Safety of oral immunotherapy for cashew nut and peanut allergy in children – a retrospective
single-centre study
DOI: https://doi.org/https://doi.org/10.57187/s.3691
Maria Breidinga,
Maarja Soomanna,
Michèle Rothb,
Johannes Trücka*,
Felicitas Bellutti Endersab*
a Divisions of Allergy and Immunology, University Children's Hospital
Zurich and the Children’s Research Center, University of Zurich, Zurich,
Switzerland
b Division of Allergy and Clinical Immunology, University Children’s
Hospital Basel, Basel, Switzerland
* These authors contributed equally as shared senior authors
Summary
AIM OF THE STUDY: Oral immunotherapy (OIT)
is increasingly used for the treatment of childhood food allergies, with limited
data available on cashew nut OIT. This real-life study investigated the safety
and feasibility of cashew nut OIT, comparing it with peanut OIT, with a focus
on the up-dosing process.
METHODS: We analysed cashew nut (n = 24)
and peanut (n = 38) OIT cases with treatment initiated between 2018 and 2022 at
the University Childrenʼs Hospital Basel. All patients who commenced therapy
within this time frame were enrolled without prior selection. Two different
starting protocols were used. Within the up-dosing protocol, the nut intake was
incrementally increased by 20–30% every 2 weeks until reaching a maintenance
dose of 1 g of nut protein. After consuming the maintenance dose regularly for
18–24 months, a second oral food challenge was performed. Patients who passed
this challenge were considered desensitised. The safety of the therapy was
evaluated based on the severity of adverse reactions during the up-dosing
phase. Symptom severity was evaluated using the validated ordinal food allergy
severity scale (o-FASS-5).
RESULTS: Over the study period, 33% of
cashew nut-allergic and 63% of peanut-allergic patients experienced mild to
moderate allergic reactions. Severe allergic reactions occurred in five
peanut-allergic children with high baseline allergen-specific IgE levels. Six
patients with peanut, and none with cashew nut OIT, discontinued the therapy
due to adverse reactions. The mean duration to reach the maintenance phase was
longer for children with asthma or another food allergy. Among children who
already underwent the second oral food challenge, desensitisation was achieved
in 91% (11 out of 12) of cashew nut- and 73% (11 out of 15) of peanut-allergic
patients.
CONCLUSION: Cashew nut OIT
had a low severity of adverse reactions and was generally well-tolerated.
However, patient characteristics influenced side effect risk and treatment
duration, emphasising the need for individualised OIT strategies.
Introduction
Cashew nuts are a common cause of food
allergies worldwide [1–3], often triggering more severe reactions than other
foods [4, 5]. The prevalence of cashew nut allergy is on the rise [6], possibly
due its increasing use in the Western diet. In Europe, peanuts are the primary
cause of anaphylaxis in children under the age of 18 years, but cashew nuts rank
first in Switzerland [7, 8]. Even a small amount (less than 1 teaspoon) of cashew
nuts or peanuts can induce an allergic reaction [8]. Notably, in only about 9%
of all tree nut allergies and 29% of peanut allergies, natural tolerance occurs
[9, 10]. Therefore, it is imperative to explore strategies to enhance reaction
threshold and minimise the risk of severe reactions [11].
In recent years, oral immunotherapy (OIT)
has emerged as a promising therapeutic option for children with food allergies
and is supported by encouraging data [12–15]. In 2018, the European Academy of
Allergy and Clinical Immunology (EAACI) officially recommended allergen
immunotherapy for peanut, milk, and egg allergies in children older than 4
years with persistent Immunoglobulin E (IgE)-mediated food allergies [16]. Furthermore,
subsequent work has shown more favourable outcomes and safety for younger age
groups [15, 17, 18]. Nevertheless, several studies have demonstrated that OIT
increases the likelihood of allergic reactions that are mostly mild in nature, though
severe reactions are possible [12, 19]. While OIT for tree nuts lacks official
endorsement, it is frequently employed, yet data on its efficacy and safety
remain scarce. The NUT CRACKER (Nut Co-Reactivity-Acquiring
Knowledge for Elimination Recommendations) study, a prospective cohort study
involving 50 patients undergoing cashew nut OIT, showed promising results with
a high rate of desensitisation and moderate incidence of adverse reactions [20].
Another real-life analysis of preschool children who underwent OIT for tree
nuts, including cashew nuts, demonstrated adverse reactions of varying degree in
70% of participants [21].
This retrospective single-centre study
aimed to evaluate the safety and feasibility of real-world cashew nut OIT, comparing
it with peanut OIT, as well as to identify factors influencing adverse
reactions and treatment duration with a focus on the up-dosing process.
Methods
Study design and population
We conducted a retrospective analysis of
OIT for cashew nut or peanut allergies initiated between October 2018 and April
2022 at the University Childrenʼs Hospital Basel, Switzerland. The option for OIT
was offered to all paediatric patients with peanut or cashew nut allergy, except
those with contraindications (uncontrolled asthma, eosinophilic oesophagitis, non-compliance
or relevant language barriers, active autoimmune diseases, or malignancies). Allergy
diagnoses were established based on a clear history of a systemic
immediate-type allergic reaction or an observed reaction during an open oral
food challenge (OFC), and either positive results from a skin prick test (SPT)
or specific immunoglobulin E levels (sIgE) exceeding 0.35 kU/l for the
respective allergen, or both. All patients who commenced therapy were included
in our study without prior selection, thereby presenting a real-life
investigation.
Oral immunotherapy protocol
Two different strategies were used to start
the OIT (see figure S1 in the appendix). In the peanut allergy group, based on the
history of their previous reactions and laboratory results, patients were
either considered low-risk or high-risk. Low-risk peanut-allergic patients and
all cashew nut-allergic patients initiated OIT with an open OFC. The OFC
protocol, following international guidelines, was stopped when symptoms
appeared, in line with the PRACTALL consensus report [22]. The dosage at
symptom onset was considered the individual reactive dose. For these patients, OIT
began with the highest tolerated OFC dose. High-risk peanut allergy patients
were started with an initial seven-step dose escalation from 0.0001 g to 0.0064
g of nut protein (figure S1, protocol slightly adapted from [23]), the dose
with which they began their OIT.
The daily intake portions were provided to
the families in pre-weighed doses, each containing the corresponding ground fresh
nut. The portions were prepared by the nursing staff within the allergy
department. Families were given the following general instructions [24]: Patients
were advised to consume the daily dose with a meal and avoid physical activity
for 2 hours after intake. In case of an infection or intake of
anti-inflammatory medication, families were instructed to reduce or temporarily
pause the daily intake after consulting with their doctor.
During the up-dosing protocol, the nut
intake was increased by 20–30% every 2 weeks, aiming for a daily maintenance
dose of 1 g protein. The decision to select 1 g protein as the maintenance dose
was based on various considerations, including our clinical experience, observations
of OFC outcomes in our patient population, and practicality for daily dietary inclusion.
While recent data may suggest the effectiveness of low-dose OIT, there is still
limited evidence to support its widespread adoption as a standard practice
beyond treatment with commercially available peanut powder. OIT up-dosing was
avoided during pollen season in case of seasonal symptoms to reduce adverse
reactions in patients, or when reactions occurred. After reaching the
maintenance dose of 1 g nut protein per day, patients continued this regimen
for 4 weeks and then were allowed to reduce the intake to 1 g protein at least every
other day for 18 to 24 months. In patients who underwent multiple nut OIT, there
was an interval of at least 3 months between starting the first OIT and
commencing the second nut OIT. During overlapping up-dosing periods, the two
doses were administered together.
Following the maintenance period of 18 to
24 months, another OFC was conducted, aiming for the consumption of a total of
4.4 g nut protein. The quantity of individual OFC doses, including the
cumulative amount of 4.4 g nut protein, corresponds to the international PRACTALL
guidelines [22]. Patients who tolerated this second OFC without experiencing
any allergic reactions were considered desensitised and were allowed to eat the
nut without limitations but were instructed to continue consuming ≥1 g nut
protein at least twice per week. For those patients without other food allergies,
the adrenaline auto-injector was then removed from their emergency medication
kit, provided they consumed the allergen regularly, independent of their sIgE
levels. They continued to have yearly follow-up appointments. In case of an
allergic reaction during this second OFC, patients returned to their previous
maintenance regimen.
Immunological parameters
Specific IgE levels to the storage proteins
were assessed at three time points: before OIT start, upon reaching the
maintenance dose, and before the second OFC. Skin prick tests were considered
positive if the wheal was more than 3 mm larger than the negative control and
were typically only conducted during the initial diagnosis of nut allergy. We opted
to analyse the specific IgE to the storage protein rather than the specific IgE
to the allergen extract due to its higher sensitivity and specificity [25]. Additional
immunological data (e.g., specific IgG4 levels) were not included in our data
collection.
