Prognostic value
of KRAS G12C in advanced non-small cell lung cancer with high PD-L1 expression
treated with upfront immunotherapy: a systematic review and meta-analysis
DOI: https://doi.org/https://doi.org/10.57187/s.3695
Caroline-Claudia
Erharta,
Marco Cefalìb,
Dylan Manganc,
Benjamin Kasendad*,
Luciano Wannessonae*
a Facoltà di Scienze Biomediche, Università
della Svizzera Italiana (USI), Lugano, Switzerland
b Istituto Oncologico della Svizzera
Italiana (IOSI), Bellinzona, Switzerland
c Division of Population Health,
University of Manchester, Manchester, United Kingdom
d Medical Oncology, University
Hospital Basel, Basel, Switzerland
e Centro Oncológico del Nordeste (CONEA),
Resistencia, Argentina
* Contributed
equally
Summary
AIM: This study aims to evaluate the prognostic role of the KRAS G12C mutation
in patients with advanced non-small cell lung cancer and PD-L1 expression ≥50% who
are treated with immune checkpoint inhibitor monotherapy.
METHODS: We conducted a systematic review of clinical studies fulfilling
the following criteria: (1) enrolling patients with advanced/metastatic non-small
cell lung cancer with high PD-L1 tumour expression receiving first-line therapy
with anti-PD-(L)1 immune checkpoint inhibitors; (2) comparing the outcomes of patients
with the KRAS G12C mutation to those without this mutation, and (3) reporting
overall survival and progression-free survival (PFS). The electronic databases
Medline, EMBASE, Cochrane and Google Scholar, along with reference lists, were systematically
searched.
RESULTS: We identified four publications that fulfilled the
inclusion criteria, comprising a total of 469 patients. Of these, two studies
reported hazard ratios (HR) for PFS, resulting in a final
pooled patient sample of 163 for the meta-analysis. In patients with non-small
cell lung cancer who received anti-PD-(L)1 monotherapy, the presence of a KRAS G12C
mutation was associated with improved PFS compared to patients with KRAS wild-type
tumours, with a pooled hazard ratio of 0.39 and a 95% Confidence Interval (CI)
of 0.25–0.63. Among all patients with KRAS mutations, those harbouring a KRAS G12C
mutation had improved PFS compared to patients with any other KRAS mutation (pooled
HR 0.33, 95% CI 0.19–0.57).
CONCLUSIONS: Patients with non-small cell lung cancer who have
the KRAS G12C mutation and high PD-L1 expression demonstrate favourable PFS with
first-line PD-(L)1 immune checkpoint inhibitor monotherapy
compared to patients with KRASwt or other KRAS mutations and high PD-L1
expression.
Abbreviations
- KRAS mutation
-
all KRAS mutations
- KRASwt
-
KRAS wild-type
- KRAS others
-
all other mutations except G12C
- non
G12C
-
KRAS others and KRASwt
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, accounting
for an estimated 1.80 million deaths in 2020 [1]. New therapeutic options for
patients with metastatic non-small cell lung cancer (NSCLC) have significantly improved
survival outcomes [2]. According to current guidelines, the selection of
first-line therapy is based on histological subtyping, molecular analysis, and
the expression of biomarkers that are predictive of immunotherapy response.
These biomarkers include epidermal growth factor receptor (EGFR), B-raf murine
sarcoma viral oncogene homolog B1 (BRAF), mesenchymal-epithelial transition
factor (MET), and gene fusions involving anaplastic lymphoma kinase (ALK),
c-ros oncogene 1 (ROS1), rearranged during transfection (RET), or neurotrophic
tyrosine receptor kinase (NTRK) 1, 2 and 3, as well as the expression level of programmed
death ligand 1 (PD-L1) on tumour cells [3–5].
The KEYNOTE-024 trial established pembrolizumab as the first-line treatment
for metastatic non-small cell lung cancer with a PD-L1 expression ≥50%, demonstrating
improved outcomes in terms of overall survival (OS) and progression-free
survival (PFS) compared to platinum-based chemotherapy [6]. Conversely, the KEYNOTE-189
and KEYNOTE-407 phase III clinical trials, involving patients with non-squamous
and squamous non-small cell lung cancer, respectively, established the
combination of chemotherapy and pembrolizumab independently of PD-L1 expression
level, thus becoming the standard treatment for patients with PD-L1 <50% [7,8].
Several other biomarkers have been proposed to guide better treatment decision-making,
including tumour mutational burden (TMB) and tumour infiltration by immune
cells [9, 10]. However, these biomarkers are rarely included in clinical
practice guidelines and are not routinely tested in all institutions [6–8].
The presence of specific driver alterations has been shown to reduce the
likelihood of response to immunotherapy, despite high PD-L1 expression. This
phenomenon can partially be explained by the fact that most driver alterations
occur among non-smokers, who often present with a disease characterised by low TMB
[11–13].
Mutations in KRAS are the most commonly reported in lung adenocarcinoma (20–25%
of cases), with the KRAS G12C variant constituting the majority [14]. At
present, the KRAS G12C variant is the only one for which targeted treatments
are available, namely sotorasib and adagrasib [15, 37]. Consequently, clarifying
the association between KRAS G12C and the efficacy of immune checkpoint
inhibitor (ICI) therapy is essential to evaluate the best combination,
integration and sequencing of treatment strategies. We conducted this
systematic review and meta-analysis to summarise the current evidence on the
prognostic role of the KRAS GC12 mutation in patients receiving first-line
treatment with checkpoint inhibitors.
Materials and methods
We aimed to summarise and assess published evidence on the prognostic
value of the KRAS G12C mutation in terms of progression-free survival and overall
survival in patients with advanced/metastatic lung cancer and PD-L1 expression
≥50% receiving their first systemic treatment with immune checkpoint inhibitor monotherapy.
