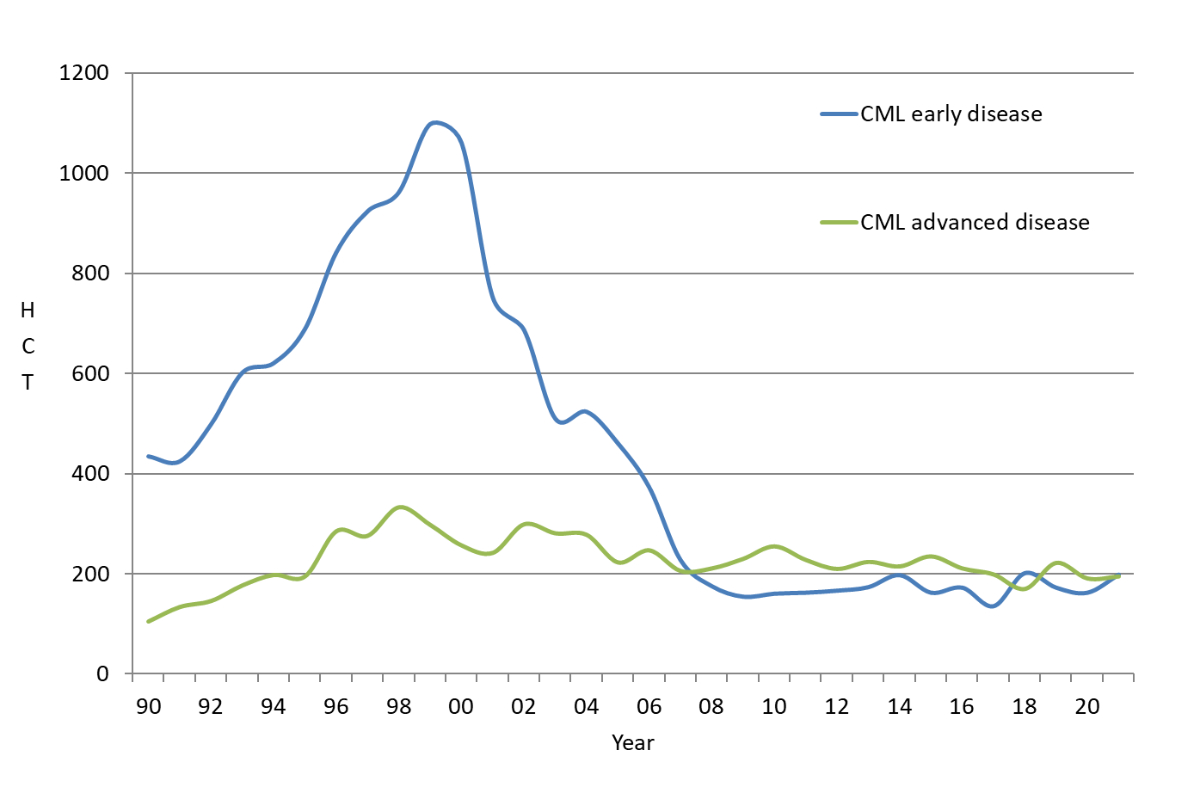
Figure 1Incidence of haematopoietic cell transplants for chronic myeloid leukaemia from 1990–2020 that were reported to the EBMT transplant activity survey.
DOI: https://doi.org/https://doi.org/10.57187/s.3754
Treatment of chronic myeloid leukaemia (CML) has changed dramatically over the years. In the last decade of the 20th century, allogeneic stem cell transplantation was the standard treatment for young and fit patients with CML in the chronic phase, as well as for patients with advanced disease [1]. CML was the main indication for allogeneic haematopoietic cell transplantation (HCT) up to 2000 (figure 1) and was replaced rapidly by the use of tyrosine kinase inhibitors once they became available. In 2000, imatinib (EMA marketing authorization: 11/2001) was introduced, followed shortly by other tyrosine kinase inhibitor drugs, such as dasatinib (11/2006), nilotinib (11/2007), bosutinib (3/2013) and ponatinib (7/2013) (https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation) [2–8]. CML exhibits clonal evolution, with the disease starting in the chronic phase and transforming to the accelerated phase and blast crisis in the majority of patients if untreated. Tyrosine kinase inhibitor treatment typically results in a response in 90% of patients, with deep and durable molecular remission in the majority. Of patients with a deep molecular response, approximately 40% retain a treatment-free remission after stopping tyrosine kinase inhibitor therapy [9]. Allogeneic HCT has remained a treatment for non-responders and for patients whose disease is transforming to the accelerated phase and blast crisis or who present at an advanced disease stage at the time of diagnosis. Therefore, allogeneic HCT has become a second- or third-line treatment although it once was a first-line treatment [10–14]. Allogeneic HCT provides a powerful targeted antitumor effect in the form of a graft-versus-host or graft-versus-tumour reaction. HCT is a complex and cost-intensive therapeutic procedure. In 1997, the Swiss Blood Stem Cell Transplantation and Cellular Therapy Group (SBST) established a central registry for all HCTs in Switzerland. As reporting is mandatory, this report includes all patients receiving an allogeneic HCT in Switzerland over the past 25 years (1997–2021). This paper describes changes in patient characteristics, the use of transplantation technology for CML and patient outcomes in the face of rapid drug development.

