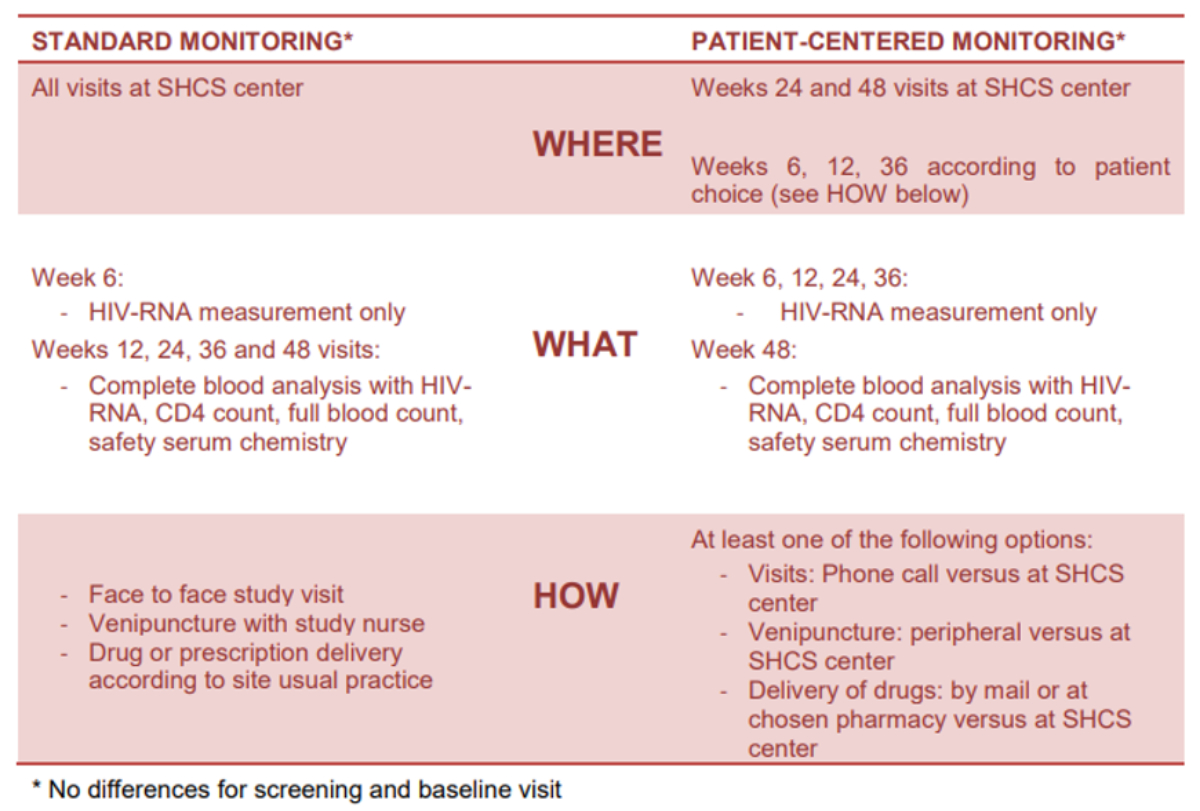
Figure 1Summary of the differences between the standard and patient-centred monitoring arms.
DOI: https://doi.org/https://doi.org/10.57187/s.3762
combined antiretroviral therapy
dolutegravir
emtricitabine
patient-centred monitoring
per-protocol
Swiss HIV Cohort Study
Antiretroviral treatments and clinical and laboratory monitoring are essential elements of HIV care for people living with HIV (PLWH) who are on antiretroviral therapy(ART). In particular, viral-load monitoring determines whether treatment is successful or needs enhanced adherence, and it allows for prompt action in instances of drug-related adverse events. Current international recommendations define regular polymerase chain reaction(PCR)-based viral load testing as the preferred approach to treatment monitoring (together with a treatment monitoring algorithm) to identify treatment failure, provide timely adherence interventions and identify the possibility of drug resistance[1, 2]. However, every monitoring strategy carries different costs and challenges. Determining the costs of a given strategy requires decision-makers to balance the health gains it provides against the health gains that could be achieved by allocating resources to other interventions. New cost-saving approaches, such as differentiated care or “differentiated service delivery”, have explored the delivery of HIV testing, care and treatment tailored to patient needs and the capacity of the health system [3, 4].
The SIMPL’HIV study is a non-inferiority, randomised, controlled clinical trial conducted among treatment-experienced HIV-infected adults in Switzerland. Participants were randomised 1:1:1:1 to switch to dolutegravir (DTG) + emtricitabine (FTC) or to continue with combination antiretroviral therapy (cART), and simplified monitoring (“patient-centred monitoring” [PCM]) versus the continuation of standard tri-monthly surveillance (SM). Randomised comparisons have previously established that dolutegravir + emtricitabine dual therapy is non-inferior in terms of viral suppression compared to standard combined antiretroviral therapy [5]. Here, we present the results of the second primary objective of the trial, which aimed to compare the costs of the patient-centred monitoring approach versus standard monitoring. We also investigated whether dual therapy and simplified monitoring were acceptable to patients in terms of safety and satisfaction.
We conducted the SIMPL’HIV study, a non-inferiority, open-label, randomised trial with a factorial design, to compare dual therapy to standard combined antiretroviral therapy and patient-centred monitoring to standard monitoring. The study was conducted in the seven main Swiss HIV Cohort Study sites [6] among adults enrolled in the study between 12 May 2017 and 30 May 2018. Patients were eligible if they were on any cART recommended by the European AIDS Clinical Society and virologically suppressed for at least 24 weeks prior to enrolment. Full inclusion and exclusion criteria are available in the trial protocol (supplement). Briefly, the trial demonstrated that the combination of dolutegravir + emtricitabine was non-inferior in terms of viral suppression compared to standard therapy, maintaining HIV-1 ribonucleic acid (RNA) <100 copies/ml through 48 weeks [5].
Participants were randomly assigned 1:1 to switch to dolutegravir + emtricitabine dual maintenance therapy or continue their combined antiretroviral therapy and 1:1 to patient-centred monitoring or standard monitoring. An independent statistician generated a computer-based, random allocation sequence stratified by study site using randomly permutated blocks of sizes four and eight to randomise patients to four arms. An independent data manager implemented the randomisation list in a web-based data management system to ensure allocation concealment.
