Implementation of evidence-based clinical nutrition: Usability of the new digital
platform clinicalnutrition.science
DOI: https://doi.org/https://doi.org/10.57187/s.3764
Valentina V. Huwilerab,
Pascal Triboletcde,
Caroline Rimensbergerf,
Christine Rotenf,
Katja A. Schönenbergerab,
Stefan Mühlebachb,
Philipp Schuetzcg,
Zeno Stangaa
a Department of Diabetes, Endocrinology, Nutritional
Medicine and Metabolism, Inselspital, Bern University Hospital, University of Bern,
Bern, Switzerland
b Division of Clinical Pharmacy and Epidemiology, Department
of Pharmaceutical Sciences, University of Basel, Basel, Switzerland
c University Department of Medicine, Division of General
Internal and Emergency Medicine, Kantonsspital Aarau, Aarau, Switzerland
d Department of Health Professions, Bern University of
Applied Sciences, Bern, Switzerland
e Department of Nutritional Sciences and Research Platform
Active Ageing, University of Vienna, Vienna, Austria
f Department of General Internal Medicine, Inselspital,
Bern University Hospital, University of Bern, Bern, Switzerland
g Faculty of Medicine, University of Basel, Basel, Switzerland
Summary
AIM OF THE STUDY: Malnutrition is a common and
complex challenge in inpatient and outpatient settings, associated with increased
risk of morbidity and mortality. Its management is often neglected, despite strong
evidence of the benefits of adequate nutritional therapy. We introduced clinicalnutrition.science
(https://clinicalnutrition.science/en/), a digital platform that provides healthcare
professionals with easy online access to evidence and streamlines the nutritional
care process. The aim of this study was to assess the usability and to validate
improvements in nutritional management when the digital platform is used by healthcare
professionals.
METHODS: The usability study, conducted from
28 September to 16 November 2023, involved 56 healthcare professionals from the
University Hospital of Bern and the Cantonal Hospital of Aarau. In an adapted cross-over
study design, participants completed key steps of nutritional management for a simulated
hepatology and oncology case both with and without the clinicalnutrition.science
platform. Usability was assessed using the validated Healthcare Systems Usability
Scale questionnaire, supplemented by collection of demographic data. Subgroup analysis
was performed for recommended protein and energy intakes by different professional
representatives.
RESULTS: Clinicalnutrition.science achieved
a good overall usability score of 71.8%. Use of the platform significantly improved
the protein intake recommendation (p = 0.03; median 96.5 and 80.0 g/d) and the basal
metabolic rate estimate (p <0.01; median 1420.8 and 1755.5 kcal/d) of the simulated
oncology case. The variance in protein and energy intake recommendations, basal
metabolic rate estimation and energy deficit estimation was reduced by using the
digital platform. These improvements were achieved without increasing the time required
to complete key steps in nutritional management for the two patient cases (median
between 10.5 and 15.0 minutes; p = 0.09 and p = 0.67) and without prior training
on the platform. There was no effect on the malnutrition detection rate, the selection
of an appropriate nutritional product or the identification of the most appropriate
guideline.
CONCLUSIONS: The use of clinicalnutrition.science
improved evidence-based clinical practice in prescribing personalised nutritional
therapy and increased the accuracy of both protein and energy intake recommendations,
without increasing the time taken to complete key steps in the nutritional management
process.
Abbreviations
- EFFORT:
-
Effect
of early nutritional support on Frailty, Functional Outcomes, and Recovery of malnourished
medical inpatients Trial
- ESPEN:
-
European Society of Clinical Nutrition and Metabolism
- HSUS:
-
Healthcare Systems Usability Scale
Introduction
Around a third of hospitalised patients are
malnourished or at high risk of malnutrition when admitted to hospital [1]. In Switzerland,
this corresponds to approximately
300,000 inpatients per year [2]. An estimated
20% of outpatients are at increased risk of malnutrition [3, 4]. Disease-related malnutrition
is triggered
by the underlying disease and can result from both inadequate nutrient intake and
the systemic inflammatory response [5]. Malnutrition
is strongly associated with an increased risk of adverse clinical outcomes. These
include increased morbidity and mortality, functional decline and prolonged hospitalisation
[1]. The two large randomised controlled trials
“Effect of early nutritional support on Frailty, Functional Outcomes, and Recovery
of malnourished medical inpatients Trial (EFFORT)” and “Nutrition effect On Unplanned
ReadmIssions and Survival in Hospitalised patients (NOURISH)” showed that individualised
nutritional intervention can improve outcomes, including reducing the risk of mortality
(number needed to treat [NNT]: 37 and 20, respectively), morbidity and non-elective
hospital readmission [6, 7]. Evidence-based
nutritional management includes nutritional risk screening, nutritional assessment,
nutritional plan and intervention, as well as nutritional monitoring and re-evaluation
[5]. The European Society of Clinical Nutrition
and Metabolism (ESPEN) and other international and national societies have published
numerous guidelines with the objective of facilitating the implementation of evidence-based
nutritional management in daily clinical practice [8–10].
Clinical nutrition is frequently overlooked
in practice due to a lack of education on the subject and inappropriate prioritisation
under time and resource pressures. Timely, straightforward and reliable access to
relevant information can assist healthcare professionals in integrating clinical
nutrition into multimodal patient care in an optimal and appropriate manner. Such
an approach can facilitate more effective performance of tasks, thereby contributing
to the long-term assurance of quality and safety in patient care. We have developed
clinicalnutrition.science (https://clinicalnutrition.science/en/) to provide evidence-based
information for nutritional management. This platform is independent, freely available
and it can be accessed remotely at the bedside. The platform consists of six tools:
NutriScreen, providing validated nutritional risk screening tools; NutriRisk, estimating
the reduction in risk of complications and short- or long-term mortality if a nutritional
intervention is initiated; NutriCalc, calculating nutritional goals based on established
equations; NutriGo, providing interactive nutritional advice for specific situations
based on current guidelines; NutriPro, a comprehensive database of nutritional products
available in Switzerland; and NutriBib, a synthesis of the most evidence-based literature
in the field of clinical nutrition.
