Real-life experience
of chronic hepatitis C treatment in Switzerland:
a retrospective analysis
DOI: https://doi.org/https://doi.org/10.57187/s.3698
Eleni Moschouria,
Gloria Salemmeb,
Adriana Basergab,
Andreas Cernyb,
Ansgar Deibelc,
Beat Müllhauptc,
Marie-Anne Meierd,
Christine Bernsmeierd,
Marie Ongaroe,
Francesco Negroe,
Marielle Grosjeanf,
Olivier Clercf,
Patrizia Künzler-Heuleg,
David Semelag,
Gabriel Hobih,
Felix Stickelch,
Adeline Mathieua,
Elise Mdawar-Baillya,
Mohamed Faouzii,
Darius Moradpoura,
Montserrat Fragaa
a Division of Gastroenterology
and Hepatology, Lausanne University Hospital and University of Lausanne,
Lausanne, Switzerland
b Epatocentro Ticino, Lugano, Switzerland
c Division
of Gastroenterology and Hepatology, University Hospital Zurich, Zurich, Switzerland
d University Centre for Gastrointestinal and Liver
Diseases (Clarunis), University Hospital Basel, Basel, Switzerland
e Division of Gastroenterology and Hepatology,
University Hospitals Geneva, Geneva, Switzerland
f Divisions of Internal Medicine and Infectious
Diseases, Hôpital Neuchâtelois-Pourtalès, Neuchâtel, Switzerland
g Division
of Gastroenterology and Hepatology,
Kantonsspital St. Gallen, St. Gallen, Switzerland
h Hirslanden Klinik Beau-Site, Bern, Switzerland
i Division of Biostatistics, Center for Primary Care and
Public Health (Unisanté), Lausanne, Switzerland
Summary
BACKGROUND AND AIM: Direct-acting
antivirals (DAAs) have revolutionised the management of chronic hepatitis C. We analysed
the use
of different generations of DAAs over time in Switzerland and investigated
factors predictive of treatment failure.
METHODS: This retrospective study was
conducted within the framework of the Swiss Association for the Study of the
Liver and the Swiss Hepatitis C Cohort Study; it included all patients with
chronic hepatitis C treated with DAAs between January 2015 and December 2019 at
eight Swiss referral centres.
RESULTS: A total of 3088 patients were
included; 57.3% were male, and the median age was 54 years. Liver cirrhosis was
present in 23.9% of the cohort, 87.8% of whom were compensated. The overall sustained
virological response (SVR) rate (defined as undetectable HCV RNA at week 12 after
the first course of DAA-based treatment) was 96.2%, with an increase over time.
The rate of treatment failure dropped from 8.3% in 2015 to 2.5% in 2019. Multivariable
analysis revealed that female sex, the use of the latest generation of pangenotypic
DAA regimens, Caucasian origin, and genotype (gt) 1 were associated with SVR, whereas
the presence of active hepatocellular carcinoma (HCC), gt 3, and increasing
liver stiffness were associated with treatment failure. Notably, the presence
of active HCC during treatment increased the risk of DAA failure by a factor of
almost thirteen.
CONCLUSIONS: SVR rates increased over time, and
the highest success rates were identified after the introduction of the latest generation
of pangenotypic DAA regimens.
Active HCC, gt 3 and increasing liver stiffness were associated with DAA
failure.
Introduction
Chronic
hepatitis C virus (HCV) infection is one of the most prevalent causes of
advanced liver disease and hepatocellular carcinoma (HCC). Approximately 10–20%
of individuals who are chronically infected develop complications over a period
of 20–30 years, including cirrhosis, liver decompensation, and HCC [1, 2]. In
2019, HCV infection led to 287,000 deaths; two-thirds of these were related to
cirrhosis and end-stage liver disease, and one-third were related to HCC [3]. An
estimated 56.8 million individuals are chronically infected worldwide [4]. In
Switzerland, an estimated 32,100 individuals were chronically infected at the
beginning of 2020 [5].
Sustained
virological response (SVR) is associated with a decrease in liver-related and
all-cause mortality [6]. According to the European Association for the Study of
the Liver (EASL), the American Association for the Study of Liver Diseases
(AASLD), and the Infectious Diseases Society of America (ISDA), antiviral
treatment should be proposed to all patients with chronic HCV infection except those
with a limited life expectancy that cannot be improved by antiviral therapy,
liver transplantation (LT), or other liver-directed therapies [7, 8]. The World
Health Organization (WHO) has stated that it aims to eliminate (i.e. control) HCV
infection by 2030 [9].
The treatment
of chronic hepatitis C entered a new era when direct-acting antivirals (DAAs)
were introduced in 2011. These drugs show excellent tolerance and a relatively
short treatment duration, and the latest pangenotypic regimens are associated
with SVR rates of >95% [10–14].
Real-life
data on DAA treatment in Switzerland are limited [15–17]. Access to DAA
treatment for chronic hepatitis C in Switzerland was restricted to patients
with advanced fibrosis or cirrhosis (Metavir stages F3 and F4) until the first
half of 2015 and a Metavir stage of at least F2 until the fall of 2017. These
restrictions were primarily due to the high cost of DAAs, prompting healthcare
authorities to prioritise treatment for individuals at the highest risk of
liver-related complications. Since the fall of 2017, DAA treatment has been reimbursed
regardless of the fibrosis stage.
This
study’s primary aim was to assess the real-life SVR rates (defined as
undetectable HCV RNA at week 12 after the end of treatment). The secondary aim
was to identify risk factors for DAA treatment failure in a large cohort of
patients followed at eight referral centres in Switzerland.
Patients and
methods
Study design and
patient population
This
observational retrospective multicentre study was conducted within the
framework of the Swiss Association for the Study of the Liver (SASL Study 44)
and the Swiss Hepatitis C Cohort Study. The study included all adult patients with
chronic HCV
infection who were treated with interferon-free DAA regimens between January 2015
and December 2019 at eight referral centres in Switzerland. Patients with HIV
coinfection were excluded.
Each centre was represented by a local
principal investigator. Data were retrieved manually from
electronic medical records and medical archives, and they were anonymised before being
transferred to the
investigators at Lausanne University Hospital.
This study was approved by the local ethics committees (CER-VD multicentric
protocol number 2020-01232). Informed consent was waived.
Data collection
and definition
The
following data were captured for each patient: age; sex; ethnicity; Metavir
fibrosis stage (F0–F4) when a liver biopsy was available or liver stiffness
measurement as assessed by transient elastography (FibroScan, Echosens, Paris,
France); Child-Turcotte-Pugh (CTP) score (A5–C15); the absence or presence of
hepatitis B virus (HBV) coinfection; viral genotype (gt); the results of
resistance-associated substitution (RAS) analyses (when available); history of HCC
or active HCC (defined as imaging evidence
of localised or metastatic disease at DAA initiation); type of DAA regimen;
and SVR (SVR group) or failure to achieve SVR after the first course of
interferon-free DAA-based treatment (non-SVR group). SVR was defined as
undetectable HCV RNA at week 12 after the end of treatment.