Outcomes
The safety of OIT was assessed by comparing
the percentage of patients experiencing adverse effects during the up-dosing
phase, as documented at each appointment. Symptoms severity was evaluated using
the validated ordinal food allergy severity scale (o-FASS-5) [26]. Grade 1 reactions
involved only the oral
cavity and were categorised as mild. Grade 2 reactions included one, while grade
3 reactions involved two of the following organ systems: skin, nose, eye, digestive,
or uterine, both considered moderate. Grade 4 reactions affected the larynx or bronchi,
while grade 5 reactions involved the cardiovascular or nervous system, both categorised
as severe reactions.
Statistics
Statistical analysis was performed using R
(version 4.2.2) with packages ggsurvfit, ggthemes, tidycmprsk, tidyverse [27].
Categorical variables were compared using Fisherʼs exact test and continuous
variables using the Mann-Whitney U-test. A p-value <0.05 was considered
significant. Time to reach maintenance in subgroups was compared using the
cumulative incidence function and Grayʼs test. Reaching maintenance was
considered to be the event of interest, stopping therapy was considered a
competing event, and the patients who either paused the up-dosing or were lost
to follow-up were right censored on their last clinical follow-up.
Ethics
General consent policy was applied for
further use of patient data, and data from patients whose parents or guardians
had denied general consent were not further analysed. Approval of this study
was granted by the ethics committee of the Canton of Basel (BASEC-Nr.
2023-00524). All aspects of the study were conducted in accordance with the
ethical principles of the Declaration of Helsinki. The privacy and
confidentiality of all study participants were strictly upheld, and all data
were coded prior to analysis to ensure the protection of their personal
information.
Results
Patient characteristics
During the study period, 67 cashew nut or peanut
OIT cases were initiated. Five patients (or their legal guardians) who did not
provide consent were excluded. The final analysis included data from 24 cashew
nut and 38 peanut OIT cases (figure 1), with four patients undergoing treatment
for both nuts; 18 peanut allergy patients (47%) had been considered high-risk.
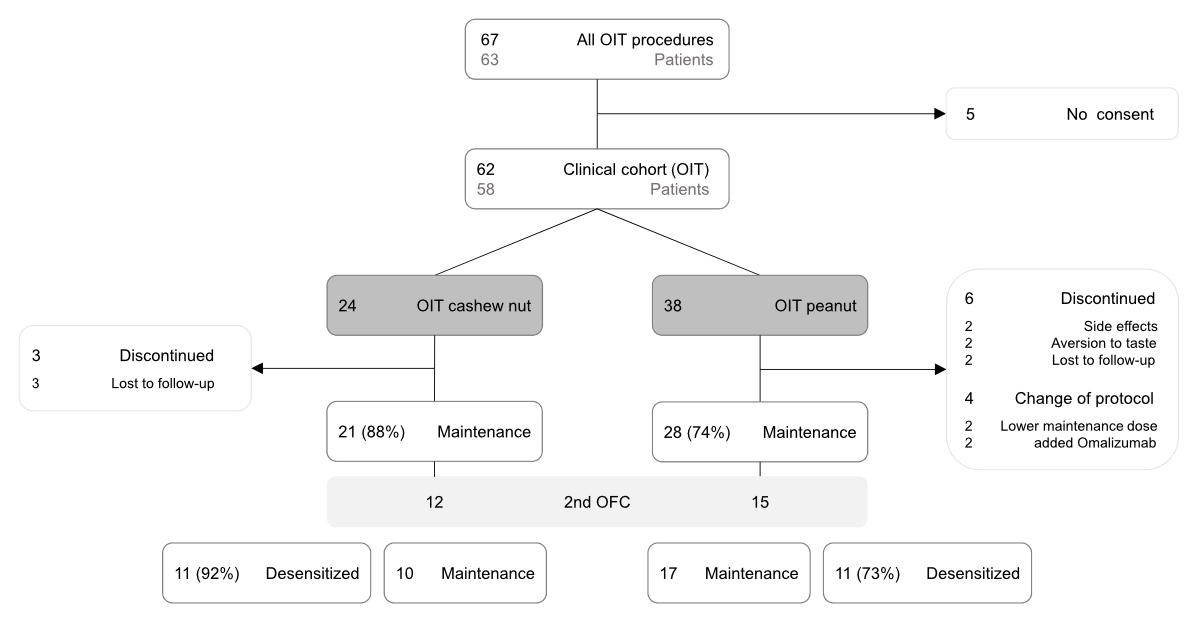
Figure 1Patient flow through the study. Approximately
half of the patients on maintenance treatment underwent a second oral food
challenge (2nd OFC). Among these, 92% cashew nut oral
immunotherapy (OIT) patients and 73% peanut oral immunotherapy patients passed
the challenge and were considered desensitised. The remaining patients either
stayed on or reverted to maintenance treatment.
Both groups displayed a similar sex
distribution, median age at initiation of the therapy, and prevalence of other
atopic diseases upon starting OIT (table 1). However, children with peanut
allergy had significantly higher specific IgE concentrations against storage
protein compared to those with cashew nut allergy (median, 25.5 kU/l vs 1.78
kU/l; p <0.001) (table 1, figure 2).
Table 1Baseline patient characteristics.
| |
Cashew
(n = 24) |
Peanut
(n = 38) |
p-Value |
| Age in years, median (range) |
6 (2, 15) |
7 (3, 17) |
0.69 |
| Female, n (%) |
13 (54) |
18 (47) |
0.79 |
| Any atopic disease, n (%) |
24 (100) |
36 (95) |
0.51 |
|
Bronchial asthma, n (%) |
11 (46) |
20 (53) |
0.79 |
| Atopic dermatitis, n (%) |
17 (71) |
32 (84) |
0.22 |
| Other food allergy, n (%) |
12 (50) |
21 (55) |
0.80 |
| Allergic rhinitis, n (%) |
12 (50) |
26 (68) |
0.19 |
| Specific immunoglobulin E to storage
protein (Ana o3/Ara h2) in kU/l, median (range) |
1.78 (0.05 to 36.9) |
25.5 (0.05 to >100) |
<0.001 |
| Reactive dose in the initial oral
food challenge (in g) nut protein, median (range) |
0.1 (0.01 to 3) |
0.3 (0.0064 to 3) |
0.97 |
| Reaction severity in the initial oral
food challenge (oFASS-5), median (range) |
2 (1 to 4) |
2 (2 to 4) |
0.37 |
| Initial standard oral food challenge,
n (%) |
24 (100) |
20 (52) |
0.19 |
|
Reaction (oFass-5), n (%)
|
Grade 1 |
1 (4) |
0 (0) |
|
| Grade 2 |
12 (50) |
10 (50) |
|
| Grade 3 |
9 (38) |
4 (20) |
|
| Grade 4 |
2 (8) |
6 (30) |
|
| Grade 5 |
0 (0) |
0 (0) |
|
| Initial dose-escalation to 0.0064 g
nut protein, n (%) |
0 (0) |
18 (47) |
|
|
Reaction (oFass-5), n (%)
|
No reaction |
0 |
15 (83) |
|
| Grade 1 |
0 |
0 (0) |
|
| Grade 2 |
0 |
2 (11) |
|
| Grade 3 |
0 |
0 (0) |
|
| Grade 4 |
0 |
1 (5) |
|
| Grade 5 |
0 |
0 (0) |
|
| Starting dose in g nut protein, median
(range) |
