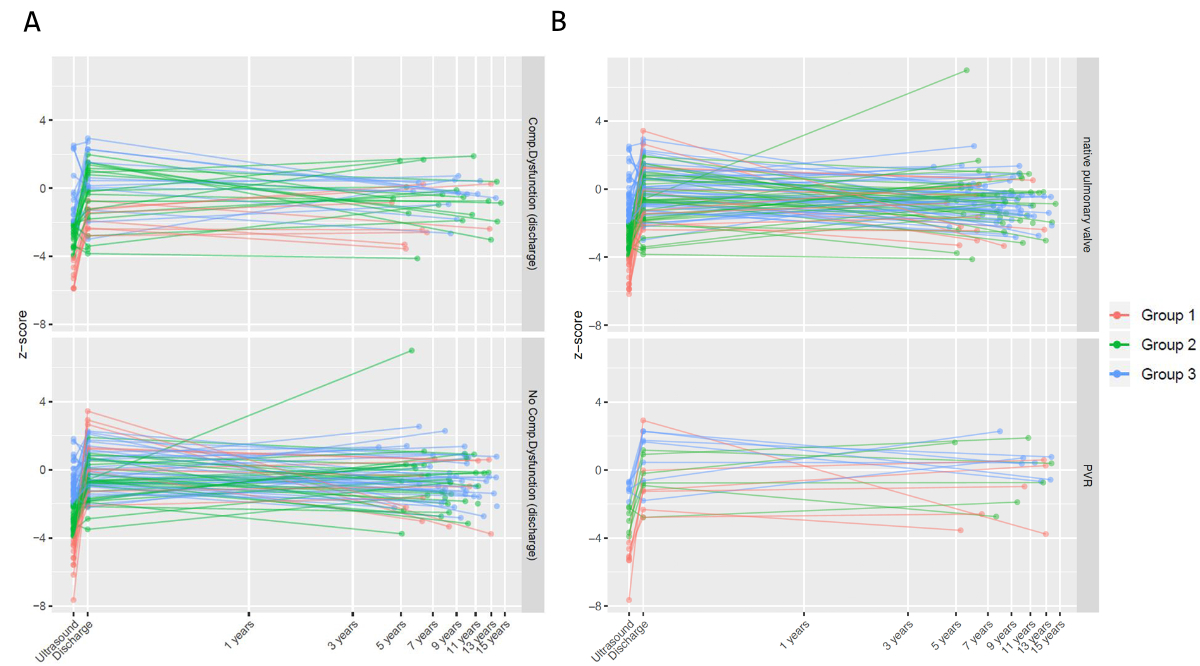
Figure 1Pulmonary annulus (PA) Z score over time. (A) Stratified by presence (above) or absence (below) of composite dysfunction at discharge. (B) Stratified by no pulmonary valve replacement (above) and need for pulmonary valve replacement (below).
DOI: https://doi.org/https://doi.org/10.57187/s.3689
Repair of tetralogy of Fallot has come a long way since Walton Lillehei performed the first repair in 1954 [1]. While survival of repaired of tetralogy of Fallot beyond the perioperative period is the norm, the focus has shifted to the residual disease load and its impact on quality of life and longevity [2]. Long-term survival is excellent; however, it is worse when compared to the normal population. The late mortality is reported to be around 0.29 per 100 patient-years; most attributed to heart failure and arrhythmias [3]. The pathophysiology underlying late mortality and sudden cardiac death in a large proportion of repaired tetralogies of Fallot can be traced back to chronic severe pulmonary regurgitation and arrhythmogenicity (dilated right ventricle, surgical scars, prolonged QRS duration). The same factors also contribute to morbidity manifesting as decreased exercise tolerance, interventions and need for medications [4].
Many surgical initiatives in the last decades have involved prevention of modifiable causes adversely affecting long-term outcome, such as operating in early infancy and use of the transatrial approach to contain right ventricular hypertrophy, myocardial fibrosis and surgical scars. Transannular patch (TAP) has appeared as a predictor of all-cause late outcome in many studies [3]. Thus, early strategies involving overwhelming enlargement of the right ventricular outflow tract (RVOT) have given way to valve- and annulus-sparing approaches while accepting some residual obstruction. However, clarity about the limits up to which a non-transannular patch (n-TAP) approach could be pushed is lacking. Consequences of residual gradients in real-life conditions in the long run, in contrast to influence of evolving pulmonary monocusp techniques [5], the unknowns about timing pulmonary valve replacement [6–8] have left the strategies for optimal repair of tetralogy of Fallot subject to individual interpretation. A middle path, striving for good pulmonary valve competence using pulmonary valve reconstructive techniques, while allowing a peak-to-peak gradient of about 20 mm Hg is gaining acceptance.
The echocardiographically derived preoperative pulmonary annular Z score [9] serves as an important guiding parameter in surgical planning, communication with the family and prognostication of the early postoperative course as well as the long-term outcome. There is little data in the literature examining the prognostic value of the pulmonary annulus Z score. This retrospective cohort study of tetralogy of Fallot repair reports a long-term primary outcome (pulmonary valve replacement and RVOT reoperations) as well as a secondary outcome (pulmonary valve dysfunction) with a focus on the body size-adjusted growth of the pulmonary annulus (Z score).
The study included 131 consecutive children undergoing tetralogy of Fallot and Double-Outlet Right Ventricle (Fallot type) repair at University Children’s Hospital Zurich, Switzerland, between 2004 and 2014. The rationale for the cohort selection was to include patients who had at least five years of follow-up. Pulmonary atresia, tetralogy of Fallot with atrioventricular septal defect (AVSD) and tetralogy of Fallot repair involving primary right ventricle to pulmonary artery conduit were excluded. Data parameters were retrieved from the hospital database or actively sought from the referring cardiologists. Data acquired included demographic data, palliation (where applicable), perioperative data during repair of tetralogy of Fallot, follow-up echo and catheter-based/surgical interventions. Follow-up was 98.5% complete. A retrospective tabularised Excel sheet is available and can be provided on demand; while the study has been registered and approved by the appropriate agency as stated below.
Since pulmonary annulus (PA) Z score > –2 is normal, this group was expected to undergo a non-transannular repair, while a PA Z score < –4 is likely to require a transannular repair. The intermediate group represents a grey zone where other factors such as number and quality of valve leaflets would determine the type of repair. This was the rationale for dividing the cohort into three groups stratified by the preoperative pulmonary annulus Z score.
Group 1: Z score < –4 (n = 20)
Group 2: Z score –4 to –2 (n = 57)
Group 3: Z score > –2 (n = 55)
Table 1 depicts the demographic features of the cohort.
Table 1Baseline characteristics. Groups stratified by preoperative pulmonary annulus Z score: group 1: Z < –4; group 2: –4 < Z ≤ –2; group 3: Z > –2.
