Figure 1Clinical phases of Chagas disease. Inspired by Echeverría LE, et al. WHF IASC Roadmap on Chagas Disease. Global Heart. 2020; 15(1): 26. https://doi.org/10.5334/gh.484 [40]
DOI: https://doi.org/https://doi.org/10.57187/s.3719
Chagas disease is a parasitic infection caused by Trypanosoma cruzi, a flagellated protozoan. Highly prevalent in many areas of continental Latin America, it has been officially recognised as a neglected tropical disease (NTD) by the World Health Organization (WHO) since 2005 [1]. According to the Swiss Federal Statistical Office (FSO) in 2022, 45,963 individuals from South America and 6,154 from Central America (including Mexico) live in Switzerland [2]. However, FSO statistics do not include undocumented Latin American migrants, estimated to be 32,800 in 2015 [3], nor people who have lived in continental Latin America but were not born there. Thus, the estimated number of people at risk of Chagas disease in Switzerland could be at least 85,000. Individuals considered ‘at risk’ are those who were born or have lived in countries where Chagas disease is endemic or who were born to Latin American mothers.
Despite its predominant prevalence in Latin America, Chagas disease presents significant global health challenges. Several factors contribute to this, including the disease’s multiple transmission routes, its under-diagnosis, a lack of awareness even among health professionals and communities at risk themselves, social stigma, absence of adequate markers of cure, extended periods of asymptomatic chronic phases, and suboptimal therapeutic options. Given the significant number of individuals at risk and the need for a more coordinated approach at a national level, the Swiss Chagas Network was formed in March 2023. The network aims to address the challenges surrounding Chagas disease and advance an agenda of improved medical care and elimination of the disease as a public health problem, in line with Switzerland's endorsement of the WHO road map for neglected tropical diseases at the 73rd World Health Assembly in November 2020.
This review summarises the scientific evidence on Chagas disease, with a specific focus on Switzerland and other countries outside Latin America. Publications from 1975 to 2024 were selected and critically reviewed to gather the most relevant clinical and epidemiological information. Additionally, the most recent national, regional, and international public health guidelines and recommendations were critically assessed. With this paper, a collaboration between experts in different fields across the country seeks to review the current knowledge about Chagas disease in Switzerland from a medical, epidemiological, and social perspective. Finally, we propose avenues to address the existing challenges to eliminate the disease as a public health problem.
Neglected tropical diseases are diseases of poverty that represent a heavy burden for over a billion people, particularly in tropical and subtropical regions. They predominantly affect vulnerable [4] and marginalised populations. Despite being largely preventable through improved diagnosis, treatment, education, hygiene, and overall development, neglected tropical diseases can be difficult to diagnose and may cause high morbidity and mortality even years after infection [5]. In non-endemic areas, they often go unnoticed due to a lack of awareness and healthcare access, even in high-income countries like Switzerland [6]. (In this article, we use the terms endemic and non-endemic to identify regions with or without the disease-carrying vector, respectively. However, the distinction is complex and debated due to evolving endemicity, and because Chagas disease should also be considered as a health challenge in countries outside Latin America.)
In 2020, the seventy-third World Health Assembly (WHA) urged member states to implement the new road map for neglected tropical diseases, entitled “Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030”. In the case of Chagas disease – one of the neglected tropical diseases with the highest prevalence and impact in high-income settings – countries are mandated to achieve interruption of transmission through the four main transmission routes (vectorial, congenital, and through transfusion or transplantation) and to increase antiparasitic treatment coverage to 75% of the eligible population by 2030 [5].
Despite being discovered more than 100 years ago, Chagas disease remains a serious public health problem worldwide [7]. In Europe, it is estimated that over 100,000 people may be infected with T. cruzi, but around 90% of cases remain undetected because of lack of access to health care, lack of awareness, and the absence of systematic screening programs [8]. In Switzerland, the estimated number of people affected by Chagas disease ranges between 2,000 and 4,000 [9]. The first case reported in the country dates back to 1979 [10, 11]. Following the introduction of a screening campaign, the Geneva University Hospital diagnosed 247 cases between 2007 and 2011 [12, 13]. Similarly, efforts in Lausanne between 2011 and 2012 revealed a 2.3% prevalence of Chagas disease among Latin American migrants living in the canton of Vaud [14]. Over the last 15 years, 480 people have been diagnosed with Chagas disease at Geneva University Hospital [personal communication, Y. Jackson, November 2023]. However, accurate estimates of the true epidemiological burden in Switzerland are lacking [8].
While Switzerland has to a certain extent addressed the challenges of Chagas disease in cantons hosting high numbers of individuals from Latin American countries, there is a need to broaden these efforts nationwide to ensure the country can achieve the WHO targets mentioned above by 2030 [5].
In the following sections, we will review key evidence on the disease transmission, pathogenesis, and diagnosis and management of Chagas disease from a medical perspective. In the final sections, we will focus on the existing challenges in tackling the disease at the national level in the Swiss context.
T. cruzi is transmitted to humans by insect vectors of the Reduviidae family (for example, Triatoma infestans and Rhodnius prolixus) [5, 15, 16]. These insects, commonly known as “kissing bugs”, live in cracks and crevices of walls and emerge at night to feed on exposed skin, often around the face (hence their name). While feeding, they defecate and urinate, and infection occurs when parasite-containing excrement is accidentally rubbed into a wound or mucous membrane. For many years, the disease has been endemic in rural and peri-urban areas of the 21 continental countries in the Americas, encompassing a vast territory from the southern United States to northern Argentina and Chile, where an estimated 6 to 8 million people are currently infected [17] and a further 70 million people are at risk [18, 19]. Parasite transmission through the vectorial route has been responsible for the majority of infections [20], but transmission can also occur through ingestion of contaminated food [20, 21], congenitally [22], through transfusion of infected blood components [20] or transplantation from an infected donor [20], or, rarely, through laboratory accident [23]. These latter four transmission routes have also been described outside Latin America, where they are the primary focus of prevention efforts.
