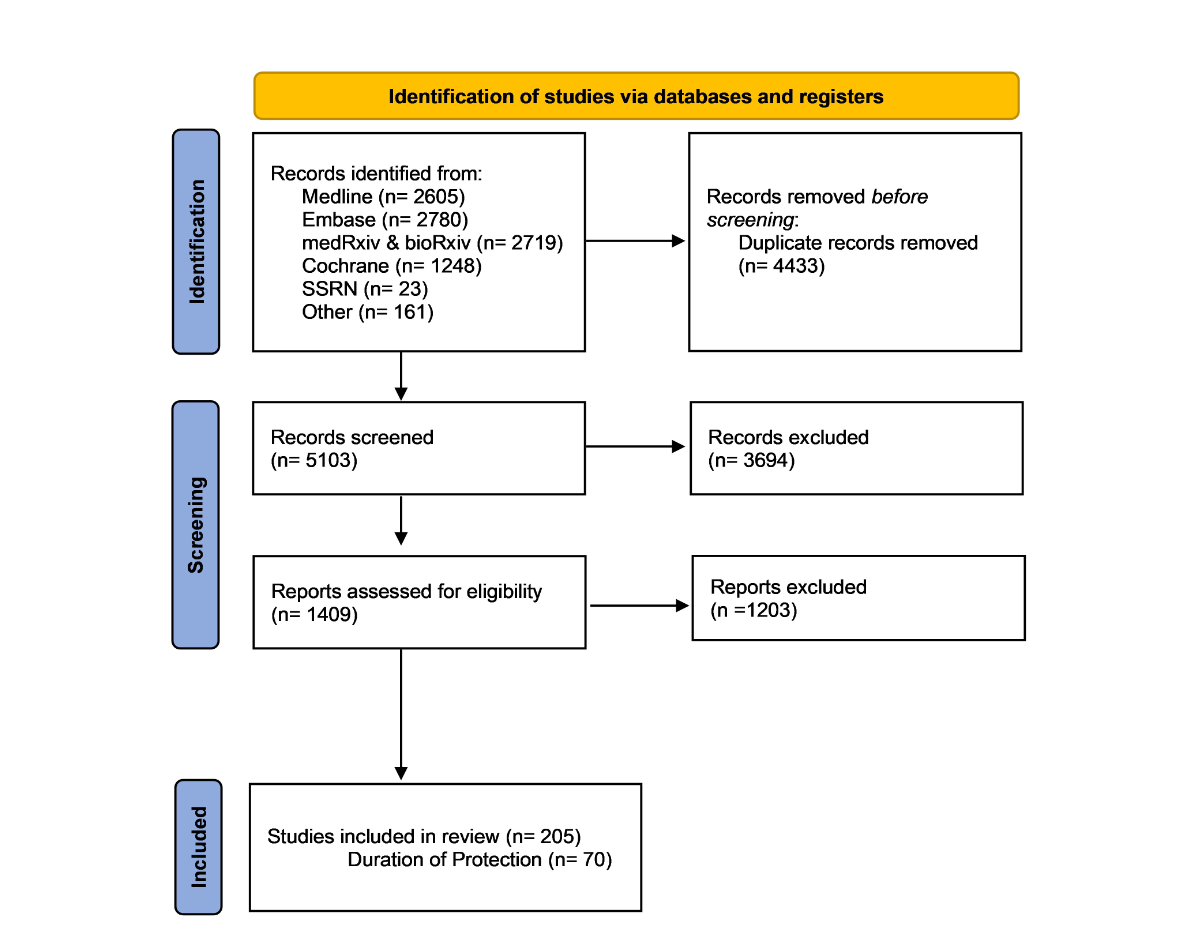
Figure 1PRISMA flowchart of study identification and selection.
DOI: https://doi.org/https://doi.org/10.57187/s.3732
As the fight against COVID-19 persists globally and the emergence of several SARS-CoV-2 variants continues to change the clinical and epidemiological course of the pandemic, an understanding of the duration of long-term protection against SARS-CoV-2 infection conferred by humoral and cellular immunity can aid in the rapid implementation of vaccine and safety guidelines. The adaptive immune response, composed of the humoral and cellular responses, can be measured by analysing antibody levels, neutralising antibodies and T cell and memory B cell responses. Antibodies that recognise receptor-binding domains (RBDs) have been considered the most important component of immunity against SARS-CoV-2 in humans due to their neutralising activity [1]. Nonetheless, the induction of SARS-CoV-2-specific memory T cells and B cells is also important because it provides long-term protection against infection [2].
Many COVID-19 vaccines have been designed to target the SARS-CoV-2 spike protein, with a focus on the receptor-binding domain as it mediates viral entry into cells [3]. However, mutations in the SARS-CoV-2 spike protein have resulted in the emergence of SARS-CoV-2 variants of concern. These variants of concern have demonstrated decreased sensitivity to convalescent sera and threaten the effectiveness and duration of protection of available COVID-19 vaccines [4]. As of December 2022, five variants of concern had been identified: Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529), including its multiple subvariants [5]. Of the five variants of concern, Omicron has accumulated the highest number of mutations and has mediated the greatest level of immune escape [6]. With its enhanced transmissibility and immune escape, Omicron, and the multiple subvariants that have emerged from it, has become the most dominant COVID-19 variant circulating by the time of writing. Omicron is comprised of various sister lineages, of which BA.4 and BA.5 have been shown to escape neutralisation to a higher degree than the original Omicron variant (B.1.1.529) and the three previous sister lineages [7].
Vaccination against SARS-CoV-2 has shown a high preventive effect worldwide, especially for reducing severe illness and death [8]. However, with newly emerging variants, especially Omicron, a high reduction in levels of humoral and cellular immunity has been observed, and vaccine effectiveness has also been affected. Several of the studies included in this review have demonstrated waning protection against the SARS-CoV-2 virus and its Omicron variant and subvariants of both humoral and cellular immunity. With each new Omicron subvariant, the ability of the antibodies elicited through vaccination to neutralise the variants is lower than that of the previous variant [9]. Consequently, these new subvariants influence vaccine effectiveness and the durability of the established protection against SARS-CoV-2 infection [10]. Therefore, analysing the effects that Omicron and its subvariants have on the levels and duration of protection against SARS-CoV-2 infection elicited by adaptive immunity is crucial to appropriately implement public health decisions. Due to the considerable number of publications shared daily, a rapid literature review was performed to assess evidence of the levels and duration of long-term protection against SARS-CoV-2 infection in adults and risk groups (immunocompromised and older individuals).
A search for literature published each week was completed using the following electronic databases: MEDLINE (PubMed), Embase, medRxiv and bioRxiv, Cochrane Library and Social Science Research Network (SSRN). Grey literature, such as information produced by government agencies and academic institutions and press releases, and journals such as the New England Journal of Medicine, The Lancet and Nature (which publish articles before they appear in search engines) were hand-searched.
We employed a search strategy composed of text words (e.g. coronavirus disease), MeSH terms (e.g. COVID-19 immunity), Boolean terms (e.g. AND, OR) and truncations (e.g. immune*) to electronically identify studies related to SARS-CoV-2 immunity in the MEDLINE, Embase and medRxiv/bioRxiv databases. The literature search was performed weekly by one of the researchers. The search strategies for MEDLINE, Embase and medRxiv/bioRxiv can be found in the appendix.