| Baseline | Overall | Missing data in % | Group 1 | Group 2 | Group 3 | Standardised mean difference* | |
| n | 131 | 20 | 56 | 54 | |||
| Female sex – n (%) | 54 (41.2%) | 0.0% | 8 (40.0%) | 23 (41.1%) | 22 (40.7%) | 0.015 | |
| Diagnosis – n (%) | 0.0% | 0.335 | |||||
| Tetralogy of Fallot | 116 (88.5%) | 15 (75.0%) | 52 (92.9%) | 48 (88.9%) | |||
| DORV | 15 (11.5%) | 5 (25.0%) | 4 (7.1%) | 6 (11.1%) | |||
| No genetic disorder – n (%) | 107 (81.7%) | 0.0% | 18 (90.0%) | 50 (89.3%) | 38 (70.4%) | 0.339 | |
| Number of palliations – n (%) | 19 (14.5%) | 3 (15.0%) | 6 (10.7%) | 10 (18.5%) | 0.148 | ||
| Age at palliation in days – median (IQR) | 24.00 (9.00–39.00) | 0.0% | 9.00 (5.50–9.50) | 29.50 (9.00–35.75) | 31.50 (16.25–46.50) | 0.985 | |
| Weight at palliation in kg – median (IQR) | 3.10 (2.75–3.63) | 5.3% | 3.20 (3.04–3.20) | 2.85 (2.62–3.75) | 3.40 (2.90–3.70) | 0.08 | |
| Age at tetralogy of Fallot repair in months – median (IQR) | 4.77 (3.20–6.28) | 0.0% | 3.65 (2.54–5.47) | 4.65 (3.09–6.14) | 5.55 (3.62–6.62) | 0.353 | |
| Weight at tetralogy of Fallot repair in kg – median (IQR) | 6.13 (5.08–7.00) | 0.8% | 5.70 (4.84–6.60) | 6.20 (5.08–7.15) | 6.03 (5.10–6.80) | 0.262 | |
| Preop pulmonary annulus in mm – median (IQR) | 6.50 (5.50–8.15) | 0.8% | 4.25 (3.92–4.50) | 6.00 (5.71–6.78) | 8.40 (7.32–9.88) | 2.311 | |
| Preop pulmonary annulus Z score – median (IQR) | −2.18 (−3.48 – −1.23) | 0.8% | −5.18 (−5.65 – −4.44) | −2.92 (−3.45 – −2.32) | −1.04 (−1.54 – −0.28) | 3.234 | |
| Preop RPA in mm – median (IQR) | 5.20 (4.40–6.00) | 1.5% | 4.05 (3.40–5.08) | 5.05 (4.07–5.70) | 5.50 (5.00–6.50) | 0.446 | |
| Preop RPA Z score – median (IQR) | −0.56 (−1.67–0.32) | 1.5% | −1.65 (−2.67 – −0.76) | −0.72 (−2.29 – −0.01) | −0.16 (−0.82–0.70) | 0.575 | |
| Preop LPA in mm – median (IQR) | 5.00 (4.00–5.90) | 1.5% | 3.75 (3.22–4.75) | 4.85 (3.70–5.82) | 5.40 (4.50–6.00) | 0.557 | |
| Preop LPA Z score – median (IQR) | −0.47 (−1.40–0.53) | 1.5% | −1.95 (−2.35 – −0.50) | −0.72 (−1.69–0.39) | 0.05 (−0.68–1.33) | 0.654 | |
| RPA and/or LPA stenosis – n (%) | 25 (19.1%) | 0.0% | 6 (30%) | 11 (19.6%) | 8 (14.8%) | 0.467 | |
| Preop max RVOT gradient – median (IQR) | 80.00 (68.50–92.55) | 0.0% | 75.00 (63.20–90.25) | 86.50 (74.00–96.25) | 79.50 (63.25–90.00) | 0.425 | |
| Preop SaO2 – median (IQR) | 91.50 (83.25–97.00) | 0.8% | 90.00 (82.50–94.50) | 91.00 (82.00–97.00) | 92.50 (85.00–97.00) | 0.2 | |
| Number of pulmonary valve cusps – n (%) | 29.8% | 0.476 | |||||
| 1 leaflet | 3 (2.3%) | 0 (0.0%) | 1 (1.8%) | 2 (3.7%) | |||
| 2 leaflets | 70 (53.4%) | 11 (55.0%) | 32 (57.1%) | 27 (50.0%) | |||
| 3 leaflets | 19 (14.5%) | 1 (5.0%) | 5 (8.9%) | 13 (24.1%) | |||
| NA | 39 (29.8%) | 8 (40.0%) | 18 (32.1%) | 12 (22.2%) | |||
| Quality of pulmonary valve cusps – n (%) | 51.1% | 0.599 | |||||
| Normal pliable | 29 (22.1%) | 3 (15.0%) | 7 (12.5%) | 19 (35.2%) | |||
| Thickened less pliable | 13 (9.9%) | 1 (5.0%) | 6 (10.7%) | 6 (11.1%) | |||
| Dysplastic | 22 (16.8%) | 7 (35.0%) | 10 (17.9%) | 5 (9.3%) | |||
| NA | 67 (51.1%) | 9 (45.0%) | 33 (58.9%) | 24 (44.4%) | |||
| Transannular patch versus non-transannular patch – n (%) | 0.31 | ||||||
| Non-transannular | 43 (32.8%) | 4 (20.0%) | 18 (32.1%) | 21 (38.9%) | |||
| Transannular patch with monocusp | 26 (19.8%) | 6 (30.0%) | 11 (19.6%) | 9 (16.7%) | |||
| Transannular patch without monocusp | 62 (47.3%) | 10 (50.0%) | 27 (48.2%) | 24 (44.4%) | |||
| Duration of mechanical ventilation – median (IQR) | 2.00 (1.00–4.00) | 0.0% | 2.50 (1.00–5.00) | 2.50 (1.00–4.00) | 1.00 (1.00–2.75) | 0.345 | |
| Duration of ICU Stay – median (IQR) | 5.00 (3.00–7.00) | 0.0% | 7.50 (4.00–9.00) | 5.00 (4.00–7.00) | 4.50 (3.00–6.00) | 0.269 | |
| Duration of hospital stay postop – median (IQR) | 14.00 (11.00–21.00) | 0.0% | 19.00 (13.25–24.25) | 14.00 (11.00–18.25) | 13.00 (10.00–20.75) | 0.237 | |
| Pulmonary annulus at discharge in mm – median (IQR) | 8.00 (6.70–9.60) | 72.5% | 7.65 (6.60–8.40) | 7.55 (6.85–8.77) | 9.60 (8.45–9.65) | 0.28 | |
| Pulmonary annulus at discharge Z score – median (IQR) | −0.38 (−1.28–0.72) | 3.8% | −1.19 (−1.67–0.62) | −0.64 (−1.68–0.53) | 0.03 (−0.88–0.81) | 0.306 | |
| RPA at discharge Z score – median (IQR) | −0.31 (−1.36–0.60) | 8.4% | −0.59 (−1.69–0.17) | −0.32 (−1.73–0.99) | −0.13 (−0.70–0.61) | 0.349 | |
| LPA at discharge Z score – median (IQR) | 0.10 (−0.96–0.99) | 7.6% | −0.68 (−1.65–0.64) | −0.09 (−1.07–0.88) | 0.31 (−0.36–1.08) | 0.441 | |
| Max RVOT gradient at discharge – median (IQR) | 20.00 (14.00–27.75) | 0.8% | 23.00 (15.00–30.00) | 22.50 (16.75–32.00) | 15.50 (10.22–25.75) | 0.317 | |
| Pulmonary regurgitation at discharge – n (%) | 0.8% | 0.876 | |||||
| ≤mild | 67 (51.1%) | 4 (20%) | 27 (48.2%) | 35 (64.8%) | |||
| moderate | 43 (32.8%) | 10 (50.0%) | 16 (28.6%) | 17 (31.5%) | |||
| severe | 20 (15.3%) | 5 (25.0%) | 13 (23.2%) | 2 (3.7%) | |||
| NA | 1 (0.8%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | |||
| Composite dysfunction at discharge – n (%) | 0.8% | 0.347 | |||||
| No composite dysfunction | 88 (67.2%) | 11 (55.0%) | 36 (64.3%) | 40 (74.1%) | |||
| Presence of composite dysfunction | 42 (32.1%) | 8 (40.0%) | 20 (35.7%) | 14 (25.9%) | |||
| NA | 1 (0.8%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | |||
| Duration of follow-up – median (IQR) | 8.44 (6.32–10.84) | 1.5% | 6.44 (5.97–11.64) | 8.20 (6.13–10.68) | 9.15 (7.92–10.82) | 0.196 | |
DORV: double outlet right ventricle; LPA: left pulmonary artery; NA: Not available; RPA: right pulmonary artery.
* Standardised mean difference (SMD) is a measure of effect size. The higher the value of SMD, the more the groups differ. (It is calculated by taking the mean difference and dividing it by the standard deviation.) Some interpret SMD values of 0.2, 0.5 and 0.8 as small, medium and large imbalance. Groups with SMD estimates below 0.1 are considered more or less balanced.
