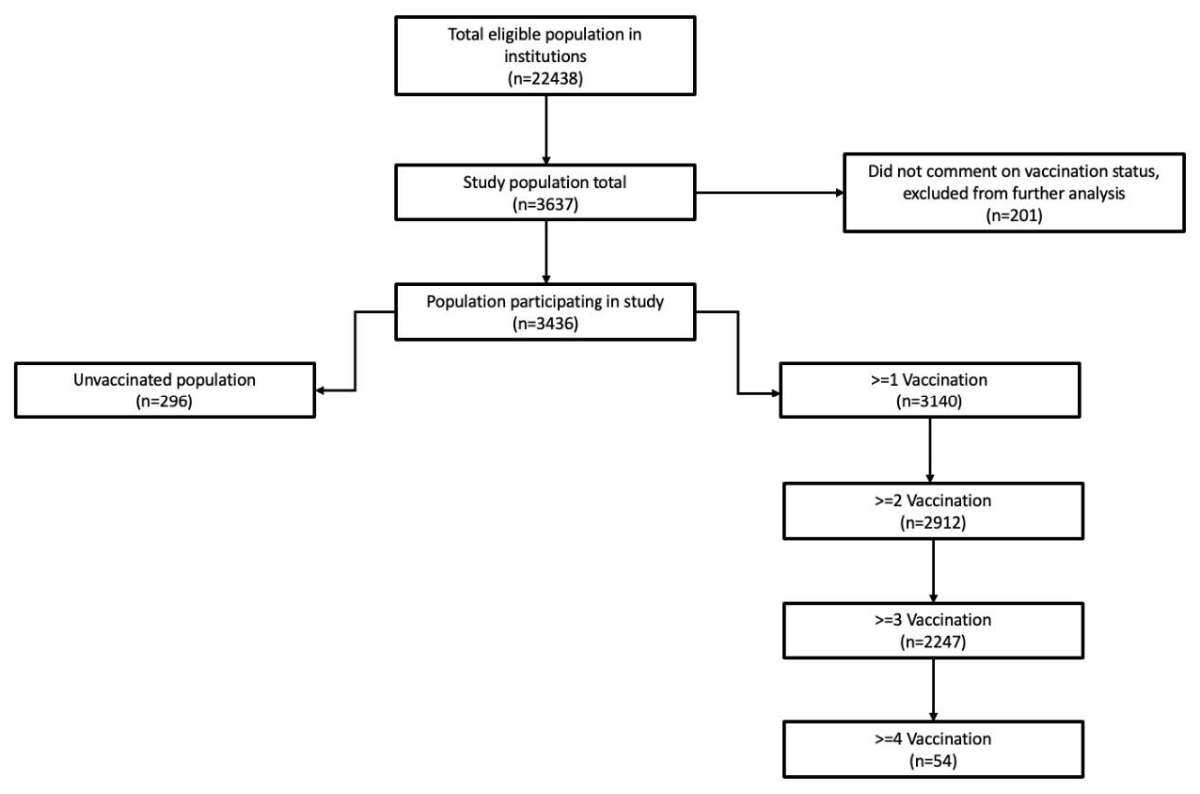
Figure 1Study flowchart.
DOI: https://doi.org/https://doi.org/10.57187/s.3734
Since the start of the COVID-19 pandemic in March 2020, 4.4 million people have tested positive for COVID-19 in Switzerland, and, as of October 2023, there have been more than 14,000 deaths due to COVID-19 in Switzerland, according to data from Swiss federal health authorities [1]. Population-based studies from primarily western and southern Switzerland have suggested that by mid-2022, a large portion of the general population had developed specific antibodies to SARS-CoV-2 [2]. However, data from other geographical regions in Switzerland, particularly from healthcare workers, are lacking. In fact, healthcare workers are highly exposed to SARS-CoV-2 and therefore have a higher risk of infection than the general population, according to data from the early phase of the pandemic [3].
Because of their high frequency of patient exposure, healthcare workers have been recommended to undergo priority vaccination against COVID-19, to ensure the delivery of essential services and to reduce the spread of infection in healthcare facilities [4, 5]. Studies have shown excellent effectiveness of both the Pfizer-BioNTech (BNT162b2) and the Moderna (mRNA-1273) mRNA vaccines against COVID-19 (89% and 96%, respectively) [6]. However, vaccination hesitancy among healthcare workers is not uncommon and is a complex issue driven by context specific factors [7]. Acceptance of a vaccine is associated with various levels of trust regarding the vaccine itself, the healthcare system, and external factors [8].
In Switzerland and in other countries, influenza vaccination uptake is significantly lower in nurses than physicians [9, 10]. An important barrier to nurses receiving seasonal influenza vaccination is the idea of maintaining a strong and healthy body, and decisional autonomy [11]. Importantly, individual vaccination status is associated with healthcare workers’ recommending vaccination to their patients [9]. Regarding SARS-CoV-2, few data are available on vaccine uptake and reasons for non-vaccination among Swiss healthcare workers. Although healthcare workers appear to be more likely to undergo vaccination than the general population [12], in a study from December 2020, fewer than half of the 3,793 participating healthcare workers were willing to receive the SARS-CoV-2 vaccine [13].
In our multicentre cohort consisting of healthcare workers from acute and non-acute healthcare settings in eastern and northern Switzerland, we aimed to assess the extent of SARS-CoV-2 humoral immunity elicited by previous infections and/or vaccination. In addition, we identified the reasons why healthcare workers were not vaccinated.
This multicentre cohort recruited volunteer healthcare workers from 14 healthcare institutions in northern and eastern Switzerland [16]. The institutions were primarily acute care hospitals (8), but also included psychiatric (3), geriatric (1), rehabilitation (1), and paediatric (1) clinics. All hospital employees were eligible to participate, regardless of patient contact, if they were 16 years of age or older. Electronic informed consent was obtained from all participants. The ethics committee of eastern Switzerland (BASEC number #2020-00502) approved the study.
Within a nested cross-sectional study performed in May and June 2022, participants answered an electronic questionnaire regarding baseline characteristics, SARS-CoV-2 and seasonal influenza vaccination status, and previous SARS-CoV-2 infections (i.e., reporting of any positive SARS-CoV-2 swab test by PCR or rapid antigen testing). A reminder was sent to non-responding participants to increase the participation rate. Participants not vaccinated against SARS-CoV-2 additionally answered a questionnaire regarding their reasons for non-vaccination, consisting of 22 statements. These statements were developed by the study team and included topics such as fear of adverse effects, concerns about vaccine efficacy, and questions about vaccine hesitancy in general, including religious beliefs, and preferring natural to vaccine-induced immunity. Participants answered with “strongly agree”, “agree”, “neutral”, “disagree”, or “strongly disagree” in response to the statements (table S1 in the appendix). In addition, in May and June 2022, participants underwent testing for SARS-CoV-2 anti-spike (anti-S) and anti-nucleocapsid (anti-N) antibodies with the Roche Elecsys® Anti-SARS-CoV-2 (Roche Diagnostics, Rotkreuz, Switzerland) electro-chemiluminescence immunoassay [14–16].