Identified literature was imported into a library in EndNote for storage and detection and deletion of duplicated articles. Screening and full-text review were completed using Rayyan systematic review software [11].
Search-identified literature was imported into Rayyan, and titles and abstracts were screened for articles related to COVID-19 immunology. At least two reviewers screened the literature and agreed on its inclusion in the full-text review. If a conflict arose during the title and abstract screening, a third, more experienced reviewer screened the study and made a final decision. Full-text reviews were performed to assess the relevancy of each selected article. Relevancy was decided based on the inclusion and exclusion criteria and topics of interest. Studies selected for full-text review were further screened in Rayyan for literature assessment and selection by two reviewers. If a conflict arose during the full-text review, then a third, more experienced reviewer reviewed the study and made a final decision.
Eligible studies were those reporting any data on immunological assays of COVID-19 (related to vaccination and/or infection) or the effectiveness of protection against SARS-CoV-2 infection (related to the effectiveness of vaccination and/or infection). The population of interest was healthy and immunocompromised people in any geographic setting. Three main exposure groups were eligible for inclusion: individuals fully vaccinated or boosted for COVID-19 (restricted to vaccines approved in Switzerland), individuals with previously confirmed infection and individuals with previously confirmed infection and documented vaccination. The outcome of interest was vaccine effectiveness or immunogenicity against SARS-CoV-2 Omicron infection, hospitalisation, severe disease and death. Test-negative case-control, cross-sectional, cohort, non-randomised controlled trial and randomised controlled trial studies were eligible for inclusion. Due to the focus on the duration of long-term protection, studies evaluating immunology over a long time period (n = 70) were categorised as “duration of protection” and emphasised in this report. No language restriction was imposed (though the search queries were in English), and we limited the search to studies published between 1 November 2021 and 30 September 2022 to capture studies covering the emergence of the Omicron variant. Underage individuals (infants, children and adolescents) and pregnant women were excluded from our search. Additionally, studies of new and second-generation vaccines and other vaccine platforms not approved in Switzerland were not included.
Our study focused on frequent screening to keep up with the rapidly growing body of COVID-19 literature and provide updates for researchers and public health experts. We acknowledge the absence of a quality assessment in our rapid review, and we understand the importance of assessing the strength of evidence in future research.
Data were extracted and imported into a common Excel table for studies that included, but were not limited to, data on the immunological surveillance of COVID-19 immunity after vaccination and/or infection. The findings were grouped and summarised by topic (e.g. humoral immunity, cellular immunity, vaccine effectiveness and risk groups).
No ethical approval was required.
A total of 9536 studies were found using the search queries; 5103 studies remained after the removal of duplicates. After title and abstract screening by two authors, 1409 studies were included for full-text review. After full-text review, 205 articles were included in this review. Of the 205 articles, 70 addressed the duration of protection against SARS-CoV-2 infection and were highlighted in the review (figure 1). Further details on the characteristics of the included studies can be found in table S1 in the appendix. The review was mandated by the Federal Office of Public Health, and detailed reports can be found on their website (https://www.bag.admin.ch/bag/en/home/das-bag/publikationen/forschungsberichte/forschungsberichte-uebertragbare-krankheiten/forschung-wissenschaft-covid-19.html) (under the “Literature searches” section and “Monitoring COVID-19 immunity” subsection).

Figure 1PRISMA flowchart of study identification and selection.
A visual summary of the general results of the levels of protection of SARS-CoV-2 infection over time for the different types of immunity is presented in figure 2.
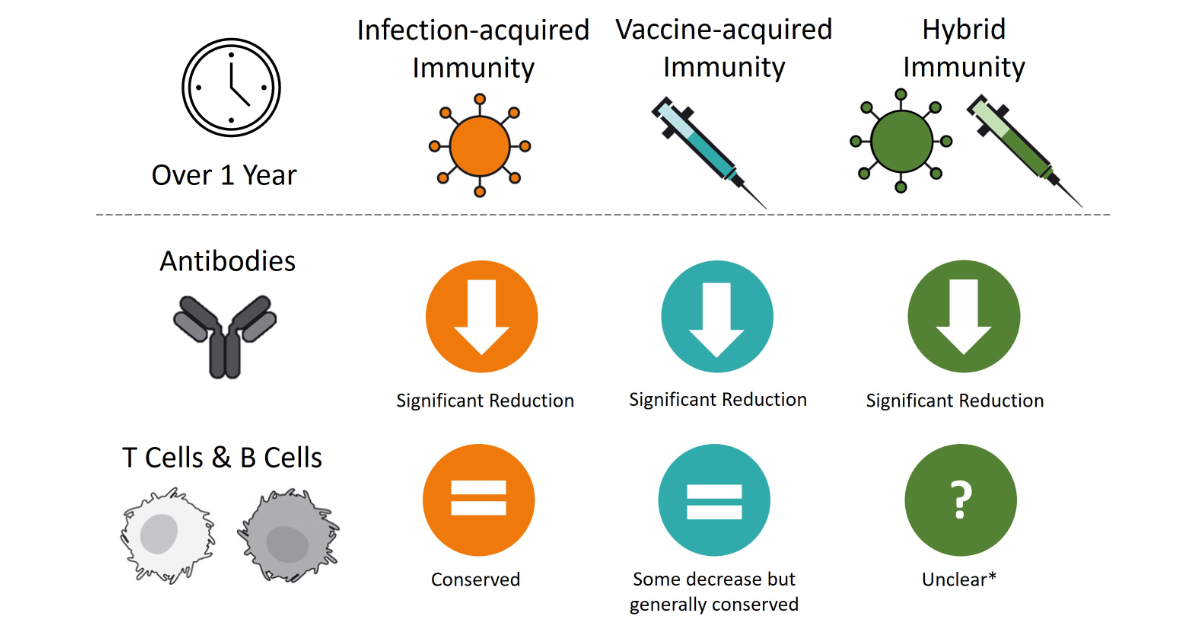
Figure 2Visual summary comparing the levels of antibodies and T and B cells from day 1 to day 365 (over a 1-year period) for infection-acquired, vaccine-acquired and hybrid immunity. This figure provides a generalised summary based on the included studies. The designs, durations and results of the included studies were very heterogenous, allowing for only a very generalised summary. * Few studies with results on the duration of the protection of hybrid immunity were included in the review and used to draw conclusions; nonetheless, it is safe to assume that similar trends in infection-acquired immunity can be expected.
The longevity and durability of protection against Omicron conferred by prior infection were assessed in three studies, in which a clear decrease in the elicited antibodies was observed after 10–12 months [1, 12, 13]. Twelve months post-infection, the humoral response in mild COVID-19 convalescents was significantly reduced and completely abrogated for the Omicron variant (B.1.1.529) [13]. In terms of the kinetics and durability of SARS-CoV-2-specific antibodies, a significant reduction in all levels of binding and neutralising antibodies was observed over 12 months; the half-life of IgG receptor-binding domain was estimated at 2.79 months, and the half-life of IgG N was estimated at 1.98 months [12]. Neutralising antibodies were shown to decay at a slower rate than binding antibodies, with an estimated half-life of 5.13 months [12]. Levels of humoral response were directly correlated with cellular response; high levels of virus-specific CD4+ T cells during the early convalescent phase were correlated with long-term neutralising antibody levels [12]. Additionally, the binding and neutralising antibodies in the low CD4+ group had a faster estimated decay rate and shorter half-life than those in the high CD4+ group [12].