Literature search
We systematically searched Medline, EMBASE, the Cochrane database and Google
Scholar from July to September 2022. Reference lists were manually checked to
identify additional studies. Named electronic databases were systematically
searched. The search performed in the MESH database from the National Institute
of Health used the terms (“KRAS protein, human” [Supplementary Concept]) AND “Immune
Checkpoint Inhibitors” [Mesh], yielding 32 articles, of which three were
selected. The search terms “G12C AND immunotherapy AND lung” retrieved 51
results, among which five met the selection criteria. Another search term
combination, “G12C AND other mutations AND pembrolizumab”, led to two results,
one of which was eligible for inclusion. Further searches using combinations of
KRAS, G12C, variants, immune checkpoint inhibitor (ICI), progression-free
survival, PFS, overall survival, OS, non-small cell lung cancer (NSCLC), PD-L1,
high-expression, pembrolizumab, nivolumab, cemiplimab, atezolizumab, durvalumab
and avelumab did not alter the number of included publications.
Studies were included if they met the following inclusion criteria: (1) patients
with advanced non-small cell lung cancer and PD-L1 ≥50%; (2) upfront single-agent
therapy with checkpoint inhibitors (pembrolizumab, nivolumab, cemiplimab, atezolizumab,
durvalumab or avelumab); and (3) presence of the KRAS G12C mutation. The
detailed search strategies are listed in figure 1, and the PICO process used to
develop a focused search strategy is shown in table S1 in the appendix.
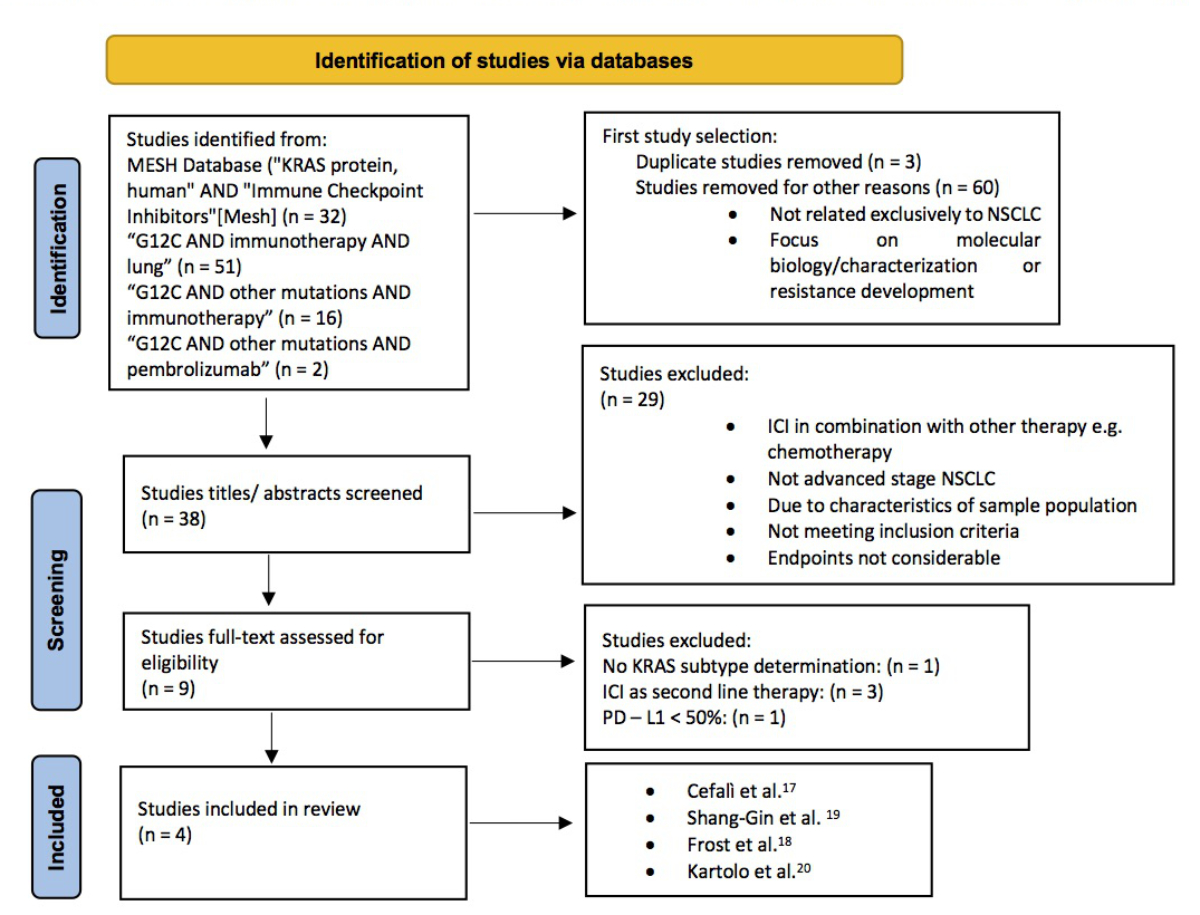
Figure 1Detailed
search strategies according to the PRISMA 2020 flow diagram (http://www.prisma-statement.org;
Page MJ, McKenzie JE, Bossuyt PM,
Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated
guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:
10.1136/bmj.n71).
Article selection
criteria
An initial screening was conducted to select all relevant publications
concerning patients with advanced non-small cell lung cancer, KRAS mutation and
first-line immunotherapy treatment. Studies that included treatments other than
single-agent immunotherapy were excluded. The second screening focused on
articles reporting diagnosis, age, PD-L1 expression status, KRAS mutation
status and subtype, type of immunotherapy used, progression-free survival
and/or overall survival. All study designs from any source (peer-reviewed
journals, non-peer-reviewed sources or scientific meeting abstracts) were
considered, provided they contained complete information as previously defined.
For studies with overlapping patient populations, only the most comprehensive publications
were included.
Data extraction
and quality assessment
Two investigators, Luciano Wannesson and Caroline Erhart, independently
extracted data and then compared and merged it. A third investigator, Benjamin
Kasenda, reviewed the results. Extracted data included study name, authors,
year of publication, sample size, patient characteristics, disease stage, PD-L1
status, KRAS mutation status and subtype, treatment, progression-free survival,
overall survival, and, if available, hazard ratios (HRs) of progression-free
survival and overall survival. The quality of the studies was evaluated using the
“Risk of Bias in Non-randomized Studies of Exposure (ROBINS-E 2022)” [16] (figure
S1 in the appendix). For this systematic review, data were synthesised into a tabulation
of characteristics and outcomes. Missing data were represented by the
abbreviation “NA”.