The surgical strategy involved palliation when the child presented with blue spells during the first 6 weeks of life. Elective total correction was undertaken at around 6 months of age. Transatrial and transinfundibular was our preferred approach; ventricular septal defect (VSD) was closed conventionally. The pulmonary annulus Z score served as a guiding parameter for RVOT reconstruction. The aim was to achieve a Z score of about –2. A dysplastic valve or an additional stenosis at the site of ductus arteriosus / shunt insertion site weighed against a valve-sparing approach for borderline pulmonary annulus. If a transannular patch was necessary, a monocusp was created or not created based on surgeon preference. The infundibulotomy was routinely closed using a xenopericardial patch.
The primary outcome was defined as RVOT reoperation or pulmonary valve replacement (surgical/catheter interventional), whichever appeared first. Secondary outcome was composite pulmonary valve dysfunction defined as peak gradient >40 mm Hg or severe pulmonary regurgitation at follow-up.
For descriptive analyses, we report median and interquartile range (IQR) for continuous variables and count and percentage for categorical variables. The standardised mean difference (SMD) was calculated between all pulmonary annulus Z score groups and averaged across all pairwise comparisons between the three groups.
The difference in the rate of growth of QRS from discharge to follow-up between the three pulmonary annulus Z score groups was compared with a one-way test assuming non-equal variances [10]. Potential differences between pulmonary regurgitation at discharge and follow-up were investigated with the McNemar test with continuity correction.
Kaplan-Meier curves were used to visually investigate RVOT reoperations, catheter interventions and the primary endpoint between the three pulmonary annulus Z score groups. The log-rank test was used to quantify potential differences between the survival curves.
The Cox proportional hazards model for the primary endpoint was calculated using age at repair of tetralogy of Fallot in months, RVOT approach (TAP/n-TAP), composite dysfunction at discharge (yes/no) and pulmonary annulus Z score groups 1–3 as explanatory variables assuming random censoring. The decision to include these variables was based on clinical knowledge and a consensus among clinical authors. Proportional hazard assumption was checked with Schoenfeldt residuals and a test for proportional hazard assumption by Grambsch and Therneau [11].
No adjustment for multiple testing was done and no subgroup analysis was conducted. Complete case analysis was performed assuming missingness completely at random for follow-up and baseline variables.
The study was approved by the Cantonal Ethics Committee of Zurich, Switzerland vide BASEC-Nr. 2017-01321. Need for consent was waived.
Table 1 details the baseline demographic and perioperative data while table 2 documents the long-term outcomes.
Table 2Long-term outcome data. Groups stratified by preoperative pulmonary annulus Z score: group 1: Z < –4; group 2: –4< Z ≤–2; group 3: Z > –2.
| Long-term outcome | Overall | Missing data in % | Group 1 | Group 2 | Group 3 | Standardised mean difference* | |
| n | 131 | 20 | 56 | 54 | |||
| Age at follow-up in years – median (IQR) | 9.01 (6.78–11.50) | 1.5% | 6.82 (6.28–12.10) | 8.63 (6.51–11.28) | 9.96 (8.39–11.13) | 0.221 | |
| Weight at follow-up in kg – median (IQR) | 27.50 (20.50–34.58) | 9.9% | 22.50 (20.00–33.00) | 26.60 (19.75–33.00) | 31.00 (22.70–36.00) | 0.165 | |
| Pulmonary annulus at follow-up – median (IQR) | 16.00 (14.00–19.00) | 10.7% | 13.50 (12.00–17.50) | 16.00 (14.00–19.00) | 17.00 (16.00–20.00) | 0.511 | |
| Pulmonary annulus Z score at follow-up – median (IQR) | −0.67 (−1.83–0.30) | 12.2% | −1.92 (−2.91–0.09) | −0.67 (−1.64–0.24) | −0.57 (−1.42–0.40) | 0.455 | |
| RPA Z score at follow-up – median (IQR) | −0.54 (−1.47–0.51) | 16.0% | −0.78 (−2.14–0.45) | −0.39 (−1.20–0.71) | −0.69 (−1.32–0.49) | 0.171 | |
| LPA Z score at follow-up – median (IQR) | 0.34 (−0.57–1.09) | 16.8% | 0.34 (−1.21–0.60) | 0.35 (−0.46–1.16) | 0.08 (−0.56–0.91) | 0.130 | |
| Max RVOT gradient at follow-up – median (IQR) | 17.00 (10.75–24.00) | 8.4% | 23.00 (20.00–37.50) | 17.00 (13.00–22.00) | 12.00 (8.00–20.00) | 0.653 | |
| Pulmonary regurgitation at follow-up – n (%) | 9.9% | 0.563 | |||||
| ≤ mild | 55 (42%) | 7 (35%) | 21 (37.5%) | 26 (48.2%) | |||
| moderate | 27 (20.6%) | 4 (20.0%) | 18 (32.1%) | 5 (9.3%) | |||
| severe | 36 (27.5%) | 8 (40.0%) | 14 (25.0%) | 14 (25.9%) | |||
| NA | 13 (9.9%) | 1 (5.0%) | 3 (5.4%) | 9 (16.7%) | |||
| Composite dysfunction at follow-up – n (%) | 10.7% | 0.432 | |||||
| No composite dysfunction | 78 (59.5%) | 10 (50.0%) | 37 (66.1%) | 30 (55.6%) | |||
| Presence of composite dysfunction | 39 (29.8%) | 9 (45.0%) | 16 (28.6%) | 14 (25.9%) | |||
| NA | 14 (10.7%) | 1 (5.0%) | 3 (5.4%) | 10 (18.5%) | |||
| Catheter intervention at follow-up – n (%) | 3.1% | 0.393 | |||||
| Catheter intervention | 25 (19.1%) | 6 (30.0%) | 11 (19.6%) | 8 (14.8%) | |||
| NA | 4 (3.1%) | 0 (0.0%) | 0 (0.0%) | 4 (7.4%) | |||
| RVOT, native or replaced – n (%) | 3.8% | 0.466 | |||||
| Replaced | 20 (15.3%) | 7 (35.0%) | 6 (10.7%) | 7 (13.0%) | |||
| Native | 106 (80.9%) | ||||||
| NA | 5 (3.8%) | 0 (0.0%) | 2 (3.6%) | 3 (5.6%) | |||
| Operation other than pulmonary valve replacement – n (%) | 0.0% | 0.542 | |||||
| No reoperation | 115 (87.8%) | 16 (80.0%) | 49 (87.5%) | 50 (92.6%) | |||
| RVOT | 5 (3.8%) | 1 (5.0%) | 0 (0.0%) | 3 (5.6%) | |||
| PM-implantation | 5 (3.8%) | 1 (5.0%) | 3 (5.4%) | 1 (1.9%) | |||
| Diaphragm plication | 2 (1.5%) | 0 (0.0%) | 2 (3.6%) | 0 (0.0%) | |||
| Pleurodesis | 1 (0.8%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | |||
| Others | 3 (2.3%) | 1 (5.0%) | 2 (3.6%) | 0 (0.0%) | |||
| Preop QRS duration in ms – median (IQR)* | 64.00 (60.00–68.00) | 11.5% | 63.00 (60.00–74.00) | 64.00 (56.50–68.00) | 66.00 (60.00–68.00) | 0.326 | |
| Discharge QRS in ms – median (IQR)* | 96.00 (76.00–102.00) | 10.7% | 100.00 (94.00–110.00) | 92.00 (72.00–102.00) | 96.00 (79.00–102.00) | 0.403 | |
| Follow-up QRS in ms – median (IQR)* | 120.00 (94.00–134.00) | 13.7% | 130.00 (124.00–150.00) | 120.00 (89.00–131.00) | 118.00 (95.50–130.00) | 0.452 | |
| Follow-up to discharge QRS slope – median (IQR) | 2.30 (1.29–3.71) | 20.6% | 3.59 (1.47–5.25) | 2.69 (1.60–3.76) | 1.93 (0.87–2.89) | 0.464 | |
LPA: left pulmonary artery; ms: milliseconds; RPA: right pulmonary artery.
Standardised mean difference (SMD) is a measure of effect size. The higher the value of SMD, the more the groups differ. (It is calculated by taking the mean difference and dividing it by the standard deviation.) Some interpret SMD values of 0.2, 0.5 and 0.8 as small, medium and large imbalance. Groups with SMD estimates below 0.1 are considered more or less balanced.
* see appendix figure S1.