Differences between standard monitoring and patient-centred monitoring are summarised in figure 1. We performed HIV-RNA measurements at baseline and in weeks 6, 12, 24 and 48 for all patients. Allocation to standard monitoring consisted of tri-monthly, routine, immunological and blood safety tests, including a CD4 cell count, lipid profile, glucose level, renal and hepatic function tests and creatinine kinase level. All visits and laboratory analyses were conducted at the affiliated Swiss HIV Cohort Study sites. Participants allocated to the patient-centred monitoring arm had immunological and blood safety monitoring only at weeks 0 and 48. In addition, participants were offered the following options: 1) to complete some of the study visits by a telephone call with a study nurse rather than a face-to-face outpatient consultation; 2) to have their drugs delivered to a specified address (e.g., home address) instead of to the pharmacy or hospital; and 3) to perform their blood tests at a location of their choice, including certified private laboratories and general practitioners.

Figure 1Summary of the differences between the standard and patient-centred monitoring arms.
The first primary outcome was the non-inferiority of dual therapy versus combined antiretroviral therapy in maintaining HIV-1 RNA <100 copies/ml through 48 weeks, which has been reported in a previously published article [5]. The second primary outcome was comparing patient-centred and standard monitoring in terms of direct costs per person between baseline and week 48. Secondary outcomes were: (a) assessments of patient monitoring satisfaction from baseline to week 48; (b) evaluations of patient treatment satisfaction at week 48; (c) satisfaction levels regarding study participation at week 48; and (d) the choice of treatment in the post-study period. Patient satisfaction was assessed using a visual analogue scale ranging from 0 to 100 (maximal satisfaction).
Costs were analysed in the patient-centred and standard monitoring arms using invoices from health insurance companies and invoices for medical services issued by the hospital where the patient was typically seen. Both invoice types were obtained after receiving written consent from the study participant. When health insurance invoices were available, this approach was preferentially used. If this was not possible, an invoice from the hospital was requested. The latter implied that costs concerning medical services provided outside the hospital were missing. We used the same average costs for antiretroviral drugs for all patients. Costs were expressed in United States dollars (US$) per person per year and classified into several categories: outpatient medical consultations (HIV/non-HIV consultations); non-medical consultations (physiotherapist, dietician, etc.); antiretroviral therapy; laboratory tests; hospitalisation; and “other” (including other diagnostic tests, drugs other than antiretroviral therapy and medical devices). The costs of all HIV-RNA measurements were also included. The Swiss franc conversion rate at the time of the analyses was 0.87 per 1 US$.
Safety endpoints included the incidence, type and seriousness of adverse events, as well as renal function, lipids and glucose values and weight over 48 weeks. Differences between treatment arms have been already shown previously [5]. Therefore, here we present the comparison between patient-centred and standard monitoring.
Patient satisfaction considered several aspects of HIV care, including type of monitoring, treatment satisfaction and study participation. Satisfaction was evaluated using visual analogue scales at baseline and week 48. Patients were asked to score their satisfaction on a quantitative scale ranging from 0 to 100. Zero corresponded to the “worst possible satisfaction” and 100 to the “best possible satisfaction”. This measure has previously been used to evaluate the quality of life among PLWH [7].
We determined the target sample size for the non-inferiority comparison between dolutegravir + emtricitabine and combined antiretroviral therapy on the primary outcome, as explained in the main paper [5]. Baseline characteristics, such as demographic, clinical and treatment variables, were summarised using descriptive statistics. To compare direct costs between the two monitoring and treatment arms, we calculated a mean difference (patient-centred monitoring – standard monitoring or dolutegravir + emtricitabine – cART, respectively) using linear regression adjusted for the type of treatment or monitoring, respectively. Secondary continuous outcomes were also analysed using linear regression adjusted for the type of treatment and the outcome value at baseline, if available. Binary outcomes were compared using the Cochran-Mantel-Haenszel test statistics and Mantel-Haenszel risk difference, stratified by treatment type. The analysis presented was in the intention-to-treat population set, including all randomised participants. All statistical analyses were performed with R software, version 3.6.1 (or higher) [8].
The study protocol was approved by both the leading and local ethics committees in Switzerland in accordance with the Helsinki Declaration and good clinical practice. Written informed consent was obtained from each participant before study initiation.
Of 873 individuals screened for eligibility, 188 were randomly assigned either to patient-centred monitoring or to continue standard monitoring (figure 2). One ineligible patient was mistakenly randomised, leading to 95 participants allocated to the patient-centred monitoring arm (cART arm, 47; dolutegravir + emtricitabine arm, 48) and 92 in the standard monitoring arm (cART arm, 47; dual therapy arm, 45). Reasons for study discontinuation have been provided in a previous publication [5].
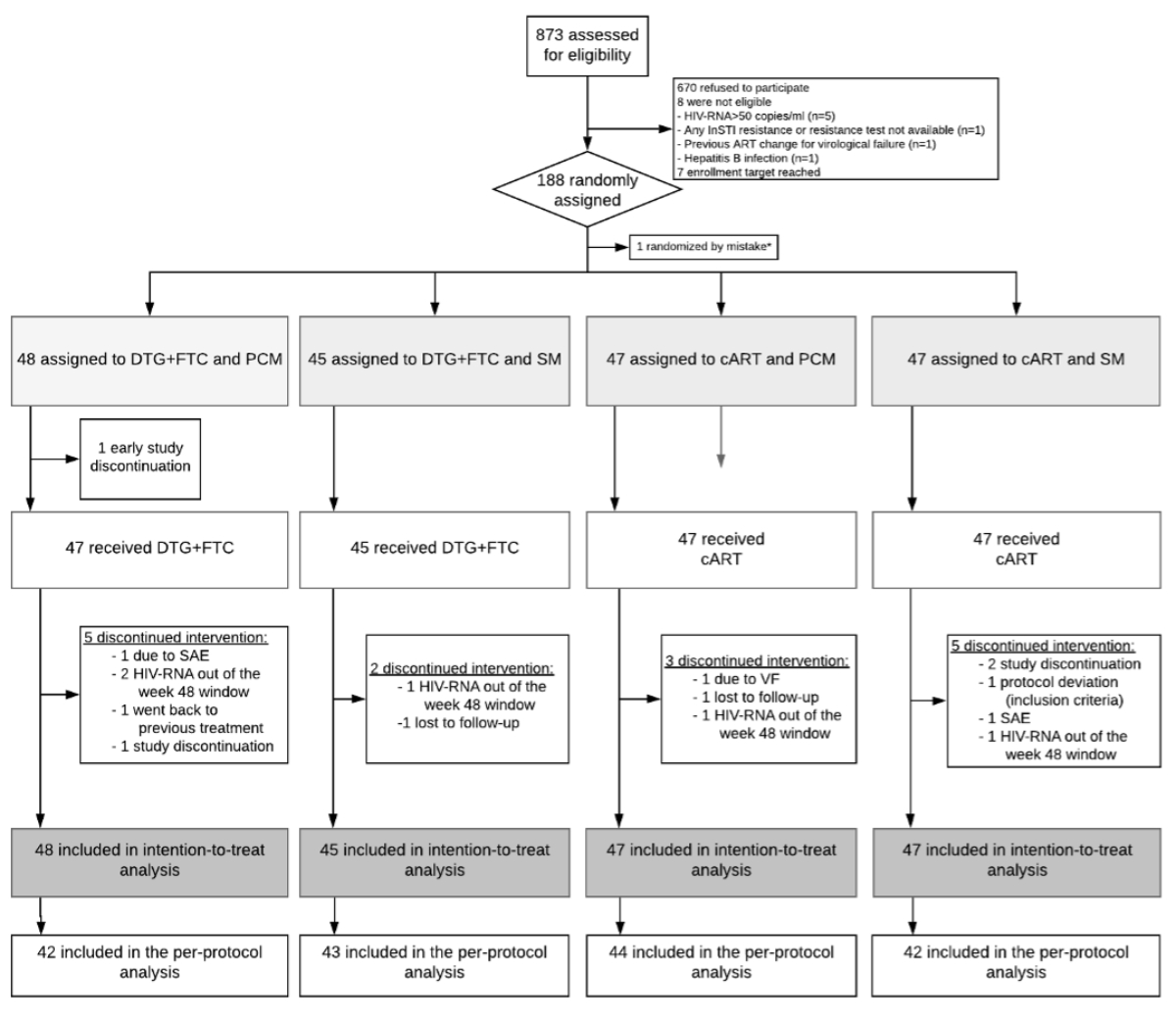
Figure 2Consort flowchart. DTG: dolutegravir; FTC: emtricitabine; cART: combined antiretroviral therapy; PCM: patient-centred monitoring; SM: standard monitoring.