Outcome measures
and statistical methods
After
selecting suitable studies, data were extracted and entered into standardised Excel
spreadsheets. The
endpoints considered were progression-free survival and overall survival. We
aimed to conduct two separate comparisons: patients with a KRAS G12C mutation
versus those with no KRAS mutation (KRASwt), and patients with a KRAS G12C
mutation versus those with other KRAS mutations (any KRAS mutation except
G12C).
One of the publications was a previous study from the same group (IOSI),
allowing us to calculate the hazard ratio (HR) based on datasets still
available to us [17]. We pooled the aggregated HR with the HR reported in the
other publications and created forest plots
using the statistical program R version 4.1.2(2021-11-01) with the
statistical package meta. We analysed the heterogeneity between studies using
the I2 statistic.
Results
Search and
selection process
After screening and full-text appraisal, we included four studies (figure
1, table 1). All four studies received no funding [17–20]. The median age of
patients ranged from 65 to 69 years. The distribution of gender, smoking status
and Eastern Cooperative Oncology Group (ECOG) performance status was relatively
similar across the studies. Patients were stratified according to their PD-L1
expression status in all trials, and progression-free survival and/or overall
survival was reported in all papers.
Two studies fully reported the hazard ratio [17, 18]. In contrast, the
other two studies had insufficient data to determine the HR of progression-free
survival among KRAS G12C, KRASwt
or other KRAS mutations [19, 20].
Table 1Baseline characteristics of the study populations.
| Author |
Cefalì
et al. [17] |
Frost
et al. [18] |
Shang-Gin
et al. [19] |
Kartolo
et al. [20] |
| Country
of origin |
Switzerland |
Germany |
Taiwan |
Canada |
| Patients,
n |
44 |
119 |
228 |
78 |
| Median
age |
69 |
68 |
66 |
>65 |
| Male, n
(%) |
25 (57%) |
68 (57%) |
159 (69%) |
37 (47%) |
| Smoker, n
(%) |
42 (95) |
98 (82) |
144 (63) |
74 (94) |
| KRAS
mutation, n |
25 |
62 |
228 |
30 |
| KRAS G12C
mutation, n (%) |
11 (44) |
32 (51) |
143 (63) |
11 (37) |
| Hazard
ratio (progression-free survival) KRAS G12C vs KRAS other (95% CI [p-value]) |
0.21
(0.06;0.72 [0.01]) |
0.37 (0.20;0.68 [0.01]) |
NA |
NA |
| Hazard
ratio (progression-free survival) KRAS G12C vs KRASwt (95 CI [p-value]) |
0.33
(0.12;0.91 [0.03]) |
0.41
(0.24; 0.69 [0.01]) |
NA |
NA |
| Included
in meta-analysis |
Yes |
Yes |
No |
No |
Narrative summary
of reported endpoints
Three of the four studies included in the systematic review suggested
that KRAS G12C mutations were associated with a better response to
immunotherapy among patients with PD-L1 expression ≥50%. Table 2 summarises the
endpoints of these studies.
Cefalì et al. identified that KRAS G12C was associated with better progression-free
survival compared to other KRAS mutations in non-small cell lung cancer
patients with PD-L1 ≥50% [17] (HR 0.27; 95% CI 0.1–0.76, p
= 0.01). In the same study, a second analysis compared PFS in patients with
any KRAS mutation versus those with wild-type KRAS gene status. The trend
towards better PFS in the KRAS-mutated subgroup was not statistically
significant (log-rank χ2(1) = 1.8, p =
0.18) [17].
A study conducted in Taiwan by Shang-Gin et al. concluded that the G12C
mutation was associated with improved immune checkpoint inhibitor treatment
effectiveness in patients with non-small cell lung cancer. For the 143 patients
with advanced-stage non-small cell lung cancer, overall survival was
significantly different between patients with the KRAS G12C mutation and those
with other KRAS mutations (7.7 months versus 6.0 months, respectively; p = 0.018).
Notably, the KRAS G12C
subgroup had a higher proportion of male individuals (80%; p = 0.018) and smokers
(81.3%; p <0.001) [19].
Another study supporting the favourable role of KRAS G12C was conducted
in Germany. Frost et al. demonstrated that KRAS subtypes and TP53 mutations differentiate
between prognostic groups (HR 0.23; 95% CI, 0.08–0,72, p = 0.01; KRAS G12C/TP53 mutant
cases against KRAS others and
TP53 wt cases) [18]. Notably, the KRAS G12C/TP53 co-mutation was frequently
associated with high PD-L1 expression [18].
In contrast, Kartolo et al. did not find a positive prognostic effect of
KRAS G12C [20]. There was no significant difference in median overall survival
between KRAS mutant and KRASwt patients (12.9 vs 19.3 months, p = 0.879). There was
a
non-significant trend towards worse outcomes in KRAS G12C cases compared to
KRAS others and KRASwt (progression-free survival 3.3 vs 8.1 vs 5.4 months, p =
0.442; and overall survival 11.4 vs 44.9 vs 19.3 months, p = 0.772). The study
population was characterised by older age and included a significant percentage
of smokers, as well as a higher proportion of patients with worse ECOG
performance status [20].
