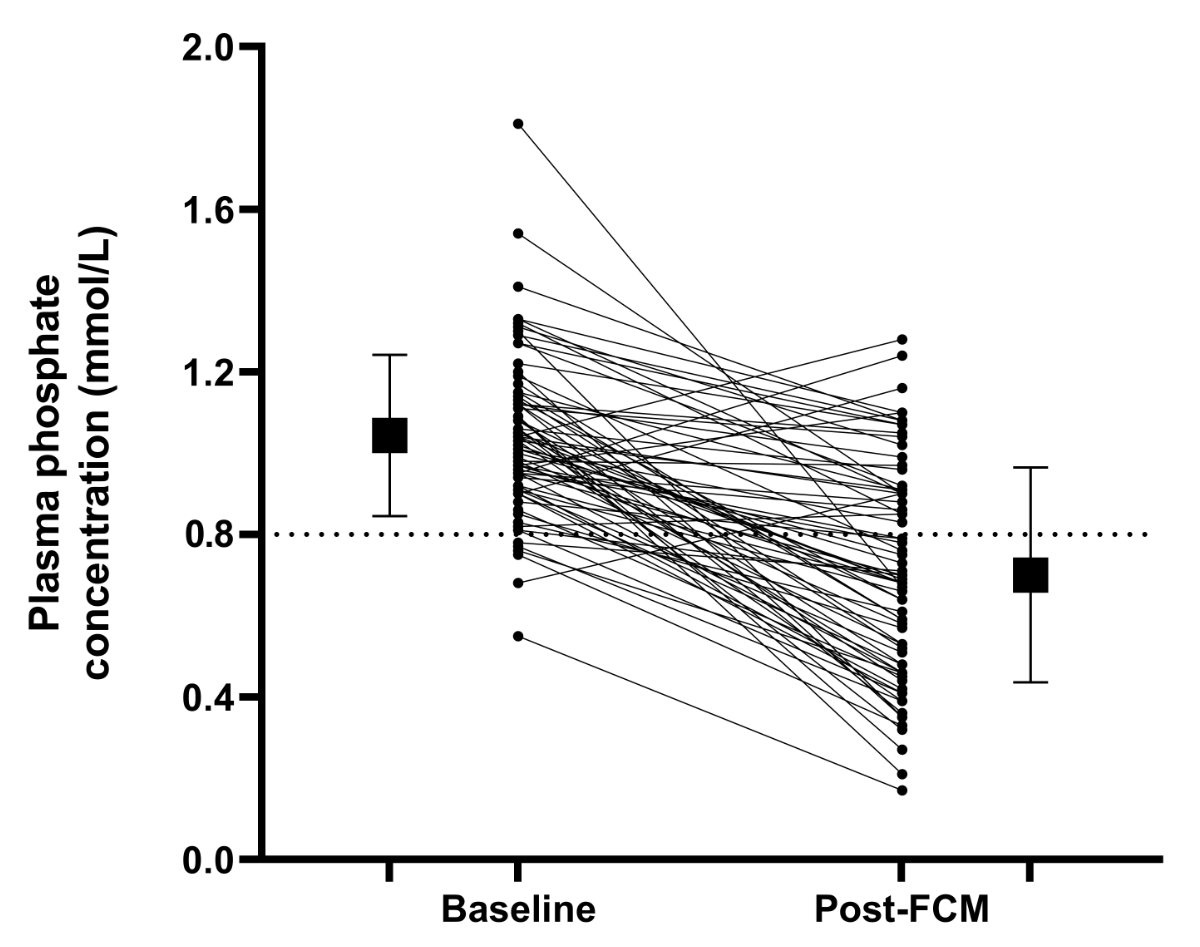
Figure 1Variation of plasma phosphate concentration at baseline and after ferric
carboxymaltose (FCM) infusion. Baseline plasma phosphate level was assessed up
to three months before FCM infusion. Post-FCM plasma phosphate level was
assessed up to one month after FCM infusion. The lower limit of normal plasma
phosphate level is indicated by a dotted line.