The aim of this project was to assess the usability
of the newly developed digital platform, clinicalnutrition.science, and to evaluate
its impact on key steps in the nutritional management process carried out by healthcare
professionals. The main outcomes of the present study were an enhancement in patient
safety, as evidenced by the identification of malnutrition and subsequent recommendation
of adequate protein and energy intake, and more accurate prescription of appropriate
nutritional products. Additionally, the study also aimed to show an improvement
in decision effectiveness, workflow integration and work efficiency.
Materials and methods
The usability study comprised three distinct
parts: (1) collection of demographic data from participants, (2) performance of
pivotal steps within nutritional management for two patient cases, one with and
one without the clinicalnutrition.science digital platform, and (3) the provision
of feedback on the usability of the platform.
Questionnaires, case vignettes and respective outcomes
1. Demographic data was collected using the
Healthcare Systems Usability Scale (HSUS) demographic questionnaire, including age,
sex, clinical experience, position, activity, place of work and use of other digital
platforms [11]. We added a question about
interest in the field of clinical nutrition and questions about current use of clinicalnutrition.science.
2. Two cases were selected as prototypes of
commonly encountered conditions/types of patients with malnutrition: one case of
a patient with liver cirrhosis (hepatology) and one case of a patient with a malignant
tumour (oncology). These cases were chosen because of their high prevalence in clinical
practice and the availability of corresponding European Society of Clinical
Nutrition and Metabolism guidelines [9, 10, 12].
The two cases were developed on the basis of two real adult cases from our clinic
and were presented in a similar format with comparable anonymised information. A
series of questions were posed to the participating healthcare professionals regarding
the simulated individualised nutritional management care of the two patients and
the rationale behind their decision:
- Presence of risk of malnutrition
- Recommendation for energy and protein
intake
- Estimation of basal metabolic rate
and energy deficit
- The selection of the optimal route
of nutrition, i.e. oral, enteral, parenteral or a combination thereof
- A list of the main objectives of
nutritional therapy
- A description of the indicated nutrition
therapy measures
- Decision of whether nutritional
products are indicated
- The identification of an appropriate
nutritional product, i.e. foods for special medical purposes or parenteral nutritional
products
- Determination of an appropriate
clinical nutrition guideline.
3. The validated HSUS questionnaire was used
to assess the usability of clinicalnutrition.science in a clinical context. The
HSUS questionnaire is based on four different categories: patient safety and decision
effectiveness, workflow integration, work effectiveness and user control. It contains
a total of 22 previously published items, which are rated on a 7-point Likert scale.
The authors of the HSUS questionnaire interpreted the overall usability score as
follows:
- 20% to <50% : “critical need
to address the system’s usability issues”
- 50% to <70% : “a need to address
the system’s usability concerns, some of which may be major”
- 70% to <90% : “a good usability
score with the potential to improve”
- 90% to 100% : “an excellent and
easy to use system” [11].
The final questionnaires were pre-tested by
a dietitian and two physicians with a medical, nutritional or educational background.
All questionnaires were completed using Google Forms [13]. Full questionnaires are provided in the appendix.
All outcomes were considered as main outcomes due to their equal relevance to improving
nutritional management.
Study design and population
Healthcare professionals were recruited by email
and personal invitation from the department of general internal medicine of two
teaching hospitals: the University Hospital of Bern (cohort A) and the Cantonal
Hospital of Aarau (cohort B). In order to be eligible for the study, participants
had to belong to one of the future user groups of healthcare professionals, including
physicians, dietitians, nurses, pharmacists and scientists, and be willing to participate
in the study. There were no limitations placed on level of clinical experience,
previous use of clinicalnutrition.science or the time taken to complete the questionnaires.
The study was conducted at the two aforementioned
centres on 28 September 2023 and 16 November 2023, respectively. Participants completed
the questionnaire form anonymously in a supervised room in order to prevent the
exchange of results. Participants were first asked to complete a background questionnaire.
Secondly, the patient case questionnaires were completed in an adapted cross-over
design.
In the initial round, cohort A responded to
the oncology case and cohort B answered the hepatology case using only the resources
normally employed in clinical practice (e.g. internet, hospital internal sheets),
excluding the platform clinicalnutrition.science. In the second round, cohort A
responded to the hepatology case, while cohort B answered the oncology case using
clinicalnutrition.science (figure 1). The
participants were unaware of the patient’s underlying conditions, yet they were
required to interpret them from the patient case. Thirdly, participants completed
the validated HSUS questionnaire. The local ethics committee decided that no ethical
approval was required for this usability study (BASEC Req-2023-01186).