Table 2Efficacy endpoints. Progression-free survival in months.
| References |
Cefalì
et al. |
Cefalì
et al. [17]retrieved data |
Frost
et al. [18] |
Shang-Gin
et al. [19] |
Kartolo
et al. [20] |
| No. of
patients |
44 |
44 |
119 |
228 |
78 |
| No. of
KRAS G12C |
11 |
11 |
32 |
143 |
11 |
| Median
age |
69 |
69 |
68 |
55 |
>65 |
| Design |
Retrospective |
Retrospective |
Retrospective |
Retrospective |
Retrospective |
| Median
overall survival KRAS G12C (months) |
Not
evaluable |
|
Not
evaluable |
7.7 |
12.9 |
| Median
overall survival KRAS others (months) |
14.7 |
|
18.9 |
6.0 |
19.3 |
| Median progression-free
survival KRAS G12C (months) |
14.6 |
|
19.8 |
4.8 |
3.3 |
| Median progression-free
survival non-G12C (months) |
12.5 |
|
NA |
NA |
NA |
| Hazard ratio for progression-free
survival KRAS G12C vs KRAS non-G12C |
0.27 |
|
NA |
NA |
NA |
| 95% CI
(p-value) |
0.1–0.76
(0.01) |
|
|
|
|
| Median progression-free
survival KRAS mutation (months) |
8.6 |
|
13.3 |
NA |
6.0 |
| Median progression-free
survival KRASwt (months) |
6.0 |
6.0 |
6.2 |
NA |
5.4 |
| Hazard ratio for KRAS mutation vs KRASwt |
0.46 |
|
0.66 |
NA |
1.184 |
| 95% CI
(p-value) |
0.22–0.95
(0.04) |
|
0.44–1.0
(0.05) |
|
0.571–2.455
(0.651) |
| Hazard ratio for KRAS G12C vs KRASwt |
NA |
0.33 |
0.41 |
NA |
NA |
| 95% CI
(p-value) |
|
0.12–0.91
(0.03) |
0.24–0.69
(0.001) |
|
|
| Median progression-free
survival KRAS others |
6.5 |
6.5 |
5.8 |
2.1 |
8.1 |
| Hazard
ratio for KRAS G12C vs KRAS others |
NA |
0.21 |
0.37 |
NA |
NA |
| 95% CI
(p-value) |
|
0.06–0.72
(0.01) |
0.20–0.68
(0.01) |
|
|
Study level
meta-analysis
Only two of the four identified studies qualified for the study-level meta-analysis.
We reused unpublished data from the study by Cefalì et al. to calculate the HR for
comparing the KRAS G12C positive group, KRASwt and other KRAS mutations. This enabled
us to pool the data from Frost et al. and Cefalì et al., resulting in the
forest plots shown in figures 2A and 2B.
The first forest plot indicates that a KRAS G12C mutation is associated
with improved progression-free survival compared to KRASwt tumours. The second
forest plot shows that a KRAS G12C mutation is associated with better
progression-free survival than other KRAS mutations.
In contrast, Shang-Gin et al. provided the hazard ratio for overall
survival for KRAS G12C versus other KRAS mutations but not for KRAS G12C versus
KRASwt. Kartolo et al. only reported the HR for progression-free survival when comparing
the KRAS mutant and KRAS wild-type groups.
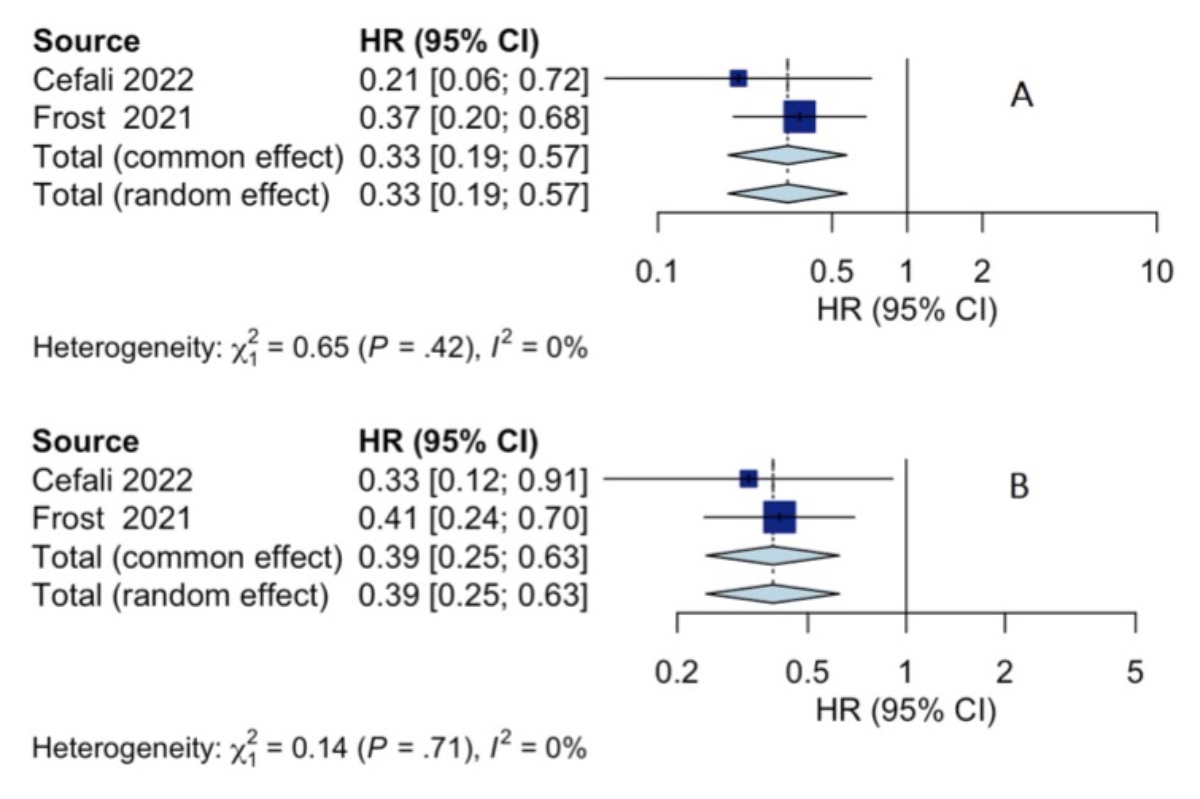
Figure 2Forest plot for KRAS G12C vs KRAS others (progression-free
survival) (A); forest plot for KRAS G12C vs KRASwt (progression-free survival)
(B)
Discussion
Our systematic review and meta-analysis suggest that patients with
advanced non-small cell lung cancer harbouring a KRAS G12C mutation and high
PD-L1 expression have a favourable prognosis when receiving first-line
immunotherapy with a checkpoint inhibitor. However, the predictive role of the KRAS
G12C mutation – specifically, whether patients with this mutation and high
PD-L1 expression benefit more from checkpoint inhibitors compared to
chemotherapy – could not be evaluated due to the absence of interaction
analysis in the identified publications. Overall, the quality of evidence is
relatively low, and most studies lack sufficient reporting to derive definitive
conclusions.