Multiple linear regression was applied to
analyse the joint influence of baseline haemoglobin, baseline ferritin,
baseline eGFR, BMI, cumulative dose of FCM, dose of FCM and time elapsed
between FCM infusion and post-FCM phosphataemia assessment, on delta phosphataemia
(outcome). An
interaction between FCM dose and the time between FCM infusion and
post-FCM phosphataemia was
also tested and founded to be not significant (not shown). The model’s assumptions
(linearity, normality and homogeneity of
variance) were verified by inspection of the residuals plotted against the model’s
fitted values and the normal probability plot of the residuals. No evidence of
assumption violation was detected. Table 2 shows the results of univariate and
of multivariable regression models. Effects represent increments in mean
phosphate serum lowering (mmol/l) associated with a one-point increase in
continuous covariates. For the FCM dose (dichotomous variable), the effect
represents the additional mean phosphate serum lowering associated with a dose
of 500 mg vs a dose of 250 mg. The dose of FCM infused was significantly
associated with the extent of phosphate serum lowering: a higher dose (500 mg vs
250 mg) was associated with a significantly greater mean decrease in phosphataemia
(difference of 0.26 mmol/l, p = 0.020). Since
the choice of the FCM dose may depend on previous hypophosphataemia post-FCM,
we re-estimated the model by adding baseline phosphate level as an additional
covariate in the model. We found that the effect of FCM dose remained
approximately the same adding baseline phosphate (0.23 mmol/l) as the one
obtained in the original model (0.26 mmol/l). Thus, at equal values of baseline
phosphate level, a higher dose of FCM will result in a greater drop in
phosphate. The interaction between the two variables was also tested and found
to be non-significant.We did not identify any other significant predictive factors
but
observed a trend for eGFR, specifically a protective effect of higher eGFR
values on phosphate serum lowering, which can be quantified as an average
reduction of 0.02 mmol/l in the phosphate lowering effect for each ten-point
increase in eGFR (ml/min/1.73 m2) (p = 0.081).
Table 2Multiple linear regression model
with delta phosphataemia as outcome.
|
Univariate
analysis |
Multivariable
analysis |
| |
Estimated
effect (mmol/l) |
95% CI |
p-value |
Estimated
effect (mmol/l) |
95% CI |
p-value |
| Dose of
FCM = 500 mg |
0.258 |
0.064 |
0.452 |
0.010 |
0.253 |
0.042 |
0.464 |
0.020 |
| Cumulative
dose of FCM >500 mg |
0.055 |
–0.109 |
0.219 |
0.505 |
0.104 |
–0.101 |
0.309 |
0.313 |
| log BMI |
–0.277 |
–0.647 |
0.094 |
0.141 |
-0.248 |
–0.702 |
0.207 |
0.279 |
| Haemoglobin
|
–0.003 |
–0.009 |
0.003 |
0.293 |
-0.004 |
–0.010 |
0.002 |
0.174 |
| eGFR |
–0.002 |
–0.006 |
0.001 |
0.185 |
-0.003 |
–0.007 |
0.000 |
0.081 |
| log
ferritin |
0.026 |
–0.074 |
0.125 |
0.611 |
0.016 |
–0.096 |
0.129 |
0.771 |
| log time
between FCM infusion and post-FCM phosphataemia |
–0.001 |
–0.215 |
0.213 |
0.992 |
-0.005 |
–0.025 |
0.014 |
0.587 |
ROC analysis was performed assessing
baseline phosphate levels as the predictor for absolute hypophosphataemia post-FCM
infusion, as shown in figure S1 and table S1 (in the appendix). Area under the
curve (AUC) was 0.678. A baseline phosphataemia of ≤1.2 mmol/l (table S1) could be
identified with Youden index as a risk
factor for absolute hypophosphataemia after FCM infusion with a sensitivity of
94%, a specificity of 37% and a positive predictive value of 73%.
Discussion
In this retrospective case series of Roux-en-Y
gastric bypass patients receiving FCM, we found that the dose of iron infused
was significantly associated with the decrease in phosphate concentration. Ninety
percent of the patients exhibited a decreased phosphate concentration post-iron
infusion and 63.6% developed hypophosphataemia within one month after infusion.
Most of the patients included were women (91%), as expected. Indeed, females
are much more represented in the general gastric bypass population than males [23].
FCM-associated hypophosphataemia is frequent,
mostly transient, and asymptomatic, with a nadir two weeks after the FCM
infusion [7, 8, 11]. However, in some extreme
cases, plasma phosphate concentration can remain low for several weeks to months
and may lead to osteomalacia and bone fractures [9].
Identification of patients at
risk of developing long-lasting hypophosphataemia following FCM infusion is essential
for preventing major health threats.
