Figure 1Patient selection chart.
DOI: https://doi.org/https://doi.org/10.57187/s.3769
Colorectal cancer is one of the most common malignancies worldwide and is associated with poor prognosis, particularly in advanced stages. The 5-year survival rate for patients with metastatic disease is below 20%. Despite numerous efforts to establish new therapeutic approaches, this situation has changed little in recent years – in stark contrast to many other cancer entities. Particularly, even checkpoint inhibitor therapies play only a minor role in the treatment of about 5% of all colorectal carcinoma patients [1].
The most effective method for early detection and tumour prevention is colonoscopy screening. This safe and highly efficient screening method has been proven to save lives [2]. Therefore, colonoscopy screening is recommended for colorectal carcinoma prevention in Switzerland specifically for adults over the age of 50 [3].
In a recent randomised trial, it was clearly demonstrated that the risk of developing colorectal carcinoma is significantly lower in patients who undergo colonoscopy screening than in those who do not [4]. However, this might have only a limited role when looking at overall mortality [4]. Nevertheless, recent studies indicated that an initial colonoscopy screen at the age of 50 might even be too late, since colorectal carcinomas are increasingly diagnosed before age 50 [5–7] even though some of those colorectal carcinoma cases occurring before the age of 50 might be due to genetic syndromes. Interestingly, when diagnosed before 50, colorectal carcinomas often show a more aggressive course than in older patients, which complicates treatment and increases the urgency for screening [8, 9].
For this reason, colonoscopy screening is now recommended as early as age 45 in the United States [10]. Our group previously showed that colorectal carcinomas are usually only detected at a more advanced disease stage in people aged below 50 [9]. It is unknown, however, whether the overall detection rate of colorectal carcinoma is also increasing in the population <50 years of age in Switzerland. Additional data to support an increased detection rate of colorectal carcinoma already before the age of 50 also in Switzerland might strongly support colonoscopy colorectal cancer screening programmes and adherence to the recommended guidelines overall, but also particularly in Switzerland.
Thus, the primary aim of our study was to investigate whether the detection rate of colorectal carcinoma was increased already in patients before the age of 50. For this purpose, we performed an exploratory, single-centre, retrospective cohort study of all patients attending the Department of Gastroenterology and Hepatology at University Hospital Zurich, Switzerland, who underwent a colonoscopy for any indication, not only for pure screening purposes in an average-risk population.
We performed a single-centre, retrospective cohort study of patients attending the Department of Gastroenterology and Hepatology of University Hospital Zurich. All patients aged 18–59 years who underwent a colonoscopy for any reason, regardless of medical history, underlying disease, current ongoing therapies and previous operations, from 1 January 2016 to 5 November 2021 and had signed a general consent were included in our study.
This yielded a total of 2846 cases in which we compared the adenoma and colorectal cancer detection rates according to different age groups. Selection criteria are summarised in the selection chart in figure 1. Medical data were retrieved by a query to our electronic patient information system (KISIM) via the Research Data Service Center (RDSC) of University Hospital Zurich using the cut-off date 5 November 2021.
Patient data were coded according to our ethics protocol. The study was approved by the local ethics committee of the Canton of Zurich (licence number KEK-ZH 2021-01742).

Figure 1Patient selection chart.
Data were analysed separately for each age group and subdivided by sex. We considered the indication for the colonoscopy, the interventions during colonoscopy, as well as the resulting histopathological diagnosis.
Each colonoscopy performed was counted as a separate case. Patients undergoing a second colonoscopy within a few years were considered new cases for the purposes of this analysis. If a colonoscopy was cancelled (e.g. due to anticoagulation or to inadequate bowel preparation), but a repeat colonoscopy was performed within the next few days, the first colonoscopy was excluded from the study. If no repeat colonoscopy was performed, the first colonoscopy was considered inconclusive. Patients were also excluded from the study if the data retrieved from KISIM were unclear. In addition, patients were excluded if the search revealed a gastroscopy instead of a colonoscopy report or if pathology reports were available without a previously performed colonoscopy in our clinic, as these data may originate from external reports. In the overall assessment, colonoscopies with inadequate quality parameters were also included (Boston Bowel Preparation Scale [BBPS] <6, caecum not reached, withdrawal time <6 min).
Cases were classified according to the indication/question given on the colonoscopy report; cases without an indication/question were grouped under “not specified”. Surveillance and screening colonoscopies were considered together in one group. By “screening” we mean all colonoscopies that were performed in asymptomatic patients in order to check for the presence of polyps or colorectal tumours. By “surveillance” we mean all colonoscopies that were performed as a follow-up colonoscopy after e.g. the removal of polyps or tumours. In addition, colonoscopies with the question of transplant, gastric bypass or bariatric pretesting were also assigned to the screening group. Patients with multiple indications such as surveillance and melena were assigned to both indication groups. Consequently, our study yielded more indications than cases. Cases with the indication faecal microbiota transplantation were grouped under the indication infectious gastroenteritis. An overview is given in table 1.
Table 1Colonoscopy: Indications and interventions by age group and sex. Figures are numbers of cases or interventions; percentages refer to respective age and patient groups in table 2 (total, female, male).