0.030 (0.001 to 1) |
0.0064 (0.0032 to 1) |
0.031 |
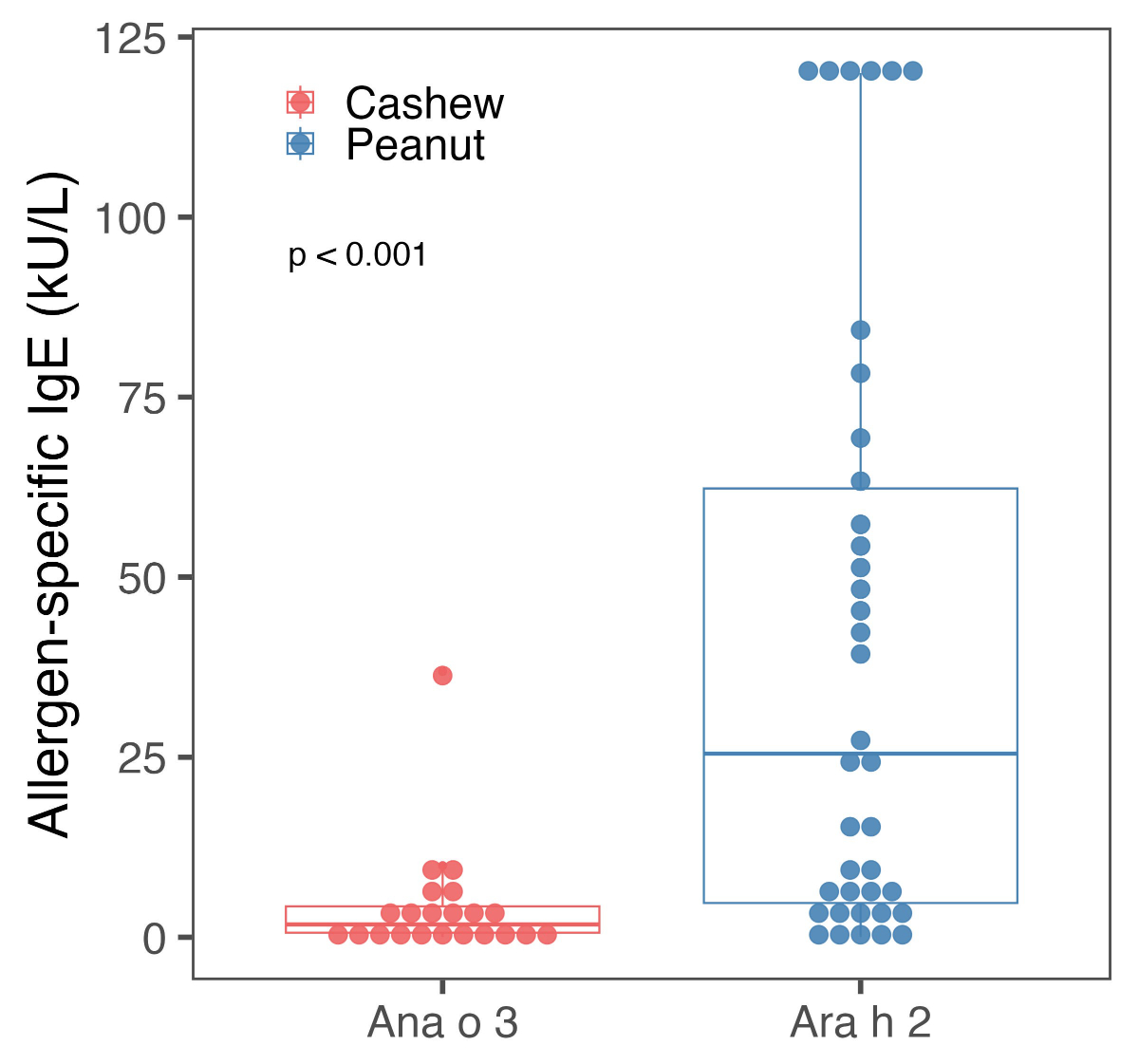
Figure 2Levels of baseline specific immunoglobulin E (sIgE)
concentrations for nut-specific storage proteins Ana o3 / Ara h2 for cashew nut
and peanut-allergic patients undergoing oral immunotherapy. Values >100
kU/l were set to 120 kU/l.
During the initial OFC, individual reactive
doses were comparable in both groups, with a median of 0.1 g in the cashew nut
allergy group and 0.3 g protein in the low-risk peanut allergy group (i.e.,
peanut cases with an initial standard OFC [p = 0.97]). Most of these children had
a moderate allergic reaction grade II, with similar median oFASS-5 scores (2 in
both groups, p = 0.37). In contrast, the median OIT starting dose in the cashew
nut allergy group was significantly higher at 0.03 g, compared to 0.0064 g
protein in the entire peanut allergy group (p = 0.031). In the peanut allergy
group, two patients (5%) stopped therapy due to taste aversion, while two
patients relocated during the up-dosing phase and were subsequently lost to
follow-up. In the cashew nut allergy group, three patients (12%) failed to come
to up-dosing appointments and were lost to follow-up (figure 1).
Safety
During up-dosing phase, mild to moderate
adverse reactions were observed in eight patients (33%) in the cashew nut
allergy group, including mild oral pruritus and moderate reactions such as abdominal
pain or acute rhino-conjunctivitis. The remaining 16 patients (66%) reported no
adverse reactions. In the entire peanut allergy group, 24 patients (63%) experienced
adverse effects during up-dosing. Nineteen of these 24 patients experienced
mild to moderate adverse reactions such as oral pruritus and abdominal pain (figure
3A), but five children experienced severe reactions, indicated by an oFASS-5
score of ≥4. Four of these severe reactions occurred at home, and one in the
outpatient clinic. A single dose of intramuscular adrenaline was administered
to three of these children, and in two patients, the symptoms resolved after
the intake of antihistamines. All severe reactions occurred during the up-dosing
phase and involved respiratory symptoms, including wheezing and/or dyspnoea. In
two of these severe reactions, an augmentation factor was present: one due to a
viral infection and one due to pollen-associated symptoms.
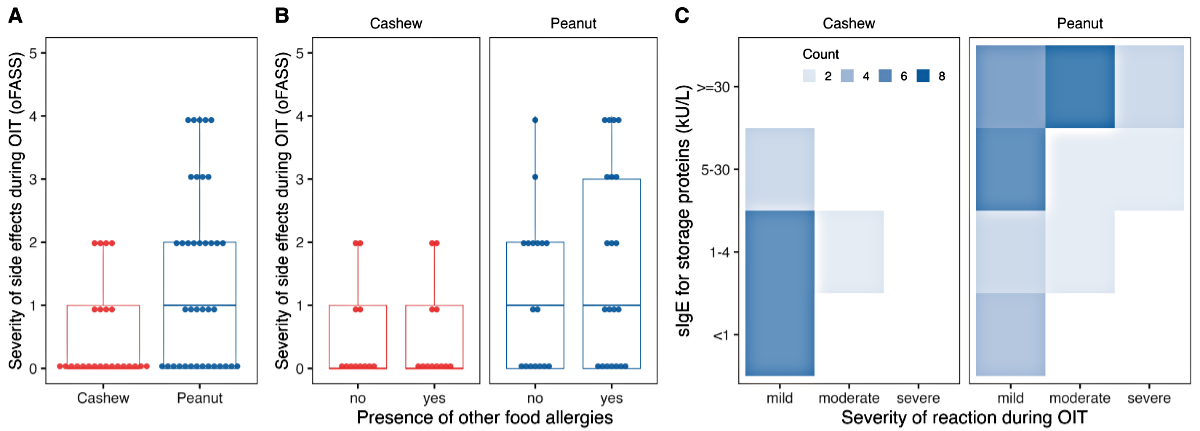
Figure 3(A) Maximum severity of allergic
reactions during oral immunotherapy (OIT) by nut allergy graded by oFASS-5. (B)
Maximum severity of adverse reactions during oral immunotherapy by nut allergy and
separated
by presence of other food allergies. (C) Heatmap of the maximum severity
of reaction during oral immunotherapy (mild: oFASS 0–1; moderate: oFASS 2–3; severe:
oFASS 4–5)
in relation to the initial levels of specific immunoglobulin E (sIgE) against
storage proteins (Ana o3, Ara h2). oFASS-5:ordinal food allergy severity scale 5.
In the peanut allergy group, two patients
(5%) reported symptoms consistent with eosinophilic oesophagitis (EoE). One
patient discontinued treatment and declined an endoscopy. The other patient
ceased therapy, but the endoscopy 4 weeks later did not meet eosinophilic oesophagitis
criteria. This
patient received omalizumab treatment due to bronchial asthma. With omalizumab
administered every 4 weeks, the OIT was successfully resumed. Additionally, in three
(13%) peanut OIT patients, the pre-defined protocol was changed due to IgE-mediated
adverse reactions: one successfully restarted OIT with omalizumab, and two continued
on a lower maintenance dose. One patient opted to discontinue therapy entirely
due to adverse reactions. Notably, none of the cashew nut OIT patients modified
their treatment due to adverse effects or had eosinophilic oesophagitis-like symptoms.
The presence of other food allergies, atopic
dermatitis, asthma/wheeze, or any allergic disease did not influence the
severity of adverse reactions during OIT (figure 3B and figure S2 in the
appendix). However, nearly all patients with severe reactions (oFASS ≥4) and
the majority of those with moderate reactions (oFASS 2–3) had high initial
specific IgE levels to the storage protein (>30 kU/l) (figure 3C). An
additional analysis showed that higher age at the start of OIT was mildly
associated with more severe adverse reactions during OIT (R = 0.4, p = 0.001; figure
S3 in the appendix). However, there was no correlation between the severity of the
index
reaction during the initial OFC and the severity of adverse reactions during
OIT.