Figure 1Incidence of haematopoietic cell transplants for chronic myeloid leukaemia from 1990–2020 that were reported to the EBMT transplant activity survey.
In accordance with the Swiss transplant law, beginning in 1997, data relating to all HCTs performed in Switzerland were collected. Patient data are updated annually. All patients receiving a transplant for CML between 1 January 1997 and 31 December 2021 were included in this analysis.
Disease stage was defined as early (first chronic phase), intermediate (accelerated phase or second or subsequent chronic phase) and late stage (blast crisis). HCT was defined according to the criteria of the European Society for Blood and Marrow Transplantation (EBMT) [10]. An HCT is the infusion of haematopoietic stem cells provided with the intention to replace the pretransplant haematopoietic system of the recipient. Patient-related outcomes are reported. Some patients received more than one transplant. Allogeneic HCTs were restricted to Basel, Geneva, Zurich University Hospital and Zurich University Children’s Hospital. All teams were required to have ethics committee approval for data collection, and all patients or their legal representatives gave written informed consent before the transplants.
Descriptive statistics included median and range for continuous variables and frequencies for categorical variables. We used the quinquennia to compare changes in the use of technology, indications and outcomes over time. Data on tyrosine kinase inhibitor use (type and duration) prior to transplantation were unavailable over the studied time period. Therefore, quinquennia were used as a surrogate of tyrosine kinase inhibitor availability, as virtually all patients in the early disease stage were receiving tyrosine kinase inhibitors once these drugs became available, and transplantation was reserved for instances of tyrosine kinase inhibitor failure. Comparisons among groups were made using Kruskal-Wallis tests for continuous variables and chi-squared tests for categorical variables. Transplant rates were calculated as the number of allogeneic HCTs per 1 million inhabitants per year for each of the quinquennia, accounting for population growth in Switzerland (https://databank.worldbank.org/reports.aspx?source=2&series=SP.POP.TOTL&country#). Non-parametric statistics were used for comparisons, as these tests are usually more conservative than parametric tests. Outcomes measured were overall survival and progression-free survival, determined as Kaplan-Meier estimates, and non-relapse mortality and relapse incidence, determined from cumulative incidence curves adjusted for competing risks as appropriate. For overall survival, death was the event; for progression-free survival, it was the time from the transplant to relapse of the original disease or death. Non-relapse mortality was defined as death without relapse. Groups were compared using the log-rank test for Kaplan-Meier estimates and the Fine-Gray model for cumulative incidence. A p-value of ≤0.05 was considered statistically significant. A multivariable model of survival was constructed using Cox regression and forcing in quinquennium as the variable of interest and age because of biological relevance. Non-significant covariates (p >0.05) were eliminated by a stepwise backward-elimination procedure.
Data were reported from four centres: Basel (89 patients, 37%), Geneva (65, 27%), Zürich adults (82, 34%) and Zürich paediatrics (3, 1%). The median follow-up period for all allograft recipients was 12.4 years (quartiles [25% and 75%]; 6.2–18.6 years). For the purpose of this analysis, time periods were analysed in quinquennia, as the observation period spanned 25 years. Q1 (1997–2001) included 96 (40.2%) patients, Q2 (2002–2006) included 56 (23.4%) patients, Q3 (2007–2011) included 25 (10.5%) patients, Q4 (2012–2016) included 34 (14.2%) patients and Q5 (2017–2021) included 28 (11.7%) patients. Median follow-up was assessed for each quinquennium and was 19.1 years (14.5–20.3) for 1997–2001, 15.6 years (14–16.1) for 2002–2006, 10 years (9.1–12.6) for 2007–2011, 6.1 years (4.2–7.3) for 2012–2016 and 1.4 years (0.3–2.4) for 2017–2021.
Overall, 239 patients received their first allogeneic transplant from 1997–2021. Fifteen of these patients received subsequent transplants, yielding a total of 254 transplants during the 25-year period. Of the allogeneic HCT recipients, 13 received a prior autologous HCT, as practiced before and around 2000. Seventy-one patients died, with the main causes of death being relapse or progression of the original disease (n = 29), secondary malignancy (n = 4) and non-relapse mortality (n = 38).