Risk factors for hypophosphataemia have
been sought in several studies [7, 18]. The
type of iron infused (FCM), lower body weight, Black ethnicity, higher baseline
haemoglobin levels and low baseline plasma phosphate levels have been
associated with the risk of developing hypophosphataemia. Other studies identified
the cumulative dose of FCM as associated with hypophosphataemia [8, 17]. The way the
underlying disease may
contribute to the risk of developing post-iron infusion hypophosphataemia or
osteomalacia is less clear, with some case reports associated with chronic
blood loss [24–26], inflammatory bowel
diseases [27–29] or malnutrition [30]. In the study by Wolf et al. [7], iron deficiency
due to abnormal uterine
bleeding compared to unspecified iron deficiency was more often associated with
post-FCM-induced hypophosphataemia.
It has not been established that the dose
of FCM is associated with hypophosphataemia. Wolf et al. [7] identified “lower body
weight” as a risk
factor and suggested that for a given dose, low body weight would expose the
patient to a higher iron concentration and therefore proposed that relative dose
may affect the magnitude of the plasma phosphate lowering effect. However, no
study confirmed this hypothesis. Our study now demonstrates that FCM infusion
dose has an impact on phosphataemia in obese patients (BMI of 30.9 kg/m2) who
underwent Roux-en-Y gastric bypass surgery. We used two relatively low doses,
250 mg (about 10% of the patients) and 500 mg of FCM (90%) and showed that the
higher the dose of FCM, the greater the probability of developing hypophosphataemia
after infusion. This dose-dependent
risk is also supported by the fact that all patients receiving FCM 250 mg were
those who previously developed hypophosphataemia with FCM 500 mg. The dose effect
suggests that the FCM dose should be adjusted in patients at risk. We cannot
speculate on higher doses (≥1000 mg) that
are often encountered in clinical practice, as these high doses were not used
in our clinic.
This study also found a trend between impaired
renal function and a decreased risk of hypophosphataemia after FCM infusion.
These results are in line with Wolf et al. [7]
and are usually explained by resistance to FGF23 action seen in chronic kidney
disease. Regarding other risk factors for decreased phosphataemia after FCM
infusion, we did not find an association with haemoglobin or ferritin levels
nor with BMI or cumulative FCM dose, as previously described. This might be due
to the limited size of our cohort or to specificities due to Roux-en-Y gastric bypass.
Patients with Roux-en-Y gastric bypass are
at risk of poor bone health. Chronic malabsorption of several micronutrients such
as calcium and vitamin D, secondary hyperparathyroidism and lower bone mineralisation
are common and increase the risk of bone fracture [31–33]. Correcting iron deficiency
with the FCM formulation may
add an additional risk factor for bone health by inducing hypophosphataemia and demineralisation.
Schoeb et al.
[13] conducted a prospective study in
this at-risk population and found that almost 30% of patients with previous Roux-en-Y
gastric bypass receiving a single dose of 500 mg of FCM developed hypophosphataemia.
However, no bone parameters were studied. We urge clinicians to carefully
investigate bone parameters in patients receiving iron infusions.
We found a median delta phosphataemia of
0.33 mmol/l, similar to what Schoeb et al. found (0.3 mmol/l), but lower
compared to the study by Wolf et al. (0.49 mmol/l) [7]. In the latter study, the FCM
dose was three times higher (1500
mg). This is in line with the dose-response effect we observed in this study and
is likely to explain the stronger effect on phosphataemia.
The weak association between low baseline
phosphataemia (≤1.2 mmol/l) and post-FCM
hypophosphataemia does not justify changes to clinical management and will need
additional confirmatory studies.
Limitations and strengths
This study has some limitations. Firstly, it
was retrospective and by definition is limited to the available data. Indeed,
there were missing data for baseline ferritin (n = 5), haemoglobin (n = 15),
eGRF (n = 11), calcium corrected for albumin (n = 19), 25-OH-vitamin D (n = 38)
and parathyroid hormone (n = 42). However, the set of data gathered here
allowed the establishment of a multiple logistic regression model with
significant results, indicating sufficient power. Secondly, we did not measure
FGF23 at baseline and after FCM infusion, as this parameter is rarely assessed
in routine clinical practice. This means that we cannot confirm the involvement
of FGF23 in the dose-dependent effect of FCM on the degree of hypophosphataemia. Thirdly,
our study did not examine the symptoms of hypophosphataemia
or other hard endpoints on bone or muscle strength. This should be done in future
prospective studies. Fourth, females were overrepresented in our study compared
to men (only 9%). However, this high proportion of women is representative of the
general gastric bypass population. Fifth, we focused only on patients with
previous Roux-en-Y gastric bypass with a recorded phosphataemia before and
after FCM infusion. Indeed, patients without data on phosphataemia were not included.