Time to reach maintenance and rates of desensitisation
In the entire study population, 21 (87%) of
cashew nut-allergic patients and 28 (73%) of peanut-allergic patients reached
the maintenance phase. Among these patients, those in the cashew nut allergy
group tended to reach maintenance faster (figure 4A). One patient in
the cashew nut allergy group and two in the peanut allergy group bypassed the
up-dosing phase as they tolerated the maintenance dose of 1 g and only showed
an allergic reaction at the last step of 3 g protein during the initial OFC. Children
with asthma (figure 4B) or another food allergy (figure 4C) needed significantly
longer to reach maintenance. There was, however, no significant difference in time
needed to reach the maintenance phase for patients with pre-existing allergic
rhinitis or atopic dermatitis compared to those without.
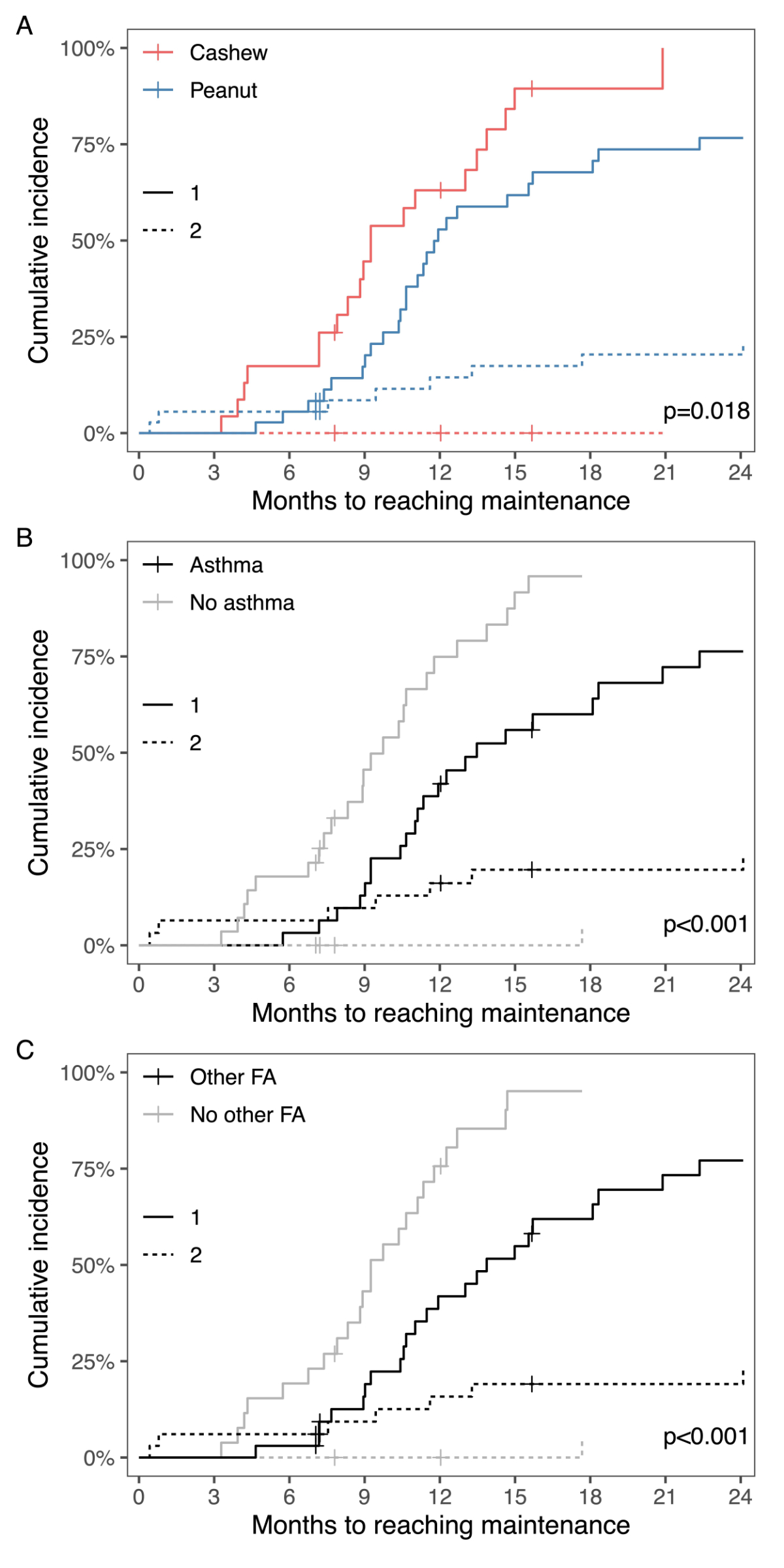
Figure 4Cumulative incidence curves for reaching
maintenance dose in patients (A) undergoing therapy in the cashew nut (n
= 24) and the peanut (n = 38) group, (B) with (n = 31) and without
asthma (n = 31), and (C) with (n = 33) and without (n = 29) other food
allergy (FA), (1) representing the patients who reached the maintenance dose
(continuous lines), and (2) the group of patients who discontinued or changed
the protocol due to adverse reactions or aversion to taste (dashed lines).
Crosses representing patients who were lost to follow-up.
The
evaluation period was extended until December 2023, with a total OIT observation
period across all patients ranging from 21 to 62 months. Hence, there were
patients (7 cashew nut- and 9 peanut-allergic patients) who had not yet
undergone a second OFC. Additionally, six patients (4 from the peanut allergy
group and 2 from the cashew nut allergy group) chose not to proceed with a
second OFC for various reasons. Four patients saw no personal benefit in
carrying out the second OFC due to a lack of interest in introducing a larger
amount of the food allergen into their diet. Another patient, faced with a
malignant tumour diagnosis, and one patient who relocated, elected to remain on
the maintenance dose. Among the cashew nut allergy group, 12 patients underwent
a second OFC, with 11 (91%) showing tolerance to a cumulative dose of 4.4 g nut
protein, indicating desensitisation. One patient experienced a mild reaction
(oral pruritus) at the last step of 3 g protein, compared to the initial OFCʼs 0.1
g reactive dose (generalised urticaria). In the peanut allergy group, 15
patients underwent a second OFC, with 11 of them (73%) showing tolerance to a
cumulative dose of 4.4 g nut protein. Among the four patients who presented an
allergic reaction during the second OFC, three experienced moderate reactions at
the last step of 3 g nut protein. These three patients had a history of severe
anaphylaxis (oFASS grade 4 with involvement of the lower respiratory tract) in the
past.
One patient had both a moderate reaction with urticaria in the initial exposure
to peanut protein and a moderate reaction with vomiting in the second OFC.
Patients who did not experience an allergic
reaction during the second OFC, and were thus considered desensitised, were then
allowed to consume the nut freely, with a minimum intake of 1 g nut protein
twice per week. To date, no allergic reactions have been reported following the
second OFC.
Changes in immunological parameters
Comparing sIgE levels to the storage
proteins (Ana o3/Ara h2) from before OIT to upon reaching the maintenance dose,
the cashew nut allergy group (n = 19) showed a clear decreasing trend in median
sIgE levels, dropping from 1.78 kU/l to 0.81 kU/l (figure 5A). In contrast, the
peanut allergy group (n = 26) exhibited more variable trajectories in sIgE
levels, resulting in relatively stable median values at both measurement points,
from 25.5 kU/l to 18.8 kU/l.
In the analysis of 27 patients with
available data who underwent a second OFC, the cashew nut allergy group showed
a continued decreasing trend in median sIgE levels to the storage protein (before
OIT 0.83 kU/l, at the start of maintenance phase 0.42 kU/l, before the second
OFC 0.29 kU/l). Similarly, the peanut allergy group exhibited an initial
increase in median sIgE levels, followed by a decrease by the time of the
second OFC (before OIT, 10.6 kU/l; at the start of maintenance phase, 19.1 kU/l;
before the second OFC, 6.99 kU/l) (figure 5B). No significant differences in
sIgE levels against storage proteins were observed when comparing patients who
tolerated the second OFC to those who did not.