Due to its structural and biochemical properties, the KRAS protein has
long been considered an “undruggable” target. The main challenges include its
high affinity for GTP, limited active binding sites and the complexity of its
downstream pathways. Additionally, there is little structural difference
between wild-type and mutant KRAS, making it difficult to target the mutant
form without affecting the normal protein [21, 26]. However, breakthrough technological advances
led to the discovery of a small inhibitory molecule (ARS-1620) capable of
binding exclusively to a site near the effector region of mutant KRAS G12C that
is not present in wild-type KRAS [22, 23]. Since this initial breakthrough,
several other small inhibitory molecules have been discovered. Sotorasib is the
first KRAS G12C inhibitor to demonstrate sustained clinical benefit in patients
with pre-treated non-small cell lung cancer harbouring a KRAS G12C mutation
[24, 25]. Based on preclinical data, a phase I/II study assessed the safety,
tolerability, pharmacokinetics and efficacy of sotorasib monotherapy in heavily
pretreated patients with locally advanced or metastatic KRAS G12C mutant solid tumours
[27].
Recent research suggests that KRAS mutations are correlated with an
inflammatory tumour microenvironment and increased immunogenicity, providing a
rationale for their superior response to PD-(L)1 inhibitors [28]. Therefore, several
clinical trials have analysed the efficacy of anti-PD-(L)1 immunotherapy in
KRAS-mutant non-small cell lung cancer. These studies indicate that patients
with KRAS mutations are more sensitive to PD-(L)1 inhibitors than those with
wild-type KRAS [29–32]. However, a systematic investigation of the prognostic
and predictive roles of the KRAS G12C mutation has not yet been conducted. Thus,
this systematic review focused exclusively on the potential prognostic and
predictive roles of the KRAS G12C mutation in relation to anti-PD-(L)1 therapy.
To avoid potential selection bias, we evaluated several relevant papers not
included in the final analysis [33–36] (table S2 in the appendix). The main
reasons for exclusion were that these papers did not focus exclusively on PD-L1
expression ≥50% and/or first-line immune checkpoint inhibitors. Moreover, studies
were excluded if they analysed KRAS mutations in general rather than specifically
concentrating on the KRAS G12C mutation.
A study conducted in Italy separated the study population of 22 patients
into two groups based on first-line (1L) and second-line (2L) immune checkpoint
inhibitor therapy [33]. In the 1L group, the median progression-free survival for
KRAS G12C mutated patients was 20 months, compared to 14.5 months for
non-KRAS G12C mutated patients (p
= 0.76) [33]. In the 2L group, better outcomes were observed in
patients with a KRAS G12C mutation compared to non-G12C, with median progression-free
survival reaching 23 months compared to only five months (p = 0.03) [33]. These results
support
our findings, considering that our study population only included first-line immune
checkpoint inhibitor therapy. This study was excluded from the analysis due to
the lack of information about PD-L1 expression [33].
In contrast, a large German prospective study using the CRISP registry
found no prognostic value for KRAS G12C [34]. This study was excluded because it
did not exclusively focus on immune checkpoint inhibitor-based first-line
therapy. The authors recruited patients with advanced non-small cell lung
cancer and KRAS mutations. Within the study population, 15.4% of patients had KRAS
G12C, 24.2% had non-G12C mutations and 60.4% had KRASwt. High PD-L1 expression,
defined as Tumor Proportion Score (TPS) >50%, was documented for each
subgroup at 43.5%, 28.9%, and 28.0%, respectively. Meanwhile, 89.3%, 87.7% and
68.8%, respectively, received first-line treatment combined with an immune checkpoint
inhibitor [34]. There were no differences in clinical outcomes between KRAS
G12C, other KRAS mutations and KRASwt. Interestingly, patients with G12C
mutations tended to have higher PD-L1 expression and were more often treated
with immune checkpoint inhibitors. This highlights the need for more extensive analyses
of patients stratified by their respective treatments to definitively elucidate
the prognostic role of PD-L1 in KRAS G12C mutant non-small cell lung cancer
[34].
A study conducted by Arbour et al. [35] involving 1,194 patients
with non-small cell lung cancer harbouring a KRAS mutation reported that, in
the subgroup with PD-L1 expression ≥50%, the median progression-free survival
for patients with KRAS G12C was 4.7 months compared with 14.4 months for
patients with non-G12C mutations (p = 0.07) [35]. Contrary to our findings,
they hypothesised a negative impact of KRAS G12C on progression-free survival
under immune checkpoint inhibitor therapy. Potential limitations of this study include
the heterogeneity of the population, particularly the conflation of first- and
second-line data without stratification [35].The statistical
analysis did not account for the balance between first- and second-line therapy
with immune checkpoint inhibitors, possibly contributing to the observed negative
impact of KRAS G12C.
Finally, Jeanson et al. analysed a French cohort of 282 patients with
advanced non-small cell lung cancer harbouring KRAS mutations without focusing
exclusively on the KRAS G12C mutation. No significant differences in response
rate, progression-free survival or overall survival were observed between the
KRAS subgroups [36]. Notably, only 9% of the patient population had PD-L1
expression ≥50%. Nevertheless, a significant trend towards improved progression-free
survival was observed in KRAS mutant NSCLC with PD-L1-positive versus PD-L1-negative
tumours, with increased benefit correlating with a higher proportion of PD-L1-positive
tumour cells (≥50%). This
association between PD-L1 expression and outcomes with immune checkpoint
inhibitors was not observed in NSCLC without KRAS mutations, suggesting that
PD-L1 overexpression is even more relevant in KRAS-mutant NSCLC [36]. Hence,
this finding strengthens our hypothesis that patients with a KRAS G12C mutation
and high PD-L1 expression benefit more from upfront immunotherapy.
Recent research is beginning to reveal the effect of co-mutations on tumour
biology and response to different therapeutic strategies [18, 37–39].
Co-mutations are significant because recent findings indicate that treatment
with KRAS G12C inhibitors (such as sotorasib) can trigger the development of
co-mutations, thereby compromising the effectiveness of immune checkpoint
inhibitors (anti-PD1/PDL1) [40].