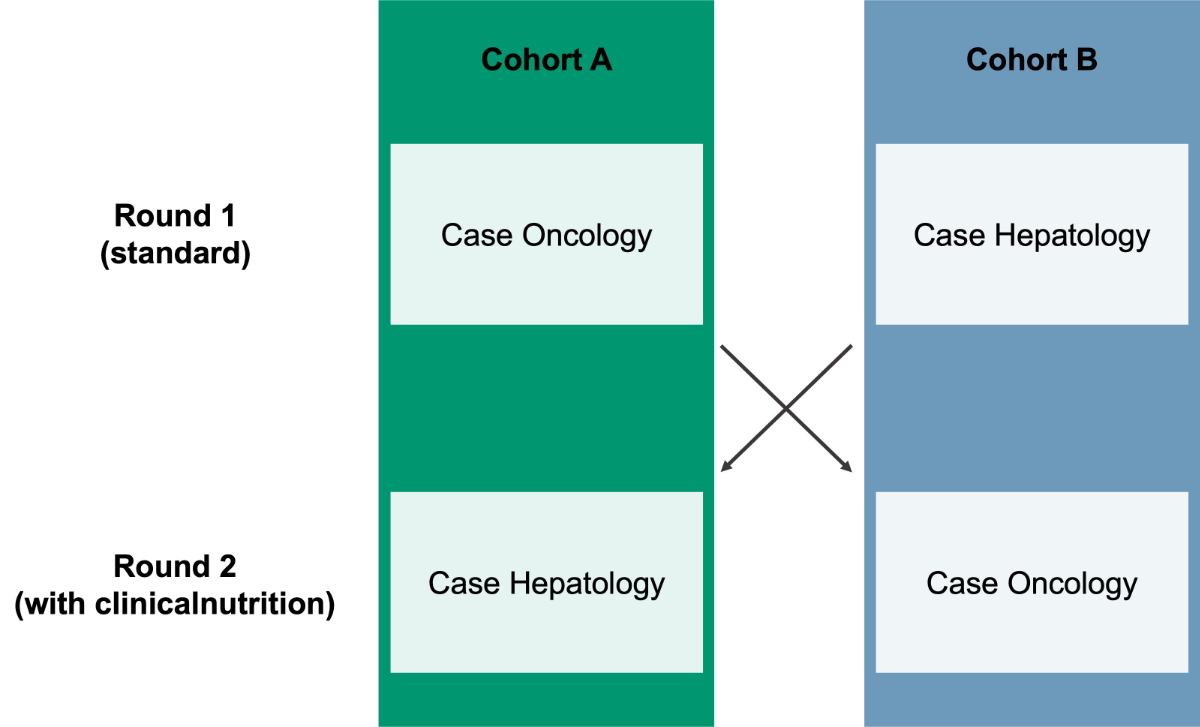
Figure 1Adapted cross-over design of the usability study with cohort A and B. In round
1, cohort A responded to the oncology case and cohort B to the hepatology case
using standard resources. In round 2, the cohorts switched cases and responded
using clinicalnutrition.science.
Statistical analysis
We performed all analyses in R version 4.3.0
(R Core Team, Austria) [14]. Visualisations
were created using the ggplot package [15]
and statistical analysis was performed using nortest [16], lme4 [17] and car packages
[18]. To allow for a self-paired
comparison between groups, only participants who completed both case vignettes were
included in the analysis. Where ranges were recommended (e.g. 1.2–1.5 g protein),
the mean of the ranges was calculated for both the ESPEN guideline recommendations
and the participants’ responses and used for analysis. Results are presented as
median and interquartile range (IQR) with quartile 1 (Q1) and quartile 3 (Q3) or
as absolute numbers, unless stated differently. No protocol was published prior
to analysis.
An unpaired Wilcoxon rank-sum test was used
to test whether the medians of the two cohorts were significantly different to account
for unequal sample sizes and non-normal distribution of the data. For categorical
outcomes, we used Fisher’s exact test (appropriate for small sample sizes) to test
whether there was a statistically significant association between the two cohorts
[19]. Statistical significance was defined
as a p <0.05. We performed a descriptive analysis to assess the difference in
variance of the results obtained with and without the use of the digital platform,
as highly divergent results and outliers should be avoided in clinical practice.
These results should be avoided in clinical practice due to the adverse effects
associated with inadequate protein and energy intake [9, 10].
For the subgroup analysis by profession, the
deviation of the participants’ recommended protein and energy intakes from the protein
and energy intakes recommended in the guidelines was calculated (i.e. recommendation
of participants – recommendation of guidelines: Oncology: Protein: 1.2–1.5 g per
kg body weight per day = 85–107 g per day [mean 96 g]; Energy: 25–30 kcal per kg
body weight per day = 1775–2130 kcal per day [mean 1952.5 kcal]; Hepatology:
Protein: 1.5 g per kg body weight per day = 105 g per day; Energy: 30–35 kcal per
kg body weight per day = 2100–2450 kcal per day [mean 2275 kcal]) [9, 10]. This allowed
for the combination of both
cases and thus increased power. All items of the HSUS questionnaire were weighted
equally to calculate the overall usability score. The overall score and the score
of each item or category were calculated as: (sum of achieved points / sum of possible
points) × 100 [%]. The responses “Not applicable” and “Statement not clear” were
excluded from the score calculation.
Results
In total, 106 healthcare professionals from
the University Hospital of Bern and 85 healthcare professionals from the Cantonal
Hospital of Aarau were contacted. Ultimately, 56 healthcare professionals from the
University Hospital of Bern (cohort A, n = 38) and from the Cantonal Hospital of
Aarau (cohort B, n = 18) completed their questionnaires, which were analysed. The
majority of included healthcare professionals were physicians (68%), followed by
dietitians (21%), clinical nutrition scientists (7%) and nurses (4%). Overall, 73%
of the participants had accumulated over one year of clinical experience, while
86% expressed a moderate or high level of interest in clinical nutrition (table
1). Three participants were unable to complete
the second case questionnaire due to clinical commitments and were therefore excluded
from all subsequent analyses.
Table 1Background information on the two cohorts included in the study.
|
Cohort A |
Cohort B |
| n (%) |
n (%) |
| Place |
|
University
Hospital Bern |
Cantonal
Hospital Aarau |
| Number of
participants |
|
38 (68%) |
18 (32%) |
| Sex |
M / F |
14 / 24 (25% /
43%) |
4 / 14 (7% /
25%) |
| Age (years) |
16–25 |
5 (9%) |
3 (5%) |
| 26–35 |
27 (48%) |
8 (14%) |
| 36–45 |
4 (7%) |
4 (7%) |
| 46–55 |
2 (4%) |
3 (5%) |
| Clinical experience |
None |
1 (2%) |
2 (4%) |
| Less than 3
months |
6 (11%) |
0 (0%) |
| 3 months to 1
year |
2 (4%) |
4 (7%) |
| 1 to 5 years |
14 (25%) |
6 (11%) |
| 5 to 10 years |
8 (14%) |
1 (2%) |
| More than 10
years |
7 (13%) |
5 (9%) |
| Personal interest
in clinical nutrition |
No interest |
1 (2%) |
0 (0%) |
| Small |
5 (9%) |
2 (4%) |
| Moderate |
23 (41%) |
4 (7%) |
| High |
9 (16%) |
12 (21%) |
| Current position |
Dietitian |
3 (5%) |
9 (16%) |
| Nurse |
2 (4%) |
0 (0%) |
| Physician |
33 (59%) |
5 (9%) |
| Scientist |
0 (0%) |
4 (7%) |
Case study
Recommended protein and energy intake
Table 2
summarises the results of the cases. The use of clinicalnutrition.science enabled
55 participants (98%) to identify patient’s malnutrition, whereas without its use,
54 participants (96%) were able to do so. The median recommended protein intake
was significantly different for the oncology case (p = 0.03; 96.5 and 80.0 g/d),
but was comparable for the hepatology case with and without use of the platform
(p = 0.76; both 94.5 g/d; figure 2). The median
recommended energy intake was similar with and without use of the platform for the
oncology case (p = 0.07, 1991.5 and 2139.0 kcal/d) and the hepatology case (p =
0.63; 2000.0 and 2100.0 kcal/d; figure 2).