| Age groups (age in years) | 1 (18–29) | 2 (30–34) | 3 (35–39) | 4 (40–44) | 5 (45–49) | 6 (50–54) | 7 (55–59) | |
| Indications for colonoscopy | ||||||||
| Haemorrhage, melena/blood in stool, anaemia | All | 11 (7.6%) | 14 (8.4%) | 16 (8.2%) | 32 (12.1%) | 47 (12.8%) | 69 (8.4%) | 79 (8.9%) |
| Women | 3 (4.7%) | 6 (6.3%) | 5 (5.1%) | 17 (14.0%) | 12 (7.7%) | 28 (8.6%) | 32 (10.1%) | |
| Men | 8 (10.0%) | 8 (11.4%) | 11 (11.3%) | 15 (10.5%) | 35 (16.7%) | 41 (8.3%) | 47 (8.2%) | |
| Radiological (incidental) findings in abdomen | All | 1 (0.7%) | – | 2 (1.0%) | 4 (1.5%) | 5 (1.4%) | 15 (1.8%) | 14 (1.6%) |
| Women | 1 (1.6%) | – | – | 3 (2.5%) | 2 (1.3%) | 5 (1.5%) | 7 (2.2%) | |
| Men | – | – | 2 (2.1%) | 1 (0.7%) | 3 (1.4%) | 10 (2.0%) | 7 (1.2%) | |
| Weight loss, fatigue, abdominal pain, intestinal stenosis/obstructions/ileus | All | 3 (2.1%) | 10 (6.0%) | 12 (6.2%) | 12 (4.5%) | 13 (3.6%) | 26 (3.2%) | 26 (2.9%) |
| Women | 2 (3.1%) | 8 (8.3%) | 9 (9.2%) | 4 (3.3%) | 11 (7.1%) | 17 (5.2%) | 11 (3.5%) | |
| Men | 1 (1.3%) | 2 (2.9%) | 3 (3.1%) | 8 (5.6%) | 2 (1.0%) | 9 (1.8%) | 15 (2.6%) | |
| Change in bowel habits (onset of diarrhoea/constipation), irritable bowel syndrome | All | 10 (6.9%) | 2 (1.2%) | 5 (2.6%) | 10 (3.8%) | 16 (4.4%) | 33 (4.0%) | 22 (2.5%) |
| Women | 5 (7.8%) | 1 (1.0%) | 5 (5.1%) | 4 (3.3%) | 6 (3.8%) | 18 (5.6%) | 10 (3.2%) | |
| Men | 5 (6.3%) | 1 (1.4%) | – | 6 (4.2%) | 10 (4.8%) | 15 (3.0%) | 12 (2.1%) | |
| Non-infectious gastroenteritis, inflammatory bowel disease (Crohn’s, ulcerative colitis), diverticulitis/diverticulosis | All | 48 (33.3%) | 44 (26.5%) | 44 (22.6%) | 49 (18.6%) | 55 (15.0%) | 41 (5.0%) | 26 (2.9%) |
| Women | 20 (31.3%) | 25 (26.0%) | 17 (17.3%) | 18 (14.9%) | 17 (10.9%) | 18 (5.6%) | 10 (3.2%) | |
| Men | 28 (35.0%) | 19 (27.1%) | 27 (27.8%) | 31 (21.7%) | 38 (18.1%) | 23 (4.6%) | 16 (2.8%) | |
| Infectious gastroenteritis (e.g. Clostridioides difficile, Salmonella, intestinal tuberculosis, etc) | All | 3 (2.1%) | 3 (1.8%) | 4 (2.1%) | – | 4 (1.1%) | 6 (0.7%) | 9 (1.0%) |
| Women | 1 (1.6%) | 1 (1.0%) | 1 (1.0%) | – | 2 (1.3%) | 3 (0.9%) | 1 (0.3%) | |
| Men | 2 (2.5%) | 2 (2.9%) | 3 (3.1%) | – | 2 (1.0%) | 3 (0.6%) | 8 (1.4%) | |
| Screening, history of polyps, benign tumours, surveillance (history of malignancy) | All | 51 (35.4%) | 87 (52.4%) | 96 (49.2%) | 146 (55.3%) | 192 (52.5%) | 546 (66.7%) | 624 (70.0%) |
| Women | 22 (34.4%) | 46 (47.9%) | 50 (51.0%) | 69 (57.0%) | 85 (54.5%) | 199 (61.4%) | 206 (65.0%) | |
| Men | 29 (36.3%) | 41 (58.6%) | 46 (47.4%) | 77 (53.8%) | 107 (51.0%) | 347 (70.1%) | 418 (72.7%) | |
| Not specified | All | 17 (11.8%) | 19 (11.4%) | 30 (15.4%) | 41 (15.5%) | 56 (15.3%) | 122 (14.9%) | 112 (12.6%) |
| Women | 10 (15.6%) | 13 (13.5%) | 17 (17.3%) | 20 (16.5%) | 29 (18.6%) | 57 (17.6%) | 44 (13.9%) | |
| Men | 7 (8.8%) | 6 (8.6%) | 13 (13.4%) | 21 (14.7%) | 27 (12.9%) | 65 (13.1%) | 68 (11.8%) | |
| Interventions during colonoscopy | ||||||||
| No intervention | All | 10 (6.9%) | 19 (11.4%) | 28 (14.4%) | 29 (11.0%) | 31 (8.5%) | 82 (10.0%) | 92 (10.3%) |
| Women | 3 (4.7%) | 12 (12.5%) | 10 (10.2%) | 17 (14.0%) | 11 (7.1%) | 35 (10.8%) | 32 (10.1%) | |
| Men | 7 (8.8%) | 7 (10.0%) | 18 (18.6%) | 12 (8.4%) | 20 (9.5%) | 47 (9.5%) | 60 (10.4%) | |
| Biopsy/polypectomy | All | 133 (92.4%) | 147 (88.6%) | 166 (85.1%) | 234 (88.6%) | 335 (91.5%) | 733 (89.5%) | 796 (89.2%) |
| Women | 61 (95.3%) | 84 (87.5%) | 87 (88.8%) | 103 (85.1%) | 145 (92.9%) | 287 (88.6%) | 282 (89.0%) | |
| Men | 72 (90.0%) | 63 (90.0%) | 79 (81.4%) | 131 (91.6%) | 190 (90.5%) | 446 (90.1%) | 514 (89.4%) | |
| Other interventions (e.g. argon plasma coagulation-treatment, stent placement, dilation) | All | 1 (0.7%) | 0 (0%) | 1 (0.5%) | 1 (0.4%) | 0 (0%) | 6 (0.7%) | 8 (0.9%) |
| Women | – | – | 1 (1.0%) | 1 (0.8%) | – | 2 (0.6%) | 5 (1.6%) | |
| Men | 1 (1.3%) | – | – | – | – | 4 (0.8%) | 3 (0.5%) | |
Biopsies and/or polypectomy were the most frequently performed interventions. Other interventions mostly concerned argon plasma coagulation treatment or faecal microbiota transplantation. In cases where a haemorrhage due to a prior polypectomy was treated with a clip, the intervention was noted as a biopsy/polypectomy only. In some cases, no polypectomy was mentioned in the colonoscopy report but the histopathology report suggested one. In these cases, a polypectomy was noted. An overview is given in table 1.