The main outcome variable was SARS-CoV-2 vaccination status as of June 2022. Participants who had received one or more vaccine doses were considered vaccinated. Those who chose not to answer the question on SARS-CoV-2 vaccination were excluded from further analyses. Other important variables were previous SARS-CoV-2 infection as of June 2022, defined by a positive SARS-CoV-2 nasopharyngeal swab test and/or positive anti-N antibodies. To compare SARS-CoV-2 anti-S and anti-N positivity across participant ages, we grouped the variable of age into six 9-year categories. Healthcare settings included adult and paediatric acute care, psychiatry, and rehabilitation/geriatric clinics. Participant professions were grouped into nurses, including radiology assistants (reference group), therapists (physiotherapists, ergotherapists, logopedists, and nutritional experts), physicians, administrative personnel (administrators or secretaries), and others (researchers, social workers, technical service personnel, facility and room management personnel, IT personnel, and laboratory personnel). Education status was grouped into those with basic education (i.e., compulsory schools or no formal education) and those with higher education (i.e., vocational or secondary education, such as a high school diploma or university degree). We also recorded whether healthcare workers used home remedies for prophylaxis against SARS-CoV-2, such as vitamin D or phytotherapeutic agents.
Immunity against SARS-CoV-2 (as of June 2022) was assessed according to the proportion of previously infected and of previously vaccinated study participants across age categories and participating institutions. Similarly, for vaccinated individuals, we compared the numbers of vaccine doses received.
We used descriptive statistics to compare baseline characteristics between vaccinated and unvaccinated participants; Fisher’s exact test was used for dichotomous variables, and the Mann-Whitney U test was used for continuous variables. We performed multivariable logistic regression (i.e., complete case analysis) to identify factors associated with SARS-CoV-2 vaccination. Variables with a p-value of 0.05 in univariable analysis were entered into the multivariable model. We performed three sensitivity analyses: first, we calculated a model by using backward variable selection; second, we included age × gender as an interaction term, to determine whether younger females might be relatively more reluctant to receive SARS-CoV-2 vaccination; and third, we applied a random effects model by using institution as a cluster variable to account for potential intra-institutional correlations. We calculated odds ratios (OR) and corresponding 95% confidence intervals (CI). Two-sided p-values <0.05 were considered significant. R statistical software version 4.0.2 was used for statistical analysis.
The entire eligible population (i.e., all employees at participating institutions) comprised 22,438 people (figure 1). The study population consisted of 3,637 individuals, of whom 201 (5.5%) did not indicate their vaccination status and were therefore excluded. Of the remaining 3,436 participants (i.e., 15% of the eligible population), 2,794 (81.3%) identified as female, and 642 (18.7%) identified as male; the mean age was 43.7 years (range 16–73 years). Most healthcare workers worked in an adult or paediatric acute care institutions (n = 2998, 87.3%), followed by psychiatric (n = 292, 8.5%) and geriatric/rehabilitation (n = 146, 4.2%) clinics.

Figure 1Study flowchart.
Of 3,436 participants, 296 (8.9%) were not vaccinated, and 3,140 had received at least one (91.4%), 2,912 had received at least two (84.7%), 2,247 had received at least three (65.4%), and 54 (1.6%) had received at least four vaccine doses (figure 1). The number of SARS-CoV-2 vaccine doses received increased with age (figure 2). Participants were administered exclusively the Comirnaty vaccine (BNT162b2; n = 2,395, 76.3%), Spikevax (mRNA-1273; n = 540, 17.2%), a combination of both Comirnaty and Spikevax (n = 142, 4.5%), or other vaccines (n = 63, 1.8%).
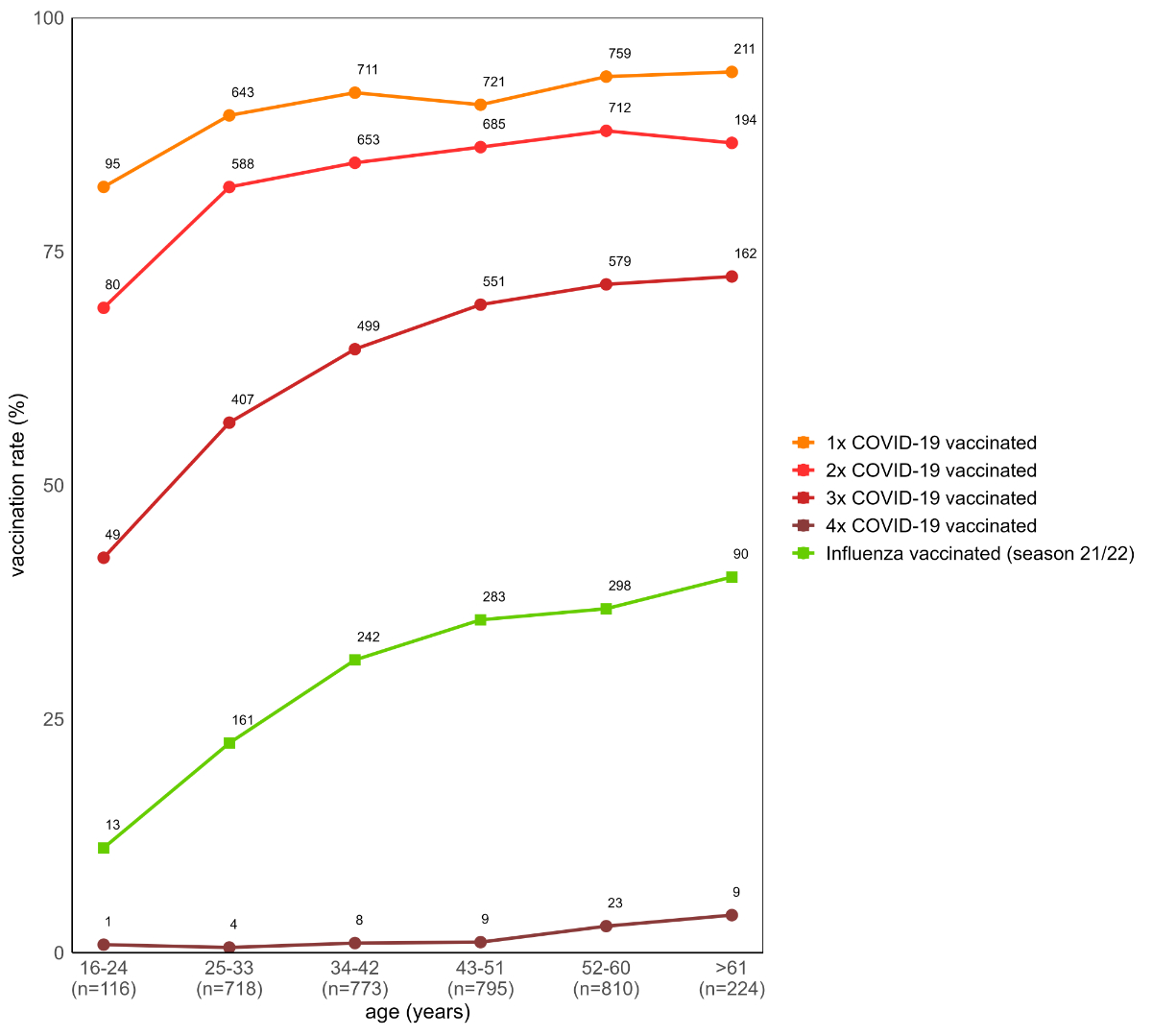
Figure 2Number of SARS-CoV-2 vaccine doses and receipt of seasonal influenza (2021/2022) vaccination among healthcare workers across age groups.
