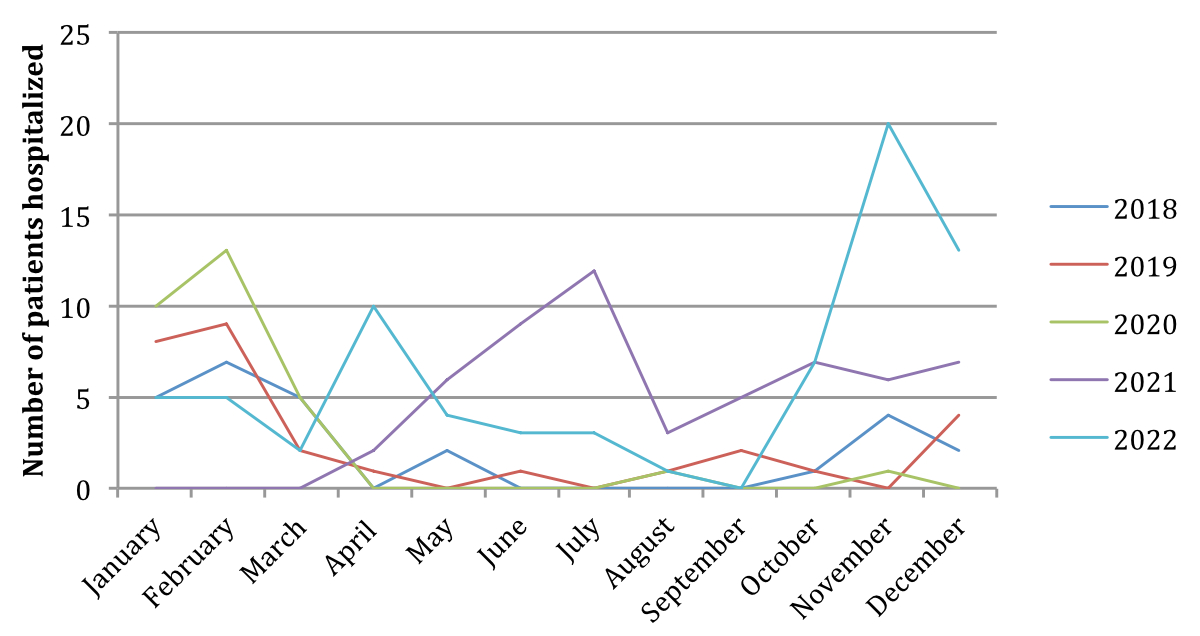
Figure 1Comparison of number of bronchiolitis hospitalisations at Hôpital du Jura according to month and year in 2018–2022.
DOI: https://doi.org/https://doi.org/10.57187/s.3768
Before the COVID-19 pandemic, respiratory syncytial virus (RSV) infections were known to peak in the autumn and winter months in temperate regions and to peak in the rainy seasons in tropical regions [1]. Since the emergence of SARS-CoV-2, a number of non-pharmacological interventions have been implemented in an attempt to slow virus transmission. These implemented measures have had important consequences on the transmission of other respiratory viruses in infants and children, most notably for RSV and influenza [2]. Specifically for bronchiolitis, measures such as lockdowns, school closures, working from home and the use of face masks have shown to be associated with a reduction of outbreaks [3]. In the southern hemisphere, the incidence of RSV and influenza was altered during the anticipated peak period in winter 2020 (June–August), with cases surging in the summer months of 2020 (September–December) [2]. The same pattern was shown in the northern hemisphere, with an increase in RSV activity in the summer months, which is unusual for this time of year. The COVID-19 pandemic outbreak has led to a clearly observable epidemiological change regarding acute bronchiolitis worldwide [4, 5].
In Switzerland, RSV EpiCH, a multicentre database managed from paediatric hospitals across Switzerland, confirmed this unusual epidemiology with an interseasonal surge of RSV infections associated with COVID-19-related non-pharmacological interventions, showing a surge in the summer months of 2020 and 2021 [6]. This database included 20 acute paediatric care hospitals; however Hôpital du Jura (H-JU) did not receive a participation request. H-JU is a regional hospital in Delémont, the capital of the canton of Jura in northwest Switzerland, with a paediatrics department of about 10 inpatient beds and 30–40 paediatric emergency consultations per day.