Finally, the design of our study induced a selection bias due to the selection
of the lowest phosphataemia after FCM infusion for individuals who had several
infusions. Due to the limited number of such cases (n = 5), a sensitivity
analysis could not be performed. However, the aim of this study was not to
determine the exact incidence of FCM-associated hypophosphataemia but rather to
identify risk factors. The strengths of our study are the real-world context
and the analysis carried out in a precisely defined population, after Roux-en-Y
gastric bypass surgery, and requiring frequent iron infusions.
Conclusion
Patients with Roux-en-Y gastric bypass are
at risk of iron deficiency and therefore receive repeated parenteral iron infusions,
mostly FCM. We found that single doses of FCM are followed by mild to critical hypophosphataemia
in this at-risk population and that the dose of FCM infused was associated with
delta phosphataemia. We thus recommend monitoring plasma phosphate levels in
patients with Roux-en-Y gastric bypass receiving FCM infusions or to switch to other
iron formulations much less associated with hypophosphataemia, such as ferric
saccharose or ferric isomaltoside.
Further studies evaluating long-term consequences
of iron-induced hypophosphataemia on target organs (heart, muscles, bone, …) are
warranted.
Acknowledgments
The authors would like to thank the
patients and the staff of the outpatient clinic for obesity of Lausanne
University Hospital. Dr O. Lamy is acknowledged for his review of the Master thesis
work of CPP.
Authors’ contribution: CPP designed the study, collected data, analysed and interpreted
data, and wrote the manuscript. OB designed the study, analysed and interpreted
data, and wrote the manuscript. IL performed statistical analysis, analysed and
interpreted data and revised the manuscript. LF was in charge of patient care,
designed the study, analysed and interpreted data and revised the manuscript.
All authors read and approved the submitted version of the manuscript.
Prof.
Olivier Bonny
Service of
Nephrology
Fribourg
State Hospital
CH-1708 Fribourg
olivier.bonny[at]h-fr.ch
References
1. Enani G, Bilgic E, Lebedeva E, Delisle M, Vergis A, Hardy K. The incidence of iron
deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: a systematic
review. Surg Endosc. 2020 Jul;34(7):3002–10. 10.1007/s00464-019-07092-3
2. Engebretsen KV, Blom-Høgestøl IK, Hewitt S, Risstad H, Moum B, Kristinsson JA, et
al. Anemia following Roux-en-Y gastric bypass for morbid obesity; a 5-year follow-up
study. Scand J Gastroenterol. 2018 Aug;53(8):917–22. 10.1080/00365521.2018.1489892
3. Rognoni C, Venturini S, Meregaglia M, Marmifero M, Tarricone R. Efficacy and Safety
of Ferric Carboxymaltose and Other Formulations in Iron-Deficient Patients: A Systematic
Review and Network Meta-analysis of Randomised Controlled Trials. Clin Drug Investig.
2016 Mar;36(3):177–94. 10.1007/s40261-015-0361-z
4. Schaefer B, Meindl E, Wagner S, Tilg H, Zoller H. Intravenous iron supplementation
therapy. Mol Aspects Med. 2020 Oct;75:100862. 10.1016/j.mam.2020.100862
5. Blumenstein I, Shanbhag S, Langguth P, Kalra PA, Zoller H, Lim W. Newer formulations
of intravenous iron: a review of their chemistry and key safety aspects - hypersensitivity,
hypophosphatemia, and cardiovascular safety. Expert Opin Drug Saf. 2021;20(7):757-69.
Epub 20210515. doi: 10.1080/14740338.2021.1912010. PubMed PMID: 33993818.
6. Schaefer B, Tobiasch M, Viveiros A, Tilg H, Kennedy NA, Wolf M, et al. Hypophosphataemia
after treatment of iron deficiency with intravenous ferric carboxymaltose or iron
isomaltoside-a systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87(5):2256-73.
Epub 20201207. doi: 10.1111/bcp.14643. PubMed PMID: 33188534; PubMed Central PMCID: PMC8247006.
7. Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of
intravenous iron-induced hypophosphatemia. JCI Insight. 2018 Dec;3(23):e124486. 10.1172/jci.insight.124486
8. Rosano G, Schiefke I, Göhring UM, Fabien V, Bonassi S, Stein J. A Pooled Analysis
of Serum Phosphate Measurements and Potential Hypophosphataemia Events in 45 Interventional
Trials with Ferric Carboxymaltose. J Clin Med. 2020;9(11). Epub 20201106. doi: 10.3390/jcm9113587. PubMed PMID: 33172157; PubMed Central PMCID: PMC7694774.
9. Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication.
Curr Opin Nephrol Hypertens. 2017 Jul;26(4):266–75. 10.1097/MNH.0000000000000329
10. Glaspy JA, Wolf M, Strauss WE. Intravenous Iron-Induced Hypophosphatemia: An Emerging
Syndrome. Adv Ther. 2021;38(7):3531-49. Epub 20210530. doi: 10.1007/s12325-021-01770-2. PubMed PMID: 34053011; PubMed Central PMCID: PMC8279965.
11. Wolf M, Rubin J, Achebe M, Econs MJ, Peacock M, Imel EA, et al. Effects of Iron Isomaltoside
vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized
Clinical Trials. JAMA. 2020 Feb;323(5):432–43. 10.1001/jama.2019.22450
12. Favrat B, Balck K, Breymann C, Hedenus M, Keller T, Mezzacasa A, et al. Evaluation
of a single dose of ferric carboxymaltose in fatigued, iron-deficient women—PREFER
a randomized, placebo-controlled study. PLoS One. 2014 Apr;9(4):e94217. 10.1371/journal.pone.0094217
13. Schoeb M, Räss A, Frei N, Aczél S, Brändle M, Bilz S. High Risk of Hypophosphatemia
in Patients with Previous Bariatric Surgery Receiving Ferric Carboxymaltose: A Prospective
Cohort Study. Obes Surg. 2020 Jul;30(7):2659–66. 10.1007/s11695-020-04544-x
14. Kassianides X, Bhandari S. Hypophosphataemia, fibroblast growth factor 23 and third-generation
intravenous iron compounds: a narrative review. Drugs Context. 2021;10. Epub 20210119.
doi: 10.7573/dic.2020-11-3. PubMed PMID: 33519940; PubMed Central PMCID: PMC7819638.
15. Bager P, Hvas CL, Dahlerup JF. Drug-specific hypophosphatemia and hypersensitivity
reactions following different intravenous iron infusions. Br J Clin Pharmacol. 2017;83(5):1118-25.
Epub 20170118. doi: 10.1111/bcp.13189. PubMed PMID: 27859495; PubMed Central PMCID: PMC5401972.
16. Coppolino G, Nicotera R, Cernaro V, Calimeri S, Leonardi G, Cosentino S, et al. Iron
Infusion and Induced Hypophosphatemia: The Role of Fibroblast Growth Factor-23. Ther
Apher Dial. 2020;24(3):258-64. Epub 20191025. doi: 10.1111/1744-9987.13435. PubMed PMID: 31483921.
17. Hardy S, Vandemergel X. Intravenous iron administration and hypophosphatemia in clinical
practice. Int J Rheumatol. 2015;2015:468675. 10.1155/2015/468675
18. Schaefer B, Würtinger P, Finkenstedt A, Braithwaite V, Viveiros A, Effenberger M,
et al. Choice of High-Dose Intravenous Iron Preparation Determines Hypophosphatemia
Risk. PLoS One. 2016 Dec;11(12):e0167146. 10.1371/journal.pone.0167146
19. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on
fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res.
2013 Aug;28(8):1793–803. 10.1002/jbmr.1923
20. Schaefer B, Tobiasch M, Wagner S, Glodny B, Tilg H, Wolf M, et al. Hypophosphatemia
after intravenous iron therapy: Comprehensive review of clinical findings and recommendations
for management. Bone. 2021;154:116202. Epub 20210915. doi: 10.1016/j.bone.2021.116202. PubMed PMID: 34534708.
21. SMOB. Directives pour le traitement chirurgical de l’obésité. 2021.
22. Jepsen P, Tapper EB, Deleuran T, Kazankov K, Askgaard G, Sorensen HT, et al. Risk
and Outcome of Venous and Arterial Thrombosis in Patients With Cirrhosis: A Danish
Nation-wide Cohort Study. Hepatology. 2021;74(5):2725-34. Epub 20210909. doi: 10.1002/hep.32019. PubMed PMID: 34137045; PubMed Central PMCID: PMC8542589.