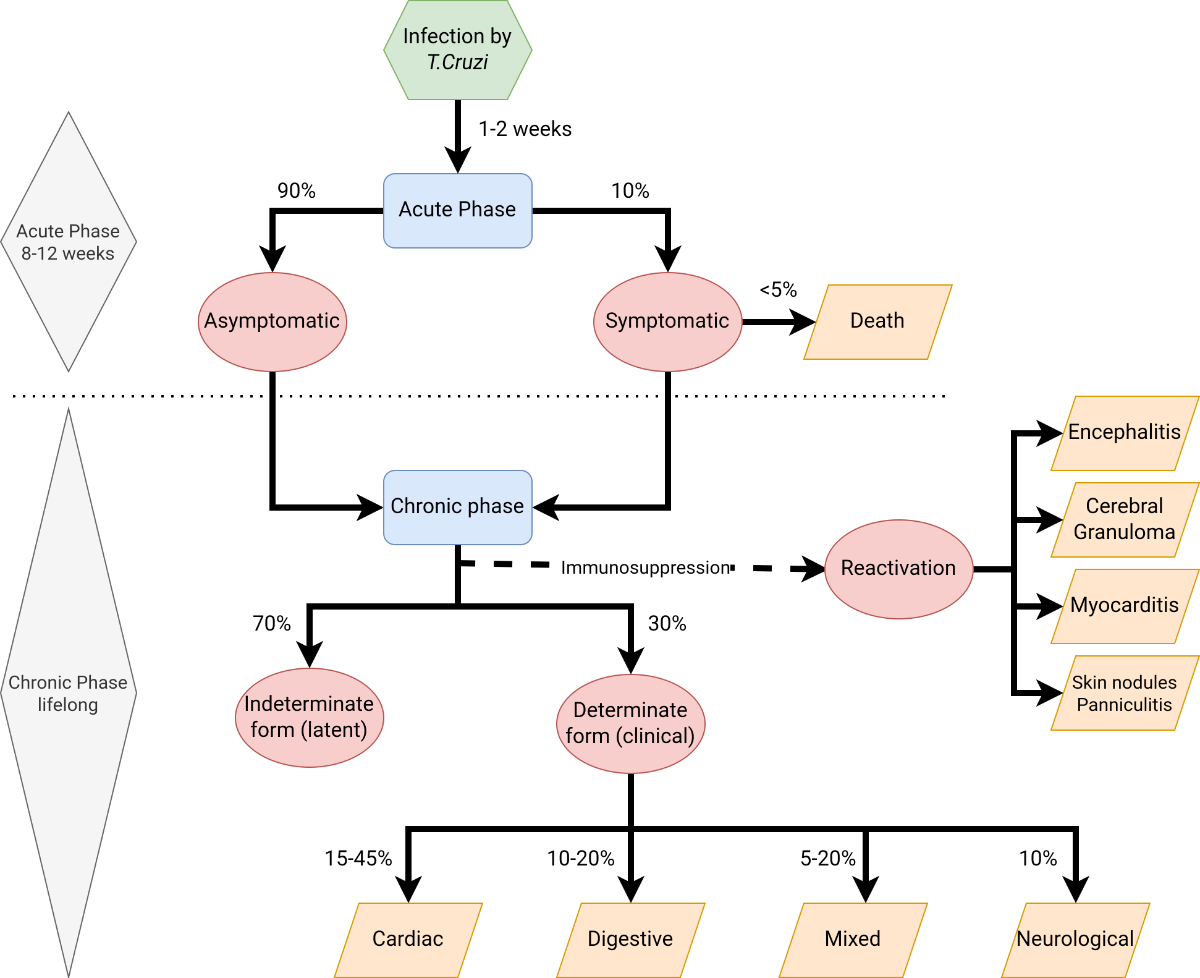
T. cruzi infection progresses through two distinct phases, acute and chronic. The incubation period following exposure typically lasts between one and two weeks, but it can extend up to four months in cases of transfusion- or transplant-associated transmission [20]. During infection, trypomastigotes invade cells near the site of inoculation and differentiate into intracellular amastigotes. Most patients at this acute stage have mild, nonspecific symptoms such as malaise, fever, and anorexia, or remain completely asymptomatic [24]. However, in a minority of patients infected through vector transmission, the acute infection may be associated with inflammation and swelling at the inoculation site, known as chagoma (on the skin) and Romaña sign (periorbital swelling, palpebral oedema, and conjunctivitis) [24]. The amastigotes multiply by binary fission in the cells and differentiate into trypomastigotes that are released into the circulation. The acute phase of the infection is marked by a high circulating load of trypomastigotes. Trypomastigotes infect cells from a variety of tissues and transform into intracellular amastigotes. Clinical manifestations can result from this infective cycle. Acute myocarditis and pericardial effusion have been reported in 1–5% of patients [20, 25, 26]. As the host's immune response gradually reduces the levels of circulating parasites, the acute symptoms begin to subside, and the patient transitions into the chronic phase, which is characterised by very low-level or undetectable parasitaemia [27].
In the absence of early antitrypanosomal therapy, this transition generally occurs at around 8 to 12 weeks after the onset of infection, and the chronic phase typically persists throughout the patient's lifetime; spontaneous remission is rare [28, 29]. The chronic phase encompasses the indeterminate (latent) form and the determinate (clinical) forms, which are marked by different degrees of organ damage (classified as cardiac, digestive, neurological, or mixed). The majority of individuals infected with T. cruzi remain in the indeterminate phase throughout their lives. Based on clinical series from the 1980s [30, 31] it is estimated that 30% to 40% of chronically infected individuals develop long-term complications (determinate form) after a latent period spanning from 10 to 30 years [32]. Recent clinical series have reported a progression rate towards cardiac involvement of 1.4–5% per year, which can vary between endemic and non-endemic areas [33, 35]. Approximately 15% to 45% of chronically infected individuals develop cardiomyopathy [34, 36, 37], and 10% to 20% present gastrointestinal damage [38, 39]; some individuals show overlapping syndromes (5–20% of those patients with cardiomyopathy). In addition, 10% of individuals present with mild sensory neuropathy [40, 41].
In immunocompromised patients, reactivation of latent T. cruzi infection is a life-threatening condition [42]. The most common complications include encephalitis, cerebral granuloma that mimics Toxoplasma gondii infection, myocarditis, and subcutaneous lesions (such as erythematous skin nodules or panniculitis) [43].

Figure 1Clinical phases of Chagas disease. Inspired by Echeverría LE, et al. WHF IASC Roadmap on Chagas Disease. Global Heart. 2020; 15(1): 26. https://doi.org/10.5334/gh.484 [40]
The clinical presentation of chronic Chagas cardiomyopathy stems from four major disorders: arrhythmias, conduction abnormalities, dilated cardiomyopathy with heart failure, and cardioembolic events (secondary to structural complications) [24]. Atrial and ventricular arrhythmias can be asymptomatic or may lead to palpitations, dizziness, dyspnoea, weakness, syncope, or sudden cardiac arrest. Heart failure progresses gradually, typically beginning with left-sided heart failure symptoms and eventually advancing to biventricular heart failure. Both systolic and diastolic heart failure can occur. In Chagas cardiomyopathy, dilated cardiac chambers and the presence of atrial fibrillation increase the risk of intraventricular thrombus formation, which can result in strokes and peripheral embolisms. Stroke secondary to Chagasic cardioembolism has been reported to occur twice as frequently as with other forms of cardiomyopathy in some highly endemic areas [9, 44]. Chest pain is also a common symptom of Chagas cardiomyopathy, although it often presents atypically [45]. Different classifications of Chagas cardiomyopathy have been created, including by the American Heart Association, the classification of Kuschnir et al. or other associations (Brazilian, Los Andes, Latin American). These classifications share similar but not exactly equivalent criteria (such as ECG and echocardiographic findings), making comparisons between clinical studies and management guidelines complicated [46].