Given that the data from our hospital was unknown, our aim was to investigate the seasonal shift in bronchiolitis hospitalisations specifically at H-JU in Delémont, Switzerland.
This study is particularly relevant to our daily practice given that H-JU, just like other hospitals in Switzerland and the northern hemisphere, will potentially need to prepare for the possibility of a substantial out-of-season respiratory syncytial virus peak and to consider the implications for hospital management and distribution of healthcare resources, including future vaccination strategies [2, 6].
Retrospective data was collected for children aged up to 2 years admitted to H-JU for bronchiolitis from 1 January 2018 to 31 December 2022. Given that bronchiolitis is defined as a disease that affects children aged up to 2 years, older children were not included in the study.
H-JU’s institutional hospitalisation criteria for bronchiolitis during the study period were: patients with major risk factors (e.g. prematurity, aged below 6 weeks, cardiopathy, …), rapidly progressing respiratory distress, high fever for over 48 hours with suspicion of secondary bacterial infection, agitation or lethargy, apnoea or malaise, dehydration or feeding difficulties (>50% decrease in oral intake), unfavourable social context or living far from the hospital. There were no changes in diagnostic or treatment policies at our hospital during our study period. Patients attending the emergency department with a diagnosis of bronchiolitis but without hospitalisation criteria were not included.
Data was retrieved by sending a request to the Medical Archives and Coding Unit for anonymised data from the electronic medical records of patients hospitalised for bronchiolitis from January 2018 to December 2022. The data included the variables age, duration of hospitalisation and virus detected by polymerase chain reaction (PCR) testing of nasopharyngeal and oropharyngeal swabs.
Data such as disease severity, risk factors and immunisation were not included due to unavailability of anonymous data, but could be potential confounders. Bias was avoided by having data supplied by the Coding Unit and not selected by a clinician involved in the study.
The following ICD10 codes were used for case identification: J21.0, RSV bronchiolitis; J21.1, Metapneumovirus bronchiolitis; J21.8, Bronchiolitis due to other microorganisms with subgroups B97.0 Adenovirus, B97.1 Enterovirus, B97.2 Coronavirus (non-SARS-CoV-2), B97.3 Retrovirus, B97.6 Parvovirus and Bocavirus, and B97.8 Other viruses; J21.9, Bronchiolitis with no virus specified. Rhinovirus was included in the Enterovirus group due to use of laboratory detection methods that do not distinguish between the two. If more than one virus was detected in a patient, this was counted as one bronchiolitis hospitalisation with coinfection and all detected viruses were counted (figure 3).
A total of 215 patients were included in the analysis. No ethical consent was required as only anonymous data was processed.
As part of our commitment to Open Science, anonymised data can be requested from the corresponding author. All data is displayed in the graphs and tables of the article.
Descriptive statistics were calculated to summarise the distribution of key variables, using means for continuous variables and percentages for categorical variables. Measures of variability such as kurtosis and skewness were interpreted for the different datasets.
This study used Microsoft Excel version 14.5.9 and RStudio for data management and descriptive statistics.
A general increase in bronchiolitis hospitalisations was observed in 2021, more than double than in 2018 (table 1). There was a mean of 2.1 hospitalisations per month in 2018 and 4.8 hospitalisations per month in 2021. This shows that there was not only a seasonal shift after the COVID-19 pandemic, but also possibly more patients infected and more severe infections leading to hospitalisation. The number of total hospitalisations and RSV hospitalisations continued to increase in 2022, with a mean of 6.1 hospitalisations per month.
Table 1Yearly percentage of respiratory syncytial virus (RSV) bronchiolitis hospitalisations.
| Year | Bronchiolitis hospitalisations with RSV detected | Total number of bronchiolitis hospitalisations | Percentage of hospitalisations with RSV detected |
| 2018 | 15 | 26 | 58% |
| 2019 | 13 | 29 | 45% |
| 2020 | 17 | 30 | 57% |
| 2021 | 37 | 57 | 65% |
| 2022 | 41 | 73 | 56% |
A clear shift in the peak of bronchiolitis is seen in 2021 compared to the three previous years (figure 1, table S1). In 2021, the start of hospitalisations began in the month of April and peaked in July, opposite to the previous years in which the peak was in the month of February. In late 2021, hospitalisations were lower than the summer months of 2021 but higher than the winter months of the previous years. The hospitalisation rates remained similar at the start of 2022. In April 2022, an increase in hospitalisation rates was observed, followed by a decrease in hospitalisation rates during the summer months, mimicking the trend of pre-pandemic years. Considering the summer months June, July and August, the mean number of hospitalisations in summer 2021 peaked at 8 per month, and decreased to 2.3 per month in 2022, trending towards what was seen in the pre-pandemic years (0 per month in summer 2018 and 0.6 per month in summer 2019). From October 2022, the hospitalisation rates markedly increased with a peak in November. Considering the winter months November to February, the mean number of hospitalisations was as high as 10.7 per month in winter 2022, compared to 3.2 per month in winter 2021.