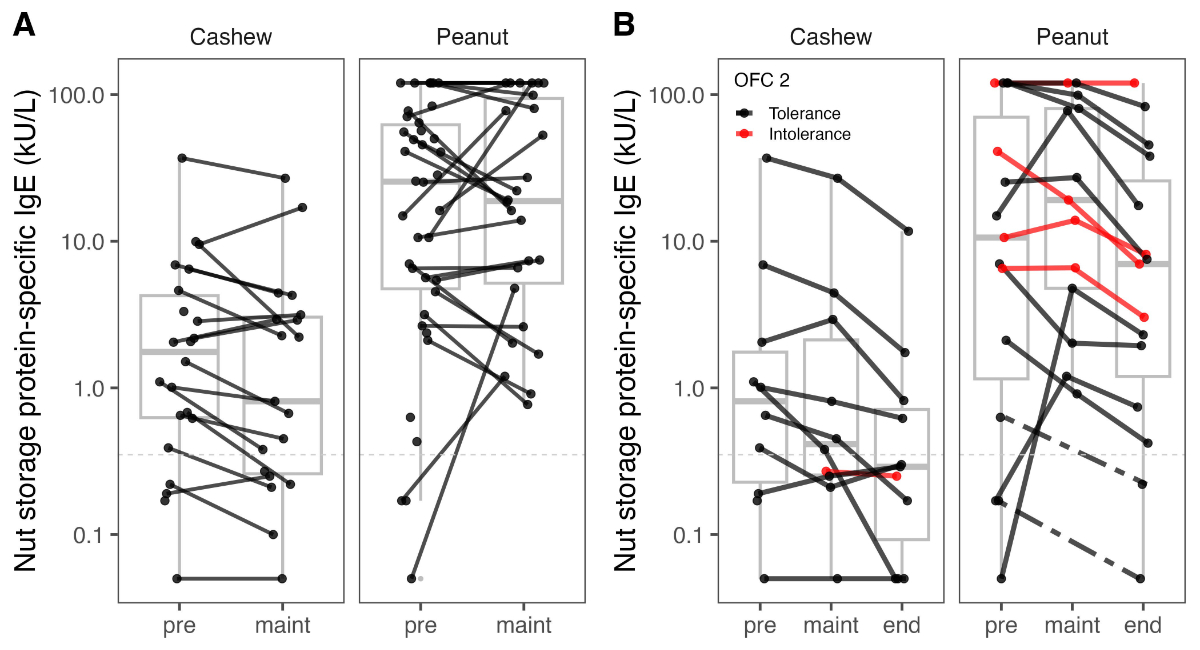
Figure 5(A) Changes in specific immunoglobulin
E (sIgE) to the storage protein (Ana o3/Ara h2) from before starting the oral
immunotherapy (OIT) to reaching maintenance dose. Complete data from two cashew
nut and two peanut allergy patients are missing. (B)
Progression over time of sIgE to the storage protein (Ana o3/Ara h2) for the
patients who underwent a second oral food challenge (OFC) for cashew nut (n = 12)
and peanut (n = 15). Black lines represent patients who had no reaction during
the second oral food challenge and were classified as desensitised; red lines
represent patients who failed to complete the second oral food challenge with
an intolerance. Complete data from two cashew nut allergy patients and two peanut
allergy patients are missing. Pre: before
starting the oral immunotherapy; maint:
start of the maintenance phase; end: end
of the maintenance phase.
Discussion
This study offers valuable insight into the
real-world application of OIT for cashew nut and peanut allergies, revealing a
low incidence of adverse reactions during the up-dosing phase in the cashew nut
allergy group, alongside with a high success rate in reaching maintenance.
Adverse reactions were generally mild to moderate, with severe reactions
primarily occurring in the peanut allergy group. Notably, a substantial
proportion of patients who underwent a second OFC demonstrated desensitisation,
especially in the cashew nut allergy group.
Ensuring safety during OIT is a significant
concern, considering the known risk of severe adverse reactions, particularly
during the up-dosing phase and especially in doses above 300 mg protein [12, 19].
In our study, we observed a low frequency and severity of allergic reactions in
patients undergoing cashew nut OIT. In contrast, the NUT CRACKER study, with a
cohort of 50 cashew nut-allergic patients and similar baseline immunological
parameters, reported a high incidence of side effects (88%), with 18% of
patients requiring adrenaline [20]. In this study, up-dosing was performed up
to 4 g nut protein, with a consecutive daily consumption of 1.2 g nut protein
daily during the maintenance regimen. Another real-world study on tree nut OIT in
58 cashew nut-allergic preschool children recorded no severe reactions, but 71%
experienced mild to moderate reactions [21]. These patients also had baseline
immunological parameters similar to those of our cashew nut allergy group, although
maintenance therapy was performed with only 0.3 g protein. Our cashew nut
allergy group had an even lower rate of mild to moderate adverse reactions (33%)
with no episodes of anaphylaxis and no patients having eosinophilic oesophagitis-like
symptoms. In
contrast to the cashew nut allergy group, the peanut allergy group exhibited a
significantly higher rate of adverse reactions, with 13% of patients
experiencing anaphylaxis and 63% encountering any adverse reaction. The
increased likelihood of severe allergic reactions during peanut OIT, compared
to strict avoidance, has been reported in other studies [19, 28], underscoring
the importance of thorough discussions with families to assess the risk-benefit
ratio.
Previous studies reported a higher
incidence and severity of reactions associated with higher sIgE levels and
larger doses during OIT [29]. In one study investigating patient factors
associated with clinical outcomes in 174 patients undergoing peanut OIT, the number
of reactions was positively correlated with baseline sIgE levels to peanut [30]. Two
other studies of peanut OIT
involving 75 and 653 patients reported baseline peanut-specific sIgE levels that
were positively correlated with the rate and severity of allergic reactions
during treatment [31, 32]. Similarly, in a sample of 270 preschoolers, higher
baseline sIgE levels to peanut were found to be more likely associated
with epinephrine use during OIT [18]. Taken together, for peanut OIT, higher
baseline specific IgE levels appear to be associated with a higher rate and
severity of allergic reactions during treatment, consistent with our study
findings (figure 3C).
Our study also observed lower nut-specific
IgE levels in cashew nut-allergic patients compared to those allergic to peanuts,
which may contribute to the lower rate and severity of adverse reactions during
cashew nut OIT. While the data supporting this observation are limited, they
align with existing literature suggesting that cashew nut-allergic individuals
generally exhibit lower allergen-specific IgE levels [33]. Importantly, our
study included unselected patients, reflecting real-world experiences and suggesting
that cashew nut OIT is generally well-tolerated. This finding underscores the
potential role of specific IgE levels in influencing the safety and feasibility
of OIT, highlighting the need for further investigation into the immunological
factors underlying allergic reactions during OIT for different allergens.
Additionally, the OFC reactive doses and reaction severity during the initial
OFC were similar in both the cashew nut and peanut allergy groups, suggesting
that cashew nut allergy patients were not inherently “less allergic” than peanut
allergy patients despite the differences in baseline specific IgE levels. This indicates
that factors beyond the initial allergic response may contribute to the
differential rates and severity of allergic reactions observed during OIT for cashew
nut and peanut allergies. Understanding these factors better will be crucial
for improving treatment outcomes and personalising OIT protocols for different
patient populations.
Of note, OIT protocols vary considerably
between centres regarding the frequency and dose increments during up-dosing, as
well as the maintenance dose and the duration of the maintenance phase.
Furthermore, there is no clear guidance on the total protein amount required in
the second food challenge to demonstrate desensitisation. We chose a
maintenance dose of 1 g protein for various reasons already discussed, which
resulted in good tolerability and safety. However, more research on this topic
is needed, and standardising protocols in the future would be preferable in
order to provide the best outcome for our patients.
In our study patients, we did not validate previous
findings that associated the severity of adverse effects during OIT up-dosing
with the presence of co-existing asthma or allergic rhinitis (figure S2 in the appendix)
[34, 35].
However, patients with asthma or other food allergies took significantly longer
to reach the maintenance phase (figure 4). It remains unclear whether this
slower progress was intentional for safety reasons as 70% of asthmatic patients
had seasonal symptoms, or if other factors influenced the time required to
reach maintenance. This suggests the possibility that seasonal triggers may
have contributed to a deceleration during the up-dosing phase, especially
during pollen season. Patients undergoing cashew nut OIT reached their
maintenance dose quicker, which might be attributed to the significantly lower
mean starting dose in the peanut allergy group. Younger patients experienced
fewer adverse reactions, consistent with existing literature [15, 17] (figure S3
in the appendix).
A small proportion (5%) of children
discontinued peanut OIT due to aversion to taste, a phenomenon not observed in
the cashew nut allergy group, suggesting a potentially better tolerance for the
taste of cashews. The rate of desensitisation in cashew nut OIT was high (91%),
in line with existing data [20, 21]. In contrast, the desensitisation rate in
the peanut allergy group was lower (73%). However, all patients who did not
pass the second OFC had mild to moderate reactions only, and increased their
individual reactive dose, indicating partial desensitisation [11]. A review of
sustained unresponsiveness through discontinuation of the therapy for a certain
period followed by a third oral food challenge was deliberately omitted, as
earlier data only promise limited success [15, 36, 37]. In contrast, after reaching
the status of desensitisation,
patients were allowed to eat the peanut or cashew nut freely but were advised
to eat 1 g protein at least twice per week.