Multiple studies analysed T cell and memory B cell responses in convalescent individuals [12–15]. In two of the studies, cellular immunity was conserved up to 1 year after infection, with T cells showing a slightly stronger memory than B cells [13, 15]. Both studies also observed a higher frequency of CD4+ T cells than CD8+ T cells, although a majority of CD8+ T cell epitopes of SARS-CoV-2 were reportedly conserved in Omicron [13, 15]. In the study by Wang et al., high percentages of virus-specific CD4+ T cells and cTfh1 cells were associated with a slower decline in humoral immunity, highlighting the importance of coordinating T cell and humoral immunity to achieve long-term protective immunity [12].
The effectiveness of previous infection waned more slowly than that of two and three doses of mRNA-1273 and BNT162b2 vaccines. Altarawneh et al. showed that the protection against SARS-CoV-2 infection after vaccination was negligible 6 months after the first and second booster doses (mRNA, 41.2%, vs BNT162b2, 44.7%, after 1 month) [16], while infection-acquired effectiveness ranged from 65–75% at 4–6 months and 32–53% at 10–12 months [16–19].
The level of neutralising antibodies against the Omicron variant B.1.1529 3–6 months after receiving the second dose of any mRNA vaccine (BNT162b2 or mRNA-1273) was lower than those against previous variants, such as the original wild strain or the Delta variant, irrespective of the recipient’s age [9, 20–25]. In addition, antibody levels after the second dose gradually decreased over time, leading fully vaccinated individuals, those receiving one dose of the Janssen vaccine or two doses of the mRNA vaccines, more vulnerable to breakthrough infections [20]. These low levels of antibody titres were observed 6 months after full administration of the primary scheduled mRNA vaccines mRNA-1273 and BNT162b2 and were not demonstrated to be sufficient for preventing breakthrough infections of the Omicron variant [26]. Nevertheless, binding antibodies against vaccine strain spike proteins and the receptor-binding domain have been shown to be significantly higher in boosted individuals compared with individuals who did not receive a booster dose, demonstrating that a booster dose increases antibody levels and inhibition against the Omicron variant [26–28].
Various studies demonstrated that, a few months after receiving a first booster dose, the neutralisation geometric mean titres (GMTs), along with the levels of binding antibodies, decreased significantly [7, 25, 27, 29]. Within 4 months after the administration of a booster dose, a 3- to 4-fold decrease in protection against SARS-CoV-2 infection was observed for all strains, including Omicron (B.1.1.529) [29], while Omicron BA.1 neutralising antibodies substantially waned by 3 months after homologous mRNA boosting and heterologous boosting with mRNA or Ad26.COV2.S vaccines [7]. Furthermore, the waning of Omicron-binding antibodies was observed as soon as 14 days after booster administration; the titres for IgA and IgG peaked on day 9 but significantly decreased by day 14 after immunisation to 66.6% for IgA (42.3% decrease) and to 30.6% for IgG antibodies (15.7% decrease) [27].
For third doses, a slower waning of the humoral response after a third BNT162b2 dose versus a second dose was reported [29]. Similar kinetics were observed in neutralising antibodies, for which a slower rate of decrease after the third vaccine dose than after the second dose was demonstrated [29]. The mean avidity – the overall strength in binding between an antibody and antigen – observed was 65.7% one month after the second vaccine dose, with no significant change 6 months after the second dose. However, avidity increased to 97.4% 1 month after the third vaccine dose and 98.04% 4 months after the third dose of BNT162b2 [29]. In other words, the binding strength between the antibody and antigen was stronger in individuals who received a third vaccine dose than in individuals who received only two doses, highlighting the stronger humoral response obtained after receiving a booster dose. Studies comparing different types of immunity revealed that the levels of neutralising antibodies in mRNA-vaccinated individuals were elevated up to 1 year after infection, while the anti-N titres amounted to only approximately 66 AU 1 year and approximately 21 AU 2 months after infection-acquired immunity [30].
A general decrease in the effectiveness of the protection offered by vaccination against SARS-CoV-2 symptomatic infection was observed, in which two doses of BNT162b2 offered a modest effectiveness of 65.5% 2–4 weeks after vaccination but later dropped to 8.8% at 25 weeks and beyond [31]. A similar decrease in the duration of protection was noted for booster doses; three doses of BNT162b2 had an effectiveness of 67.2% at 2–4 weeks, which then plunged to 45.7% at 10 weeks and beyond [31]. The duration of the protection of vaccine-acquired immunity was stronger against severe outcomes and hospitalisation; the estimated mRNA booster effectiveness (BNT162b2 and mRNA-1273 combined) was 87.4% 15–60 days after boosting and 87.2% 5–6 months after boosting, with no significant differences among various vaccine combinations [32]. With the spread of the more infectious Omicron subvariants BA.1/BA.2 and BA.4/BA.5, the duration of vaccine protection was compromised, and rapid waning of vaccine effectiveness in protecting against hospitalisation due to current sub-lineages of the Omicron variant was observed [10].
Waning of memory T cell responses for both the Delta and Omicron variants was observed 3 months after vaccination [33]. Similar to CD4+ T cell responses, CD8+ T cells were consistently detected in more vaccinees than in non-vaccinated controls, though CD4+ T cell responses were stronger up to 6 months after all vaccination regimens [34]. Both CD8+ and CD4+ T cells were maintained after 8 months with some decline and were restored to initial levels by booster vaccination [8]. Additionally, more than one-third of resting memory B cells bound Beta and Omicron variants and steadily increased the B cell receptor breadth up to 4.9 months after vaccination [35]. T cell responses declined between 28 days and 5 months after booster vaccination towards levels similar to those detected prior to vaccination with the Ad26.COV2.S booster [36]. Furthermore, the durability of cellular immune responses in individuals who received a primary vaccination of Pfizer BNT162b2 and were boosted with either Ad26.COV2.S or BNT162b2 was assessed [37]. At 16 weeks, median Omicron T cell responses generated by the Ad26.COV2.S booster were higher than those generated by the BNT162b2 booster [37].
The cellular immune responses after a fourth vaccine dose compared to those after breakthrough infections reached peak frequencies approximately 60 days after the second dose; a substantial but short-lived booster effect after the third dose was reported [38]. Within 30–60 days after the third dose, the CD8+ T cell response was reduced back to pre-third dose levels. When analysing the CD8+ T cell responses after the fourth antigen contact, either by a fourth vaccine dose or by breakthrough infection with Omicron or Delta after three doses, the T cell response was rapidly and robustly induced at similar frequencies. Furthermore, 1 or 2 months after breakthrough infection and second booster vaccination, a fully functional T cell memory was present with similar reactivation capacities [38]. Additionally, the spike-specific CD8+ T cells elicited by a fourth vaccine had a substantial response towards variants of concern, including Omicron.