Modified Blalock Taussig Shunt, RVOT patch or RVOT / Persistent Ductus Arteriosus Botalli stent palliation was performed in 19 (15%) children (15%, 11% and 19% in groups 1, 2 and 3, respectively). Details are enumerated in table 1. All of them survived the total correction.
Due to the retrospective character of the data harvest, information about the number and quality of native pulmonary leaflets was available only in 64 cases, thus precluding a statistical analysis. On descriptive analysis, 36 (55%) leaflets were documented as thickened, dysplastic and less pliable. The prevalence of abnormal leaflets was highest in group 1 and lowest in group 3. The number of pulmonary valve leaflets was evenly spread across groups.
Overall a transannular patch was used in 88 (67.2%) children; in 16 (80%), 38 (67.9%) and 33 (61.1%) children in groups 1, 2 and 3, respectively. While 62/131 (47.3%) received a simple transannular patch, 26 (19.8%) received a transannular patch with monocusp.
There were no perioperative deaths. The duration of ventilation and ICU stay were longer in group 1, but there was no evidence that the hospital stay differed between groups (table 1). One patient received a pacemaker due to congenital complete AV block; 4 (2.3%) patients received a pacemaker due to a iatrogenic complete AV block post-repair of tetralogy of Fallot.
The pulmonary annulus Z score normalised at discharge: median –1.2 (IQR –1.7–0.6), –0.6 (–1.7–0.5) and 0.03 (–0.9–0.8) for groups 1, 2 and 3, respectively. The preoperative distinctly smaller Z scores of right pulmonary artery and left pulmonary artery between groups normalised after total correction. The left pulmonary artery Z score remained borderline smaller in group 1 compared to the rest (table 1). The repair involved correction of side branch stenosis in 25 (19%) children. The postoperative peak RVOT gradient was a median of 20 (14–28) mm Hg. It was greater in group 1 >2 >3, with a standardised mean difference of 0.32 (an SMD below 0.1 indicates very balanced data). Severe pulmonary regurgitation at discharge was noted in 20 patients (15%): 25%, 23% and 4% in groups 1, 2 and 3, respectively. Composite dysfunction at discharge occurred in 42 patients (32%): 40%, 36% and 26% in groups 1, 2 and 3, respectively.
All patients were alive at a median follow-up of 9.6 (95% confidence interval [CI] 9–10.4) years. None of the patients suffered from severe left ventricular or right ventricular dysfunction at follow-up (table 2). Univariate analysis of long-term outcome data compared between groups are depicted in table 2.
The median weight of the cohort increased from 6.1 (IQR 5.1–7) kg at operation to 28 (21–35) kg at follow-up. The median pulmonary annulus, right pulmonary artery and left pulmonary artery Z scores remained larger than –2; that is they grew proportionately with the growth of the child and did not differ between groups (table 2). Figure 1 (A–B) demonstrates the evolution of pulmonary annulus Z score on a log scale of time.

Figure 1Pulmonary annulus (PA) Z score over time. (A) Stratified by presence (above) or absence (below) of composite dysfunction at discharge. (B) Stratified by no pulmonary valve replacement (above) and need for pulmonary valve replacement (below).
Composite dysfunction at follow-up was prevalent in 31% (compared to 32% at discharge). Figure 2 depicts distribution of composite dysfunction at discharge, as well as its evolution over a log scale of time.
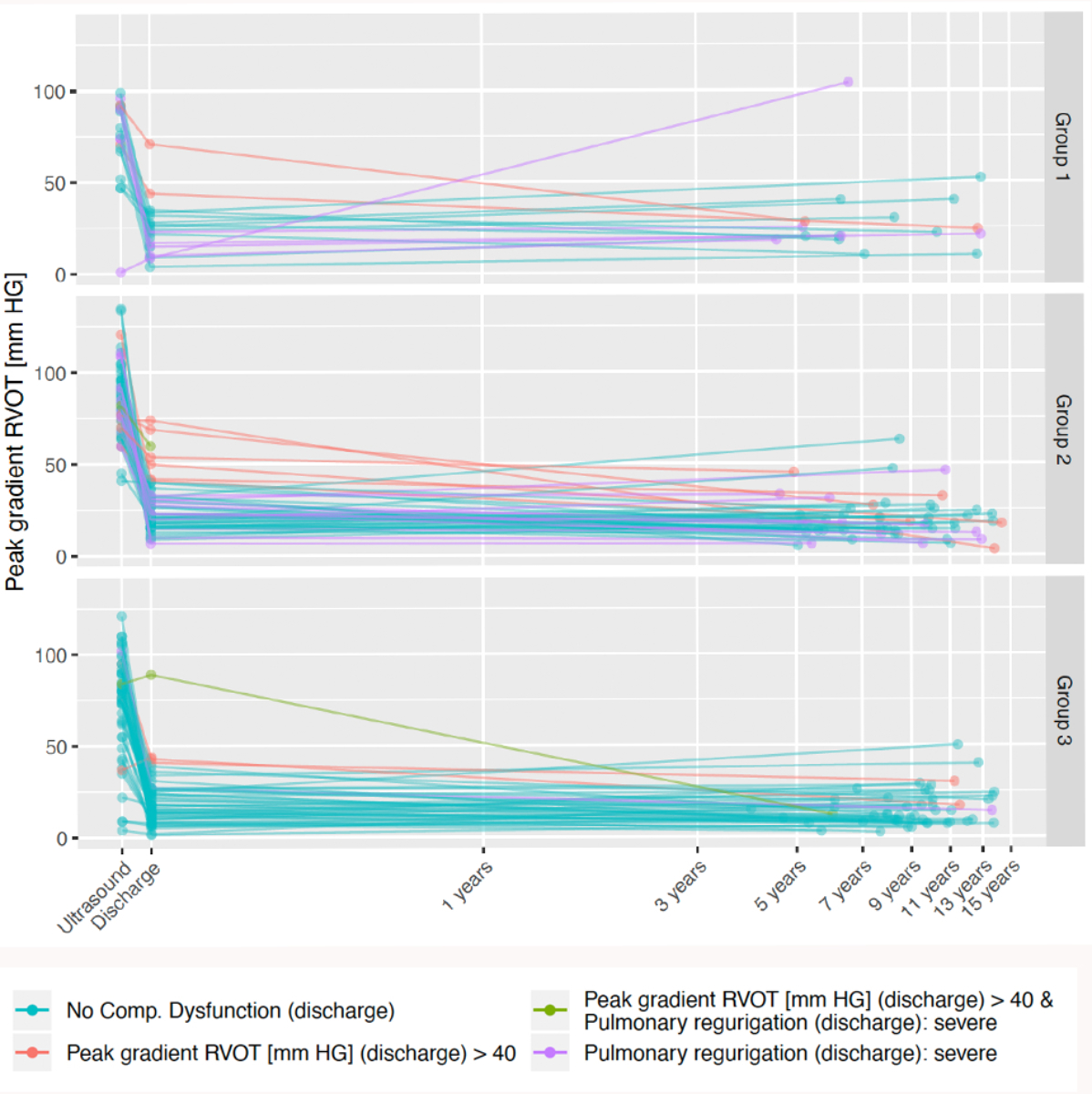
Figure 2Distribution of composite dysfunction at discharge between groups 1–3 over a log of time. Please note the colour distribution demonstrating various types of composite dysfunction (below in the legends). RVOT: right ventricular outflow tract.
The time of discharge was considered time point 0. The three patients who had an event before discharge were deleted from this analysis. When event rate is so low so as not to reach the 50% mark, median event time cannot be estimated; this was the case for many of the outcomes addressed in this study.
Overall median follow-up time for primary outcome was 9.6 (95% CI 9–10.4) years, median event time for group 1 was 12.3 (95% CI 7.3 to xx) years (upper bound of CI does not exist and median event time for group 2 and 3 does not exist). Freedom from primary outcome at 10 years was 69% (46–100%), 91% (82–100%) and 84% (74–95%) for groups 1, 2 and 3, respectively (log-rank p = 0.16), see figure 3.