Liver
cirrhosis was defined by any of the following: Metavir fibrosis stage 4 on
liver histology, a liver stiffness measurement of ≥12 kPa, or clinical evidence of
cirrhosis based on an evaluation by the referring hepatologist.
Treating
physicians chose the type and duration of DAA regimens according to the regularly
updated Expert Opinion Statements by the Swiss Association for the Study of the
Liver, the Swiss Society of Gastroenterology, and the Swiss Society for
Infectious Diseases (available at https://www.sasl.ch). In general, these are in line with the EASL
Recommendations [8] as well as the AASLD-IDSA Guidance [7] and consider
the approval and reimbursement of the different regimens in Switzerland.
This study
divided DAA regimens into two categories: nonpangenotypic and latest generation
pangenotypic DAA regimens.
The nonpangenotypic regimen category comprised sofosbuvir + ribavirin (SOF + RBV),
simeprevir/sofosbuvir ± ribavirin (SMV/SOF ± RBV),
ledipasvir/sofosbuvir ± ribavirin (LDV/SOF ± RBV), ritonavir-boosted
paritaprevir/ombitasvir ± dasabuvir ± ribavirin (PTV/r/OBV ± DSV ± RBV),
grazoprevir/elbasvir ± ribavirin (GZR/EBR ± RBV), and the early pangenotypic
regimen daclatasvir/sofosbuvir ± ribavirin (DCV/SOF ± RBV).
The latest
generation pangenotypic regimen category consisted of velpatasvir/sofosbuvir ±
ribavirin (VEL/SOF ± RBV), glecaprevir/pibrentasvir ± ribavirin (GLE/PIB ±
RBV), glecaprevir/pibrentasvir + sofosbuvir ± ribavirin (GLE/PIB + SOF ± RBV), and
voxilaprevir/velpatasvir/sofosbuvir ± ribavirin (VOX/VEL/SOF ± RBV).
Statistical
analyses
Descriptive
statistics are presented as the mean and standard deviation (SD) or the median
and range for continuous variables and frequencies or percentages for
categorical variables. The non-SVR group was compared with the SVR group using
a logistic regression model. To account for the multicentre
design, a robust standard error was calculated for parameter estimation. Univariable
analysis was performed to identify factors associated with DAA failure. The
strength of the association was measured using the odds ratio (OR) and the
calculated p-value. The factors significantly associated with the outcome (i.e.
those with a p-value of <10%) were tested in a stepwise backward selection
procedure to fit a multivariable model. Standard goodness-of-fit tests for
logistic regression were performed to assess the calibration of the fitted
model. Statistical analyses were performed using Stata
software (Stata Statistical Software: Release 16, StataCorp 2023, College
Station, TX, USA).
Results
Study population
Between
January 2015 and December 2019, 3088 patients with chronic HCV infection were
treated with interferon-free DAA regimens at the eight participating centres. The
patients’ characteristics are presented in table 1.
Table 1Baseline
characteristics of patients in the SVR and non-SVR groups and univariable and multivariable
logistic regression analyses. Variables
in bold were significantly associated with DAA failure in the univariable or multivariable
analysis. The retained covariables used for the multivariable analysis were sex,
liver stiffness, genotype, active hepatocellular carcinoma (HCC), and the latest
generation of pangenotypic regimens.
|
|
SVR, n (%) |
Non-SVR, n (%) |
Univariable analysis, OR (p-value) |
Multivariable analysis, OR (p-value) |
| Total |
|
2972 (96.2%) |
116 (3.8%) |
|
|
| Age (years); mean (SD) |
|
54.4 (12.0) |
54.8 (10.5) |
1.00 (0.82) |
|
| Sex (female)1 |
|
1256 (42.3%) |
31 (27.0%) |
0.50 (0.02) |
0.1 (0.004) |
| Ethnicity2 |
Caucasian |
2769 (93.3%) |
100 (86.2%) |
0.45 (<10-4) |
0.35 (0.001) |
| Asian |
72 (2.4%) |
4 (3.5%) |
1.44 (0.47) |
|
| African |
112 (3.8%) |
10 (8.6%) |
2.41 (0.001) |
|
| Latin American |
16 (0.5%) |
2 (1.7%) |
3.24 (0.24) |
|
| Metavir fibrosis
score |
F0 (reference) |
121 (8.1%) |
5 (7.5%) |
|
|
| F1 |
410 (27.4%) |
|
0.65 (0.22) |
|
| F2 |
356 (23.8%) |
15 (22.4%) |
1.02 (0.95) |
|
| F3 |
215 (14.4%) |
3 (4.5%) |
0.33 (0.01) |
|
| F4 |
393 (26.%3) |
33 (49.2%) |
2.03 (0.04) |
|
| Liver stiffness
(kPa); mean (SD) |
10.4 (9.8%) |
17.3 (17.9%) |
1.03 (<10-4) |
1.02 (0.003) |
| Cirrhosis3 4 |
678 (23.1%) |
51 (44.4%) |
2.65 (<10-4) |
|
| Child-Pugh score4 |
A |
546 (87.8%) |
44 (86.2%) |
1.02 (0.94) |
|
| B |
70 (11.2%) |
6 (11.8%) |
1.07 (0.79) |
|
| C |
6 (1.0%) |
0 |
|
|
| Genotype4 5 |
1 |
1678 (56.6%) |
36 (31.6%) |
0.35 (<10-4) |
0.36 (0.01) |
| 2 |
241 (8.1%) |
5 (4.4%) |
0.52 (0.07) |
|
| 3 |
647 (21.8%) |
52 (45.6%) |
3.00 (<10-4) |
2.22 (0.003) |
| 4 |
388 (13.1%) |
21 (18.4%) |
1.49 (0.16) |
|
| 5 |
5 (0.2%) |
0 |
|
|
| 6 |
5 (0.2%) |
0 |
|
|
| HBV coinfection |
31 (1.1%) |
2 (1.8%) |
1.64 (0.54) |
|
| HCC4 |
No HCC |
2868 (97.4%) |
98 (85.2%) |
0.15 (<10-4) |
|
| HCC in remission |
47 (1.6%) |
5 (4.3%) |
2.80 (0.06) |
|
| Active HCC |
30 (1.0%) |
12 (10.4%) |
11.32 (<10-4) |
12.99 (0.001) |
| Latest generation pangenotypic regimen |
1221 (39.5%) |
20 (19.4%) |
0.35 (0.003) |
0.17 (<10-4) |
The median
age was 54 years (range, 18–88 years); 1287 patients (42.7%) were female, and most
(93.0%) were Caucasian.