Table 2Responses of the case vignette questionnaire completed by cohort A and B with
and without the clinicalnutrition.science digital platform. Bold p-values
indicate statistical significance (<0.05). Significance of differences in
medians was assessed using the Wilcoxon test for numerical outcomes and Fisher’s
exact test for categorical outcomes. Significance of differences in variance
was assessed using the F test for numerical outcomes.
|
Use of clinicalnutrition |
Without use of clinicalnutrition |
p-value median |
| Oncology case |
|
n = 18 |
n = 38 |
|
| Rate of detection of malnutrition (%)] |
17/18 (94%) |
37/38 (97%) |
0.54 |
| Recommended protein intake (g/d) |
96.5 (0.5, 96.0–96.5)
(1 NA) |
80.0 (35.2, 61.0–96.15) (3 NA) |
0.03 |
| Recommended energy intake (kcal/d) |
1991.5 (170.3, 1959.0–2129.3) (0
NA) |
2139.0 (460.3, 1900.0–2360.3) (2 NA) |
0.07 |
| Estimated basal metabolic rate (kcal/d) |
1420.8 (99.3, 1399.3–1498.5) (0 NA) |
1755.5 (497.0, 1503.0–2000.0) (6 NA) |
<0.01 |
| Estimated energy deficit (kcal/d) |
708.0 (324.0, 500.0–824.0) (1 NA) |
800.0 (657.0, 468.0–1125.0) (3 NA) |
0.32 |
| Appropriate product selection |
10/18 (56%) |
16/38 (42%) |
0.40 |
| Suitable guideline selection |
6/18 (33%) |
7/38 (18%) |
0.31 |
| Completion duration (min) |
14.5 (5.5,
12.0–17.5) |
12.0 (5.0, 10.0–15.0) |
0.09 |
| Hepatology case |
|
n = 38 |
n = 18 |
|
| Rate of detection of malnutrition (%) |
38/38 (100%) |
17/18 (94%) |
0.32 |
| Recommended protein intake (g/d) |
94.5 (10.0, 90.0–100.0) (1 NA) |
94.5 (30.0, 70.0–100.0) (1 NA) |
0.76 |
| Recommended energy intake (kcal/d) |
2000.0 (456.0, 1819.0–2275.0) (1
NA) |
2100.0 (525.0, 1750.0–2275.0) (1 NA) |
0.63 |
| Estimated basal metabolic rate (kcal/d) |
1400.0 (97.0, 1399.0–1496.0) (2 NA) |
1500.0 (350.0, 1400.0–1750.0) (1 NA) |
0.08 |
| Estimated energy deficit (kcal/d) |
1000.0 (521.0, 800.0–1321.0) (4 NA) |
1200.0 (537.5, 937.5–1475.0) (3 NA) |
0.29 |
| Appropriate product selection |
17/38 (45%) |
8/18 (44%) |
>0.99 |
| Suitable guideline selection |
10/38 (26%) |
6/18 (33%) |
0.75 |
| Completion duration (min) |
15.0 (6.5, 10.3–16.8) |
10.5 (8.5, 10.0–18.5) |
0.67 |
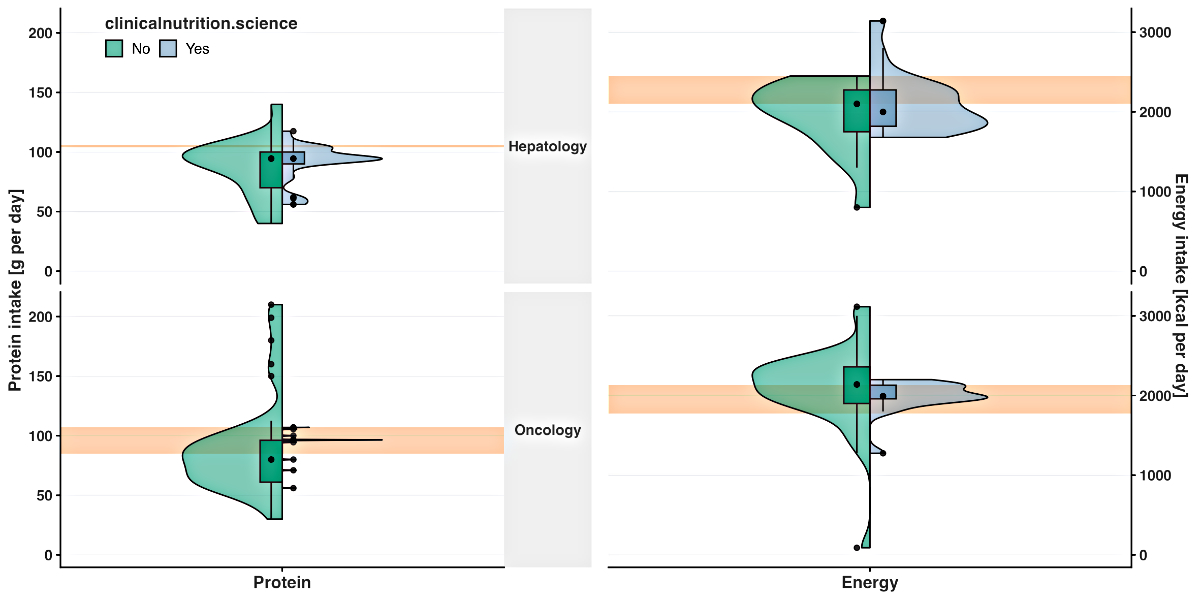
Figure 2Recommended protein intake (g per day) and recommended energy intake (kcal per
day) for the hepatology and oncology patient without (green) and with (blue)
use of the clinicalnutrition.science digital platform. The green and blue boxes
represent the lower and upper quartiles. The point within the box represents
the median. The vertical lines are the whiskers extending to the maximum and
minimum values, excluding the outliers (black points outside the box). The
orange rectangle indicates the intake recommended by the corresponding European
Society of Clinical Nutrition and Metabolism guideline [9, 10].