Among 3,414 study participants with available blood samples, 3,379 (99.0%) showed anti-S antibodies (3,357, 98.3%), anti-N antibodies (2,396, 70.1%), or both (2,370, 69.4%). Among vaccinated healthcare workers, 51.6% had a positive COVID-19 test at some point (before or after vaccination) during the study, compared with 81.1% of unvaccinated healthcare workers (p <0.001). Among 1,889 participants with self-reported positive SARS-CoV-2 swab tests, 1,675 (88.7%) had anti-N positivity; in contrast, among all participants with anti-N positivity, 77% (1,675 of 2,173) reported a positive SARS-CoV-2 swab test. Whereas previous infection was more common in younger participants, vaccination rates increased with age (figure 3).
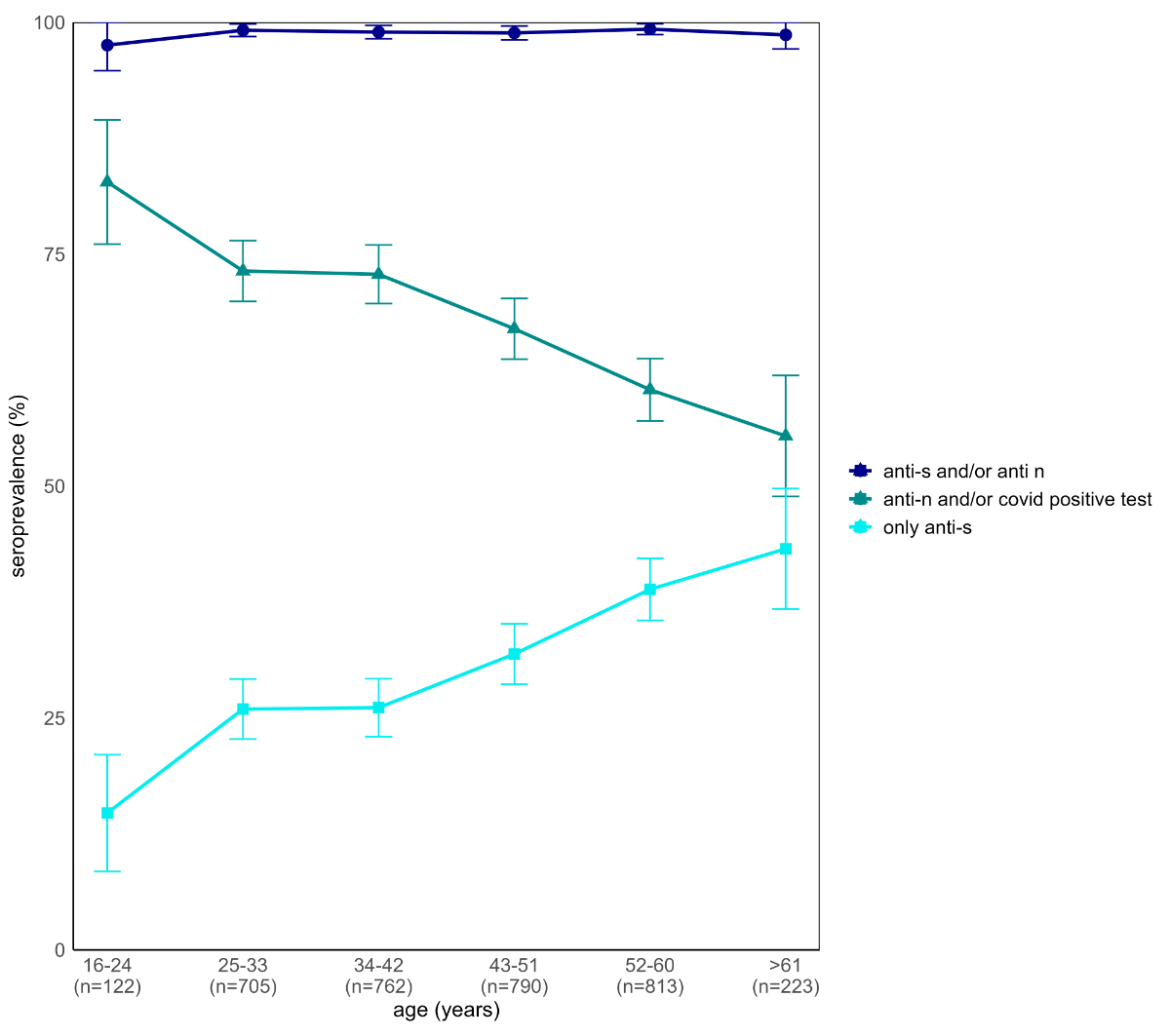
Figure 3Seroprevalence percentages among healthcare workers for only anti-spike positivity (turquoise), anti-nucleocapsid positivity and/or a positive COVID-19 test (dark green), and any (anti-spike or anti-nucleocapsid) SARS-CoV-2 antibody (dark blue), across age groups.