Gastrointestinal manifestations of T. cruzi infection are less common than cardiac involvement, and occur more frequently in the southern Amazon Basin, with a correlation between the affected organ system and geographical areas (for instance, colonic dilation is more frequent in Andean countries, while oesophageal dilation is more common in Central Brazil). The destruction of submucosal motor neurons leads to a range of motility disorders, from mild symptoms to severe dilatation of the oesophagus and colon [24]. Patients may experience regurgitation and progressive dysphagia, initially with solids, but eventually with liquids as well, mimicking achalasia. When the colon is affected, patients typically present with gradually worsening constipation, eventually reaching a point where stool evacuation becomes nearly impossible. Mild abdominal discomfort or rectal fullness are common in these cases. As constipation worsens, symptoms can become severe and may lead to intestinal obstruction, sometimes requiring surgical intervention. In advanced stages of the disease, malnutrition and anorexia may develop. It is worth noting that most cases of megacolon are associated with megaoesophagus, meaning both the oesophagus and colon show significant dilation. In rare instances, other parts of the digestive tract can also be impacted by the infection, including the stomach, small bowel, biliary tree, and salivary glands. However, digestive forms of the disease generally have a better prognosis than cardiac forms [47, 48].
The cardiac and gastrointestinal manifestations associated with chronic Chagas disease can lead to a high rate of long-term complications for infected individuals, resulting in a significant burden of disease that requires costly medical interventions. Economic evaluations have provided evidence that screening for Chagas disease in asymptomatic Latin American migrants living in Europe is a cost-effective strategy [49]. Early diagnosis and adequate care (including treatment when necessary) are crucial not only for the well-being of the patient but also to mitigate the financial burden of managing advanced disease. As most individuals infected with Chagas disease remain asymptomatic for many years, it is essential to adopt a proactive approach to detect cases. Early detection is of paramount importance as it significantly influences the effective management of Chagas disease [50]. This is particularly important when considering the risk of congenital transmission and management of infected children.
Antiparasitic treatment of infected women of childbearing age – outside the periods of pregnancy and lactation – nearly eliminates the risk of transmission during subsequent pregnancies [51, 52]. This reinforces the importance of offering Chagas disease screening to girls and women of childbearing age to prevent transmission in future pregnancies.
Pregnant women at risk of Chagas disease represent another crucial population group that should be systematically screened. This would ensure appropriate follow-up for newborns and rapid access to treatment for those infected. Indeed, antiparasitic treatments are most effective at the onset of infection – with a cure rate exceeding 95% in infants treated before reaching one year of age [53]. The effectiveness of these treatments diminishes as the child ages. Undiagnosed children are at risk of developing fatal Chagas-related health problems later in life [54].
The rate of vertical transmission is estimated to be around 5% [54], with variation reported across different geographical areas. Most infected pregnant women are in the asymptomatic chronic phase, and the majority of infected newborns also remain asymptomatic [55]. This can result in undiagnosed cases if proactive screening is not performed. The risk of parasite transmission during breastfeeding is generally considered to be low, and only appears to exist in cases of acute infection and bleeding nipples. Therefore, breastfeeding is not considered a contraindication for mothers with chronic Chagas disease infection [55]. Screening should be performed for all at-risk pregnant women to ensure proper follow-up for the mothers and their newborns. The infection status of their siblings should also be investigated, particularly in cases where the mother tests positive [56].
Early diagnosis and treatment of infected children are crucial, as antiparasitic therapy is more effective and better tolerated in this group compared to adults in the chronic disease stage [55]. With treatment becoming less effective over time, undiagnosed children may develop fatal Chagas-related health problems later in life that could have been avoided [53, 57].
Healthcare professionals play an essential role in promoting early detection, especially gynaecologists – who work with women before and during pregnancy – and paediatricians who care for children.
Chronically infected individuals also require close attention. Early screening is essential to ensure comprehensive clinical management and initiate therapies targeting infection and complications. Moreover, this helps protect patients against the reactivation of the disease in the event of immunosuppression.
Chagas disease screening should target a range of different groups to enhance prevention and control efforts. In countries outside Latin America, like Switzerland, chronic Chagas disease should be suspected in individuals who have lived in Latin America and those born to women from endemic areas. Epidemiological studies conducted in Europe show that the prevalence of Chagas disease infection is particularly high among the Bolivian population; however, it is also prevalent among other migrant groups [58]. Given the significant underdiagnosis in Europe [10] and that screening for T. cruzi infection in all Latin American adults living in Europe has been calculated to be cost-effective [49], a global and inclusive approach is appropriate. This includes not only recent migrants but also those who have been residing in Switzerland for longer periods, as individuals can remain asymptomatic for decades. Therefore, screening should also be considered for individuals who have resided in rural parts of Latin America for at least 6 months, independent of their administrative identity (i.e., their migrant and/or legal status). Screening is advisable for adopted children who were born in Latin America or from a mother of Latin American origin. If patients with epidemiological risk factors present with cardiac or gastrointestinal symptoms evocative of T. cruzi infection, Chagas disease should be investigated as part of the differential diagnosis. Additionally, immunocompromised patients or patients considered for immunosuppressive therapy (e.g., pre-transplant or receiving chemotherapy or monoclonal antibodies) with risk factors should undergo screening for Chagas disease [59]. To date, cases of infection among tourists are rarely reported, as the risk of infection is primarily associated with long-term exposure to the triatomine in endemic areas or through oral transmission via contaminated food.
The laboratory confirmation of Chagas disease is based on different tests, depending on the phase of the disease. During the acute phase, motile trypomastigotes may be detectable through microscopy in fresh buffy coat or blood smear preparations after centrifugation. These methods require high loads of circulating parasites due to their low sensitivity. When available, polymerase chain reaction (PCR) is a more sensitive diagnostic method in the acute phase.
In the chronic phase, diagnosis relies on serological tests targeting distinct T. cruzi antibodies. To confirm infection, two tests using different techniques are recommended. Various assays are available in Switzerland, including enzyme-linked immunosorbent assay (ELISA), chemiluminescent immunoassays, immunoblot transfer or immune-fluorescent antibodies assay (IFA). Immunochromatography-based assays, available as rapid tests, are valuable as community screening tools due to their high sensitivity [60] or as complementary diagnostic methods. However, they require confirmation with more specific tests such as ELISA or IFA [61, 62].