Estimated basal metabolic rate and energy
deficit and time to complete case
Estimated basal metabolic rate was significantly
reduced for the oncology case (p = 0.03; 1420.8 and 1755.5 kcal/d) and was comparable
for the hepatology case when using the platform (p = 0.08; 1400.0 and 1500.0 kcal/g;
figure S1). The estimated energy deficit was similar with and without use of the
platform for the oncology and the hepatology case (p = 0.32 [oncology case] and
0.29 kcal/d [hepatology case]; figure S1). Time to complete the cases was similar
with and without use of the platform (p = 0.15 [oncology case] and p = 0.52 [hepatology
case], figure S2).
Variance of outcomes with and without clinicalnutrition.science
The variance was reduced by using the digital
platform for recommended protein intake (oncology: IQR 0.5 [with platform] and 35.2
g/d [without platform]; hepatology: IQR 10.0 [with platform] and 30.0 g/d [without
platform]; figure 2), for the recommended
energy intake (oncology: IQR 170.3 [with platform] and 460.3 kcal/d [without platform];
hepatology: IQR 456.0 [with platform] and 525.0 kcal/d [without platform]; figure
2), for the estimated basal metabolic rate
(oncology: IQR 99.3 [with platform] and 497.0 g/d [without platform]; hepatology:
IQR 97.0 [with platform] and 350.0 g/d [without platform]; figure 2) and for the estimated
energy deficit (oncology:
IQR 324.0 [with platform] and 657.0 g/d [without platform]; hepatology: IQR 521.0
[with platform] and 537.5 g/d [without platform]; figure 2).
Subgroup analysis
The difference between the median recommended
protein intake and the corresponding ESPEN guideline was similar with and without
use of clinicalnutrition.science for physicians (p = 0.49; −10.5 [with
platform], −16.0 g/d [without platform]) and significantly increased for dietitians
(p = 0.02; 0.0 [with platform]; −9.25 g/d [without platform]; figure 3). The median
difference between the recommended
energy intake and the corresponding ESPEN guideline recommendation increased significantly
with use of the platform for physicians (p = 0.02; −181.0 [with platform],
97.5 kcal/d [without platform]) and was comparable for dietitians (p = 0.64; 28.3
[with platform]; 23.8 kcal/d [without platform]; figure 3). The variance in the recommended
protein intake was lower with the
platform compared to without the platform for physicians (IQR 14.0 and 36.2 g/d),
dietitians (IQR 1.3 and 20.3 g/d) and the others (IQR 30.5 and 32.6 g/d; figure
3). The variance in the recommended energy
intake was lower when using the platform compared to not using the platform for
physicians (IQR 503.5 and 585.3 kcal/d), dietitians (IQR 157.3 and 350.0 kcal/d)
and the others (IQR 386.8 and 950.0 kcal/d; figure 3).
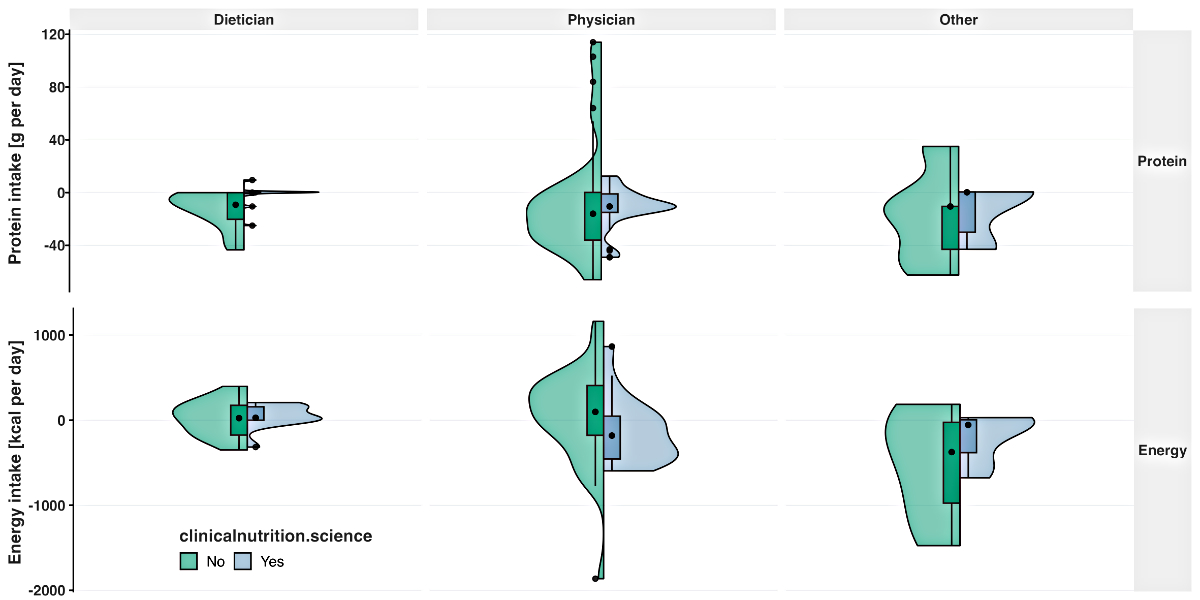
Figure 3Deviation of the recommended protein intake (g per day) and recommended energy
intake (kcal per day) from the corresponding European Society of Clinical
Nutrition and Metabolism guideline [9, 10]
for the hepatology and oncology patient without (green) and with (blue) use of
the clinicalnutrition.science digital platform, by profession. The green and
blue boxes represent the lower and upper quartiles. The point within the box
represents the median.