Consistent with other studies, a
substantial number of patients in the peanut allergy group experienced an
initial increase in sIgE levels after completion of the up-dosing phase [38, 39],
with some showing persistent high levels even after years of therapy. This
complexity in sIgE dynamics makes it challenging to rely on IgE levels for
prognostic purposes.
This study has several limitations, including
its retrospective design, which leads to potential biases. Additionally, there are
missing immunological data such as specific IgG4 levels that could serve as a
helpful biomarker during OIT. The heterogeneity in starting and up-dosing
protocols further complicates therapy duration comparisons. But this
heterogeneity in protocols mirrors the real-life setting and provides important
insights into OIT handling. The open OFC format may introduce bias, and the smaller
number of patients undergoing a second OFC after the maintenance phase limits
statistical power and generalisability. Further evaluations, especially
considering the association of low specific IgE with a higher remission rate, may
offer additional insights into the study population [15, 36]. To optimise and
standardise cashew nut OIT, prospective studies are needed to evaluate safety, feasibility,
and long-term outcomes, enhancing the effectiveness and reliability of this
treatment.
In conclusion, cashew nut OIT shows promise
as a treatment option, demonstrating a low rate of severe adverse reactions and
good feasibility in a real-world setting. However, careful consideration of
immunological parameters and other allergic diseases is crucial when informing
families and planning therapy. Further prospective studies will help enhance
the safety and effectiveness of OIT as a treatment option for cashew nut-allergic
children.
Acknowledgments
Author contributions: Study design and concept was realised by MB, FB,
and JT; data collection was conducted by MB, FB, and MR; data analysis and
interpretation was carried out by JT and MS; manuscript was written by MB, FB, and
JT; all authors reviewed and edited the manuscript; all authors approved the
final version of the manuscript.
Maria Breiding
Steinwiesstrasse 75
CH-8032 Zurich
maria.breiding[at]kispi.uzh.ch
References
1. McWilliam V, Koplin J, Lodge C, Tang M, Dharmage S, Allen K. The Prevalence of Tree
Nut Allergy: A Systematic Review. Curr Allergy Asthma Rep. 2015 Sep;15(9):54. doi: https://doi.org/10.1007/s11882-015-0555-8
2. Mendes C, Costa J, Vicente AA, Oliveira MB, Mafra I. Cashew Nut Allergy: Clinical
Relevance and Allergen Characterisation. Clin Rev Allergy Immunol. 2019 Aug;57(1):1–22.
doi: https://doi.org/10.1007/s12016-016-8580-5
3. Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Golobov K, et al. NUT Co
Reactivity - ACquiring Knowledge for Elimination Recommendations (NUT CRACKER) study.
Allergy. 2018 Mar;73(3):593–601. doi: https://doi.org/10.1111/all.13353
4. Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis
in children and adolescents: The European Anaphylaxis Registry. J Allergy Clin Immunol.
2016 Apr;137(4):1128–1137.e1. doi: https://doi.org/10.1016/j.jaci.2015.11.015
5. Clark AT, Anagnostou K, Ewan PW. Cashew nut causes more severe reactions than peanut:
case-matched comparison in 141 children. Allergy. 2007 Aug;62(8):913–6. doi: https://doi.org/10.1111/j.1398-9995.2007.01447.x
6. Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported
peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010 Jun;125(6):1322–6.
doi: https://doi.org/10.1016/j.jaci.2010.03.029
7. Brough HA, Caubet JC, Mazon A, Haddad D, Bergmann MM, Wassenberg J, et al. Defining
challenge-proven coexistent nut and sesame seed allergy: A prospective multicenter
European study. J Allergy Clin Immunol. 2020 Apr;145(4):1231–9. doi: https://doi.org/10.1016/j.jaci.2019.09.036
8. Dölle-Bierke S, Höfer V, Francuzik W, Näher AF, Bilo MB, Cichocka-Jarosz E, et al. Food-Induced
Anaphylaxis: Data From the European Anaphylaxis Registry. J Allergy Clin Immunol Pract.
2023 Jul;11(7):2069–2079.e7. doi: https://doi.org/10.1016/j.jaip.2023.03.026
9. Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut
allergy. J Allergy Clin Immunol. 2005 Nov;116(5):1087–93. doi: https://doi.org/10.1016/j.jaci.2005.09.002
10. Peters RL, Guarnieri I, Tang ML, Lowe AJ, Dharmage SC, Perrett KP, et al. The natural
history of peanut and egg allergy in children up to age 6 years in the HealthNuts
population-based longitudinal study. J Allergy Clin Immunol. 2022 Sep;150(3):657–665.e13.
doi: https://doi.org/10.1016/j.jaci.2022.04.008
11. Baumert JL, Taylor SL, Koppelman SJ. Quantitative Assessment of the Safety Benefits
Associated with Increasing Clinical Peanut Thresholds Through Immunotherapy. J Allergy
Clin Immunol Pract. 2018;6(2):457–465.e4. doi: https://doi.org/10.1016/j.jaip.2017.05.006
12. Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, Hourihane JO, et al.; PALISADE
Group of Clinical Investigators. AR101 Oral Immunotherapy for Peanut Allergy. N Engl
J Med. 2018 Nov;379(21):1991–2001. doi: https://doi.org/10.1056/NEJMoa1812856
13. de Silva D, Rodríguez Del Río P, de Jong NW, Khaleva E, Singh C, Nowak-Wegrzyn A,
et al.; GA2LEN Food Allergy Guidelines Group. Allergen immunotherapy and/or biologicals
for IgE-mediated food allergy: A systematic review and meta-analysis. Allergy. 2022 Jun;77(6):1852–62.
doi: https://doi.org/10.1111/all.15211
14. Loke P, Hsiao KC, Lozinsky A, et al. Probiotic and Peanut OIT leads to long-lasting
sustained unresponsiveness and quality-of-life improvement in peanut-allergic children. Authorea.
July 31, 2021. DOI: 10.22541/au.162769428.85138587/v1
15. Jones SM, Kim EH, Nadeau KC, Nowak-Wegrzyn A, Wood RA, Sampson HA, et al.; Immune
Tolerance Network. Efficacy and safety of oral immunotherapy in children aged 1-3
years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised
placebo-controlled study. Lancet. 2022 Jan;399(10322):359–71. doi: https://doi.org/10.1016/S0140-6736(21)02390-4
16. Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al.;
EAACI Allergen Immunotherapy Guidelines Group. EAACI Guidelines on allergen immunotherapy:
IgE-mediated food allergy. Allergy. 2018 Apr;73(4):799–815. doi: https://doi.org/10.1111/all.13319
17. Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy
in peanut-allergic preschool children is safe and highly effective. J Allergy Clin
Immunol. 2017 Jan;139(1):173–181.e8. doi: https://doi.org/10.1016/j.jaci.2016.05.027
18. Soller L, Abrams EM, Carr S, Kapur S, Rex GA, Leo S, et al. First Real-World Safety
Analysis of Preschool Peanut Oral Immunotherapy. J Allergy Clin Immunol Pract. 2019;7(8):2759–2767.e5.
doi: https://doi.org/10.1016/j.jaip.2019.04.010
19. Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy
for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety.
Lancet. 2019 Jun;393(10187):2222–32. doi: https://doi.org/10.1016/S0140-6736(19)30420-9
20. Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Koren Y, et al. Cashew oral
immunotherapy for desensitizing cashew-pistachio allergy (NUT CRACKER study). Allergy.
2022 Jun;77(6):1863–72. doi: https://doi.org/10.1111/all.15212
21. Erdle SC, Cook VE, Cameron SB, Yeung J, Kapur S, McHenry M, et al. Real-World Safety
Analysis of Preschool Tree Nut Oral Immunotherapy. J Allergy Clin Immunol Pract. 2023 Apr;11(4):1177–83.
doi: https://doi.org/10.1016/j.jaip.2023.01.031
22. Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW,
et al. Standardizing double-blind, placebo-controlled oral food challenges: American
Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology
PRACTALL consensus report. J Allergy Clin Immunol. 2012 Dec;130(6):1260–74. doi: https://doi.org/10.1016/j.jaci.2012.10.017
23. Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized
controlled study of peanut oral immunotherapy: clinical desensitization and modulation
of the allergic response. J Allergy Clin Immunol. 2011 Mar;127(3):654–60. doi: https://doi.org/10.1016/j.jaci.2010.12.1111
24. Tirumalasetty J, Barshow S, Kost L, Morales L, Sharma R, Lazarte C, et al. Peanut
allergy: risk factors, immune mechanisms, and best practices for oral immunotherapy
success. Expert Rev Clin Immunol. 2023;19(7):785–95. doi: https://doi.org/10.1080/1744666X.2023.2209318
25. Santos AF, Riggioni C, Agache I, Akdis CA, Akdis M, Alvarez-Perea A, et al. EAACI
guidelines on the diagnosis of IgE-mediated food allergy. Allergy. 2023 Dec;78(12):3057–76.
doi: https://doi.org/10.1111/all.15902
26. Fernández-Rivas M, Gómez García I, Gonzalo-Fernández A, Fuentes Ferrer M, Dölle-Bierke S,
Marco-Martín G, et al. Development and validation of the food allergy severity score.