Males were more likely to be vaccinated than females (94.2% vs 90.7%, p <0.01). Across participating institutions, the vaccination rates ranged from 81.6% to 94.8%, with 92% in acute care and 86% in rehabilitation/geriatric clinics. Among professions, vaccination rates were highest among physicians (97%) and lowest in therapists (88%). Participants with patient contact had slightly lower vaccination rates than those without (90.7% vs 93.1%, p <0.05). The vaccination rate was higher in participants who had been vaccinated against influenza in the past (96.7% vs 82.8%, p <0.001) (table 1).
Table 1Baseline characteristics between healthcare workers without (n = 296) or with (n = 3,140) at least one SARS-CoV-2 vaccination.
| Missing values | Unvaccinated | Vaccinated | p-value | ||
| n = 296 | n = 3140 | ||||
| Age (in years), median (range) | n = 1 | 40.9 (16–73) | 43.9 (16–69) | <0.001 | |
| BMI (in kg/m2), median (IQR) | n = 33 | 24.2 (20.74–26.41) | 24.4 (21.26–26.53) | 0.45 | |
| Male gender (vs female) | n = 0 | 37 (5.76%) | 605 (94.24%) | <0.05 | |
| European ethnicity (vs other) | n = 51 | 284 (8.56%) | 3035 (91.44%) | 0.50 | |
| Setting | n = 0 | ||||
| Adult/paediatric acute care | 251 (8.37%) | 2747 (91.63%) | 0.20 | ||
| Psychiatric clinic | 25 (8.56%) | 267 (91.44%) | 1.00 | ||
| Rehabilitation/geriatric clinic | 20 (13.70%) | 126 (86.30%) | <0.05 | ||
| ICU | n = 27 | 33 (7.14%) | 429 (92.86%) | 0.25 | |
| Profession | n = 27 | ||||
| Physician | 12 (2.94%) | 396 (97.06%) | <0.001 | ||
| Nurse | 179 (11.01%) | 1447 (88.99%) | <0.001 | ||
| Therapist | 18 (12.24%) | 129 (87.76%) | 0.13 | ||
| Administrator | 32 (6.18%) | 486 (93.82%) | <0.05 | ||
| Other | 55 (7.75%) | 655 (92.25%) | 0.41 | ||
| Active smoker (vs non-smoker) | n = 27 | 69 (11.96%) | 508 (88.04%) | <0.05 | |
| Household members, median (range) | n = 29 | 1.71 (0–6) | 1.67 (0–9) | 0.63 | |
| Higher education (vs basic education) | n = 62 | 285 (8.58%) | 3035 (91.42%) | 0.02 | |
| Any home remedy | n = 27 | 76 (15.64%) | 410 (84.36%) | <0.001 | |
| Patient contact | n = 27 | 237 (9.28%) | 2318 (90.72%) | <0.01 | |
| Seasonal influenza vaccination | n = 108 | 60 (3.28%) | 1769 (96.72%) | <0.001 | |
BMI: body mass index; IQR: interquartile range; ICU: intensive care unit.
In multivariable analysis, age (adjusted OR [aOR] 1.02 per year, 95% CI 1.01–1.03), being a physician (aOR 3.22, 95% CI 1.75–5.92) or administrator (aOR 1.74, 95% CI 1.10–2.77), as compared with being a nurse, and having higher education (aOR 2.23, 95% CI 1.09–4.57), were positively associated with vaccine uptake, whereas working in non-acute care (aOR 0.58, 95% CI 0.34–0.97), active smoking (aOR 0.68, 95% CI 0.51–0.91), and taking any prophylactic home remedy against SARS-CoV-2 (aOR 0.42, 95% CI 0.31–0.56) were negatively associated (table 2).
Table 2Results of univariable and multivariable logistic regression analysis of SARS-CoV-2 vaccine uptake among healthcare workers.
| Univariable analysis | Multivariable analysis | ||||
| OR (95% CI) | p-value | aOR (95% CI) | p-value | ||
| Age (per year) | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 | |
| BMI (per one kg/m2) | 1.00 (1.00–1.00) | 0.54 | NA | ||
| Male gender (ref. female) | 1.67 (1.17–2.38) | <0.01 | 1.24 (0.85–1.80) | 0.26 | |
| European ethnicity (ref. other) | 1.47 (0.70–3.12) | 0.31 | NA | ||
| Setting (ref. acute care) | Psychiatric clinic | 0.98 (0.63–1.50) | 0.91 | 0.94 (0.61–1.47) | 0.79 |
| Rehabilitation/geriatric clinic | 0.58 (0.35–0.94) | <0.05 | 0.58 (0.34–0.97) | <0.05 | |
| ICU (ref. non-ICU) | 1.27 (0.88–1.86) | 0.21 | NA | ||
| Profession (ref. nurse) | Physician | 4.08 (2.25–7.40) | <0.001 | 3.22 (1.75–5.92) | <0.001 |
| Therapist | 0.89 (0.53–1.50) | 0.65 | 0.96 (0.56–1.66) | 0.89 | |
| Administrator | 1.88 (1.27–2.80) | <0.01 | 1.74 (1.10–2.77) | <0.05 | |
| Other | 1.47 (1.07–2.00) | <0.05 | 1.32 (0.92–1.90) | 0.13 | |
| Active smoker (ref. non-smoker) | 0.64 (0.48–0.85) | <0.01 | 0.68 (0.51–0.91) | <0.01 | |
| Household members (per person) | 1.00 (0.99–1.02) | 0.82 | NA | ||
| Higher education (ref. basic education) | 2.46 (1.23–4.94) | <0.05 | 2.23 (1.09–4.57) | <0.05 | |
| Any home remedy (ref. none) | 0.44 (0.33–0.58) | <0.001 | 0.42 (0.31–0.56) | <0.001 | |
| Patient contact (ref. no patient contact) | 0.73 (0.54–0.98) | <0.05 | 1.02 (0.70–1.50) | 0.90 | |
(a)OR: (adjusted) odds ratio; BMI: body mass index; ICU: intensive care unit; NA: not applicable.
Backward selection of variables and inclusion of institutions as cluster variable yielded similar results; including age × gender as interaction term showed no interaction effect between these two variables (table S2 in the appendix).
The most commonly indicated reasons for non-vaccination were a lack of belief in long-lasting immunity conferred by the vaccine (268/296, 92.1%), belief that vaccination does not stop SARS-CoV-2 transmission (255/294, 86.7%), and preferring naturally acquired to vaccine-induced immunity (236/284, 83.1%). Fear of adverse effects was reported by 68.4% (197/288), and wanting more information regarding vaccine effectiveness was reported by 52.2% (151/289) of the participants (figure 4).