In newborns, diagnosis [51] should be based on microscopic blood observation and/or T. cruzi PCR, as passive transfer of maternal antibodies during pregnancy interferes with the accuracy of serological tests. If a test at birth is negative, it should be repeated at one month of age, the typical peak of parasitaemia [51]. For infants who test negative at birth and one month, serological testing should be performed between 9 and 12 months of age to confirm the absence of infection, once maternal antibodies have been cleared [55].
The management of Chagas disease varies depending on the patient’s characteristics as well as the phase and form of the disease.
The treatment objectives comprise two main goals. The first is the use of antiparasitic drugs to clear (or at least reduce) parasitic load, to stop clinical progression and vertical transmission. Second is the essential management and treatment of complications during the chronic stage. In cases of chronic heart disease, this could include the treatment of arrhythmias and heart failure, and evaluation for pacemaker implantation, for example. For gastrointestinal involvement, motility disorder therapies, including surgery, may be necessary for advanced disease.
Currently, only two antitrypanosomal medicines, benznidazole (BZN) and nifurtimox (NFX), are available for first-line treatment. Importantly, assessing a cured state after treatment remains challenging, due to the absence of reliable biomarkers and the significant geographical variation in response rates [63]. Circulating antibodies can persist for decades following treatment, complicating the accurate evaluation of treatment efficacy [64]. Highly sensitive PCR may show the decline or disappearance of parasitaemia after treatment, but the exact clinical significance and effect on prognosis remains unclear [65–68]. Treatment may allow for a complete cure in acute and early infections, eliminating the potential risk of future blood-borne transmission. It can also reduce the risk of organ damage developing in asymptomatic patients; however, it has shown no effect on the progression of pre-existing cardiomyopathy [64]. Furthermore, data from randomised trials indicate that benznidazole therapy does not significantly reduce mortality in patients with established Chagas cardiomyopathy [69].
Nifurtimox and benznidazole therapy can lead to significant side effects in adults, most commonly related to the skin, nervous system, liver, and blood [40]. Benznidazole causes adverse reactions in 44% of patients, while nifurtimox has an even higher incidence, ranging from 80% to 100%; the side effect profiles are different between the two drugs [64]. A study conducted among adults in Geneva revealed that nearly 90% of patients experienced adverse effects following treatment with either nifurtimox or benznidazole, and almost 40% of patients did not complete their treatment [70]. Among children, adverse drug-related events are rare, except for mild rashes (more common with benznidazole), anorexia, irritability, and headache (more common with nifurtimox) [55, 64]. Overall, this underscores the importance of collecting relevant medical information, close clinical monitoring during treatment, and the discussion of strategies to identify and manage events early [70].
Treatment should be administered to all patients with acute or congenital T. cruzi infection, as well as for most patients with the indeterminate form of chronic Chagas disease, both children and adults [57]. However, the decision to treat adults over 50 years old should be made on a case-by-case basis, due to the lack of data on real-life efficacy and the significant potential for side effects. According to WHO and PAHO (Pan American Health Association) 2019 guidelines, systematic prescription of treatment is not recommended for adult patients with chronic T. cruzi infection complicated by advanced organ damage. However, these guidelines are based on moderate certainty regarding the effects of the intervention and may evolve with further research. For instance, recent data seem to indicate a potential positive effect of benznidazole treatment, with a reduction of cardiac inflammation and fibrosis as assessed by magnetic resonance imaging [71]. A joint decision-making process between the patient and physician is crucial to discuss the potential benefits and risks of treatment.
In immunosuppressed patients, antitrypanosomal therapy is recommended for all patients with reactivated or acute donor-derived T. cruzi infection. Treatment is not recommended during pregnancy. Maternal treatment should be started after the breastfeeding period [55]. After birth, children must be monitored for the first year to detect any infection, and treatment should be initiated in infected children as early as possible.
In addition to parasitological treatment, yearly ECG and clinical follow-up should be performed in all patients during the indeterminate phase of the disease.
Over the past 15 years, a group of healthcare providers in Geneva and Lausanne have implemented successful strategies to identify individuals with Chagas disease in the community, as well as those attending maternity wards of university hospitals [72]. However, under-diagnosis remains a problem in Switzerland, with the majority of cases going undetected. Currently, Chagas disease is not a notifiable disease in the country. Addressing Chagas disease remains a significant challenge, with numerous obstacles at the clinical and public health levels, as well as among affected individuals. The lack of standardised protocols for Chagas disease management leads to complex and inconsistent implementation of clinical strategies, with healthcare providers adopting varied approaches.
The lack of directives for Chagas disease management, coupled with limited awareness among healthcare providers, presents a significant hurdle in the diagnosis and management of the disease. Due to limited exposure and education, healthcare professionals might not readily recognise its symptoms or risk factors, leading to delayed or missed diagnoses. Concurrently, communities affected by Chagas disease often underestimate its severity and prevalence, contributing to a low level of concern and delays in seeking medical care. This underestimation can stem from a lack of knowledge about the disease within these communities [73]. Additionally, there is evidence that affected individuals fear the potential adverse effects of treatment [14, 74]. This fear acts as a barrier to initiating or adhering to treatment regimens, impeding efforts to effectively control and manage the disease. Furthermore, stigma plays an important role, as the infection carries negative stereotypes, such as being a disease of poverty and rurality. Overcoming these challenges necessitates comprehensive educational campaigns, targeted at both healthcare professionals and affected communities. Such campaigns must improve awareness, understanding, and trust in available treatments, ultimately fostering better health outcomes for those impacted by Chagas disease.
Access to and use of appropriate medications can be challenging. The import and use of nifurtimox and benznidazole requires specific approval from Swissmedic [75]. The WHO distributes nifurtimox and benznidazole free of charge in all countries with reported Chagas disease cases. However, for benznidazole, this scheme is limited to patients under 19; nifurtimox distribution has no age limit, but is considered a second-line treatment. At the Geneva University Hospital, for example, benznidazole is ordered directly from the Argentinian producer (ELEA) at high cost and with sometimes limited availability. This cross-border procurement adds logistical complexities, highlighting the urgent need for improved accessibility and recognition of the importance of Chagas disease treatments within Switzerland. Nevertheless, with the support of WHO and the creation of the Swiss Chagas collaborative network, treatment availability might improve.
While several public health measures have been put in place to prevent transmission, there are still gaps that must be addressed. Systematic screening of at-risk blood donors has been implemented throughout the country since 2013 [76], and the risk of transmission in the context of transplants is usually carefully monitored in both donors and recipients [personal communication, M. Oriol, CHUV]. However, to our knowledge, systematic congenital screening has only been implemented in the maternity wards of Geneva and Lausanne [77]. Therefore, congenital transmission remains a risk for children of infected mothers living elsewhere in Switzerland. In areas where the vectors are absent and protocols of blood transfusion prevention have been implemented, mother-to-child is currently the main mode of T. cruzi transmission [51]. The absence of a comprehensive Chagas disease management program contributes to potential ongoing congenital transmission in many cantons; the aggregated prevalence of Chagas disease infection among pregnant women from Latin America is estimated to be around 4.4%, with wide variation depending on the country of origin [78]. This highlights a gap that should be urgently addressed through the widespread implementation of systematic screening protocols for at-risk girls and women of childbearing age, complemented by screening of at-risk pregnant women.
