Investigations of an increased incidence of non-Aspergillus invasive
mould infections
in an onco-haematology unit
DOI: https://doi.org/https://doi.org/10.57187/s.3730
Elisavet Stavropouloua,
Anne Huguenina,
Giorgia Caruanaab,
Onya Opotab,
Nancy Perrottetc,
Dominique S. Blancd,
Bruno Grandbastiend,
Laurence Sennd,
Pierre-Yves Bochuda,
Frederic Lamothab
a Infectious diseases service, Department of medicine,
Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
b Institute of microbiology, Department of laboratory medicine and
pathology, Lausanne University Hospital and University of Lausanne, Lausanne,
Switzerland
c Unit of clinical pharmacy, Lausanne University Hospital and University
of Lausanne, Lausanne, Switzerland
d Infection prevention and control unit, Infectious diseases Service,
Department of medicine, Lausanne University Hospital and University of
Lausanne, Lausanne, Switzerland
* Equal
contribution
Summary
AIMS OF THE STUDY: Invasive mould infections are
life-threatening complications in patients with haematologic cancer and
chemotherapy-induced neutropenia. While invasive aspergillosis represents the
main cause of invasive mould infections, non-Aspergillus mould
infections, such as mucormycosis, are increasingly reported. Consequently, their
local epidemiology should be closely monitored. The aim of this study was to
investigate the causes of an increased incidence of non-Aspergillus mould
infections in the onco-haematology unit of a Swiss tertiary care hospital.
METHODS: All cases of proven and probable invasive mould
infections were retrospectively identified via a local registry for the period
2007–2021 and their incidence was calculated per 10,000 patient-days per year.
The relative proportion of invasive aspergillosis and non-Aspergillus mould
infections was assessed. Factors that may affect invasive mould infections’ incidence,
such as antifungal drug consumption, environmental contamination and changes in
diagnostic approaches, were investigated.
RESULTS: A significant increase of the incidence of non-Aspergillus
mould infections (mainly mucormycosis) was observed from 2017 onwards (Mann and
Kendall test p = 0.0053), peaking in 2020 (8.62 episodes per 10,000
patient-days). The incidence of invasive aspergillosis remained stable across
the period of observation. The proportion of non-Aspergillus mould
infections increased significantly from 2017 (33% vs 16.8% for the periods 2017–2021
and 2007–2016, respectively, p = 0.02).
Building projects on the hospital site were identified as possible contributors of
this increase in non-Aspergillus
mould infections. However, novel diagnostic procedures may have improved their
detection.
CONCLUSIONS: We report a
significant increase in non-Aspergillus mould infections, and mainly in
mucormycosis infections, since 2017. There seems to be a multifactorial origin
to this increase. Epidemiological trends of invasive mould infections should be
carefully monitored in onco-haematology units in order to implement potential
corrective measures.
Introduction
Invasive mould
infections (IMI) affect between 5% and 10% of patients with haematologic
malignancies and chemotherapy-induced neutropenia. Aspergillus spp.
accounts for the majority of cases [1, 2].
Albeit less frequent, non-Aspergillus
moulds, and particularly the Mucorales, are often refractory to standard
antifungal therapies and associated with high mortality rates (40–50%) [3, 4]. A selection
of recent reports suggest
that their incidence and relative proportion are increasing [5–8]. This epidemiological
shift might be explained
by various factors, such as the
development of novel anti-cancer therapies, or the selective pressure of
antifungal agents. Environmental changes, including the contamination of hospital
rooms by airborne spores, may also play a role [9].
Moreover, the development of diagnostic tools with increased sensitivity, such
as PCR, may lead to a better recognition of these fungi that are fastidious to
grow in cultures [3].
We observed
an increased incidence of non-Aspergillus
invasive mould infections (NAIMI) in a single Swiss tertiary care hospital between
2017-2021. The aim of this study was to investigate the potential causes of
this epidemiological shift.