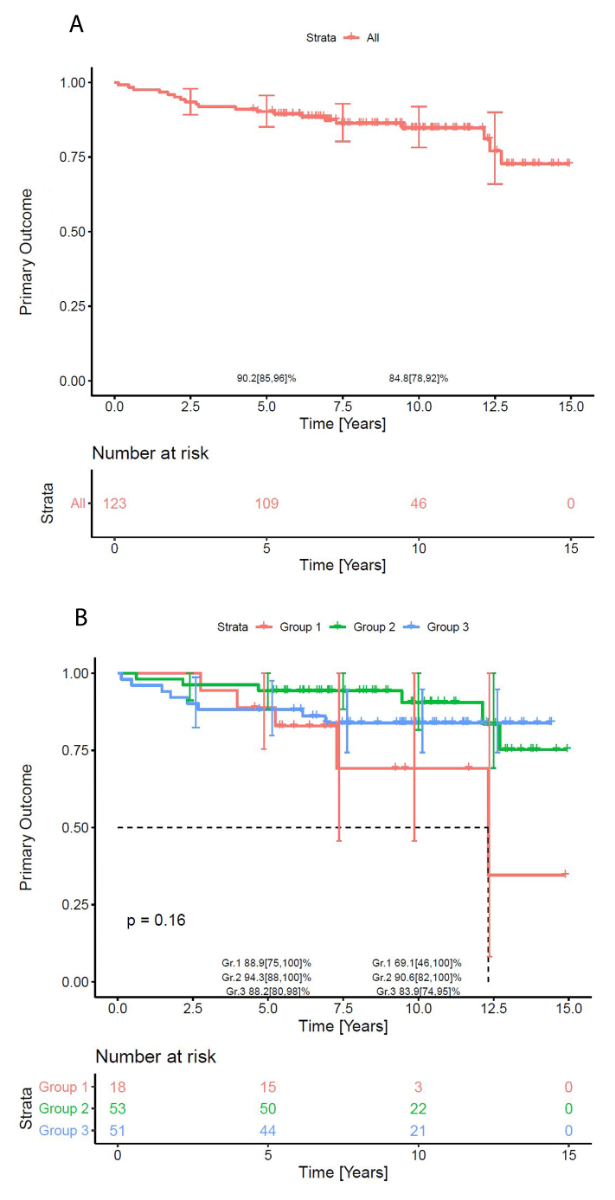
Figure 3Freedom from primary endpoint (right ventricular outflow tract reoperation or pulmonary valve replacement). (A) Overall. (B) Stratified according to pulmonary annulus Z score groups.
Overall median follow-up time for catheter intervention was 9.5 (95% CI 9–10.3) years. Median event time for group 1 was 12.8 (95% CI 7.4 to xx) years (upper bound of CI does not exist and median event time does not exist for other groups). 10-year freedom from catheter intervention was 54% (27–100%), 81% (71–93%) and 83% (72–94%) for groups 1, 2 and 3, respectively (log-rank p = 0.2).
The multiple Cox regression model estimates the hazard ratio (HR) associated with the event for the primary endpoint. In detail, this model (figure 4) estimated the following associations:
(Interpretation assumes holding all the other variables constant.)
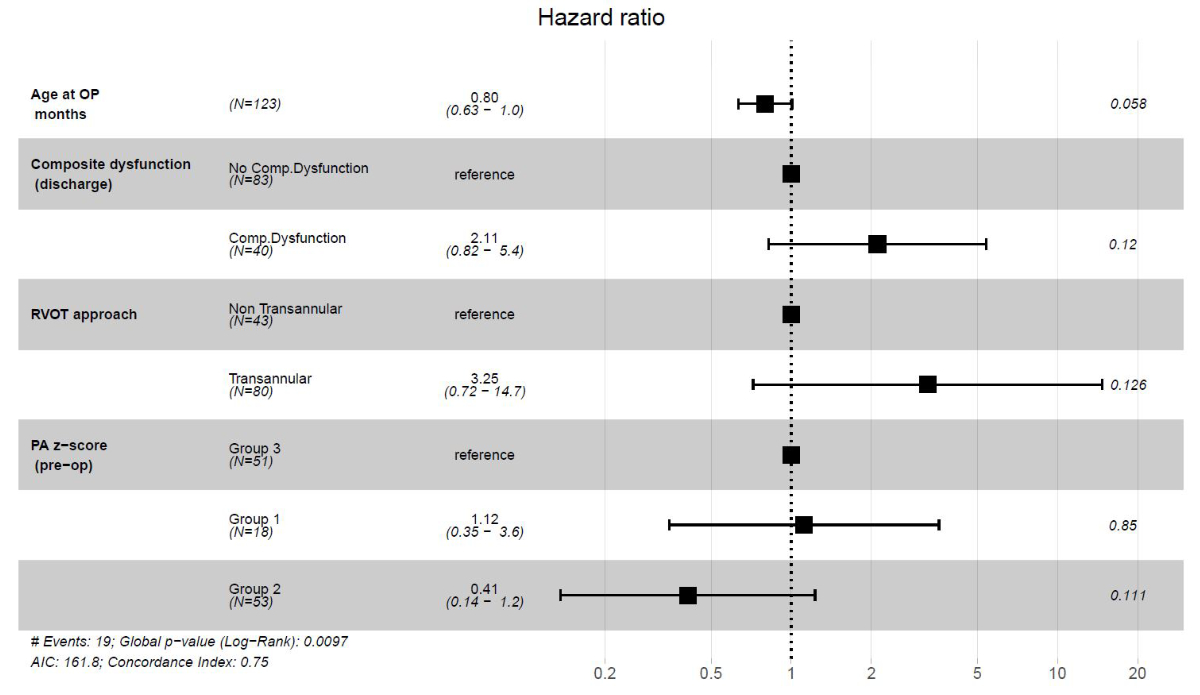
Figure 4Cox proportional-hazards model for primary endpoint (right ventricular outflow tract [RVOT] reoperation or pulmonary valve replacement). Cox proportional-hazards model for the primary endpoint was calculated using age at tetralogy of Fallot repair, RVOT approach (transannular patch versus non-transannular patch), presence or absence of composite dysfunction at discharge and pulmonary annulus (PA) Z score groups 1–3 as explanatory variables assuming random censoring.
With increasing numbers of patients with tetralogy of Fallot repair surviving to adulthood, the focus has naturally shifted to their long-term survival and wellbeing. Late death after repair of tetralogy of Fallot has been attributed to cardiac causes (predominantly sudden cardiac death and congestive cardiac failure) in around two thirds of cases [2]. With studies indicating that pulmonary regurgitation may indeed be responsible for many of the late consequences in repair of tetralogy of Fallot [12], pulmonary valve- or annulus-preserving techniques have flourished [13, 14]. The impact of these strategies on late outcome may only become evident after decades of follow-up [15]. Recent MRI data has demonstrated the presence of diffuse myocardial fibrosis in repair of tetralogy of Fallot, which may be triggered by extreme volume overload or pressure overload, in addition to those resulting from surgical insults (patches, stitching scars, etc.) [16]. The intensity of myocardial fibrosis is impacted by the duration and intensity of volume, pressure or mixed overload. The natural variations in the severity and type of RVOT obstruction make it difficult to envisage a one-size-fits-all approach. Few studies have explored surgical decision-making based on the pulmonary annulus Z score [17–19]. Our study adds to this body of data about the prognostic value of preoperative pulmonary annulus size in long-term follow-up of repaired tetralogy of Fallot.
A recent study by Bosch et al. has shown greater age and weight at tetralogy of Fallot repair to be associated with lower mortality [20]. It is logical that the more severe the RVOT obstruction, the earlier the child needs repair. Despite early age at repair in group 1 of our study, their body weight was comparable to those of groups 2 and 3. This could be explained by the inverse relationship between pulmonary and systemic output. Older age at repair showed a tendency to be protective (HR 0.8). Whether delaying tetralogy of Fallot repair beyond 6 months of age would help late outcome is open to debate. Any such decision should be tempered by the fact that operating too late may lead to increased right ventricular hypertrophy and diastolic dysfunction. Indeed, while some reports do plead for later repair [20], there is an evolving consensus that repair not be delayed beyond 12 months of age [21].