Figure 1Comparison of number of bronchiolitis hospitalisations at Hôpital du Jura according to month and year in 2018–2022.
For RSV specifically, a similar pattern was observed, with a peak in the summer months of 2021 and a shift of the peak towards the final months of the year in 2022 (figure 2, table S2). The trend observed for 2020 and 2021 is similar to what has been shown in the Swiss national reporting system (RSV Epi-CH) [6].
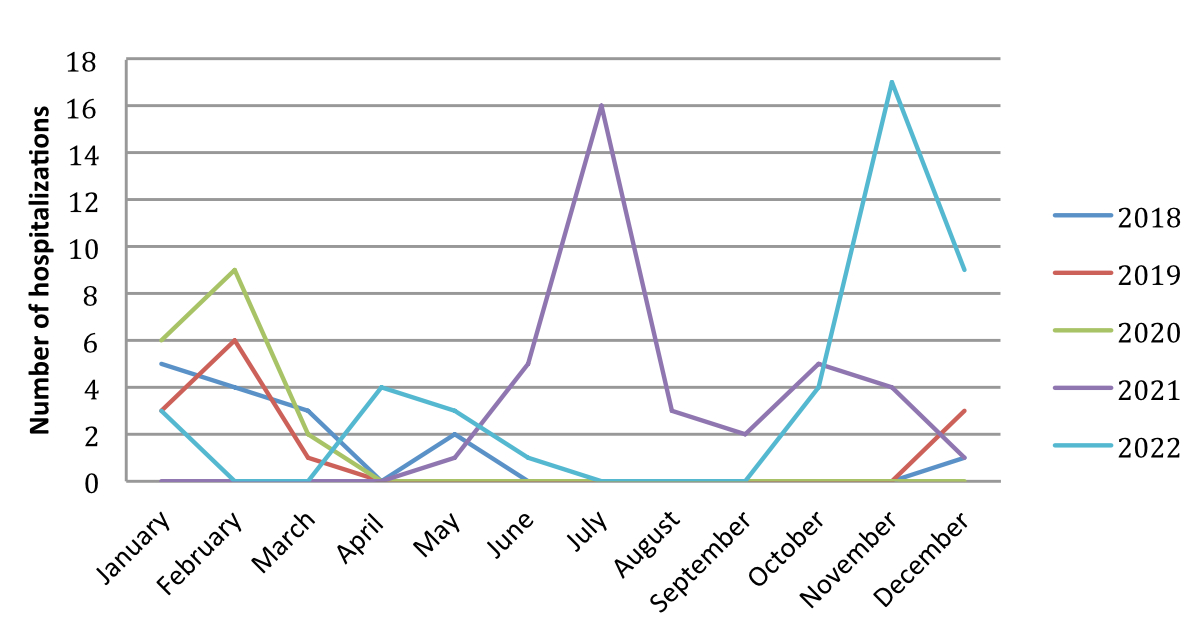
Figure 2Comparison of number of respiratory syncytial virus bronchiolitis hospitalisations in 2018–2022.
The bronchiolitis hospitalisations were coded according to the virus detected: Respiratory syncytial virus, Human metapneumovirus, Rhino/Enterovirus, Coronavirus (non-SARS-CoV-2), Adenovirus and Parvovirus/Bocavirus. Those in which a different virus was detected were classified as “Other viruses” and those in which there was no virus detected were classified as “No virus specified”. Throughout the five years included in the study, RSV was the most frequently detected virus, with a mean of 24.6 bronchiolitis hospitalisations per year. Coronavirus (non-SARS-CoV-2) was the least frequently detected virus, with a mean of 1 bronchiolitis hospitalisation per year (figure 3, table S3).