Materials and
methods
Patients and
setting
The
Lausanne University Hospital is a 1500-bed hospital in which there is an 18-bed
isolation unit both for patients undergoing chemotherapy for acute leukaemia
and for those receiving autologous haematopoietic stem cell transplantation for
other haematologic malignancies. In 2019, the isolation unit had been moved
from a satellite building to a renovated unit in the main hospital building. This
unit had positive-pressure
rooms as well as the high efficiency particulate air (HEPA) filtration system. Also
in 2019, important renovation works have been
undertaken close to the main hospital building in the form of ground excavation for
the
construction of a new hospital building.
Patients
undergoing myeloablative chemotherapies with an expected duration of
neutropenia >10 days receive fluconazole prophylaxis and are monitored twice
weekly for galactomannan and (1→3)-β-d-glucan in serum. Fluconazole may
be substituted by posaconazole for individual cases who are considered at
higher risk or for patients included in a study protocol requiring posaconazole
prophylaxis. Patients with persisting or recurrent neutropenic fever undergo a chest
CT-scan and, in case of lung CT abnormality, a bronchoscopy. In addition to
standard fungal cultures, the microbiology laboratory provides an Aspergillus fumigatus-specific PCR and a
panfungal PCR, as previously described [10].
Moreover, a pan-Mucorales PCR has been implemented in 2018, as previously
described [11].
Incidence of invasive
mould infections
All
episodes of proven or probable invasive mould infections, according to the European
Organisation for Research and Treatment of Cancer and Mycoses Study Group
Education and Research Consortium (EORTC-MSGERC) [12], were extracted from the ward
registry for the period 2007–2021.
Incidence of proven or probable invasive aspergillosis, invasive mucormycosis
and other NAIMI were expressed for each year
in the number of cases per 10,000 patient-days with expression of the 95%
confidence interval (CI).
Room air analyses
The
contamination of room air with mould was investigated by passive air sampling
using open Sabouraud agar plates that were dispatched at different locations
within each room (3 per room) for 4 h. The plates were then closed and incubated
for 5 days at 20 °C.
Antifungal drug
consumption
Consumption
of antifungals was extrapolated from the
hospital pharmacy’s delivery data for
posaconazole and other antifungal agents. The total mass of compounds (in grams)
was converted into defined daily dose (DDD) according to the Anatomical
Therapeutic Chemical (ATC) code / DDD index (https://www.whocc.no/atc_ddd_index/ last
accessed June 19th 2023). An adjustment was made for the
posaconazole suspension formulation (used before 2015) with a DDD defined at
0.6 g (0.2 g three times daily) instead of 0.3 g for the tablet formulation
(0.3 g once daily).
Statistical
analyses
Two time
periods (2007–2016 and 2017–2021) were compared. The Fisher exact test was used
to compare proportions of NAIMI. Temporal
variations of the incidence rates of invasive aspergillosis and NAIMI have been investigated.
The Mann and Kendall test was used to assess
the significance of the trend in incidence. The significance level was set at p ≤0.05.
Analyses were performed on Microsoft Excel with XLSTAT program (Microsoft
Corporation, Redmont WA)
Ethical statement
The
patients included in this study have signed informed consent for inclusion in a
previous cohort study of epidemiological survey during febrile neutropenia,
which was approved by the local ethics committee (CER-VD, protocol number
2017-01975). No protocol has been publicly deposited for the present study,
which was part of the routine investigations of the infection control unit within
our institution.
Results
Incidence of non-Aspergillus
mould infections over years
From 2007
to 2021, a total of 103 episodes of proven or probable invasive mould
infections occurred in the isolation unit. This includes 79 invasive
aspergillosis and 24 NAIMI (including 18
invasive mucormycosis). The incidence of invasive mould infections, invasive
aspergillosis and NAIMI over years is shown
in figure 1. A significant increase of the incidence of NAIMI was observed from 2017
onwards (Mann and Kendall test, p = 0.0053), while
the incidence of invasive aspergillosis remained stable (p = 0.77). While NAIMI represented
16.8% of invasive mould infection cases for the
period 2007–2016, their proportion was 33% for the period 2017–2021 (p =
0.02), with the peak observed in 2020 (8.62 episodes per 10,000 patient-days, 95%
CI:
3.74–18.64, 45% of all invasive mould infections). The increased incidence was
significant for mucormycosis (p = 0.003), which represented the majority of NAIMI.