In Switzerland, health insurance covers the expenses for clinical care, diagnostic procedures, and treatments of Chagas disease among insured individuals. However, significant financial challenges persist for the majority of affected people. Indeed, Chagas disease disproportionately affects migrants living below the Swiss national poverty line, who often lack health insurance. In Switzerland, undocumented migrants have the right to purchase health insurance [79]. However, these groups often encounter significant administrative, legal, and financial barriers that restrict their access to health insurance and healthcare; less than 10% are adequately insured [80]. As many affected individuals do not have insurance, it falls upon specialised medical-social units to ensure these patients find ways to secure the necessary coverage.While documented migrants are less exposed to the precarious situation described above, they are still at risk of remaining undiagnosed and not receiving care for Chagas disease. Furthermore, the Swiss federal system is characterised by a high degree of cantonal autonomy in the management of healthcare, leading to significant inconsistencies in access to care for both documented and undocumented migrants across different cantons [80, 81]. These disparities have measurable effects on migrant health, with mortality rates among undocumented migrants being higher in less inclusive cantons [81]. In this context, the social vulnerability of a high proportion of people affected by Chagas disease in Switzerland, combined with the difficulties they may encounter in accessing care, calls for increased and urgent attention from the healthcare system.
Since Chagas disease can be prevented and treated, or at least closely monitored for clinical manifestations and complications, improving access to Chagas disease diagnosis and medical follow-up for at-risk individuals in Switzerland is essential. Enhancing Chagas disease management nationwide, ensuring universal access to diagnosis and proper medical care, especially – but not only – for vulnerable populations like undocumented individuals, is necessary for Switzerland to align with its international commitment to eliminating Chagas disease transmission and improving care coverage of affected populations.
Interrupt congenital transmission:
Pregnant women:
Newborns, siblings and children:
Chronically infected adults:
In March 2023, we established the Swiss Chagas Network, a consortium comprising prominent institutions and experts in the field of Chagas disease screening, diagnosis, treatment, prevention and control. The network includes the WHO Control of Neglected Tropical Diseases, Swiss Tropical and Public Health Institute (Swiss TPH) in Basel, the Geneva University Hospital (HUG), the School of Health Sciences (HESAV), the University of Applied Sciences and Arts Western Switzerland (HES-SO), the University of Lausanne (UNIL) and Unisanté in Lausanne, the Department of Public & Global Health at the University of Zurich, and Mundo Sano Foundation (Spain office), a Latin American-born NGO that has worked on Chagas disease since 1993. This collaboration reflects a shared commitment to confront the complex challenges posed by this neglected tropical disease in Switzerland. The overarching objective of the network is to deepen our understanding of the current landscape of Chagas disease epidemiology, research, and public health interventions in Switzerland. The ultimate goal is to eliminate Chagas disease as a public health problem in Switzerland by 2030, aligning with the neglected tropical disease roadmap targets [81]. This objective is not to be understood as the complete eradication or elimination of this chronic infection, but rather the "achievement of measurable targets set by the WHO" [82]. In other words, eliminating Chagas disease as a public health concern involves implementing public health measures to reach the following goals:
To implement our mission, we are currently working on assessing the current situation in Switzerland – considering epidemiological and socio-economic aspects – and identifying gaps and efforts required to overcome these. We aim to raise public awareness among healthcare providers, public health actors, stakeholders, at-risk communities, and potentially infected individuals. Additionally, we aim to act as a central hub for information and resources across various domains. Finally, we are dedicated to providing evidence for decision-making to effectively combat Chagas disease and ultimately achieve our objective. To fulfil this aim, the following specific objectives are outlined:
The establishment of the Swiss Chagas Network and the articulation of these objectives mark the crucial first steps in a coordinated endeavour to combat Chagas disease in Switzerland. This collaboration leverages the collective expertise and resources of diverse institutions, promising a more robust and effective response to this global health challenge. In addition, this network facilitates coordination at different levels: cantonal, national and international. In a forthcoming article, we plan to delve deeper into the specific initiatives and projects undertaken by the Network that will shape the future of Chagas disease control in Switzerland.
We are currently in the process of expanding and enhancing the Swiss Chagas Network. We extend an open invitation to all individuals and organisations involved or interested in Chagas disease research, prevention, and treatment to join us in this crucial endeavour. Together, we can strengthen our collective efforts to combat this neglected tropical disease and support the realisation of the 2030 Swiss government and WHO objectives.
Chagas disease remains a concerning yet neglected tropical disease, particularly prevalent among populations of Latin American origin. Despite its high prevalence in this group, it often goes undetected and untreated, leading to serious morbidity, costly long-term complications, and even death. The key to mitigating these dire consequences lies in early screening, diagnosis, treatment, and follow-up, which can facilitate timely intervention and prevent the progression of the disease.
Chagas disease in Switzerland is significantly underdiagnosed due to several key factors, including a lack of awareness among both health professionals and the Latin American immigrant population, limited access and availability of treatment, and barriers to adequate medical care. The situation is exacerbated by the absence of systematic congenital screening programs, with mother-to-child representing the primary mode of transmission in countries outside Latin America.
In Switzerland, efforts to address Chagas disease have taken a significant step forward with the establishment of the Swiss Chagas Network. This collaborative initiative aims to comprehensively map the current epidemiological landscape of Chagas disease in the country and promote effective public health interventions. By pooling expertise and resources, the Swiss Chagas Network seeks not only to raise awareness of the disease but also to support improved prevention, diagnosis, and treatment.
Ultimately, this proactive approach will be essential in combating Chagas disease and reducing its impact on affected individuals and communities. We hope that continued research and public health initiatives will facilitate early detection and intervention, providing a brighter future for those at risk, and contributing to the global effort to reduce the burden of this neglected tropical disease.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Chagas disease (also known as American trypanosomiasis). https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
2. Federal Statistical Office. Federal Statistical Office. https://www.bfs.admin.ch/bfs/en/home.html
3. Morlok M, Meier H, Oswald A, Efionayi-Mäder D. Les sans-papiers en Suisse en 2015, rapport final à l’attention du Secrétariat d’Etat aux migrations (SEM). https://www.sem.admin.ch/dam/sem/fr/data/internationales/illegale-migration/sans_papiers/ber-sanspapiers-2015-f.pdf.download.pdf/ber-sanspapiers-2015-f.pdf
4. Garrett CM, Altman R. Vulnerabilized: revisiting the language of the vulnerable populations framework. Am J Public Health. 2024 Feb;114(2):177–9. doi: https://doi.org/10.2105/AJPH.2023.307532
5. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. World Health Organization; 2021. 196.