Incidence data report CML at 0.8–1.0 cases per 100,000/year, thus expecting approximately 65–100 cases per annum in Switzerland. In Q1, approximately 20–30% of diagnosed patients underwent allogeneic HCT, compared to 4–7% in Q5. Median CML transplant rates for allogeneic HCT per 1 million inhabitants per quinquennium ranged from 2.52 in Q1 to 0.47 in Q5 (Q1: 2.52; Q2: 1.49; Q3: 0.63; Q4: 0.73; Q5: 0.47). Table 1 shows patient demographic data, as well as transplant technology, for the entire cohort and for each quinquennium. Comparisons across quinquennia showed that patient characteristics changed over time. Recent patients were older and had a longer interval from diagnosis to transplantation, likely because of treatment with tyrosine kinase inhibitors, but proportions of patients who received transplants in an early versus advanced disease stage differed minimally and in a skewed manner (table 1). Transplant technology changed, as well. Patients received intensive conditioning regimens less often due to higher age and/or comorbidities and more commonly had peripheral blood as opposed to bone marrow transplants. However, the type of stem cell donor selected did not differ significantly across quinquennia. Table 2 shows univariable outcomes for the quinquennia. There were no significant differences in survival, progression-free survival, non-relapse mortality, relapse incidence or the incidence of acute and chronic graft-versus-host disease over time (table 2, figures 2A–2D). Other factors were significantly associated with outcomes. For survival, disease stage (figure 3A) was a major driver of mortality. To a lesser extent, type donor (figure 3B), patient age (figure 3C) and interval from diagnosis to transplantation for all patients (figure 3d) and for patients transplanted in first chronic phase were also drivers of mortality. Figure 4 shows overall survival for the entire cohort conditional on surviving to 2 years, which was 85 ± 7% after 20 years. Results of the multivariable analysis of survival are shown in table 3. Patient age (p = 0.065), type of donor (p = 0.011) and disease stage (p <0.0001), but not quinquennium of treatment (p = 0.37), were significantly associated with the risk of death. Other variables, such as the interval from diagnosis to transplantation, stem cell source, conditioning intensity and graft manipulation, were not significantly associated with survival.
Table 1Allogeneic haematopoietic cell transplantation (HCT) for chronic myeloid leukaemia in Switzerland from 1997–2021 by quinquennium. Patient characteristics are shown overall and by quinquennium.
| Quinquennium | Total patients | ||||||||
| 1997–2001 | 2002–2006 | 2007–2011 | 2012–2016 | 2017–2021 | |||||
| N (% within time span) | N (% within time span) | N (% within time span) | N (% within time span) | N (% within time span) | p-value | N | % | ||
| Age at time of HCT | <20 | 6 (6%) | 1 (2%) | 1 (4%) | 1 (3%) | 2 (7%) | 0.001 | 11 | 4.6 |