All NAIMI other than
mucormycosis, except one, occurred in the later period (2017–2021).
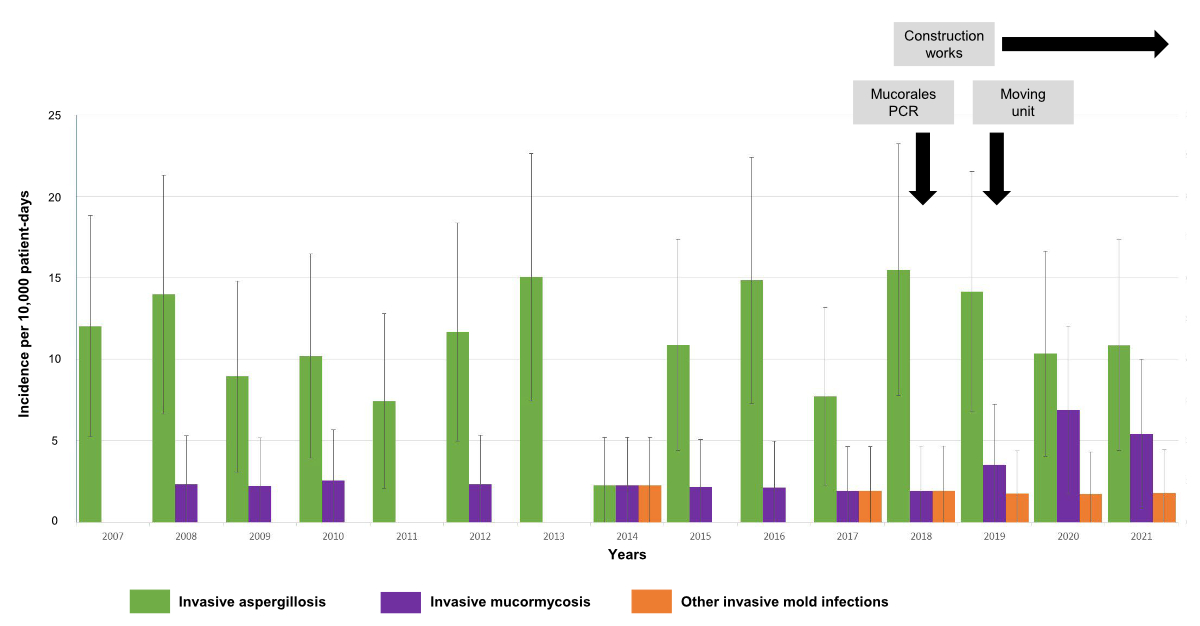
Figure 1Incidence and
proportions of different invasive mould infections in the isolation unit of
onco-haematology. Incidences are
represented in the number of cases per 10,000 patient-days for invasive
aspergillosis (green), invasive mucormycosis (violet) and other invasive mould
infections (red) for each year. Bars represent the 95% confidence interval. The
black arrows and grey boxes indicate the different events with a potential
impact on invasive mould infections incidence. “Construction works”: construction
of a new
hospital building near the main building; “Mucorales PCR”: introduction of a
new PCR for detection of Mucorales; “Moving unit”: re-localization of the isolation
unit in another hospital building.
Investigations of
potential causes of non-Aspergillus mould infections outbreak
Analyses of
antifungal drug consumption did not reveal a significant increase in the use of
posaconazole (median 661.3 DDD/year for the period 2017–2021, vs 934.9 DDD/year
for the period 2007–2016, p = 0.13) or other anti-mould active
antifungal drugs (data not shown).