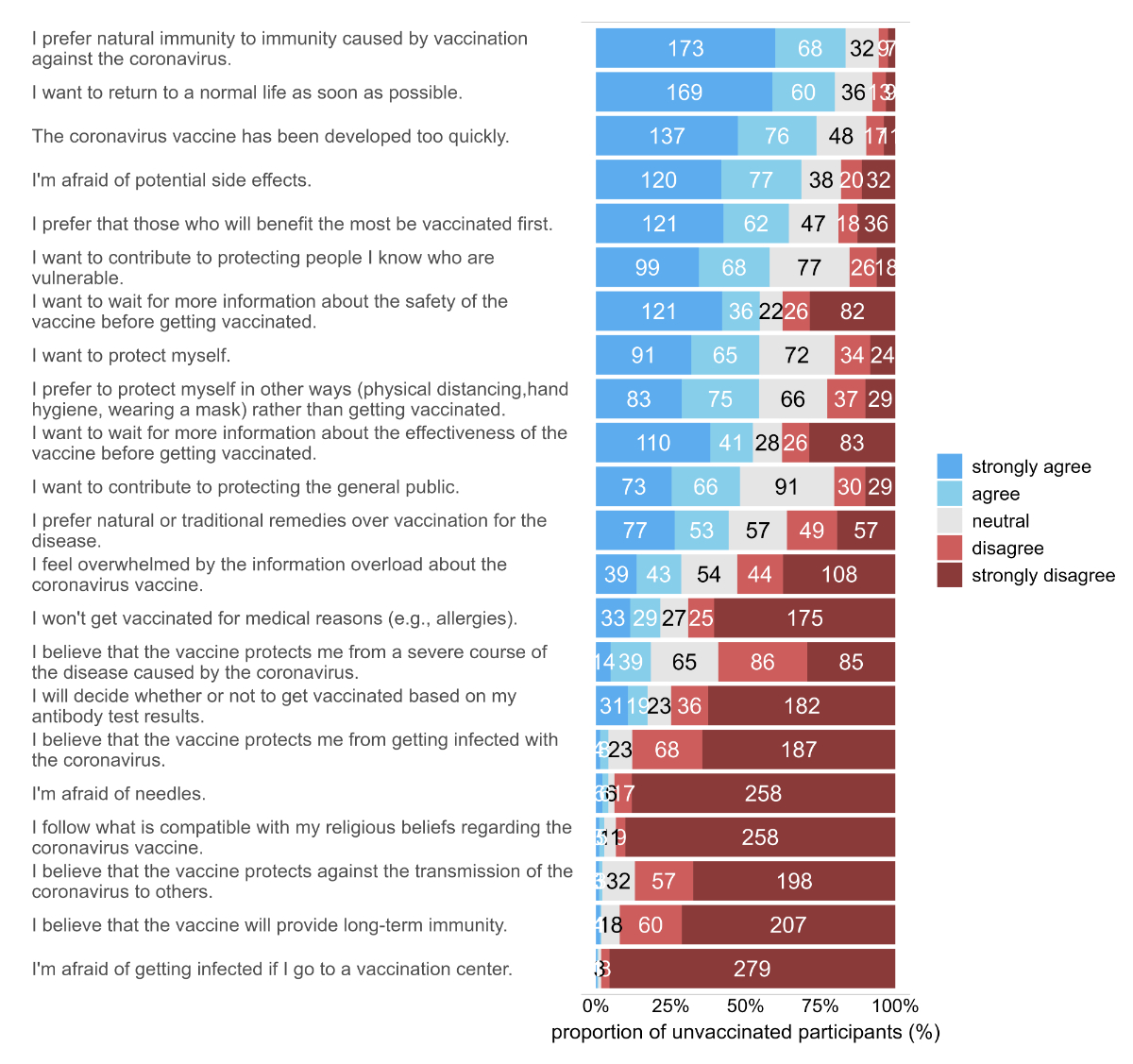
Figure 4Reasons for non-vaccination among 296 unvaccinated healthcare workers (multiple answers per participant were possible).
In this multicentre cohort of healthcare workers with more than 3,400 participants from German-speaking Switzerland, 99% had evidence of natural and/or vaccine-induced humoral immunity against SARS-CoV-2. Approximately 9% of healthcare workers remained unvaccinated as of mid-2022. Non-vaccination was observed primarily among younger healthcare workers, therapists, nurses, and those in non-acute healthcare settings. A lack of belief in vaccine effectiveness and preferring naturally acquired to vaccine-induced immunity were the most important reasons for non-vaccination.
The high prevalence of humoral immunity found in our study was in line with previous data. In a meta-analysis, SARS-CoV-2 seroprevalence has been estimated to be 95% in the general population in European high-income countries as of December 2021 [17]. In Switzerland, these high numbers have been confirmed in population-based seroprevalence studies performed in some cantons in June 2022 [2]. Although no recent data are available for Swiss healthcare workers specifically, data from western Switzerland have shown an anti-S seroprevalence of 45% after the second pandemic wave in March 2021 [18].
Of note, almost 70% of our population showed hybrid immunity, i.e., had signs of a previous SARS-CoV-2 infection and were vaccinated against the disease. Hybrid immunity has been shown to be associated with better and prolonged protection [19–22] for newly emerging variants against which vaccination with monovalent mRNA vaccines alone is relatively less effective [23, 24].
The vaccination rate in our cohort was 91%, a percentage considerably higher than the 40% vaccination willingness (assessed before initiation of the vaccination campaign in Switzerland) reported by Zürcher et al. [13]. Reasons for this discrepancy may include the different timing of the two studies (December 2020 vs June 2022), given that more data on efficacy and safety became available over time, and highly motivated individuals might potentially have been overrepresented in our cohort. However, younger therapists and nurses working in non-acute care were less likely to be vaccinated in our population. This finding is notable, because non-acute settings such as geriatric and rehabilitation clinics often care for frail populations at high-risk of severe COVID-19 [25]. In addition, COVID-19 outbreaks have frequently been reported in these settings [25–27]. However, whether vaccination of healthcare workers would indeed decrease the burden of COVID-19 in these settings remains unknown.