6. Jackson Y, Varcher Herrera M, Gascon J. Economic crisis and increased immigrant mobility: new challenges in managing Chagas disease in Europe. Bull World Health Organ. 2014 Oct;92(10):771–2. doi: https://doi.org/10.2471/BLT.13.134072
7. de Sousa AS, Vermeij D, Ramos AN Jr, Luquetti AO. Chagas disease. Lancet. 2024 Jan;403(10422):203–18. doi: https://doi.org/10.1016/S0140-6736(23)01787-7
8. Jackson Y, Basile L, Chappuis F. Chagas Disease in Europe. Neglected Tropical Diseases - Europe and Central Asia; 2021. pp. 101–22.
9. Basile L, Jansa JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, et al.; Working Group on Chagas Disease. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011 Sep;16(37):19968. doi: https://doi.org/10.2807/ese.16.37.19968-en
10. Navarro M, Reguero L, Subirà C, Blázquez-Pérez A, Requena-Méndez A. Estimating chagas disease prevalence and number of underdiagnosed, and undertreated individuals in Spain. Travel Med Infect Dis. 2022;47:102284. doi: https://doi.org/10.1016/j.tmaid.2022.102284
11. Liechti M, Baur HR, Gurtner HP, Straub PW. [Cardiac complications of American trypanosomiasis (Chagas disease). Various case reports and general observations]. Schweiz Med Wochenschr. 1990 Oct;120(41):1493–6.
12. Jackson Y, Chappuis F, Loutan L. [Chagas disease in Switzerland: managing an emerging infection and interrupting its transmission]. Rev Med Suisse. 2008 May;4(157):1212–4.
13. Jackson Y, Chappuis F. Chagas disease in Switzerland: history and challenges. Euro Surveill. 2011 Sep;16(37):19963. doi: https://doi.org/10.2807/ese.16.37.19963-en
14. Da Costa-Demaurex C, Cárdenas MT, Aparicio H, Bodenmann P, Genton B, D’Acremont V. Screening strategy for Chagas disease in a non-endemic country (Switzerland): a prospective evaluation. Swiss Med Wkly. 2019 Apr;149:w20050. doi: https://doi.org/10.4414/smw.2019.20050
15. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. World Health Organization; 2021. 196 pp.
16. Justi SA, Galvão C. The Evolutionary Origin of Diversity in Chagas Disease Vectors. Trends Parasitol. 2017 Jan;33(1):42–52. doi: https://doi.org/10.1016/j.pt.2016.11.002
17. Guidelines for the diagnosis and treatment of Chagas disease. World Health Organization; 2018.
18. Chagas disease (American trypanosomiasis). https://www.who.int/health-topics/chagas-disease#tab=tab_1
19. Jan 27. Factsheet: Chagas Disease in the Americas for Public Health Workers. https://www.paho.org/en/documents/factsheet-chagas-disease-americas-public-health-workers
20. Pérez-Molina JA, Molina I. Chagas disease. Lancet. 2018 Jan;391(10115):82–94. doi: https://doi.org/10.1016/S0140-6736(17)31612-4
21. de Noya B, González O, Robertson LJ. Trypanosoma cruzi as a Foodborne Pathogen. Springer; 2015. 92 pp. doi: https://doi.org/10.1007/978-3-319-23410-6
22. Klein MD, Proaño A, Noazin S, Sciaudone M, Gilman RH, Bowman NM. Risk factors for vertical transmission of Chagas disease: A systematic review and meta-analysis. Int J Infect Dis. 2021 Apr;105:357–73. doi: https://doi.org/10.1016/j.ijid.2021.02.074
23. Herwaldt BL. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev. 2001 Oct;14(4):659–88. doi: https://doi.org/10.1128/CMR.14.3.659-688.2001
24. Bern C. Chagas’ Disease. N Engl J Med. 2015 Jul;373(5):456–66. doi: https://doi.org/10.1056/NEJMra1410150
25. Pinto AY, Valente SA, Valente VC, Ferreira Junior AG, Coura JR. [Acute phase of Chagas disease in the Brazilian Amazon region: study of 233 cases from Pará, Amapá and Maranhão observed between 1988 and 2005]. Rev Soc Bras Med Trop. 2008;41(6):602–14. doi: https://doi.org/10.1590/S0037-86822008000600011
26. Laranja FS, Dias E, Nobrega G, Miranda A. Chagas’ Disease. Circulation. 1956. https://www.ahajournals.org/doi/abs/10.1161/01.cir.14.6.1035
27. Alba Soto CD, Cappa SM. Trypanosoma cruzi Journey from the Insect Vector to the Host Cell. Chagas Disease; 2019. pp. 25–59. doi: https://doi.org/10.1007/978-3-030-00054-7_2
28. Francolino SS, Antunes AF, Talice R, Rosa R, Selanikio J, de Rezende JM, et al. New evidence of spontaneous cure in human Chagas’ disease. Rev Soc Bras Med Trop. 2003;36(1):103–7. doi: https://doi.org/10.1590/S0037-86822003000100014
29. Dias JC, Dias E, Martins-Filho OA, Vitelli-Avelar D, Correia D, Lages E, et al. Further evidence of spontaneous cure in human Chagas disease. Rev Soc Bras Med Trop. 2008;41(5):505–6. doi: https://doi.org/10.1590/S0037-86822008000500014
30. Bittencourt AL, Sadigursky M, Barbosa HS. [Congenital Chagas’ disease. Study of 29 cases]. Rev Inst Med Trop São Paulo. 1975;17(3):146–59.