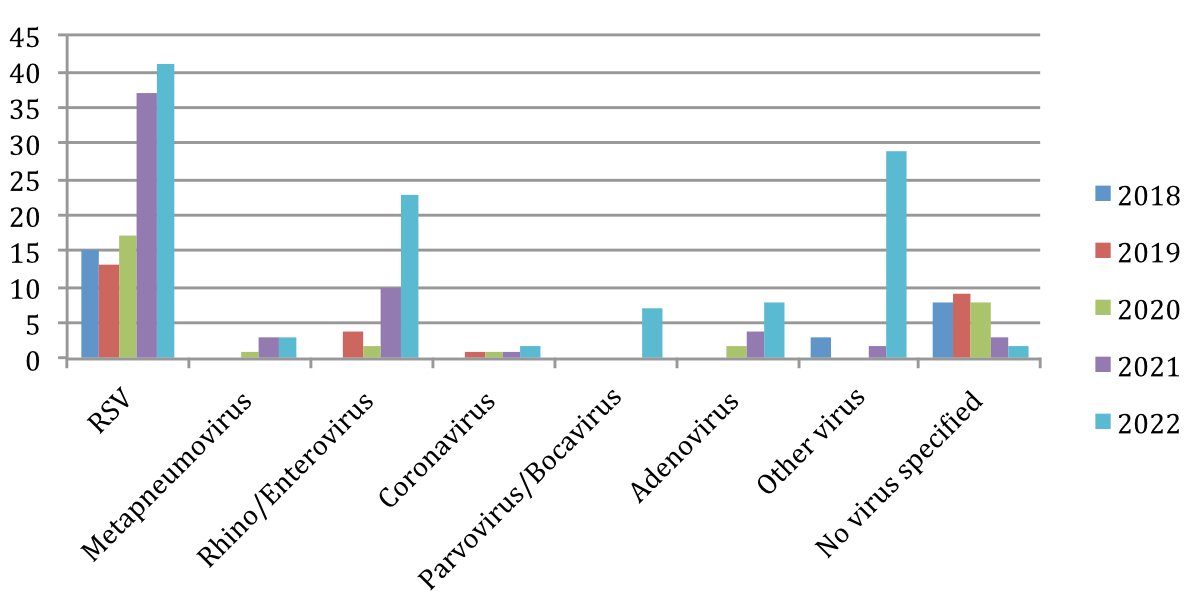
Figure 3Comparison of number of bronchiolitis hospitalisations in 2018–2022 according to virus detected (including coinfections). RSV: respiratory syncytial virus.
Missing data includes disease severity level, immunisation status and comorbidities. Including this data would have entailed reading patient records, which would not be consistent with our anonymisation policy.
The SARS-CoV-2 outbreak has led to an epidemiological change regarding acute bronchiolitis.
As hypothesised, we have seen a clear shift in the peak of bronchiolitis in 2021 compared to the three previous years. This change can likely be attributed to the non-pharmacological measures implemented due to the COVID-19 pandemic in the winter months, and the consequent relaxation of measures in spring and summer months.
During the winter months of 2020, there were little to no hospitalisations, corresponding to the period in which the non-pharmacological measures were strongest, as has been shown in other countries [7].
Regarding the increase in number of hospitalisations in 2021, one possible explanation is the continuous non-pharmacological measures in 2020 leading to a more susceptible immune system after the lifting of these measures in 2021.
Seemingly, the opening of child day-care centres did not have an impact in the epidemic shift of bronchiolitis hospitalisations. Complete opening of day-care centres in the canton of Jura occurred on 11 May 2020 [8]. However we did not see a summer peak after the opening of day-care centres in 2020. In 2021, the day-care centres remained open all year, and no peak was seen until the summer months. This illustrates the relevance of older children and especially of the adult population as important factors in promoting the RSV epidemic.
In 2021, a sharp drop in the peak was seen in the month of August, with an increase in the month of October, which could possibly be explained by the majority of patients being on holiday during the month of August, corresponding to the school holiday period. However, due to the small sample size this drop is not easily explained.