Similar to previous results on the duration of protection conferred by infection-acquired or vaccine-acquired immunity, hybrid immunity showed signs of waning. To evaluate the lifespan of the antibodies elicited against the Omicron variant, Chang et al. [39] divided study participants into two groups: one representing a short interval (6 months after recovery from infection) and one representing a long interval (12 months after recovery from infection). Antibodies elicited against Omicron significantly decreased in neutralisation ability 6 months after recovery from infection, when the GMT ratio was 2.6; the GMT ratio 12 months after recovery was 1.7 [39]. When comparing different immunities (vaccine-acquired and hybrid), a greater decay was found in vaccinated and uninfected individuals than in previously infected and vaccinated individuals, in which the neutralising antibodies waned more than the binding antibodies (11.5- and 10.2-fold decreases in uninfected individuals vs 2.9- and 2.5-fold decreases in previously infected individuals in neutralising and binding antibodies, respectively) [40]. Nonetheless, the neutralising antibody titres against all variants tested, including Omicron, declined 1–6 months after the second mRNA vaccine dose [41]. The sera from naïve vaccinated participants demonstrated no neutralising activity against the Omicron variant, while fully vaccinated individuals who recovered from COVID-19 showed a 22-fold reduction, with most participants retaining their neutralising antibody response [42].
Similar kinetics were found in boosted individuals; booster durability waned more substantially in uninfected individuals than in those who had acquired a breakthrough infection [43]. In contrast, two studies observed that the decay of the humoral response was faster in infected and vaccinated participants than uninfected and vaccinated participants [29, 40].
The effectiveness of hybrid immunity 7–59 days post-vaccination with two doses of an mRNA vaccine was 89% and eventually decreased to 68% at 6–12 months. Similar protection over time was noted in participants who received three doses and had a previous infection [17]. In terms of severe outcomes, multiple studies found that hybrid immunity conferred more durable protection against deaths and hospitalisation than against infections over time [44, 45] (84.5% vs 52.8% at 3–5 months, 89.5% vs 32.7% at 6–12 months, 80.3% vs 14.7% after 1 year [44]). A similar pattern was found for BNT162b2 and Ad26.COV2.S, whose effectiveness after the respective booster dose was higher against severe outcomes than against infection (booster dose BNT162b2 after 2–9 weeks, 95.7% vs 70%; Ad26.COV2.S after 2–9 weeks, 97.5% vs 47.2%) and higher against infection for BNT162b2 than for Ad26.COV2.S [44].
A third or fourth dose of an mRNA vaccine enhanced and sustained the antibody response against the Omicron variant in a high proportion of immunocompromised groups [46–59]. Nonetheless, lower levels of neutralising antibodies against Omicron after three doses of an mRNA vaccine and subsequent breakthrough infections in both immunocompromised and healthy groups were observed compared to the levels against the wild-type and Delta variant [50]. Additionally, a cohort study on solid organ transplant recipients found that, even after four doses of an mRNA vaccine, participants still had poor neutralising antibody responses against Omicron, particularly compared to healthy controls [52], and immunosuppressive treatments such as infliximab in inflammatory bowel disease patients were associated with attenuated and waning antibody responses [53]. Despite booster doses increasing the humoral response against Omicron, neutralisation of Omicron was still substantially weaker than that of earlier variants. Moreover, waning of the neutralising antibody response in immunocompromised individuals with time after vaccination was demonstrated [48, 60–62]. One month after a third dose of an mRNA vaccine, 18.3% of organ transplant recipients had detectable levels of neutralising antibodies against Omicron; this decreased to 15.7% 3 months after vaccination [60]. Similarly, antibody responses waned significantly 6 months after second and third doses of mRNA vaccines in kidney transplant recipients and cancer patients, respectively, in whom neutralisation against the Omicron variant was significantly attenuated or completely missing 6 months after vaccination [61, 62].
Spike-specific CD4+ and CD8+ T cells against all variants, including Omicron, were sustained in 45–60% of multiple sclerosis patients taking B cell depleting drugs 6 months after their second vaccination, albeit at lower median frequencies against the Delta and Omicron variants than against the original SARS-CoV-2 vaccine strain [63]. Furthermore, the spike-specific T cell response up to 3 months after two doses of an mRNA vaccine was comparable between inflammatory bowel disease (IBD) patients and healthy individuals [64]. Similar results were demonstrated in heart transplant and solid tumour patients, in whom a durable T cell response was maintained 6 months after a third dose of BNT162b2 vaccine [61, 65]. Additionally, the T cell response was sustained at a higher magnitude, particularly in those treated with tumour necrosis factor (TNF) inhibitor therapy. The T cell response against mutations present in the Omicron variant was found to be mainly preserved in these patients [64]. Similarly, primary antibody deficiency patients were still able to mount a durable CD4+ T cell response specific to SARS-CoV-2 that was similar to that of healthy groups after mRNA vaccination [66].
Cellular immunity increased upon receipt of third and fourth doses of an mRNA vaccine [48, 63, 67, 68]. In addition, a third dose enhanced the number of responders to all variants (55–75% of patients) and significantly increased CD8+ T cell responses [63]. However, even after a third mRNA vaccine dose, SARS-CoV-2-specific interferon(IFN)-γ responses were much lower in kidney transplant recipients, although interleukin(IL)-2 responses remained similar to those of healthy participants [69]. T cell responses deteriorated significantly in immunocompromised groups 6 months after mRNA vaccination. Notably, SARS-CoV-2 T cells became undetectable in a significant proportion of dialysis patients and the majority of kidney transplant recipients 6 months post-vaccination [62].
Decreasing levels of neutralising antibodies over time were observed in older groups. A 4.9-fold decrease in neutralising antibody titres was detected 3–20 weeks after mRNA vaccination, while a greater decline in Omicron neutralisation was reported among older patients 6 months after a third dose of an mRNA vaccine [70, 71]. In contrast, hybrid immunity resulted in a more sustained humoral response over time in older groups of individuals aged 80 years and older 15 months after COVID-19 infection; they were able to sustain their SARS-CoV-2 spike-specific IgG antibody response [72]. Additionally, vaccination with a single dose of an mRNA vaccine enhanced the antibody response in previously infected individuals more significantly than in naïve individuals receiving two doses [72]. Infected patients were able to sustain high levels of anti-receptor-binding domain antibodies 7 months after their second dose of an mRNA vaccine, and, upon receiving the third dose, both anti-receptor-binding domain and neutralising antibody titres against Omicron increased more notably in previously infected groups than in SARS-CoV-2-naïve individuals [73]. In contrast, Gilboa et al. [29] observed that, in participants aged 65 years and older, IgG and neutralising antibodies declined more rapidly in infected individuals.
Few studies focused on cellular immunity in high-risk groups. Gimenez et al. [73] assessed cellular immunity following a third dose of the Pfizer vaccine in nursing home residents. However, while they found that most of the assessed residents had a detectable T cell response at baseline, changes in SARS-CoV-2 S-specific T cells after a third dose of an mRNA vaccine were negligible [73]. In contrast, another study [74] found that, while baseline CD4 TH1 was substantially lower in an older group pre-vaccination, TH1 response was similar to that of younger groups post-vaccination. Additionally, this same study found that older adults produced more IFN-γ than younger adults post-vaccination [74].