In 2020, a
passive air sample was taken from all 18
rooms of the isolation unit. No Mucorales were recovered by this method; only Cladosporium spp. grew on 2 out of 54 of
the plates.
We also assessed
the impact of the novel Mucorales PCR, which may have resulted in increased
recognition of proven or probable mucormycosis among cases that would otherwise
have been classified only as possible invasive mould infections, according to EORTC-MSGERC
criteria. Since its introduction in 2018, the Mucorales PCR was performed in 9 out
of 10 cases of mucormycosis. There were positive results in 8 (89%) cases and
one negative result (one invasive mould infections due to Cunninghamella,
not included in the PCR panel) (table 1). In 3 out of 10 cases (30%), the diagnosis
of mucormycosis was obtained via a positive result of the Mucorales PCR only
(i.e. negative histopathology, culture and panfungal PCR).
Table 1Characteristics of patients with non-Aspergillus invasive mould
infections. Numbers are
total number (percentage) for proportions and median (range) for continuous
variables.
| N
total |
24 |
| Demographic characteristics |
Female
/ Male |
8 (33%) / 16 (67%) |
| Age
(years) |
55 (19–72) |
| Underlying haematologic disease |
Acute
myeloid leukaemia |
16
(67%) |
| Other
haematologic cancer* |
8
(33%) |
| Predisposing conditions** |
Neutropenia*** |
22 (92%) |
| Recent
chemotherapy |
21 (87.5%) |
|
Induction |
9 (43%) |
| Re-induction
/ consolidation |
5 (24%) |
| Salvage / maintenance |
7 (33%) |
| Allogeneic haematopoietic cell
transplantation |
6 (25%) |
|
Graft versus host disease |
5 (83%) |
Characteristics of patients and non-Aspergillus invasive mould infections episodes
The demographic characteristics of
the 24 patients with a documented NAIMI are
described in table 1. Most patients (n = 21, 87.5%) had chemotherapy-induced
prolonged neutropenia. Eight (33%) patients were receiving antifungal prophylaxis
that was considered to be mould-active (posaconazole n = 5, voriconazole n = 2,
isavuconazole n = 1) for ≥7 days when they developed breakthrough NAIMI (table 2).
The characteristics of all 24 NAIMI
episodes are shown in table 2. Most cases (n = 20, 83%) were proven invasive fungal
infections. The lungs were the primary site of infection in most cases (n
= 22, 92%) and disseminated disease (≥2 affected organs) was observed in 8
(33%) patients. Extra-pulmonary sites of infection were intra-abdominal
abscesses (liver or spleen, n = 5, 21% of patients), skin or soft tissue
lesions (n = 5, 21%) and brain lesions (n = 2, 8%). Among episodes of
mucormycosis (n = 18), the most frequent pathogen was Rhizomucor spp. (n
= 7, 39%) followed by Lichtheimia spp. (n = 5, 28%), Mucor or Rhizopus
spp. (n = 4, 22%) and Cunninghamella (n = 1, 6%). In one case, the
diagnosis of mucormycosis relied on histopathological documentation only (no
genus or species identification occured). Other NAIMI were attributed to Hormographiella aspergillata (n = 3), Alternaria
spp. (n = 2) and Conidiobolus spp. (n = 1). First-line antifungal
therapy of mucormycosis consisted of liposomal amphotericin B alone (n = 8) or
combined with an echinocandin (n = 6) or isavuconazole (n = 1), posaconazole (n
= 2) and isavuconazole (n = 1). A triazole was used as second-line, or "maintenance",
therapy in 12 cases (isavuconazole, n = 7, and posaconazole, n = 5). Different
drugs were used for the treatment of other NAIMI (liposomal amphotericin B, posaconazole,
isavuconzole,
voriconazole). Overall, surgical interventions for therapeutic purposes were
performed in 18 out of 24 (75%) cases. Response to therapy was assessed at week
12 according to recommended criteria [13].