Patients with dual pathological diagnoses were assigned to multiple groups. However, in cases showing multiple relevant precursors of colorectal carcinoma, only the diagnosis of the most advanced precursor lesion was included in the evaluation. For example, in cases where a hyperplastic polyp as well as an adenoma with low-grade dysplasia were found in the same report, only the adenoma with low-grade dysplasia was evaluated. If multiple pathology reports were found for a colonoscopy, the most recent and comprehensive report was analysed.
The primary objective of our study was to investigate whether the detection rate of colorectal carcinoma is increased in people already before the age of 50 years and thus comparable to the rate in people aged over 50. As a secondary endpoint, we assessed whether this potential increase in detection rate was concentrated in certain age intervals. For this purpose, we analysed the number of patients diagnosed with colorectal carcinoma after colonoscopy in the respective age groups. For the purpose of analysis, the patients were assigned to the following pre-defined age groups (age strata were predefined as per our ethics protocol): 18–29 (1), 30–34 (2), 35–39 (3), 40–44 (4), 45–49 (5), 50–54 (6) and 55–59 (7). The main focus of this study was on data from groups 5 (45–49) and 6 (50–54). Furthermore, to identify possible sex-specific differences, data were also analysed separately by sex. The numbers of cases in each group are detailed in table 2.
Table 2Study population. Figures are numbers of cases; percentages refer to the total case number in the respective age group.
| Age groups (age in years) | 1 (18–29) | 2 (30–34) | 3 (35–39) | 4 (40–44) | 5 (45–49) | 6 (50–54) | 7 (55–59) |
| All | 144 | 166 | 195 | 264 | 366 | 819 | 892 |
| Women | 64 (44.4%) | 96 (57.8%) | 98 (50.3%) | 121 (45.8%) | 156 (42.6%) | 324 (39.6%) | 317 (35.5%) |
| Men | 80 (55.6%) | 70 (42.2%) | 97 (49.7%) | 143 (54.2%) | 210 (57.4%) | 495 (60.4%) | 575 (64.5%) |
The study population included 2846 cases aged 18–59 who underwent a colonoscopy from 1 January 2016 to 5 November 2021 (table 2). The detection rate of colorectal carcinoma was determined for each defined age group and subdivided by sex.
Table 1 summarises the indications and interventions during colonoscopies by each age group and sex. Table 3 summarises the respective histopathological diagnoses.
The detection rate of colorectal carcinoma confirmed by histopathological diagnosis was 5/366 cases (1.4%) in age group 5 (45–49 years) and 9/819 cases (1.1%) in age group 6 (50–54 years). In addition, the number of cases of adenoma with high-grade dysplasia was slightly higher, albeit nonsignificantly, in age group 5 (45–49) with 5/366 cases (1.4%) compared to age group 6 (50–54) with 11/819 cases (1.3%). The biggest increase in colorectal cancer detection rate in cases younger than 50 years was found between age group 4 (40–44 years) with 0.8% and age group 5 (45–49 years) with 1.4% (table 3).
Table 3Histopathological findings by age group and sex. Figures represent numbers of cases; percentages refer to respective age and patient groups in table 2 (total, female, male).
| Age groups (age in years) | 1 (18–29) | 2 (30–34) | 3 (35–39) | 4 (40–44) | 5 (45–49) | 6 (50–54) | 7 (55–59) | ||
| Cancers | Colon carcinoma, neuroendocrine tumours | All | 1 (0.7%) | 2 (1.2%) | 1 (0.5%) | 2 (0.8%) | 5 (1.4%) | 9 (1.1%) | 8 (0.9%) |
| Women | 1 (1.6%) | 2 (2.1%) | 1 (1.0%) | 1 (0.8%) | 2 (1.3%) | 4 (1.2%) | 4 (1.3%) | ||
| Men | – | – | – | 1 (0.7%) | 3 (1.4%) | 5 (1.0%) | 4 (0.7%) | ||
| Polyps | Not further specified | All | 1 (0.7%) | 1 (0.6%) | – | 1 (0.4%) | 1 (0.3%) | 6 (0.7%) | – |
| Women | 1 (1.6%) | 1 (1.0%) | – | 1 (0.8%) | 1 (0.6%) | 2 (0.6%) | – | ||
| Men | – | – | – | – | – | 4 (0.8%) | – | ||