Preoperative RVOT gradient considerably differed between groups. Interestingly, despite greater severity of obstruction, the gradient was lower in group 1 compared to groups 2 and 3. This can be explained by presence of collateral flow or a Blalock Taussig shunt. This may also explain the similar incidence of palliation across groups. In fact, the need for palliation in group 3 with adequate pulmonary annulus may be explained by dynamic infundibular obstruction leading to blue spells. The Chicago group [19] showed that 50% of transannular patch patients (Z –4.8±1.7) as also 18% of non-transannular patch patients (Z –1.7±1.2) needed a shunt.
The use of transannular patch in the contemporary era may range from 5–88% [14, 17]. Interoperator variability may be considerable, with use of transannular patch ranging from 55–79% in our own cohort. Various surgical strategies using a Z score cut-off of –1.3 to –4 have been followed [17, 22]. While the pulmonary annulus Z score provides a rough guide, pulmonary valve morphology also plays an important role in decision-making. Since transannular patch repairs represent the prognostically worse group, a higher risk of reoperation is to be expected. Various groups [23, 24] have argued that even if the transannular patch carries a higher risk of reoperation, it has no impact on late survival. This is counterintuitive and needs further long-term data.
Vida et al. [25] have described complex pulmonary valvuloplasty including delamination and balloon dilation to achieve nearly 100% freedom from transannular patch (excluding the monocuspid pulmonary valves).
Logoteta et al. [26], while pursuing a valve-sparing strategy for pulmonary annulus Z score > –4, reported a transannular patch in 32% of patients, and reoperations in 14% at a median follow-up of 13 years. They demonstrated that MRI-diagnosed pulmonary regurgitation fraction was significantly less (21% patients) and the right ventricular mass not any greater than those in a controlled nationwide German registry.
Using an aggressive approach, Kim et al. [14] performed valve-sparing repair in 95% of repairs of tetralogy of Fallot; their freedom from significant RVOT obstruction was 83% at 1 year. In their experience, peak systolic right ventricular/left ventricular pressure ratio (pRV/LV) >0.59 had a high probability of developing RVOT obstruction; every 0.01 increase in pRV/LV being associated with a slightly increased hazard (HR 1.12).
Indeed, while the push for valve-sparing repair is understandable, Bacha et al. have warned about the limits of valve-sparing repair [27] citing freedom from reoperation of 82% at 1 year [14]. The Alabama group reported RV/LV pressure ratio of >0.85 to be associated with 2.5 times increased risk of death, and 7.3 times increased risk of reoperation [28]. Our pRV/LV ratio threshold of 0.65–0.7 and freedom from RVOT reoperation / pulmonary valve replacement of 84.8 (78–92) % at 10 years compares favourably with published reports.
With an incidence of transannular patch of 67% in our cohort, we have been more permissive compared to abovementioned groups, while being careful to avoid overzealous pulmonary annulus enlargement. Body surface area-proportional PA enlargement was achieved across the three groups and their growth has remained stable over a median follow-up of 10 years (figure 1). Early reoperations were seldom required. Also, perioperative outcome parameters in terms of duration of hospital stay did not differ between severity groups.
Variable time to reoperation, replacement and composite dysfunction can be attributed to the surgical strategy employed as also to the differences in native valve tissue available or to differences in reconstructive ability of the surgeon. The Osaka group [18], reporting results of their pulmonary annulus-sparing strategy, showed freedom from moderate-severe pulmonary regurgitation of 50% at 5 years and 36% at 20 years. Our freedom from moderate/severe pulmonary regurgitation of around 42% at 9 years compares well with the above study.
The functional outcomes of our cohort (figure 2) indicate that RVOT gradient and severe regurgitation at discharge contributed equally to late reoperations and replacements, indicating a balanced reconstructive strategy. Pulmonary valve function remained stable during 9+ years of follow-up, suggesting growth proportional to the 5-fold increase in the weight of the child. RVOT gradient was seldom the reason for reoperation or pulmonary valve replacement.
Most patients had less than moderate regurgitation. The deterioration to severe regurgitation in some can be attributed to the use of monocusp in only 29% of transannular patches; this suggests room for improvement.
While paediatric cardiac care consortium data found the transannular patch approach to be a hazard (HR 3.76) for mortality/transplant [29], we had no mortality in our cohort. However, multiple Cox regression showed transannular patch (HR 3.25), composite dysfunction at discharge (HR 2.11) and Z score group 1 (HR 1.12) to be associated with the primary outcome in our study. This reinforces the need to reduce composite dysfunction at discharge with all means at one’s disposal. The 10-year Kaplan-Meier freedom from primary outcome of 85 (78–92) % (with no evidence of difference between groups) compares well with 72% event-free survival from the Netherlands [20]. Logoteta et al. [26], pursuing an aggressive valve-sparing strategy, in contrast have reported excellent 10-year freedom from reoperation of 92%.
QRS duration [30] and fragmentation [31] have been proposed to be prognostic of ventricular arrhythmias in repair of tetralogy of Fallot [8]. Ventricular arrhythmias were found to occur in about 16% of subjects followed up for 30 years and correlated with QRS duration >160 ms. Marked fibrosis consequent to outflow stenosis as well as regurgitation is thought to underlie this finding. In our study, median QRS duration was 64 (60–68), 96 (76–102) and 120 (94–134) milliseconds preoperatively, postoperatively and at last follow-up, respectively. The median QRS duration was longer in group 1 and in patients with primary outcome (table 2). This progression should be seen in the context of the fact that QRS duration is in the range 70–85 ms in neonates and increases in a linear fashion to about 90–110 ms in adolescents [32]. Occurrence of right bundle branch block in repair of tetralogy of Fallot contributes to prolonged QRS in the first place. Correlating the slope of increase in QRS to morphology, surgical technique and late functional outcome would help place QRS duration in the right perspective. This was beyond the scope of the present study.
Comparing our outcomes with contemporary studies, a more aggressive valve-sparing approach using delamination, balloon dilation and leaflet augmentation is warranted; however, how small a pulmonary annulus Z score < –2 can be conserved is unclear. There is a worldwide tendency to set a ceiling of post-repair RV:LV pressure ratio of around 0.7. Pursuing an aggressive strategy may invite early reoperations. Even if this is acceptable, how high residual gradients can be accepted, and for how long a duration, remains unclear. Enough literature exists alluding to the damaging influence of chronic pulmonary regurgitation [6, 33]. Newer data, however, also suggests a possible damaging role of right ventricular afterload and mass. Geva et al. [34] from Boston, while investigating determinants of poor outcome post-pulmonary valve replacement, have demonstrated that right ventricular mass/volume ≥0.45 g/ml (HR 4.1), pulmonary valve replacement ≥28 years (HR 3.1) and right ventricular ejection fraction (RVEF) <40% (HR 2.4) are multivariable predictors of mortality and sudden cardiac death. Significantly, right ventricular systolic pressure ≥40 mm Hg (HR 3.4) also correlated with negative outcome. Latus et al. [35] in another MRI study have shown that while RVOT obstruction in the presence of pulmonary regurgitation appears to be protective with preserved RV strain and interventricular interaction, it is associated with decreased left ventricular strain and intraventricular synchrony. Peak RVOT gradient has also been proved to be a significant predictor of impaired exercise capacity [36]. While leaving mild residual gradients (peak-to-peak of around 20 mm Hg) could probably be advantageous [37], sound evidence should precede any idea of stretching these limits. Frigiola et al. [38] in their study characterising “good repair of tetralogy of Fallot” demonstrated that patients with repaired tetralogies of Fallot >35 years of age with normal exercise capacity, mild residual gradient and pulmonary annulus Z score <0.5 may probably be considered cured. It appears that the last word about an optimum RVOT strategy has yet to be written.
This study has all the limitations inherent to a retrospective cohort observational study. It involves heterogeneous morphology of tetralogy of Fallot patients involving multiple surgeons over a time frame of nearly two decades. It is obvious that surgical strategies and perioperative care may have undergone static change which may introduce biases. Retrospective data acquisition means that available data about various standard variables, e.g. pulmonary valve morphology, is limited. A longitudinal dataset of the evolution of ECG, echocardiographic and exercise test parameters is not available for each patient. These shortcomings make it difficult to draw substantive conclusions from the study.