The study population’s demographic, clinical and treatment characteristics are presented in table 1. The overall mean age (± standard deviation) at randomization was 48 ± 11 years. Approximately two-thirds of the participants were male, and nearly 80% were Caucasian. Most had started antiretroviral therapy seven years prior to study inclusion and were on two nucleoside reverse transcriptase inhibitors plus an integrase strand transferase inhibitor when included in the trial. The median nadir CD4 was relatively low (246 cells/mm3), and the median body mass index was 25 kg/m2. The most common comorbidity was hypertension, affecting approximately one in five participants (19%), followed by cardiovascular disorders (8%), osteoporosis (8%), diabetes (3.2%) and chronic kidney disease (3.2%). The median number of concomitant medications, other than antiretroviral therapy drugs, was two.
Table 1Baseline demographic and clinical characteristics of participants (percentages may not total 100 because of missing values).
| Characteristic | Total (n = 187) | PCM/DTG+FTC (n = 48) | SM/DTG+FTC (n = 45) | PCM/cART (n = 47) | SM/cART (n = 47) | |
| Male gender, n (%) | 155 (83%) | 42 (88%) | 37 (82%) | 36 (77%) | 40 (85%) | |
| Age at screening (y): mean (SD) | 48 (11) | 49 (12) | 46 (8.3) | 45 (10) | 51 (12) | |
| Duration since first ARV treatment (y): median [lq, µq] | 7.3 [4.0, 12] | 7.8 [5.3, 12] | 7.9 [3.7, 12] | 6.4 [4.1, 12] | 8.5 [3.3, 15] | |
| HIV-RNA zenith, n (%) | <100000 copies | 83 (44%) | 20 (42%) | 21 (47%) | 21 (45%) | 21 (45%) |
| ≥100000 copies | 92 (49%) | 25 (52%) | 20 (44%) | 23 (49%) | 24 (51%) | |
| Nadir CD4 count: median [lq, µq] x 106/l | 246 [147, 340] | 271 [155, 351] | 206 [84, 284] | 247 [156, 338] | 258 [192, 367] | |
| Combined antiretroviral therapy regimen before inclusion, n (%) | 2 NRTI + 1 boosted PI | 11 (5.9%) | 4 (8.3%) | 1 (2.2%) | 4 (8.5%) | 2 (4.3%) |
| 1 NRTI + 1 boosted PI | 1 (0.53%) | 0 (0.00%) | 1 (2.2%) | 0 (0.00%) | 0 (0.00%) | |
| 2 NRTI + 1 NNRTI | 50 (27%) | 13 (27%) | 13 (29%) | 10 (21%) | 14 (30%) | |
| 2 NRTI + 1 InSTI | 121 (65%) | 29 (60%) | 30 (67%) | 32 (68%) | 30 (64%) | |
| Cardiovascular disease: n (%) | 15 (8.0%) | 1 (2.1%) | 5 (11%) | 3 (6.4%) | 6 (13%) | |
| Hypertension (%) | 35 (19%) | 10 (21%) | 9 (20%) | 6 (13%) | 10 (21%) | |
| Diabetes mellitus: n (%) | 6 (3.2%) | 0 (0.00%) | 1 (2.2%) | 2 (4.3%) | 3 (6.4%) | |
| Chronic kidney disease (GFR <60 ml/min/1.72 m2): n (%) | 6 (3.2%) | 0 (0.00%) | 2 (4.4%) | 1 (2.1%) | 3 (6.4%) | |
| Cirrhosis: n (%) | 1 (0.53%) | 0 (0.00%) | 0 (0.00%) | 1 (2.1%) | 0 (0.00%) | |
| Chronic obstructive pulmonary disease: n (%) | 4 (2.1%) | 3 (6.3%) | 0 (0.00%) | 0 (0.00%) | 1 (2.1%) | |
| Osteoporosis: n (%) | 15 (8.0%) | 6 (13%) | 2 (4.4%) | 1 (2.1%) | 6 (13%) | |
| Body mass index (kg/m2): Median [lq, µq] | 25 [23, 27] | 25 [23, 27] | 24 [23, 25] | 25 [23, 27] | 24 [22, 28] | |
| Concomitant medications: Median [lq, µq] | 2.0 [0.00, 3.0] | 1.5 [0.00, 4.0] | 2.0 [0.00, 3.0] | 1.0 [0.00, 2.0] | [1.0, 4.0] | |
DTG: dolutegravir; FTC: emtricitabine; PCM: patient-centred monitoring; SM: standard monitoring; cART: combined antiretroviral therapy; SD: standard deviation; ARV: antiretroviral; NRTI: nucleoside reverse transcriptase inhibitors; PI: protease inhibitors; NNRTI: non-nucleoside reverse transcriptase inhibitors; InSTI: integrase strand transfer inhibitors.