In 2022, the trends observed correspond to a “backwards shift” of what is observed in 2021, with a spring peak in April 2022 as opposed to a summer peak. In October and November 2022, a high peak of hospitalisations is observed, demonstrating the reappearance of a winter peak, but earlier in the winter (October–December) compared to pre-pandemic years (January–March). This shows that the non-pharmacological interventions implemented during 2020 and early 2021 did not cause a long-lasting seasonal shift of bronchiolitis. In 2022, when the non-pharmacological interventions were no longer in place in the non-hospital setting, the peak of bronchiolitis hospitalisations is once again in the winter months. The immunisation of the population after the summer 2021 peak could also play a role in the shift of the peak back to the winter months.
For hospitalisations according to virus detected, no specific pattern was observed. Before 2021, more patients were classified as “No virus specified” because the respiratory panel started to be widely used in 2021.There were no hospitalisations due to SARS-CoV-2 and only a few non-SARS-CoV-2 coronavirus hospitalisations. These findings support what has been shown in other studies, such as the one by Andina-Martinez et al. [9] that demonstrated that SARS-CoV-2 does not cause either frequent or severe bronchiolitis.
This study has several limitations including the small sample size and population, with data from only one regional hospital. Many variables such as comorbidities, vaccination status and disease severity including need for oxygen therapy or need for nasogastric tube feeding were not analysed, and could help us determine if the bronchiolitis infections were more or less severe before and after the COVID-19 pandemic. The data on oxygen therapy was not reliable due to a lack of precision on the type of oxygen therapy (high-flow nasal cannula or standard oxygen nasal cannula) and the exact duration of oxygen therapy. Bronchiolitis phenotyping could also provide interesting data for prognosis and future treatment opportunities. Data regarding non-bronchiolitis RSV admissions in older children were not analysed but could have been useful in order to compare the epidemiology of other paediatric respiratory diseases before vs after the COVID-19 pandemic.
However, although the sample size was small and thus could entail significant variability, the data corresponds to what has been shown by other studies such as RSV Epi-CH [6] with much larger sample sizes, indicating that our findings could be extrapolated to a larger region and thus are likely not due to chance.
With the results shown in this study, we can conclude that the summer peak observed in 2021 was only transient and will not cause a permanent shift in the seasonal epidemiology of bronchiolitis. We predict that the hospitalisation patterns will gradually revert to what was seen in pre-pandemic years, with the hospitalisation peak shifting to the months of January and February in the coming years.
The findings of this study shed light on the potential consequences of a delayed RSV season in the context of a large RSV-naïve population. By understanding the impact of this scenario, we can better prepare to address the associated healthcare challenges. However, it is important to note that this research only scratches the surface of this complex issue. Future studies could delve deeper into the specific mechanisms underlying the impact of a delayed RSV season. By continuing to investigate this area, we can develop more comprehensive and effective measures to safeguard public health. This paves the way for an exciting avenue of research that holds great promise for improving our ability to manage RSV outbreaks in the future.
Christelle Beuchat, Medical Archives and Coding Unit, Hôpital du Jura, Hôpital du Jura Department of Pediatrics