The median liver
stiffness measurement was 7.3 kPa (range, 2.8-75 kPa). Of the 3088 patients,
1562 (50.6%) had a liver biopsy before DAA treatment; 126 (8.1%) had Metavir
fibrosis stage F0, F1 in 421 (26.9%) had stage F1, 371 (23.7%) had stage F2,
218 (14.0%) had stage F3, and 426 (27.3%) had stage F4. A total of 729 patients
(23.9%) had cirrhosis, and the CTP score could be calculated in 672 patients;
590 (87.8%) had CTP A, 76 (11.3%) had CTP B, and 6 (0.9%) had CTP C.
Information on cirrhosis diagnosis and CTP score was missing for 38 and 57
patients, respectively.
The HCV
genotype distribution was as follows: 1714 patients (55.7%) had gt 1, 246
(8.0%) had gt 2, 699 (22.7%) had gt 3, 409 (13.3%) had gt 4, 5 (0.2%) had gt 5,
and 5 (0.2%) had gt 6. Genotype information was missing for 10 patients (0.3%).
A total of
33 patients (1.2%) had HBV coinfection, 42 (1.4%) had active HCC at the time of
DAA treatment, and 52 (1.7%) had a history of HCC.
During the
study period, 10 DAA regimens associated or not associated with RBV were used
according to the investigators’ discretion (see Patients and methods). A total
of 1866 patients (60.5%) were treated with nonpangenotypic DAA regimens, and
1221 (39.5%) were treated with latest generation of pangenotypic DAA regimens.
Information regarding the DAA regimen was missing for one patient. The treatment
duration ranged from 1.5 to 24 weeks, with a median of 12.0 weeks and a mean of
12.5 weeks. One patient with genotype 1a HCV infection who was considered
chronically infected stopped treatment with GLE/PIB after 1.5 weeks. However,
this patient achieved SVR (confirmed 24 weeks after treatment), suggesting that
it was an acute infection.
Treatment outcome
Among the
3088 patients, 2972 (96.2%) achieved SVR after the first course of DAA
treatment. SVR rates increased throughout this study, from 91.7% in 2015 to
97.5% in 2019 (figure 1). DAA failure was reported in 116 (3.8%) patients (table 1).
Patients in the non-SVR group were mostly male (73.0%) with a median age of 55
years (range, 22–88 years). The median liver stiffness measurements were
7.3 kPa and 9.9 kPa in the SVR and non-SVR groups, respectively. Fifty-one
patients in the non-SVR group (44.4%) had cirrhosis, with a CTP score of A in
86.2% and B in 11.8%. The CTP score could not be calculated for one patient in
this group.
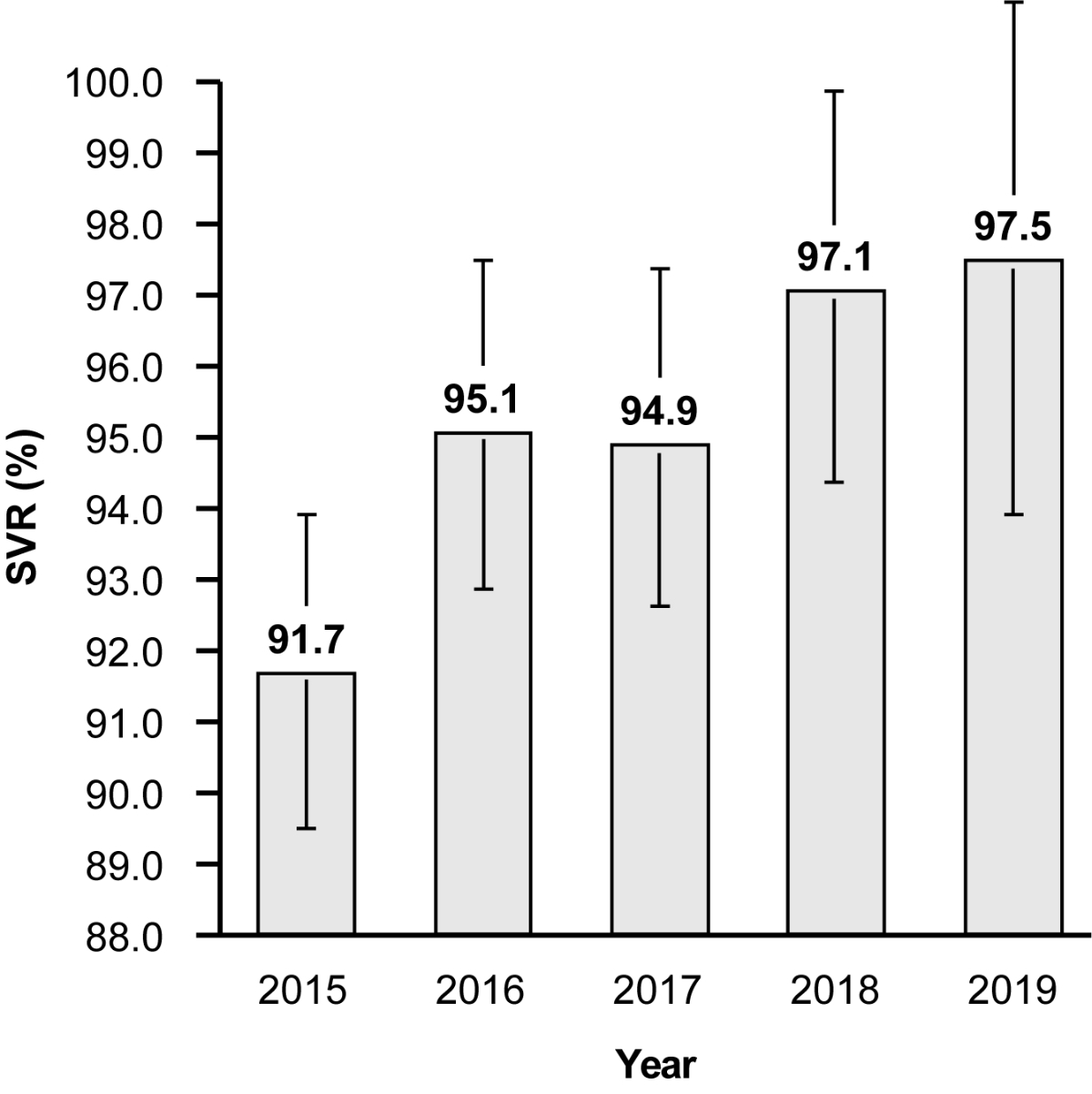
Figure 1Sustained virological response (SVR) rates achieved with interferon-free,
direct-acting antiviral (DAA)-based treatment of chronic hepatitis C from 2015
to 2019. The vertical bars represent
95% confidence intervals for each year (2015, 89.5–93.9; 2016, 92.8–97.5; 2017,
92.6–97.3; 2018, 94.3–99.9; 2019, 94.0–101.1).