| 20–40 | 52 (54%) | 22 (39%) | 7 (28%) | 8 (24%) | 7 (25%) | 96 | 40.2 | ||
| 40–60 | 38 (40%) | 32 (57%) | 14 (56%) | 20 (59%) | 14 (50%) | 118 | 49.4 | ||
| >60 | 0 | 1 (2%) | 3 (12%) | 5 (15%) | 5 (18%) | 14 | 5.8 | ||
| Patient sex | Male | 62 (65%) | 33 (59%) | 14 (56%) | 22 (65%) | 18 (64%) | 0.9 | 149 | 62.3 |
| Female | 34 (35%) | 23 (41%) | 11 (44%) | 12 (35%) | 10 (36%) | 90 | 37.7 | ||
| Disease stage at time of HCT | Early | 70 (73%) | 39 (70%) | 8 (32%) | 18 (53%) | 20 (71%) | 0.01 | 155 | 64.9 |
| Intermediate | 21 (22%) | 15 (27%) | 14 (56%) | 12 (35%) | 5 (18%) | 67 | 28 | ||
| Late | 5 (5%) | 2 (3%) | 3 (12%) | 4 (12%) | 3 (11%) | 17 | 7.1 | ||
| Interval from diagnosis to HCT: all disease stages | <365 days | 60 (63%) | 29 (52%) | 7 (28%) | 12 (35%) | 10 (36%) | 0.003 | 118 | 49.4 |
| >365 days | 36 (37%) | 27 (48%) | 18 (72%) | 22 (65%) | 18 (64%) | 121 | 50.6 | ||
| Interval from diagnosis to HCT: early stage only | <365 days | 48 (69%) | 19 (49%) | 4 (50%) | 3 (17%) | 4 (20%) | <0.001 | 78 | 50 |
| >365 days | 22 (31%) | 20 (51%) | 4 (50%) | 15 (83%) | 16 (80%) | 77 | 50 | ||
| Risk score | 0–1 | 17 (18%) | 3 (5%) | 2 (8%) | 1 (3%) | 1 (4%) | <0.001 | 24 | 10 |
| 2–3 | 53 (55%) | 34 (61%) | 5 (20%) | 14 (41%) | 10 (36%) | 116 | 48.5 | ||
| 4–5 | 25 (26%) | 19 (34%) | 17 (68%) | 17 (50%) | 16 (57%) | 94 | 39.3 | ||
| 6–7 | 1 (1%) | 0 | 1 (4%) | 2 (6%) | 1 (4%) | 5 | 2.1 | ||
| Donor type | HLA-ID sibling | 56 (58%) | 30 (54%) | 10 (40%) | 15 (44%) | 7 (25%) | 0.25 | 118 | 49.4 |
| Twin | 1 (1%) | 1 (2%) | 0 | 0 | 0 | 2 | 0.8 | ||
| Mismatched relative | 6 (6%) | 2 (4%) | 2 (8%) | 3 (9%) | 4 (14%) | 17 | 7.1 | ||
| Unrelated | 33 (34%) | 23 (41%) | 13 (52%) | 16 (47%) | 17 (61%) | 102 | 42.7 | ||
| CMV status (donor-recipient) | Neg-neg | 19 (33%) | 7 (26%) | 11 (48%) | 8 (24%) | 7 (25%) | 0.62 | 52 | 21.8 |
| Pos-neg | 12 (21%) | 5 (19%) | 1 (4%) | 5 (15%) | 7 (25%) | 30 | 12.6 | ||
| Pos-pos | 20 (35%) | 9 (33%) | 7 (30%) | 14 (42%) | 11 (39%) | 61 | 25.5 | ||
| Neg-pos | 6 (11%) | 6 (22%) | 4 (17%) | 6 (18%) | 3 (11%) | 25 | 10.5 | ||
| Sex match | Male-male | 39 (41%) | 22 (39%) | 10 (40%) | 16 (47%) | 10 (36%) | 0.37 | 97 | 40.6 |
| Female-male | 23 (24%) | 11 (20%) | 4 (16%) | 6 (18%) | 8 (29%) | 52 | 21.8 | ||
| Male-female | 21 (22%) | 13 (23%) | 2 (8%) | 7 (21%) | 8 (29%) | 51 | 21.3 | ||
| Female-female | 13 (14%) | 10 (18%) | 9 (36%) | 5 (15%) | 2 (7%) | 39 | 16.3 | ||
| Stem cell source | Bone marrow | 50 (52%) | 23 (41%) | 4 (16%) | 7 (21%) | 3 (11%) | <0.001 | 87 | 36.4 |
| Peripheral blood | 45 (47%) | 33 (59%) | 20 (80%) | 27 (79%) | 25 (89%) | 150 | 62.8 | ||
| Cord blood | 1 (1%) | 0 | 1 (4%) | 0 | 0 | 2 | 0.8 | ||
| Conditioning | Non-myeloablative | 5 (7%) | 4 (7%) | 4 (16%) | 10 (29%) | 7 (25%) | 0.003 | 30 | 12.6 |
| Myeloablative | 72 (94%) | 52 (93%) | 21 (84%) | 24 (71%) | 21 (75%) | 190 | 79.5 | ||