Allergy. 2022 May;77(5):1545–58. doi: https://doi.org/10.1111/all.15165
27. R Core Team. R: A language and environment for statistical computing. [Internet].
Vienna, Austria; 2022. Available from: https://www.R-project.org
28. Nachshon L, Schwartz N, Levy MB, Goldberg MR, Epstein-Rigbi N, Katz Y, et al. Factors
associated with home epinephrine-treated reactions during peanut and tree-nut oral
immunotherapy. Ann Allergy Asthma Immunol. 2023 Mar;130(3):340–346.e5. doi: https://doi.org/10.1016/j.anai.2022.12.001
29. Wasserman RL, Hague AR, Pence DM, Sugerman RW, Silvers SK, Rolen JG, et al. Real-World
Experience with Peanut Oral Immunotherapy: Lessons Learned From 270 Patients. J Allergy
Clin Immunol Pract. 2019 Feb;7(2):418–426.e4. doi: https://doi.org/10.1016/j.jaip.2018.05.023
30. Guarnieri KM, Slack IF, Gadoury-Lévesque V, Eapen AA, Andorf S, Lierl MB. Peanut oral
immunotherapy in a pediatric allergy clinic: patient factors associated with clinical
outcomes. Ann Allergy Asthma Immunol. 2021 Aug;127(2):214–222.e4. doi: https://doi.org/10.1016/j.anai.2021.04.003
31. Blackman AC, Staggers KA, Anagnostou A. Anaphylaxis during Peanut Oral Immunotherapy:
looking beyond dose escalation. Pediatr Allergy Immunol. 2022 Dec;33(12):e13888. doi: https://doi.org/10.1111/pai.13888
32. Karunakaran D, Chan ES, Zhang Q, Bone JN, Carr S, Kapur S, et al. Risk factors associated
with safety of preschool peanut oral immunotherapy. J Allergy Clin Immunol Glob. 2023 Mar;2(2):100094.
doi: https://doi.org/10.1016/j.jacig.2023.100094
33. Sindher S, Long AJ, Purington N, Chollet M, Slatkin S, Andorf S, et al. Analysis of
a Large Standardized Food Challenge Data Set to Determine Predictors of Positive Outcome
Across Multiple Allergens. Front Immunol. 2018 Nov;9:2689. doi: https://doi.org/10.3389/fimmu.2018.02689
34. Nachshon L, Schwartz N, Tsviban L, Levy MB, Goldberg MR, Epstein-Rigby N, et al. Patient
Characteristics and Risk Factors for Home Epinephrine-Treated Reactions During Oral
Immunotherapy for Food Allergy. J Allergy Clin Immunol Pract. 2021 Jan;9(1):185–192.e3.
doi: https://doi.org/10.1016/j.jaip.2020.07.034
35. Virkud YV, Burks AW, Steele PH, Edwards LJ, Berglund JP, Jones SM, et al. Novel baseline
predictors of adverse events during oral immunotherapy in children with peanut allergy.
J Allergy Clin Immunol. 2017 Mar;139(3):882–888.e5. doi: https://doi.org/10.1016/j.jaci.2016.07.030
36. Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained
unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy.
J Allergy Clin Immunol. 2014 Feb;133(2):468–75. doi: https://doi.org/10.1016/j.jaci.2013.11.007
37. Lodge CJ, Waidyatillake N, Peters RL, Netting M, Dai X, Burgess J, et al. Efficacy
and safety of oral immunotherapy for peanut, cow’s milk, and hen’s egg allergy: A
systematic review of randomized controlled trials. Clin Transl Allergy. 2023 Jul;13(7):e12268.
doi: https://doi.org/10.1002/clt2.12268
38. Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy
and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009 Aug;124(2):292–300.
doi: https://doi.org/10.1016/j.jaci.2009.05.022
39. Barshow SM, Kulis MD, Burks AW, Kim EH. Mechanisms of oral immunotherapy. Clin Exp
Allergy. 2021 Apr;51(4):527–35. doi: https://doi.org/10.1111/cea.13824
Appendix: supplementary figures
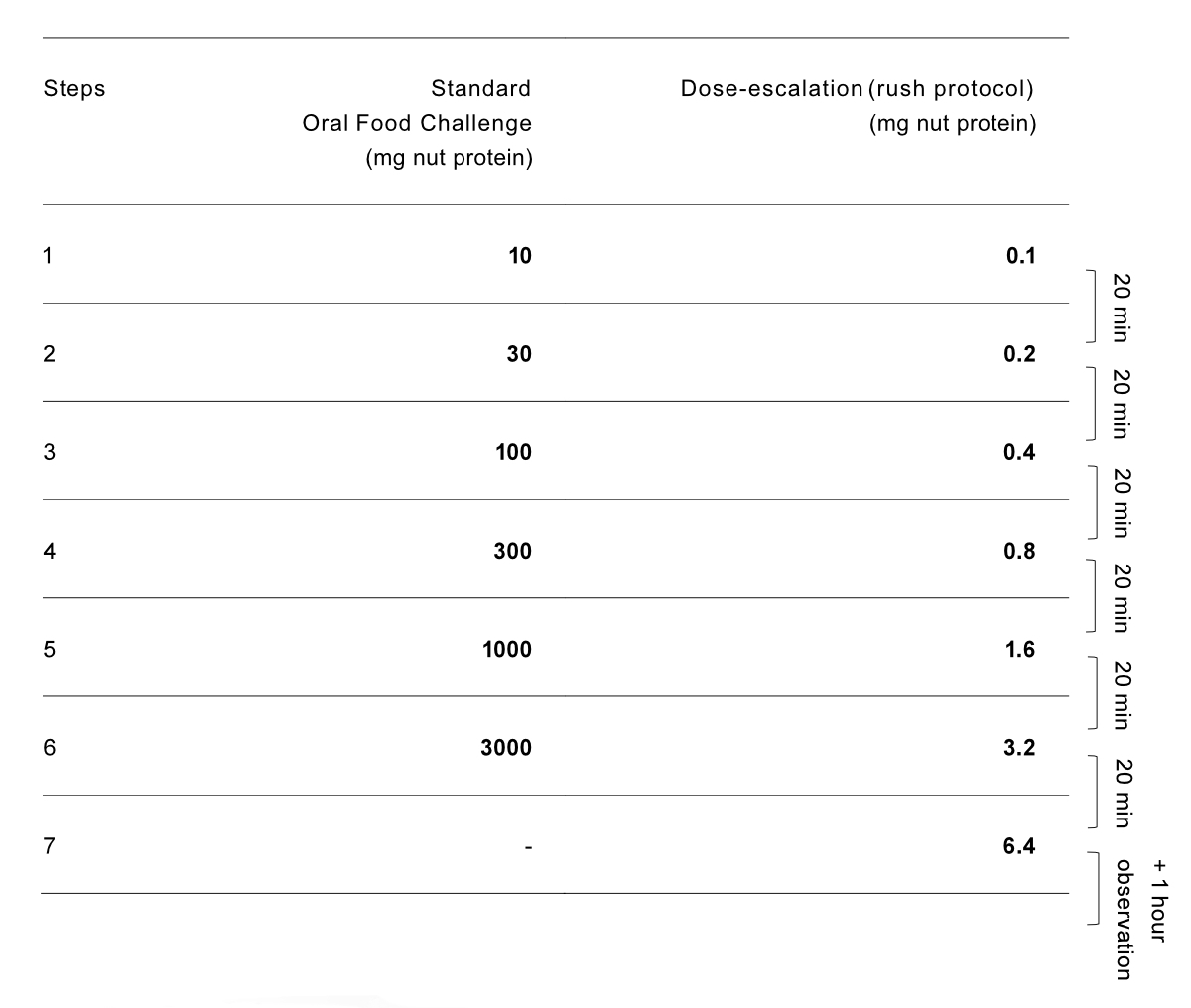
Figure S1The two starting protocols for nut oral
immunotherapy (OIT) at the outpatient clinic, conducted on 1 day. Single
highest tolerated dose or 6.4 mg was after eaten daily at home until first
up-dosing appointment.