Success (complete or partial response) was observed in 15 out of 24 (63%)
cases. The overall mortality rate at week 12 was 2 out of 24 (8%). Two patients
subsequently died between week 12 and 24. The mortality rate for
mucormycosis was 11% and 22% at week 12 and 24, respectively. All patients with
other NAIMI were alive at week 24.
Table 2Characteristics
of episodes of non-Aspergillus invasive mould infections.
| Patient |
Period1 |
EORTC-MSGERC criteria |
Site(s) of infection |
Fungal species |
Method of identification |
Prior anti-fungal2 |
Anti-fungal therapy3 |
Outcome at week 12
(response)4 |
| Cases of mucormycosis |
| 1 |
1 |
Proven |
Lung |
Lichtheimia corymbifera |
Histology / Panfungal
PCR |
CAS |
L-AMB, then POS / Surgery |
Alive (Complete) |
| 2 |
1 |
Proven |
Lung |
Rhizopus microsporus |
Histology / Panfungal
PCR |
No |
POS / Surgery |
Alive (Partial) |
| 3 |
1 |
Proven |
Lung, liver, spleen |
Not identified |
Histology |
No |
L-AMB5 |
Deceased |
| 4 |
1 |
Proven |
Lung |
Rhizopus spp. |
Histology / Panfungal
PCR |
No |
L-AMB / Surgery |
Alive (Complete) |
| 5 |
1 |
Proven |
Lung, soft tissue |
Lichtheimia spp. |
Histology / Panfungal
PCR |
No |
L-AMB, then POS / Surgery |
Alive (Complete) |
| 6 |
1 |
Proven |
Lung, liver, spleen, soft tissues |
Rhizomucor miehei / pusillus |
Histology / Panfungal
PCR |
FLU |
L-AMB / Surgery |
Alive (Progression) |
| 7 |
1 |
Proven |
Lung |
Lichtheimia corymbifera |
Histology / Panfungal
PCR |
FLU |
L-AMB, then POS / Surgery |
Alive (Partial) |
| 8 |
2 |
Proven |
Lung |
Rhizomucor pusillus / microsporus |
Histology / Culture |
FLU |
L-AMB, then POS / Surgery |
Alive (Progression) |
| 9 |
2 |
Proven |
Lung |
Rhizomucor spp. |
Histology / Mucorales
PCR |
VOR |
L-AMB, then ISA / Surgery |
Alive (Partial) |
| 10 |
2 |
Proven |
Lung, liver |
Rhizomucor spp. |
Histology / Panfungal
PCR |
FLU |
ISA, then POS /
Surgery |
Alive (Stable) |
| 11 |
2 |
Probable |
Lung |
Lichteimia spp. |
Mucorales PCR |
POS |
POS |
Alive (Complete) |
| 12 |
2 |
Probable |
Lung |
Mucor / Rhizopus spp. |
Mucorales PCR |
ISA |
L-AMB and ISA, then ISA |
Alive (Stable) |
| 13 |
2 |
Probable |
Lung |
Mucor / Rhizopus spp. |
Mucorales PCR |
ANI |
L-AMB and CAS, then ISA / Surgery |
Alive (Progression) |
| 14 |
2 |
Proven |
Lung |
Cunninghamella spp. |
Histology / Panfungal
PCR |
FLU |
L-AMB and CAS, then ISA / Surgery |
Alive (Progression) |
| 15 |
2 |
Proven |
Lung, brain |
Rhizomucor spp. |
Histology / Panfungal PCR / Mucorales PCR |
POS |
L-AMB and CAS, then L-AMB and ISA, then ISA / Surgery |
Alive (Complete) |
| 16 |
2 |
Proven |
Lung |
Rhizomucor spp. |
Histology / Panfungal PCR / Mucorales PCR |
FLU |
L-AMB and CAS, then ISA / Surgery |
Alive (Complete) |
| 17 |
2 |
Proven |
Lung, liver, intestine |
Rhizomucor spp. |
Histology / Panfungal PCR / Mucorales PCR |
AND |
L-AMB and ANI, then ISA |
Alive (Progression) |
| 18 |
2 |
Probable |
Lung, brain, spleen |
Lichtheimia corymbifera |
Panfungal PCR / Mucorales PCR |
POS |
L-AMB and CAS |
Deceased |
| Cases of other non-mould infections |
| 19 |
1 |
Proven |
Skin |
Alternaria alternata |
Histology / Culture /
Panfungal PCR |
FLU |
POS / Surgery |
Alive (Complete) |
| 20 |
2 |
Proven |
Lung |
Hormographiella aspergillata |