However, studies have demonstrated that vaccine effectiveness wanes over time [75]. As observed with the first booster (third dose), the protection against infection was short lived. However, protection against severe illness did not disappear during the study period (i.e. 6 weeks after receiving the fourth dose) [75, 76]. Baum et al. [77] found that, 91–180 days after a second dose, vaccine effectiveness against hospitalisation had decreased from 91% to 76%. Similar results were observed by Gazit et al. [78] after a fourth dose of an mRNA vaccine. Relative effectiveness of the fourth dose of the Pfizer vaccine waned significantly by the tenth week after peaking 3 weeks post-vaccination. However, in the same study, relative effectiveness of the fourth dose against severe COVID-19 was sustained throughout the study period [78]. This is consistent with the findings of Bar-On et al. [75].
Although quantifying the exact duration of protection against SARS-CoV-2 infection poses a great challenge, numerous articles have found a significant decrease in the levels of protection, especially against the Omicron variant (B.1.1.529) and its subvariants, 3–6 months after primary, first booster and second booster vaccination. A list of all the included studies discussing duration of protection is available in the appendix (Data Protection Sheet). In a systematic review and meta-regression study on the duration of effectiveness of vaccination against COVID-19 caused by the Omicron variant, similar results were reported [79]. Overall, 6 months after vaccination, the primary vaccine series led to little protection against symptomatic infections and to a more rapid decrease in vaccine effectiveness during the Omicron period (47.6% decrease [95% CI, 36.6–60.2]) than the pre-Omicron period (24.9% decrease [95% CI, 13.4–41.6]), while decreases in vaccine effectiveness for severe diseases remained relatively similar [79]. With the first booster vaccination, the waning of protection against Omicron was generally higher than after the primary vaccine series for all outcomes; the mean decrease in vaccine effectiveness against symptomatic disease from 1–4 months was 24.3% (95% CI, 19.9–29.1) and projected to 6 months was 28.5% (95 CI, 18.3–40.5) [79]. For second booster doses (fourth doses), the durability of protection against Omicron infections remains relatively uncertain, although a study analysing the protection of a fourth dose over time demonstrated that, for confirmed infections, a fourth dose appeared to provide only short-term protection and a modest absolute benefit [75].
When estimating the half-lives and decay rates of humoral responses, neutralising antibodies were found to wane at higher rates than binding antibodies in vaccinated individuals [40], while total neutralising antibodies had longer lifespans in convalescent individuals [12]. A correlation between cellular and humoral immunity has also been found, where increased levels of CD4+ T cells in cellular immunity are associated with prolonged neutralising antibody responses, crucial for long-term defense against SARS-CoV-2 infection. In the study by Wang et al. [12], high levels of virus-specific CD4+ T cells at baseline were correlated with long-term neutralising levels, indicating the possible role of CD4+ T cells in regulating long-term humoral immunity in patients with previous COVID-19 infection. Additional differences in the duration and waning of humoral immunity were also noted among the diverse types of antibodies. For instance, the decay of IgG-binding antibodies against variants 30 days after the third or fourth vaccine dose was more pronounced than the decay of the IgA response, suggesting possible long-term advantages of IgA antibodies [80]. These results provide evidence that next-generation vaccines targeting the mucosal immunity driven by IgA antibodies could be a solution to the continuous waning of protection against newly emerged variants. In fact, the highest levels of humoral and even cellular immunity were observed in cases of hybrid immunity in which infections triggered a strong IgA response and detectable Omicron-neutralising activity [81]. While the current COVID-19 vaccines continue to demonstrate durable protection against severe outcomes and hospitalisation, their performance at reducing mild illness and transmission, especially with Omicron variants, is suboptimal. With the potential use of nasal vaccines, the thin mucous membrane that lines the nose, mouth and lungs could, in theory, prevent even mild cases of illness and block transmission to other people – something the first-generation COVID-19 vaccines have been unable to achieve – while triggering a strong and durable IgA response. As of October 2022, two nasal COVID-19 vaccines have been approved for use as booster doses in India and China [82, 83]. Although evidence of the effectiveness and duration of protection of such vaccines is scarce, a phase II trial of CanSino’s inhaled vaccines found that they raised blood-serum antibody levels significantly more than an intramuscular booster dose [83].
To our knowledge, this is the largest review of humoral and cellular immunity against SARS-CoV-2 in adults and risk groups. The studies presented covered the duration of protection of three types of humoral and cellular immunity: infection-acquired, vaccine-acquired and hybrid. However, due to the high complexity of investigating T cell responses of individuals, there were fewer studies analysing such immune responses than evaluating humoral immunity. Consequently, literature addressing cellular immunity against SARS-CoV-2 was less prevalent than literature on humoral immunity in our review. This was particularly apparent for high-risk groups; this report was able to identify only two relevant studies assessing cellular immunity in older populations. The results of this study do not apply to other important populations, such as underage individuals (infants, children and adolescents) and pregnant women, since those populations were excluded from our search. Additionally, studies of new and second-generation vaccines and other vaccine platforms not approved in Switzerland were not included. In addition to the identified gaps in literature, our report did not assess the risk of bias or quality of the studies included. Although we attempted to ensure that our search was as exhaustive as possible, the current report provides a narrative summary of current data in the literature that was limited by our eligibility criteria. Any specific research questions may require further analysis of data.
Hybrid immunity was shown to elicit the highest levels of humoral and cellular immunity while lasting longer than infection-acquired and vaccine-acquired immunity. However, with the emergence of new Omicron subvariants, the levels and duration of protection were greatly affected, leaving immunised individuals prone to infection or reinfection. Boosting maintains vaccine effectiveness against severe disease caused by the current Omicron sub-lineages; nonetheless, the evidence of rapid waning of durability may indicate that at best, there is a need for regular boosting as early as 4 months after the last dose and the need for vaccines to incorporate variants of concern to maintain protection. In terms of CD8+ T cell responses, mRNA boosters induced a temporary T effector cell response, while spike-specific CD8+ T cell memory was conserved for targeting variants of concern. Infection with the Omicron variant or Omicron variant-specific vaccination may induce a memory T cell response sufficient for protecting against an Omicron infection. Despite humoral response defects, vaccine-induced T cell responses might still provide a layer of protection for patients undergoing immune-modifying therapies.
The data that support the findings of this review were gathered from publicly available databases.
We would like to express our sincere appreciation and gratitude to the entire team at the GRAPH Network (https://thegraphnetwork.org/), the Federal Office of Public Health of Switzerland (FOPH), the Swiss School of Public Health (SSPH+), and Jorgen Bauwens for guiding and supporting us along the entirety of this review.
Author contributions: SRV and SBM conceived and designed the study. MA, and OK made substantial contributions in reviewing the design of the study. SRV, LEB, and SMH conducted the literature search. SRV, LEB, and SMH conducted the title and abstract screening. SRV, LEB, SMH, IT, and JD conducted the full-text review. SRV, LEB, SMH, IT, and JD conducted the data extraction. SRV, LEB, SMH, IT, and JD drafted the manuscript. MA, SBM, CBGV, and OK contributed by revising the manuscript critically for important intellectual content. MA and OK critically reviewed the manuscript. All authors contributed to final approval of the version to be submitted.