Overall costs related to patient care expressed in US$ per person per year are shown in tables 2 and 3. We obtained invoices from health insurance companies for 52 participants, hospital invoices for 53 individuals, and both invoice types for 59 persons. Data were missing for 21 participants. An additional sensitivity analysis was performed for the overall costs by applying multiple imputations (table S1). Total costs did not differ when comparing patient-centred monitoring to standard monitoring, corresponding to US$ 20,635 (±5676) in the patient-centred monitoring arm and US$ 21,060 (±6956) in the standard monitoring arm (95% CI –2292 to 1451; p = 0.658) (tables 2 and 3). Antiretroviral therapy represented the main expense in both arms, followed by costs of outpatient consultations (US$ 1917 [±2020] in the patient-centred monitoring arm compared to US$ 1834 [±1670] in the standard monitoring arm) and laboratory tests (US$ 1363 [± 683] in the patient-centred monitoring arm compared to US$ 1549 [+/– 801] in the standard monitoring arm). Hospitalisations constituted a marginal portion of total costs. Overall, we observed no significant difference in any category costs between the patient-centred monitoring and standard monitoring arms. However, total costs per person per year were significantly lower for the dolutegravir + emtricitabine arm, with US$ 19,102 (±6738) in the dual therapy arm compared to US$ 22,485 (±5377) in the cART arm (95% CI –5251 to –1514; p <0.001). This was driven by the reduced costs of dual therapy, which was, on average, US$ 2726 (95% CI –3449 to –2004) less expensive than combined antiretroviral therapy.
Table 2Costs per person per year stratified by monitoring arms are expressed in US$. Medians with the interquartile range IQR [lower and upper quartile] are shown, and the adjusted median difference with 95% CIs are displayed. The adjustment of the median was done for the treatment group or monitoring group, respectively, depending on the comparison.
| Patient-centred monitoring | Standard monitoring | Adjusted median difference (95% CI) | p-value | |
| Total costs (US$) | 19388 [16868;23713] | 19474 [17269;23457] | 40 (–1021.5 to 1059.22) | 0.962 |
| Outpatient consultations | 1298 [673;2227] | 1255 [889;2436] | 51.5 (–336.7 to 326.35) | |
| Other (non-medical consultations) | 61 [0;402] | 102 [42;485] | –39.8 (–92.1 to 40.96) | |
| Antiretroviral drugs | 14860 [13753;16556] | 14860 [13712;17619] | 203.4 (–337.3 to 305.18) | |
| Laboratory tests | 1293 [898;1746] | 1457 [920;2224] | –183 (–464.9 to 179.91) | |
| Hospitalisations | 0 [0;0] | 0 [0;0] | 0 (0 to 0) | |
| Other* | 393 [68;2051] | 249 [21;1224] | 110 (–322.7 to 641.24) |
Table 3Costs per person per year stratified by treatment arms are expressed in US$. Medians with the interquartile range (IQR) [lower and upper quartile] are shown, and the adjusted median difference with 95% CIs are displayed. The adjustment of the median was done for the treatment or monitoring group, respectively, depending on the comparison.
| Dolutegravir | Combined antiretroviral therapy | Adjusted median difference (95% CI) | p-value | |
| Total costs (US$) | 17486 [15709;21043] | 22117 [18480;25250] | –4643.6 (–5128 to –3160.11) | <0.001 |
| Outpatient consultations | 1175 [693;2206] | 1339 [974;2459] | –138.4 (–448.8 to 208.5) | |
| Other (non-medical consultations) | 79 [24;384] | 92 [24;549] | 2.9 (–92.8 to 38.62) | |
| Antiretroviral drugs | 13753 [13631;14860] | 16392 [15853;17957] | –2620.4 (–2864.3 to –2331.4) | |
| Laboratory tests | 1350 [925;1869] | 1311 [898;2122] | –71.2 (–307.3 to 282.14) | |
| Hospitalisations | 0 [0;0] | 0 [0;0] | 0 (0 to 0) | |
| Other* | 261 [45;1427] | 492 [21;1686] | –183.5 (–767.8 to 173.93) |
* Including other diagnostic tests, drugs other than antiretroviral therapy and medical devices.
Participants in the patient-centred monitoring arm were offered the possibility to initiate one or more options of simplified monitoring. Approximately 50% of participants selected only one monitoring option among three variations, one-third chose two, and a few patients opted for all three options (table 4). Nearly 80% of individuals chose to complete visits via telephone calls, approximately 50% opted for their drugs to be delivered to a specific address, and less than 20% decided to perform their blood tests at alternative locations. Most participants did not require supplementary visits in addition to those already scheduled as part of the study plan. Apart from one patient in the patient-centred monitoring arm who required 45 additional visits due to a newly diagnosed cancer, the number of visits per person and type of consultation did not differ between the two study arms (table S2).
Regarding safety endpoints, we only detected one virological failure, as reported in the main article of the study [5]. We observed blips, defined as HIV-RNA <200 copies/ml, in seven participants (six in the patient-centred monitoring and one in the standard monitoring arm). The HIV-RNA values of these participants are shown in figure 3. We observed no differences in the biological profiles apart from total cholesterol and weight (table S3). Total cholesterol was significantly lower in the patient-centred monitoring arm than in the cART arm (adjusted difference –0.2; 95% CI −0.4 to 0.0; p = 0.037). We observed a slight increase in weight in the patient-centred monitoring arm (adjusted difference +1.1; 95% CI 0.1 to 2.1; p = 0.032). Patient satisfaction related to monitoring, treatment and study participation was moderately high in both study arms, with no significant difference observed between baseline and week 48. Treatment satisfaction remained elevated in both monitoring groups at the 48-week follow-up (figure S1). At study termination (week 48), 85.6% of participants in the dual therapy arm and 32.2% in the cART arm opted for dolutegravir + emtricitabine or the recommended European Aids Clinical Society dual therapy of dolutegravir/lamivudine (figure 4a). At week 48, 6/17 (35%) patients decided to discontinue laboratory tests at alternative locations, 18/48 (37.5%) discontinued the delivery of drugs, and 22/75 (29%) suspended visits by telephone (figure 4b).
Table 4Type and number of monitoring options selected by participants at baseline.
| Outcome | DTG+FTC, PCM (n = 48) | cART, PCM (n = 47) | Risk/adjusted difference (95% CI)* | |
| Type of monitoring option chosen at baseline | Peripheral laboratory | 8 (17.0%) | 9 (19.1%) | –2.1% [–17.6;13.5] |
| Drug supplied by mail | 25 (53.2%) | 23 (48.9%) | +4.3% [–16.1;24.3] | |
| Telephone call visits | 38 (80.9%) | 37 (78.7%) | +2.1 % [–14.2; +18.3] | |
| Number of monitoring options chosen at baseline | One option | 23 (48.9%) | 23 (48.9%) | NA |
| Two options | 15 (31.9%) | 17 (36.2%) | ||
| Three options | 6 (12.8%) | 4 (8.5%) | ||
* Obtained from a chi-square test. DTG: dolutegravir; FTC: emtricitabine; PCM: patient-centred monitoring; cART: combined antiretroviral therapy; NA: not available.