In total,
42 patients (1.4%) had active HCC at the time of DAA treatment, 30 (1.0%) of
whom were in the SVR group and 12 (10.4%) of whom were in the non-SVR group.
Furthermore, 52 patients (1.7%) had a history of HCC, 47 (1.6%) of whom were in
the SVR group and 5 (4.3%) of whom were in the non-SVR group.
Risk factors for DAA failure
Several parameters were tested for
possible association with DAA failure (table 1), including age, sex, ethnicity,
Metavir score, liver stiffness measurement, the presence or absence of
cirrhosis, CTP score, genotype, HBV coinfection, active HCC or history of HCC, and
DAA regimen.
The univariable
analysis revealed that the presence of active HCC at the time of antiviral
treatment (OR 11.3, 95% confidence interval [CI] 4.8–26.2), gt 3 (OR 3.00, 95%
CI 2.14–4.20), cirrhosis (OR 2.65, 95% CI 2.02–3.48), African ethnicity (OR
2.41 95% CI 1.45–3.99), Metavir stage F4 (OR 2.03, 95% CI 1.04–3.94), and liver
stiffness measurement (OR 1.03, 95% CI 1.02–1.04) were significantly associated
with DAA failure. Conversely, the absence of HCC (OR 0.15, 95% CI 0.07–0.36),
Metavir stage F3 (OR 0.33, 95% CI 0.15–0.76), the use of the latest generation of
pangenotypic DAA regimens (OR 0.35, 95% CI 0.15–0.76), gt 1 (OR 0.35, 95% CI
0.28–0.43), Caucasian origin (OR 0.45, 95% CI 0.37–0.54), and female sex (OR
0.50, 95% CI 0.28–0.87) were significantly associated with increased chances of
achieving SVR.
Of these parameters, seven were
associated with response to treatment in the multivariable analysis. Active HCC during
treatment (OR 12.99, 95% CI
2.80–60.13, p = 0.001), gt 3 (OR
2.22, 95% CI 1.30–3.78, p = 0.003), and liver stiffness measurement (OR
1.02, 95% CI 1.00–1.04, p = 0.003) were
independent predictors of an increased risk of DAA failure. The presence of active
HCC during treatment
increased the risk of DAA failure by a factor of almost 13. Liver
stiffness measurement (as assessed by transient elastometry) was significantly
correlated with the risk of DAA failure; this correlation was nearly linear
starting at 40 kPa (figure 2). Conversely, female sex (OR 0.1, 95% CI 0.02–0.48, p
= 0.004), the use of the latest
generation of pangenotypic DAA regimens (OR 0.17, 95% CI 0.08–0.34 p <10−4), Caucasian ethnicity (OR 0.35, 95% CI 0.19–0.64,
p = 0.001), and gt 1 (OR 0.36, 95% CI 0.16–0.82, p = 0.01) were significantly
associated with SVR.
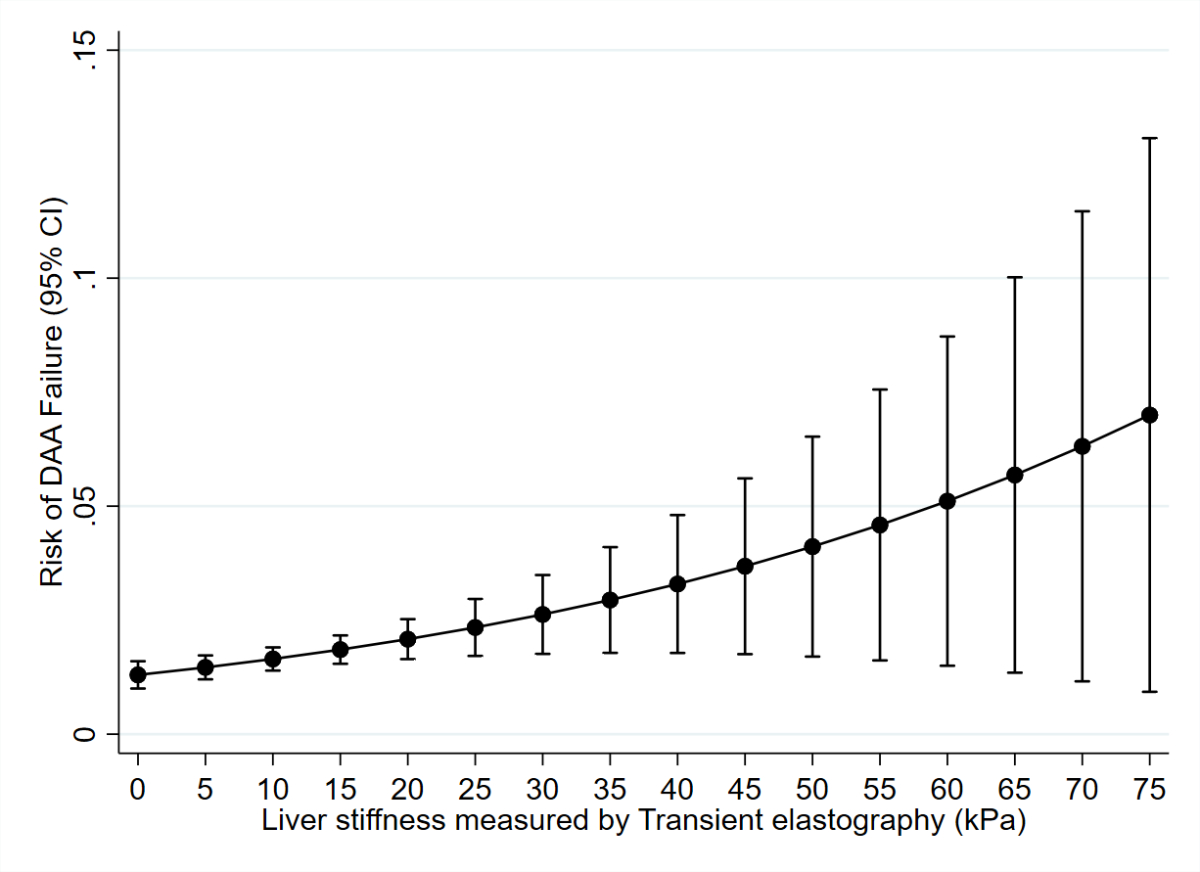
Figure 2The adjusted predicted risk of DAA failure
as a function of liver stiffness. Liver stiffness (as assessed by
transient elastography) was significantly correlated with the risk of DAA
failure, with a near-linear correlation starting at 40 kPa. For the multivariable
model, see table 1.