Data from a Spanish study suggest that the most frequent KRAS
co-mutations are in TP53 (39%), serine/threonine kinase 11 (STK11) (20%) and
kelch-like ECH-associated protein 1 (KEAP1) (13%) [37]. Interestingly, the
study by Frost et al. found significantly better outcomes in patients receiving
first-line immune checkpoint inhibitors who harboured a KRAS G12C/TP53
co-mutation [18].Another study by Assoun et al. (2019) hypothesised
that TP53 mutational status may correlate with response to immune checkpoint
inhibitors and suggested a synergistic interaction between PD-L1 expression,
KRAS mutation, TMB and TP53 mutation. Their study population included non-small
cell lung cancer patients treated with immune checkpoint inhibitors in the
first line and subsequent lines of therapy. Their data showed that a
TP53-mutated status predicted an overall survival benefit in advanced NSCLC
treated with immunotherapy [13].
Further investigation is necessary to clarify the influence of
co-mutations in KRAS G12C-positive non-small cell lung cancer [41], identify the
optimal combination of predictive biomarkers for immune checkpoint inhibitor
therapies and reevaluate and improve the current therapy allocation process. Moreover,
new pan-KRAS inhibitors are under investigation and may offer broader
therapeutic options because they do not distinguish between different KRAS
mutants [42].
Conclusion
In conclusion, preliminary evidence suggests that the presence of a KRAS
G12C mutation is associated with a favourable prognosis in patients with
advanced non-small cell lung cancer and high PD-L1 expression treated with
upfront immunotherapy. However, this observation needs validation in additional,
well-designed studies. Future treatment sequencing or combination strategies
may be explored based on these results.
Acknowledgments
Author contributions: Caroline-Claudia Erhart: Conceptualization; Data curation;
Investigation; Writing – original draft; Writing – review and editing. Marco
Cefalì: Investigation; Writing – review and editing; Supervision. Dylan Mangan:
Formal analysis; Writing – review and editing. Benjamin Kasenda: Formal
analysis; Writing – review and editing; Visualization. Luciano Wannesson:
Conceptualization; Supervision; Writing – review and editing.
Caroline-Claudia
Erhart
Facoltà di
Scienze Biomediche
Università della
Svizzera Italiana (USI)
CH-6900 Lugano
caroline-claudia.erhart[at]usi.ch
References
1. WHO: Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer#:~:text=lung%20(1.80%20million%20deaths)%3B,rectum%20(916%20000%20deaths)%3B
2. Elkrief A, Joubert P, Florescu M, Tehfe M, Blais N, Routy B. Therapeutic landscape
of metastatic non-small-cell lung cancer in Canada in 2020 [Erratum in: Curr Oncol.
2020 Jun;27] [3] [:e349. PMID: 32218661; PMCID: PMC7096203]. Curr Oncol. 2020 Feb;27(1):52–60.
10.3747/co.27.5953
3. Annals of Oncology 29 (Supplement 4): iv192–iv237, 2018. doi:10.1093/annonc/mdy275, Published online 3 October 2018; updated 26 January 2019
4. National Comprehensive Cancer Network. Non-Small cell lung cancer (Version 3.2022).
https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed July 27, 2022.
5. Hanna NH, Robinson AG, Temin S, Baker S Jr, Brahmer JR, Ellis PM, et al. Therapy for
Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint
Guideline Update. J Clin Oncol. 2021 Mar;39(9):1040–91. 10.1200/JCO.20.03570
6. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated Analysis
of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell
Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019 Mar;37(7):537–46.
10.1200/JCO.18.00149
7. Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed
plus platinum with or without pembrolizumab in patients with previously untreated
metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189.
Ann Oncol. 2021 Jul;32(7):881–95. 10.1016/j.annonc.2021.04.008
8. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A
Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients
With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407.
J Thorac Oncol. 2020 Oct;15(10):1657–69. 10.1016/j.jtho.2020.06.015
9. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al.; ESMO Guidelines
Committee. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up [Erratum in: Ann Oncol. 2019 May;30] [5] [:863-870.
PMID: 30285222]. Ann Oncol. 2018 Oct;29 Suppl 4:iv192–237. 10.1093/annonc/mdy275
10. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-Small
Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J
Natl Compr Canc Netw. 2022 May;20(5):497–530. 10.6004/jnccn.2022.0025
11. Assoun S, Theou-Anton N, Nguenang M, Cazes A, Danel C, Abbar B, et al. Association
of TP53 mutations with response and longer survival under immune checkpoint inhibitors
in advanced non-small-cell lung cancer. Lung Cancer. 2019 Jun;132:65–71. 10.1016/j.lungcan.2019.04.005
12. Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with
uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade
in non-small cell lung cancer. OncoImmunology. 2017 Jul;6(11):e1356145. 10.1080/2162402X.2017.1356145
13. Dantoing E, Piton N, Salaün M, Thiberville L, Guisier F. Anti-PD1/PD-L1 Immunotherapy
for Non-Small Cell Lung Cancer with Actionable Oncogenic Driver Mutations. Int J Mol
Sci. 2021 Jun;22(12):6288. 10.3390/ijms22126288
14. Tao L, Miao R, Mekhail T, Sun J, Meng L, Fang C, et al. The prognostic value of KRAS
mutation subtypes and PD-L1 expression in patients with lung adenocarcinoma. Clin
Lung Cancer. 2020; 10.1016/j.cllc.2020.07.00
15. Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, et al. Adagrasib in Non-Small-Cell
Lung Cancer Harboring a KRASG12C Mutation. N Engl J Med. 2022 Jul;387(2):120–31. 10.1056/NEJMoa2204619
16. ROBINS-E Development Group (Higgins J, Morgan R, Rooney A, Taylor K, Thayer K, Silva
R, Lemeris C, Akl A, Arroyave W, Bateson T, Berkman N, Demers P, Forastiere F, Glenn
B, Hróbjartsson A, Kirrane E, LaKind J, Luben T, Lunn R, McAleenan A, McGuinness L,
Meerpohl J, Mehta S, Nachman R, Obbagy J, O'Connor A, Radke E, Savović J, Schubauer-Berigan
M, Schwingl P, Schunemann H, Shea B, Steenland K, Stewart T, Straif K, Tilling K,
Verbeek V, Vermeulen R, Viswanathan M, Zahm S, Sterne J). Risk Of Bias In Non-randomized
Studies - of Exposure (ROBINS-E). Launch version, 20 June 2023.