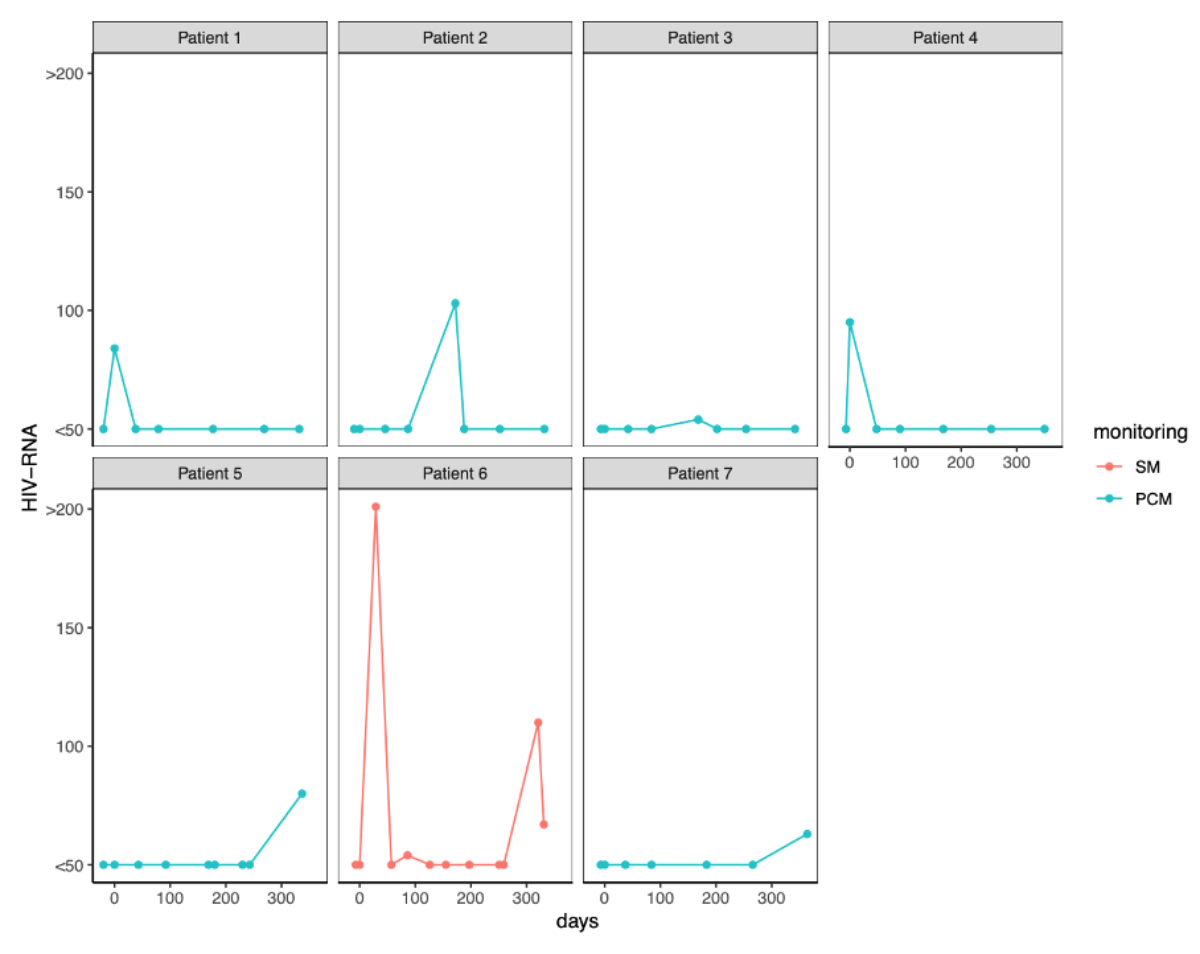
Figure 3HIV-RNA values throughout the study in participants with blips. Patient 6 was the only virological failure of the study. PCM: patient-centred monitoring; SM: standard monitoring.
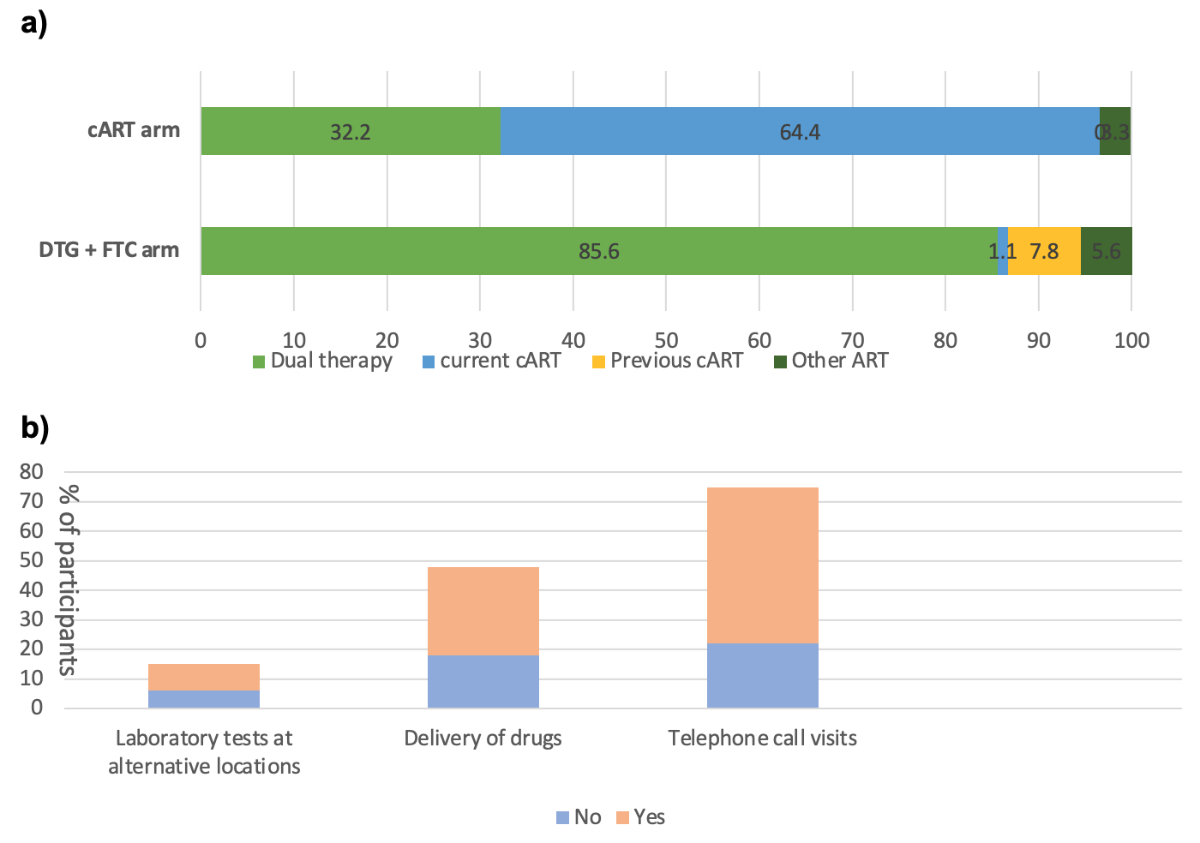
Figure 4(A) Choice of antiretroviral therapy (ART) at the end of the study (week 48) for all patients. (B) Proportions of participants in the patient-centred monitoring (PCM) arm who continued one or more monitoring options after week 48. DTG: dolutegravir; FTC: emtricitabine; cART: combined antiretroviral therapy.