| Low-grade dysplasia | All | 21 (14.6%) | 32 (19.3%) | 55 (28.2%) | 88 (33.3%) | 150 (41.0%) | 356 (43.5%) | 421 (47.2%) | |
| Women | 7 (10.9%) | 21 (21.9%) | 30 (30.6%) | 33 (27.3%) | 58 (37.2%) | 122 (37.7%) | 144 (45.4%) | ||
| Men | 14 (17.5%) | 11 (15.7%) | 25 (25.8%) | 55 (38.5%) | 92 (43.8%) | 234 (47.3%) | 277 (48.2%) | ||
| High-grade dysplasia | All | 2 (1.4%) | 2 (1.2%) | 2 (1.0%) | 1 (0.4%) | 5 (1.4%) | 11 (1.3%) | 18 (2.0%) | |
| Women | 1 (1.6%) | 1 (1.0%) | 1 (1.0%) | – | 1 (0.6%) | 5 (1.5%) | 3 (0.9%) | ||
| Men | 1 (1.3%) | 1 (1.4%) | 1 (1.0%) | 1 (0.7%) | 4 (1.9%) | 6 (1.2%) | 15 (2.6%) | ||
| Hyperplastic polyps | All | 23 (16.0%) | 29 (17.5%) | 32 (16.4%) | 44 (16.7%) | 68 (18.6%) | 121 (14.8%) | 112 (12.6%) | |
| Women | 8 (12.5%) | 16 (16.7%) | 12 (12.2%) | 17 (14.0%) | 30 (19.2%) | 51 (15.7%) | 41 (12.9%) | ||
| Men | 15 (18.8%) | 13 (18.6%) | 20 (20.6%) | 27 (18.9%) | 38 (18.1%) | 70 (14.1%) | 71 (12.3%) | ||
| Sessile serrated adenomas | All | 27 (18.8%) | 26 (15.7%) | 21 (10.8%) | 29 (11.0%) | 33 (9.0%) | 62 (7.6%) | 54 (6.1%) | |
| Women | 17 (26.6%) | 14 (14.6%) | 16 (16.3%) | 17 (14.0%) | 20 (12.8%) | 36 (11.1%) | 20 (6.3%) | ||
| Men | 10 (12.5%) | 12 (17.1%) | 5 (5.2%) | 12 (8.4%) | 13 (6.2%) | 26 (5.3%) | 34 (5.9%) | ||
| Inflammatory polyps | All | 6 (4.2%) | 7 (4.2%) | 3 (1.5%) | 3 (1.1%) | – | 4 (0.5%) | 8 (0.9%) | |
| Women | 3 (4.7%) | 2 (2.1%) | 2 (2.0%) | 1 (0.8%) | – | 1 (0.3%) | 1 (0.3%) | ||
| Men | 3 (3.8%) | 5 (7.1%) | 1 (1.0%) | 2 (1.4%) | – | 3 (0.6%) | 7 (1.2%) | ||
| Hamartomatous polyps | All | 2 (1.4%) | 2 (1.2%) | 1 (0.5%) | 1 (0.4%) | 1 (0.3%) | 1 (0.1%) | 1 (0.1%) | |
| Women | 1 (1.6%) | 1 (1.0%) | – | 1 (0.8%) | – | – | – | ||
| Men | 1 (1.3%) | 1 (1.4%) | 1 (1.0%) | – | 1 (0.5%) | 1 (0.2%) | 1 (0.2%) | ||
| Other histological findings | Inflammation, inflammatory bowel disease (Crohn’s, ulcerative colitis), lipoma, spirochaetosis, Melanosis coli and other findings | All | 36 (25.0%) | 36 (21.7%) | 47 (24.1%) | 63 (23.9%) | 63 (17.2%) | 103 (12.6%) | 104 (11.7%) |
| Women | 13 (20.3%) | 20 (20.8%) | 24 (24.5%) | 26 (21.5%) | 21 (13.5%) | 41 (12.7%) | 26 (8.2%) | ||
| Men | 23 (28.8%) | 16 (22.9%) | 23 (23.7%) | 37 (25.9%) | 42 (20.0%) | 62 (12.5%) | 78 (13.6%) | ||
| No abnormal histological findings | All | 11 (7.6%) | 9 (5.4%) | 7 (3.6%) | 16 (6.1%) | 10 (2.7%) | 38 (4.6%) | 40 (4.5%) | |
| Women | 7 (10.9%) | 7 (7.3%) | 4 (4.1%) | 10 (8.3%) | 7 (4.5%) | 17 (5.2%) | 21 (6.6%) | ||
| Men | 4 (5.0%) | 2 (2.9%) | 3 (3.1%) | 6 (4.2%) | 3 (1.4%) | 21 (4.2%) | 19 (3.3%) | ||
| No biopsy | All | 31 (21.5%) | 36 (21.7%) | 49 (25.1%) | 47 (17.8%) | 61 (16.7%) | 172 (21.0%) | 202 (22.6%) | |
| Women | 14 (21.9%) | 20 (20.8%) | 17 (17.3%) | 25 (20.7%) | 27 (17.3%) | 71 (21.9%) | 79 (24.9%) | ||
| Men | 17 (21.3%) | 16 (22.9%) | 32 (33.0%) | 22 (15.4%) | 34 (16.2%) | 101 (20.4%) | 123 (21.4%) | ||
In age group 1, the detection rate of colorectal carcinoma confirmed by histopathological diagnosis was 1/144 (0.7%) colonoscopies for both sexes combined. The single case was diagnosed in a woman; no colorectal carcinoma was diagnosed in men in this group. The detection rates of adenoma with low-grade dysplasia and high-grade dysplasia were 21 (14.6%) and 2 (1.4%), respectively. In men, the detection rates of adenoma with low-grade dysplasia and high-grade dysplasia were 14 (17.5%) and 1 (1.3%), respectively. In women the detection rates of low-grade dysplasia and high-grade dysplasia adenoma were 7 (10.9%) and 1 (1.6%), respectively. The detection rate of “Other histopathological diagnosis” was 36 (25.0%) for both sexes combined. In this age group, colonoscopy was most commonly performed for questions concerning non-infectious gastroenteritis, inflammatory bowel disease and diverticulosis/diverticulitis (48 cases, 33.3%), and as screening/surveillance (51 cases, 35.4%).