With gradually evolving surgical techniques, even extreme forms of tetralogy of Fallot continue to enjoy a growth-appropriate functioning pulmonary valve in the majority of cases of repaired tetralogy of Fallot during the first decade of life. Use of transannular patch and composite dysfunction at discharge, although not statistically significant at 5% level, may be associated with need of RVOT reoperation and pulmonary valve replacement. Considering this, a more aggressive pulmonary annulus-sparing approach is justified. Applying valve reconstructive techniques to achieve a better composite dysfunction at discharge, if transannular patch is necessary, could reduce the primary outcome. If clinically permissible, tetralogy of Fallot repair at an older age but within the first year of life, may be considered. A middle path aiming at a mild residual gradient and mild regurgitation appears achievable and best suited for an event-free survival in repair of tetralogy of Fallot.
Presented at the 53rd Association of European Pediatric Cardiology Meeting 2019, Seville, Spain.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Warnes CA. The adult with congenital heart disease: born to be bad? J Am Coll Cardiol. 2005 Jul;46(1):1–8. doi: https://doi.org/10.1016/j.jacc.2005.02.083
2. Nollert G, Fischlein T, Bouterwek S, Böhmer C, Klinner W, Reichart B. Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol. 1997 Nov;30(5):1374–83. doi: https://doi.org/10.1016/S0735-1097(97)00318-5
3. Cuypers JA, Menting ME, Konings EE, Opić P, Utens EM, Helbing WA, et al. Unnatural history of tetralogy of Fallot: prospective follow-up of 40 years after surgical correction. Circulation. 2014 Nov;130(22):1944–53. doi: https://doi.org/10.1161/CIRCULATIONAHA.114.009454
4. Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Evans SP, Gatzoulis M, et al. Rationale and design of an International Multicenter Registry of patients with repaired tetralogy of Fallot to define risk factors for late adverse outcomes: the INDICATOR cohort. Pediatr Cardiol. 2013 Jan;34(1):95–104. doi: https://doi.org/10.1007/s00246-012-0394-5
5. Turrentine MW, McCarthy RP, Vijay P, McConnell KW, Brown JW. PTFE monocusp valve reconstruction of the right ventricular outflow tract. Ann Thorac Surg. 2002 Mar;73(3):871–9. doi: https://doi.org/10.1016/S0003-4975(01)03441-5
6. Therrien J, Siu SC, McLaughlin PR, Liu PP, Williams WG, Webb GD. Pulmonary valve replacement in adults late after repair of tetralogy of fallot: are we operating too late? J Am Coll Cardiol. 2000 Nov;36(5):1670–5. doi: https://doi.org/10.1016/S0735-1097(00)00930-X
7. Geva T. Tetralogy of Fallot repair: ready for a new paradigm. J Thorac Cardiovasc Surg. 2012 Jun;143(6):1305–6. doi: https://doi.org/10.1016/j.jtcvs.2012.01.076
8. Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995 Jul;92(2):231–7. doi: https://doi.org/10.1161/01.CIR.92.2.231
9. Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008 Aug;21(8):922–34. doi: https://doi.org/10.1016/j.echo.2008.02.006
10. Welch BL. On the Comparison of Several Mean Values - an Alternative Approach. Biometrika. 1951;38(3-4):330–6. doi: https://doi.org/10.1093/biomet/38.3-4.330
11. Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81(3):515–26. doi: https://doi.org/10.1093/biomet/81.3.515
12. Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005 Mar;95(6):779–82. doi: https://doi.org/10.1016/j.amjcard.2004.11.037
13. Rao V, Kadletz M, Hornberger LK, Freedom RM, Black MD. Preservation of the pulmonary valve complex in tetralogy of fallot: how small is too small? Ann Thorac Surg. 2000 Jan;69(1):176–9. doi: https://doi.org/10.1016/S0003-4975(99)01152-2
14. Kim DH, Lee JH, Choi ES, Park CS, Yun TJ. Optimal Pulmonary Valve Annulus Diameter for Annulus Preservation in Tetralogy of Fallot May Be Far Smaller Than Normal Annulus Size. Semin Thorac Cardiovasc Surg. 2019;31(2):253–63. doi: https://doi.org/10.1053/j.semtcvs.2018.10.014
15. Geva T. Diffuse Myocardial Fibrosis in Repaired Tetralogy of Fallot: Linking Pathophysiology and Clinical Outcomes. Circ Cardiovasc Imaging. 2017 Mar;10(3):e006184. doi: https://doi.org/10.1161/CIRCIMAGING.117.006184
16. Yim D, Riesenkampff E, Caro-Dominguez P, Yoo SJ, Seed M, Grosse-Wortmann L. Assessment of Diffuse Ventricular Myocardial Fibrosis Using Native T1 in Children With Repaired Tetralogy of Fallot. Circ Cardiovasc Imaging. 2017 Mar;10(3):e005695. doi: https://doi.org/10.1161/CIRCIMAGING.116.005695
17. Awori MN, Mehta NP, Mitema FO, Kebba N. Optimal Use of Z-Scores to Preserve the Pulmonary Valve Annulus During Repair of Tetralogy of Fallot. World J Pediatr Congenit Heart Surg. 2018 May;9(3):285–8. doi: https://doi.org/10.1177/2150135118757991
18. Hoashi T, Kagisaki K, Meng Y, Sakaguchi H, Kurosaki K, Shiraishi I et al. Long-term outcomes after definitive repair for tetralogy of Fallot with preservation of the pulmonary valve annulus. J Thorac Cardiovasc Surg 2014;148:802-8; discussion 08-9.
19. Stewart RD, Backer CL, Young L, Mavroudis C. Tetralogy of Fallot: results of a pulmonary valve-sparing strategy. Ann Thorac Surg. 2005 Oct;80(4):1431–8. doi: https://doi.org/10.1016/j.athoracsur.2005.04.016