Author contributions: Clara Hayes Vidal-Quadras and Isshak Mrabet Deraoui contributed equally to the data analysis, writing and revision of the manuscript. Vincent Muehlethaler supervised the study and critically reviewed the manuscript. All authors approved the final manuscript as submitted.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Chadha M, Hirve S, Bancej C, Barr I, Baumeister E, Caetano B, et al.; WHO RSV Surveillance Group. Human respiratory syncytial virus and influenza seasonality patterns-Early findings from the WHO global respiratory syncytial virus surveillance. Influenza Other Respir Viruses. 2020 Nov;14(6):638–46. 10.1111/irv.12726
2. Williams TC, Sinha I, Barr IG, Zambon M. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID-19 pandemic. Euro Surveill. 2021 Jul;26(29):2100186. 10.2807/1560-7917.ES.2021.26.29.2100186
3. Lenglart L, Ouldali N, Honeyford K, Bognar Z, Bressan S, Buonsenso D, et al.; EPISODES Study Group. Respective roles of non-pharmaceutical interventions in bronchiolitis outbreaks: an interrupted time-series analysis based on a multinational surveillance system. Eur Respir J. 2023 Feb;61(2):2201172. 10.1183/13993003.01172-2022
4. Van Brusselen D, De Troeyer K, Ter Haar E, Vander Auwera A, Poschet K, Van Nuijs S, et al. Bronchiolitis in COVID-19 times: a nearly absent disease? Eur J Pediatr. 2021 Jun;180(6):1969–73. 10.1007/s00431-021-03968-6
5. Britton PN, Hu N, Saravanos G, Shrapnel J, Davis J, Snelling T, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health. 2020 Nov;4(11):e42–3. 10.1016/S2352-4642(20)30307-2
6. von Hammerstein AL, Aebi C, Barbey F, Berger C, Buettcher M, Casaulta C, et al. Interseasonal RSV infections in Switzerland - rapid establishment of a clinician-led national reporting system (RSV EpiCH). Swiss Med Wkly. 2021 Sep;151(3536):w30057. 10.4414/SMW.2021.w30057
7. Flores-Pérez P, Gerig N, Cabrera-López MI, de Unzueta-Roch JL, Del Rosal T, Calvo C; COVID-19 Study Group in Children. Acute bronchiolitis during the COVID-19 pandemic. Enferm Infecc Microbiol Clin (Engl Ed). 2022 Dec;40(10):572-5. 10.1016/j.eimce.2021.06.005
8. Info Covid-19 Canton du Jura [Internet]. Coronavirus -Communiqués de presse du Canton. [cited Mar 03 2023]. Available from:https://www.jura.ch/fr/Autorites/Coronavirus/Infos-Actualites/Communiques-de-presse-JU.html
9. Andina-Martinez D, Alonso-Cadenas JA, Cobos-Carrascosa E, Bodegas I, Oltra-Benavent M, Plazaola A, et al.; EPICO-AEP Working Group. SARS-CoV-2 acute bronchiolitis in hospitalized children: neither frequent nor more severe. Pediatr Pulmonol. 2022 Jan;57(1):57–65. 10.1002/ppul.25731
Table S1Bronchiolitis hospitalisations at Hôpital du Jura according to month of year.
| 2018 | 2019 | 2020 | 2021 | 2022 | |
| January | 5 | 8 | 10 | 0 | 5 |
| February | 7 | 9 | 13 | 0 | 5 |
| March | 5 | 2 | 5 | 0 | 2 |
| April | 0 | 1 | 0 | 2 | 10 |
| May | 2 | 0 | 0 | 6 | 4 |
| June | 0 | 1 | 0 | 9 | 3 |
| July | 0 | 0 | 0 | 12 | 3 |
| August | 0 | 1 | 1 | 3 | 1 |
| September | 0 | 2 | 0 | 5 | 0 |
| October | 1 | 1 | 0 | 7 | 7 |
| November | 4 | 0 | 1 | 6 | 20 |
| December | 2 | 4 | 0 | 7 | 13 |
Table S2Bronchiolitis hospitalisations with respiratory syncytial virus detected.
| 2018 | 2019 | 2020 | 2021 | 2022 | |
| January | 5 | 3 | 6 | 0 | 3 |
| February | 4 | 6 | 9 | 0 | 0 |
| March | 3 | 1 | 2 | 0 | 0 |
| April | 0 | 0 | 0 | 0 | 4 |
| May | 2 | 0 | 0 | 1 | 3 |
| June | 0 | 0 | 0 | 5 | 1 |
| July | 0 | 0 | 0 | 16 | 0 |
| August | 0 | 0 | 0 | 3 | 0 |
| September | 0 | 0 | 0 | 2 | 0 |
| October | 0 | 0 | 0 | 5 | 4 |
| November | 0 | 0 | 0 | 4 | 17 |
| December | 1 | 3 | 0 | 1 | 9 |
Table S3Bronchiolitis hospitalisations according to virus detected.
| 2018 | 2019 | 2020 | 2021 | 2022 | |
| Respiratory syncytial virus | 15 | 13 | 17 | 37 | 41 |
| Metapneumovirus | 0 | 0 | 1 | 3 | 3 |
| Rhino/Enterovirus | 0 | 4 | 2 | 10 | 23 |
| Coronavirus (non-SARS-CoV-2) | 0 | 1 | 1 | 1 | 2 |
| Parvovirus/Bocavirus | 0 | 0 | 0 | 0 | 7 |
| Adenovirus | 0 | 0 | 2 | 4 | 8 |
| Other virus | 3 | 0 | 0 | 2 | 29 |
| No virus specified | 8 | 9 | 8 | 3 | 2 |