Retreatments
Of the 116 patients in the non-SVR
group, 103 (88.8%) were retreated with a second DAA regimen, and 13 were not
retreated for various reasons (three declined retreatment, one developed
advanced-stage HCC, one died, and eight were lost to follow-up). Retreatment consisted
of VOX/VEL/SOF in 43 patients
(41.7%), VEL/SOF in 33 patients (32.0%), GLE/PIB in 10 patients (9.7%), GLE/PIB
+ SOF in 1 patient (1.0%), and nonpangenotypic DAAs in 16 patients (15.5%).
Of the 103 retreated patients, 88
(85.4%) achieved SVR, whereas 13 (12.6%) did not. Information on retreatment outcomes
was not available for two patients (1.9%) because of loss to follow-up.
The characteristics of the 13
patients who experienced a second DAA failure are summarised in table S1 in the
Appendix. These patients had a median age of 57 years (range, 35–73 years) and
were mostly male (69.2%) and Caucasian (84.6%). Six patients (46.2%) had
cirrhosis with a median CTP score of 5 (range, A5–B9). Five patients had gt 3
infection (38.5%).
Of the 13 patients who presented a
second DAA failure, 11 received a third course of DAA treatment; 10 received VOX/VEL/SOF
± RBV, and 1 had missing
information on the treatment used. One patient
with decompensated cirrhosis and one patient with advanced HCC were not
retreated.
Nine of the eleven patients achieved SVR after a third course of DAAs, whereas two
experienced a third DAA failure. One of the
latter patients was retreated with a fourth course of DAAs (GLE/PIB + SOF + RBV
for 24 weeks) and achieved SVR. No decision regarding further treatment was
made for the second patient during the study period. The results of RAS
analyses regarding those patients are presented in table S1 (patients 5 and
10).
The multivariable analysis revealed
that the use of VOX/VEL/SOF ± RBV was
associated with SVR when used as a second DAA regimen compared with other
regimens (p = 0.005). Treatment success was not significantly affected by other
baseline features, such as sex (p = 0.73), ethnicity (p = 0.65), liver
stiffness measurement (p = 0.57), the presence of cirrhosis (p = 0.76), gt (p
= 0.2), or the presence of active
HCC (p = 0.63).
Resistance-associated substitution
analyses
The results of RAS analyses were
available for 86 of the 116 patients
with DAA failure (74.1%). The analysis of the nonstructural protein 5A (NS5A) region
was most frequently available (table 2). A total of 57 patients (66.2%) presented
a
significant RAS in the NS5A
region, and 24 patients (27.9%) had no detectable RAS.
Table 2Most frequently reported RAS in NS5A
region after first DAA treatment.
| Genotype (n) |
NS5A |
| 1a (22) |
Q30R > L31M > Y93H > H58D |
| 1b (13) |
Y93H > Q30R, L31M/V > H58S |
| 2 (5) |
|
| 3 (52) |
Y93H > A30K |
| 4 (21) |
L31M/V/S > Q30R > L28F/S > T58P > Y93H |
| RAS,
resistance-associated substitution; DAA, direct-acting antiviral |
In NS5A, Q30R was predominant in
patients infected with gt 1a, whereas Y93H was predominant in those with gt 1b
and gt 3 infections. Overall, Y93H was
the most frequently identified RAS in NS5A, identified in 31 patients (36.0%).
Discussion
This is the largest real-life study evaluating
the efficacy of interferon-free, DAA-based treatment for chronic hepatitis C in
Switzerland to date; it included 3088 consecutive patients from eight referral centres.
The overall SVR rate after the first treatment course across all HCV genotypes
and the study period ranging from 2015 to 2019 was 96.2%. SVR rates increased during
the first interferon-free, DAA-based treatments (a period of 5 years) until the
latest generation of pangenotypic regimens, which are still and will likely
remain standard-of-care. The introduction of the latter regimens may have
contributed substantially to increasing SVR rates across the study period.
However, patients with advanced liver disease, especially in the cirrhosis
stage, were initially prioritised for access to DAAs and were at higher risk of treatment
failure. In 2019,
the SVR rate reached 97.5%, which is comparable to the SVR rate of phase 3 and
real-life studies performed in other countries using the latest generation of pangenotypic
regimens [10-14, 17, 18].
As expected, gt 1 was the predominant
genotype observed in our study (55.7%), followed by gt 3 (22.7%). However, gt 3 was
the predominant
genotype among patients who failed to achieve SVR after the first course of DAA-based
treatment (44.8%), followed by gt 1 (31.0%)
[19].
Our secondary aim was to characterise
baseline demographic,
clinical, and virological parameters associated with DAA failure. In a study published
by Arias et al.
[20], a total of 363 patients with chronic hepatitis C had completed a course
of all-oral DAA treatment outside of clinical trials. Based on their multivariable
analysis, only advanced liver fibrosis (Metavir stages F3 and F4) and HIV
coinfection were significantly associated with an increased risk of treatment
failure. Patients with HIV coinfection were excluded from our study.
In our univariable analysis, the
presence of active HCC at the time of antiviral treatment, gt 3, cirrhosis,
African ethnicity, Metavir stage F4, and increasing liver stiffness were
significantly associated with DAA failure. Conversely, the absence of HCC,
Metavir stage F3, the use of the latest generation of pangenotypic DAA
regimens, gt 1, Caucasian origin, and female sex were significantly associated
with increased chances of achieving SVR.
The multivariable analysis revealed
that active HCC at treatment, gt
3, and liver stiffness
measurement were independent predictors of an increased risk of DAA failure.
Conversely, female sex, the use of the latest
generation of pangenotypic DAA regimens, Caucasian ethnicity, and gt 1 were
significantly associated with SVR.
The observation that gt 3 infection is independently associated with a
reduced probability of achieving SVR compared with other genotypes is in line
with previous reports
[21, 22].
Another
important factor associated with an increased risk of DAA failure on multivariable
analysis was an increased liver stiffness measurement as assessed by transient
elastography. Importantly, an almost linear progression of DAA failure risk was
observed above a liver stiffness measurement of 40 kPa. This observation is
consistent with previous reports of an increased risk of DAA failure in the
presence of cirrhosis and portal hypertension [23–26].
Notably,
the presence of active HCC at the time of DAA treatment was independently
associated with DAA failure. Patients with active HCC at the time of DAA
initiation had an almost 13-fold increased risk of DAA failure compared with those
without HCC. This is in line with previously published data [27-30]. In a study
by Prenner et al., DAA failure was observed in 21% of patients with HCC,
whereas it was only observed in 12% of patients without HCC (p = 0.009) [27].