Histology / Panfungal
PCR |
POS |
L-AMB, then VOR / Surgery |
Alive (Complete) |
| 21 |
2 |
Proven |
Skin |
Alternaria spp. |
Culture / Panfungal
PCR |
VOR |
L-AMB, then VOR |
Alive (Complete) |
| 22 |
2 |
Proven |
Lung |
Conidiobolus pachyzygosporus |
Histology / Panfungal
PCR |
FLU |
L-AMB, then ISA / Surgery |
Alive (Partial) |
| 23 |
2 |
Proven |
Lung, soft tissue |
Hormographiella aspergillata |
Histology / Culture /
Panfungal PCR |
FLU |
POS, then VOR /
Surgery |
Alive (Complete) |
| 24 |
2 |
Proven |
Lung |
Hormographiella aspergillata |
Histology / Panfungal
PCR |
POS |
L-AMB, then ISA, then VOR / Surgery |
Alive (Partial) |
Discussion
We have
documented a significant burden of NAIMI over the last 5 years in our onco-haematology
unit. These
epidemiological changes appear to result from diverse factors. We hypothesize
that the relocation of the unit (2019) and occurrence of building work nearby
the hospital (from 2019 to present) may
have been a major factor. The timing of events is strongly suggestive of a
cause-effect association, as these events corresponded to the peaks of NAIMI incidence
between 2019 and 2021. Although we could not
document airborne contamination by moulds in the rooms, the lack of sensitivity
of the method does not rule out this hypothesis. Of note, we did not perform
quantification of airborne fungal spores in the rooms by sampling via an air
impactor during the study period. However, the presence of positive air
pressure and HEPA filters makes mould contamination
of the rooms unlikely. The patients may have been in contact with airborne
spores when outside of their isolation rooms during transfers to examination
rooms (e.g. radiology, bronchoscopy).
Several
outbreaks of invasive mould infections have been associated with airborne
contamination arising from building work occurring near hospitals, although the
link could not always be clearly demonstrated [9,
14]. These outbreaks were mainly caused by Aspergillus spp. Recently,
a cluster of mucormycosis has been reported in a French intensive care unit
among patients with Coronavirus disease 2019 (COVID-19), which was also
possibly linked to building works [15].
Our results
also suggest that improved diagnostic procedures may have contributed to an
apparent rise of NAIMI incidence and that the
disease could have gone underdiagnosed in the past. Indeed, the newly
implemented Mucorales-specific PCR has been estimated to increase the yield of mucormycosis’
detection by about 30%. Although this possible artefact should be considered, it
is not the unique explanation of this epidemiological shift, as we observed a
near 2-fold increase of mucormycosis incidence rates from 2018 to 2019 and a 3.5-fold
increase from 2018 to 2020 (figure 1).
Although
about one third of these NAIMI corresponded
to breakthrough infections in patients receiving mould-active prophylaxis, we
did not observe a significant change in the consumption of posaconazole or
other mould-active drugs over the study period.
Finally, whether
other factors, such as the global warming, may have contributed to this burden
of NAIMI is unknown. Similarly to other
European countries, Switzerland has experienced a constant rise of mean
temperatures over the last few years [16].