Usability questionnaire
The HSUS questionnaire was completed by 55 participants.
Overall, 46 participants (83%) had never used the platform before. A total of 9
participants (16%) had used at least one of the clinicalnutrition.science tools
(NutriScreen, NutriCalc, NutriGo, NutriPro, NutriBib) prior to the usability study.
Seven (13%) had used them for less than 3 months and 2 (4%) for between 3 months
and 1 year. Previous users used the platform between 1 and 3 hours per week.
The overall usability score of the HSUS questionnaire
for clinicalnutrition.science was 71.8%. By category, scores were 71.8% for Patient
safety and decision effectiveness, 74.1% for Workflow integration, 71.4% for Work
effectiveness and 69.8% for User control (figure 4). The four highest scoring items
on the HSUS questionnaire were:
- “I believe the recommendations are
reliable” (score 79.6%, Work effectiveness category question 5)
- “I can easily remember how to use
clinicalnutrition.science” (score 78.8%, Workflow integration category question
4)
- “Clinicalnutrition.science supports
my decision-making rather than dictating it” (score 75.3%, User control category
question 1)
- “I am able to provide better quality
of care for patients by using clinicalnutrition.science” (score 75.1%, Patient safety
and decision effectiveness category question 3).
The four lowest scoring items were:
- “Clinicalnutrition.science helps
me prioritise my daily workload” (score 59.2%, Work effectiveness category question
1)
- “Clinicalnutrition.science makes
it easier to collaborate with colleagues” (score 65.1%, Patient safety and decision
effectiveness question 2)
- “Clinicalnutrition.science highlights
potential data entry errors” (score 66.2%, User control category question 3)
- “Recommendations do not unnecessarily
interrupt my workflow” (score 66.4%, User control category question 4).
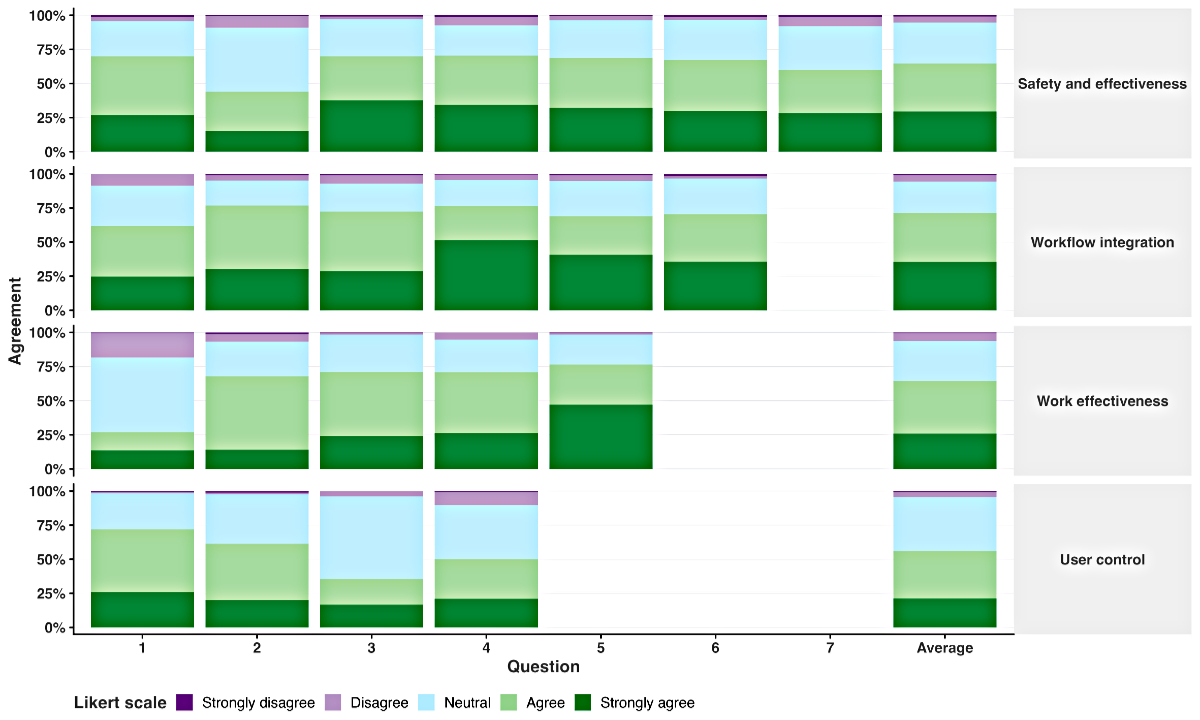
Figure 4Usability of the clinicalnutrition.science digital platform based on the
validated Healthcare Systems Usability Scale (HSUS) questionnaire. For each of
the four categories (Patient safety and decision effectiveness, Workflow
integration, Work effectiveness, User control), 4–7 items were rated on a 5-point
Likert scale (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 =
strongly agree; see the appendix for detailed questions).
Discussion
Key findings of the study
The results of this study indicate that the
overall usability of the newly developed clinicalnutrition.science platform is satisfactory,
with a positive score of 71.8%. Use of the platform led to a notable enhancement
in the precision of the recommended protein intake and the estimation of the basal
metabolic rate for the oncology patient. Furthermore, the variance of the recommended
protein and energy intake as well as the estimated basal metabolic rate and energy
deficit was reduced when using the clinicalnutrition.science platform. It is of
significant importance to note that these improvements were achieved without an
increase in the time required to complete the key steps in the nutritional management
process. The use of the platform did not result in a significant impact on the detection
rate of malnutrition, the provision of appropriate product recommendations or the
identification of suitable guidelines for the oncology and hepatology patient cases.