The vast majority of nonresponders in this study presented active HCC.
Similarly, Beste et al. included 482 patients with HCC and 16,863 patients
without HCC with chronic HCV infection who were treated with DAAs; they reported
significantly lower SVR rates in patients with HCC across all genotypes [28].
The HCV-TARGET study also compared patients with treated to untreated HCC and reported
similar results [29]. SVR was achieved in 87% of the non-HCC group compared with
78% of the treated HCC group and 72% of the untreated HCC group. Finally, a
recent meta-analysis by Ji et al., including 3341 patients with HCC and 35,701
without HCC, found a 4.8% SVR reduction in patients with HCC compared with those
without HCC. The largest SVR rate reduction (18.8%) occurred in patients with
active HCC (SVR 73.1% vs. 92.6%, p = 0.002) [30].
In our
multivariable analysis, African ethnicity was not associated with an increased
risk of DAA failure. However, this demographic variable was associated with
significantly increased failure rates in the univariable analysis. A high
prevalence of unusual subtypes, namely gt 1 non-1a, non-1b, 3b/3g, and 4r, has
been reported in African patients, contributing to increased failure rates [31–33].
Information
on these rare subtypes was not available in our real-life study. A
previous subanalysis of patients from Lausanne University Hospital showed that
African patients with HCV subtype 4r were overrepresented among patients who relapsed
[34].
Finally,
the favourable effect of female sex on SVR rates in our study also aligns with
previous reports [24].
Prior studies have attempted to identify factors
predictive of treatment failure. Most of them initially included patients
treated with interferon-based regimens that are no longer prescribed [35]. Few
studies have specifically assessed risk factors for DAA failure [20, 21, 30–33]. Based
on our analysis
of a large cohort of patients, active HCC, gt 3 and increasing liver
stiffness measurement as a correlate of
cirrhosis and portal hypertension appear as the
strongest risk factors while subtype analyses were not available in our
real-life study.
Although DAA-based treatment has become simple and highly
successful, with SVR rates approaching 100%, some patients remain difficult to
treat. Therefore, the risk factors associated with treatment
failure identified in this study may allow clinicians to select patients who could
benefit from first-line antiviral therapy with a triple DAA regimen, such as
VOX/VEL/SOF or GLE/PIB + SOF [36]. Further randomised controlled studies employing
this approach should explore this.
In our study, 116 of 3088 (3.7%)
patients failed to achieve SVR. Most (103 patients) were retreated with a second
course of DAA, and 84.5% of them achieved SVR. The overall SVR rate after DAA
retreatment was high in this real-life study. Hence, very few patients failed
more than two treatment courses. Notably, gt 3 was predominant among patients
who failed a second course of DAA treatment, confirming that attention must still
paid to this genotype. VOX/VEL/SOF as the second DAA regimen was the only
variable significantly associated with SVR.
Results from RAS analyses were available
in roughly three-quarters of patients who experienced failure of the first
course of DAA-based treatment in this real-life study. The NS5A region was
sequenced in almost all cases; the sequencing of the NS3 protease domain and
NS5B was available only in some patients. As expected, and in line with overall
good treatment adherence, significant NS5A RASs were detected in many patients
who were tested.
Overall, our RAS analysis results are
concordant with those of previous reports. A recent publication by SHARED, an
international consortium studying HCV drug resistance, highlighted the clinical
relevance of RAS [37]. Among 730 virologic failures, 94% had resistance
against at least one DAA class. In a European multicentre study involving 938
patients with DAA failure, similar to our study, Q30R in the NS5A region was
predominant in patients with gt 1a, whereas L31M and Y93H were the most
frequent in patients with gt 1b and Y93H was the most frequent in those with gt
3a [38].
Our study presents some limitations, principally due to its real-life
and retrospective nature as well as the exclusion of patients with HIV coinfection.
All patients diagnosed with
chronic hepatitis C within each centre during the study period were included in
the analysis. However, some patients may not have been accounted for, although
their absence is not anticipated to substantially affect the overall results. RAS
analyses were not performed at baseline or in all non-SVR patients. However, our study
has several strengths, including the
large number and wide spectrum of patients as well as the representation of
referral university and non-university hospitals in different regions across
the country. In addition, only a few data points were missing, highlighting
the high quality of medical documentation and close follow-up offered in the
eight Swiss referral centres.
In conclusion,
our study, which included over 3000 patients, confirms the high rates of HCV
cure with current DAA regimens outside of clinical trials. Our study did not include
patients coinfected with HIV. Nevertheless, our findings align with results previously
reported by the Swiss HIV Cohort Study [39]. Our study also revealed factors
associated with treatment failure, providing unique large-scale real-life data
for Switzerland.
Although these
high cure rates offer substantial benefits, they can only be achieved in patients
who are diagnosed. Hence, our study underscores the importance of HCV testing
in key populations and aligns with ongoing efforts towards the elimination of
viral hepatitis and HIV infection in Switzerland.
Acknowledgments
The authors gratefully acknowledge endorsement by and
support from the Swiss Association for the Study of the Liver and the Swiss
Hepatitis C Cohort Study.
The authors confirm
contribution to the paper as follows: study conception and design: Eleni
Moschouri, Darius Moradpour, Montserrat Fraga; data collection: Eleni
Moschouri, Gloria Salemme, Adriana Baserga, Ansgar Deibel, Marie-Anne Meier,
Marie Ongaro, Marielle Grosjean, Patrizia Künzler-Heule, Gabriel Hobi, Adeline
Mathieu, Elise Mdawar-Bailly; analysis and interpretation of results: Eleni
Moschouri, Andreas Cerny, Beat Müllhaupt, Christine Bernsmeier, Francesco Negro, Olivier
Clerc, David Semela, Mohamed Faouzi, Darius
Moradpour, Montserrat Fraga; draft manuscript preparation: Eleni Moschouri,
Darius Moradpour, Montserrat Fraga. All authors reviewed the results and
approved the final version of the manuscript.
Montserrat Fraga
Division of
Gastroenterology and Hepatology
CHUV
Rue du Bugnon 44
CH-1011 Lausanne
Montserrat.Fraga[at]chuv.ch
References
1. Bruden DJ, McMahon BJ, Townshend-Bulson L, Gounder P, Gove J, Plotnik J, et al. Risk
of end-stage liver disease, hepatocellular carcinoma, and liver-related death by fibrosis
stage in the hepatitis C Alaska Cohort. Hepatology. 2017 Jul;66(1):37–45. 10.1002/hep.29115
2. Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection
and HCV-related disease. Nat Rev Gastroenterol Hepatol. 2017 Feb;14(2):122–32. 10.1038/nrgastro.2016.176
3. Cui F, Blach S, Manzengo Mingiedi C, Gonzalez MA, Sabry Alaama A, Mozalevskis A, et
al. Global reporting of progress towards elimination of hepatitis B and hepatitis
C. Lancet Gastroenterol Hepatol. 2023 Apr;8(4):332–42. 10.1016/S2468-1253(22)00386-7
4. Blach S, Terrault NA, Tacke F, Gamkrelidze I, Craxi A, Tanaka J, et al.; Polaris Observatory
HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care
between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022 May;7(5):396–415.