In this age group, the colorectal cancer detection rate had increased slightly with 2/166 colonoscopies (1.2%). Both cases were diagnosed in women. The detection rates of adenomas with low-grade dysplasia and high-grade dysplasia were 32 (19.3%) and 2 (1.2%), respectively. In women, 21 cases of adenoma with low-grade dysplasia (21.9%) and 1 case of adenoma with high-grade dysplasia (1.0%) were diagnosed. In men, 11 cases of adenoma with low-grade dysplasia (15.7%) and 1 case of adenoma with high-grade dysplasia (1.4%) were diagnosed. An “Other histopathological diagnosis” was detected in 36 (21.7%) cases. Irrespective of sex, colonoscopy in this age group was most commonly performed as screening/surveillance (87 cases, 52.4%, likely due to the genetic risk syndromes), followed by questions concerning non-infectious gastroenteritis, inflammatory bowel disease and diverticulosis/diverticulitis (44 cases, 26.5%). In 14 cases (8.4%), the colonoscopy was performed following haemorrhage, melena or anaemia.
In both sexes combined, the detection rate of colorectal carcinoma in this age group was 1/195 cases (0.5%). The colorectal carcinoma case was diagnosed in a woman. The detection rates of adenoma with low-grade dysplasia and high-grade dysplasia were 55 (28.2%) and 2 (1.0%), respectively. The diagnosis of hyperplastic polyp remained stable with 32 cases (16.4%). An “Other histopathological diagnosis” was detected in 47 cases (24.1%). Regarding the reason for colonoscopy, the result was similar to the previous age groups: screening/surveillance in 96 cases (49.2%), non-infectious gastroenteritis, inflammatory bowel disease and diverticulosis/diverticulitis were assessed in 44 cases (22.6%) and haemorrhage, melena or anaemia were examined in 16 cases (8.2%).
In this age group, we observed a colorectal cancer detection rate of 2/264 (0.8%) cases. One colorectal cancer case was diagnosed in a man, the other one in a woman. With regard to colorectal cancer precursors, there was a slight increase in adenoma with low-grade dysplasia to 88 cases (33.3%) compared to previous groups. The detection rate of adenoma with high-grade dysplasia was 1 case (0.4%) (figure 2). Similarly, the rate of “Other histopathological diagnosis” was comparable to the previous age groups with 63 cases (23.9%). Regarding the question addressed with the colonoscopy, we observed a similar result as in the previous groups, with screening/surveillance being the most common indication.
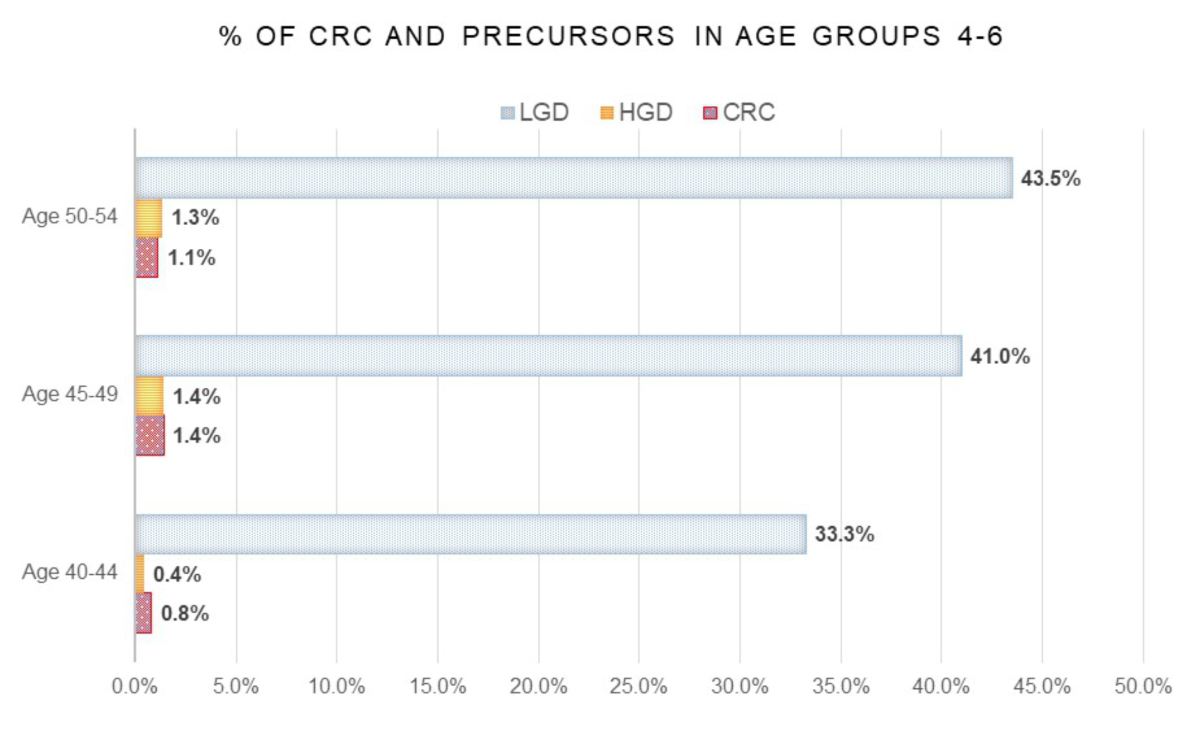
Figure 2Percentage of cases of colorectal cancer (CRC), adenoma with high-grade dysplasia (HGD) and adenoma with low-grade dysplasia (LGD) detected in age groups 4 (40–44 years), 5 (45–49 years) and 6 (50–54 years). The total number of cases in the respective age groups are considered 100%.
In this age group, we observed an increase in the colorectal cancer detection rate to 5/366 colonoscopies (1.4%). A relevant increase was also observed in the detection rate of adenoma with low-grade dysplasia to 150 cases (41.0%). The detection rate of adenoma with high-grade dysplasia was slightly higher with 5 cases (1.4%) (figure 2). In men, the colorectal cancer detection rate increased to 3 cases (1.4%). A similar result was also observed for colorectal cancer precursors in men, where the detection rates of adenoma with low-grade dysplasia and high-grade dysplasia were 92 (43.8%) and 4 cases (1.9%), respectively. In women, we observed a similar trend: the detection rate for colorectal carcinoma was 2 cases (1.3%), for adenoma with low-grade dysplasia 58 cases (37.2%) and for adenoma with high-grade dysplasia 1 case (0.6%) (figure 3). The detection rate of “Other histopathological diagnosis” was 63 cases (17.2%). Regarding the question addressed with the colonoscopy, we did not observe any differences between the sexes with respect to the previous groups.