Our findings showed that a simplified HIV care approach (patient-centred monitoring) generated no substantial reductions or increases in provider care costs compared to standard monitoring in terms of costs, safety or patient satisfaction. However, dual therapy was significantly less expensive than standard triple-drug antiretroviral therapy. Among participants randomised to patient-centred monitoring, 50% selected only one patient-centred monitoring option, with a marked preference for telephone call visits, followed by home drug delivery. The number of additional visits per person outside the study schedule did not differ by the type of monitoring. We demonstrated a good safety profile of the patient-centred monitoring arm, with few blips, followed by suppressed HIV-RNA. Furthermore, our results demonstrated an already high level of satisfaction among participants towards HIV care and treatment services, with patient-centred monitoring having neither a positive or negative impact.
Costs related to HIV care were primarily driven by antiretroviral drugs, which accounted for approximately 65% of the total costs, thus confirming the findings of previous studies in different high- and low-income countries [9–13]. Our results are also similar to those observed in a study that matched the Swiss HIV Cohort Study and claims data from the largest Swiss health insurer for HIV-related and non-HIV-related conditions. In that study, antiretroviral therapy was the primary driving cost, but patient profiling enabled the identification of factors related to higher resource use [14]. Despite developing new models of care to meet the needs of PLWH [15], we identified only a few studies providing primary data about costs, particularly in developed countries. In one model of care that integrated community-based pharmacists with primary medical providers was shown to be a cost-saving intervention that assisted patients in achieving viral suppression and preventing HIV transmission [16]. The United States national HIV/AIDS strategy attempted a new technological approach to enhance the rapid and effective treatment of HIV to achieve sustained viral suppression, but with no effect on cost-effectiveness [17]. In South Africa, two differentiated models of care for stable HIV patients, including adherence clubs and decentralised medication delivery, were unable to reduce provider costs [18].However, a comparison of results with other HIV care options remains challenging due to differences between health systems, particularly in low- and middle-income countries, as well as the heterogeneity of alternative models.
Previous studies have reported that patient satisfaction with HIV care is high in developed countries [19]. Notably, patient satisfaction with healthcare services is related to retention in care programmes and adherence to antiretroviral therapy, the latter being a critical factor in HIV suppression [20]. Effective communication between the patient and healthcare provider is also crucial. For example, at the time of regular medical check-ups, it is important that doctors are perceived as listening carefully to patient needs and promptly responding to their requests [21], as envisaged in clinical trial procedures. We expected to observe more consultations in the patient-centred monitoring arm due to the reduced number of medical visits and laboratory tests. However, the number of additional visits was similar in both arms (except for one patient in the patient-centred monitoring arm) as well as the type of requested visits.
The strengths of the current study include the innovative approach to finding new and potentially less costly models of care for HIV-infected patients in a high-income country and the representativeness and comprehensiveness of cost and clinical data on a national level, covering not only antiretroviral therapy costs but also ambulatory and in-hospital resource use. Our study also has some limitations. Importantly, we investigated a select HIV population, including long-term antiretroviral therapy recipients with excellent virological control and few comorbidities. Thus, we observed low inpatient costs attributable to HIV‐related hospitalisations. Tri-monthly monitoring is the typical care practiced by most hospital centres in Switzerland, but this should not be considered the standard approach to routine HIV care in Europe or elsewhere, where medical controls are less frequent. Furthermore, the study was designed to demonstrate the non-inferiority of a dual treatment regimen, with multiple HIV-RNA measurements for all participants. Therefore, trial design may have influenced laboratory costs by overestimating them in the patient-centred monitoring strategy.Finally, there were missing values in cost covariates for a limited number of patients. In particular, this concerned medical services provided outside the hospital. However, we estimated that these services represented a minor part of the costs as participants in our setting frequently went to the study sites for medical problems other than HIV.
Patient-centred monitoring did not reduce the overall costs of medical care in HIV-suppressed patients switching to dual therapy or continuing combined antiretroviral therapy compared to standard monitoring. In this representative sample of stable HIV patients in a high-income country, antiretroviral therapy was the primary driving cost. Costs could be further reduced by optimising generic formulations used to mitigate the high cost of lifelong HIV care.Alternative models of care for treatment‐experienced, stable patients remain a priority to improve patient satisfaction and decrease associated costs. Although our simplified HIV monitoring model was unlikely to decrease health costs, it could inform strategies to effectively support continued high‐quality care in the context of a future public health emergency of international concern, such as the COVID-19 pandemic.
Data underlying the reported findings are provided upon request. Instructions with contact information as well as further relevant documents are available at the Bern Open Repository (BORIS).
The authors would like to thank the study participants for their cooperation in this study.
They also thank the following persons for their contributions: Rosemary Sudan (review and editing); Charlotte Barbieux, Tamara Da Silva, Christelle Martin (investigation, data curation, validation); Christina Grube and Louise Seiler (investigation); Dominique Rubi (software); and the Clinical Research Centre, Geneva University Hospitals and the University of Geneva Faculty of Medicine (validation).
Members of the Swiss HIV Cohort Study
Aebi-Popp K, Anagnostopoulos A, Battegay M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Günthard HF (President of the Swiss HIV Cohort Study), Haerry D (Deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (Chairman of the Scientific Board), Rudin C, Scherrer AU (Head of Data Centre), Schmid P, Speck R, Stöckle M (Chairman of the Clinical and Laboratory Committee), Tarr P, Trkola A, Vernazza P, Wandeler G, Weber R, Yerly S.