17. Cefalì M, Epistolio S, Ramelli G, Mangan D, Molinari F, Martin V, et al. Correlation
of KRAS G12C Mutation and High PD-L1 Expression with Clinical Outcome in NSCLC Patients
Treated with Anti-PD1 Immunotherapy. J Clin Med. 2022 Mar;11(6):1627. 10.3390/jcm11061627
18. Frost N, Kollmeier J, Vollbrecht C, Grah C, Matthes B, Pultermann D, et al. KRASG12C/TP53
co-mutations identify long-term responders to first line palliative treatment with
pembrolizumab monotherapy in PD-L1 high (≥50%) lung adenocarcinoma. Transl Lung Cancer
Res. 2021 Feb;10(2):737–52. 10.21037/tlcr-20-958
19. Wu SG, Liao WY, Su KY, Yu SL, Huang YL, Yu CJ, et al. Prognostic Characteristics and
Immunotherapy Response of Patients With Nonsquamous NSCLC With Kras Mutation in East Asian Populations: A Single-Center Cohort Study in Taiwan. JTO Clin
Res Rep. 2020 Dec;2(2):100140. 10.1016/j.jtocrr.2020.100140
20. Kartolo A, Feilotter H, Hopman W, Fung AS, Robinson A. A single institution study
evaluating outcomes of PD-L1 high KRAS-mutant advanced non-small cell lung cancer
(NSCLC) patients treated with first line immune checkpoint inhibitors. Cancer Treat
Res Commun. 2021;27:100330. 10.1016/j.ctarc.2021.100330
21. Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018 Jul;8(7):822–35. 10.1158/2159-8290.CD-18-0099
22. Spagnuolo A, Maione P, Gridelli C. The treatment of advanced non-small cell lung cancer
harboring KRAS mutation: a new class of drugs for an old target-a narrative review.
Transl Lung Cancer Res. 2022 Jun;11(6):1199–216. 10.21037/tlcr-21-948
23. Tani T, Kitajima S, Conway EB, Knelson EH, Barbie DA. KRAS G12C inhibition and innate
immune targeting. Expert Opin Ther Targets. 2021 Mar;25(3):167–74. 10.1080/14728222.2021.1902991
24. Reck M, Carbone DP, Garassino M, Barlesi F. Targeting KRAS in non-small-cell lung
cancer: recent progress and new approaches. Ann Oncol. 2021 Sep;32(9):1101–10. 10.1016/j.annonc.2021.06.001
25. Zhang SS, Nagasaka M. Spotlight on Sotorasib (AMG 510) for KRAS G12C Positive Non-Small Cell Lung Cancer. Lung Cancer (Auckl). 2021 Oct;12:115–22.
10.2147/LCTT.S334623
26. Li JX, Li RZ, Ma LR, Wang P, Xu DH, Huang J, et al. Targeting Mutant Kirsten Rat Sarcoma
Viral Oncogene Homolog in Non-Small Cell Lung Cancer: Current Difficulties, Integrative
Treatments and Future Perspectives. Front Pharmacol. 2022 Apr;13:875330. 10.3389/fphar.2022.875330
27. Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C)
inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019 Nov;575(7781):217–23.
10.1038/s41586-019-1694-1
28. Chen N, Fang W, Lin Z, Peng P, Wang J, Zhan J, et al. KRAS mutation-induced upregulation
of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother.
2017 Sep;66(9):1175–87. 10.1007/s00262-017-2005-z
29. Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, et al. The superior efficacy of anti-PD-1/PD-L1
immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory
phenotype and increased immunogenicity. Cancer Lett. 2020 Feb;470:95–105. 10.1016/j.canlet.2019.10.027
30. Torralvo J, Friedlaender A, Achard V, Addeo A. The Activity of Immune Checkpoint Inhibition
in KRAS Mutated Non-small Cell Lung Cancer: A Single Centre Experience. Cancer Genomics
Proteomics. 2019;16(6):577–82. 10.21873/cgp.20160
31. Sun L, Hsu M, Cohen RB, Langer CJ, Mamtani R, Aggarwal C. Association Between KRAS
Variant Status and Outcomes With First-line Immune Checkpoint Inhibitor-Based Therapy
in Patients With Advanced Non-Small-Cell Lung Cancer. JAMA Oncol. 2021 Jun;7(6):937–9.
10.1001/jamaoncol.2021.0546
32. Landre T, Justeau G, Assié JB, Chouahnia K, Davoine C, Taleb C, et al. Anti-PD-(L)1
for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled
trials. Cancer Immunol Immunother. 2022 Mar;71(3):719–26. 10.1007/s00262-021-03031-1
33. Sciortino C, Viglialoro V, Nucci M, Polito MG, Cortesi E, Gelibter A, et al. Response
to immunotherapy in KRAS G12C mutated NSCLC: a single-centre retrospective observational
study. Oncotarget. 2022 May;13(1):686–93. 10.18632/oncotarget.28230
34. Sebastian M, Eberhardt WE, Hoffknecht P, Metzenmacher M, Wehler T, Kokowski K, et
al.; CRISP Registry Group. KRAS G12C-mutated advanced non-small cell lung cancer:
A real-world cohort from the German prospective, observational, nation-wide CRISP
Registry (AIO-TRK-0315). Lung Cancer. 2021 Apr;154:51–61. 10.1016/j.lungcan.2021.02.005
35. Arbour KC, Rizvi H, Plodkowski AJ, Hellmann MD, Knezevic A, Heller G, et al. Treatment
Outcomes and Clinical Characteristics of Patients with KRAS-G12C-Mutant Non-Small
Cell Lung Cancer. Clin Cancer Res. 2021 Apr;27(8):2209–15. 10.1158/1078-0432.CCR-20-4023
36. Jeanson A, Tomasini P, Souquet-Bressand M, Brandone N, Boucekine M, Grangeon M, et
al. Efficacy of Immune Checkpoint Inhibitors in KRAS-Mutant Non-Small Cell Lung Cancer
(NSCLC). J Thorac Oncol. 2019 Jun;14(6):1095–101. 10.1016/j.jtho.2019.01.011
37. Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for Lung
Cancers with KRAS p.G12C Mutation. N Engl J Med. 2021 Jun;384(25):2371–81. 10.1056/NEJMoa2103695
38. Corral de la Fuente E, Olmedo Garcia ME, Gomez Rueda A, Lage Y, Garrido P. Targeting
KRAS in Non-Small Cell Lung Cancer. Front Oncol. 2022 Jan;11:792635. 10.3389/fonc.2021.792635
39. Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, et al. Potential Predictive Value
of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma.