Figure 3Sex distribution of cases of colorectal cancer (CRC), adenoma with high-grade dysplasia (HGD) and adenoma with low-grade dysplasia (LGD) detected in age groups 4 (40–44 years), 5 (45–49 years) and 6 (50–54 years). The total number of cases in the respective age and sex group are considered 100%.
In this age group, the results were similar to those of the previous group. The detection rate of colorectal carcinoma was 9/819 (1.1%), of adenoma with low-grade dysplasia 356 cases (43.5%) and of adenoma with high-grade dysplasia 11 cases (1.3%) (figure 2). For both sexes combined, the colonoscopy in this age group was most commonly performed as screening/surveillance (546 cases, 66.7%), followed by questions concerning haemorrhage, melena and anaemia (69 cases, 8.4%). In men, the detection rate of colorectal carcinoma was 5 cases (1.0%), while those of adenoma with low-grade dysplasia and high-grade dysplasia were 234 cases (47.3%) and 6 cases (1.2%), respectively. In women, the colorectal cancer detection rate was 4 cases (1.2%). The detection rate of adenoma with low-grade dysplasia and high-grade dysplasia was 122 cases (37.7%) and 5 cases (1.5%), respectively (figure 3). The detection rate of “Other histopathological diagnosis” was clearly lower than in the age group before, with 103 cases (12.6%). For both sexes combined, the colonoscopy in this age group was mostly performed as screening/surveillance (546 cases, 66.7%).
Regardless of sex, we observed a stable colorectal cancer detection rate compared to the previous group, which was 8/892 (0.9%). The detection rates of adenoma with low-grade dysplasia and high-grade dysplasia increased to 421 cases (47.2%) and 18 cases (2.0%), respectively. We did not observe any differences regarding the question addressed with the colonoscopy. In men, the colorectal cancer detection rate was 4 cases (0.7%), while the detection rates of adenoma with low-grade dysplasia and high-grade dysplasia were 277 cases (48.2%) and 15 cases (2.6%), respectively. In women, the colorectal cancer detection rate was 4 cases (1.3%), the detection rate of adenoma with low-grade dysplasia increased to 144 cases (45.4%) and the detection rate of adenoma with high-grade dysplasia decreased to 3 cases (0.9%). The detection rate of “Other histopathological diagnosis” was 104 cases (11.7%). For both sexes combined, the colonoscopy in this age group was mostly performed as screening/surveillance (624 cases, 70%).
The number of detected sessile serrated adenomas decreased from 27/144 (18.8%) in age group 1 (18–29) to 26/166 (15.7%) in age group 2 (30–34) and further to 21/195 (10.8%) in age group 3 (35–39). Thereafter, the detection rates were 29/264 (11.0%) in age group 4, 33/366 (9.0%) in age group 5, 62/819 (7.6%) in age group 6 and 54/892 (6.1%) in age group 7. In women, the detection rate of sessile serrated adenomas was also highest in age group 1 with 26.6% and lowest in age group 7 with 6.3%. In men, sessile serrated adenomas were detected in 12.5% of age group 1 and 5.9% of age group 7.
Of note, particularly in the age groups of 50 years and above, the rates of colonoscopies performed for screening/surveillance were higher in men compared to women. This was opposite in the age groups 35–49 years. However, detection rates of colorectal carcinoma were comparable between males and females within the respective age groups. In particular, colorectal cancer detection rate within age group 5 (45–49) was 1.4% (3/210) in men and 1.3% (2/156) in women. In the age group 50–54, colorectal cancer detection rate was 1.2% in females and 1.0% in males. These findings however were contrasted by data for high-grade and low-grade adenomas. Particularly for low-grade adenomas, the detection rate in men was clearly higher than in females in all three age groups (40–44, 45–49 and 50–54; figure 3).
The majority of colonoscopies performed in age group 1 (18–29 years) were for indications such as non-infectious gastroenteritis (mainly patients with chronic abdominal symptoms and altered bowel habits), inflammatory bowel disease and diverticulosis/diverticulitis (33.3%) as well as for screening and surveillance purposes (35.4%). In contrast, colonoscopies performed in group 6 (50–54 years) were only rarely performed for non-infectious gastroenteritis, inflammatory bowel disease and diverticulosis/diverticulitis (5%). Here, the majority of colonoscopies (66.7%) were performed for screening purposes, which is comparable to the data for age group 7. Colonoscopies indicated by incidental radiological findings were performed more frequently in older age groups.
In our present hypothesis-generating study, we found a comparable, slightly higher detection rate of colorectal carcinoma as well as of pre-malignant adenomas in patients of age group 5 (45–49) compared to those of age group 6 (50–54). This supports the finding that colorectal carcinoma might already occur in a significant number of patients at the age of 45 or at least before the age of 50. In addition, quite a relevant number of precursor lesions with low- and high-grade dysplasia were found. Performing polypectomy of non-malignant polyps has an impact on prognosis and mortality as well. In this regard, our data from a Swiss tertiary centre are well in line with recent data from the US and the Netherlands [5–7].
All those data suggest that people under the age of 50 could benefit from screening colonoscopy. This particularly is due to the fact that early-onset colorectal carcinoma, meaning colorectal cancer diagnosis before the age of 50, accounts for about 10% of all colorectal carcinoma cases [11]. The importance of this early-onset colorectal carcinoma is further highlighted by the observation that not only incidence but also mortality due to colorectal carcinoma increases in this group of patients aged below 50 [12, 13]. Unfortunately, these observations contrast the beneficial effects of colorectal cancer screening in older patients [14].