The most frequently reported reason for non-vaccination was a lack of belief in vaccine effectiveness. This finding was unexpected, because the scientific literature is unequivocal regarding findings demonstrating vaccine protection against severe SARS-CoV-2 infection [28]. Reports of breakthrough infections after previous vaccination might potentially have contributed to this widespread misbelief [29]. Although not directly investigated in our survey, fear of vaccine adverse effects, such as infertility, might have constituted an implicit barrier for vaccination among healthcare workers. Infertility fears were raised early in the pandemic as potential adverse effect of mRNA vaccines, although such a connection has not been demonstrated to date [30]. In multivariable analysis, neither gender nor the interaction between age and gender (in sensitivity analysis) had any significant effect, and young women were not particularly hesitant to receive the vaccine in our cohort. Unsurprisingly, seasonal influenza vaccine uptake correlated strongly with SARS-CoV-2 vaccination uptake in our study. This finding suggests that scepticism against vaccination in general, and not selectively against SARS-CoV-2 vaccination, might also have played a role. Accordingly, insights gained from previous research on seasonal influenza vaccine uptake in Swiss healthcare workers could potentially be used to promote SARS-CoV-2 vaccination.
On the basis of our results, information and education among younger healthcare workers regarding the benefits and adverse effects of SARS-CoV-2 vaccination might be an approach to further improve vaccine uptake in unvaccinated individuals. A survey in 1,933 Swiss healthcare workers has described training and information campaigns as potential measures to increase SARS-CoV-2 vaccine uptake, whereas vaccine mandates, not unexpectedly, were rather unpopular [31]. For example, the motivational-interview technique, an individually tailored intervention considering personal knowledge and beliefs, has been successfully used to decrease vaccine hesitancy in the paediatric setting [32].
However, the cost-effectiveness of interventions aimed at increasing SARS-CoV-2 vaccine uptake among healthcare workers remains unknown, particularly given the high SARS-CoV-2 background immunity, the low risk of severe disease in this population, and the circulation of clinically less aggressive and increasingly immune-evasive SARS-CoV-2 variants such as Omicron lineage XBB [33]. In addition, future research should evaluate whether repetitive booster vaccination of healthcare workers is necessary to sustain protection against newly emerging SARS-CoV-2 variants and to mitigate transmission of the virus to patients.
The large sample size of participants from different healthcare settings and the broad-scale serological testing are among the strengths of this work. In addition, the results were robust in sensitivity analyses. An important limitation of our study is a potential lack of representativeness, because participation in the study was not mandatory, and most healthcare workers at the participating institutions did not participate. We believe that the high vaccination rate might therefore be an overestimation, because unvaccinated healthcare workers might have been less likely than vaccinated healthcare workers to participate in the cohort or to report their vaccination status. Another limitation is that most of our data were self-reported. However, broad-scale serology testing allowed us to validate at least part of the study data. For instance, self-reported SARS-CoV-2 infection showed excellent correlation with anti-N positivity in the blood; therefore, we are confident that our self-reported data are reliable. In this regard, some individuals show a waning anti-N response over time, or may not mount a complete anti-N response [34]. Therefore, undetected SARS-CoV-2 infection might have been underestimated with our assay. Finally, another important limitation of your work is that the questions regarding reasons for non-vaccination were not previously assessed for reliability or validity.
Seroprevalence against SARS-CoV-2, either naturally acquired infection or vaccine-induced, is very high among Swiss healthcare workers. Young age, professions other than physicians, and working in a non-acute setting are factors associated with diminished SARS-CoV-2 vaccination uptake. The added benefit of further promoting SARS-CoV-2 vaccination in this low-risk population with high background immunity remains to be assessed.
De-identified raw data and statistical codes are available from the authors upon reasonable request.
We thank the participants of the SURPRISE study and the members of the study group (in alphabetical order): Ulrike Besold, MD (Geriatric Clinic St. Gallen), Angela Brucher, MD (Psychiatry Services South, St. Gallen), Alexia Cusini, MD (Cantonal Hospital Graubünden), Thomas Egger (Cantonal Hospital St. Gallen), Andrée Friedl, MD (Cantonal Hospital Baden), Stephan Goppel, MD (Psychiatry Services North, St. Gallen), Fabian Grässli, MSc (Cantonal Hospital St. Gallen), Christian R. Kahlert, MD (Children's Hospital of Eastern Switzerland, St. Gallen), Joelle Keller (Hirslanden Clinic Zurich), Simone Kessler (Cantonal Hospital St. Gallen), Philipp Kohler, MD MSc (Cantonal Hospital St. Gallen), Stefan P. Kuster, MD MSc (Cantonal Hospital St. Gallen), Onicio Leal, PhD (University of Zurich), Eva Lemmenmeier, MD (Clienia Littenheid), Allison McGeer, MD MSc (Mount Sinai Hospital, Toronto), Dorette Meier Kleeb, MD (Cantonal Hospital Baden), Elisabeth Möller (Clienia Littenheid), J. Carsten Möller, MD (Clinic Zihlschlacht), Maja F. Müller (Hirslanden Clinic Zurich), Vaxhid Musa (Cantonal Hospital St. Gallen), Manuela Ortner (Rheintal Werdenberg Sarganserland Hospital Group, Grabs), Philip Rieder, PhD (Hirslanden Clinic Zurich), Lorenz Risch, MD PhD (Laboratory Risch Buchs), Markus Ruetti, MD (Fuerstenland Toggenburg Hospital Group Wil), Matthias Schlegel, MD (Cantonal Hospital St. Gallen), Hans-Ruedi Schmid, PhD (Cantonal Hospital Baden), Reto Stocker, MD (Hirslanden Clinic Zurich), Pietro Vernazza, MD (Cantonal Hospital St. Gallen), Matthias von Kietzell MD (Clinic Stephanshorn St. Gallen), Danielle Vuichard-Gysin, MD MSc (Thurgau Hospital Group Muensterlingen), and Benedikt Wiggli, MD (Cantonal Hospital Baden).
This work was supported by the Swiss National Sciences Foundation (grant number 31CA30_196544; grant number PZ00P3_179919 to P.K.), the Federal Office of Public Health (grant number 20.008218/421-28/1), and the Health Department of the Canton of St. Gallen.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Covid-19 Schweiz Coronavirus Dashboard [Internet]. [cited 2023 May 27]. Available from: https://www.covid19.admin.ch/de/epidemiologic/death
2. Swiss School of Public Health. (SSPH+). Resultate von Corona Immunitas [Internet]. Corona Immunitas. 2023. Available from: https://www.corona-immunitas.ch/programm/resultate/
3. Dörr T, Haller S, Müller MF, Friedl A, Vuichard D, Kahlert CR, et al. Risk of SARS-CoV-2 Acquisition in Health Care Workers According to Cumulative Patient Exposure and Preferred Mask Type. JAMA Netw Open. 2022 Aug;5(8):e2226816. 10.1001/jamanetworkopen.2022.26816
4. Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, et al.; Vaccine Effectiveness Among Healthcare Personnel Study Team. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel - 33 U.S. Sites, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021 May;70(20):753–8. 10.15585/mmwr.mm7020e2
5. Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, et al. COVID-19 in Healthcare Workers: A Living Systematic Review and Meta-analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am J Epidemiol. 2020Sep;kwaa191.