Recommendation on protein and energy intake
The results of our study indicated that use
of the clinicalnutrition.science platform was associated with a reduction in the
variability of recommended protein and energy intakes, and a decrease in the discrepancy
between the ESPEN guidelines and recommended intake in this study [9, 10]. It is of
paramount importance to prevent
overfeeding and underfeeding to ensure the safety of patients. This can, for instance,
help to mitigate cancer cachexia and prevent glycogen store depletion in cirrhosis
patients [9, 10]. The EFFORT trial demonstrated
that meeting protein and energy targets significantly reduced adverse outcomes and
mortality in malnourished patients [6]. It
is notable that the reduction in variance and adherence to guideline recommendations
were more pronounced in the oncology patient case compared to the hepatology case.
The reported acute kidney injury in addition to liver cirrhosis in the hepatology
case may have influenced the formulation of nutritional therapy, as it complicated
the process. In patients with kidney injury, guidelines recommend that, for example,
the protein intake should be reduced [20–22].
Subgroup analysis by profession
A subgroup analysis revealed that dietitians
and physicians recommended similar median protein and energy intakes, both with
and without use of clinicalnutrition.science. Although dietitians were already more
accurate than physicians in recommending protein and energy intakes using their
standard resources, both were able to reduce deviations from the guideline recommendations
when using the platform. It is of paramount importance to address deviations from
the guideline recommendations to minimise the occurrence of treatment errors and
adverse clinical outcomes. The results of this study indicate that the digital platform
is advantageous for both dietitians and physicians.
Added value of the platform clinicalnutrition.science
and strength of study
In comparison to other clinical nutrition resources,
clinicalnutrition.science guides the majority of the nutritional management process,
from nutritional risk screening to the prescription of nutritional products. It
is an independent, content-proven and applicable resource that can be used directly
at the bedside. While the ESPEN guidelines are of great importance for evidence-based
clinical nutrition, their complex workflow integration constrains their impact and
implementation in daily practice. The recently launched ESPEN interactive guideline
app only covers a limited number of steps in the nutritional management process
[23]. Previous studies have demonstrated that
a simple online training tool or multifaceted nutritional education, when employed
alone, is insufficient to improve nutritional management [24, 25].
The clinicalnutrition.science platform received
an overall usability score of 71.8% based on the HSUS questionnaire, which states
that a score between 70% and 90% reflects good usability, with room for improvement
[11]. Poor usability of health information
systems must be prevented as they are associated with reduced efficiency, workflow
disruption, increased risk of medical treatment errors and increased incidence of
adverse events [11]. It is of significant
importance to note that participants found our digital platform to be straightforward
to use, intuitively structured and efficacious in enhancing their nutritional management
skills. The questionnaire item with the lowest score (59%) was “prioritisation of
daily workload”, which was not the aim of the platform. All other items scored above
65%. While the HSUS score may underestimate usability by equally weighting all items,
it provides a comprehensive assessment. The validation phase involved healthcare
professionals with diverse backgrounds, skills and experiences, which is essential
for a robust and reliable validation process [26].
Limitations of the current study
The current study has several limitations. The
sample size was limited to 56 participants, with a preponderance of physicians and
dietitians with at least a moderate interest in clinical nutrition. This may limit
the generalisability of the results. Furthermore, the evaluation based on two patient
cases that merely mimicked the key steps of the nutritional management process may
have introduced a degree of bias in the results. For example, the malnutrition detection
rate of over 90% in our study contrasts strongly with malnutrition detection rates
in clinical practice, which usually range from 20% to 60% [27]. The participants were
not randomly assigned
to the study cohort; rather, they were allocated based on their working hospital
due to time and resource constraints. In addition, the participants were new to
the clinicalnutrition.science platform, which may have increased the time needed
for the nutritional plan formulation. Consequently,
it is of paramount importance to subject the clinicalnutrition.science platform
to rigorous testing in a genuine clinical setting, including comprehensive training
and a diverse range of participants.
Current state and outlook
We are currently engaged in a collaborative
endeavour with the Swiss Society of Clinical Nutrition and Metabolism (SSNC) with
the objective of raising awareness about the free resource clinicalnutrition.science
throughout Switzerland. We will actively seek feedback from frequent users and distribute
regular questionnaires such as the one presented here, to ascertain the effectiveness
of the platform and to identify areas for improvement and enhancement. Furthermore,
the content will be updated on a regular basis to guarantee the reliability and
currency of the information provided.
Conclusion
The new and independent digital platform clinicalnutrition.science
was found to be intuitive and exhibited a high positive degree of usability. The
platform supported the recommendation of accurate protein and energy intake and
optimised the nutritional management process without increasing the time required
compared to standard resources. In collaboration with the SSNC, we will raise
awareness about this digital platform across Switzerland and will ensure that it
is continuously updated and improved.
Data availability statement
Questionnaires are present
in the appendix. The corresponding author will provide access to raw data and full
analysis code in this manuscript upon request. Case vignettes are not
published for reasons of patient protection, but were available to the academic
editor and reviewers during the manuscript revision process.
Acknowledgments
We would like to express our gratitude to all
the clinical nutrition experts who have supported us in the development of the digital
platform. We would like to thank all the healthcare professionals who participated
in the validation and took the time to answer the questionnaire.
Author contributions: Conceptualisation and methodology: Valentina V. Huwiler, Pascal Tribolet,
Caroline Rimensberger, Christine Roten, Philipp Schuetz, Zeno Stanga – Formal
analysis and visualisation: Valentina V. Huwiler – Original draft preparation:
Valentina V. Huwiler – Funding acquisition: Stefan Mühlebach, Philipp Schuetz, Zeno
Stanga
– Critical revision and editing of draft manuscript: Valentina V. Huwiler,
Pascal Tribolet, Katja A. Schönenberger, Stefan Mühlebach, Philipp Schuetz, Zeno
Stanga – Supervision: Stefan Mühlebach, Philipp Schuetz, Zeno Stanga.
All authors have read and agreed to the final version of
the manuscript.