| T-cell depletion | No depletion | 70 (73%) | 42 (75%) | 23 (92%) | 29 (85%) | 24 (86%) | 0.15 | 188 | 78.7 |
| In vitro T-cell depletion | 26 (27%) | 14 (25%) | 2 (8%) | 5 (15%) | 4 (14%) | 51 | 21.3 | ||
Table 2Allogeneic haematopoietic cell transplantation (HCT) for chronic myeloid leukaemia in Switzerland from 1997–2021. Outcome analysis is by quinquennium with a 95% confidence interval. Outcome overview at 5 years post-transplant by overall survival (OS), progression-free survival (PFS), non-relapse mortality (NRM), relapse or progression, and incidence of acute graft-versus-host disease (aGvHD) at 180 days and chronic graft-versus-host disease (cGvHD).
| OS | PFS | NRM | Relapse/progression | aGvHD – grade 2–4 | cGvHD – present | ||
| 5 years | 5 years | 5 years | 5 years | 180 days | 5 years | ||
| All cases | Overall | 73% (67–79) | 55% (48–62) | 13% (10–19) | 32% (27–95%) | 26% (21–33) | 56% (50–64) |
| Quinquennium | 1997–2001 | 73% (64–82) | 52% (42–62) | 19%(12–29) | 29% (21–40) | 24% (16–36) | 58% (48–70) |
| 2002–2006 | 82% (72–92) | 59% (46–72) | 12% (6–25) | 29% (19–43) | 15% (8–30) | 61% (49–76) | |
| 2007–2011 | 56% (36–76) | 40% (20–60) | 8% (2–30) | 52% (36–76) | 38% (22–66) | 54% (37–78) | |
| 2012–2016 | 69% (53–85) | 57% (39–75) | 6% (2–23) | 37% (24–58) | 27% (15–48) | 52% (37–74) | |
| 2017–2021 | 76% (57–95) | 75% (57–93) | 9% (2–33) | 16% (7–40) | 41% (26–65) | 46% (26–79) | |
| p-value | 0.31 | 0.33 | 0.53 | 0.31 | 0.17 | 0.57 | |
| Age at time of HCT | <20 | 90% (71–100) | 77% (48–100) | 10% (2–64) | n/a | 22% (7–75) | 38% (15–95) |
| 20–40 | 77% (68–86) | 60% (50–70) | 15% (9–24) | 26% (18–36) | 29% (20–41) | 58% (49–70) | |
| 40–60 | 72% (64–80) | 50% (40–60) | 12% (7–20) | 38% (30–48) | 19% (13–29) | 57% (48–67) | |
| >60 | 42% (13–71) | 36% (8–64) | 16% (4–58) | 48% (27–87) | 64% (44–95) | 57% (33–98) | |
| p-value | 0.025 | 0.04 | 0.94 | 0.05 | 0.01 | 0.60 | |
| Donor type | HLA-ID sibling | 82% (75–89) | 57% (48–66) | 2% (1–8) | 40% (32–51) | 15% (9–24) | 55% (46–65) |
| Mismatched relative | 49% (21–77) | 30% (4–56) | 18% (6–49) | 52% (31–88) | 54% (33–89) | 45% (23–88) | |
| Unrelated | 67% (57–77) | 56% (46–66) | 24% (17–34) | 20% (13–30) | 36% (27–48) | 61% (51–73) | |
| p-value | 0.01 | 0.04 | <0.001 | 0.001 | <0.001 | 0.39 | |
| Interval from diagnosis to transplant: all disease stages | <365 days | 79% (71–87) | 58% (49–67) | 8% (4–15) | 34% (26–44) | 20% (13–30) | 60% (51–70) |
| >365 days | 67% (58–76) | 51% (41–61) | 19% (13–27) | 30% (23–40) | 32% (24–41) | 53% (43–64) | |
| p-value | 0.03 | 0.22 | 0.02 | 0.56 | 0.07 | 0.07 | |
| Interval from diagnosis to transplant: early disease stage | <365 days | 91% (84–98) | 66% (55–77) | 4% (1–12) | 30%(21–42) | 10% (5–21) | 59% (49–71) |
| >365 days | 79% (69–89) | 65% (54–76) | 15% (8–25) | 20% (13–32) | 30% (21–43) | 49% (38–64) | |
| p-value | 0.096 | 0.76 | 0.04 | 0.21 | 0.007 | 0.03 | |