31. Coura JR, de Abreu LL, Pereira JB, Willcox HP. Morbidade da doenĉa de Chagas. IV. Estudo longitudinal de dez anos emPains e Iguatama, Minas Gerais, Brasil. Mem Inst Oswaldo Cruz. 1985;80(1):73–80. doi: https://doi.org/10.1590/S0074-02761985000100011
32. Rassi A Jr, Rassi A, Marcondes de Rezende J. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am. 2012 Jun;26(2):275–91. doi: https://doi.org/10.1016/j.idc.2012.03.002
33. Sabino EC, Ribeiro AL, Salemi VM, Di Lorenzo Oliveira C, Antunes AP, Menezes MM, et al.; National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II), International Component. Ten-year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi-seropositive former blood donors. Circulation. 2013 Mar;127(10):1105–15. doi: https://doi.org/10.1161/CIRCULATIONAHA.112.123612
34. Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006 May;144(10):724–34. doi: https://doi.org/10.7326/0003-4819-144-10-200605160-00006
35. Machado-de-Assis GF, Diniz GA, Montoya RA, Dias JC, Coura JR, Machado-Coelho GL, et al. A serological, parasitological and clinical evaluation of untreated Chagas disease patients and those treated with benznidazole before and thirteen years after intervention. Mem Inst Oswaldo Cruz. 2013 Nov;108(7):873–80. doi: https://doi.org/10.1590/0074-0276130122
36. Dias JC. The indeterminate form of human chronic Chagas’ disease A clinical epidemiological review. Rev Soc Bras Med Trop. 1989;22(3):147–56. doi: https://doi.org/10.1590/S0037-86821989000300007
37. Coura JR, de Abreu LL, Pereira JB, Willcox HP. [Morbidity in Chagas’ disease. IV. Longitudinal study of 10 years in Pains and Iguatama, Minas Gerais, Brazil]. Mem Inst Oswaldo Cruz. 1985;80(1):73–80. doi: https://doi.org/10.1590/S0074-02761985000100011
38. Salvador F, Treviño B, Sulleiro E, Pou D, Sánchez-Montalvá A, Cabezos J, et al. Trypanosoma cruzi infection in a non-endemic country: epidemiological and clinical profile. Clin Microbiol Infect. 2014 Jul;20(7):706–12. doi: https://doi.org/10.1111/1469-0691.12443
39. de Oliveira RB, Troncon LE, Dantas RO, Menghelli UG. Gastrointestinal manifestations of Chagas’ disease. Am J Gastroenterol. 1998 Jun;93(6):884–9. doi: https://doi.org/10.1111/j.1572-0241.1998.270_r.x
40. Echeverría LE, Marcus R, Novick G, Sosa-Estani S, Ralston K, Zaidel EJ, et al. WHF IASC Roadmap on Chagas Disease. Glob Heart. 2020 Mar;15(1):26. doi: https://doi.org/10.5334/gh.484
41. Sica RE, Gonzalez Cappa SM, Sanz OP, Mirkin G. Peripheral nervous system involvement in human and experimental chronic American trypanosomiasis. Bull Soc Pathol Exot. 1995 Mar;88(4):156–63.
42. Bern C. Chagas disease in the immunosuppressed host. Curr Opin Infect Dis. 2012 Aug;25(4):450–7. doi: https://doi.org/10.1097/QCO.0b013e328354f179
43. Martinez-Perez A, Norman FF, Monge-Maillo B, Perez-Molina JA, Lopez-Velez R. An approach to the management of Trypanosoma cruzi infection (Chagas’ disease) in immunocompromised patients. Expert Rev Anti Infect Ther. 2014 Mar;12(3):357–73. doi: https://doi.org/10.1586/14787210.2014.880652
44. Cardoso RN, Macedo FY, Garcia MN, Garcia DC, Benjo AM, Aguilar D, et al. Chagas cardiomyopathy is associated with higher incidence of stroke: a meta-analysis of observational studies. J Card Fail. 2014 Dec;20(12):931–8. doi: https://doi.org/10.1016/j.cardfail.2014.09.003
45. Bestetti RB, Restini CB. Precordial chest pain in patients with chronic Chagas disease. Int J Cardiol. 2014 Sep;176(2):309–14. doi: https://doi.org/10.1016/j.ijcard.2014.07.112
46. Saraiva RM, Mediano MF, Mendes FS, Sperandio da Silva GM, Veloso HH, Sangenis LH, et al. Chagas heart disease: an overview of diagnosis, manifestations, treatment, and care. World J Cardiol. 2021 Dec;13(12):654–75. doi: https://doi.org/10.4330/wjc.v13.i12.654
47. Swett MC, Rayes DL, Campos SV, Kumar RN. Chagas Disease: Epidemiology, Diagnosis, and Treatment. Curr Cardiol Rep. 2024 Oct;26(10):1105–12. doi: https://doi.org/10.1007/s11886-024-02113-7
48. Rassi A Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010 Apr;375(9723):1388–402. doi: https://doi.org/10.1016/S0140-6736(10)60061-X
49. Requena-Méndez A, Bussion S, Aldasoro E, Jackson Y, Angheben A, Moore D, et al. Cost-effectiveness of Chagas disease screening in Latin American migrants at primary health-care centres in Europe: a Markov model analysis. Lancet Glob Health. 2017 Apr;5(4):e439–47. doi: https://doi.org/10.1016/S2214-109X(17)30073-6
50. Gascón J, Albajar P, Cañas E, Flores M, Gómez i Prat J, Herrera RN, et al.; Working Group of the second workshop on “Imported Chagas’ Disease, a New Challenge in Public Health”; Spanish Society of Tropical Medicine and International Health. [Diagnosis, management and treatment of chronic Chagas’ heart disease in areas where Trypanosoma cruzi infection is not endemic]. Enferm Infecc Microbiol Clin. 2008 Feb;26(2):99–106.
51. Carlier Y, Altcheh J, Angheben A, Freilij H, Luquetti AO, Schijman AG, et al. Congenital Chagas disease: updated recommendations for prevention, diagnosis, treatment, and follow-up of newborns and siblings, girls, women of childbearing age, and pregnant women. PLoS Negl Trop Dis. 2019 Oct;13(10):e0007694. doi: https://doi.org/10.1371/journal.pntd.0007694
52. Moraes FC, Souza ME, Dal Moro L, Donadon IB, da Silva ER, de Souza DD, et al. Prevention of congenital chagas disease by trypanocide treatment in women of reproductive age: A meta-analysis of observational studies. PLoS Negl Trop Dis. 2024 Sep;18(9):e0012407. doi: https://doi.org/10.1371/journal.pntd.0012407
53. Edwards MS, Montgomery SP. Congenital Chagas disease: progress toward implementation of pregnancy-based screening. Curr Opin Infect Dis. 2021 Oct;34(5):538–45. doi: https://doi.org/10.1097/QCO.0000000000000769
54. Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014 Jan;121(1):22–33. doi: https://doi.org/10.1111/1471-0528.12396
55. Altcheh JM. Congenital Chagas Disease. Chagas Disease; 2019. pp. 179–98. doi: https://doi.org/10.1007/978-3-030-00054-7_9
56. Wagner N, Jackson Y, Posfay Barbe C. Maladie de Chagas: le point pour le praticien. Paediatrica. 2015;23(3):25–8.
57. Moscatelli G, Moroni S, García Bournissen F, González N, Ballering G, Schijman A, et al. Longitudinal follow up of serological response in children treated for Chagas disease. PLoS Negl Trop Dis. 2019 Aug;13(8):e0007668. doi: https://doi.org/10.1371/journal.pntd.0007668
58. Requena-Méndez A, Aldasoro E, de Lazzari E, Sicuri E, Brown M, Moore DA, et al. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015 Feb;9(2):e0003540. doi: https://doi.org/10.1371/journal.pntd.0003540
59. Eperon G, Bühler S, Enriquez N, Vaudaux B. [The immunosuppressed traveler : vaccination guidelines]. Rev Med Suisse. 2018 May;14(605):922–33.