23. Young MT, Phelan MJ, Nguyen NT. A Decade Analysis of Trends and Outcomes of Male vs
Female Patients Who Underwent Bariatric Surgery. J Am Coll Surg. 2016;222(3):226-31.
Epub 20151217. doi: 10.1016/j.jamcollsurg.2015.11.033. PubMed PMID: 26782151.
24. Callejas-Moraga EL, Casado E, Gomez-Nuñez M, Caresia-Aroztegui AP. Severe osteomalacia
with multiple insufficiency fractures secondary to intravenous iron therapy in a patient
with Rendu-Osler-Weber syndrome. Bone Rep. 2020 Aug;13:100712. 10.1016/j.bonr.2020.100712
25. Sangrós Sahún MJ, Goñi Gironés E, Camarero Salazar A, Estébanez Estébanez C, Lozano
Martínez ME. Symptomatic hypophosphataemic osteomalacia secondary to the treatment
with iron carboxymaltose detected in bone scintigraphy. Rev Esp Med Nucl Imagen Mol.
2016;35(6):391–3. 10.1016/j.remn.2016.04.006 10.1016/j.remnie.2016.09.002
26. Moore KL, Kildahl-Andersen O, Kildahl-Andersen R, Tjønnfjord GE. Uncommon adverse
effect of a common medication. Tidsskr Nor Laegeforen. 2013 Jan;133(2):165. 10.4045/tidsskr.12.0494
27. Bartko J, Roschger P, Zandieh S, Brehm A, Zwerina J, Klaushofer K. Hypophosphatemia,
Severe Bone Pain, Gait Disturbance, and Fatigue Fractures After Iron Substitution
in Inflammatory Bowel Disease: A Case Report. J Bone Miner Res. 2018 Mar;33(3):534–9.
10.1002/jbmr.3319
28. Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23-based hypophosphataemic osteomalacia
due to ferric carboxymaltose administration. BMJ Case Rep. 2018 Jan;2018:bcr2017222851.
10.1136/bcr-2017-222851
29. Schaefer B, Glodny B, Zoller H. Blood and Bone Loser. Gastroenterology. 2017 May;152(6):e5–6.
10.1053/j.gastro.2016.09.050
30. Fierz YC, Kenmeni R, Gonthier A, Lier F, Pralong F, Coti Bertrand P. Severe and prolonged
hypophosphatemia after intravenous iron administration in a malnourished patient.
Eur J Clin Nutr. 2014 Apr;68(4):531–3. 10.1038/ejcn.2014.20
31. Axelsson KF, Werling M, Eliasson B, Szabo E, Näslund I, Wedel H, et al. Fracture Risk
After Gastric Bypass Surgery: A Retrospective Cohort Study. J Bone Miner Res. 2018 Dec;33(12):2122–31.
10.1002/jbmr.3553
32. Yu EW, Kim SC, Sturgeon DJ, Lindeman KG, Weissman JS. Fracture Risk After Roux-en-Y
Gastric Bypass vs Adjustable Gastric Banding Among Medicare Beneficiaries. JAMA Surg.
2019 Aug;154(8):746–53. 10.1001/jamasurg.2019.1157
33. Fashandi AZ, Mehaffey JH, Hawkins RB, Schirmer B, Hallowell PT. Bariatric surgery
increases risk of bone fracture. Surg Endosc. 2018 Jun;32(6):2650–5. 10.1007/s00464-017-5628-4
34. Summary of Recommendation Statements. Kidney Int Suppl (2011). 2013;3(3):263-5. doi:
10.1038/kisup.2013.31. PubMed PMID: 25018998; PubMed Central PMCID: PMC4089618.