| Disease stage at time of HCT | Early | 85% (79–91) | 66% (58–74) | 9% (6–15) | 25% (19–33) | 21% (15–29) | 54% (46–63) |
| Intermediate | 58% (46–70) | 40% (28–52) | 20% (12–32) | 40% (30–54) | 38% (27–53) | 66% (54–80) | |
| Late | 16% (0–36) | 10% (0–27) | 25% (10–58) | 66% (45–95) | 29% (12–65) | 38% (19–76) | |
| p-value | <0.001 | ≤0.001 | 0.10 | ≤0.001 | 0.03 | 0.19 | |
| Conditioning | Non-myeloablative | 50% (31–69) | 33% (13–53) | 14% (6–34) | 53% (37–76) | 31% (18–53) | 44% (28–69) |
| Myeloablative | 78% (72–84) | 58% (46–65) | 12% (8–18) | 30% (24–38) | 25% (19–33) | 59% (52–67) | |
| p-value | <0.001 | ≤0.001 | 0.9 | 0.001 | 0.65 | 0.16 | |
| Stem cell source | Bone marrow | 77% (68–87) | 67% (57–77) | 19% (12–29) | 14% (8–24) | 23% (15–35) | 62% (52–74) |
| Peripheral blood | 72% (64–80) | 48% (39–57) | 10% (6–16) | 42% (35–52) | 27% (21–36) | 53% (45–63) | |
| p-value | 0.16 | 0.002 | 0.33 | ≤0.001 | 0.58 | 0.11 | |
| T-cell depletion | None | 71% (65–77) | 61% (54–68) | 14% (10–20) | 25% (19–32) | 27% (21–36) | 62% (54–70) |
| In vitro T-cell depletion | 80% (69–91) | 32% (19–45) | 10% (4–23) | 58% (46–74) | 22% (13–37) | 39% (27–56) | |
| p-value | 0.12 | ≤0.001 | 0.3 | ≤0.001 | 0.42 | 0.004 | |
Table 3Allogeneic haematopoietic cell transplantation (HCT) for chronic myeloid leukaemia in Switzerland 1997–2021. Multivariate relative risk and 95% confidence interval analysis of survival adjusted for all significant covariates: quinquennium, age at time of transplant, donor type and disease stage at time of transplant.
| Multivariate relative risk and 95% confidence interval | p-value | ||
| Quinquennium | 1997–2001 | 1.0 | 0.37 |
| 2002–2006 | 0.67 (0.36–1.28) | ||
| 2007–2011 | 0.59 (0.27–1.28) | ||
| 2012–2016 | 0.46 (0.20–1.07) | ||
| 2017–2021 | 0.51 (0.19–1.39) | ||
| Age at time of HCT | <20 | 1.0 | 0.065 |
| 20–40 | 2.0 (0.27–15.0) | ||
| 40–60 | 3.1 (0.41–22.89) | ||
| >60 | 6.59 (0.75–57.75) | ||
| Donor type | HLA-ID sibling | 1.0 | 0.011 |
| Mismatched relative | 2.79 (1.2–6.49) | ||
| Unrelated | 1.99 (1.17–3.39) | ||
| Disease stage | Early | 1.0 | 0.0001 |
| Intermediate | 3.33 (1.92–5.76) | ||
| Late | 12.4 (6.08–25.29) | ||
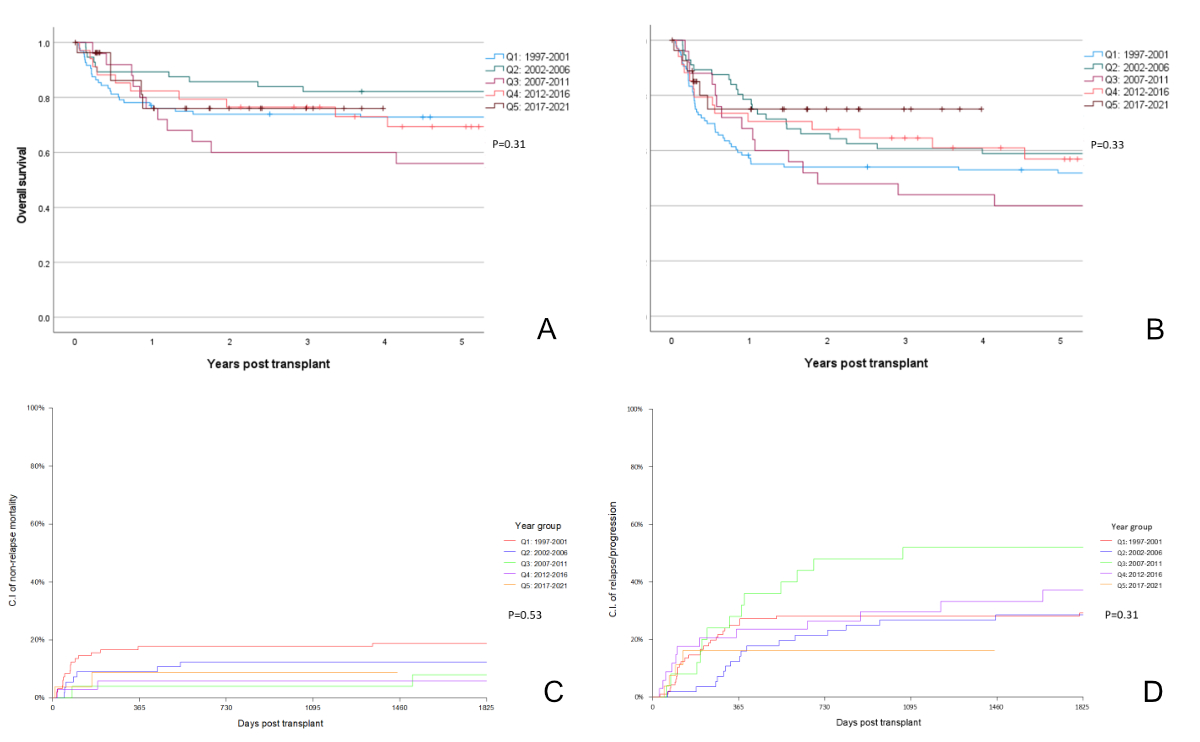
Figure 2Allogeneic haematopoietic cell transplantation for chronic myeloid leukaemia in Switzerland from 1997–2021. A: Overall survival at 5 years by quinquennium. B: Progression-free survival at 5 years by quinquennium. C: Non-relapse mortality at 5 years by quinquennium. D: Relapse/progression at 5 years by quinquennium.
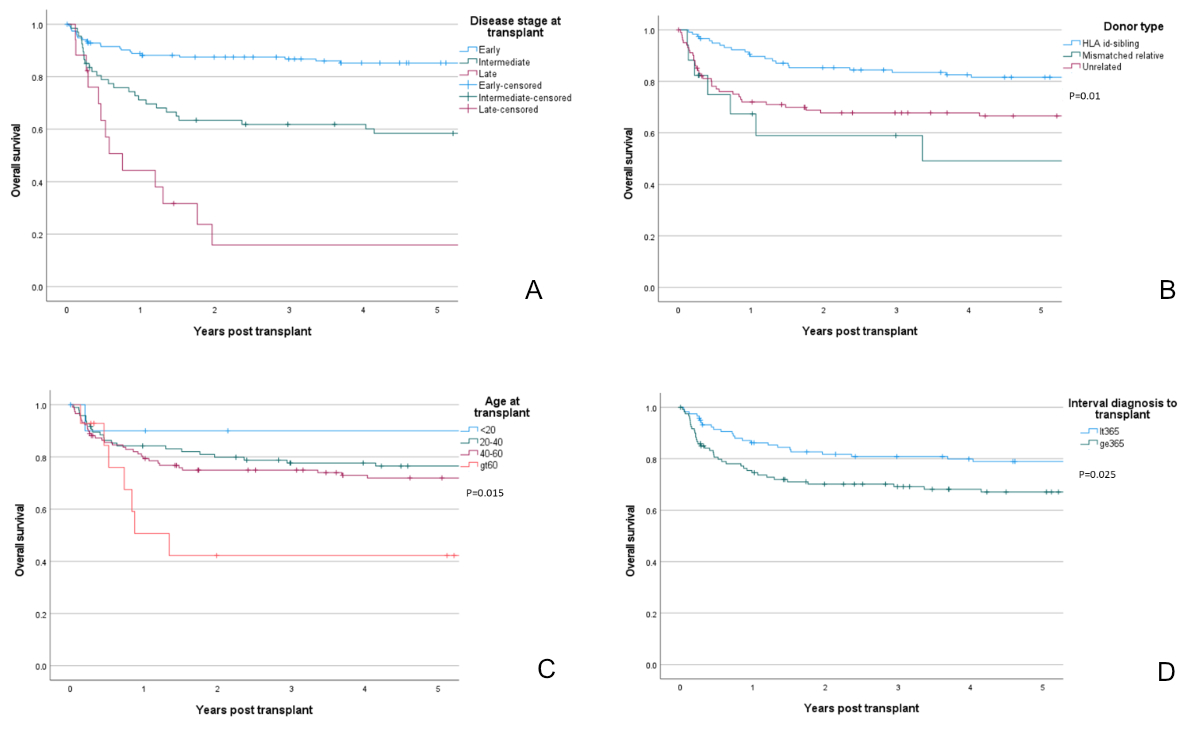
Figure 3Allogeneic haematopoietic cell transplantation for chronic myeloid leukaemia in Switzerland from 1997–2021. A: Overall survival at 5 years by disease stage at time of transplant. B: Overall survival at 5 years by donor type. C: Overall survival at 5 years by age at time of transplant. D: Overall survival at 5 years by interval from diagnosis to transplant.