6. Pilishvili T, Gierke R, Fleming-Dutra KE, Farrar JL, Mohr NM, Talan DA, et al. Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N Engl J Med. 2021 Sep 22;NEJMoa2106599.
7. Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. 2014 Apr;32(19):2150–9. 10.1016/j.vaccine.2014.01.081
8. Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021 Feb;27(2):225–8. 10.1038/s41591-020-1124-9
9. Lang P, Wu CT, Le-Nguyen AF, Czock A. Influenza Vaccination Behaviour of Healthcare Workers in Switzerland: A Cross-Sectional Study. Int J Public Health. 2023 Mar;68:1605175. 10.3389/ijph.2023.1605175
10. Influenza Vaccination Coverage Among Health-Care Personnel — 2011–12 Influenza Season, United States [Internet]. [cited 2023 Nov 5]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a1.htm
11. Pless A, McLennan SR, Nicca D, Shaw DM, Elger BS. Reasons why nurses decline influenza vaccination: a qualitative study. BMC Nurs. 2017 Apr;16(1):20. 10.1186/s12912-017-0215-5
12. Fadda M, Bezani K, Amati R, Fiordelli M, Crivelli L, Albanese E, et al. Decision-making on COVID-19 vaccination: A qualitative study among health care and social workers caring for vulnerable individuals. SSM Qual Res Health. 2022 Dec;2:100181. 10.1016/j.ssmqr.2022.100181
13. Zürcher K, Mugglin C, Egger M, Müller S, Fluri M, Bolick L, et al. Vaccination willingness for COVID-19 among healthcare workers: a cross-sectional survey in a Swiss canton. Swiss Med Wkly. 2021 Sep;151(3738):w30061–30061. 10.4414/SMW.2021.w30061
14. Baron RC, Risch L, Weber M, Thiel S, Grossmann K, Wohlwend N, et al. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: a population-based study. Clin Chem Lab Med. 2020 Aug;58(12):2131–40. 10.1515/cclm-2020-0978
15. Mahase E. Covid-19: two antibody tests are “highly specific” but vary in sensitivity, evaluations find. BMJ. 2020 May;369:m2066. 10.1136/bmj.m2066
16. Schaffner A, Risch L, Aeschbacher S, Risch C, Weber MC, Thiel SL, et al. Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study. J Clin Med. 2020 Dec;9(12):3989. 10.3390/jcm9123989
17. Bergeri I, Whelan MG, Ware H, Subissi L, Nardone A, Lewis HC, et al.; Unity Studies Collaborator Group. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022 Nov;19(11):e1004107. 10.1371/journal.pmed.1004107
18. Jacot D, von Rotz U, Blondet F, Aebischer O, Matthieu P, De Rham M, et al. SARS-CoV-2 seroprevalence in hospital healthcare workers in Western Switzerland at the end of the second pandemic wave. J Med Microbiol. 2022 Aug;71(8):001558. 10.1099/jmm.0.001558
19. Carazo S, Skowronski DM, Brisson M, Barkati S, Sauvageau C, Brousseau N, et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. Lancet Infect Dis. 2023 Jan;23(1):45–55. 10.1016/S1473-3099(22)00578-3
20. Hui DS. Hybrid immunity and strategies for COVID-19 vaccination. Lancet Infect Dis. 2023 Jan;23(1):2–3. 10.1016/S1473-3099(22)00640-5
21. Babouee Flury B, Güsewell S, Egger T, Leal O, Brucher A, Lemmenmeier E, et al.; SURPRISE Study Group. Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and Omicron waves in Switzerland-A multicentre cohort study. PLoS Med. 2022 Nov;19(11):e1004125. 10.1371/journal.pmed.1004125
22. Kohler P, Babouee Flury B, Güsewell S, Egger T, Leal O, Brucher A, et al.; SURPRISE Study Group. Clinical symptoms of SARS-CoV-2 breakthrough infection during the Omicron period in relation to baseline immune status and booster vaccination-A prospective multicentre cohort of health professionals (SURPRISE study). Influenza Other Respir Viruses. 2023 Jun;17(6):e13167. 10.1111/irv.13167
23. Irving SA, Buchan SA. Considerations of hybrid immunity and the future of adolescent COVID-19 vaccination. Lancet Infect Dis. 2023 Apr;23(4):382–3. 10.1016/S1473-3099(22)00759-9
24. Spinardi JR, Srivastava A. Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination-Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines. 2023 Jan;11(2):370. 10.3390/biomedicines11020370
25. Abbas M, Robalo Nunes T, Cori A, Cordey S, Laubscher F, Baggio S, et al. Explosive nosocomial outbreak of SARS-CoV-2 in a rehabilitation clinic: the limits of genomics for outbreak reconstruction. J Hosp Infect. 2021 Nov;117:124–34. 10.1016/j.jhin.2021.07.013
26. Cormier H, Brangier A, Lefeuvre C, Asfar M, Annweiler C, Legeay C. Lessons learnt from a nosocomial COVID-19 outbreak in a geriatric acute care ward with a high attack rate. Maturitas. 2021 Jul;149:34–6. 10.1016/j.maturitas.2021.05.001
27. Abbas M, Cori A, Cordey S, Laubscher F, Robalo Nunes T, Myall A, et al. Reconstruction of transmission chains of SARS-CoV-2 amidst multiple outbreaks in a geriatric acute-care hospital: a combined retrospective epidemiological and genomic study. eLife. 2022 Jul;11:e76854. 10.7554/eLife.76854
28. Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022 Mar;399(10328):924–44. 10.1016/S0140-6736(22)00152-0
29. Dzinamarira T, Tungwarara N, Chitungo I, Chimene M, Iradukunda PG, Mashora M, et al. Unpacking the Implications of SARS-CoV-2 Breakthrough Infections on COVID-19 Vaccination Programs [Internet]. Vaccines (Basel). 2022 Feb;10(2):252. [cited 2023 Nov 1] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8879800/ 10.3390/vaccines10020252
30. Zaçe D, La Gatta E, Petrella L, Di Pietro ML. The impact of COVID-19 vaccines on fertility-A systematic review and meta-analysis. Vaccine. 2022 Oct;40(42):6023–34. 10.1016/j.vaccine.2022.09.019
31. Politis M, Sotiriou S, Doxani C, Stefanidis I, Zintzaras E, Rachiotis G. Healthcare Workers’ Attitudes towards Mandatory COVID-19 Vaccination: A Systematic Review and Meta-Analysis. Vaccines (Basel). 2023 Apr;11(4):880. 10.3390/vaccines11040880
32. Gagneur A, Battista MC, Boucher FD, Tapiero B, Quach C, De Wals P, et al. Promoting vaccination in maternity wards ─ motivational interview technique reduces hesitancy and enhances intention to vaccinate, results from a multicentre non-controlled pre- and post-intervention RCT-nested study, Quebec, March 2014 to February 2015. Euro Surveill. 2019 Sep;24(36):1800641. 10.2807/1560-7917.ES.2019.24.36.1800641
33. Shrestha NK, Burke PC, Nowacki AS, Simon JF, Hagen A, Gordon SM. Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine. Open Forum Infect Dis. 2023 Apr;10(6):ofad209. 10.1093/ofid/ofad209
34. Nakagama Y, Komase Y, Kaku N, Nitahara Y, Tshibangu-Kabamba E, Tominaga T, et al. Detecting Waning Serological Response with Commercial Immunoassays: 18-Month Longitudinal Follow-up of Anti-SARS-CoV-2 Nucleocapsid Antibodies. Microbiol Spectr. 2022 Aug;10(4):e0098622. 10.1128/spectrum.00986-22
Table S1Questionnaire with 22 statements regarding reasons for vaccine hesitancy. Answer options are “strongly agree”, “agree”, “neutral”, “disagree”, “strongly disagree”.