This research was funded by the Federal Office of Public Health of Switzerland in collaboration with the Swiss School of Public Health (SSPH+). One author (OK) was supported by the Swiss National Science Foundation (grants no 31CA30_196270 and PP00P3_202660).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Chen CP, Huang KA, Shih SR, Lin YC, Cheng CY, Huang YC, et al. Anti-Spike Antibody Response to Natural Infection with SARS-CoV-2 and Its Activity against Emerging Variants. Microbiol Spectr. 2022 Aug;10(4):e0074322.
2. Cox RJ, Brokstad KA. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat Rev Immunol. 2020 Oct;20(10):581–2.
3. Mengist HM, Kombe Kombe AJ, Mekonnen D, Abebaw A, Getachew M, Jin T. Mutations of SARS-CoV-2 spike protein: implications on immune evasion and vaccine-induced immunity. Semin Immunol. 2021 Jun;55:101533.
4. Hu J, Peng P, Wang K, Fang L, Luo FY, Jin AS, et al. Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies. Cell Mol Immunol. 2021 Apr;18(4):1061–3.
5. Hirabara SM, Serdan TD, Gorjao R, Masi LN, Pithon-Curi TC, Covas DT, et al. SARS-COV-2 Variants: Differences and Potential of Immune Evasion. Front Cell Infect Microbiol. 2022 Jan;11:781429.
6. McCallum M, Czudnochowski N, Rosen LE, Zepeda SK, Bowen JE, Walls AC, et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022 Feb;375(6583):864–8.
7. Lyke KE, Atmar RL, Islas CD, Posavad CM, Szydlo D, Paul Chourdhury R, et al.; DMID 21-0012 Study Group. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med. 2022 Jul;3(7):100679.
8. Mise-Omata S, Ikeda M, Takeshita M, Uwamino Y, Wakui M, Arai T, et al. Memory B Cells and Memory T Cells Induced by SARS-CoV-2 Booster Vaccination or Infection Show Different Dynamics and Responsiveness to the Omicron Variant. J Immunol. 2022 Dec;209(11):2104–13.
9. Kurhade C, Zou J, Xia H, Cai H, Yang Q, Cutler M, et al. Neutralization of Omicron BA.1, BA.2, and BA.3 SARS-CoV-2 by 3 doses of BNT162b2 vaccine. Nat Commun. 2022 Jun;13(1):3602.
10. Collie S, Nayager J, Bamford L, Bekker LG, Zylstra M, Gray G. Effectiveness and Durability of the BNT162b2 Vaccine against Omicron Sublineages in South Africa. N Engl J Med. 2022 Oct;387(14):1332–3.
11. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016 Dec;5(1):210.
12. Wang Z, Yang X, Mei X, Zhou Y, Tang Z, Li G, et al. SARS-CoV-2-specific CD4+ T cells are associated with long-term persistence of neutralizing antibodies. Signal Transduct Target Ther. 2022 Apr;7(1):132.
13. Garcia-Valtanen P, Hope CM, Masavuli MG, Yeow AE, Balachandran H, Mekonnen ZA, et al. SARS-CoV-2 Omicron variant escapes neutralizing antibodies and T cell responses more efficiently than other variants in mild COVID-19 convalescents. Cell Rep Med. 2022 Jun;3(6):100651.
14. Guo L, Zhang Q, Zhang C, Huang T, Ren L, Cao B, et al. Assessment of Antibody and T-Cell Responses to the SARS-CoV-2 Virus and Omicron Variant in Unvaccinated Individuals Recovered From COVID-19 Infection in Wuhan, China. JAMA Netw Open. 2022 Apr;5(4):e229199.
15. Li Y, Wang X, Shen XR, Geng R, Xie N, Han JF, et al. A 1-year longitudinal study on COVID-19 convalescents reveals persistence of anti-SARS-CoV-2 humoral and cellular immunity. Emerg Microbes Infect. 2022 Dec;11(1):902–13.
16. Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N Engl J Med. 2022 Jul;387(1):21–34.
17. Carazo S, Skowronski DM, Brisson M, Sauvageau C, Brousseau N, Gilca R, et al. Estimated Protection of Prior SARS-CoV-2 Infection Against Reinfection With the Omicron Variant Among Messenger RNA-Vaccinated and Nonvaccinated Individuals in Quebec, Canada. JAMA Netw Open. 2022 Oct;5(10):e2236670.
18. Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, et al. Protection from previous natural infection compared with mRNA vaccination against SARS-CoV-2 infection and severe COVID-19 in Qatar: a retrospective cohort study. Lancet Microbe. 2022 Dec;3(12):e944–55.
19. Šmíd M, Berec L, Přibylová L, Májek O, Pavlík T, Jarkovský J, et al. Protection by Vaccines and Previous Infection Against the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 [Erratum in: J Infect Dis. 2023 Apr 18;227] [8] [:1021-1022. PMID: 35482442; PMCID: PMC9129207]. J Infect Dis. 2022 Oct;226(8):1385–90.
20. Belik M, Jalkanen P, Lundberg R, Reinholm A, Laine L, Väisänen E, et al. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat Commun. 2022 May;13(1):2476.
21. Furukawa K, Tjan LH, Kurahashi Y, Sutandhio S, Nishimura M, Arii J, et al. Assessment of Neutralizing Antibody Response Against SARS-CoV-2 Variants After 2 to 3 Doses of the BNT162b2 mRNA COVID-19 Vaccine. JAMA Netw Open. 2022 May;5(5):e2210780.
22. Lu L, Mok BW, Chen LL, Chan JM, Tsang OT, Lam BH, et al. Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant by Sera From BNT162b2 or CoronaVac Vaccine Recipients. Clin Infect Dis. 2022 Aug;75(1):e822–6.
23. Muik A, Lui BG, Wallisch AK, Bacher M, Mühl J, Reinholz J, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022 Feb;375(6581):678–80.
24. Seki Y, Yoshihara Y, Nojima K, Momose H, Fukushi S, Moriyama S, et al. Safety and immunogenicity of the Pfizer/BioNTech SARS-CoV-2 mRNA third booster vaccine dose against the BA.1 and BA.2 Omicron variants. Med (N Y). 2022 Jun;3(6):406–421.e4.
25. Xia H, Zou J, Kurhade C, Cai H, Yang Q, Cutler M, et al. Neutralization of Omicron SARS-CoV-2 by 2 or 3 doses of BNT162b2 vaccine. bioRxiv 2022.01.21.476344; doi: https://doi.org/
26. Adachi E, Nagai E, Saito M, Isobe M, Konuma T, Koga M, et al. Anti-spike protein antibody titer at the time of breakthrough infection of SARS-CoV-2 omicron. J Infect Chemother. 2022 Jul;28(7):1015–7.
27. Hein S, Mhedhbi I, Zahn T, Sabino C, Benz NI, Husria Y, et al. Quantitative and Qualitative Difference in Antibody Response against Omicron and Ancestral SARS-CoV-2 after Third and Fourth Vaccination. Vaccines (Basel). 2022 May;10(5):796.