Clin Cancer Res. 2017 Jun;23(12):3012–24. 10.1158/1078-0432.CCR-16-2554
40. Rosell R, Codony-Servat J, González J, Santarpia M, Jain A, Shivamallu C, et al. KRAS
G12C-mutant driven non-small cell lung cancer (NSCLC). Crit Rev Oncol Hematol. 2024 Mar;195:104228.
10.1016/j.critrevonc.2023.104228
41. Illini O, Fabikan H, Hochmair MJ, Weinlinger C, Krenbek D, Brcic L, et al. Characteristics
and Treatment Outcomes in Advanced-Stage Non-Small Cell Lung Cancer Patients with
a KRAS G12C Mutation: A Real-World Study. J Clin Med. 2022 Jul;11(14):4098. 10.3390/jcm11144098
42. Kim D, Herdeis L, Rudolph D, Zhao Y, Böttcher J, Vides A, et al. Pan-KRAS inhibitor
disables oncogenic signalling and tumour growth. Nature. 2023 Jul;619(7968):160–6.
10.1038/s41586-023-06123-3
Appendix
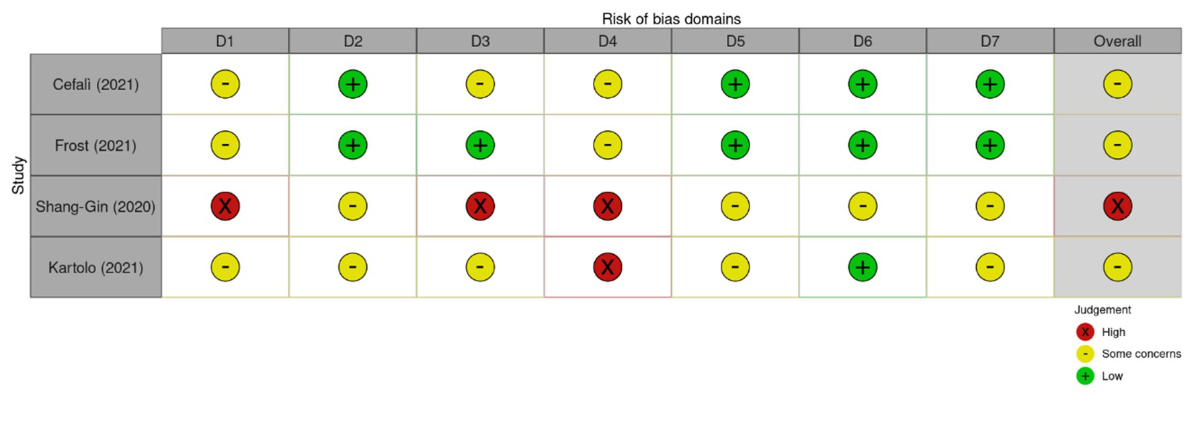
Figure S1Risk Of Bias In Non-randomized Studies – of Exposure (ROBINS-E), 2023 [16]. Domains:
D1: Bias
due to confounding. D2: Bias arising from measurement of the exposure. D3: Bias
in selection of participants into the study (or into the analysis). D4: Bias
due to post-exposure interventions. D5: Bias due to missing data. D6: Bias
arising from measurement of the outcome. D7: Bias in selection of the reported
result.
| P |
Advanced non-small
cell lung cancer with PD-L1 >50% receiving upfront immunotherapy |
| I |
KRAS G12C mutation |
| C |
Non-G12C KRAS mutations and KRAS wt |
| O |
Progression-free survival (PFS) and overall survival
(OS) |
Table S2Additional studies.
| Authors |
Results
|
Reasons
for exclusion |
| Sciortino
et al. (2022) [33] |
In the subgroup treated with second-line immune
checkpoint inhibitors, patients with KRAS-G12C mutations had a median progression-free
survival of 23 months compared to 5 months for non-KRAS G12C mutated cases (p
= 0.03) |
No explicit mention of PD-L1 expression ≥50%; only
9 patients with G12C mutation treated in first line |
| Sebastian
et al. (2019) [34] |
No differences in clinical outcomes between
patients with KRASwt, G12C and non-G12C mutations, with progression-free
survival of 5.7 months (95% CI 4.9–6.6) for KRASwt non-squamous, 6.0 months
(95% CI 3.2–8.4) for KRASwt squamous, 5.7 months (95% CI 4.2–8.2) for KRAS
G12C, and 5.4 months (95% CI 4.5–6.5) for KRAS non-G12C |
Did not exclusively focus on first-line immune
checkpoint inhibitor-based therapy |
| Arbour et
al. (2021)[35] |
mPFS was 3.7 months in patients with G12C vs
3.3 months in those with non-G12C mutations (p = 0.89) |
Statistical analysis does not separate first-
and second-line immune checkpoint inhibitors |
| Jeanson
et al. (2019) [36] |
Trend towards better ORR and longer progression-free
survival was observed for KRAS mutant non-small cell lung cancer with
PD-L1–positive versus PD-L1-negative tumours, with increased benefit for a
higher rate of PD-L1-positive tumour cells (≥50%) |
Analysis of PD-L1 expression between the KRAS
mutation groups but no statistical analysis correlating with immune
checkpoint inhibitors |
| Statistical analysis of immune checkpoint
inhibitor effect focusing only on KRASwt and KRAS mutations |