10.1016/S2468-1253(21)00472-6
5. Bihl F, Bruggmann P, Castro Batänjer E, Dufour JF, Lavanchy D, Müllhaupt B, et al. HCV
disease burden and population segments in Switzerland. Liver Int. 2022 Feb;42(2):330–9.
10.1111/liv.15111
6. Ogawa E, Chien N, Kam L, Yeo YH, Ji F, Huang DQ, et al. Association of Direct-Acting
Antiviral Therapy With Liver and Nonliver Complications and Long-term Mortality in
Patients With Chronic Hepatitis C. JAMA Intern Med. 2023 Feb;183(2):97–105. 10.1001/jamainternmed.2022.5699
7. Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019
Update: American Association for the Study of Liver Diseases-Infectious Diseases Society
of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection.
Hepatology. 2020 Feb;71(2):686–721. 10.1002/hep.31060
8. Pawlotsky JM, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al.; European
Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu;
Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel
members. EASL recommendations on treatment of hepatitis C: final update of the series.
J Hepatol. 2020 Nov;73(5):1170–218. 10.1016/j.jhep.2020.08.018
9. Thomas DL. Global Elimination of Chronic Hepatitis. N Engl J Med. 2019 May;380(21):2041–50.
10.1056/NEJMra1810477
10. Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, et al.; ASTRAL-1 Investigators.
Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J
Med. 2015 Dec;373(27):2599–607. 10.1056/NEJMoa1512610
11. Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, et al.; ASTRAL-2 Investigators;
ASTRAL-3 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection.
N Engl J Med. 2015 Dec;373(27):2608–17. 10.1056/NEJMoa1512612
12. Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, et al. Glecaprevir plus pibrentasvir
for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated
cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet
Infect Dis. 2017 Oct;17(10):1062–8. 10.1016/S1473-3099(17)30496-6
13. Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, et al. Glecaprevir-Pibrentasvir
for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N Engl J Med. 2018 Jan;378(4):354–69.
10.1056/NEJMoa1702417
14. Berg T, Naumann U, Stoehr A, Sick C, John C, Teuber G, et al. Real-world effectiveness
and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection:
data from the German Hepatitis C-Registry. Aliment Pharmacol Ther. 2019 Apr;49(8):1052–9.
10.1111/apt.15222
15. Müllhaupt B, Semela D, Ruckstuhl L, Magenta L, Clerc O, Torgler R, et al. Real-world
effectiveness and safety of glecaprevir/pibrentasvir therapy in patients with chronic
hepatitis C virus infection in Switzerland. Swiss Med Wkly. 2021 Jan;151(304):w20399.
10.4414/smw.2021.20399
16. Bachofner J, Valli PV, Bergamin I, Kröger A, Künzler P, Baserga A, et al.; The Swiss
Hepatitis C Cohort Study. Excellent outcome of direct antiviral treatment for chronic
hepatitis C in Switzerland. Swiss Med Wkly. 2018 Jan;148:w14560.
17. Béguelin C, Suter A, Bernasconi E, Fehr J, Kovari H, Bucher HC, et al.; Swiss HIV
Cohort Study. Trends in HCV treatment uptake, efficacy and impact on liver fibrosis
in the Swiss HIV Cohort Study. Liver Int. 2018 Mar;38(3):424–31. 10.1111/liv.13528
18. Scotto R, Buonomo AR, De Pascalis S, Nerilli M, Pinchera B, Staiano L, et al. Changing
epidemiology of patients treated with direct acting antivirals for HCV and persistently
high SVR12 in an endemic area for HCV infection in Italy: real-life ‘LIver Network
Activity’ (LINA) cohort update results. Expert Rev Gastroenterol Hepatol. 2021 Sep;15(9):1057–63.
10.1080/17474124.2021.1890029
19. Prasad L, Spicher VM, Zwahlen M, Rickenbach M, Helbling B, Negro F; Swiss Hepatitis
C Cohort Study Group. Cohort Profile: the Swiss Hepatitis C Cohort Study (SCCS). Int
J Epidemiol. 2007 Aug;36(4):731–7. 10.1093/ije/dym096
20. Arias A, Aguilera A, Soriano V, Benítez-Gutiérrez L, Lledó G, Navarro D, et al. Rate
and predictors of treatment failure to all-oral HCV regimens outside clinical trials.
Antivir Ther. 2017;22(4):307–12. 10.3851/IMP3061
21. Chen CY, Huang CF, Cheng PN, Tseng KC, Lo CC, Kuo HT, et al. Factors associated with
treatment failure of direct-acting antivirals for chronic hepatitis C: A real-world
nationwide hepatitis C virus registry programme in Taiwan. Liver Int. 2021 Jun;41(6):1265–77.
10.1111/liv.14849
22. Feld JJ, Maan R, Zeuzem S, Kuo A, Nelson DR, Di Bisceglie AM, et al. Effectiveness
and Safety of Sofosbuvir-Based Regimens for Chronic HCV Genotype 3 Infection: results
of the HCV-TARGET Study. Clin Infect Dis. 2016 Sep;63(6):776–83. 10.1093/cid/ciw387
23. Buggisch P, Vermehren J, Mauss S, Günther R, Schott E, Pathil A, et al. Real-world
effectiveness of 8-week treatment with ledipasvir/sofosbuvir in chronic hepatitis
C. J Hepatol. 2018 Apr;68(4):663–71. 10.1016/j.jhep.2017.11.009
24. Daniel KE, Saeian K, Rizvi S. Real-world experiences with direct-acting antiviral
agents for chronic hepatitis C treatment. J Viral Hepat. 2020 Feb;27(2):195–204. 10.1111/jvh.13218
25. Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al.; ASTRAL-4
Investigators. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis.
N Engl J Med. 2015 Dec;373(27):2618–28. 10.1056/NEJMoa1512614
26. Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, et al.; SOLAR-1
Investigators. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection
in Patients With Advanced Liver Disease. Gastroenterology. 2015 Sep;149(3):649–59.
10.1053/j.gastro.2015.05.010
27. Prenner SB, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, Kulik L. Hepatocellular
carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting
antivirals. J Hepatol. 2017 Jun;66(6):1173–81. 10.1016/j.jhep.2017.01.020
28. Beste LA, Green PK, Berry K, Kogut MJ, Allison SK, Ioannou GN. Effectiveness of hepatitis
C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma.