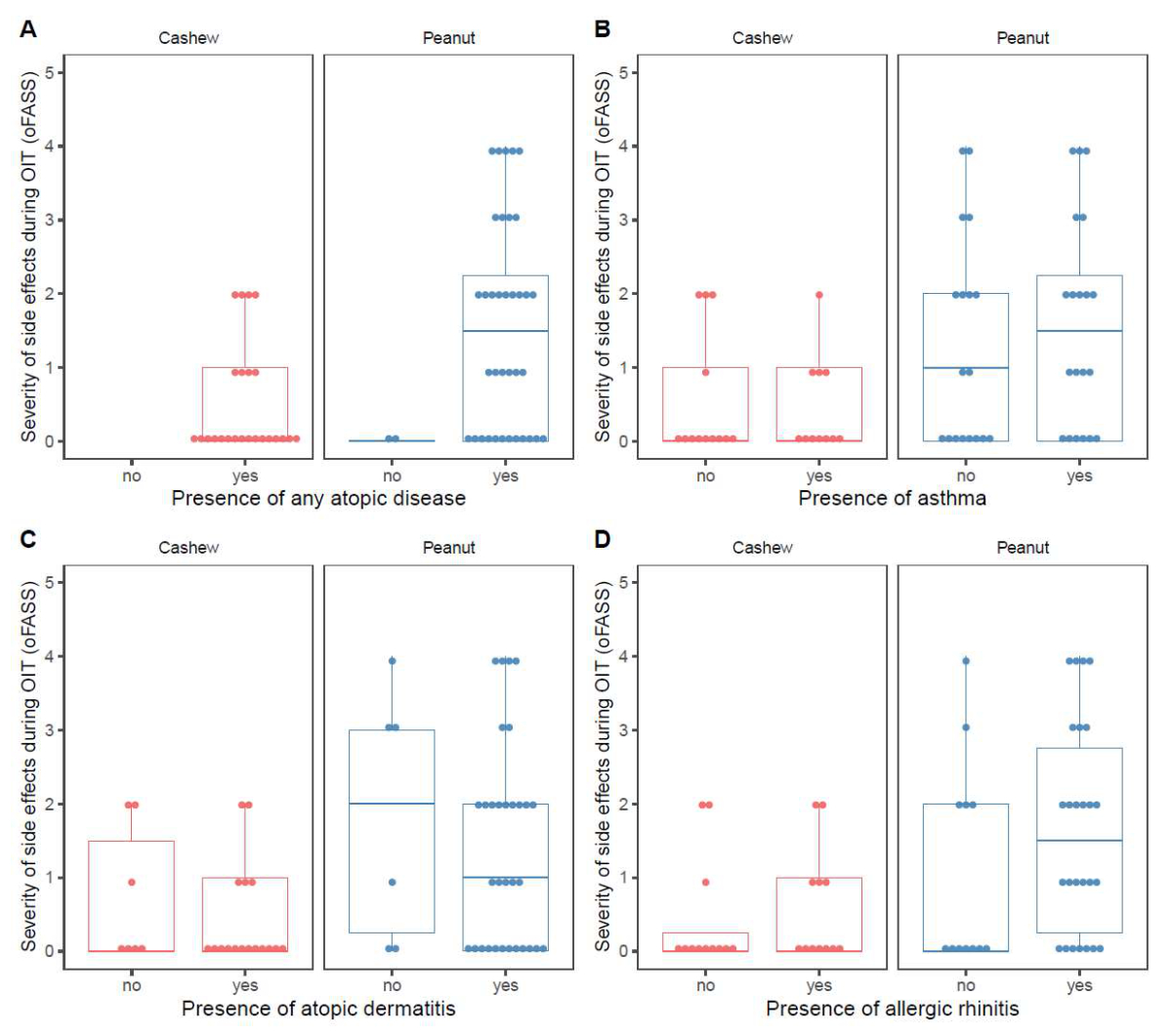
Figure S2(A) Maximum severity of adverse
reactions during oral immunotherapy (OIT) by nut allergy and separated by
presence of any atopic disease. (B)
Maximum severity of adverse reactions during oral immunotherapy by nut allergy
and separated by presence of asthma. (C) Maximum severity of adverse reactions during oral
immunotherapy by nut allergy and separated by presence of atopic dermatitis. (D)
Maximum severity of adverse reactions during oral immunotherapy by nut allergy
and separated by presence of allergic rhinitis. oFASS-5: ordinal food allergy severity
scale 5.
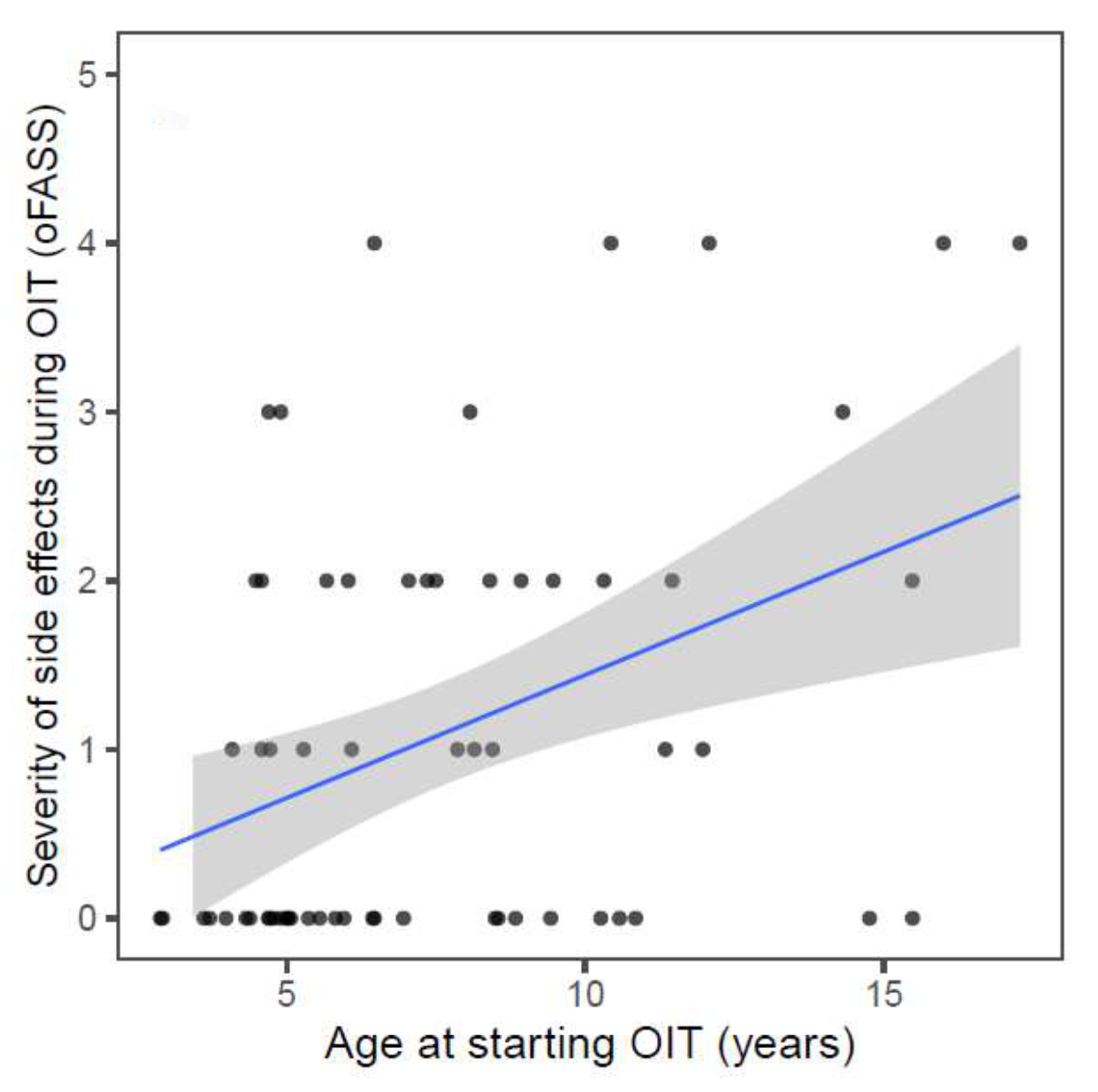
Figure S3Maximum
severity of side effects during oral immunotherapy (OIT) in relation to age at start
of the oral immunotherapy. R = 0,4; p = 0.001. oFASS-5:ordinal food allergy severity
scale 5.