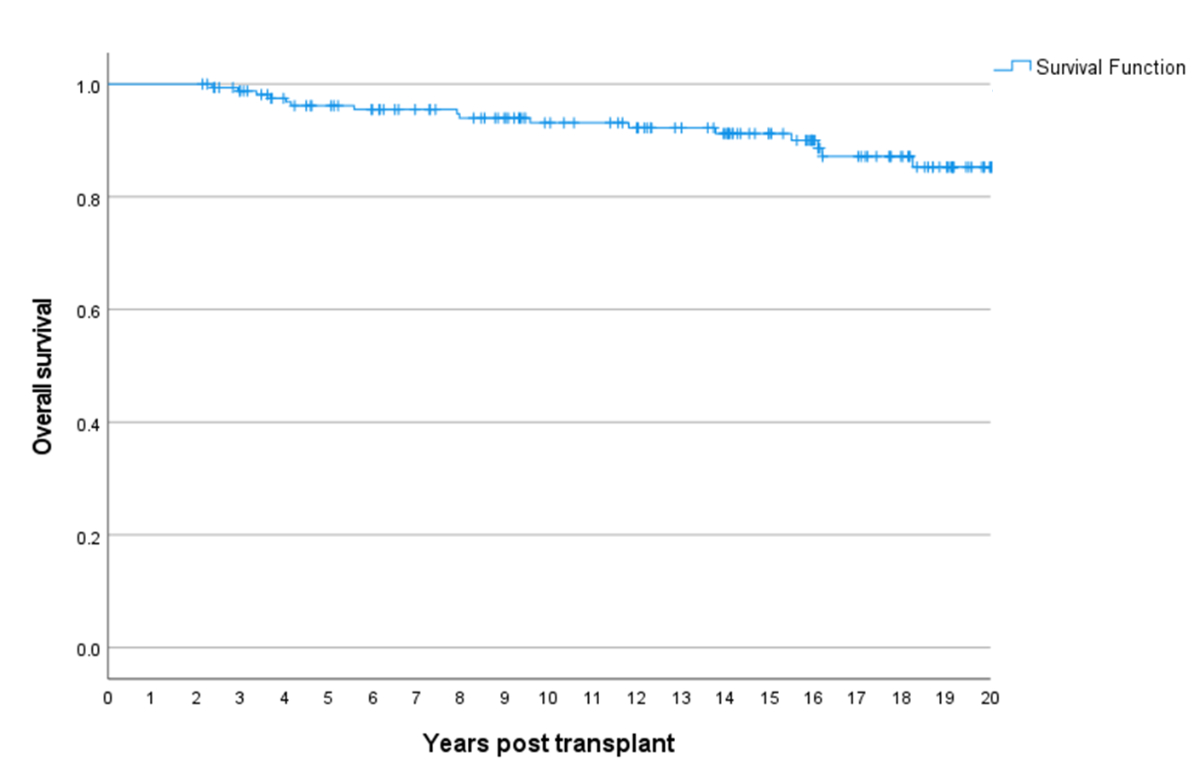
Figure 4Allogeneic haematopoietic cell transplantation for chronic myeloid leukaemia in Switzerland from 1997–2021. Overall survival at 20 years in 2-year survivors.
Treatment of CML has changed dramatically over time. In 2000, tyrosine kinase inhibitors were introduced, and second- and third-generation tyrosine kinase inhibitors were developed rapidly. CML was the main indication for allogeneic HCT until 2000, when it was replaced rapidly by the use of tyrosine kinase inhibitors as first-line and subsequently second-line treatment. Allogeneic HCT has remained a treatment for non-responders to tyrosine kinase inhibitors, for patients experiencing unacceptable toxicities and for patients whose disease is transforming to the accelerated phase and blast crisis or who present with an advanced disease stage at the time of diagnosis. Established indications for HCT since 2007 (Q3) for chronic phase CML are failure of first-line tyrosine kinase inhibitors, predicted poor response to second-line tyrosine kinase inhibitors, failure to respond to second-line tyrosine kinase inhibitors, presence of the T315I mutation and/or failure to respond to ponatinib, and presence of repeated grade 4 cytopenias despite appropriate dose reduction and cytokine support. For the advanced phase, the indications are being tyrosine kinase inhibitor naïve, tyrosine kinase inhibitor naïve with suboptimal response to tyrosine kinase inhibitors and tyrosine kinase inhibitor resistant. For the blast phase, the indications are progression to the second chronic phase after use of tyrosine kinase inhibitors or chemotherapy. We show here that tyrosine kinase inhibitors have led to a rapid decrease in the use of stem cell transplantation for CML, with the transplant rate dropping from 2.52 per million inhabitants per annum in Switzerland in Q1 to 0.47 per million inhabitants per annum in Q5.