By analysing the detection rates of colorectal cancer precursor lesions, namely adenoma with low- or high-grade dysplasia, we detected a similar tendency as for colorectal carcinoma, showing an increase of detection rate with increasing age. The peak of the detection rate of adenoma with low-grade dysplasia in our cohort was reached in age group 7 (55–59, 47.2%); however the detection rate had already reached values above 30% and 40.0% in age groups 4 (40–44) and 5 (45–49), respectively. This suggests that screening of younger patients could help detect colorectal cancer precursors and even colorectal carcinoma itself at an early stage, which in turn would allow for preventive/curative therapy and increased life expectancy of affected patients.
In age groups 4 (40–44) and 5 (45–49), the colonoscopy was performed as screening or surveillance in 55.3% and 52.5% of cases, respectively. In 12.1% and in 12.8%, respectively, of patients in these age groups, endoscopy was performed following a rectal haemorrhage/melena. This suggests that young adults usually undergo a colonoscopy in the presence of symptoms, which might be caused by a colorectal carcinoma. This could partly explain why the detection rate of colorectal carcinoma in these groups was higher.
As a single-centre study, our analysis has its limitations. Our retrospective, exploratory analysis is not a population-based screening study and may not be representative of the overall population, since the patients treated at our tertiary hospital certainly comprise a selected patient group and the number of incident cases are limited. Nevertheless the observed trend can be seen in other European and US studies too [5–7]. In addition, the lack of data regarding the history and indication for colonoscopy, as well as of the information about the histopathological result of some of our patients led to the exclusion of these patients and should be considered when evaluating the study results. Patients with a predisposition were not treated separately because the number of patients was limited and the diagnosis was not always clear from the reports. Therefore, such patients were included under the indication screening. However, this could explain a confounding effect especially in the younger patients. Lastly, another limitation of our work is the lack of follow-up in patients diagnosed with adenoma or colorectal carcinoma, thereby precluding predictions of the expected gain in life expectancy. We might state that our analysis was mainly exploratory, with the aim of reporting and comparing the number of colorectal carcinoma and colorectal cancer precursor lesions in the different age groups, without further research on possible risk factors or causes, thus showing a trend that has been observed in previous studies, strengthening the hypothesis that screening at the age of 45 can help in the early detection of colorectal carcinoma and adenoma. One possibility for future studies would be to use randomisation or control groups to draw causal conclusions or to accurately assess the impact of specific measures or factors on colonoscopy outcomes. All these aspects should be included in future analyses to better study the consequences of earlier screening initiation on a population-wide level. Based on data from a US study considering the respective circumstances in the US, it can be anticipated that lowering the screening age to 45 would likely be cost-effective. However, the most efficient impact would likely be to increase participation rates in screening and surveillance programmes particularly for persons at higher age and with high-risk features [15]. The most cost-effective screening method compared to no screening, at least in the population aged 50–75 years, seems to indeed be colonoscopy [16].
Interestingly, the prevalence of colorectal carcinoma is lower in European countries with a long-standing and well-established colorectal cancer screening programme such as the Netherlands or Slovenia. Such countries also feature clearly lower colorectal cancer mortality rates, highlighting the benefits of a colorectal cancer screening programme [17]. Furthermore, screening by colonoscopy seems to be more efficient with respect to colorectal cancer detection rate and reducing colorectal carcinoma incidence than screening by sigmoidoscopy, particularly in women [18–21] and stool-based tests for occult blood [22–25].
In conclusion, the observed slightly higher detection rate of colorectal carcinomas in age group 5 (45–49) compared to age group 6 (50–54) suggests that lowering the screening age to 45 years might help detect an increasing number of patients with colorectal carcinoma or its precursors. Early-stage detection could increase curative therapy and improve life expectancy. This is supported by the recent change in endoscopy practice recommendations for example in the US, where the United States Preventive Services Task Force (USPSTF) published an updated guideline suggesting earlier colon cancer screening from age 45 in May 2021 [10], which was endorsed by all national gastroenterology societies (AGA, ACG and ASGE) [26]. Of note, such screening recommendations should then also be accompanied by improved patient education and promotion of healthier lifestyles [27]. In summary, current screening recommendations starting at age 50 should be critically questioned and the potential benefits of lowering the screening age to 45 should be evaluated.
No financial support to be reported.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. MS has no competing interests regarding the content of this article, but declares the following: MS has shares and is co-founder of Recolony AG, Zurich, Switzerland and has shares in PharmaBiome AG, Zurich, Switzerland, served as Advisor for AbbVie, Gilead, Fresenius, Topadur, Takeda, Roche and Celltrion, received speaker’s honoraria from Falk Pharma, Vifor Pharma, Janssen, Pileje and Bromatech and received research grants from AbbVie, Takeda, Gilead, Gnubiotics, Roche, Axalbion, Pharmabiome, Topadur, Basilea, MBiomics, Storm Therapeutics, LimmaTech, Zealand Pharma, NodThera, Calypso Biotech, Pileje, Herbodee and Vifor. GR has no competing interests regarding the content of this article, but declares the following: GR has shares in, is co-founder of and scientific advisor for PharmaBiome AG, Zurich, Switzerland; GR has consulted to AbbVie, Arena, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, Lilly, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions and Zeller; GR has received speaker’s honoraria from AbbVie, AstraZeneca, BMS, Celgene, FALK, Janssen, MSD, Pfizer, Phadia, Takeda, Tillots, UCB, Vifor and Zeller; GR has received educational grants and research grants from AbbVie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB and Zeller. RF has served as Advisor for Bristol-Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Pierre Fabre, and has received speaker’s fees from BMS, Servier and Pierre Fabre. MT has served as advisor for Takeda and Topadur and has received speaker fees from Janssen, Takeda and Intuitive Surgical. The other authors have no competing interests regarding the content of this article.