20. van den Bosch E, Bogers AJ, Roos-Hesselink JW, van Dijk AP, van Wijngaarden MH, Boersma E, et al. Long-term follow-up after transatrial-transpulmonary repair of tetralogy of Fallot: influence of timing on outcome. Eur J Cardiothorac Surg. 2020 Apr;57(4):635–43. doi: https://doi.org/10.1093/ejcts/ezz331
21. Gerling C, Rukosujew A, Kehl HG, Tjan TD, Hoffmeier A, Vogt J, et al. Do the age of patients with tetralogy of fallot at the time of surgery and the applied surgical technique influence the reoperation rate? a single-center experience. Herz. 2009 Mar;34(2):155–60. doi: https://doi.org/10.1007/s00059-009-3169-x
22. Sinha R, Gooty V, Jang S, Dodge-Khatami A, Salazar J. Validity of Pulmonary Valve Z-Scores in Predicting Valve-Sparing Tetralogy Repairs-Systematic Review. Children (Basel). 2019 May;6(5):67. doi: https://doi.org/10.3390/children6050067
23. Bacha EA, Scheule AM, Zurakowski D, Erickson LC, Hung J, Lang P, et al. Long-term results after early primary repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001 Jul;122(1):154–61. doi: https://doi.org/10.1067/mtc.2001.115156
24. Ylitalo P, Nieminen H, Pitkänen OM, Jokinen E, Sairanen H. Need of transannular patch in tetralogy of Fallot surgery carries a higher risk of reoperation but has no impact on late survival: results of Fallot repair in Finland. Eur J Cardiothorac Surg. 2015 Jul;48(1):91–7. doi: https://doi.org/10.1093/ejcts/ezu401
25. Vida VL, Angelini A, Guariento A, Frescura C, Fedrigo M, Padalino M, et al. Preserving the pulmonary valve during early repair of tetralogy of Fallot: anatomic substrates and surgical strategies. J Thorac Cardiovasc Surg. 2015 May;149(5):1358–63.e1. doi: https://doi.org/10.1016/j.jtcvs.2015.01.030
26. Logoteta J, Dullin L, Hansen JH, Rickers C, Salehi Ravesh M, Al Bulushi A, et al. Restrictive enlargement of the pulmonary annulus at repair of tetralogy of Fallot: a comparative 10-year follow-up study. Eur J Cardiothorac Surg. 2017 Dec;52(6):1149–54. doi: https://doi.org/10.1093/ejcts/ezx143
27. Bacha EA. Increased Reinterventions After Valve-Sparing Tetralogy of Fallot Repair: Is It the Price to Pay? Semin Thorac Cardiovasc Surg. 2019;31(2):264–5. doi: https://doi.org/10.1053/j.semtcvs.2018.11.008
28. Katz NM, Blackstone EH, Kirklin JW, Pacifico AD, Bargeron LM Jr. Late survival and symptoms after repair of tetralogy of Fallot. Circulation. 1982 Feb;65(2):403–10. doi: https://doi.org/10.1161/01.CIR.65.2.403
29. Smith CA, McCracken C, Thomas AS, Spector LG, St Louis JD, Oster ME, et al. Long-term outcomes of tetralogy of Fallot: a study from the pediatric cardiac care consortium. JAMA Cardiol. 2019 Jan;4(1):34–41. doi: https://doi.org/10.1001/jamacardio.2018.4255
30. Bassareo PP, Mercuro G. QRS Complex Enlargement as a Predictor of Ventricular Arrhythmias in Patients Affected by Surgically Treated Tetralogy of Fallot: A Comprehensive Literature Review and Historical Overview. ISRN Cardiol. 2013;2013:782508. doi: https://doi.org/10.1155/2013/782508
31. Egbe AC, Miranda WR, Mehra N, Ammash NM, Missula VR, Madhavan M, et al. Role of QRS Fragmentation for Risk Stratification in Adults With Tetralogy of Fallot. J Am Heart Assoc. 2018 Dec;7(24):e010274. doi: https://doi.org/10.1161/JAHA.118.010274
32. Dickinson DF. The normal ECG in childhood and adolescence. Heart. 2005 Dec;91(12):1626–30. doi: https://doi.org/10.1136/hrt.2004.057307
33. Bouzas B, Kilner PJ, Gatzoulis MA. Pulmonary regurgitation: not a benign lesion. Eur Heart J. 2005 Mar;26(5):433–9. doi: https://doi.org/10.1093/eurheartj/ehi091
34. Geva T, Mulder B, Gauvreau K, Babu-Narayan SV, Wald RM, Hickey K, et al. Preoperative Predictors of Death and Sustained Ventricular Tachycardia After Pulmonary Valve Replacement in Patients With Repaired Tetralogy of Fallot Enrolled in the INDICATOR Cohort. Circulation. 2018 Nov;138(19):2106–15. doi: https://doi.org/10.1161/CIRCULATIONAHA.118.034740
35. Latus H, Hachmann P, Gummel K, Khalil M, Yerebakan C, Bauer J, et al. Impact of residual right ventricular outflow tract obstruction on biventricular strain and synchrony in patients after repair of tetralogy of Fallot: a cardiac magnetic resonance feature tracking study. Eur J Cardiothorac Surg. 2015 Jul;48(1):83–90. doi: https://doi.org/10.1093/ejcts/ezu396
36. Freling HG, Willems TP, van Melle JP, van Slooten YJ, Bartelds B, Berger RM, et al. Effect of right ventricular outflow tract obstruction on right ventricular volumes and exercise capacity in patients with repaired tetralogy of fallot. Am J Cardiol. 2014 Feb;113(4):719–23. doi: https://doi.org/10.1016/j.amjcard.2013.10.049
37. van der Hulst AE, Hylkema MG, Vliegen HW, Delgado V, Hazekamp MG, Rijlaarsdam ME, et al. Mild residual pulmonary stenosis in tetralogy of fallot reduces risk of pulmonary valve replacement. Ann Thorac Surg. 2012 Dec;94(6):2077–82. doi: https://doi.org/10.1016/j.athoracsur.2012.06.065
38. Frigiola A, Hughes M, Turner M, Taylor A, Marek J, Giardini A, et al. Physiological and phenotypic characteristics of late survivors of tetralogy of fallot repair who are free from pulmonary valve replacement. Circulation. 2013 Oct;128(17):1861–8. doi: https://doi.org/10.1161/CIRCULATIONAHA.113.001600
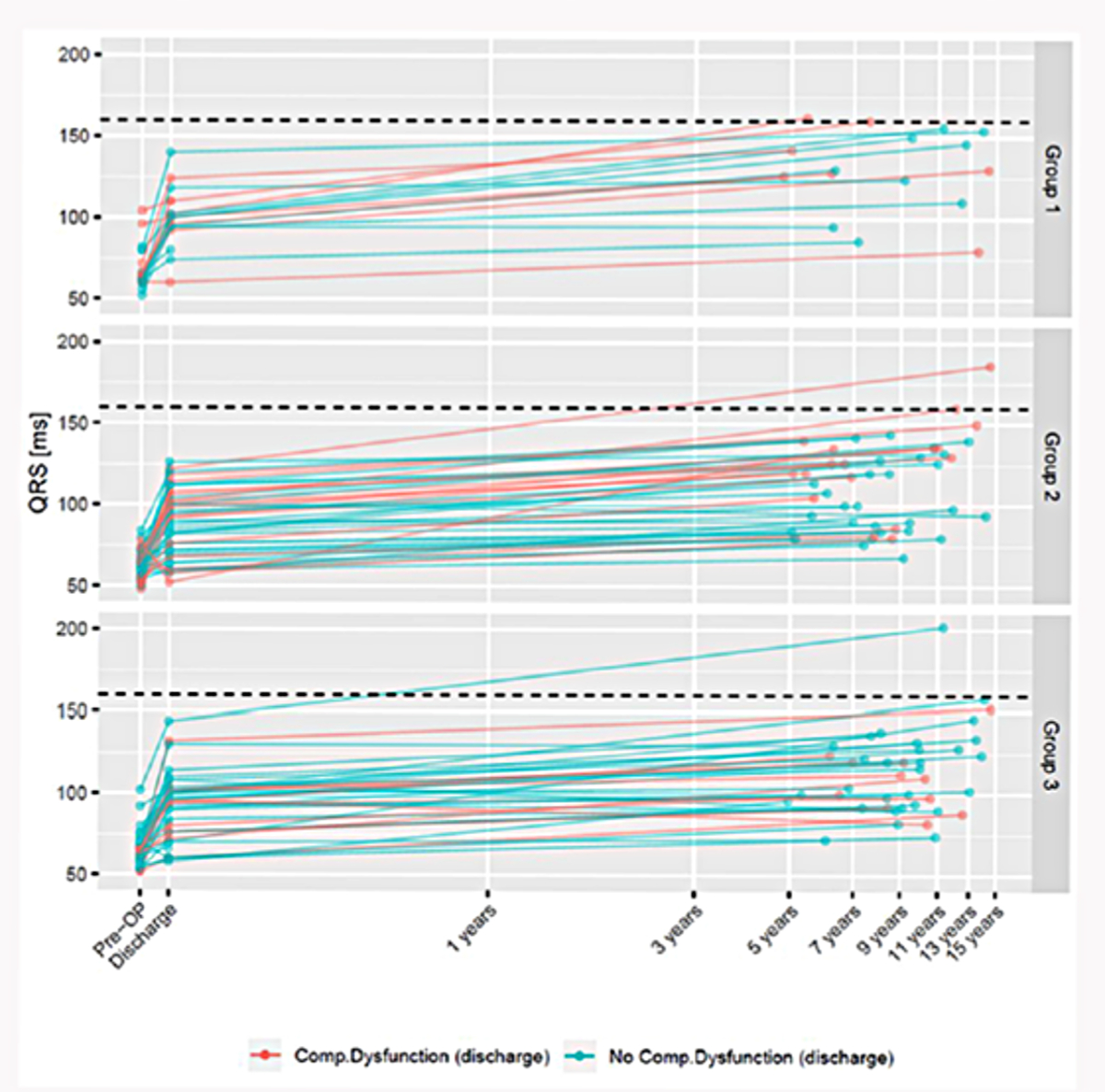
Figure S1Evolution of QRS duration in milliseconds (ms) over time stratified by groups. Red lines represent patients with composite dysfunction at discharge. Green lines represent patients not having composite dysfunction at discharge.