Overall, the long-term outcome of transplantation for CML has not changed significantly over the decades, but the results of the last 5-year period, with estimated overall survival of 76% and progression-free survival of 75%, are encouraging. This can be viewed positively given that patients were older and had longer disease duration in later time periods and given that allogeneic HCT for CML has been moved from first-line treatment to second- or third-line treatment or even further.
The most important driver of the outcome was disease stage, with transplants for blast phase CML resulting in poor survival outcomes, while first chronic phase transplants had a good prognosis. This leads to recommendations to plan for transplants in patients refractory to tyrosine kinase inhibitors or experiencing unacceptable toxicity due to tyrosine kinase inhibitors prior to disease transformation to the accelerated or blast phase. The number of patients with intermediate stage disease receiving transplants increased from the second to the third quinquennium and then decreased. This pattern is best explained by enthusiasm for the possibilities of tyrosine kinase inhibitors followed by a readjustment involving guiding patients to transplantation while still in the first chronic phase. Compared to European data published recently [13], the number of transplants in patients with advanced disease in Switzerland has not increased over time, which may indicate that patients are monitored closely and that transplantation is proposed prior to reaching advanced phases.
This study had several limitations. We did not have data on the type and duration of tyrosine kinase inhibitor treatment or reasons for transplant (e.g. not obtaining deep molecular remission or toxicity induced by tyrosine kinase inhibitor treatment). However, we did have data on all patients receiving transplants to treat CML in Switzerland over a long time period, and we had an adequate follow-up period for evaluating long-term outcomes after transplantation. However, the long time period of the study meant that follow-up periods differed greatly by quinquennium. Therefore, we limited the graphical outcome curves to 5 years post-transplant, with adequate follow-up for patients in each quinquennium.
Since introduction of tyrosine kinase inhibitors, HCT has been used less frequently to treat CML. Patients in recent cohorts received transplants at an older age and later in the disease course. Despite these higher risks, the outcome of allogeneic HCT has neither worsened nor improved over time. The major factor affecting the outcome of allogeneic HCT remains the disease stage at time of transplantation. Therefore, it is of the utmost importance to monitor CML patients closely and direct them to transplant centres prior to disease transformation to the accelerated or blast phases.
Special thanks go to the cooperation of the participating centers and their staff at the University Hospital Basel, University Hospital of Geneva, University and University Hospital of Zurich and University Children's Hospital Zurich.
Author’s contributions: HB and JRP were responsible for the analysis. DH, HB, MM, SM-L, US, GN, TG, JH, YC and JRP contributed to the writing of the manuscript.
No funding to declare.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. YC has received consulting fees from MSD, Novartis, Incyte, BMS, Pfizer, Abbvie, Roche, Jazz, Gilead, Amgen, Astra-Zeneca, Servier and travel support from MSD, Roche, Gilead, Amgen, Incyte, Abbvie, Janssen, Astra-Zeneca, Jazz. The other authors have not disclosed any potential conflicts of interest.
1. Speck B, Bortin MM, Champlin R, Goldman JM, Herzig RH, McGlave PB, et al. Allogeneic bone-marrow transplantation for chronic myelogenous leukaemia. Lancet. 1984 Mar;1(8378):665–8. 10.1016/S0140-6736(84)92179-2
2. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al.; IRIS Investigators. Long-term outcomes of Imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017 Mar;376(10):917–27. 10.1056/NEJMoa1609324
3. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018 Mar;93(3):442–59. 10.1002/ajh.25011
4. Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011 Jan;117(4):1141–5. 10.1182/blood-2010-03-277152
5. Shah NP, Guilhot F, Cortes JE, Schiffer CA, le Coutre P, Brümmendorf TH, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014 Apr;123(15):2317–24. 10.1182/blood-2013-10-532341
6. Khoury HJ, Cortes JE, Kantarjian HM, Gambacorti-Passerini C, Baccarani M, Kim DW, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012 Apr;119(15):3403–12. 10.1182/blood-2011-11-390120
7. Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010 Jun;362(24):2260–70. 10.1056/NEJMoa1002315
8. Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al.; ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010 Jun;362(24):2251–9. 10.1056/NEJMoa0912614
9. Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J Clin Oncol. 2017 Jan;35(3):298–305. 10.1200/JCO.2016.68.2914
10. Hehlmann R, Berger U, Pfirrmann M, Heimpel H, Hochhaus A, Hasford J, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007 Jun;109(11):4686–92. 10.1182/blood-2006-11-055186
11. Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al.; German CML Study Group. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010 Mar;115(10):1880–5. 10.1182/blood-2009-08-237115
12. Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al.; European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022 Aug;57(8):1217–39. 10.1038/s41409-022-01691-w
13. Gratwohl A, Pfirrmann M, Zander A, Kröger N, Beelen D, Novotny J, et al.; SAKK; German CML Study Group. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016 Mar;30(3):562–9. 10.1038/leu.2015.281
14. Chalandon YS, Bianchi G, Gras L, Koster L, Apperley J, Byrne J, et al. Allogeneic hematopoietic cell transplantation in patients with chronic phase chronic myeloid leukemia in the era of third generation tyrosine kinase inhibitors: A retrospective study by the chronic malignancies working party of the EBMT. Am J Hematol. 2022 Oct; Online ahead of print.