Valentina V. Huwiler
Department of Diabetes, Endocrinology, Nutritional Medicine and
Metabolism
Inselspital, Bern University Hospital
University of Bern
CH-3010 Bern
valentina.huwiler[at]extern.insel.ch
References
1. Schuetz P, Seres D, Lobo DN, Gomes F, Kaegi-Braun N, Stanga Z. Management of disease-related
malnutrition for patients being treated in hospital. Lancet. 2021 Nov;398(10314):1927–38.
doi: https://doi.org/10.1016/S0140-6736(21)01451-3
2. Bundesamt für Statistik. Patient/innen, Hospitalisierungen. 2021; Available from:
https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitswesen/spitaeler/patienten-hospitalisierungen.html
3. Cawood AL, Elia M, Sharp SK, Stratton RJ. Malnutrition self-screening by using MUST
in hospital outpatients: validity, reliability, and ease of use. Am J Clin Nutr. 2012 Nov;96(5):1000–7.
doi: https://doi.org/10.3945/ajcn.112.037853
4. Álvaro Sanz E, Garrido Siles M, Rey Fernández L, Villatoro Roldán R, Rueda Domínguez A,
Abilés J. Nutritional risk and malnutrition rates at diagnosis of cancer in patients
treated in outpatient settings: early intervention protocol. Nutrition. 2019 Jan;57:148–53.
doi: https://doi.org/10.1016/j.nut.2018.05.021
5. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN
guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017 Feb;36(1):49–64.
doi: https://doi.org/10.1016/j.clnu.2016.09.004
6. Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional
support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet.
2019 Jun;393(10188):2312–21. doi: https://doi.org/10.1016/S0140-6736(18)32776-4
7. Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al.; NOURISH Study
Group. Readmission and mortality in malnourished, older, hospitalized adults treated
with a specialized oral nutritional supplement: A randomized clinical trial. Clin
Nutr. 2016 Feb;35(1):18–26. doi: https://doi.org/10.1016/j.clnu.2015.12.010
8. Wunderle C, Gomes F, Schuetz P, Stumpf F, Austin P, Ballesteros-Pomar MD, et al. ESPEN
guideline on nutritional support for polymorbid medical inpatients. Clin Nutr. 2023 Sep;42(9):1545–68.
doi: https://doi.org/10.1016/j.clnu.2023.06.023
9. Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, et al. ESPEN practical
guideline: clinical nutrition in liver disease. Clin Nutr. 2020 Dec;39(12):3533–62.
doi: https://doi.org/10.1016/j.clnu.2020.09.001
10. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN
practical guideline: clinical Nutrition in cancer. Clin Nutr. 2021 May;40(5):2898–913.
doi: https://doi.org/10.1016/j.clnu.2021.02.005
11. Ghorayeb A, Darbyshire JL, Wronikowska MW, Watkinson PJ. Design and validation of
a new Healthcare Systems Usability Scale (HSUS) for clinical decision support systems:
a mixed-methods approach. BMJ Open. 2023 Jan;13(1):e065323. doi: https://doi.org/10.1136/bmjopen-2022-065323
12. Aubert CE, Fankhauser N, Marques-Vidal P, Stirnemann J, Aujesky D, Limacher A, et
al. Patterns of multimorbidity in internal medicine patients in Swiss university hospitals:
a multicentre cohort study. Swiss Med Wkly. 2019 Jun;149:w20094. doi: https://doi.org/10.4414/smw.2019.20094
13. Google. Google Forms. 2023 05. [January 2024]; Available from: https://docs.google.com/forms
14. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria:
R Foundation for Statistical Computing; 2023.
15. Wickham H. ggplot2: Elegant graphics for data analysis. 2016, New York: Springer-Verlag.
11-31.
16. Gross, J. and U. Ligges, nortest: Tests for Normality. R package version, 2015. 1(4).
17. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using
lme4. J Stat Softw. 2015;67(1):1–48. doi: https://doi.org/10.18637/jss.v067.i01
18. Fox J, Weisberg S. An R companion to applied regression. 3rd ed. Sage publications;
2018.
19. Fisher RA. Statistical methods for research workers. Breakthroughs in statistics:
Methodology and distribution. Springer; 1970. pp. 66–70.
20. Sobotka L. Basics in clinical nutrition. 5th ed. Galen; 2020.[S.l.].
21. Cano N, Fiaccadori E, Tesinsky P, Toigo G, Druml W, Kuhlmann M, et al.; DGEM (German
Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral
Nutrition). ESPEN Guidelines on Enteral Nutrition: adult renal failure. Clin Nutr.
2006 Apr;25(2):295–310. doi: https://doi.org/10.1016/j.clnu.2006.01.023
22. Fiaccadori E, Sabatino A, Barazzoni R, Carrero JJ, Cupisti A, De Waele E, et al. ESPEN
guideline on clinical nutrition in hospitalized patients with acute or chronic kidney
disease. Clin Nutr. 2021 Apr;40(4):1644–68. doi: https://doi.org/10.1016/j.clnu.2021.01.028
23. Bischoff SC, Cuerda C, Barazzoni R. Practical guidelines and apps for improvement
of guideline implementation. Clin Nutr. 2020 Oct;39(10):2943–4. doi: https://doi.org/10.1016/j.clnu.2020.07.031
24. Aeberhard C, Birrenbach T, Joray M, Mühlebach S, Perrig M, Stanga Z. Simple training
tool is insufficient for appropriate diagnosis and treatment of malnutrition: A pre-post
intervention study in a tertiary center. Nutrition. 2016 Mar;32(3):355–61. doi: https://doi.org/10.1016/j.nut.2015.09.012
25. Reber E, Messmer Ivanova A, Cadisch P, Stirnimann J, Perrig M, Roten C, et al. Does
multifaceted nutritional education improve malnutrition management? Nutrition. 2020 Oct;78:110810.
doi: https://doi.org/10.1016/j.nut.2020.110810
26. Wyatt JC, Wyatt SM. When and how to evaluate health information systems? Int J Med
Inform. 2003 Mar;69(2-3):251–9. doi: https://doi.org/10.1016/S1386-5056(02)00108-9
27. Imoberdorf R, Meier R, Krebs P, Hangartner PJ, Hess B, Stäubli M, et al. Prevalence
of undernutrition on admission to Swiss hospitals. Clin Nutr. 2010 Feb;29(1):38–41.
doi: https://doi.org/10.1016/j.clnu.2009.06.005
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3764.