Interestingly, in addition to the increased incidence of mucormycosis, we also
observed some NAIMI attributed to rare moulds
(Hormographiella aspergillata) or supposed tropical moulds (Conidiobolus
spp.) since 2017, which was not the case during the early period. The
COVID-19 pandemic has been associated with a significant burden of mucormycosis
in some parts of the world, notably in India [17].
However, we did not observe any case of COVID-19-associated mucormycosis in our
institution during the study period. Other factors, such as novel anti-cancer
chemotherapy protocols, may have played a role in NAIMI incidence, but have not yet
been investigated.
The
analysis of the characteristics of these NAIMI cases also provided interesting epidemiological
data about these
rare infections. Interestingly, we observed relatively low overall mortality
rates (11% for mucormycosis and 0% for other NAIMI at week 12) when compared to previous
cohorts reporting mortality
rates of 40–50% [4, 18, 19]. While
diagnostic and therapeutic approaches have improved over time with the
introduction of the Mucorales PCR and the approval of novel antifungal
therapies (e.g. isavuconazole) [11, 20],
we did not observe any trend in mortality rates between periods 1 and 2 of our
study. Longer survey and larger datasets are needed to assess mortality trends
of NAIMI. Importantly, other recent advances,
such as the development of international guidelines and of the European QUALity
(EQUAL) score for the management of mucormycosis, may also contribute to
improved outcomes of this severe disease in the future [21, 22].
In
conclusion, continuous monitoring of the incidence of invasive mould infections,
in particular NAIMI, may be helpful to detect
possibly emerging mould diseases and, consequently, to trigger further
investigations and potential preventive measures.
Acknowledgments
We are grateful to the
study nurses Aurélie Guillet and Corine Guyaz for data management.
Prof.
Frederic Lamoth
Infectious
Diseases Service and Institute of Microbiology
Lausanne
University Hospital
University
of Lausanne
Rue du
Bugnon 48
CH-1011
Lausanne
Frederic.Lamoth[at]chuv.ch
References
1 Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology
of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study.
Haematologica. 2006 Aug;91(8):1068–75.
2 Rodríguez-Veiga R, Montesinos P, Boluda B, Lorenzo I, Martínez-Cuadrón D, Salavert M,
et al. Incidence and outcome of invasive fungal disease after front-line intensive
chemotherapy in patients with acute myeloid leukemia: impact of antifungal prophylaxis.
Ann Hematol. 2019 Sep;98(9):2081–8. 10.1007/s00277-019-03744-5
3 Lamoth F, Kontoyiannis DP. Therapeutic Challenges of Non-Aspergillus Invasive Mold Infections in Immunosuppressed Patients. Antimicrob Agents Chemother.
2019 Oct;63(11):e01244-19. 10.1128/AAC.01244-19
4 Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al.; European Confederation
of Medical Mycology Working Group on Zygomycosis. Zygomycosis in Europe: analysis
of 230 cases accrued by the registry of the European Confederation of Medical Mycology
(ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect.
2011 Dec;17(12):1859–67. 10.1111/j.1469-0691.2010.03456.x
5 Bitar D, Van Cauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, et al. Increasing
incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis. 2009 Sep;15(9):1395–401.
10.3201/eid1509.090334
6 Lamoth F, Chung SJ, Damonti L, Alexander BD. Changing Epidemiology of Invasive Mold
Infections in Patients Receiving Azole Prophylaxis. Clin Infect Dis. 2017 Jun;64(11):1619–21.
10.1093/cid/cix130
7 Parra Fariñas R, Alonso-Sardón M, Velasco-Tirado V, Pérez IG, Carbonell C, Álvarez
Artero E, et al. Increasing Incidence of mucormycosis in Spanish inpatients from 1997
to 2018. Mycoses. 2022 Mar;65(3):344–53. 10.1111/myc.13418
8 Saegeman V, Maertens J, Meersseman W, Spriet I, Verbeken E, Lagrou K. Increasing incidence
of mucormycosis in University Hospital, Belgium. Emerg Infect Dis. 2010 Sep;16(9):1456–8.