This study has been financed within the framework of the Swiss National Science Foundation (grant nos. 166819 and 17481) and the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant no. 177499), by the Swiss HIV Cohort Study project no. 826 and by the Swiss HIV Cohort Study research foundation. Data were gathered by the five Swiss university hospitals, two cantonal hospitals, 15 affiliated hospitals and 36 private physicians (listed in http://www.shcs.ch/180-health-care-providers).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2016. Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/
2. The state of the HIV market in low- and middle-income countries. Clinton Health Access Initiative. 2020. Available from: https://www.clintonhealthaccess.org/the-state-of-the-hiv-market-in-low-and-middle-income-countries-3/
3. Grimsrud A, Bygrave H, Doherty M, Ehrenkranz P, Ellman T, Ferris R, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc. 2016 Dec;19(1):21484. 10.7448/IAS.19.1.21484
4. Roy M, Bolton Moore C, Sikazwe I, Holmes CB. A review of differentiated service delivery for HIV treatment: effectiveness, mechanisms, targeting, and scale. Curr HIV/AIDS Rep. 2019 Aug;16(4):324–34. 10.1007/s11904-019-00454-5
5. Sculier D, Wandeler G, Yerly S, Marinosci A, Stoeckle M, Bernasconi E, et al.; Swiss HIV Cohort Study (SHCS). Efficacy and safety of dolutegravir plus emtricitabine versus standard ART for the maintenance of HIV-1 suppression: 48-week results of the factorial, randomized, non-inferiority SIMPL’HIV trial. PLoS Med. 2020 Nov;17(11):e1003421. 10.1371/journal.pmed.1003421
6. Swiss HIV Cohort Study, Schoeni-Affolter F, Ledergerber B, Rickenbach M, Rudin C, Günthard HF, et al. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39:1179–89.
7. Verolet CM, Delhumeau-Cartier C, Sartori M, Toma S, Zawadynski S, Becker M, et al.; LIPO Group Metabolism. Lipodystrophy among HIV-infected patients: a cross-sectional study on impact on quality of life and mental health disorders. AIDS Res Ther. 2015 Jun;12(1):21. 10.1186/s12981-015-0061-z
8. R: The R Project for Statistical Computing. Available from: https://www.r-project.org/
9. Sloan CE, Champenois K, Choisy P, Losina E, Walensky RP, Schackman BR, et al.; Cost-Effectiveness of Preventing AIDS Complications (CEPAC) investigators. Newer drugs and earlier treatment: impact on lifetime cost of care for HIV-infected adults. AIDS. 2012 Jan;26(1):45–56. 10.1097/QAD.0b013e32834dce6e
10. Nakagawa F, Miners A, Smith CJ, Simmons R, Lodwick RK, Cambiano V, et al. Projected lifetime healthcare costs associated with HIV infection. PLoS One. 2015 Apr;10(4):e0125018. 10.1371/journal.pone.0125018
11. Treskova M, Kuhlmann A, Bogner J, Hower M, Heiken H, Stellbrink HJ, et al. Analysis of contemporary HIV/AIDS health care costs in Germany: driving factors and distribution across antiretroviral therapy lines. Medicine (Baltimore). 2016 Jun;95(26):e3961. 10.1097/MD.0000000000003961
12. Tucker A, Tembo T, Tampi RP, Mutale J, Mukumba-Mwenechanya M, Sharma A, et al. Redefining and revisiting cost estimates of routine ART care in Zambia: an analysis of ten clinics. J Int AIDS Soc. 2020 Feb;23(2):e25431. 10.1002/jia2.25431
13. Krentz HB, Vu Q, Gill MJ. Updated direct costs of medical care for HIV-infected patients within a regional population from 2006 to 2017. HIV Med. 2020 May;21(5):289–98. 10.1111/hiv.12824
14. Leon-Reyes S, Schäfer J, Früh M, Schwenkglenks M, Reich O, Schmidlin K, et al. Cost estimates for human immunodeficiency virus (HIV) care and patient characteristics for health resource use from linkage of claims data with the Swiss HIV Cohort Study. Clin Infect Dis. 2019 Feb;68(5):827–33. 10.1093/cid/ciy564
15. Tran H, Saleem K, Lim M, Chow EP, Fairley CK, Terris-Prestholt F, et al. Global estimates for the lifetime cost of managing HIV: a systematic review. AIDS. 2021 Mar;35(8):1273–81. 10.1097/QAD.0000000000002887
16. Larson BA, Pascoe SJ, Huber A, Long LC, Murphy J, Miot J, et al. Will differentiated care for stable HIV patients reduce healthcare systems costs? J Int AIDS Soc. 2020 Jul;23(7):e25541. 10.1002/jia2.25541
17. Shrestha RK, Schommer JC, Taitel MS, Garza OW, Camp NM, Akinbosoye OE, et al.; Patient-centered HIV Care Model Team. Costs and cost-effectiveness of the patient-centered HIV care model: a collaboration between community-based pharmacists and primary medical providers. J Acquir Immune Defic Syndr. 2020 Nov;85(3):e48–54. 10.1097/QAI.0000000000002458
18. Cooper V, Clatworthy J, Youssef E, Llewellyn C, Miners A, Lagarde M, et al. Which aspects of health care are most valued by people living with HIV in high-income countries? A systematic review. BMC Health Serv Res. 2016 Nov;16(1):677. 10.1186/s12913-016-1914-4
19. Préau M, Protopopescu C, Raffi F, Rey D, Chêne G, Marcellin F, et al.; Anrs Co8 Aproco-Copilote Study Group. Satisfaction with care in HIV-infected patients treated with long-term follow-up antiretroviral therapy: the role of social vulnerability. AIDS Care. 2012;24(4):434–43. 10.1080/09540121.2011.613909
20. Dang BN, Westbrook RA, Black WC, Rodriguez-Barradas MC, Giordano TP. Examining the link between patient satisfaction and adherence to HIV care: a structural equation model. PLoS One. 2013;8(1):e54729. 10.1371/journal.pone.0054729
21. Pérez-Salgado D, Compean-Dardón MS, Staines-Orozco MG, Ortiz-Hernández L. Satisfaction with healthcare services and adherence to antiretroviral therapy among patients with HIV attending two public nstitutions. Rev Invest Clin. 2015;67(2):80–8.
22. Flickinger TE, Saha S, Moore RD, Beach MC. Higher quality communication and relationships are associated with improved patient engagement in HIV care. J Acquir Immune Defic Syndr. 2013 Jul;63(3):362–6. 10.1097/QAI.0b013e318295b86a
All statistical analyses were performed using R, version 4.3.1. The packages and functions used for the main analysis are: statistics (linear regression using the lm function, chi-square test using the chisq.test function), quantreg (quantile regression using the rq function) and mice (mice function to compute the multiple imputation).