28. Woldemeskel BA, Garliss CC, Aytenfisu TY, Johnston TS, Beck EJ, Dykema AG, et al. SARS-CoV-2-specific immune responses in boosted vaccine recipients with breakthrough infections during the Omicron variant surge. JCI Insight. 2022 May;7(10):e159474.
29. Gilboa M, Regev-Yochay G, Mandelboim M, Indenbaum V, Asraf K, Fluss R, et al.; Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection, JAMA Netw. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw Open. 2022 Sep;5(9):e2231778.
30. Richardson JR, Götz R, Mayr V, Lohse MJ, Holthoff HP, Ungerer M. SARS-CoV2 wild type and mutant specific humoral and T cell immunity is superior after vaccination than after natural infection. PLoS One. 2022 Apr;17(4):e0266701.
31. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022 Apr;386(16):1532–46.
32. Wang X, Chang H, Tian H, Zhu Y, Li J, Wei Z, et al. Epidemiological and clinical features of SARS-CoV-2 infection in children during the outbreak of Omicron variant in Shanghai, March 7-31, 2022. Influenza Other Respir Viruses. 2022 Nov;16(6):1059–65.
33. Peng Q, Zhou R, Wang Y, Zhao M, Liu N, Li S, et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine. 2022 Mar;77:103904.
34. GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S, et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022 Mar;7(69):eabo2202.
35. Kotaki R, Adachi Y, Moriyama S, Onodera T, Fukushi S, Nagakura T, et al. SARS-CoV-2 Omicron-neutralizing memory B cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci Immunol. 2022 Apr;7(70):eabn8590.
36. Sablerolles RS, Rietdijk WJ, Goorhuis A, Postma DF, Visser LG, Schmitz KS, et al.; SWITCH Research Group. Durability of Immune Responses After Boosting in Ad26.COV2.S-Primed Healthcare Workers. Clin Infect Dis. 2023 Feb;76(3):e533–6.
37. Tan CS, Collier AY, Yu J, Liu J, Chandrashekar A, McMahan K, et al. Durability of Heterologous and Homologous COVID-19 Vaccine Boosts. JAMA Netw Open. 2022 Aug;5(8):e2226335.
38. Reinscheid M, Luxenburger H, Karl V, Graeser A, Giese S, Ciminski K, et al. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat Commun. 2022 Aug;13(1):4631.
39. Chang MR, Ke H, Coherd CD, Wang Y, Mashima K, Kastrunes GM, Huang CY, Marasco WA. Analysis of a SARS-CoV-2 convalescent cohort identified a common strategy for escape of vaccine-induced anti-RBD antibodies by Beta and Omicron variants. EBioMedicine. 2022 Jun;80:104025. doi: . Epub 2022 May 6. PMID: 35533497; PMCID: PMC9073271.https://doi.org/..
40. Favresse J, Gillot C, Bayart JL, David C, Simon G, Wauthier L, et al. Vaccine-induced binding and neutralizing antibodies against Omicron 6 months after a homologous BNT162b2 booster. J Med Virol. 2023 Jan;95(1):e28164.
41. Evans JP, Zeng C, Carlin C, Lozanski G, Saif LJ, Oltz EM, et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med. 2022 Mar;14(637):eabn8057.
42. Edara VV, Manning KE, Ellis M, Lai L, Moore KM, Foster SL, et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep Med. 2022 Jan;3(2):100529.
43. Qu P, Faraone JN, Evans JP, Zheng YM, Yu L, Ma Q, et al. Durability of Booster mRNA Vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 Subvariants. N Engl J Med. 2022 Oct;387(14):1329–31.
44. Cerqueira-Silva T, de Araujo Oliveira V, Paixão ES, Florentino PT, Penna GO, Pearce N, et al. Vaccination plus previous infection: protection during the omicron wave in Brazil. Lancet Infect Dis. 2022 Jul;22(7):945–6.
45. McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EH, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study [Erratum in: Lancet Infect Dis. 2022 Sep;22] [9] [:e239. PMID: 35850128; PMCID: PMC9286709]. Lancet Infect Dis. 2022 Oct;22(10):1435–43.
46. Al Jurdi A, Gassen RB, Borges TJ, Lape IT, Morena L, Efe O, et al. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int. 2022 Jun;101(6):1282–6.
47. Carr EJ, Wu M, Harvey R, Billany RE, Wall EC, Kelly G, et al.; Haemodialysis COVID-19 Consortium, Crick COVID Immunity Pipeline. Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet. 2022 Feb;399(10327):800–2.
48. Becker M, Cossmann A, Lürken K, Junker D, Gruber J, Juengling J, et al. Longitudinal cellular and humoral immune responses after triple BNT162b2 and fourth full-dose mRNA-1273 vaccination in haemodialysis patients. Front Immunol. 2022 Oct;13:1004045.
49. Cheng CC, Platen L, Christa C, Tellenbach M, Kappler V, Bester R, et al. Improved SARS-CoV-2 Neutralization of Delta and Omicron BA.1 Variants of Concern after Fourth Vaccination in Hemodialysis Patients. Vaccines (Basel). 2022 Aug;10(8):1328.
50. Di Giacomo AM, Giacobini G, Anichini G, Gandolfo C, D’alonzo V, Calabrò L, et al. SARS-CoV-2 infection in cancer patients on active therapy after the booster dose of mRNA vaccines. Eur J Cancer. 2022 Aug;171:143–9.
51. Haggenburg S, Hofsink Q, Lissenberg-Witte BI, Broers AE, van Doesum JA, van Binnendijk RS, et al.; COBRA KAI Study Team. Antibody Response in Immunocompromised Patients With Hematologic Cancers Who Received a 3-Dose mRNA-1273 Vaccination Schedule for COVID-19. JAMA Oncol. 2022 Oct;8(10):1477–83.
52. Karaba AH, Johnston TS, Aytenfisu TY, Akinde O, Eby Y, Ruff JE, et al. A Fourth Dose of COVID-19 Vaccine Does Not Induce Neutralization of the Omicron Variant Among Solid Organ Transplant Recipients With Suboptimal Vaccine Response. Transplantation. 2022 Jul;106(7):1440–4.
53. Kennedy NA, et al. Vaccine escape, increased breakthrough and reinfection in infliximab-treated patients with IBD during the Omicron wave of the SARS-CoV-2 pandemic. Gut. 2022 Jul:
54. Kontopoulou K, Nakas CT, Belai C, Papazisis G. Antibody titers after a third dose of the SARS-CoV-2 BNT162b2 vaccine in immunocompromised adults in Greece: is a fourth dose necessary? J Med Virol. 2022 Oct;94(10):5056–60.
55. Montez-Rath ME, Garcia P, Han J, Cadden L, Hunsader P, Morgan C, et al. SARS-CoV-2 Infection during the Omicron Surge among Patients Receiving Dialysis: The Role of Circulating Receptor-Binding Domain Antibodies and Vaccine Doses. J Am Soc Nephrol. 2022 Oct;33(10):1832–9.