10.3201/eid1609.100276
9 Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and
infection prevention in healthcare settings during construction and renovation. Clin
Infect Dis. 2015 Aug;61(3):433–44. 10.1093/cid/civ297
10 Greub G, Sahli R, Brouillet R, Jaton K. Ten years of R&D and full automation in molecular
diagnosis. Future Microbiol. 2016;11(3):403–25. 10.2217/fmb.15.152
11 Millon L, Larosa F, Lepiller Q, Legrand F, Rocchi S, Daguindau E, et al. Quantitative
polymerase chain reaction detection of circulating DNA in serum for early diagnosis
of mucormycosis in immunocompromised patients. Clin Infect Dis. 2013 May;56(10):e95–101.
10.1093/cid/cit094
12 Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al.; Revision
and Update of the Consensus Definitions of Invasive Fungal Disease From the European
Organization for Research and Treatment of Cancer and the Mycoses Study Group Education
and Research Consortium. Revision and Update of the Consensus Definitions of Invasive
Fungal Disease From the European Organization for Research and Treatment of Cancer
and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020 Sep;71(6):1367–76.
10.1093/cid/ciz1008
13 Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, et al. Defining
responses to therapy and study outcomes in clinical trials of invasive fungal diseases:
Mycoses Study Group and European Organization for Research and Treatment of Cancer
consensus criteria. Clin Infect Dis. 2008 Sep;47(5):674–83. 10.1086/590566
14 Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect.
2006 Jul;63(3):246–54. 10.1016/j.jhin.2006.02.014
15 Guemas E, Cassaing S, Malavaud S, Fillaux J, Chauvin P, Lelièvre L, et al. A Clustered
Case Series of Mucorales Detection in Respiratory Samples from COVID-19 Patients in
Intensive Care, France, August to September 2021. J Fungi (Basel). 2022 Mar;8(3):258.
10.3390/jof8030258
16 Begert M, Frei C. Long-term area-mean temperature series for Switzerland-Combining
homogenized station data and high resolution grid data. Int J Climatol. 2018;38(6):2792–807.
10.1002/joc.5460
17 John TM, Jacob CN, Kontoyiannis DP. When Uncontrolled Diabetes Mellitus and Severe
COVID-19 Converge: The Perfect Storm for Mucormycosis. J Fungi (Basel). 2021 Apr;7(4):298.
10.3390/jof7040298
18 Lamoth F, Damonti L, Alexander BD. Role of Antifungal Susceptibility Testing in Non-Aspergillus
Invasive Mold Infections. J Clin Microbiol. 2016 Jun;54(6):1638–40. 10.1128/JCM.00318-16
19 Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, et al. A
global analysis of mucormycosis in France: the RetroZygo Study (2005-2007). Clin Infect
Dis. 2012 Feb;54 Suppl 1(S35-43.
20 Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR 3rd,
et al.; VITAL and FungiScope Mucormycosis Investigators. Isavuconazole treatment for
mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect
Dis. 2016 Jul;16(7):828–37. 10.1016/S1473-3099(16)00071-2
21 Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, et
al.; Mucormycosis ECMM MSG Global Guideline Writing Group. Global guideline for the
diagnosis and management of mucormycosis: an initiative of the European Confederation
of Medical Mycology in cooperation with the Mycoses Study Group Education and Research
Consortium. Lancet Infect Dis. 2019 Dec;19(12):e405–21. 10.1016/S1473-3099(19)30312-3
22 Koehler P, Mellinghoff SC, Lagrou K, Alanio A, Arenz D, Hoenigl M, et al. Development
and validation of the European QUALity (EQUAL) score for mucormycosis management in
haematology. J Antimicrob Chemother. 2019 Jun;74(6):1704–12. 10.1093/jac/dkz051