1. Dekker E, Tanis PJ, Vleugels JL, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019 Oct;394(10207):1467–80. 10.1016/S0140-6736(19)32319-0
2. Guo F, Chen C, Holleczek B, Schöttker B, Hoffmeister M, Brenner H. Strong Reduction of Colorectal Cancer Incidence and Mortality After Screening Colonoscopy: Prospective Cohort Study From Germany. Am J Gastroenterol. 2021 May;116(5):967–75. 10.14309/ajg.0000000000001146
3. Krebsliga. https://www.krebsliga.ch/ueber-krebs/frueherkennung/darmkrebs/darmkrebs-screening-programm [
4. Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al.; NordICC Study Group. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022 Oct;387(17):1547–56. 10.1056/NEJMoa2208375
5. Sehgal M, Ladabaum U, Mithal A, Singh H, Desai M, Singh G. Colorectal Cancer Incidence After Colonoscopy at Ages 45-49 or 50-54 Years. Gastroenterology. 2021 May;160(6):2018–2028.e13. 10.1053/j.gastro.2021.02.015
6. Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019 Dec;68(12):2179–85. 10.1136/gutjnl-2019-319511
7. Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019 Oct;68(10):1820–6. 10.1136/gutjnl-2018-317592
8. Siegel RL, Miller KD, Jemal A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970-2014. JAMA. 2017 Aug;318(6):572–4. 10.1001/jama.2017.7630
9. Mueller M, Schneider MA, Deplazes B, Cabalzar-Wondberg D, Rickenbacher A, Turina M. Colorectal cancer of the young displays distinct features of aggressive tumor biology: A single-center cohort study. World J Gastrointest Surg. 2021 Feb;13(2):164–75. 10.4240/wjgs.v13.i2.164
10. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al.; US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021 May;325(19):1965–77. 10.1001/jama.2021.6238
11. Zaborowski AM, Abdile A, Adamina M, Aigner F, d’Allens L, Allmer C, et al.; REACCT Collaborative. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021 Sep;156(9):865–74. 10.1001/jamasurg.2021.2380
12. Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med. 2017 Feb;65(2):311–5. 10.1136/jim-2016-000229
13. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020 May;70(3):145–64. 10.3322/caac.21601
14. Araghi M, Soerjomataram I, Bardot A, Ferlay J, Cabasag CJ, Morrison DS, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019 Jul;4(7):511–8. 10.1016/S2468-1253(19)30147-5
15. Ladabaum U, Mannalithara A, Meester RG, Gupta S, Schoen RE. Cost-Effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-Risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology. 2019 Jul;157(1):137–48. 10.1053/j.gastro.2019.03.023
16. Gheysariyeha F, Rahimi F, Tabesh E, Hemami MR, Adibi P, Rezayatmand R. Cost-effectiveness of colorectal cancer screening strategies: A systematic review. Eur J Cancer Care (Engl). 2022 Nov;31(6):e13673. 10.1111/ecc.13673
17. Cardoso R, Guo F, Heisser T, Hackl M, Ihle P, De Schutter H, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021 Jul;22(7):1002–13. 10.1016/S1470-2045(21)00199-6
18. Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, et al.; NORCCAP Study Group†. Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann Intern Med. 2018 Jun;168(11):775–82. 10.7326/M17-1441
19. Ko CW, Doria-Rose VP, Barrett MJ, Kamineni A, Enewold L, Weiss NS. Screening flexible sigmoidoscopy versus colonoscopy for reduction of colorectal cancer mortality. Int J Colorectal Dis. 2019 Jul;34(7):1273–81. 10.1007/s00384-019-03300-7
20. Cheng TI, Wong JM, Hong CF, Cheng SH, Cheng TJ, Shieh MJ, et al. Colorectal cancer screening in asymptomaic adults: comparison of colonoscopy, sigmoidoscopy and fecal occult blood tests. J Formos Med Assoc. 2002 Oct;101(10):685–90.
21. Segnan N, Senore C, Andreoni B, Azzoni A, Bisanti L, Cardelli A, et al.; SCORE3 Working Group-Italy. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007 Jun;132(7):2304–12. 10.1053/j.gastro.2007.03.030
22. Baldacchini F, Bucchi L, Giuliani O, Mancini S, Ravaioli A, Vattiato R, et al.; Emilia-Romagna Region Workgroup for Colorectal Screening Evaluation. Effects of Attendance to an Organized Fecal Immunochemical Test Screening Program on the Risk of Colorectal Cancer: An Observational Cohort Study. Clin Gastroenterol Hepatol. 2022 Oct;20(10):2373–82. 10.1016/j.cgh.2022.01.053
23. Shaukat A, Kaalby L, Baatrup G, Kronborg O, Duval S, Shyne M, et al. Effects of Screening Compliance on Long-term Reductions in All-Cause and Colorectal Cancer Mortality. Clin Gastroenterol Hepatol. 2021 May;19(5):967–975.e2. 10.1016/j.cgh.2020.06.019
24. Buskermolen M, Cenin DR, Helsingen LM, Guyatt G, Vandvik PO, Haug U, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a microsimulation modelling study. BMJ. 2019 Oct;367:l5383. 10.1136/bmj.l5383
25. Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013 Sep;369(12):1106–14. 10.1056/NEJMoa1300720
26. Patel SG, May FP, Anderson JC, Burke CA, Dominitz JA, Gross SA, et al. Updates on Age to Start and Stop Colorectal Cancer Screening: Recommendations From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022 Jan;162(1):285–99. 10.1053/j.gastro.2021.10.007
27. Sinicrope FA. Increasing Incidence of Early-Onset Colorectal Cancer. N Engl J Med. 2022 Apr;386(16):1547–58. 10.1056/NEJMra2200869