56. Otto C, Schwarz T, Jeworowski LM, Schmidt ML, Walper F, Pache F, et al. Humoral immune responses remain quantitatively impaired but improve qualitatively in anti-CD20 treated patients with multiple sclerosis after three or four COVID-19 vaccinations. medRxiv 2022.08.10.22278639; doi: https://doi.org/
57. Thakkar A, Pradhan K, Duva B, Carreno JM, Sahu S, Thiruthuvanathan V, et al. Study of efficacy and longevity of immune response to third and fourth doses of COVID-19 vaccines in patients with cancer: A single arm clinical trial. eLife. 2023 Mar;12:e83694.
58. Tillmann FP, Figiel L, Ricken J, Still H, Korte C, Plaßmann G, et al. Effect of Third and Fourth mRNA-Based Booster Vaccinations on SARS-CoV-2 Neutralizing Antibody Titer Formation, Risk Factors for Non-Response, and Outcome after SARS-CoV-2 Omicron Breakthrough Infections in Patients on Chronic Hemodialysis: A Prospective Multicenter Cohort Study. J Clin Med. 2022 Jun;11(11):3187.
59. Vergori A, Cozzi-Lepri A, Matusali G, Colavita F, Cicalini S, Gallì P, et al.; HIV-VAC Study Group. SARS-CoV-2 Omicron Variant Neutralization after Third Dose Vaccination in PLWH. Viruses. 2022 Aug;14(8):1710.
60. Kumar D, Hu Q, Samson R, Ferreira VH, Hall VG, Ierullo M, et al. Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine. Am J Transplant. 2022 Aug;22(8):2089–93.
61. Lasagna A, Bergami F, Lilleri D, Percivalle E, Quaccini M, Serra F, et al. Six-month humoral and cellular immune response to the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with solid tumors: a longitudinal cohort study with a focus on the variants of concern. ESMO Open. 2022 Oct;7(5):100574.
62. Sanders JF, Messchendorp AL, de Vries RD, Baan CC, van Baarle D, van Binnendijk R, et al.; VACcination Immune Response Study (RECOVAC) Collaborators. Antibody and T-Cell Responses 6 Months After Coronavirus Disease 2019 Messenger RNA-1273 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Clin Infect Dis. 2023 Feb;76(3):e188–99.
63. Madelon N, Heikkilä N, Sabater Royo I, Fontannaz P, Breville G, Lauper K, et al. Omicron-Specific Cytotoxic T-Cell Responses After a Third Dose of mRNA COVID-19 Vaccine Among Patients With Multiple Sclerosis Treated With Ocrelizumab. JAMA Neurol. 2022 Apr;79(4):399–404.
64. Qui M, Le Bert N, Chan WP, Tan M, Hang SK, Hariharaputran S, et al. Favorable vaccine-induced SARS-CoV-2-specific T cell response profile in patients undergoing immune-modifying therapies. J Clin Invest. 2022 Jun;132(12):e159500.
65. Peled Y, Afek A, Kreiss Y, Rahav G, Nemet I, Kliker L, et al. Kinetics of cellular and humoral responses to third BNT162B2 COVID-19 vaccine over six months in heart transplant recipients - implications for the omicron variant. J Heart Lung Transplant. 2022 Oct;41(10):1417–25.
66. Lin FJ, Doss AM, Davis-Adams HG, Adams LJ, Hanson CH, VanBlargan LA, et al. SARS-CoV-2 booster vaccination rescues attenuated IgG1 memory B cell response in primary antibody deficiency patients. Front Immunol. 2022 Dec;13:1033770.
67. Lasagna A, Bergami F, Lilleri D, Percivalle E, Quaccini M, Alessio N, et al. Immunogenicity and safety after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with solid tumors on active treatment: a prospective cohort study. ESMO Open. 2022 Apr;7(2):100458.
68. Sannier G, Nicolas A, Dubé M, Marchitto L, Nayrac M, Tastet O, et al. A third SARS-CoV-2 mRNA vaccine dose in people receiving hemodialysis overcomes B cell defects but elicits a skewed CD4+ T cell profile. Cell Rep Med. 2023 Mar;4(3):100955.
69. Thümmler L, Gäckler A, Bormann M, Ciesek S, Widera M, Rohn H, et al. Cellular and Humoral Immunity against Different SARS-CoV-2 Variants Is Detectable but Reduced in Vaccinated Kidney Transplant Patients. Vaccines (Basel). 2022 Aug;10(8):1348.
70. Newman J, Thakur N, Peacock TP, Bialy D, Elrefaey AM, Bogaardt C, et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat Microbiol. 2022 Aug;7(8):1180–8.
71. Mwimanzi F, Lapointe HR, Cheung PK, Sang Y, Yaseen F, Umviligihozo G, et al. Older Adults Mount Less Durable Humoral Responses to Two Doses of COVID-19 mRNA Vaccine but Strong Initial Responses to a Third Dose. J Infect Dis. 2022 Sep;226(6):983–94.
72. Lee HK, Knabl L, Moliva JI, Knabl L Sr, Werner AP, Boyoglu-Barnum S, et al. mRNA vaccination in octogenarians 15 and 20 months after recovery from COVID-19 elicits robust immune and antibody responses that include Omicron. Cell Rep. 2022 Apr;39(2):110680.
73. Giménez E, Albert E, Zulaica J, Torres I, Rusu L, Moreno AR, et al.; Valencian Vaccine Research Program (ProVaVac) Study Group. Severe Acute Respiratory Syndrome Coronavirus 2 Adaptive Immunity in Nursing Home Residents Following a Third Dose of the Comirnaty Coronavirus Disease 2019 Vaccine. Clin Infect Dis. 2022 Aug;75(1):e865–8.
74. Renia L, Goh YS, Rouers A, Le Bert N, Chia WN, Chavatte JM, et al.; SCOPE Cohort Study Group. Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose. Nat Commun. 2022 Aug;13(1):4615.
75. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, et al. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022 May;386(18):1712–20.
76. Magen O, Waxman JG, Makov-Assif M, Vered R, Dicker D, Hernán MA, et al. Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2022 Apr;386(17):1603–14.
77. Baum U, Poukka E, Leino T, Kilpi T, Nohynek H, Palmu AA. High vaccine effectiveness against severe COVID-19 in the elderly in Finland before and after the emergence of Omicron. BMC Infect Dis. 2022 Nov;22(1):816.
78. Gazit S, Saciuk Y, Perez G, Peretz A, Pitzer VE, Patalon T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ. 2022 May;377:e071113.
79. Higdon MM, Baidya A, Walter KK, Patel MK, Issa H, Espié E, et al. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect Dis. 2022 Aug;22(8):1114–6.
80. Hertz T, Levy S, Ostrivsky D, Oppenheimer H, Zismanov S, Ku A, et al. Correlates of protection for booster doses of the BNT162b2 vaccine. MedRxiv 2022.07.16.22277626; doi: https://doi.org/
81. Planas D, Staropoli I, Porot F, Guivel-Benhassine F, Handala L, Prot M, et al. Duration of BA.5 neutralization in sera and nasal swabs from SARS-CoV-2 vaccinated individuals, with or without omicron breakthrough infection. Med (N Y). 2022 Dec;3(12):838–847.e3.
82. Waltz E. How nasal-spray vaccines could change the pandemic. Nature. 2022 Sep;609(7926):240–2.
83. Waltz E. China and India approve nasal COVID vaccines - are they a game changer? Nature. 2022 Sep;609(7927):450.
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3732.