J Hepatol. 2017 Jul;67(1):32–9. 10.1016/j.jhep.2017.02.027
29. Radhakrishnan K, Di Bisceglie AM, Reddy KR, Lim JK, Levitsky J, Hassan MA, et al. Treatment
Status of Hepatocellular Carcinoma Does Not Influence Rates of Sustained Virologic
Response: an HCV-TARGET Analysis. Hepatol Commun. 2019 Aug;3(10):1388–99. 10.1002/hep4.1412
30. Ji F, Yeo YH, Wei MT, Ogawa E, Enomoto M, Lee DH, et al. Sustained virologic response
to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular
carcinoma: A systematic review and meta-analysis. J Hepatol. 2019 Sep;71(3):473–85.
10.1016/j.jhep.2019.04.017
31. Childs K, Davis C, Cannon M, Montague S, Filipe A, Tong L, et al. Suboptimal SVR rates
in African patients with atypical genotype 1 subtypes: implications for global elimination
of hepatitis C. J Hepatol. 2019 Dec;71(6):1099–105. 10.1016/j.jhep.2019.07.025
32. Fourati S, Rodriguez C, Hézode C, Soulier A, Ruiz I, Poiteau L, et al. Frequent Antiviral
Treatment Failures in Patients Infected With Hepatitis C Virus Genotype 4, Subtype
4r. Hepatology. 2019 Feb;69(2):513–23. 10.1002/hep.30225
33. Nguyen D, Smith D, Vaughan-Jackson A, Magri A, Barnes E, Simmonds P; STOP-HCV Consortium.
Efficacy of NS5A inhibitors against unusual and potentially difficult-to-treat HCV
subtypes commonly found in sub-Saharan Africa and South East Asia. J Hepatol. 2020 Oct;73(4):794–9.
10.1016/j.jhep.2020.05.029
34. Cottagnoud S, Mathieu A, Perreau M, Moradpour D, Fraga M. Out of Africa: Hepatitis
C virus subtype 4r as troublemaker. Schweiz Med Forum 2019;149 [Suppl 240]:22S)
35. Heim MH. 25 years of interferon-based treatment of chronic hepatitis C: an epoch coming
to an end. Nat Rev Immunol. 2013 Jul;13(7):535–42. 10.1038/nri3463
36. Graf C, D’Ambrosio R, Degasperi E, Paolucci S, Llaneras J, Vermehren J, et al. Real-world
effectiveness of voxilaprevir/velpatasvir/sofosbuvir in patients following DAA failure.
JHEP Rep Innov Hepatol. 2024 Feb;6(3):100994. 10.1016/j.jhepr.2023.100994
37. Howe AY, Rodrigo C, Cunningham EB, Douglas MW, Dietz J, Grebely J, et al.; SHARED
Collaborators. Characteristics of hepatitis C virus resistance in an international
cohort after a decade of direct-acting antivirals. JHEP Rep Innov Hepatol. 2022 Feb;4(5):100462.
10.1016/j.jhepr.2022.100462
38. Popping S, Cento V, Seguin-Devaux C, Boucher CA, de Salazar A, Heger E, et al. The
European Prevalence of Resistance Associated Substitutions among Direct Acting Antiviral
Failures. Viruses. 2021 Dec;14(1):16. 10.3390/v14010016
39. Baumann L, Braun DL, Cavassini M, Stoeckle M, Bernasconi E, Schmid P, et al. Long-term
trends in hepatitis C prevalence, treatment uptake and liver-related events in the
Swiss HIV Cohort Study. Liver Int. 2024 Jan;44(1):169–79. 10.1111/liv.15754
Appendix
Table S1Characteristics of patients who experienced a second DAA failure.
| Patient |
Geno-type |
Cirrhosis |
Active HCC |
Previous IFN-based
treatment |
1st DAA
treatment |
2nd DAA
treatment |
3rd DAA
treatment |
SVR after 3rd
DAA treatment |
RAS analysis after
1st DAA treatment |
RAS analysis after
2nd DAA treatment |
| 1 |
3 |
Yes |
No |
PegIFN + RBV |
DCV/SOF + RBV |
VEL/SOF + RBV |
None1 |
NA |
NS5A: Y93H |
NS5A: Y93H |
| 2 |
1a |
Yes |
Yes |
PegIFN + RBV |
VEL/SOF + RBV |
VEL/SOF |
None2 |
NA |
NS5A: Q30R/L31M |
NA |
| 3 |
4 |
Yes |
No |
PegIFN + RBV |
PTV/r/OBV + RBV |
SOF + RBV |
VOX/VEL/SOF |
Yes |
NA |
NS5A: M28V |
| 4 |
4 |
Yes |
No |
IFN |
LDV/SOF |
SOF + RBV up to LT |
VOX/VEL/SOF + RBV
after LT |
Yes |
NS5A:L28F/Q30R/Y93H |
NA |
| 5 |
3 |
F3 |
No |
No |
DCV + SOF |
VEL/SOF + RBV |
VOX/VEL/SOF3 |
No |
NS5A: Y93H |
NA |
| 6 |
3 |
Yes |
No |
PegIFN + RBV |
SOF + RBV |
VEL/SOF + RBV |
VOX/VEL/SOF |
Yes |
NA |
NA |
| 7 |
1a |
Νο |
No |
PegIFN + RBV |
LDV/SOF |
SMV + SOF + RBV |
VOX/VEL/SOF |
Yes |
NA |
NA |
| 8 |
2 |
Νο |
No |
No |
SOF + RBV |
VEL/SOF |
VOX/VEL/SOF |
Yes |
NA |
NA |
| 9 |
2 |
Νο |
No |
No |
SOF + RBV |
VEL/SOF + RBV |
VOX/VEL/SOF |
Yes |
No RAS |
NA |
| 10 |
1b |
F3 |
No |
PegIFN + RBV |
LDV/SOF |
VEL/SOF + RBV |
VOX/VEL/SOF + RBV4 |
No |
NS5A: Y93H |
NS5A: Y93H/L31M |
| 11 |
1a |
No |
No |
PegIFN + RBV |
GZR/EBR |
VOX/VEL/SOF |
NA |
Third-line
treatment during study period |
NS5A: no RAS |
NS5A: no RAS |
| 12 |
3a |
Yes |
No |
Peg-IFN + RBV |
SOF+RBV |
DCV/SOF + RBV |
VOX/VEL/SOF |
Yes |
NA |
NS5A: A30K |
| 13 |
3h |
Yes |
No |
PegIFN + RBV |
SOF/DCV+RBV |
VEL/SOF + RBV |
VOX/VEL/SOF |
Third-line
treatment during study period |
NS5A: no RAS |
NA |