Appendix
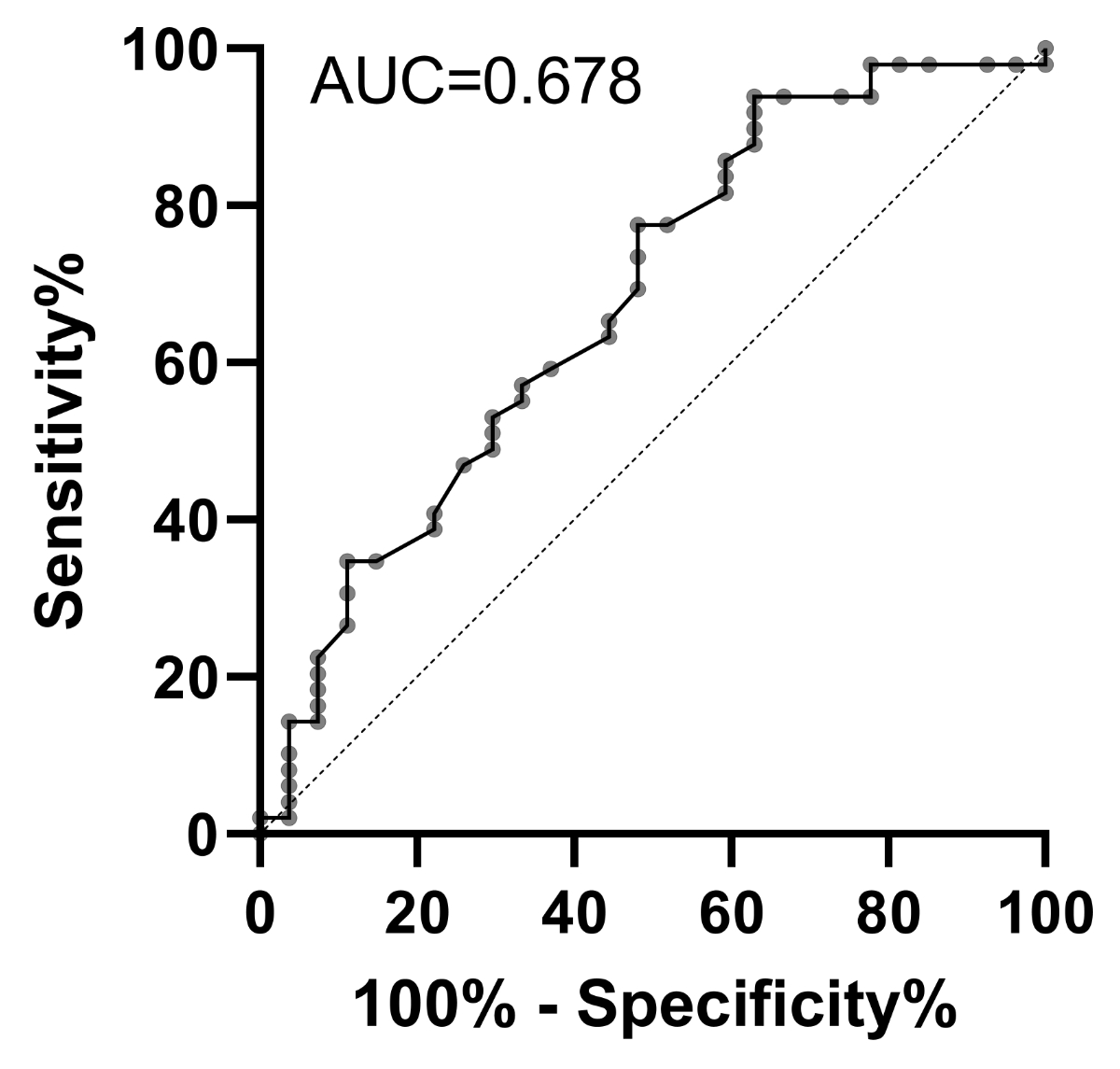
Figure S1Receiver operating characteristic (ROC) curve
of the absolute risk of hypophosphataemia as a function of baseline phosphate
level. AUC: area under the curve.
Table S1Cutoffs of baseline phosphataemia
as a risk factor for post-ferric carboxymaltose hypophosphataemia according to
ROC analysis.
| Cutoffs |
Positive* |
Negative** |
Likelihood ratio |
95% CI |
| 0.4–0.8 |
5 |
1 |
2.755 |
0.339–22.388 |
| 0.8–1.0 |
21 |
7 |
1.653 |
0.809–3.379 |
| 1.0–1.2*** |
20 |
9 |
1.224 |
0.651–2.302 |
| 1.2–1.4 |
2 |
8 |
0.138 |
0.0315–0.603 |
| 1.4–2.0 |
1 |
2 |
0.276 |
0.0262–2.901 |
| Total |
49 |
27 |
|
|
| Sensitivity*** |
94% |
|
|
|
| Specificity*** |
37% |
|
|
|
| PPV*** |
73% |
|
|
|
| NPV |
78% |
|
|
|