60. Sánchez-Camargo CL, Albajar-Viñas P, Wilkins PP, Nieto J, Leiby DA, Paris L, et al. Comparative evaluation of 11 commercialized rapid diagnostic tests for detecting Trypanosoma cruzi antibodies in serum banks in areas of endemicity and nonendemicity. J Clin Microbiol. 2014 Jul;52(7):2506–12. doi: https://doi.org/10.1128/JCM.00144-14
61. Chappuis F, Mauris A, Holst M, Albajar-Vinas P, Jannin J, Luquetti AO, et al. Validation of a rapid immunochromatographic assay for diagnosis of Trypanosoma cruzi infection among Latin-American Migrants in Geneva, Switzerland. J Clin Microbiol. 2010 Aug;48(8):2948–52. doi: https://doi.org/10.1128/JCM.00774-10
62. D’Acremont V, Greub G, Genton B. [Rapid diagnostic tests (RDT): the cure-all for the practitioner?]. Rev Med Suisse. 2011 May;7(294):984–6.
63. Yun O, Lima MA, Ellman T, Chambi W, Castillo S, Flevaud L, et al. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10-year experience of Médecins Sans Frontières. PLoS Negl Trop Dis. 2009 Jul;3(7):e488. doi: https://doi.org/10.1371/journal.pntd.0000488
64. Pérez-Molina JA, Crespillo-Andújar C, Bosch-Nicolau P, Molina I. Trypanocidal treatment of Chagas disease. Enferm Infecc Microbiol Clin (Engl Ed). 2021 Nov;39(9):458–70. doi: https://doi.org/10.1016/j.eimce.2020.04.012
65. De Salazar PM, Sosa-Estani S, Salvador F, Sulleiro E, Sánchez-Montalvá A, Ribeiro I, et al. Human Trypanosoma cruzi chronic infection leads to individual level steady-state parasitemia: implications for drug-trial optimization in Chagas disease. PLoS Negl Trop Dis. 2022 Nov;16(11):e0010828. doi: https://doi.org/10.1371/journal.pntd.0010828
66. Grossmann U, Rodriguez ML. Chagas disease treatment efficacy markers: experiences from a Phase III study with nifurtimox in children. Front Parasitol. 2023 Sep;2:1229467. doi: https://doi.org/10.3389/fpara.2023.1229467
67. Parrado R, Ramirez JC, de la Barra A, Alonso-Vega C, Juiz N, Ortiz L, et al. Usefulness of Serial Blood Sampling and PCR Replicates for Treatment Monitoring of Patients with Chronic Chagas Disease. Antimicrob Agents Chemother. 2019 Jan;63(2):e01191-18. doi: https://doi.org/10.1128/AAC.01191-18
68. Basquiera AL, Sembaj A, Aguerri AM, Omelianiuk M, Guzmán S, Moreno Barral J, et al. Risk progression to chronic Chagas cardiomyopathy: influence of male sex and of parasitaemia detected by polymerase chain reaction. Heart. 2003 Oct;89(10):1186–90. doi: https://doi.org/10.1136/heart.89.10.1186
69. Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, et al.; BENEFIT Investigators. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N Engl J Med. 2015 Oct;373(14):1295–306. doi: https://doi.org/10.1056/NEJMoa1507574
70. Jackson Y, Wyssa B, Chappuis F. Tolerance to nifurtimox and benznidazole in adult patients with chronic Chagas’ disease. J Antimicrob Chemother. 2020 Mar;75(3):690–6. doi: https://doi.org/10.1093/jac/dkz473
71. Silva S, Barros MV, Nunes MD, Silva L, Edvardsen T, Araujo F, et al. Abstract 11768: Regression of Inflammation by Cardiac Magnetic Resonance in Chronic Chagasic Patients Treated With Benznidazole. Circulation. 2023; https://www.ahajournals.org/doi/10.1161/circ.148.suppl_1.11768
72. Rapp E. Chagas Congenital Screening in Switzerland: Processes of Recognition and Knowledge-Sharing. Med Anthropol. 2021;40(6):557–71. doi: https://doi.org/10.1080/01459740.2021.1922900
73. Romay-Barja M, Iglesias-Rus L, Boquete T, Benito A, Blasco-Hernández T. Key Chagas disease missing knowledge among at-risk population in Spain affecting diagnosis and treatment. Infect Dis Poverty. 2021 Apr;10(1):55. doi: https://doi.org/10.1186/s40249-021-00841-4
74. Ventura-Garcia L, Roura M, Pell C, Posada E, Gascón J, Aldasoro E, et al. Socio-cultural aspects of Chagas disease: a systematic review of qualitative research. PLoS Negl Trop Dis. 2013 Sep;7(9):e2410. doi: https://doi.org/10.1371/journal.pntd.0002410
75. Swissmedic: https://www.swissmedic.ch/swissmedic/en/home.html
76. Swissmedic. Hémovigilance Rapport Annuel 2013.
77. Requena-Méndez A, Albajar-Viñas P, Angheben A, Chiodini P, Gascón J, Muñoz J; Chagas Disease COHEMI Working Group. Health policies to control Chagas disease transmission in European countries. PLoS Negl Trop Dis. 2014 Oct;8(10):e3245. doi: https://doi.org/10.1371/journal.pntd.0003245
78. Colombo V, Giacomelli A, Casazza G, Galimberti L, Bonazzetti C, Sabaini F, et al. Trypanosoma cruzi infection in Latin American pregnant women living outside endemic countries and frequency of congenital transmission: a systematic review and meta-analysis. J Travel Med. 2021 Jan;28(1):taaa170. doi: https://doi.org/10.1093/jtm/taaa170
79. Bilger V, Hollomey C, Efionayi-Mäder D, Wyssmüller C. Health care for undocumented migrants in Switzerland : policies, people, practices. 2011.
80. Jackson Y, Paignon A, Wolff H, Delicado N. Health of undocumented migrants in primary care in Switzerland. PLoS One. 2018 Jul;13(7):e0201313. doi: https://doi.org/10.1371/journal.pone.0201313
81. Piccoli L, Wanner P. The political determinants of the health of undocumented immigrants: a comparative analysis of mortality patterns in Switzerland. BMC Public Health. 2022 Apr;22(1):804. doi: https://doi.org/10.1186/s12889-022-13188-8
82. Ending the neglect to attain the sustainable development goals. One Health: approach for action against neglected tropical diseases 2021-2030. Executive summary. World Health Organization; 2022. 4 pp.