| Statements |
| 1. Ich möchte mit der Impfung warten, bis mehr über die Wirksamkeit des Impfstoffs bekannt ist. |
| 2. Ich möchte mit der Impfung warten, bis mehr über die Sicherheit des Impfstoffs bekannt ist |
| 3. Ich glaube, dass mich die Impfung vor einer Infektion mit dem Coronavirus schützt. |
| 4. Ich glaube, dass mich die Impfung vor einem schweren Verlauf der Erkrankung durch das Coronavirus schützt. |
| 5. Ich glaube, dass die Impfung vor einer Übertragung des Coronavirus auf andere schützt. |
| 6. Ich habe Angst vor möglichen Nebenwirkungen. |
| 7. Ich folge dem, was bezüglich Coronavirus-Impfung mit meinem religiösen Glauben vereinbar ist. |
| 8. Ich ziehe eine natürliche Immunität gegen das Coronavirus einer durch die Impfung hervorgerufenen Immunität vor. |
| 9. Ich ziehe natürliche oder traditionelle Heilmittel gegen die Krankheit einer Impfung vor. |
| 10. Ich habe Angst vor Spritzen. |
| 11. Ich habe Angst mich anzustecken, wenn ich in ein Impfzentrum gehe. |
| 12. Ich schütze mich lieber auf eine andere Weise (körperlicher Abstand, Händehygiene, Tragen einer Maske) als geimpft zu werden. |
| 13. Ich glaube, dass der Impfstoff eine langfristige Immunität bieten wird. |
| 14. Ich möchte mich selbst schützen. |
| 15. Ich möchte zum Schutz der Allgemeinheit beitragen. |
| 16. Ich möchte zum Schutz von Personen beitragen, die ich kenne und die gefährdet sind. |
| 17. Ich möchte so schnell wie möglich zu einem normalen Leben zurückkehren. |
| 18. Ich ziehe es vor, dass diejenigen, die am meisten davon profitieren werden, zuerst geimpft werden. |
| 19. Ich lasse mich aus medizinischen Gründen (z. B. Allergien) nicht impfen. |
| 20. Ich werde mich aufgrund meines Resultats des Antikörpertests entscheiden, ob ich mich impfen lasse oder nicht. |
| 21. Der Coronavirus-Impfstoff ist zu schnell entwickelt worden. |
| 22. Ich fühle mich von der Informationsflut zum Coronavirus-Impfstoff überfordert. |
Table S2Sensitivity analyses (multivariable logistic regression) regarding SARS- CoV-2 vaccine uptake in healthcare workers. Sensitivity analysis I: Backward selection of variables. Sensitivity analysis II: Inclusion of age*gender as interaction term. Sensitivity analysis III: Random effects model (institutions as cluster variable).
| Analysis I | Analysis II | Analysis III | |||||
| aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | ||
| Age (per year) | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.01 | 1.02 (1.01–1.03) | <0.001 | |
| Male gender (ref. female) | NA | 0.57 (0.13–2.50) | 0.46 | 1.21 (0.83–1.77) | 0.31 | ||
| Setting (ref. acute care) | Psychiatric clinic | 0.96 (0.61–1.49) | 0.84 | 0.94 (0.60–1.46) | 0.77 | 1.01 (0.55–1.85) | 0.98 |
| Rehabilitation / geriatric clinic | 0.57 (0.34–0.96) | <0.05 | 0.57 (0.34–0.97) | <0.05 | 0.62 (0.31–1.26) | 0.18 | |
| Profession (ref. nurse) | Physicians | 3.43 (1.88–6.24) | <0.001 | 3.22 (1.75–5.91) | <0.001 | 3.26 (1.77–6.01) | <0.001 |
| Therapists | 0.98 (0.57–1.69) | 0.93 | 0.96 (0.55–1.66) | 0.88 | 0.94 (0.55–1.64) | 0.84 | |
| Administration | 1.72 (1.16–2.56) | <0.01 | 1.75 (1.10–2.79) | <0.05 | 1.74 (1.10–2.76) | <0.05 | |
| Other | 1.35 (0.98–1.86) | 0.07 | 1.32 (0.83–2.11) | 0.13 | 1.26 (0.87–1.81) | 0.22 | |
| Active smoker (ref. non-smoker) | 0.68 (0.51–0.92) | <0.05 | 0.68 (0.50–0.91) | <0.01 | 0.66 (0.49–0.88) | <0.01 | |
| Higher education (ref. basic education) | 2.27 (1.11–4.64) | <0.05 | 2.23 (1.09–4.58) | <0.05 | 2.19 (1.06–4.52) | <0.05 | |
| Any home remedy (ref. none) | 0.42 (0.31–0.56) | <0.001 | 0.42 (0.32–0.56) | <0.001 | 0.41 (0.30–0.55) | <0.001 | |
| Patient contact (ref. no patient contact) | NA | 1.03 (0.70–1.50) | 0.89 | 1.03 (0.70–1.50) | 0.88 | ||
| Gender × age (interaction) | NA | 1.02 (0.98–1.05) | 0.30 | NA | |||
(a)OR: (adjusted) Odds Ratio; NA: not applicable.