Table S1Multiple imputation to impute the values of the missing total costs (Multiple imputation was performed on 50 different imputed datasets, applying predictive mean matching based on baseline characteristics, treatment, monitoring groups and centres).
| Adjusted median difference (95% CI) | p-value | |
| Total costs (US$) PCM versus SM | 286.6 (–1474.2 to 2047.315) | 0.750 |
| Total costs (US$) DTG versus cART | –3914.8 (–5700.811 to –2128.688) | <0.001 |
| PCM: patient-centred monitoring; SM: standard monitoring; cART: combined antiretroviral therapy. | ||
Table S2Additional visits outside the study plan throughout 48 weeks.
| Extra-visits performed throughout the 48 weeks | Patient-centred monitoring, n = 95 | Standard monitoring, n = 92 |
| Total | n = 132 (%) | n = 90 (%) |
| None | 70 (73.7) | 68 (73.9) |
| 1 visit | 9 (9.5) | 9 (9.8) |
| 2–3 visits | 9 (9.5) | 5 (5.4) |
| 4–5 visits | 2 (2.1) | 6 (6.5) |
| >5 visits | 5 (5.3) | 4 (4.3) |
| Type of visit (only descriptive) | 7 urgent consultations; 2 GP; 32 HIV specialist/GP; 59 other specialists; 30 other health appointments; 2 unknowns | 4 urgent consultations; 5 GP; 26 HIV specialist/GP; 33 other specialists; 22 other health appointments |
PCM: patient-centred monitoring; SM: standard monitoring; GP: general practitioner; NA: not available.
Table S3Safety outcomes of the patient-centred and standard monitoring arms: comparison of changes between baseline and week 48.
| Mean (±SD) change between baseline and week 48 | |||
| patient-centred monitoring | Standard monitoring | Adjusted difference (95% CI) | |
| Glucose profile, mmol/l | n = 91, –0.2 (±1.2) | n = 90, +0.0 (±1.2) | –0.1[–0.4; +0.2] |
| Framingham-calculated cardiovascular risk | n = 91, +0.2 (±3.0) | n = 88, +0.2 (±2.0) | –0.0 [–0.8; +0.7] |
| Estimated creatinine clearance (CKD-EPI), ml/min/1.7 3m2 | n = 92, –0.5 (±11.0) | n = 91, –0.8 (±10.7) | +0.7 [–2.3; +3.7] |
| Weight, kg | n = 92, +1.3 (±3.5) | n = 89, +0.2 (±3.4) | +1.1 [+0.1; +2.1] |
| Total cholesterol, mmol/l | n = 91, –0.3 (±0.6) | n = 89, –0.1 (±0.7) | –0.2 [–0.4; –0.0] |
| HDL, mmol/l | n = 91, –0.1 (±0.2) | n = 89, +0.0 (±0.4) | –0.1 [–0.2; –0.0] |
| LDL, mmol/l | n = 89, –0.1 (±0.6) | n = 88, –0.0 (±0.6) | –0.1 [–0.2; +0.1] |
| Triglycerides, mmol/l | n = 91, –0.2 (±0.8) | n = 89, –0.0 (±0.8) | –0.2 [–0.4; +0.0] |
| Proportion of patients with at least one adverse event throughout 48 weeks | n = 95, 62 (65.3%) | n = 92, 60 (65.2%) | –0.1% [–13.8%; +13.6%] |
| Proportion of patients with at least one serious adverse event throughout 48 weeks | n = 95, 10 (10.5%) | n = 92, 11 (12.0%) | –1.3% [–10.2%; +7.7%] |
PCM: patient-centred monitoring; SM: standard monitoring; LDL: low density lipoprotein; HDL: high density lipoprotein
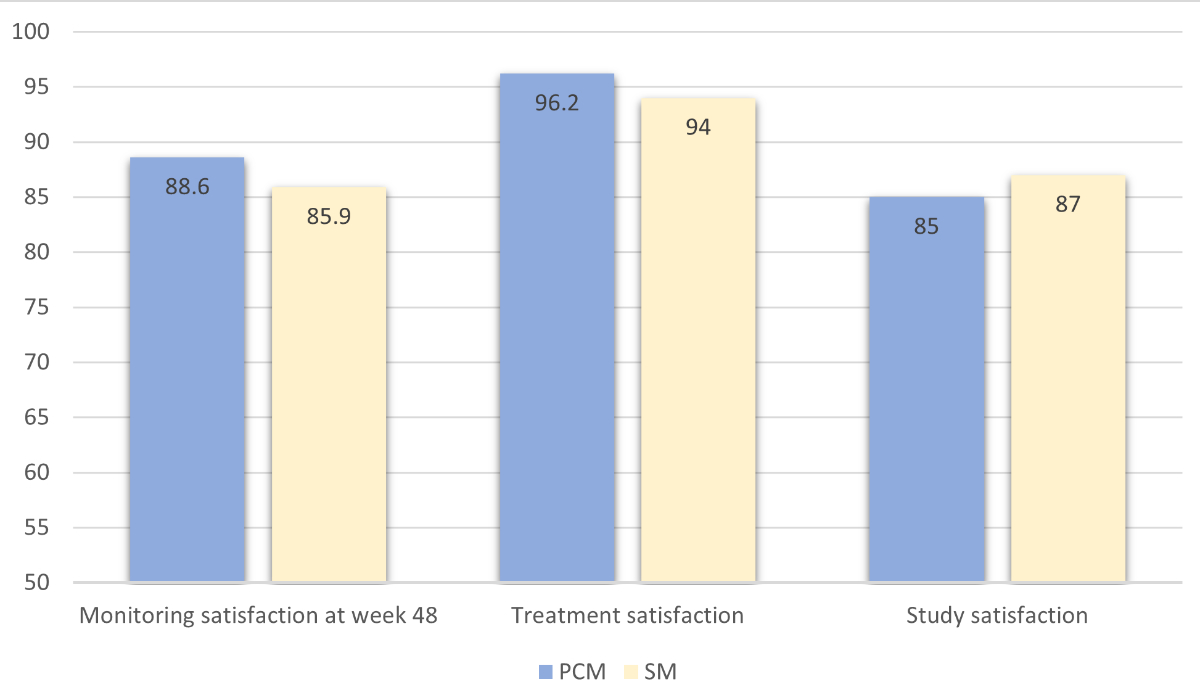
Figure S1Monitoring and treatment satisfaction, including satisfaction level with regards to study participation, were assessed at week 48 using a visual analogue scale ranging from 0 to 100 (“maximal satisfaction”).